Summary
Introduction
Diabetes mellitus and obesity are important health issues; increasing in prevalence, both in the US and globally. There are only limited pharmacological treatments, and although bariatric surgery is effective, new effective pharmacologic treatments would be of great value. This review covers one area of increasing interest that could yield new novel treatments of obesity/diabetes mellitus. It involves recognition of the central role the G-protein-coupled receptor, bombesin receptor subtype 3(BRS-3) plays in energy/glucose metabolism.
Areas covered
Since the initial observation that BRS-3-knockout mice develop obesity, hypertension, impaired glucose-metabolism, and hyperphagia, there has been numerous studies of the mechanisms involved and the development of selective BRS-3-agonists/ antagonists which have marked effects on body weight, feeding, and glucose/insulin homeostasis. In this review each of these areas will be briefly reviewed.
Expert opinion
BRS-3 plays an important role in glucose/energy homeostasis. The development of potent, selective BRS-3 agonists demonstrates promise as a novel approach to treat obesity/diabetic states. One important question that needs to be addressed is whether BRS-3 agonists need to be centrally-acting. This is particular important in light of recent animal and human studies that report transient cardiovascular side-effects with centrally acting oral BRS-agonists.
1. Introduction
It has been well established by various experimental approaches, including the use of selective agonists and antagonists[1,2] and more recently, by receptor knockout studies[1,3–5], that two original members of the mammalian bombesin receptor(BnR) family, the gastrin releasing peptide receptor(GRPR,BB2) and the neuromedin B receptor(NMBR, BB1), play an important role in satiety. A third member of the mammalian BnR receptor family has been described[6,7], also a G-protein-coupled receptor, but, as will be reviewed in the next section, it is an orphan receptor at present, but because of close homology to the other two mammalian BnRs(i.e. the GRPR, NMBR), it is also classified in the BnR family and named bombesin receptor, subtype 3(BRS-3, BB3)[6–9]. Until recently because of lack of a native ligand, few pharmacological tools existed to explore its role in physiological or pathophysiological processes[8–11]. However, this receptor is now receiving considerable attention, because mice with the BRS-3 removed by targeted deletion[12], were found to become obese, develop mild hypertension and demonstrate impaired glucose metabolism, with reduced metabolic rates, increased feeding efficiency and hyperphagia, leading the authors[12] to suggest, they could be a new model to study human obesity and associated diseases, such as diabetes. Numerous subsequent studies have supported the importance of BRS-3 in energy balance, glucose homeostasis, regulation of feeding, as well as a number of other processes that can affect these, such as alterations of various behaviors. Furthermore, recently, both selective agonists (nonpeptide) and antagonists (peptide) have been described which may provide insights into therapeutically important approaches to treat both obesity and diabetes. In this short review advances in each of these areas will be briefly covered. Before this is undertaken it is important to first understand a few aspects of the mammalian BnR family and how the BRS-3-receptor relates to this family, as well as some aspects of the specific biology/pharmacology of the BRS-3-receptor.
2. BRS-3 and the mammalian BnR family (Table 1)
Table 1.
Comparison of characteristics of human BRS-3 to other human bombesin (Bn) receptors
| Variable | Human bombesin(Bn) receptor subtype | ||
|---|---|---|---|
|
| |||
| IUPHAR Code | BB3 | BB1 | BB2 |
|
| |||
| Other Names | Bn receptor subtype 3, BRS-3 | NMB preferring receptor NMBR |
GRP preferring receptor GRPR |
|
| |||
| Gene location(Chr) | Xq25 | 6p21 | Xp22 |
|
| |||
| Structural information | 399 aa | 390 aa | 384 aa |
|
| |||
| Natural ligands | Unknown | NMB>GRP | GRP>NMB |
|
| |||
| Nonselective agonists | [DPhe6,βAla11,Phe13] Bn (6–14) | DPhe6,βAla11,Phe13] Bn (6–14) Bn, litorin, ranatensin, alytesin |
DPhe6,βAla11,Phe13] Bn (6–14) Bn, litorin, ranatensin, alytesin |
|
| |||
| Selective agonists | Low affinity all natural Bn’s MK5046 Chiral diazepines 9f,9g,17c [D-Tyr6, Apa-4Cl11, Phe13, Nle14] Bn 6–14 |
NMB, NMB30 | GRP |
|
| |||
| Selective antagonists | Bantag-1 | PD 168368 | Ac-GRP20–26 methylester,[D-Phe6,Cpa14, ψ13–14]Bn6–14, JMV641, JMV594, BW2258U89 |
|
| |||
| Principal transduction | PLC activation, Tyr kinase cascades | PLC, Tyr kinase cascades | PLC, Tyr kinase cascades |
|
| |||
| Principal Receptor location | CNS (restricted)(Hi-hypothalamus), testis, pancreatic islets, GI tract Some tumors |
CNS (Hi olfactory bulb), GI and urogenital tract, testis, some tumors | CNS (hi-hypothalamus, amygdala), GI and urogenital tract, pancreas, respiratory tract, many tumors |
| Tissue functions | glucose/insulin regulation energy homeostasis, satiety; lung development and response to injury: in, cells of Cajal proposed involved GI motility, some tumor growth | CNS (regulate TSH release, satiety), GI tract (motility); regulate stress responses, tumor growth-autocrine growth factor.; | CNS (satiety, thermoregulation, regulate circadian rhythm,); regulate secretions [pancreas, gastric acid, islets]). GI tract (hormone release, motility), immunologic (chemo-attractant, lymphocyte function); fetal development (lung), tumor growth-autocrine growth factor. Mediate pruritis |
Abbreviations: NMB, neuromedin B; GRP, gastrin-releasing peptide; PLC-phospholipase C; Tyr kinase, tyrosine kinase cascades.
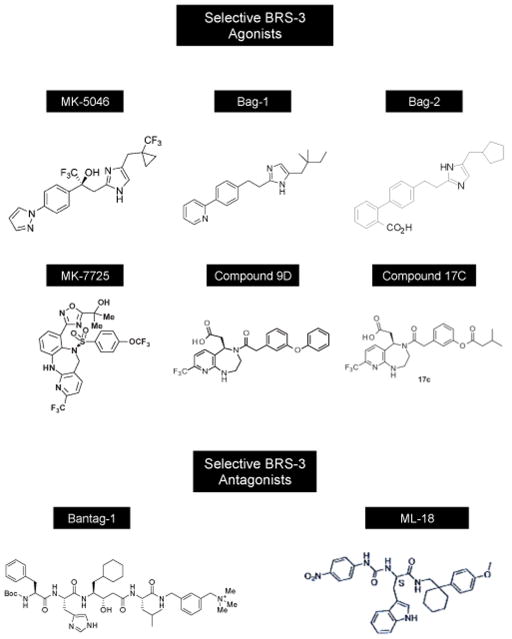
Agonist/Antagonist structures: structureMK-5046,(2S)-1,1,1-trifluoro-2-[4-(1H-pyrazol-1-yl)phenyl]-3-(4-[[1-(trifluoromethyl)cyclopropyl]methyl]-1H-imidazol-2-yl)propan-2-ol[79];9f, [(5R)-4-((3-[(6-methylpyridin-3-yl)oxy]phenyl)acetyl)-8-(trifluoromethyl)-2,3,4,5-tetrahydro-1H-pyrido[2,3-e][1,4]diazepin-5-yl]acetic acid [77]; 9g, [(5R)-4-([3-(2-methylpropoxy)phenyl]acetyl)-8-(trifluoromethyl)- 2,3,4,5-tetrahydro-1H-pyrido[2,3-e][1,4]diazepin-5-yl]acetic acid (9g)[77];17c, 2-[(5R)-4-[2-[3-(3-Methylbutanoyloxy)phenyl]acetyl]-8-(trifluoromethyl)-1,2,3,5-tetrahydropyrido[2,3-e][1,4]diazepin-5-yl]acetic acid [76]; Bantag-1, Boc-Phe-His-4-amino-5-cyclohexyl-2,4,5-trideoxypentonyl-Leu-(3-dimethylamino) benzylamide N-methylammonium trifluoroacetate [31,34]; PD168368, (3-(1H-indol-3-yl)-2-methyl-2-[3(4-nitro-phenyl)- ureido]-N-(1-pyridin-2-yl-cyclohexylmethyl)-propionamide) [27,99,132]. Alytesin, pGlu-Gln-Arg-Leu-Gly-Thr-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 ; Bn, pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 ; GRP, Ala-Pro-Val-Ser-Val-Gly-Gly-Gly-Thr-Val-Leu-Ala-Lys-Met-Tyr-Pro-Arg-Gly-Asn-His-Trp-Ala-Val-Gly-His-Leu-Met-NH2 ;Litorin, pGlu- Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 ; NMB, Gly-Asn-Leu-Trp-Ala-Thr-Gly-His-Phe-Met-NH2; ; Ranatensin, pGlu-Val-Pro-Gln-Trp-Ala-Val-Gly-His-Phe-Met-NH2 ; pGlu denotes pyroglutamate
2.1.GRP/NMB and their receptors: Cell biology, pharmacology and function
GRP is a 27 amino acid peptide that occurs widely in both peripheral tissues and in the central nervous system (CNS)[8,13]. GRP has a COOH terminus, which is the biologically active portion, ending in Gly-His-Leu-Met-NH2 (Table 1), and it has very high homology to a number of amphibian peptides (Table 1), including bombesin (Table 1), with which it shares the same last 7 COOH terminal amino acids. GRP interacts preferentially with the GRPR, which is also widely distributed in both peripheral tissue and the CNS, and has an 650-fold higher affinity for GRPR than NMBR (Table 1,2)[8,9,14,15]. NMB (Table 1), is a decapeptide formed from a 30 and 32 amino acid precursor (NMB30, NMB32) and has a COOH terminus ending in Gly-His-Phe-Met-NH2 (Table 1),,which shares 6 of the 7 COOH terminal amino acid from the amphibian peptide, ranatensin (Table 1)[8,13]. NMB interacts preferentially with the NMBR having an 670-fold higher affinity for NMBR than GRPR[14] (Table 1,2). The NMBR also occurs widely in peripheral tissues as well as the CNS[8,9,13,15]. In the monkey CNS both GRPR and NMBR mRNA were found in the hypothalamus, thalamus, amygdala, caudate nucleus, cerebellum, hippocampus and spinal cord[15]. In rat CNS binding studies a particularly high density of NMBR was found in the central thalamic nuclei and olfactory regions with the highest density of GRPRs in the hypothalamus, particularly the suprachiasmatic and paraventricular nuclei, which both agree with the mRNA distribution studies of these two receptors in rat brain[16]. Numerous studies demonstrate that the GRPR and NMBR are involved in a wide range of physiological and pathophysiological processes which include: in the CNS (circadian rhythm, TSH release, behavior control, thermoregulation, satiety), in the immune system [effects on macrophages, lymphocytes, leukocytes, dendritic cells], endocrine effects [release of numerous hormones/neurotransmitters], GI tract [motility, secretion, growth], as well as urogenital tract and respiratory system (Table 1)[8–11,13]. GRPR and NMBR also have important pathophysiological roles in such processes as mediation of various pruritic disorders, abnormalities of thyroid function, inflammatory disorders, and having profound effects on growth and differentiation of a number of important human tumors [colon, prostate, lung, head/neck squamous cell, CNS, pancreatic and some gynecologic cancers] and in some cases function as autocrine growth factors (Table 1)[8,9,13,17,18].
Table 2.
Affinity of BRS-3 and other human BnR subtypes for various agonist/antagonists.
| Variable | Affinity (nM)
|
||
|---|---|---|---|
| hNMBR (BB1) | hGRPR (BB2) | hBRS-3 (BB3) | |
|
| |||
| I. Natural occurring Agonist a | |||
|
| |||
| GRP | 148 | 0.19 | >3,000 |
| NMB | 0.052 | 35 | >3,000 |
| Bombesin (Bn) | 2 | 0.07 | >3,000 |
| Litorin | 0.16 | 0.54 | >3,000 |
| Ranatesin | 1.5 | 2.2 | >3,000 |
| Alytesin | 1.2 | 0.56 | >3,000 |
|
| |||
| II. Synthetic Agonists a | |||
| [D-Phe6, β-Ala11, Phe13, Nle14] Bn(6–14) | 0.21 | 0.048 | 1.3 |
| [D-Phe6] Bn(6–14) | 1.3 | 0.17 | >3,000 |
| [Cmp 16a j | >10,000 | >10,000 | 6026 |
| [D-Tyr6, (R)-Apa11, Phe13, Nle14] Bn (6–14) b | 160 | 110 | 8.2 |
| [D-Tyr6, Apa-4Cl11, Phe13, Nle14] Bn (6–14) b | 590 | 258 | 2.8 |
| Ac-Phe,Trp, Ala, His,(tBzl),Nip,Gly,Arg-NH2b | >10,000 | >10,000 | 63 |
| MK-5046 c | >10,000 | >10,000 | 18 |
| Bag-1 f | 7,000 | 4,400 | 18 |
| Bag-2 f | >7,500 | 6,400 | 82 |
| Compound 1 g | >10,000 | >10,000 | 1.4 |
| Compound 9 l | 7,000 | 6,400 | 18 |
| MK-7725 h | >10,000 | >10,000 | 3.6 |
| RY-337 I | >10,000 | >10,000 | 3676 |
| Compound 9G d | >10,000 | >10,000 | 70 |
| Compound 9D d | >10,000 | >10,000 | 121 |
| Compound 1t k | >30,000 k | >30,000 k | 240 k |
| Compound 17c | >10,000 | >10,000 | 12 m |
|
| |||
| II. Antagonists d | |||
| [D-Phe6] Bn 6–13 methyl ester | >10,000 | 4 | 5300 |
| [D-Tpi6, Leu13, Leu14, ψ13–14]Bn6–14 [RC3095] | 870 | 1 | >10,000 |
| PD 168368 | 0.51 | 1700 | >10,000 |
| PD 176252 | 0.53 | 170 | >3,000 |
| D-Nal, Cys,Tyr,D-Trp,Lys,Val,Cys,Nal-NH2 | 2500 | 605 | 340 |
| [[Tyr4, D-Phe12] Bn6–14 | 3100 | 912 | >10,000 |
| [Leu13, ψ13–14,Leu14]Bn6–14 | >10,000 | 8 | >10,000 |
| [D-Phe6, Leu13,Cpa14, ψ13–14]Bn6–14 | >10,000 | 1 | >10,000 |
| (3Ph-Pr6),His7, D -Ala11, D -Pro12, ψ13–14,Phe14]Bn(6–14)(BW2258U89) | 5,000 | 0.23 | 7,000 |
| [D-Arg1, D-Trp7,9, Leu11]substance P | >4,500 | 1,800 | >10,000 |
| Bantag-1 b | >10,000 | >10,000 | 1 |
| ML-18 e | >100,000 | 16,000 | 4800 |
Data for selective BRS-3 ligands is shown in shaded boxes
Data are from [25]
Data are from [30]
Data are from [53]
Data are from [34]
Data are from [86]
Data are from [81]
Data are from [83]
Data are from [72]
Data are from [88]: These data are not Ki’s determining by binding studies, but EC50’s determining by using a cellular FLIPR dye Calcium assay.
Data are from [85]:
Data are from [76]: These data are not Ki’s determining by binding studies, but EC50’s determining by using a IP-One HTRF assay which assesses phospholipase C activity and accumulation of IP1.
Abbreviations: See Table 1 for structures of 9f, 9g, 17c, Alytesin, Bantag-1, Bn, GRP, Litorin, MK-5046, PD168368, Ranatensin. Ac, acetyl; Apa, 3-amino,propionic acid; Apa-4Cl, 4-chloro,3-amino,propionic acid; Bag-1, (2-(4-(2-[5-(2,2-dimethylbutyl)-1H-imidazol-2-yl]ethyl)phenyl)pyridine[34]; Bag-2 (4′-(2-[5-(cyclopentylmethyl)- 1H-imidazol-2-yl]-1,2-ditritium-ethyl)biphenyl-2-carboxylic acid[34]; Cpa, chlorophenylalamine,; His(tBzl), histidine(tBenzl); Cmpd 9, 2-(2-[4-(pyridin-2-yl)phenyl] ethyl)-5-(2,2-dimethylbutyl)- 1H-imidazole; Cmpd 16, phenyacetyl-Ala,DTrp, phenthylamide[68,69]; ML-18, S-enantiomer of PD176252; Cpd 1t [88]: MK-7725,2-(3-(6-((4-(trifluoromethoxy)phenyl)sulfonyl)-2-(trifluoromethyl)-6,11-dihydro-5H-benzo[b]pyrido[2,3-e][1,4]diazepin-7-yl)-1,2,4-oxadiazol-5-yl)propan-2-ol[80,81]; Nip, piperidine-3 carboxylic acid; Nal, β-napthylalanine; Orn, ornithine; ψ; pseudopeptide bond, (i.e., CONH changed to CH2NH); Ph-Pr, phenylpropanolamine; PD176252, (3-(1H-Indol-3-yl)-N-[1-(5-methoxy-pyridin-2-yl)-cyclohexylmethyl]-2-methyl-2-[3-(4-nitro-phenyl)-ureido]-propionamide); RY-337[83].
2.2.BRS-3: Cell biology
In Table 1 various important aspects of the cell biology of BRS-3 are contrasted with the GRPR and NMBR and discussed primarily in the sections on BRS-3 below.
BRS-3 has been characterized in humans[7] and a number of other species including mouse, rat, sheep, guinea pig and monkeys[6,15,19–22]. The gene for BRS-3 in humans is localized to chromosomal regions Xq25–q26 and thus resembles the GRPR in its X chromosome localization (Table 1)[6,20]. In humans the BRS-3 contains 399-amino acids (Table 1) and hydropathy plots demonstrate it belongs to the G-protein coupled hepta-helical family of receptors[6,8]. BRS-3 is included in the BnR family of Bn receptors, even though at present it is categorized also as an orphan receptor, because the native ligand is unknown, due to the fact that in all species characterized it has high homology to the GRPR and the NMBR[6,7,15,19–22]. In the case of the human BRS-3, it has 51% amino acid identities with a the hGRPR, and 47% with the hNMBR, demonstrating close similarity to these receptors[6–8,10,11,23]. In contrast the human BRS-3 has only a 25% amino acid homology with the unrelated human substance P receptor[7].
In comparison to the GRPR and the NMBR, the distribution of the BRS-3-receptor has not been as well studied primarily because the native ligand is unknown and until recently no selective ligands were available. The synthetic Bn peptide analog, [DTyr6,βAla11,Phe13]Bn(6–14) can be radiolabeled and used for binding studies to localize BRS-3 in human tissues because it has high affinity for human BRS-3 (Table 2).[14,24–29]; however, it is not useful in rodents, because it has a very low affinity for the mouse or rat BRS-3[21,30]. Furthermore, its utility for BRS3 receptor localization is limited because it has high affinity for GRPR and NMBR in all species examined[14,24–29]. Using RT-PCR the relative abundance of BRS-3 mRNA in different areas of the monkey brain was assessed and it was found throughout the CNS with the greatest amounts in the hypothamus and in lower amounts in most peripheral tissues with the highest amount in the testis followed by lower amounts in the pancreas, thyroid, ovary and pituitary gland (Table 1)[15]. Because of its importance in islet cell function (reviewed below) it has been carefully examined in islets of different species and BRS-3 mRNA is reported to be present in the islets of mice, human, rhesus monkey and dog, but not in rat islets[31]. Detailed immunohistochemical studies for BRS-3-receptor localization have been performed in the rat CNS[32]and gastrointestinal tract[33], with the former study demonstrating BRS-3 is strongly present in cerebral cortex, hippocampal formation, hypothalamus and thalamus in the CNS[32]. In the GI tract BRS-3 was found throughout the GI tract in nerves and non-neuronal tissues, including enteric and submucosal ganglia, myenteric ganglia and in cell bodies of c-KIT interstitial cells of Cajal, which are important in regulating GI motility[33]. Two more recent studies have provided additional information on the localization of BRS-3-receptors. One recent study[34]made use of the availability of recently described BRS-3 selective ligands, and synthesized a specific BRS-3 radiolabeled agonist([3H]Bag-2) to examine the distribution of BRS-3-receptors in mouse brain. They found[34] moderate to abundant BRS-3 binding in a number of hypothalamic nuclei(PVN, DMH, ARC, VMH, PH, Pe, MPA, NHA), forebrain areas, caudal brain(PBN, NTS), the amygdala and the thalamus. The strong binding in hypothalamic nuclei support the importance of BRS-3 in feed behavior and energy metabolism which was first reported in BRS-3 knockout mice which developed obesity, hypertension and impairment of glucose metabolism[12]. In a second recent study in rat and mouse brain the regional distribution of BRS-3 mRNA localization was examined using in situ hybridization and its co-localization with various neurotransmitters was examined[35]. BRS-3 was found in many brain regions with the highest concentrations in the amygdala and hypothalamus[35]. In terms of co-localization with various brain neurotransmitters, many BRS-3 expressing neurons were glutamatergic, a few GABAergic or cholingergic, and also a small number co-localize with corticotropin-releasing factor (CRF) and growth hormone-releasing factor (GHRH) raising the possibility or interaction of BRS-3 with stress- and growth-hormone endocrine systems[35]. In these various BRS-3 CNS localization studies, all showed high localization in number of hypothalamic nuclei, however in other brain areas there was differences. In the case of the immunohistochemical receptor localization, high antibody specificity is essential, which can be difficult to achieve in all cases with such closely related receptors such as BRS-3, GRPR and NMBR.
The principal signaling cascade for BRS-3, similar to all BnR’s, is the activation of the phospholipase C (PLC) cascade resulting in the stimulation of changes in intracellular Ca2+ concentrations, the generation of diacylglycerol, and activation of various protein kinases C’s (PKCs) (Table 1)[8,10,11,28,36–40]. In the case of GRPR and NMBR, PLC activation has been studied in more detail and is primarily accomplished through coupling to the Gq/11 and G12/13 families of heterotrimeric G-proteins[8,9,41]. Activation of GRPR and NMBR, as well as in many cases also BRS-3, have been shown to stimulate a number of other signaling cascades including activation of Phospholipase D and A2, small GTP binding proteins such as Rho, protein kinase D and various ion channels[8,9,28,41–44].
Similar to other G-protein coupled receptors, numerous studies demonstrate after activation, GRPR and NMBR undergo a number of receptor modulatory processes including receptor phosphorylation[8,9,45], internalization and recycling[8,9,46–50], down-regulation[8,9,48–50,50] and desensitization[8,9,48–50]. Similar studies have not been reported with BRS-3-receptor, although it would be expected because of their close homology to the GRPR and NMBR, that similar receptor modulation would be seen with activation of BRS-3.
Two signaling cascades receiving increased attention is the recognition that, similar to many growth factor receptors, BRS-3 as well as the other BnR’s, when activated, stimulate a number of tyrosine kinase cascades including the MAPK cascade, including the transactivation of the epidermal growth factor receptor (EGFR), which is an important mediator of many of the growth related effects of each of the BnR’s, especially in neoplastic tissues[8,17,25,36,37,40,42,43,51–58]. Studies on normal and neoplastic tissues demonstrate that stimulation of BRS-3, as well as GRPR and NMBR, can result in potent stimulation of focal adhesion kinases (p125FAK, PYK-2); MAPK kinases especially ERK; EGFR; Src kinases; and paxillin[8–10,37,38,40,43,52,55–57,59]. Transactivation of the EGFR by GRPR and NMBR, has been shown to be an important signaling cascade mediating growth effects of these receptors on a number of different neoplasms including cancers of the lung (especially nonsmall cell tumors), head and neck squamous cells, neuroendocrine cells and the prostate[8,17,37,52,56–58,60–62]. Studies with BRS-3 in this area are more limited, although recently a number of studies of BRS-3 growth stimulatory effects in lung cancer cells report a similar mechanism with its growth stimulatory effects occurring primarily via EGFR transactivation[53,57]. In a number of studies the transactivation of EGFR by BRS-3 and the other mammalian BnRs has been shown to involve activation of phospholipase C, Src kinases, stimulation of matrix metalloproteinases and shedding of EGF-related ligands, as well as the stimulation of reactive oxygen species[8,9,17,52,54,57].
Recently detailed studies have been reported of the comparative signaling cascades involved in BRS-3 activation in myocytes of diabetic, obese and normal subjects[59,63,64], and this will be discussed in a later section on role of BRS-3 in obesity, energy metabolism and glucose homeostasis.
2.3.BRS-3: Receptor pharmacology
2.3.A. Pharmacology of BR-3 receptor agonists
As pointed out in previous sections, the BRS-3-receptor remains an orphan receptor so the natural ligand is at present unknown, and therefore there are no structure-function studies of the natural ligand. However, it has been possible to examine BRS-3’s pharmacology and cell biology using the synthetic peptide agonist, [DPhe6,βAla11,Phe13] Bn(6–14) or its DTyr6 analogue, which were discovered to have high affinity/potency for activating the human BRS-3 (Table 2)[24,27–29,38], however these synthetic Bn analogues have two disadvantages. These include a lack of specificity, because they also bind to, and activate in man and all species examined GRPR and NMBR, with high affinity and potency (Tables 1,2) and also because they have low affinity for rat and mouse BRS-3, the most commonly used laboratory animals[8,21,25–27,30].
A number of other peptide agonists have been described with reported increased selectivity for BRS-3 over GRPR and NMBR, some using [DPhe6,βAla11,Phe13] Bn(6–14) as a template (Table 2)[39,65–71]. In general these have relatively low selectivity (i.e.,<150 fold) for the BRS-3 over the NMBR or GRPR with the most potent being [DTyr6, Apa-4Cl11,Phe13,Nle14]Bn(6–14)(92 –fold selective) Table 2)[65,71–73] and the most selective being Ac-Phe, Trp, Ala, His, (tBzl), Nip, Gly, Arg-NH2 (Table 2)[67,71,72].
With the observation that mBRS-3-receptor knockout mice develop obesity, diabetes and metabolic disturbances[12],there has recently been increased interest in developing BRS-3 agonists for possible treatment of obesity [9,34,60,74–78]. Three pharmaceutical companies [Merck Research Laboratories, Daiichi Sankyo Co. Ltd, Glaxo SmithKline)] have recently reported efforts to develop selective nonpeptide agonists that activate BRS-3 with high potency (Table 2)[34,75–79,79–88]. A number of the nonpeptide agonists have selectivity >100-fold for the BRS-3 over the GRPR and NMBR and some >1000-fold selectivity (Table 2). Only a few of these have been assessed in vivo and those will be discussed in more detail in the next section related to energy and glucose homeostasis. Of these different nonpeptide agonists the most extensively studied, not only in terms of pharmacology, but also in energy and glucose homeostasis in normals and altered states such as obesity, is MK-5046 (Table 2). MK-5046 [(2S)-1,1,1-trifluoro-2- [4-(1H-pyrazol-1-yl) phenyl]-3-(4-[[1-(trifluoromethyl) cyclopropyl] methyl]-1H-imidazol-2-yl) propan-2-ol] is an orally active, selective and potent BRS-3 agonist with activity in rat, mouse, dog, and human (Table 2)[25,75,78,79]. MK-5046 has high affinity for human BRS-3-receptor (IC50-18-28nM), rhesus (IC50-50nM), mouse, rat and dog BRS-3 (IC50-1.2-6.5nM) and very low affinity for GRPR and NMBR (IC50>10,000nM) in humans as well as in other species (Table 2)[25,30,79]. MK-5046’s interaction/activation of the hBRS-3-receptor had both similarities and differs from the nonselective, high affinity BnR universal peptide agonist, [DPhe6,βAla11,Phe13]Bn(6–14)[25]. Both MK-5046 and the peptide agonist interacted with high and low affinity sites on the hBRS-3, however they differed in aspect of activation of hBRS-3[25]. The dose-response curves for phospholipase C were biphasic with MK-5046 and not with the peptide agonist; they differed in receptor occupation-activation relationships with MK-5046 having a greater receptor coupling spareness; in having a different pattern of activation of MAPK, p125FAK, Akt and paxillin and they differed in their kinetics of cellular activation with MK-5046 demonstrating slower kinetics of activation and a longer duration of action[25]. These results[25] demonstrate the peptide and MK-5046’s activation of hBRS were not always concordant raising the possibility they could lead to different cellular responses. Investigators at Daiichi Sankyo Co. Ltd[76,77] recently report a series of chiral diazepine analogues with low brain penetration, which function as high affinity BRS-3 agonists. Results assessing affinity of three of these chiral diazepine analogues (9G, 9D, compound 17c) are shown in Table 2 demonstrating that they function as selective BRS-3 ligands[30,76,77]. As will be discussed in more detail in the next section, it is proposed that because of their low brain penetrance, they may lack side-effects seen in trials with MK-5046, and be potentially useful anti-obesity agents[76,77].
2.3.A. Pharmacology of BRS-3-receptor antagonists
Extensive studies have identified six different chemical classes of GRPR antagonists including both peptide analogues and non-peptides, and a limited number of NMBR antagonists (Table 2)[8,36,89–95]. Many of these have high affinity and selectivity for the hGRPR or hNMBR, but do not interact with the hBRS3[8,24,28,36,89]. One important finding that arose from these studies with GRPR and NMBR is that there are marked species differences in the BnR family in their pharmacology as well as the structural requirement of the ligand for cell activation[8,14,90–92,96]. While this is only limited comparative species studies on BRS-3 pharmacology, the results with [DPhe6,βAla11,Phe13]Bn(6–14)[21] showing marked different pharmacology between human and rat or mouse BRS-3, as well as lower affinities of some nonpeptide analogues for human BRS-3, than rat or mouse BRS-3[79] suggest important species differences may exist also for the BRS-3-receptor. This is particularly important in evaluating whether a given ligand behaves as a BnR agonist or antagonist, because studies especially with GRPR containing tissues from different species, show not only can the pharmacophore of rodent receptors differ markedly from human, but the coupling can be markedly different in different species as well as with overexpressing transfected receptors[8,90,96–98].
Until recently no potential useful selective antagonists existed for the BRS-3-receptor. Two different BRS-3 antagonists have recently been described: ML-18[53] is a peptoid(S-enantiomer of PD176252, a GRP preferring antagonist[89,99]) and the other is a peptide antagonist, Bantag-1(Table 2)[31,34]. ML-18 has both a low affinity and low (<5-fold) selectivity for hBRS-3 over GRPR or NMBR (Table 2)[53]. In contrast, Bantag-1 (Boc-Phe-His-4-amino-5-cyclohexyl-2,4,5-trideoxypentonyl-Leu-(3-dimethylamino)benzylamide N-methylammonium trifluoroacetate) was reported to be a potent, selective peptide antagonist for the BRS-3-receptor in human, rat and mouse[31,34]. In a recent detailed pharmacologic study[25] in which the pharmacology of Bantag-1 was studied in both native hBRS-3 and transfected hBRS-3 cells, Bantag-1 had a high affinity for hBRS-3(1–1.6nM) and had >3000-fold selectivity for hBRS-3 over hGRPR or hNMBR-containing cells (Table 2). Furthermore, in this study[25], Bantag-1 was found to be a competitive hBRS-3-receptor antagonist, showing no agonist activity at this receptor and causing a parallel rightward-shift in the cell activating dose-responses curves by either MK-5046 or the peptide agonist,[DPhe6 βAla11,Phe13] Bn(6–14). The availability of these selective BRS-3 agonists (Table 2) and the peptide antagonist, Bantag-1, should prove to be valuable tools to studying the role of BRS-3 in both normal physiological and pathophysiological processes.
3. BRS-3 in obesity; energy and glucose homeostasis and diabetes
3.1. Studies in BRS-3 knockout mice
As discussed above, until recently there were no agonists or antagonists sufficiently potent and selective enough available to allow exploration of BRS-3’s role in physiological or pathophysiological processes. Much of what is known of its role in physiological or pathophysiological process has come from the study of BRS-3 knockout mice. Similar to the other mammalian BnRs (i.e. GRPR and NMBR)[2–4,8,9], it was found that BRS-3 knockout mice had alterations in feeding including increased feeding efficiency and hyperphagia[1,12,70,100–102]. In addition they developed mild obesity, hyperleptinemia, demonstrated impaired glucose metabolism, and even though they show reduced metabolic rates[1,12,70,100–102], which is not seen in many studies on human obesity, nevertheless, some authors[12] suggest, because of the other similarities, they could be a new model to study human obesity and associated diseases, such as diabetes. In addition to effects on energy and glucose homeostasis, BRS-3 knockout mice demonstrated behavioral changes[5,8,9,103,104], which possibly could affect feeding behavior. Specifically, BRS-3 deficient mice show changes in the taste preference test including elevated preference for sweets, and increased aversion for bitterness, while they had a decreased nonaggressive social response[5,103]. They also showed decreased spontaneous activity in isolated conditions that usually lead to increased activity[5]. In addition, in contrast to the GRPR and NMBR knockout animals, which showed no alteration in anxiety tests, BRS-3 knockout mice exhibited reduced levels of anxiety[104].
In the initial studies of BRS-3 knockout mice it was found that in addition to the development of obesity, hypertension, impaired glucose metabolism and insulin resistance, they also exhibited a reduced metabolic rate, hyperphagia and increased feeding efficacy[12]. At 20–24 weeks, when obesity progressed, blood contents of thyroid hormone and glucagon were not modified, but secretion of growth hormone was reduced by 46%, while insulin level was elevated significantly, suggesting alterations in glucose transport, lipogenesis and lipolysis[12]. At 27–34 weeks, when the weight difference was clear, the increased amount of white adipose tissue mass-epididymal, inguinal and retroperitoneal fat pads- in BRS-3-deficient mice became relevant[12].
Subsequent studies attempting to elucidate the potential mechanisms for obesity, as well as the alterations in glucose/insulin homeostasis in BRS-3 knockout (KO) mice, have been conducted (Table 3)[100–102,105,106]. In one study[100] female heterozygous BRS-3-KO mice were mated with another model of obesity, male KK-Ay mice, a strain with a mutation at the agouti locus, which has been shown to play and important role in appetite/food intake regulation[100,107,108]. KK-Ay mice exhibit hyperphagia, hyperinsulinemia, maturity onset obesity, and diabetes, due to antagonism to the melanocortin 4 receptor by ectopic expression of agouti protein[100,107–109]. Sibutramine, which affects the central monoamine system to regulate feeding behavior, markedly decreased food intake in BRS-3-KO mice and normals, but was much less effective in KK-Ay mice[100,110]. Because sensitivity to sibutramine is considered to reflect in an inverse fashion the degree to which hyperphagia contributes to the development of obesity in rodents[100,110], the above data indicate the obesity in KK-AY mice have a different origin for their obesity than in BRS-3-KO mice, and that the contribution of hyperphagia to the development of obesity is greater in the KK-AY mice than the BRS-3-KO mice[100]. During the period which obesity progressed the energy efficiencies of the BRS-3-KO mice was 2.7-fold higher that the wild type animals, suggesting that the obesity in these animals was largely dependent on these animals having higher energy efficiencies[100]. Hybrid mice were significantly heavier than either genotype alone, displayed insulin levels that were higher than those with just the Ay gene, and showed impairments in glucose-induced insulin secretion implicating BRS-3 in the regulation of plasma insulin concentration sensitivity[100]. The authors[100] hypothesize that BRS-3 deficiency induces changes in the autonomic nervous system and subsequently affects both insulin secretion and peripheral insulin sensitivity.
Table 3.
Summary of the physiological, pathophysiological effects of BRS-3.
|
A. Changes in BRS-3-KO-mice: Develop obesity[5,12,84,100,101,103,106,110] Hypertension (systolic and diastolic)[12] Glucose intolerance/hyperglycemia[12,100] Increased feeding efficiency[12] Hyperphagia[12,84,100,101,103,110] Hyperinsulinemia[12,100,101,106] Plasma Total cholesterol and triglycerides are increased[100] Decreased growth hormone secretion[12] Abnormal OGTT with higher insulin and glucose[12,100] In basal state mutant mice have lower oxygen consumption and lower metabolic rate[12,84,103] Hyperleptinemia suggesting leptin sensitivity is reduced in mutant mice[12,101] Hyperresponsiveness to palatable and aversive taste stimuli[5,111] Decreased social behavior[5,103,104] Mutant mice show reduced anxiety, non-aggressive social behavior, increased risk assessment[5,104] Mutant mice have enhanced hyperphagia response to melanin-concentrating hormone(MCH)[106] Level of MCH-R and prepro-MCH mRNAs elevated in hypothalamus[106] Develop both leptin resistance and insulin resistance[106] The anorexigenic peptide, CART had greater effect in mutant mice[106] Body temperature of mutant mice slightly lower[84,106,116] Mutant mice weight gain(36 wks) accompanied by gain in subcut and epididmyl fat, and brown fat[101] The gene, Urb(upregulated in BRS-3-deficient mice) in brown adipose tissue was 4-fold higher in BRS-3 mutant mice and was also increased in brain, digestive tissues, kidney and lung[105] |
|
B. Energy homeostasis: important areas of no change in BRS-3-KO-mice: Brown-fat thermogenesis was not unchanged from control animals[12] No change in physical activity of mutant mice[12] Feeding response to bombesin similar in wild type and mutant mice[5,106] No different in mutant and wild type mice in cumulative food intakes after administration of neuropeptide Y, orexins, and agouti-related protein(AGRP)[106] No different in mutant and wild type mice in inhibition of cumulative food intakes after α MSH[106] Body temperature of mutant mice decreased with Bn administration in a similar fashion[106] Mutant mice had similar sensitivity to sibutramine, a cmpd commonly used for human obesity[100,110] No difference in corticosterone levels between wild type and mutant mice[101] No difference in the sensitivity to an inhibitory dose of CCK on intake in mutant mice[101] |
|
C. Other important functions of BRS-3 related to energy/glucose homeostasis BRS-3 pathway contributes to the regulation of plasma insulin concentrations[100] BRS-3 antagonist, Bantag-1, increases food intake and body weight in wild type mice[34] and BRS-3 agonists decrease both and increase metabolic rate[34,75,79,83,86] BRS-3 agonist’s decreased body weight in NPY-1-,,AGRP-1-,,MCR-1-,Cnr1-1-, Leprdb/db mice[34] BRS-3 found in human, mice, rhesus, dog islets(not rat) and β-cell lines; silencing BRS-3 decreases glucose stimulated insulin secretion, but had no effect on GLP-1 stimulated insulin release[31]. BRS-3 agonists augment insulin release from human isolated islets[31] BRS-3 agonists reduced glucose levels during OGTT in mice[31] With chronic dosing in wild type mice the BRS-3 agonist, MK-5046-inducd weight loss was mediated primarily by increased metabolic rate rather than decreased food intake[75] BRS-3 agonist, MK-5046 also increases body temperature, heart rate, blood pressure[75,78,84] Skeletal muscle myocytes express BRS-3, which is BRS-3, is underexpressed in human myocytes from patients with diabetes, obesity or both[59,63,64] Skeletal muscle myocytes showed increased sensitivity to activation BRS-3(increased for glucose transport, MAPK, p90RSK,PKB,p70s6K ) from patients with diabetes, obesity or both[59,63,64] |
In a study of BRS-3-KO mice in which they were allowed to have ad libitum- feeding or their caloric intake was matched to wild-type ad libitum-feeding mice over a 21 week period, the BRS-3-KO mice had similar body weights to the wild type mice and showed less than the 29% increase over controls seen in BRS-3 ad libitum-feeding mice, However, in the pair-fed BRS-3-KO mice, the plasma insulin normalized, but it failed to completely reverse adiposity and hyperleptinemia[101], suggesting that hyperinsulinemia is not a primary deficit in ad libitum-fed KO mice. In this study[101], no differences in feeding suppression by GI hormones (CCK, bombesin, GRP) was found in BRS-3-KO mice, although BRS-3-KO mice had some deficits in the arcuate nucleus energy response signals. This study[101] concluded that hyperphagia is a major factor contributing to the increased body weight and the hyperinsulinemia in BRS-3-KO mice. It also concluded[101] that because the pair feeding did not completely normalize hyperleptinemia or the fat distribution, there also may be and underlying metabolic dysregulation that contributes to the development and possibility sustaining of the obesity seen in BRS-3-KO animals.
In addition to hyperleptinemia, BRS-3-KO mice also demonstrate leptin resistance[106], which is not seen in GRPR KO mice. Furthermore, BRS-3-KO mice, but not GRPR KO mice, have an enhanced feeding response to melanin-concentrating hormone (MCH), however this is not a generalized increased responsiveness to orexigenic neuropeptides, because no differences in feeding behavior in BRS-3-KO mice from normals, was seen with administration of NPY, orexin or agouti-related peptide[106]. The BRS-3-KO mice also have increased levels of both prepro-MCH and MCH-R mRNA in their hypothalamus[106]. These results led the authors[106] to suggest that the disruption of the BRS-3 gene leads to an upregualtion of hypothalamic MCH-R and MCH expression, which in turn leads to hyperphagia. Furthermore, it is speculated[106] that the BRS-3-KO upsets homeostatic mechanisms by which leptin decreases the expression of MCH-R and that this effect is mediated through neural mechanisms that are independent of those activated by other Bn-related peptides such as GRPR activating via the GRPR.
BRS-3-KO animals have a number of alterations in behavior that could also directly or indirectly effect energy homeostasis or feeding behavior (Table 3). [5,103,104,111]. It has been documented that food restriction activates the hypothalamic-pituitary-adrenal (HPA) axis resulting in an elevation in plasma corticosterone levels[112]; however, this effect was not detected in BRS-3-KO mice who were food restricted[101], suggesting a deficit in HPA activation or that food restriction is not perceived as a stressor in these animals. The last possibility has been pointed out by studies demonstrating a decrease in anxiety-related behaviours in BRS-3-KO mice[5,103,104]. When anxiety levels were assessed in an elevated plus maze, BRS-3-KO mice showed less anxiety than wild type as well as a decrease in the behavioural response to social isolation and a novel environment[5,103,104]. Another altered behavioral feature related to BRS-3 gene deletion that could possibly effect feeding behavior or energy homeostasis is changes in the sense of taste[111]. BRS-3 deficient mice not only showed a stronger preference for sweet taste —saccharin solution—, but also a stronger aversive response to quinine solution, relative to wild-type littermates[5,111]. The BRS-3-receptor is expressed in the parabrachial nucleus, centromedial nucleus of the amygdala and in hypothalamus nuclei such as the paraventricular nucleus[111], all of which are known to be involved in taste perception[111]. The authors[111] speculate that the obesity that BRS-3-KO mice develop and the functional alterations of taste preference/learning that is seen in these animals could be related but the exact manner or mechanism by which the altered tasted preference is related to the hyperphagia seen in these animals is unclear at present.
To gain insight into the pathogenesis of the alterations in glucose homeostasis/diabetes that occur in BRS-3-KO mice, studies of islet cell function, as well as the action of insulin on peripheral targets such as adipocytes in these animals have been performed[100,102,105]. In BRS-3-KO mice the number of islets and the size of the islets was decreased compared to normals[102], however no alteration in glucose-stimulated insulin secretion or in proinsulin biosynthesis was found between the BRS-3-KO mice and the wild type controls[102]. In contrast, in adipocytes from BRS-3-KO mice a number of alterations were found compared to controls[102]. These differences included the observation that insulin failed to stimulate the uptake of deoxyglucose into adipocytes from BRS-3-KO mice in contrast to adipocytes from control animals, and that the glucose transporter, GLUT-4, was only minimally detected in membranes from the BRS-3-KO mouse adipocytes after insulin stimulation, in contrast to their abundance in control adipocytes[102]. However, using quantitative RT-PCR, the mRNA level in BRS-3-KO and control adipocytes were similar for GLUT-4, insulin receptor, IRS-1, IRS2, syntaxin 4, SNAP23 and VAMP-2, leading to the conclusion that impaired glucose metabolism is occurring in BRS-3-KO mice mainly because of impaired GLUT-4 transport in adipocytes[102]. These results combined with the results of the study of BRS-3-KO mice crossed with male KK-AY mice, which was reviewed above[100], demonstrate the importance of the BRS-3 pathway in regulating plasma insulin concentrations and therefore glucose homeostasis.
To identify possible novel obesity related genes in adipose tissue that could be contributing to the development of obesity in BRS-3, a differential display analysis was undertaken to identify genes that were upregulated or downregulated in BRS-3 adipose tissue[105]. This resulted in the cloning of a 3-KB cDNA called Urb (for upregulated in BRS-3 deficient mice), yielding a protein of 949 amino acids, which had homology to the sushi protein superfamily[105]. In brown adipose tissue, but not white adipose tissue, of BRS-3-KO mice, Urb mRNA was increased 4-fold, and was also enhanced in brain, kidney, lung and various digestive tissues[105]. Brown adipose tissue is characterized by its thermogenic activity with dissipation of energy and providing heat; raising the possibility the Urb could be a candidate molecule contributing to the energy phenotype of BRS-3-KO mice[105]. At present the exact role of Urb plays in the regulation of body with and energy metabolism is unknown.
3.2. Studies in BRS-3 containing cells
To attempt to better define the mechanisms of the alterations in glucose and metabolic homeostasis that occur in BRS-3-KO mice, a number of studies have been reported investigating the role of BRS-3 in normal cellular/tissue function and cells/tissues from animals or human subjects with pathological conditions such as diabetes and obesity (Table 3)[31,59,63,64,113]. These investigations include the role of BRS-3 in islet/insulin responses, as well as responses of important insulin target tissues such as adipose tissue and skeletal muscle myocytes in normals as well as disease states[31,59,63,64,113].
Studies show that human, rhesus, mice, and dog pancreas (but not rat pancreas) all have high expression of BRS-3 in their islets[31,114,115]. Using siRNA to silence BRS-3 or using a specific antagonist (Bantag-1)[31] reduced glucose-stimulated insulin-secretion, and the selective agonist (Bag-1) increased it, in an insulinoma cell line INS-1 832/6), which also possessed BRS3 receptors. BAG-1 augmentation of glucose-stimulated insulin-release was dependent on activation of phospholipase C, was also seen in isolated wild type islets, but not islets from BRS-3-KO-mice[31]. In vivo in normal mice, but not in BRS-3-KO-mice, the BRS-3 agonist, Bag-1, reduced plasma glucose levels during an oral glucose tolerance test, demonstrating that this was occurring in a BRS-3-receptor-dependent manner[31]. Bag-1 also increased glucose-stimulated insulin-secretion from human islets. The authors concluded that these results demonstrate the important role of BRS-3 in islet regulation and glucose homeostasis, with BRS-3 stimulation directly promoting glucose-stimulated insulin-release, suggesting it may have an important potential role in the treatment of obesity as well as in tratment of diabetes mellitus[31]. These suggestions are supported by studies demonstrating that activation of BRS-3 has important effects on both adipose tissue[102](reviewed above) and skeletal muscle (myocytes)(reviewed in next section)[59,63,64].
In addition to these effects of BRS-3-receptor activation on glucose/energy homeostasis in peripheral tissues, BRS-3-receptors in the CNS, are found in neurons containing orexin A and B, which are produced in lateral hypothalamic nuclei and are key regulators of feeding behavior, the sleep and wake state, as well as the reward system[113]. A BRS-3-selective agonist stimulated increases in cytosolic calcium in the orexin neurons[113] as well as caused hyperpolarization of these neurons. However, in the presence of tetrodotoxin, or GABA receptor antagonists, the BRS-3 agonist stimulated depolarization and increased firing frequency of orexin neurons. The authors concluded that with CNS BRS-3-receptor activation, both indirect inhibitions of orexin neurons occur though a GABAergic input, as well as direct activation of orexin neurons[113]. Furthermore, the authors suggest that inhibition of orexin neuronal activity through the BRS-3 pathway could be a novel approach to treatment of obesity[113].
3.3. In vivo Studies on BRS-3
3.3.1 In vivo studies on BRS-3 in animals: feeding/appetite/weight loss studies
Several selective BRS-3 agonists and the antagonist, Bantag-1, have been studied in animals for their effect on body weight/food intake. Cmp MK-7725, a highly selective, potent BRS-3 agonist, which is a chiral benzodiazepine sulfonamide analogue[81] (Table 2), caused a dose-dependent decrease in food intake and body weight loss in mice, whereas in BRS-3-KO mice it had no effect[81]. A more chronic 12-day study in dogs with Cmp MK-7725 showed also robust weight loss with no evidence of tachyphlaxis, which was due to a combination of reduction in food intake and increase in metabolic rate[81]. Similarly, in wild type mice, the selective BRS-3 agonist, Bag-1[34](Table 2) when given orally(by gavage), reduced food intake in dose-dependent manner at 1 and 2 hours, and this was not seen in BRS-3-KO mice[34]. With Bag-1 treatment, mice demonstrated an increased metabolic rate, which persisted for up to 10 hours, which was not seen in BRS-3-KO mice[34]. Mice treated for longer periods of time (4 days) demonstrated significant weight loss, with significantly lower fat mass, which was not seen in BRS-KO mice, and Bag-1’s effectiveness complemented the changes in energy homeostasis caused by alterations in the MCR4 and CB1T pathways[34]. Rats treated for longer periods of time also demonstrated a continued decreased in food intake[34]. Wild type mice treated with the potent, selective BRS-3 agonist, MK-5046[75,79](Table 2) demonstrated decreases in overnight food intake and increased metabolic rats after a single dose, which was not seen in BRS-3-KO mice[75]. After MK-5046 dosing for 14 days, wild type, but not BRS-3-KO mice demonstrated a 5% decrease in body weight, and this was primarily due to an effect on increased metabolic rate, rather than decreased food intake [75]. Similarly in rats and dogs, MK-5046 caused significant weight loss, which was accompanied in both species by a transient increase in body temperature and heart rate, with also transient increased blood pressure in rats[75]. A subsequent study [84] found that BRS-3 regulation of body temperature is via a central mechanism, upstream of sympathetic efferents, and that the lower body temperature seen in BRS-3-KO mice[116], is due to altered regulation of energy homeostasis affecting the centers of body temperature regulation, rather than due to an intrinsic defect in the brown fat tissue of these animals[84]. Other selective BRS-3 agonists have also been examined for their effect on food intake/body weight and were generally less effective than the compounds reviewed above. This include the substituted biphenyl imidazole compound 22c[83] which when dosed intra-cerebroventricularly in rats, reduced food intake by 29%, however it was not effective in diet-induced obese mice orally, likely do to low blood levels. Similarly, the 2-biaryethylimidazole analogue, compound 9[85](Table 2), had limited oral bioavailability and a short half-life in mice. However, with oral dosing after a single dose reduced food intake was seen in mice and after 14-day dosing body weight reduction was seen in diet-induced obese mice[85]. Most of the BRS-3 selective agonists discussed above including MK-5046 and MK-7725, are brain penetrant[75–77,81]. Because of the possibility that the cardiovascular side-effects seen with MK-5046[75,78] could be due to brain penetrance and activation of CNS sympathetic cascades, low brain penetrant BRS-3 selective agonists were developed(ie.19d,19g, compound 17c,Table 2)[76,77]. A novel chiral diazepine analogue with a labile carboxylic ester with antedrug functionality introduced onto the terminal position to yield only a peripheral acting compound, compound 17c(Table 2)[76] was selected and tested on food intake in B6 mice. In wild type mice but not BRS-3-KO mice, compound 17c demonstrated anorectic activity without cardiovascular side-effects.
The specific, potent BRS-3 receptor antagonist, Bantag-1 (Table 2)[34], when given to rats for 12 days, increased food intake 12% over controls and caused a progressive increase in body weight gain which reached a 38% increase by 12 days[34]. This increase in body weight by Bantag-1 was due to an increase in adipose mass, and thus the BRS-3 antagonist reproduced the phenotype seen in BRS-3-KO mice[34]. Because Bantag-1 is a specific BRS-3 receptor agonist, this increase in body weight suggests that it is inhibiting an endogenous unknown natural agonist, which as yet is unidentified.
3.3.2 In vivo studies on BRS-3 in humans
Only one study[78] has been performed using a BRS-3 selective agonist in humans. This was pharmacokinetic and pharmodynamic study of the potent, selective BRS-3 agonist, MK-5046[78](Table 2) in healthy(n=17) and obese(n=9) male volunteers performed in a double-blind, randomized, placebo-controlled manner. Plasma MK-5046 AUC0-∞ and Cmax increased dose proportionately over the MK-5046, oral dose range studied (10–160 mg) with a terminal half-life of 1.5–3.5hrs. Single doses transiently increased blood pressure and patients reported some adverse events including erections, felling hot, cold and/or jittery[78]. No changes in body weight, heart rate, hunger/satiety or plasma glucose occurred during this short-term study. It was concluded that MK-5046 achieved blood levels that are projected to activate BRS-3 and therefore could be suitable for human studies[78].
3.43. Other Studies on BRS-3 in humans
In humans, studies have been performed to identify genetic alterations in genes encoding BnR’s and their potential association to specific diseases. Specifically, sequencing of the three exons of BRS-3 in104 Japanese obese men did not detect any mutations or polymorphisms[117]. These results are in contrast to findings with another mammalian BnR gene, the NMBR gene. Two SNPs-rs1414839 y rs967790-in the gene encoding the NMBR have been associated with higher weight loss in patients undergoing Roux-en-Y gastric bypass surgery[118]. Furthermore, a rs3809508 polymorphism of the gene encoding the peptide NMB, as well as other mutations in the NMB gene, is associated with increased risk of obesity[119–121].
Lower than normal levels of BRS-3 mRNA/protein were detected in skeletal muscle from obese or patients with type 2 diabetes mellitus (T2D)(Table 3)[59,64]. Furthermore, in patients simultaneously diagnosed with obesity and type 2 diabetes, much lower BRS-3 mRNA levels in skeletal muscle are found, than that previously observed in patients affected by either T2D or obesity, which suggest a potential synergy in the negative impact of these two conditions on BRS—receptor expression[63]. In skeletal muscle myocytes from normals, patients with obesity or with type 2 diabetes mellitus, BRS-3-receptor activation results in stimulation of glucose transport, MAP kinase, p90SRSKI, protein kinase B, PI3K, p70s6 kinase, glycogen synthetase α activity and glycogen synthesis[59,63,64]. In addition the myocytes from patients with the two altered metabolic states alone or together, had increased sensitivity to BRS-3 stimulated glucose uptake and these intracellular cascades (Table 3) [59,63,64]. At present it is unclear whether these changes are related to insulin resistance that is commonly seen in these metabolic states, or what part, if any, it plays in the pathogenesis of these metabolic states. Nevertheless, these alterations, combined with the prominent effect of BRS-3 on energy/glucose homeostasis, insulin secretion and metabolism, all support an important role for the BRS-3-receptor in obesity and diabetes mellitus.
4. Conclusions
Similar to the two well-established bombesin receptors (BnR)[GRPR and NMBR], recent evidence strongly supports a role for the Bn-related orphan receptor, BRS-3 in effecting feeding behavior, as well as, even a wider range of physiological and pathological functions effecting energy and glucose homeostasis, metabolic rate and insulin regulation (Table 3). Each of these areas is reviewed in detail above, and supports the unique role of BRS-3 in energy and glucose homeostasis both in its peripheral and central actions. In animal models, the lack of the BRS-3 gene unleashes a cascade of hormone dysregulation, which are now being defined, which lead to obesity, diabetes, hypertension and various metabolic abnormalities. The increased understanding of the cellular signaling cascades involved in mediating BRS-3’s actions, as well as, increased insights into the mechanisms invoked, coupled with the development of BRS-3 selective agonists and antagonists, which have effects on appetite control as well as body weight, and glucose homeostasis, raises the increased possibility of pharmacological manipulation of BRS-3 activity, as a potential novel approach to the treatment of both body weight disorders much as obesity, as well as type 2 diabetes.
5. Expert Opinion
Both obesity and diabetes mellitus are serious medical conditions that are major global health issues worldwide. It is estimated that 30% of the adult US population has prediabetes mellitus with impaired fasting glucose levels or impaired glucose tolerance and that 13% has type 2 diabetes mellitus (T2D)[122]. In 2014, the International Diabetes Federation estimated 387 million people worldwide have diabetes mellitus, and by 2035 this number will rise to 592 million[123]. T2D, characterized by insulin resistance and relative deficiency of insulin, accounts for 90% of all cases of diabetes[124]. This increased occurrence of T2D both in the United States and worldwide, is primarily driven by rapidly increasing occurrence of obesity, which has almost reached epidemic proportions[125]. Obesity rates for children have tripled in the United States and rates for adults have doubled over the last thirty years, with the result that greater that two-thirds of adults are now considered obese or overweight[124]. Obesity is a multi-system disorder, which is associated with increased occurrence of diabetes mellitus, cancer, coronary heart disease and a number of other complications, which result in an increased mortality [124]. At present there is only limited pharmacological success at treating obesity, with the most effective therapies, being bariatric surgical procedures [126–128]. Pharmacological therapies that could treat both obesity and diabetes mellitus would be particularly desirable. Although glucagon-like peptides, which are widely used to treat the T2D[129,130], and also, have beneficial effects on body weight[131], there is still a great need for additional therapies with combined treatment of these two major health disorders.
As reviewed in this manuscript, possible pharmacological manipulation of the activity of the BRS-3-receptor is a very attractive target that could fulfill this requirement of an effect on both obesity and diabetes mellitus, because of it central role in both glucose and energy homeostasis, as well as its important role in effects on metabolic rate and insulin secretion (Table 3). The clearest insight into the physiological and pathophysiological effects of altered BRS-3 activity, are best shown in the original study of BRS-3-KO-mice[12], and subsequently collaborated in number of more recent studies(Table 3). These studies consistently demonstrate that the BRS-3-KO-mice become obese, develop mild hypertension and demonstrate impaired glucose metabolism, with reduced metabolic rates, increased feeding efficiency and hyperphagia. In the BRS-3-KO-mice the hyperphagia that develops, occurs despite the presence of hyperinsulinemia and hyperleptinemia at 23 weeks[12] suggesting that peripheral hormonal signals related to appetite may be improperly relayed to the brain[106]. These animals have both insulin and leptin resistance[106]. It has been suggested[106] that these results in BRS-3-KO animals are in part, because the targeted BRS-3 deletion upsets the mechanism by which leptin decreases the central MCH-R, resulting in MCH-R overexpression in the hypothalamus of the mutant mice. In BRS-3-KO-mice, the overall findings were so similar to the human conditions that they lead the authors of the initial study[12] to suggest, BRS-3-KO-mice could be a new model to study human obesity and associated diseases, such as diabetes. That these changes were indeed due to the loss of the BRS-3 receptor and not some other mechanism, are supported by recent studies using the BRS-3 receptor antagonist which reproduced and number of these findings, as well as numerous studies using potent, selective BRS-3 agonists, which have the opposite effects[34,75,83,85].
Subsequent studies reviewed above have strongly supported the importance of BRS-3 in energy balance, glucose homeostasis, regulation of feeding, as well as a number of other processes that can affect these, such as alterations of various behaviors (Table 3). These results as well as the encouraging results with the use of BRS-3 agonists, in numerous animals including mice, rats and dogs (Table 3)[34,75,83,85] all attest to the prominent role of the BRS-3 receptor in energy and glucose homeostasis and support the approach of using it as therapeutic target for each of these disorders. One area of prominent concern is the side effect of BRS-3 activation on the cardiovascular system[75,78,84]. Specifically, MK-5046, the best-studied BRS-3 agonist in vivo, demonstrated transient cardiovascular effects in both human and mice consisting of increased heart rate and increased blood pressure[75,78,84]. With continued treatment this was not seen. This side effect because of possible safety concerns is an important issue that needs to be better studied. Is it due to the CNS penetrant properties of MK-5046 and the activation of sympathetic nervous system neurons centrally? If this is circumvented, by peripheral acting agents, is the BRS-3 agonist still as effective at causing weight loss? This is an important issue because many of the BRS-3 agonists increase metabolic rate (Table 3) and in some studies, this is the most prominent effect contributing to the weight loss Table 3). The recent development of peripherally acting BRS-3 agonists without CNS penetration(compounds 9D,G,cmpd 17c, Table 2, Fig. 1)[76,77], and the demonstration that one of these, compound 17c, (Fig. 1, Table 2) can cause weight loss in animals[76], suggests that the peripheral action of BRS-3 may also be therapeutic, without a central component. One would expect that the peripheral effects on glucose and insulin homeostasis seen with the central acting BRS-3 agonists (Table 3), would also be seen with the peripheral acting agonists, but as, of now, this has not been studied. Also at present it is not apparent whether the transient cardiovascular changes with BRS-3 activation by central penetrant formulations will not be a major determinate to their possible use. It is also unknown whether the central activity is essential for potent effects on glucose/energy metabolism, or whether equal potency is seen with the peripheral BRS-3 acting formulations. In conclusion the above results suggest that the use of BRS-3 agonists could be a promising novel area to further explore for the treatment of obesity and/or diabetes.
Fig. 1. Structures of selective BRS-3 agonists and antagonists.
Chemical composition and references are listed in Table legends 1 and 2.
Article Highlights.
BRS-3 remains an orphan, G-protein receptor, but it is included in the bombesin receptor family because of its close homology to other mammalian members of this family
Studies of BRS-3 function using pharmacological approaches, molecular receptor deletion and in vivo studies have confirmed BRS-3’s important role in energy and glucose homeostasis.
BRS-3 is widely expressed in both the central nervous system (particularly the hypothalamic nuclei) and in peripheral tissues including the pancreatic islets of dog, mice, rhesus and human (not rat)
Deletion of BRS-3 results in animals, which are obese, demonstrate impaired glucose metabolism, with reduced metabolic rates, increased feeding efficiency, hyperphagia, mild hypertension, a number of metabolic abnormalities (hyperinsulinemia, hyperleptinemia, decreased growth hormone secretion, lower oxygen consumption), and a number of behavior changes. BRS-3 receptor antagonists reproduce a number of these effects in wild-type mice.
Development of potent, nonpeptide selective BRS-3 agonists has allowed animal and human studies to be performed. These show promise with weight loss, decreased feeding, stimulation of insulin release and effects on glucose homeostasis. However, transient cardiovascular side-effects are seen with central acting formulations, but may be absent in peripheral-acting formulations.
Acknowledgments
This study was partially supported by intramural funds of the NIDDK, NIH
Footnotes
Declaration of interest
Drs N. González, P. Moreno and R.T. Jensen declare that there is not conflict of interest that would prejudice the impartiality of the reported research
References
- 1.Ladenheim EE. Bombesin. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2013. pp. 1064–1070. [Google Scholar]
- 2.Ladenheim EE, Wirth KE, Moran TH. Receptor subtype mediation of feeding suppression by bombesin-like peptides. Pharmacol Biochem Behav. 1996;54:705–711. doi: 10.1016/0091-3057(96)00023-8. [DOI] [PubMed] [Google Scholar]
- 3.Paula GS, Souza LL, Cabanelas A, et al. Female mice target deleted for the neuromedin B receptor have partial resistance to diet-induced obesity. J Physiol. 2010;588:1635–1645. doi: 10.1113/jphysiol.2009.185322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladenheim EE, Hampton LL, Whitney AC, et al. Disruptions in feeding and body weight control in gastrin-releasing peptide receptor deficient mice. J Endocrinol. 2002;174:273–281. doi: 10.1677/joe.0.1740273. [DOI] [PubMed] [Google Scholar]
- 5•.Yamada K, Wada E, Wada K. Bombesin-like peptides: studies on food intake and social behaviour with receptor knock-out mice. Ann Med. 2000;32:519–529. doi: 10.3109/07853890008998831. Summary of behavior changes in GRPR-. NMBR- and BRS-3 receptor-KO mice. [DOI] [PubMed] [Google Scholar]
- 6•.Gorbulev V, Akhundova A, Buchner H, et al. Molecular cloning of a new bombesin receptor subtype expressed in uterus during pregnancy. Eur J Biochem. 1992;208(2):405–410. doi: 10.1111/j.1432-1033.1992.tb17201.x. Initial cloning of BRS from guinea pig. [DOI] [PubMed] [Google Scholar]
- 7•.Fathi Z, Corjay MH, Shapira H, et al. BRS-3: novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. J Biol Chem. 1993;268(8):5979–5984. Initial cloning of human BRS. [PubMed] [Google Scholar]
- 8.Jensen RT, Battey JF, Spindel ER, et al. International Union of Pharmacology. LVIII. Mammalian Bombesin Receptors: Nomenclature, distribution, pharmacology, signaling and functions in normal and disease states. Pharmacol Rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Ramos-Alvarez I, Moreno P, Mantey SA, Nakamura T, Nuche-Berenguer B, Moody TW, Coy DH, Jensen RT. Insights into bombesin receptors and ligands: highlighting recent advances. Peptides. 2015 doi: 10.1016/j.peptides.2015.04.026. Ref Type: In Press detailed update on recent advances with all bombesin receptors: GRPR, NMBR and BRS-3 including cell biology, pharmacology and role in disease states. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez N, Moody TW, Igarashi H, et al. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majumdar ID, Weber HC. Biology and pharmacology of bombesin receptor subtype-3. Curr Opin Endocrinol Diabetes Obes. 2012;19:3–7. doi: 10.1097/MED.0b013e32834ec77d. [DOI] [PubMed] [Google Scholar]
- 12••.Ohki-Hamazaki H, Watase K, Yamamoto K, et al. Mice lacking bombesin receptor subtype-3 develop metabolic defects and obesity. Nature. 1997;390(6656):165–169. doi: 10.1038/36568. Original study revealing important effects of BRS-3 on energy and glucose metabolism by assessing BRS-3-KO mice. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RT, Moody TW. Bombesin-Related Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2013. pp. 1188–1196. [Google Scholar]
- 14.Uehara H, Gonzalez N, Sancho V, et al. Pharmacology and selectivity of various natural and synthetic bombesin related peptide agonists for human and rat bombesin receptors differs. Peptides. 2011;32:1685–1699. doi: 10.1016/j.peptides.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Sano H, Feighner SD, Hreniuk DL, et al. Characterization of the bombesin-like peptide receptor family in primates. Genomics. 2004;84:139–146. doi: 10.1016/j.ygeno.2004.01.008. Detailed quantitative assessment of the distribution of GRPR, NMBR and BRS-3 in rhesus monkey. [DOI] [PubMed] [Google Scholar]
- 16.Wada E, Way J, Lebacq-Verheyden AM, et al. Neuromedin B and gastrin-releasing peptide mRNAs are differentially distributed in the rat nervous system. J Neurosci. 1990;10:2917–2930. doi: 10.1523/JNEUROSCI.10-09-02917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: effects on cancer growth/proliferation and cellular signaling in cancer. In: Kastin AJ, editor. Handbook of Biologically active peptides. Amsterdam: Elsevier; 2006. pp. 429–434. [Google Scholar]
- 18.Weber HC. Gastrointestinal peptides and itch sensation. Curr Opin Endocrinol Diabetes Obes. 2015;22:29–33. doi: 10.1097/MED.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 19.Ohki-Hamazaki H, Wada E, Matsui K, et al. Cloning and expression of the neuromedin B receptor and the third subtype of bombesin receptor genes in the mouse. Brain Res. 1997;762:165–172. doi: 10.1016/s0006-8993(97)00380-6. [DOI] [PubMed] [Google Scholar]
- 20.Weber HC, Hampton LL, Jensen RT, et al. Structure and chromosomal localization of the mouse bombesin receptor subtype 3 gene. Gene. 1998;211:125–131. doi: 10.1016/s0378-1119(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Lao ZJ, Zhang J, et al. Molecular basis of the pharmacological difference between rat and human bombesin receptor subtype-3 (BRS-3) Biochemistry (Mosc) 2002;41:8954–8960. doi: 10.1021/bi0202777. [DOI] [PubMed] [Google Scholar]
- 22.Whitley JC, Moore C, Giraud AS, et al. Molecular cloning, genomic organization and selective expression of bombesin receptor subtype 3 in the sheep hypothalamus and pituitary. J Mol Endocrinol. 1999;23:107–116. doi: 10.1677/jme.0.0230107. [DOI] [PubMed] [Google Scholar]
- 23.Spindel ER. Bombesin Peptides. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2013. pp. 325–330. [Google Scholar]
- 24•.Mantey SA, Weber HC, Sainz E, et al. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. J Biol Chem. 1997;272(41):26062–26071. doi: 10.1074/jbc.272.41.26062. First description of a potent, nonselective BRS-3 agonist. [DOI] [PubMed] [Google Scholar]
- 25•.Moreno P, Mantey SA, Nuche-Berenguer B, et al. Comparative pharmacology of bombesin receptor subtype-3, nonpeptide agonist MK-5046, a universal peptide agonist, and peptide antagonist Bantag-1 for human bombesin receptors. J Pharmacol Exp Ther. 2013;347:100–116. doi: 10.1124/jpet.113.206896. Detailed description of pharmacology of one of most selective nonpeptide BRS-3 agonists, MK-5046 and peptide antagonists, Bantag-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pradhan TK, Katsuno T, Taylor JE, et al. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. Eur J Pharmacol. 1998;343:275–287. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- 27.Ryan RR, Katsuno T, Mantey SA, et al. Comparative pharmacology of a nonpeptoid neuromedin B antagonist PD 168368. J Pharmacol Exp Ther. 1999;290:1202–1211. [PubMed] [Google Scholar]
- 28.Ryan RR, Weber HC, Mantey SA, et al. Pharmacology and intracellular signaling mechanisms of the native human orphan receptor BRS-3 in lung cancer cells. J Pharmacol Exp Ther. 1998;287:366–380. [PubMed] [Google Scholar]
- 29.Reubi JC, Wenger S, Schumuckli-Maurer J, et al. Bombesin receptor subtypes in human cancers: detection with the universal radoligand (125)I-[D-TYR(6), beta-ALA(11),PHE(13), NLE(14)] bombesin(6–14) Clin Cancer Res. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 30.Ramos-Alvarez I, Nakamura T, Mantey SA, Jensen RT. Chiral diazepine analogs are selective, potent agonists for the orphan bombesin receptor, BRS-3. Gastroenterology. 2015;148(5) in press. Ref Type: Abstract. [Google Scholar]
- 31•.Feng Y, Guan XM, Li J, et al. Bombesin Receptor Subtype-3 (BRS-3) Regulates Glucose-Stimulated Insulin Secretion in Pancreatic Islets across Multiple Species. Endocrinology. 2011;152:4106–4115. doi: 10.1210/en.2011-1440. Detailed study of expression of BRS-3 in islets and effect on islet function. [DOI] [PubMed] [Google Scholar]
- 32.Jennings CA, Harrison DC, Maycox PR, et al. The distribution of the orphan bombesin receptor subtype-3 in the rat CNS. Neuroscience. 2003;120:309–324. doi: 10.1016/s0306-4522(03)00260-4. [DOI] [PubMed] [Google Scholar]
- 33.Porcher C, Juhem A, Peinnequin A, et al. Bombesin receptor subtype-3 is expressed by the enteric nervous system and by interstitial cells of Cajal in the rat gastrointestinal tract. Cell Tissue Res. 2005;320:21–31. doi: 10.1007/s00441-004-1032-1. [DOI] [PubMed] [Google Scholar]
- 34•.Guan XM, Chen H, Dobbelaar PH, et al. Regulation of Energy Homeostasis by Bombesin Receptor Subtype-3: Selective Receptor Agonists for the Treatment of Obesity. Cell Metab. 2010;11:101–112. doi: 10.1016/j.cmet.2009.12.008. Detailed study of effect of a BRS-3 antagonist on food intake and body weight as well as effect of a BRS-3 agonist on feeding/body weight in mice with KO’s of genes effecting energy/feeding behaviors. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Parks GS, Wang Z, et al. Anatomical characterization of bombesin receptor subtype-3 mRNA expression in the rodent central nervous system. J Comp Neurol. 2013;521:1020–1039. doi: 10.1002/cne.23216. [DOI] [PubMed] [Google Scholar]
- 36.Jensen RT, Battey J, Benya RV, Moody TW. Bombesin Receptors. IUPHAR/BPS guide to pharamcology (WEBSITE) 2014 http;//guidetophramacolgy.org/GRAC/FamilyDisplayForward?familyld=9. Ref Type: Generic.
- 37.Rozengurt E. Mitogenic signaling pathways induced by G protein-coupled receptors. J Cell Physiol. 2007;213:589–602. doi: 10.1002/jcp.21246. [DOI] [PubMed] [Google Scholar]
- 38.Ryan RR, Weber HC, Hou W, et al. Ability of various bombesin receptor agonists and antagonists to alter intracellular signaling of the human orphan receptor BRS-3. J Biol Chem. 1998;273:13613–13624. doi: 10.1074/jbc.273.22.13613. [DOI] [PubMed] [Google Scholar]
- 39.Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16:66–71. doi: 10.1097/med.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- 40.Qin X, Qu X, Coy D, et al. A selective human bombesin receptor subtype-3 peptide agonist mediates CREB phosphorylation and transactivation. J Mol Neurosci. 2012;46:88–99. doi: 10.1007/s12031-011-9675-3. [DOI] [PubMed] [Google Scholar]
- 41.Patel M, Kawano T, Suzuki N, et al. Galpha13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol Pharmacol. 2014;86:252–262. doi: 10.1124/mol.114.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuda T, Kusui T, Jensen RT. Neuromedin B receptor activation causes tyrosine phosphorylation of p125FAK by a phospholipase C independent mechanism which requires p21rho and integrity of the acini cytoskeleton. Biochemistry (Mosc) 1997;36(51):16328–16337. doi: 10.1021/bi971448o. [DOI] [PubMed] [Google Scholar]
- 43.Berna MJ, Hoffmann KM, Tapia JA, et al. CCK causes PKD1 activation in pancreatic acini by signaling through PKC-delta and PKC-independent pathways. Biochim Biophys Acta. 2007;1773:483–501. doi: 10.1016/j.bbamcr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pace A, Tapia JA, Garcia-Marin LJ, et al. The Src family kinase, Lyn, is activated in pancreatic acinar cells by gastrointestinal hormones/neurotransmitters and growth factors which stimulate its association with numerous other signaling molecules. Biochim Biophys Acta. 2006;1763:356–365. doi: 10.1016/j.bbamcr.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Williams BY, Wang Y, Schonbrunn A. Agonist binding and protein kinase C activation stimulate phosphorylation of the gastrin-releasing peptide receptor at distinct sites. Mol Pharmacol. 1996;50:716–727. [PubMed] [Google Scholar]
- 46.Benya RV, Akeson M, Mrozinski J, et al. Internalization of the gastrin-releasing peptide receptor is mediated by phospholipase C-dependent and -independent processes. Mol Pharmacol. 1994;46:495–501. [PubMed] [Google Scholar]
- 47.Benya RV, Fathi Z, Battey JF, et al. Serines and threonines in the gastrin-releasing peptide receptor carboxyl terminus mediate internalization. J Biol Chem. 1993;268:20285–20290. [PubMed] [Google Scholar]
- 48.Benya RV, Fathi Z, Pradhan T, et al. Gastrin-releasing peptide receptor-induced internalization, down-regulation, desensitization and growth: Possible role of cAMP. Mol Pharmacol. 1994;46(2):235–245. [PubMed] [Google Scholar]
- 49.Benya RV, Kusui T, Battey JF, et al. Desensitizaton of neuromedin B receptors (NMB-R) on native and NMB-R transfected cells involves down-regulation and internalization. J Biol Chem. 1994;269:11721–11728. [PubMed] [Google Scholar]
- 50.Benya RV, Kusui T, Battey JF, et al. Chronic desensitization and down-regulation of the gastrin-releasing peptide receptor are mediated by a protein kinase C-dependent mechanism. J Biol Chem. 1995;270:3346–3352. doi: 10.1074/jbc.270.7.3346. [DOI] [PubMed] [Google Scholar]
- 51.Moody TW, Chan D, Fahrenkrug J, et al. Neuropeptides as autocrine growth factors in cancer cells. Curr Pharm Des. 2003;9:495–509. doi: 10.2174/1381612033391621. [DOI] [PubMed] [Google Scholar]
- 52.Jensen RT, Moody TW. Bombesin Peptides (Cancer) In: Kastin AJ, editor. Handbook of Biologically Active Peptides. Amsterdam: Elsevier; 2013. pp. 506–511. [Google Scholar]
- 53.Moody TW, Mantey SA, Moreno P, et al. ML-18 is a non-peptide bombesin receptor subtype-3 antagonist which inhibits lung cancer growth. Peptides. 2014 doi: 10.1016/j.peptides.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moody TW, Nuche-Berenguer B, Nakamura T, et al. EGFR Transactivation by Peptide G Protein-Coupled Receptors in Cancer. Curr Drug Targets. 2015 doi: 10.2174/1389450116666150107153609. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Carlisle DL, Swick MC, et al. Gastrin-releasing peptide activates Akt through the epidermal growth factor receptor pathway and abrogates the effect of gefitinib. Exp Cell Res. 2007;313:1361–1372. doi: 10.1016/j.yexcr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Moody TW, Berna MJ, Mantey S, et al. Neuromedin B receptors regulate EGF receptor tyrosine phosphorylation in lung cancer cells. Eur J Pharmacol. 2010;637:38–45. doi: 10.1016/j.ejphar.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moody TW, Sancho V, Di Florio A, et al. Bombesin receptor subtype-3 agonists stimulate the growth of lung cancer cells and increase EGF receptor tyrosine phosphorylation. Peptides. 2011;32:1677–1684. doi: 10.1016/j.peptides.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Florio A, Sancho V, Moreno P, et al. Gastrointestinal hormones stimulate growth of Foregut Neuroendocrine Tumors by transactivating the EGF receptor. Biochim Biophys Acta. 2013;1833:573–582. doi: 10.1016/j.bbamcr.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramos-Alvarez I, Moreno-Villegas Z, Martin-Duce A, et al. Human BRS-3 receptor: functions/role in cell signaling pathways and glucose metabolism in obese or diabetic myocytes. Peptides. 2014;51:91–99. doi: 10.1016/j.peptides.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Majumdar ID, Weber HC. Biology of mammalian bombesin-like peptides and their receptors. Curr Opin Endocrinol Diabetes Obes. 2011;18:68–74. doi: 10.1097/MED.0b013e328340ff93. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Q, Bhola NE, Lui VW, et al. Antitumor mechanisms of combined gastrin-releasing peptide receptor and epidermal growth factor receptor targeting in head and neck cancer. Mol Cancer Ther. 2007;6:1414–1424. doi: 10.1158/1535-7163.MCT-06-0678. [DOI] [PubMed] [Google Scholar]
- 62.Sancho V, Di Florio A, Moody TW, et al. Bombesin receptor-mediated imaging and cytotoxicity: review and current status. Curr Drug Deliv. 2011;8:79–134. doi: 10.2174/156720111793663624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63•.Gonzalez N, Martin-Duce A, Martinez-Arrieta F, et al. Effect of bombesin receptor subtype-3 and its synthetic agonist on signaling, glucose transport and metabolism in myocytes from patients with obesity and type 2 diabetes. Int J Mol Med. 2015 doi: 10.3892/ijmm.2015.2090. Recent report of altered BRS-3 expression levels and signaling alterations of human BRS-3 in skeletal muscle cells of patients with obesity and diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramos-Alvarez I, Martin-Duce A, Moreno-Villegas Z, et al. Bombesin receptor subtype-3 (BRS-3), a novel candidate as therapeutic molecular target in obesity and diabetes. Mol Cell Endocrinol. 2013;367:109–115. doi: 10.1016/j.mce.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 65.Mantey SA, Coy DH, Entsuah LK, et al. Development of bombesin analogs with conformationally restricted amino acid substitutions with enhanced selectivity for the orphan receptor human bombesin receptor subtype 3. J Pharmacol Exp Ther. 2004;310:1161–1170. doi: 10.1124/jpet.104.066761. [DOI] [PubMed] [Google Scholar]
- 66.Mantey SA, Coy DH, Pradhan TK, et al. Rational design of a peptide agonist that interacts selectively with the orphan receptor, bombesin receptor subtype 3. J Biol Chem. 2001;276:9219–9229. doi: 10.1074/jbc.M008737200. [DOI] [PubMed] [Google Scholar]
- 67.Boyle RG, Humphries J, Mitchell T, et al. The design of a new potent and selective ligand for the orphan bombesin receptor subtype 3 (BRS3) J Pept Sci. 2005;11:136–141. doi: 10.1002/psc.599. [DOI] [PubMed] [Google Scholar]
- 68.Weber D, Berger C, Heinrich T, et al. Systematic optimization of a lead-structure identities for a selective short peptide agonist for the human orphan receptor BRS-3. J Pept Sci. 2002;8:461–475. doi: 10.1002/psc.407. [DOI] [PubMed] [Google Scholar]
- 69.Zhang L, Nothacker HP, Wang Z, et al. Pharmacological characterization of a selective agonist for bombesin receptor subtype-3. Biochem Biophys Res Commun. 2009;387:283–288. doi: 10.1016/j.bbrc.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majumdar ID, Weber HC. Appetite-modifying effects of bombesin receptor subtype-3 agonists. Handb Exp Pharmacol. 2012:405–432. doi: 10.1007/978-3-642-24716-3_19. [DOI] [PubMed] [Google Scholar]
- 71.Mantey SA, Gonzalez N, Schumann M, et al. Identification of bombesin receptor subtype-specific ligands: effect of N-methyl scanning, truncation, substitution, and evaluation of putative reported selective ligands. J Pharmacol Exp Ther. 2006;319:980–989. doi: 10.1124/jpet.106.107011. [DOI] [PubMed] [Google Scholar]
- 72.Sancho V, Moody TW, Mantey SA, et al. Pharmacology of putative selective hBRS-3 receptor agonists for human bombesin receptors (BnR): Affinities, potencies and selectivity in multiple native and BnR transfected cells. Peptides. 2010;31:1569–1578. doi: 10.1016/j.peptides.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonzalez N, Hocart SJ, Portal-Nunez S, et al. Molecular basis for agonist selectivity and activation of the orphan bombesin receptor subtype 3 receptor. J Pharmacol Exp Ther. 2008;324:463–474. doi: 10.1124/jpet.107.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coll AP. Treating Obesity? It’s in the Bag! Cell Metab. 2010;11:95–96. doi: 10.1016/j.cmet.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 75••.Guan XM, Metzger JM, Yang L, et al. Antiobesity effect of MK-5046, a novel bombesin receptor subtype-3 agonist. J Pharmacol Exp Ther. 2011;336:356–364. doi: 10.1124/jpet.110.174763. Detailed description of the effect of the oral, nonpeptide BRS-3 agonist, MK-5046 on energy metabolism, feeding body weight in animals. [DOI] [PubMed] [Google Scholar]
- 76.Matsufuji T, Shimada K, Kobayashi S, et al. Synthesis and biological evaluation of novel chiral diazepine derivatives as bombesin receptor subtype-3 (BRS-3) agonists incorporating an antedrug approach. Bioorg Med Chem. 2015;23:89–104. doi: 10.1016/j.bmc.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 77•.Matsufuji T, Shimada K, Kobayashi S, et al. Discovery of novel chiral diazepines as bombesin receptor subtype-3 (BRS-3) agonists with low brain penetration. Bioorg Med Chem Lett. 2014;24:750–755. doi: 10.1016/j.bmcl.2013.12.106. Description of peripheral acting BRS-3 agonists with effects on body weight/feeding. [DOI] [PubMed] [Google Scholar]
- 78••.Reitman ML, Dishy V, Moreau A, et al. Pharmacokinetics and pharmacodynamics of MK-5046, a bombesin receptor subtype-3 (BRS-3) agonist, in healthy patients. J Clin Pharmacol. 2012;52:1306–1316. doi: 10.1177/0091270011419854. First human study of BRS-3 agonist: A pharmacokinetic and pharmacodynamic study of MK-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sebhat IK, Franklin C, Lo MC, et al. Discovery of MK-5046, a potent, selective bombesin recptor subtype-3 agonist for the treatment of obesity. ACS Medicinal Chemistry Letters. 2011;2:43–47. doi: 10.1021/ml100196d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chobanian HR, Guo Y, Liu P, et al. The design and synthesis of potent, selective benzodiazepine sulfonamide bombesin receptor subtype 3 (BRS-3) agonists with an increased barrier of atropisomerization. Bioorg Med Chem. 2012;20:2845–2849. doi: 10.1016/j.bmc.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 81.Chobanian HR, Guo Y, Liu P, et al. Discovery of MK-7725, A Potent, Selective Bombesin Receptor Subtype-3 Agonist for the Treatment of Obesity. ACS Med Chem Lett. 2012;3:252–256. doi: 10.1021/ml200304j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hadden M, Goodman A, Guo C, et al. Synthesis and SAR of heterocyclic carboxylic acid isosteres based on 2-biarylethylimidazole as bombesin receptor subtype-3 (BRS-3) agonists for the treatment of obesity. Bioorg Med Chem Lett. 2010;20:2912–2915. doi: 10.1016/j.bmcl.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 83.He S, Dobbelaar PH, Liu J, et al. Discovery of substituted biphenyl imidazoles as potent, bioavailable bombesin receptor subtype-3 agonists. Bioorg Med Chem Lett. 2010;20:1913–1917. doi: 10.1016/j.bmcl.2010.01.154. [DOI] [PubMed] [Google Scholar]
- 84.Lateef DM, breu-Vieira G, Xiao C, et al. Regulation of body temperature and brown adipose tissue thermogenesis by bombesin receptor subtype-3. Am J Physiol Endocrinol Metab. 2014;306:E681–E687. doi: 10.1152/ajpendo.00615.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J, He S, Jian T, et al. Synthesis and SAR of derivatives based on 2-biarylethylimidazole as bombesin receptor subtype-3 (BRS-3) agonists for the treatment of obesity. Bioorg Med Chem Lett. 2010;20:2074–2077. doi: 10.1016/j.bmcl.2010.02.076. [DOI] [PubMed] [Google Scholar]
- 86.Liu P, Lanza TJ, Jr, Chioda M, et al. Discovery of benzodiazepine sulfonamide-based bombesin receptor subtype 3 agonists and their unusual chirality. ACS Med Chem Lett. 2011;2:933–937. doi: 10.1021/ml200207w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lo MM, Chobanian HR, Palyha O, et al. Pyridinesulfonylureas and pyridinesulfonamides as selective bombesin receptor subtype-3 (BRS-3) agonists. Bioorg Med Chem Lett. 2011;21:2040–2043. doi: 10.1016/j.bmcl.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Carlton DL, Collin-Smith LJ, Daniels AJ, et al. Discovery of small molecule agonists for the bombesin receptor subtype 3 (BRS-3) based on an omeprazole lead. Bioorg Med Chem Lett. 2008;18:5451–5455. doi: 10.1016/j.bmcl.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 89.Gonzalez N, Mantey SA, Pradhan TK, et al. Characterization of putative GRP- and NMB-receptor antagonist’s interaction with human receptors. Peptides. 2009;30:1473–1486. doi: 10.1016/j.peptides.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jensen RT, Coy DH. Progress in the development of potent bombesin receptor antagonists. Trends Pharmacol Sci. 1991;12(1):13–19. doi: 10.1016/0165-6147(91)90483-9. [DOI] [PubMed] [Google Scholar]
- 91.Wang LH, Coy DH, Taylor JE, et al. Desmethionine alkylamide bombesin analogues: a new class of bombesin receptor antagonists with a potent antisecretory activity in pancreatic acini and antimitotic activity in Swiss 3T3 cells. Biochemistry (Mosc) 1990;29(3):616–622. doi: 10.1021/bi00455a004. [DOI] [PubMed] [Google Scholar]
- 92.Wang LH, Coy DH, Taylor JE, et al. Des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J Biol Chem. 1990;265(26):15695–15703. [PubMed] [Google Scholar]
- 93.Heinz-Erian P, Coy DH, Tamura M, et al. [D-Phe12]bombesin analogues: a new class of bombesin receptor antagonists. Am J Physiol. 1987;252:G439–G442. doi: 10.1152/ajpgi.1987.252.3.G439. [DOI] [PubMed] [Google Scholar]
- 94.Coy DH, Taylor JE, Jiang NY, et al. Short-chain pseudopeptide bombesin receptor antagonists with enhanced binding affinities for pancreatic acinar and Swiss 3T3 cells display strong antimitotic activity. J Biol Chem. 1989;264:14691–14697. [PubMed] [Google Scholar]
- 95.von Schrenck T, Wang LH, Coy DH, et al. Potent bombesin receptor antagonists distinguish receptor subtypes. Am J Physiol. 1990;259:G468–G473. doi: 10.1152/ajpgi.1990.259.3.G468. [DOI] [PubMed] [Google Scholar]
- 96.Maina T, Nock BA, Zhang H, et al. Species differences of bombesin analog interactions with GRP-R define the choice of animal models in the development of GRP-R-targeting drugs. J Nucl Med. 2005;46:823–830. [PubMed] [Google Scholar]
- 97.Coy DH, Wang LH, Jiang NZ, et al. Short chain bombesin pseudopeptides which are potent and more general bombesin receptor antagonists. Eur J Pharmacol. 1990;190(#1/2):31–38. doi: 10.1016/0014-2999(90)94109-b. [DOI] [PubMed] [Google Scholar]
- 98.Tsuda T, Kusui T, Hou W, et al. Effect of gastrin-releasing peptide receptor number on receptor affinity, coupling, degradation and receptor modulation. Mol Pharmacol. 1997;51(5):721–732. doi: 10.1124/mol.51.5.721. [DOI] [PubMed] [Google Scholar]
- 99.Ashwood V, Brownhill V, Higginbottom M, et al. PD 176252 - The first high affinity non-peptide gastrin-releasing peptide (BB2) receptor antagonist. Bioorg Med Chem. 1998;8:2589–2594. doi: 10.1016/s0960-894x(98)00462-4. [DOI] [PubMed] [Google Scholar]
- 100.Matsumoto K, Yamada K, Wada E, et al. Bombesin receptor subtype-3 modulates plasma insulin concentration. Peptides. 2003;24:83–90. doi: 10.1016/s0196-9781(02)00279-6. [DOI] [PubMed] [Google Scholar]
- 101•.Ladenheim EE, Hamilton NL, Behles RR, et al. Factors contributing to obesity in bombesin receptor subtype-3-deficient mice. Endocrinology. 2008;149:971–978. doi: 10.1210/en.2007-1319. Detailed study of the mechanisms involved in BRS-3-KO mice resulting in altered energy, body weight and glucose metabolism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakamichi Y, Wada E, Aoki K, et al. Functions of pancreatic beta cells and adipocytes in bombesin receptor subtype-3-deficient mice. Biochem Biophys Res Commun. 2004;318:698–703. doi: 10.1016/j.bbrc.2004.04.081. [DOI] [PubMed] [Google Scholar]
- 103.Yamada K, Ohki-Hamazaki H, Wada K. Differential effects of social isolation upon body weight, food consumption, and responsiveness to novel and social environment in bombesin receptor subtype-3 (BRS-3) deficient mice. Physiol Behav. 2000;68:555–561. doi: 10.1016/s0031-9384(99)00214-0. [DOI] [PubMed] [Google Scholar]
- 104.Yamada K, Santo-Yamada Y, Wada E, et al. Role of bombesin (BN)-like peptides/receptors in emotional behavior by comparison of three strains of BN-like peptide receptor knockout mice. Mol Psychiatry. 2002;7:113–7. 6. doi: 10.1038/sj.mp.4000974. [DOI] [PubMed] [Google Scholar]
- 105.Aoki K, Sun YJ, Aoki S, et al. Cloning, expression, and mapping of a gene that is upregulated in adipose tissue of mice deficient in bombesin receptor subtype-3. Biochem Biophys Res Commun. 2002;290:1282–1288. doi: 10.1006/bbrc.2002.6337. [DOI] [PubMed] [Google Scholar]
- 106.Maekawa F, Quah HM, Tanaka K, et al. Leptin resistance and enhancement of feeding facilitation by melanin-concentrating hormone in mice lacking bombesin receptor subtype-3. Diabetes. 2004;53:570–576. doi: 10.2337/diabetes.53.3.570. [DOI] [PubMed] [Google Scholar]
- 107.Miltenberger RJ, Mynatt RL, Wilkinson JE, et al. The role of the agouti gene in the yellow obese syndrome. J Nutr. 1997;127:1902S–1907S. doi: 10.1093/jn/127.9.1902S. [DOI] [PubMed] [Google Scholar]
- 108.Ollmann MM, Wilson BD, Yang YK, et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 109.Voisey J, Carroll L, van Daal A. Melanocortins and their receptors and antagonists. Curr Drug Targets. 2003;4:586–597. doi: 10.2174/1389450033490858. [DOI] [PubMed] [Google Scholar]
- 110.Matsumoto K, Iijima H. Sibutramine sensitivity assay revealed a unique phenotype of bombesin BB3 receptor-deficient mice. Eur J Pharmacol. 2003;473:41–46. doi: 10.1016/s0014-2999(03)01908-3. [DOI] [PubMed] [Google Scholar]
- 111.Yamada K, Wada E, Imaki J, et al. Hyperresponsiveness to palatable and aversive taste stimuli in genetically obese (bombesin receptor subtype-3-deficient) mice. Physiol Behav. 1999;66:863–867. doi: 10.1016/s0031-9384(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 112.Garcia-Belenguer S, Oliver C, Mormede P. Facilitation and feedback in the hypothalamo-pituitary-adrenal axis during food restriction in rats. J Neuroendocrinol. 1993;5:663–668. doi: 10.1111/j.1365-2826.1993.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 113.Furutani N, Hondo M, Tsujino N, et al. Activation of bombesin receptor subtype-3 influences activity of orexin neurons by both direct and indirect pathways. J Mol Neurosci. 2010;42:106–111. doi: 10.1007/s12031-010-9382-5. [DOI] [PubMed] [Google Scholar]
- 114.Waser B, Eltschinger V, Linder K, et al. Selective in vitro targeting of GRP and NMB receptors in human tumours with the new bombesin tracer (177)Lu-AMBA. Eur J Nucl Med Mol Imaging. 2007;34:95–100. doi: 10.1007/s00259-006-0229-9. [DOI] [PubMed] [Google Scholar]
- 115.Fleischmann A, Laderach U, Friess H, et al. Bombesin receptors in distinct tissue compartments of human pancreatic diseases. Lab Invest. 2000;80:1807–1817. doi: 10.1038/labinvest.3780192. [DOI] [PubMed] [Google Scholar]
- 116.Metzger JM, Gagen K, Raustad KA, et al. Body temperature as a mouse pharmacodynamic response to bombesin receptor subtype-3 agonists and other potential obesity treatments. Am J Physiol Endocrinol Metab. 2010;299:E816–E824. doi: 10.1152/ajpendo.00404.2010. [DOI] [PubMed] [Google Scholar]
- 117.Hotta K, Matsukawa Y, Nishida M, et al. Mutation in bombesin receptor subtype-3 gene is not a major cause of obesity in the Japanese. Horm Metab Res. 2000;32:33–34. doi: 10.1055/s-2007-978582. [DOI] [PubMed] [Google Scholar]
- 118.Rinella ES, Still C, Shao Y, et al. Genome-wide association of single-nucleotide polymorphisms with weight loss outcomes after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2013;98:E1131–E1136. doi: 10.1210/jc.2012-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spalova J, Zamrazilova H, Vcelak J, et al. Neuromedin beta: P73T polymorphism in overweight and obese subjects. Physiol Res. 2008;57(Suppl 1):S39–S48. doi: 10.33549/physiolres.931488. [DOI] [PubMed] [Google Scholar]
- 120.Bouchard L, Drapeau V, Provencher V, et al. Neuromedin beta: a strong candidate gene linking eating behaviors and susceptibility to obesity. Am J Clin Nutr. 2004;80:1478–1486. doi: 10.1093/ajcn/80.6.1478. [DOI] [PubMed] [Google Scholar]
- 121.Pigeyre M, Bokor S, Romon M, et al. Influence of maternal educational level on the association between the rs3809508 neuromedin B gene polymorphism and the risk of obesity in the HELENA study. Int J Obes (Lond) 2010;34:478–486. doi: 10.1038/ijo.2009.260. [DOI] [PubMed] [Google Scholar]
- 122.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.International Diabetes Federation. IDF Diabetes Atlas upadte poster. 6. Brussels, Belgium: International Diabetes Foundation; 2014. [Google Scholar]
- 124.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 125.Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 126.Bariatric surgery: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5:1–148. [PMC free article] [PubMed] [Google Scholar]
- 127.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8:iii–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 128.Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011:CD006423. doi: 10.1002/14651858.CD006423.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lund A, Knop FK, Vilsboll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25:407–414. doi: 10.1016/j.ejim.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 131.Vilsboll T, Christensen M, Junker AE, et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tokita K, Hocart SJ, Katsuno T, et al. Tyrosine 220 in the fifth transmembrane domain of the neuromedin B receptor is critical for the high selectivity of the peptoid antagonist PD168368. J Biol Chem. 2001;276:495–504. doi: 10.1074/jbc.M006059200. [DOI] [PubMed] [Google Scholar]
- 133.Benya RV, Kusui T, Pradhan TK, et al. Expression and characterization of cloned human bombesin receptors. Mol Pharmacol. 1995;47:10–20. [PubMed] [Google Scholar]