Abstract
Purpose
Although bronchoscopy has conventionally been performed using conscious sedation, advanced diagnostic techniques like endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA), peripheral EBUS, and electromagnetic navigational bronchoscopy add to procedural complexity. The adaptation of these techniques by bronchoscopists of varied backgrounds is expanding. It is not clear how patients will tolerate these advanced procedures when they are performed using traditional conscious sedation.
Methods
We prospectively studied patients that underwent diagnostic bronchoscopic procedures using conscious sedation over a 1-year period. The primary outcome was patient tolerability measured with four questions soliciting subjective responses. Secondary outcomes included required dosage of medications, thoroughness of the procedure, diagnostic yield, and occurrence of complications.
Results
A total of 181 patients were enrolled. Compared to patients in whom conventional bronchoscopy with transbronchial biopsies were performed, there was no difference in patient tolerability using the advanced techniques. Although some of the advanced procedures added to the procedure time, the required amount of medication was within commonly accepted dosages. When EBUS-TBNA was performed, a mean of 2.8 lymph node stations per patient were sampled. A specific diagnosis was obtained in 55.9 % of patients who solely underwent EBUS-TBNA. The diagnostic yield increased to 75.7 % when a parenchymal abnormality prompted additional biopsies. One patient required sedation reversal. Complications were minimal.
Conclusions
This study suggests that advanced diagnostic bronchoscopic procedures are well tolerated using conscious sedation with no compromise of thoroughness, diagnostic yield, or safety. This may be useful for bronchoscopists using these techniques who do not have ready access to general anesthesia.
Keywords: Bronchoscopy, Endobronchial ultrasound (EBUS), Navigational bronchoscopy
Introduction
The introduction of new technology has brought about a new era for diagnostic bronchoscopy. Prospective studies comparing types of sedation used during diagnostic bronchoscopic procedures are lacking, as are studies evaluating the tolerability of performing multiple advanced procedures in one session using only conscious sedation. The most recent consensus statement by the American College of Chest Physicians (ACCP) purports that “optimal procedural conditions are achieved when patients are comfortable, physicians are able to perform the procedure, and risk is minimized [1].” The combination of topical lidocaine, a benzodiazepine, and an opioid is commonly used. A recent multi-institutional prospective analysis using the ACCP Quality Improvement Registry, Evaluation and Education (AQuIRE) database demonstrated that only 36.8 % of convex probe endobronchial ultrasound-guided transbronchial needle aspiration (CP-EBUS TBNA) cases were performed using moderate sedation. This study also reported that the use of deep sedation or general anesthesia were associated with an increased probability of escalation of care [2]. A separate study evaluating the diagnostic yield of peripheral EBUS (pEBUS) demonstrated no significant difference in diagnostic yield for patients who underwent sedation with midazolam and fentanyl compared to propofol [3]. Recently, a study using propofol and intubation during electromagnetic navigational bronchoscopy (ENB) demonstrated a high yield but noted the lack of prospective studies comparing different sedation strategies [4].
Relative to single station lymph node sampling during bronchoscopy with TBNA, procedural time is increased in several common scenarios. These include when full mediastinal evaluation is performed, when trainees are involved, and when multiple diagnostic techniques are used. For instance, it has been shown that medication requirements and complications are increased when trainees participate in interventional pulmonary procedures [5]. Many physicians believe anecdotally that increased procedural time and complexity mandate general anesthesia to ensure either patient tolerability or procedural accuracy. It is in this context that we address whether it is feasible to thoroughly evaluate the lung parenchyma and mediastinum using advanced bronchoscopic techniques with only conscious sedation in an academic institution.
Methods
A prospective observational cohort study was conducted between December 2010 and December 2011 on all patients who were referred to the interventional pulmonary program at Yale-New Haven Hospital (YNHH) for diagnostic bronchoscopic procedures. Conventional transbronchial biopsies (TBBx) were performed using fluoroscopic guidance. Advanced techniques included CP-EBUS TBNA, pEBUS, and ENB.
YNHH is a tertiary care 1,000-bed urban, academic medical center. Patients were excluded if they declined enrollment in the study, were <18 years of age, or if they required other procedures during the bronchoscopy (tumor ablation, pleural procedures, etc.). The Institutional Review Board of Yale University School of Medicine approved this study. Consent was obtained from the patient or their designated surrogate if the patient was unable to consent.
Procedure
Bronchoscopy was performed using conscious sedation in our procedure center, which was staffed by an attending interventional pulmonologist, a nurse, a technician, and often a pulmonary fellow in-training. Two attending physicians trained in interventional pulmonary medicine performed all of the procedures in this study. The majority of the study patients had bronchoscopies performed by the last author of the manuscript. Up to ten fellows in-training also participated, although their level of participation was often limited to airway inspection, BAL, biopsies, and CP-EBUS sampling of one lymph node. For the sake of time, they did not perform sampling of all lymph nodes during the procedure. Our practice is to perform these procedures using the nurse to administer conscious sedation and monitor vital signs and the technician to assist with specimen processing. The nurse monitoring the patient typically advises when additional midazolam, fentanyl, or lidocaine are necessary due to patient discomfort or excess cough if not recognized by the physician. We do not use an anesthesiologist, medications requiring an anesthesiologist at our institution (propofol, traditional anesthetic agents), or ventilatory support during our procedures. Furthermore, although we may use oxygen via face mask during our procedures, we do not use non-invasive ventilatory support (CPAP, BiPap), intubation or laryngeal mask airways (LMA). Patients in this study were not selected to undergo one form of sedation over any other. The techniques described are our practice pattern.
The patient was brought into the bronchoscopy room and attached to a cardiac monitor and nasal cannula oxygen. The fellow, attending, nurse, and technician were present at all times the patient was in the room. Airway anesthesia was achieved with lidocaine using a combination of a laryngeal nerve block, spray atomizer, and direct topical administration. Intravenous medications including fentanyl, midazolam, and diphenhydramine were used to achieve sedation in a nurse-administered, physician-driven protocol described above. Diphenhydramine was only rarely administered as an adjunct when midazolam, fentanyl, and lidocaine failed to provide adequate comfort as indicated by persistent cough or agitation. Following a time-out for safety, the patient was administered 1 mg of midazolam. A laryngeal nerve block was performed by the attending pulmonologist by identifying the hyoid bone on both sides of the neck. In this location, the neck was cleaned with an alcohol swab and a 25 gauge needle was inserted approximately 1 cm in depth aiming toward the lateral edge of the hyoid bone. Following aspiration to ensure no vascular puncture, 1 ml of 1 % lidocaine was injected on each side. Thereafter, an atomizer was used to spray 1 % lidocaine into the back of the mouth and hypopharnx. Upon cessation of cough, McGill forceps were used to place a cotton ball soaked in 1 % lidocaine to the posterior pharynx until the patient’s gag reflex and cough were absent. The bronchoscope was then passed through the mouth with a bite block in place. A spray catheter (Olympus PW-6C-1) was used to administer atomized 1 % lidocaine at and below the vocal cords. A total of 6 ml was typically used at the vocal cords, followed by 4 ml in the trachea and 2 ml in each mainstem bronchi. If the patient developed an intolerable cough during the procedure, additional lidocaine or additional sedatives to regain comfort were administered. The total amount of lidocaine used included all of these doses. A complete bronchoscopic airway inspection was performed, followed by CP-EBUS TBNA of the mediastinal or hilar lymph nodes, with or without parenchymal biopsies and other advanced techniques. The tools we used included the CP-EBUS bronchoscope (Olympus BF-UC160F-0L8) with 22 g TBNA needle, flexible bronchoscope (Olympus BF-1T180), radial ultrasound probes (Olympus UM-S20-17S, UM-S20-20R, UM-BS20-26R), and other accessories (19 g TBNA needle, cytologic brush, biopsy forceps).
When EBUS-TBNA was performed, we consistently traversed the diameter of the lymph node 20–30 times using the “jabbing” method of aspiration and then rinsed the specimen into a cytologic preservative. The syringe was fully retracted to apply suction for the EBUS samples. This process was repeated for a total of three passes per lymph node station. For lung cancer staging, the N3 lymph node was biopsied first, followed by sampling of the lower stage lymph nodes. All nodes ≥5 mm were sampled, including those patients with isolated mediastinal lymphadenopathy. Rapid on-site cytology (ROSE) was not routinely used.
For parenchymal biopsies using pEBUS in which a nodule or mass is present, we typically performed a combination of forceps biopsy, brush biopsy, transbronchial needle aspiration (TBNA), and bronchoalveolar lavage (BAL) of the parenchymal lesion(s) using fluoroscopic guidance. When pEBUS was used, a metal ring was placed on the patient’s chest to identify the lesion targeted by the ultrasound probe. A guide sheath was used in less than five cases and only when the radial probe failed to easily reach the target. In these circumstances, the bronchoscope required significant rotation and turns into the bronchial tree. Rotational fluoroscopy was not performed as the operators felt pEBUS provided a more accurate location. A total of six forceps biopsies, one to two TBNA biopsies, one brush biopsy, and a BAL using 120 ml of saline were performed when a nodule or mass was present in the parenchyma. When an infiltrate or parenchymal changes such as presumed sarcoidosis or organizing pneumonia was the predominant indication, a BAL and transbronchial biopsies were performed. Diffuse changes did not require the use of pEBUS but did include a BAL and TBBx.
ENB was accomplished using the iLogic system from Superdimension. It is our practice to restrict the use of ENB to those cases in which the operator felt a low likelihood of diagnostic success would be present if using pEBUS alone. In these circumstances, lesions tended to be smaller, located peripherally, and did not have a straight airway leading directly to them. ENB was a new procedure for the primary operator, and thus a higher learning curve was present during this study. While the sheath was typically left in place after ENB guidance, a metal ring was placed on the patient’s chest when pEBUS was performed.
At the conclusion of the case, the patient was brought to the recovery bay. Vital signs were monitored for at least 1 h. If transbronchial biopsies were performed, a chest X-ray was performed at the bedside. Once the patient was awake with adequate oxygen saturations, he or she was discharged with an adult responsible for driving the patient home.
Data Collection
Demographic and historical information was obtained from the patient or their surrogate. Laboratory data, imaging, and pathology results were abstracted from medical records. Self-reported tolerability of the procedures was assessed by interviews conducted at 1-day post-procedure by telephone. These interviews were performed by a research assistant who was not part of the bronchoscopy team and who was blinded regarding which procedure had been performed or how much sedation was given. The research assistant received training in the conduct of phone interviews and was observed at scheduled intervals to assure reliability. We asked each patient to separately rate their subjective tolerability of the lidocaine administration, the bronchoscopic insertion, and the procedure as a whole, with a four point Likert response scale consisting of poorly, not well, well, and very well. For descriptive purposes, we tabulated the proportions of persons responding with either very well or well, and those of persons responding with either not well or poorly. A fourth question asked whether they would repeat the procedure with possible answers of no, unsure, and yes. The proportions of persons in the four procedural subgroups responding in each of the response categories were compared for all four questions with patients receiving TBBx-only serving as the reference.
The amount of time and medications required for completion of the procedure were also recorded. Timing of the procedure started when the bronchoscope first entered the mouth and ended when the bronchoscope was withdrawn at the conclusion of the case. It included any scope changes and all techniques performed throughout the exam. The timing did not include the numbing prior to the insertion of the bronchoscope. Thoroughness of the procedure was determined by assessing the number of lymph node stations biopsied, number of passes completed, and whether parenchymal sampling was performed. Yield was calculated based on whether a specific diagnosis was obtained as a result of the procedure. Lymphocytes obtained in nodes were not classified as a specific diagnosis but were routinely present and confirmed appropriate sampling. Examples of specific diagnoses included malignant cells, granulomas, and infectious organisms such as acid fast bacilli.
Safety was determined by assessing complications up to 24-h post-procedure. The procedures were grouped as sampling of lymph nodes (CP-EBUS TBNA) alone, or in combination with parenchymal biopsies using either pE-BUS or ENB. Patients undergoing fluoroscopically guided TBBx by the same operators served as a comparison group in our cohort.
Statistical Analysis
Patient characteristics from the three subgroups of advanced procedures were separately compared with those of patients who underwent TBBx alone. Continuous variables were tested with either a T test (normal variables) or a Wilcoxon rank-sum statistic (non-normal variables) and binary variables with a χ2 statistic. Subjective tolerance of each of three subgroups of advanced procedures was tested against that of the reference group using a χ2 statistic. In the same manner, the total amount of each of the four types of medications was tested, as were procedural duration and other procedural details. Finally, in order to test whether there were significant differences in overall tolerability between any of the three groups of advanced procedures, relative to the reference group of TBBx-only, we combined the scores of all four questions into a count scale with possible values ranging from 0 to 11. The first three questions were scored between counts of 0 and 3, for the responses poorly, not well, well, and very well, respectively. The last question regarding whether the patient would repeat the procedure was scored as either 0 (no), 1 (unsure), or 2 (yes). A multivariate Poisson model was used to test for significant differences, with adjustment for the covariates after adjustment for age, asthma, COPD, weight, sex, and history of cancer. All analysis was performed with SAS version 9.3 and a two-tailed P value ≤ 0.05 was considered to be statistically significant.
Results
We enrolled a total of 181 patients, of which 45 patients underwent TBBx-only and whom comprise the reference group against which all comparisons are made. The second group consisted of 104 patients, and is a combination of the 75 patients that underwent CP-EBUS alone and the 29 patients that underwent TBBx in addition to CP-EBUS. Because the addition of TBBx without pEBUS or ENB did not significantly change subjective tolerability, procedure time or the amount of medications used, we have combined these patients to form one group (106 patients). Patients who underwent additional parenchymal sampling with pEBUS (18 patients) and combined ENB and pEBUS (14 patients) form groups 3 and 4, respectively. All 136 patients undergoing advanced diagnostic procedures underwent CP-EBUS, out of which 64 patients also underwent additional parenchymal sampling. Indications for parenchymal sampling included a mass or nodule(s) and/or other parenchymal abnormalities. The indications for the procedure included a solitary nodule in 9 % of patients, a mass in 21 % of patients, multiple nodules or masses in 5 % of patients. The other patients had procedures to determine the nature of abnormal radiographs, including infectious and non-infectious parenchymal changes or adenopathy not related to masses or nodules. CP-EBUS was performed either for isolated mediastinal lymphadenopathy or lymphadenopathy associated with these parenchymal changes. Lymph nodes were not always enlarged (>1 cm) on radiographs, but nodes≥5 mm were typically biopsied. Patient characteristics are summarized in Table 1.
Table 1.
Participant characteristics (N = 181)
| TBBx-only n = 45 |
CP-EBUS only and EBUS with TBBx n = 104 |
CP-EBUS, pEBUS, and TBBx n = 18 |
CP-EBUS, ENB, pEBUS, and TBBx n = 14 |
|
|---|---|---|---|---|
| Demographics | ||||
| Age in years, mean (SD) | 58.5 (15.1) | 64.9 (14.9)* | 66.7 (9.0)* | 63.2 (11.0) |
| Male gender, n (%) | 17 (38) | 50 (48) | 6 (33) | 10 (71)* |
| Non-white race, n (%) | 8 (18) | 12 (12) | 1 (6) | 1 (7) |
| BMI, mean (SD) | 25.7 (5.8) | 26.5 (6.1) | 27.2 (6.3) | 25.6 (7.6) |
| Smoking status, n (%) | ||||
| Never | 10 (22) | 22 (21) | 3 (17) | 1 (7) |
| Current | 4 (9) | 18 (17) | 0 (0) | 7 (50)* |
| Former | 31 (69) | 64 (62) | 15 (83) | 6 (43) |
| Inpatient, n (%) | 24 (53) | 26 (25)* | 4 (22)* | 4 (29) |
| History of medical comorbidities, n (%) | ||||
| Asthma | 8 (18) | 7 (7) | 0 (0) | 2 (14) |
| Chronic obstructive pulmonary disease (COPD) | 7 (16) | 22 (21) | 5 (28) | 6 (43) |
| Sleep apnea | 2 (4) | 8 (8) | 2 (11) | 2 (14) |
| Cancer | 24 (53) | 66 (63) | 9 (50) | 6 (43) |
| Heart disease (includes MI, hypertension, hyperlipidemia) | 24 (53) | 67 (64) | 14 (78) | 10 (71) |
Demographic information and patient comorbidities for those undergoing bronchoscopic procedures in this cohort. Missing data: non-white race (n = 9), asthma (n = 1), COPD (n = 1), sleep apnea (n = 1), coronary artery disease (n = 1)
TBBx transbronchial biopsy using traditional flexible bronchoscopy, CP-EBUS convex probe endobronchial ultrasound, pEBUS peripheral EBUS; Transbronchial biopsy using pEBUS, ENB electromagnetic navigational bronchoscopy
Significant differences at 0.05 level, referent to TBBx-only. BMI and age tested with T test and all other variables with a χ2 statistic
The post-procedure interview for subjective tolerability was completed in 172 (95 %) out of 181 patients. Of those not completed (n = 9), two patients were intubated and sedated while three were otherwise unable to answer questions due to medical conditions or a lack of understanding of the questions asked. In this situation, consent for the procedure and study was obtained from the surrogate and data was predominantly collected for procedural data, such as number of stations sampled or diagnostic yield. In the group only undergoing TBBx, the proportion of positive responses (very well or well) to questions about lidocaine tolerance, scope insertion tolerance, and overall bronchoscopy tolerance were 82, 84, and 82 %, respectively. In this group, 78 % were willing to repeat the procedure if ever needed. For patients undergoing CP-EBUS with or without TBBx, the proportion of positive responses to the questions was 85, 90, and 85 %, and 88 % would agree to a repeat procedure. Of those who underwent CP-EBUS with pEBUS-guided parenchymal biopsies, 100, 100, and 94 % responded positively, and 94 % would agree to a repeat procedure. Of those who underwent CP-EBUS with ENB and pEBUS-guided parenchymal sampling, the proportion of positive responses was 93, 93, and 86 %. Of these, 79 % agreed to a repeat procedure, if needed. These results are summarized in Table 2. No procedure was terminated due to patient intolerability.
Table 2.
Tolerance of individual components of procedures under conscious sedation with laryngeal block
| Patient’s subjective response, n (%) | TBBx-only n = 45 |
CP-EBUS only and EBUS with TBBx n = 104 |
CP-EBUS, pEBUS, and TBBx n = 18 |
CP-EBUS, ENB, pEBUS, and TBBx n = 14 |
|---|---|---|---|---|
| How did you tolerate the medicine used to numb your throat for the procedure? | ||||
| Very well or well | 37 (82) | 88 (85) | 18 (100) | 13 (93) |
| Not well or Poorly | 4 (9) | 11 (10) | 0 (0) | 1 (7) |
| How did you tolerate the insertion of the bronchoscope? | ||||
| Very well or well | 38 (84) | 94 (90) | 18 (100) | 13 (93) |
| Not well or Poorly | 3 (7) | 5 (5) | 0 (0) | 1 (7) |
| How did you tolerate the actual bronchoscopy when the scope was in your lungs? | ||||
| Very well or well | 37 (82) | 88 (85) | 17 (94) | 12 (86) |
| Not well or Poorly | 4 (9) | 11 (11) | 1 (6) | 2 (14) |
| Would you agree to repeat procedure? | ||||
| Yes | 35 (78) | 92 (88) | 17 (94) | 11 (79) |
| No | 5 (11) | 3 (3) | 0 (0) | 2 (14) |
| Unsure | 2 (4) | 4 (4) | 0 (0) | 1 (7) |
Referent to TBBx-only, there were no significant differences at 0.05 level. All variables tested with χ2 statistic. Although represented as TBBx, additional techniques such as brush biopsies, peripheral TBNA or BAL were frequently included along with the TBBx
TBBx transbronchial biopsy using traditional flexible bronchoscopy, CP-EBUS convex probe endobronchial ultrasound, pEBUS transbronchial biopsy using peripheral EBUS guidance, ENB electromagnetic navigational bronchoscopy guidance for parenchymal biopsies
Table 3 summarizes the medication requirements, procedural duration, and lymph node sampling details. Medication requirements were within recommended guidelines and were similar among all groups, although the amount of midazolam increased with the addition of pE-BUS and ENB guidance to biopsy both the parenchyma and the lymph nodes. The mean procedure time for parenchymal sampling with TBBx was 33 min. The mean time for mediastinal and hilar lymph node sampling with CP-EBUS with or without TBBx was 59 min. The addition of pEBUS to guide parenchymal sampling did not add significantly to the overall procedure time. However, when both pEBUS and ENB-guided parenchymal biopsies were combined with lymph node biopsies using CP-EBUS, the mean procedure time increased to 95 min.
Table 3.
Procedural information
| TBBx-only n = 45 |
CP-EBUS only and CP-EBUS with TBBx n = 104 |
CP-EBUS, pEBUS and TBBx n = 18 |
CP-EBUS, pEBUS, ENB, and TBBx n = 14 |
|
|---|---|---|---|---|
| Medications used (mg/kg), median (IQR) | ||||
| Lidocaine total (mg/kg) | 5.3 (3.6) | 4.6 (2.3) | 5.3 (3.1) | 7.1 (3.1) |
| Midazolam (mg/kg) | 0.07 (0.07) | 0.08 (0.04) | 0.09 (0.04)* | 0.14 (0.04)* |
| Fentanyl (mcg/kg) | 1.9 (1.7) | 2.2 (1.7) | 3.1 (1.5) | 2.0 (4.9) |
| Benadryl (mg/kg) | 0.0 (0.0) | 0.0 (0.58) | 0.42 (0.38) | 0.64 (0.19) |
| Procedure time, mean (SD) | 34.2 (24.3) | 59.5 (25.0)* | 60.6 (17.1)* | 95.6 (18.5)* |
| Procedural informationa | ||||
| Number of lymph node stations sampled mean (SD) | NA | 2.8 (1.2) | 2.6 (1.2) | 2.9 (1.4) |
| Lymph node station size (mm) mean (SD) | NA | 12.3 (6.1) | 11.1 (4.6) | 8.9 (3.2) |
| Number of passes per lymph node station mean (SD) | NA | 3.1 (0.8) | 3.0 (0.3) | 3.0 (0.35) |
TBBx transbronchial biopsy using traditional flexible bronchoscopy, CP-EBUS convex probe endobronchial ultrasound, pEBUS transbronchial biopsy using peripheral EBUS guidance, ENB electromagnetic navigational bronchoscopy guidance for parenchymal biopsies, IQR interquartile range, SD standard deviation
Significantly different at 0.05 level: medication values compared with Wilcoxon rank-sum statistic and procedure time with T test where TBBx-Only served as reference group in each case
Because values for reference group were not available, procedural information not statistically tested
For the 136 patients undergoing CP-EBUS TBNA, the mean number of lymph nodes sampled per patient was 2.8 with a mean of 3.1 passes per lymph node station. The mean size of each lymph node station varied from 12.3 mm in group 2 to 8.9 mm in group 4. Three (2.2 %) out of 136 patients undergoing advanced diagnostic procedures developed a pneumothorax. All of these patients had undergone parenchymal biopsies and two of the three required chest tube placement. There were no complications (pneumothorax, serious bleeding, or step-up of care) in the 75 patients that underwent lymph node sampling alone. One patient who underwent CP-EBUS and trans-bronchial biopsies received naloxone and flumazenil due to bradycardia during the procedure. CP-EBUS alone yielded a specific pathological diagnosis in 76 out of 136 patients (55.9 %). When combined with parenchymal sampling, a specific pathological diagnosis was established in 103 out of 136 patients (75.7 %).
The multivariate analysis of the results compared the overall tolerability score on a count scale from 0 to 11 of each of the advanced procedural subgroups to the common reference group of TBBx-only and is presented in Table 4. This Poisson model included adjustment for age, sex, history of asthma, COPD or cancer, and body mass index (BMI). Relative to TBBx-only and with adjustment for covariates, each of the advanced procedural subgroups exhibited no statistical difference in tolerability.
Table 4.
Multivariate Poisson associations with overall subjective tolerance of advanced thoracic procedures relative to TBBx alone (N = 181)
| Procedures or patient characteristics | Relative risk | (95 % CI) | p value |
|---|---|---|---|
| CP-EBUS only and EBUS with TBBxa | 1.00 | (0.88, 1.14) | 0.99 |
| CP-EBUS, pEBUS, and TBBxa | 1.05 | (0.87, 1.27) | 0.60 |
| CP-EBUS, ENB, pEBUS, and TBBxa | 0.96 | (0.78, 1.19) | 0.74 |
| Age in years | 1.00 | (1.00, 1.01) | 0.56 |
| Asthma | 0.89 | (0.74, 1.07) | 0.21 |
| BMI | 1.00 | (0.99, 1.00) | 0.24 |
| Cancer | 0.99 | (0.89, 1.10) | 0.82 |
| COPD | 0.98 | (0.86, 1.11) | 0.75 |
| Male sex | 1.04 | (0.94, 1.15) | 0.49 |
Multivariate associations from Poisson model with each term tested against a null relative risk of 1 with the Wald χ2 statistic. Missing data: non-white race (n = 9), asthma (n = 1), COPD (n = 1), sleep apnea (n = 1), coronary artery disease (n = 1)
TBBx Transbronchial biopsy using traditional flexible bronchoscopy, CP-EBUS convex probe endobronchial ultrasound, pEBUS peripheral EBUS, transbronchial biopsy using pEBUS, ENB electromagnetic navigational bronchoscopy, BMI body mass index, COPD chronic obstructive pulmonary disease
Procedural associations referent to TBBx-only
Discussion
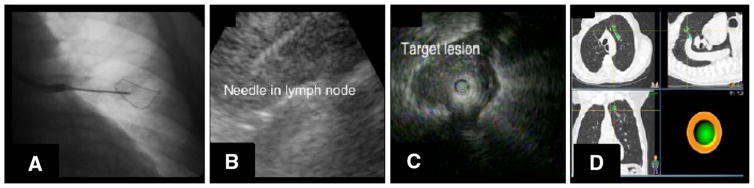
In this study, we have demonstrated that the safety and tolerability of advanced diagnostic bronchoscopy using CP-EBUS, pEBUS, and ENB performed under conscious sedation is similar to that of bronchoscopy with TBBx. The literature has shown that diagnostic bronchoscopy yields are higher with the use of new technology compared to that of conventional bronchoscopy. Convex probe EBUS-TBNA has become a well-established diagnostic modality for evaluation of mediastinal and hilar lymph nodes. It is a highly sensitive initial diagnostic tool that performs equal to, if not better, than mediastinoscopy for mediastinal lymph node sampling in patients with suspected non-small cell cancer [6, 7], isolated mediastinal lymphadenopathy [8], and other conditions. It is considered less invasive, safer, and less expensive than mediastinoscopy [9, 10]. Newer bronchoscopic technologies have enhanced the diagnostic yield of parenchymal sampling as well. Whereas the sensitivity of traditional transbronchial biopsies have been reported as low as 14 %, the yield for nodules and other pathologic entities increases with pEBUS and EMN guidance [11]. A meta-analysis for guided bronchoscopy in the evaluation of a solitary pulmonary nodule reported a pooled diagnostic yield of 70 % [12]. Examples of the intraprocedural images obtained during these advanced techniques are shown in Fig. 1.
Fig. 1.
Compared to conventional fluoroscopic-guided TBBx (a), CP-EBUS (b), pEBUS (c), and ENB (d) improve the diagnostic yield during bronchoscopy. In each of these newer techniques, technology facilitates guidance to the area to be biopsied more accurately than the conventional two-dimension fluoroscopy-guided approach
Tolerability
Parenchymal sampling using conscious sedation during conventional bronchoscopy is a well-established practice with an excellent patient tolerability and safety profile [13]. Recent technologic improvements have provided means that offer higher diagnostic yields in both mediastinal and lymph node biopsies and in parenchymal sampling compared to the era prior to the introduction of CP-EBUS, pEBUS, and ENB. There is limited data regarding the subjective tolerance of CP-EBUS, although satisfaction is felt to be very high [14]. To our knowledge, there is no data about patient tolerability when a thorough mediastinal evaluation using CP-EBUS is combined with other advanced parenchymal procedures such as pEBUS and ENB. In the first study of its kind, we demonstrate that CP-EBUS, pEBUS, and ENB, used in isolation or combination, are well tolerated using conscious sedation and can be performed without compromising the thoroughness of the procedure, patient safety, or diagnostic yield. Although procedural time increases with the addition of more techniques and more biopsies, tolerability is not sacrificed and the procedural yield is improved. In our study, a satisfactory level of conscious sedation was achieved throughout the entirety of the procedure, and no procedure was aborted due to inadequate levels of sedation.
Medication Requirements
Guidelines exist regarding safe doses of medications for bronchoscopy. As described in the most recent ACCP guidelines [1], a lidocaine dose of <7 mg/kg is recommended and midazolam doses of 0.06–0.07 mg/kg are typical. Fentanyl is the most frequently used opioid due to its short half-life and rapid absorption. We attempted to optimize the use of lidocaine by combining a laryngeal nerve block with a spray atomizer, both of which have been shown to be efficacious [15, 16]. Our medication doses were highest and broached the recommended limits for conscious sedation only for the most prolonged procedures. We intentionally avoid terminology such as “moderate” and “deep” sedation to avoid subjectivity. Instead, we provided sedation without airway support (LMA, endotracheal tubes) or medications that required us to use anesthesiologists (propofol, inhaled anesthetics). It should be noted that the medications given were temporally spaced throughout the procedure. One patient required reversal agents due to bradycardia that developed during the procedure. Notably, by using conscious sedation, we avoided the additional costs inherent with the use of general anesthesia or propofol. As referenced earlier, the patients in the AQuIRE database that required a step-up of care tended to receive deep levels of sedation [2].
Minimal studies compare sedation techniques for advanced bronchoscopic procedures. Yarmus et al. described a two-center retrospective review in which sedation using a combination of benzodiazepines and opioids was compared with deep sedation using propofol. This study used on-site cytology and demonstrated that more lymph nodes (2.2 vs. 1.4 lymph nodes per patient) could be biopsied using deep sedation and the diagnostic yield was higher (80 vs. 66 %). They noted that prospective studies including patient selection and cost are needed [17]. The mere fact that we averaged 2.8 lymph node stations per patient without deep sedation argues that other factors may be involved than the type of sedation used. For example, as one gains experience with procedures, technical acumen improves. In our experience, the time of the procedure has decreased markedly since this study was performed as the primary endoscopist has become more experienced. The type of setting may determine sedation strategies and are factors that are not controlled by the bronchoscopist. Within our own hospital system, the largest hospital uses conscious sedation yet other hospitals use propofol and anesthesiology for all bronchoscopy cases based on “tradition.” Theories regarding operator experience, patient factors, lymph node size, anticipated pathology, and other factors related to diagnostic yield have been proposed but none studied prospectively using different sedation strategies. In our opinion, and as shown by our results, we believe that conscious sedation is feasible and advocate that it be used as a first choice of sedation, opting for other techniques if this regimen does not work in a satisfactory manner.
Thoroughness
The AQuIRE registry represented 1,317 patients in six centers across the country. In that study, the mean number of lymph nodes sampled per patient ranged from 1.65 in low volume centers to 2.45 in high volume institutions. In our cohort, the mean number of lymph nodes sampled was 2.8 per patient. Also, the AQuIRE registry showed that risk-adjusted diagnostic yield from the EBUS procedures ranged from 38 % in low volume centers to 58 % in high volume centers. Our results are similar to high volume centers in AQuIRE. In our cohort, we were able to make a specific diagnosis from lymph node sampling in 56 % of the patients. Lymph node tissue was present in all patients. The mean number of passes per lymph node station was 3.1, which has previously been shown to be a sufficient number of passes [18]. In the literature, the pooled diagnostic yield for the evaluation of solitary nodules with advanced techniques is reported to be 70 % [12]. Although we sampled more than solitary nodules, our diagnostic yield was 77 %.
Safety
In keeping with other investigators, we found CP-EBUS, pEBUS, and ENB to be extremely safe. The only complications resulted when parenchymal biopsies were also performed and pneumothoraces occurred as a result. Our frequency of complications is similar to other centers [12]. As described above, only one patient required reversal of sedation despite the thoroughness of the procedures.
Limitations
This study was designed to assess the tolerability of advanced bronchoscopic diagnostic procedures performed using conscious sedation over a 1-year period of time. As this was an observational study, we did not compare techniques using different types of anesthesia, nor did we seek to include a specific number of procedures per group. A primary limitation of this study is that the majority of the procedures were performed in an academic setting by bronchoscopists with specialized training in interventional pulmonary. We also used a laryngeal nerve block to assist with topical anesthesia. These results may not be generalizable. We did not routinely use ROSE, however, ROSE might actually help reduce the procedure times [19], the amount of medications used, and even complications from parenchymal biopsies when the lymph node on-site diagnosis is conclusive. The majority of the cases were performed with fellow involvement, which may have increased the total procedure times. However, the fellows’ role was limited in the procedure to the basic procedures (inspection, parenchymal biopsies) and the final lymph node sampled by CP-EBUS.
The number of patients that underwent additional parenchymal sampling with use of ENB was relatively small. The procedural time, particularly for ENB, was long. Reasons for increased procedural time for ENB may be related to a learning curve of the bronchoscopist and ancillary staff as well as the involvement of trainees in the procedure. It is important to emphasize that the use of ENB was generally reserved for “more difficult cases,” namely those with small lesions that were clinically deemed necessary to biopsy and in which a bronchoscopic route was felt to be most appropriate. In these circumstances, the operator thought the chances of a definitive diagnosis without ENB were low enough to justify the additional expense of the ENB equipment. Furthermore, very aggressive biopsy attempts were made in these scenarios, as often the patients had no other options for diagnosis due to severity of their underlying lung disease and inherent risks of either transthoracic biopsies or surgery to evaluate the parenchymal lesions. Despite the procedural duration, patient tolerability remained high.
Finally, this study does not attempt to compare sedation techniques or methods of performing advanced diagnostic procedures. It provides a description of the combination of high patient tolerability with that of high diagnostic yields while performing multiple bronchoscopic techniques using medications commonly used for diagnostic bronchoscopy. The authors hope to demonstrate that these techniques do not necessarily require propofol, general anesthesia, or assisted breathing with intubation or LMA. This may be beneficial for those practitioners who do not have easy access to anesthesiologists or the operating room. As these latter practices invoke more costs, the techniques described in this study may invoke discussions about reducing costs in this age of cost-containment.
Conclusions
Thorough mediastinal lymph node evaluation can be combined with parenchymal sampling using conscious sedation and the laryngeal nerve block without compromising patient tolerability, thoroughness, diagnostic yield, or safety. This approach is useful for those bronchoscopists who perform advanced procedures such as EBUS, pEBUS, and ENB without general anesthesia or the operating room.
Acknowledgments
The authors would like to express gratitude to Anna Kookoolis, MA, for her ongoing contributions to patient enrollment and follow-up and for her diligence in data entry. This work was supported in part by the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (2P30AG021342-06 for KLBA and TEM).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Geetinder Goyal, Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA.
Margaret A. Pisani, Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA
Terrence E. Murphy, Claude D. Pepper Older Americans Independence Center at Yale, Program on Aging, Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA
Katy L. Araujo, Claude D. Pepper Older Americans Independence Center at Yale, Program on Aging, Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA
Jonathan T. Puchalski, Pulmonary, Critical Care and Sleep Medicine, Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA. The Thoracic Interventional Program (TIP), Yale University School of Medicine, 15 York Street, LCI 100, New Haven, CT 06510, USA
References
- 1.Wahidi MM, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140(5):1342–1350. doi: 10.1378/chest.10-3361. [DOI] [PubMed] [Google Scholar]
- 2.Eapen GA, et al. Complications, consequences, and practice patterns of endobronchial ultrasound-guided transbronchial needle aspiration. Chest. 2013;143(4):1044–1053. doi: 10.1378/chest.12-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsia DW, et al. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchol Interv Pulmonol. 2012;19(1):5–11. doi: 10.1097/LBR.0b013e31823fcf11. [DOI] [PubMed] [Google Scholar]
- 4.Mohanasundaram U, Ho LA, Kuschner WG, Chitkara RK, Canfield J, Canfield LM, Krishna G. The diagnostic yield of naviagational bronchoscopy performed with propofol deep sedation. ISRN Endosc. 2013 doi: 10.5402/2013/824693. [DOI] [Google Scholar]
- 5.Stather DR, et al. Trainee impact on procedural complications: an analysis of 967 consecutive flexible bronchoscopy procedures in an interventional pulmonology practice. Respiration. 2013;85(5):422–428. doi: 10.1159/000346650. [DOI] [PubMed] [Google Scholar]
- 6.Yasufuku K, et al. Endobronchial ultrasound guided transbronchial needle aspiration for staging of lung cancer. Lung Cancer. 2005;50(3):347–354. doi: 10.1016/j.lungcan.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Ernst A, et al. Diagnosis of mediastinal adenopathy-real-time endobronchial ultrasound guided needle aspiration versus mediastinoscopy. J Thorac Oncol. 2008;3(6):577–582. doi: 10.1097/JTO.0b013e3181753b5e. [DOI] [PubMed] [Google Scholar]
- 8.Navani N, et al. Endobronchial ultrasound-guided trans-bronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012;186(3):255–260. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharples LD, et al. Clinical effectiveness and cost-effectiveness of endobronchial and endoscopic ultrasound relative to surgical staging in potentially resectable lung cancer: results from the ASTER randomised controlled trial. Health Technol Assess. 2012;16(18):1–75. Iii–iv. doi: 10.3310/hta16180. [DOI] [PubMed] [Google Scholar]
- 10.Steinfort DP, et al. Cost-benefit of minimally invasive staging of non-small cell lung cancer: a decision tree sensitivity analysis. J Thorac Oncol. 2010;5(10):1564–1570. doi: 10.1097/JTO.0b013e3181e8b2e6. [DOI] [PubMed] [Google Scholar]
- 11.Steinfort DP, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J. 2011;37(4):902–910. doi: 10.1183/09031936.00075310. [DOI] [PubMed] [Google Scholar]
- 12.Memoli W, Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest. 2012;142(2):385–393. doi: 10.1378/chest.11-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lechtzin N, et al. Patient satisfaction with bronchoscopy. Am J Respir Crit Care Med. 2002;166(10):1326–1331. doi: 10.1164/rccm.200203-231OC. [DOI] [PubMed] [Google Scholar]
- 14.Steinfort DP, Irving LB. Patient satisfaction during endobronchial ultrasound-guided transbronchial needle aspiration performed under conscious sedation. Respir Care. 2010;55(6):702–706. [PubMed] [Google Scholar]
- 15.Lee HJ, et al. Pilot randomized study comparing two techniques of airway anaesthesia during curvilinear probe endobronchial ultrasound bronchoscopy (CP-EBUS) Respirology. 2011;16(1):102–106. doi: 10.1111/j.1440-1843.2010.01861.x. [DOI] [PubMed] [Google Scholar]
- 16.Gotta AW, Sullivan CA. Anaesthesia of the upper airway using topical anaesthetic and superior laryngeal nerve block. Br J Anaesth. 1981;53(10):1055–1058. doi: 10.1093/bja/53.10.1055. [DOI] [PubMed] [Google Scholar]
- 17.Yarmus LB, et al. Comparison of moderate versus deep sedation for endobronchial ultrasound transbronchial needle aspiration. Ann Am Thorac Soc. 2013;10(2):121–126. doi: 10.1513/AnnalsATS.201209-074OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HS, et al. Real-time endobronchial ultrasound-guided transbronchial needle aspiration in mediastinal staging of non-small cell lung cancer: how many aspirations per target lymph node station? Chest. 2008;134(2):368–374. doi: 10.1378/chest.07-2105. [DOI] [PubMed] [Google Scholar]
- 19.Wohlschlager J, et al. Rapid on-site evaluation (ROSE) in cytological diagnostics of pulmonary and mediastinal diseases. Pathologe. 2012;33(4):308–315. doi: 10.1007/s00292-012-1578-8. [DOI] [PubMed] [Google Scholar]