Abstract
The continued evolution of biomedical nanotechnology has enabled clinicians to better detect, prevent, manage, and treat human disease. In order to further push the limits of nanoparticle performance and functionality, there has recently been a paradigm shift towards biomimetic design strategies. By taking inspiration from nature, the goal is to create next-generation nanoparticle platforms that can more effectively navigate and interact with the incredibly complex biological systems that exist within the body. Of great interest are cellular membranes, which play essential roles in biointerfacing, self-identification, signal transduction, and compartmentalization. In this review, we explore the major ways in which researchers have directly leveraged cell membrane-derived biomaterials for the fabrication of novel nanotherapeutics and nanodiagnostics. Such emerging technologies have the potential to significantly advance the field of nanomedicine, helping to improve upon traditional modalities while also enabling novel applications.
Keywords: cell membrane, biomimetic nanoparticle, drug delivery, immunotherapy, detoxification, imaging
Graphical abstract
1. Introduction
Nanotechnology has had a significant impact on medicine, helping to create better solutions for addressing some of the most pressing human health needs [1,2]. While nanoparticle technology was initially applied towards the design of cancer therapies, a wide range of applications, from the treatment of antibiotic resistant bacteria to creating immunological tolerance, are being explored both in the lab and in the clinic [3,4]. When designing effective nanoformulations, it is of the utmost importance that they perform well in the complex environments that are present in the human body. As such, a significant amount of effort has been placed on exploring methods to make nanoparticles interact and interface more efficiently with biological systems [5].
This can be daunting task, however, as most nanoparticulate platforms are synthetic. Their foreign nature can present major hurdles because the body is adept at detecting and eliminating anything that it does not recognize as endogenous [6]. Further, it can be hard to artificially replicate the biological interactions that naturally occur in the body, which often have exceptional sensitivity and specificity. Instead of creating new solutions from the ground-up, it behooves researchers working in the field of nanomedicine to look towards nature for inspiration, taking design cues directly from biological systems. Such a strategy can enable the facile discovery and streamlined development of new ways in which to boost nanoparticle performance. Indeed, an entire area of research dedicated towards biomimetic nanotechnologies has spawned in recent years [7,8].
One area towards which researchers have shown considerable interest is the mimicry of cellular functions [4]. As one of the most fundamental biological units, cells are tasked with carrying out many of the basic functions necessary for life. They can often be specialized, and each type of cell has its own set of unique characteristics. For example, red blood cells (RBCs) are responsible to delivering oxygen and circulate for extended periods of time, while platelets maintain hemostasis and can adhere to many disease-relevant substrates. Ultimately, a significant portion of these cell-specific functions are governed by their membranes, a collection of biomacromolecules embedded into a lipid bilayer that is responsible for the many levels of cellular compartmentalization. This complex biological assembly is capable of governing how a cell physically interacts with others, takes in signals, anchors binding interactions, and modulates immunity among many others.
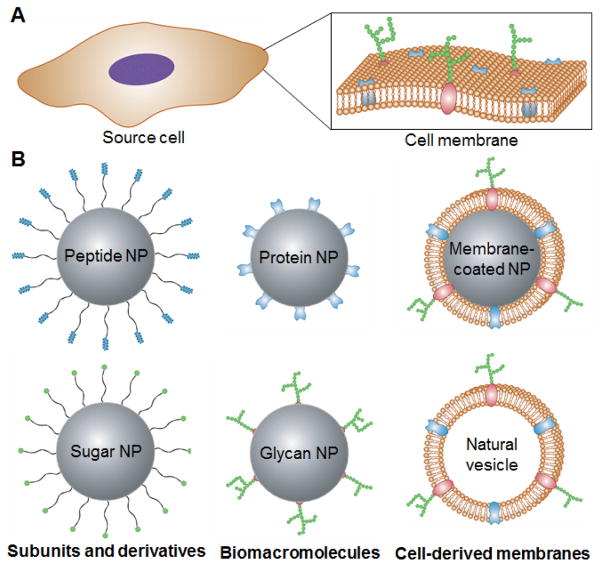
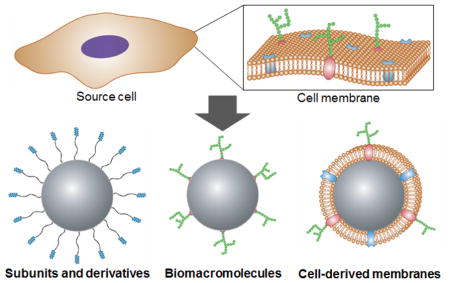
In this review, we discuss nanoscale platforms that directly leverage cell membrane-derived materials and their applications (Fig. 1). Regarding individual membrane components, two main classes, namely proteins and glycans, are covered. We start with an overview of their single molecules or subunit derivatives, and this is followed by discussion on the direct use of these biomacromolecules. The final section covers how researchers have employed natural cell membrane structures for nanoparticle design. This includes vesicular structures, such as exosomes or outer membrane vesicles (OMVs), as well as newly reported cell membrane-coated nanoparticles, which combine the advantageous of both natural membrane materials and synthetic nanoparticulate cores.
Fig. 1. Cell membrane-based strategies for nanoparticle functionalization.
A) Many cellular properties are determined by membrane composition, including the different proteins and glycan structures that are present. B) Examples of different cell membrane-based design strategies for fabricating biomimetic nanoparticles. Single molecule or subunit derivatives of cell membrane glycans and proteins can be used as functional ligands. Biomacromolecule components of the cell membrane, such as proteins and glycans, can be used to add specific functionalities. Further, entire cell membranes can be leveraged either directly as vesicular structures or as the coating material for a nanoparticulate core.
2. Membrane-derived molecules and subunits
Simple sugars and peptides generally represent the smallest functional derivatives of glycans and proteins, respectively, and both have been widely explored for introducing nanoparticle functionality. Unlike their more complex counterparts, these smaller compounds can often be faithfully replicated by synthetic means. Especially for peptides, many workflows exist for the identification of high affinity ligands that are not found in nature [9]. In this section, however, we will focus exclusively on naturally occurring derivatives that have their origins on the cell membrane.
2.1 Simple sugars
Carbohydrates are important components of the cell membrane that generally occupy 2 to 10 percent of its mass [10]. Cell surface carbohydrates are usually present in the form of glycoconjugates with either proteins or lipids [11]. These glycoproteins and glycolipids are abundant on the cell membrane, and their saccharide components play important roles in a variety of cell signaling events, particularly in specific cell recognition processes [12]. Common monosaccharides found on the cell membrane include mannose, galactose, sialic acid, glucose, and fucose [10]. Researchers have employed these molecules for nanoparticle functionalization due to their ability to target specific cells and tissues, as well as for their ability to mediate certain cellular responses (Fig. 2).
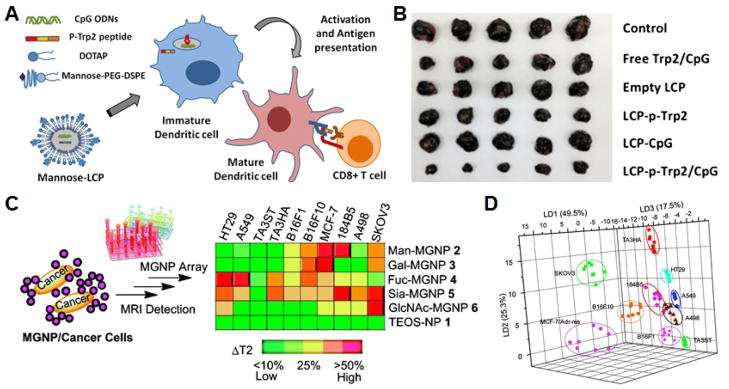
Fig. 2. Sugar-functionalized nanoparticles.
A) Schematic of a nanoparticle-based vaccine formulation using mannose to target dendritic cells for cancer immunotherapy. B) Imaging of tumors on day 20 after tumor inoculation demonstrates efficacy of the different nanoformulation variants. C) Schematic of magnetic glyconanoparticles for use in the detection of cancer cells with different carbohydrate binding affinities. D) Linear discriminant analysis plot demonstrating the ability to fully differentiate 10 different cancer cell lines bound with the nanoformulations according to their magnetic resonance signature. A, B adapted with permission from [42]. Copyright Elsevier, 2013. C, D adapted with permission from [55]. Copyright American Chemical Society, 2010.
2.1.1 Delivery
The use of simple sugars as ligands for targeted nanoparticle delivery has been widely explored. A common target is the liver, where various lectins that bind carbohydrates are present. One example is the asialoglycoprotein receptor, which is present on hepatocytes and binds to galactose [13]. In vitro experiments using nanoparticles employing galactose as the targeting moiety have demonstrated uptake via receptor-mediated endocytosis in healthy hepatocytes but not in the HeLa cell line, which is not of hepatic origin [14,15]. After systemic administration, glycosylated nanoparticles have shown preferential distribution to the liver compared with non-glycosylated particles [16,17]. In a recent study, nanocomplexes combining galactose-conjugated polymers with antiviral peptides against hepatitis C achieved preferential biodistribution to liver, resulting in decreased expression of intracellular viral protein [18]. In terms of gene delivery, galactosylated nanoparticles have been used to protect DNA and RNA from degradation in vivo and have likewise demonstrated effective hepatic delivery [19,20]. Of particular interest is the use of glyconanoparticles for liver cancer treatment, some types of which are highly lethal with 5-year survival rates of less than 20% [21]. Paclitaxel-loaded polymeric nanoparticles endcapped with galactose showed specific cytotoxicity on asialoglycoprotein receptor-expressing HepG2 cells compared with cell lines that did not express the receptor [22]. In a different example, galactose decorated theranostic nanogels loaded with imaging and radiotherapy sensitizing agents have been used for delivery to hypoxic liver cancer cells, although further in vivo investigations are required [23].
Other than the liver, sugar-based functionalization has also shown utility for modulating delivery to other targets. Fucose-conjugated nanoparticles developed in recent years display increased uptake by multiple carcinoma cell lines, particularly pancreatic cancer cell [24,25]. The binding and internalization of glucose by their protein-based transporters has likewise been leveraged. As an example, glucose-modified nanoparticles can be taken up by muscle cells expressing glucose transporter type 4 on their cell membrane. This effect can be upregulated by insulin in a dose-dependent manner and is considerably suppressed by competition from free ligand [26]. Brain targeting is highly desirable given that most drug formulations cannot cross the blood-brain barrier. Taking advantage of the fact that certain carbohydrate receptors are upregulated in cells of the central nervous system, sugar functionality can be employed to improve brain localization. In one example, glucose-coated gold nanoparticles could traverse a layer of brain endothelial cells in vitro [27]. In another case, sialic acid was combined with a blood-brain barrier-targeting peptide to enable increased uptake in the brain [28].
Using sugar-based ligands to aid in delivery is attractive for imaging applications where they can help to offer targeting specificity, physical stability, and biocompatibility [29]. Many types of carbohydrate-coated iron oxide nanoparticles have been reported [30,31]. In one example, a galactose-carrying polymer was coated onto superparamagnetic iron oxide nanoparticles to form a liver-targeted magnetic resonance imaging contrast agent [32]. The carbohydrate coating helped to increase solubility in fluids, and significant signal was observed after injection in a rat model. Quantum dots are also often used and have unique photophysical properties that make them well-suited as imaging probes. However, these inorganic nanoparticles generally exhibit cytotoxicity and short lifetime in the absence of additional surface functionality [33]. To address this, sialic acid residues have demonstrated the capacity to prolong their lifetime [34]. In terms of targeted delivery, mannosylated quantum dots showed greatly improved affinity for macrophages with minimal cytotoxicity [35]. In vivo, quantum dots functionalized with polyethylene glycol with capping by mannose and galactosamine showed enhanced liver targeting compared with nanoparticles without the carbohydrate functional groups [36].
2.1.2 Immune modulation
Carbohydrate-conjugated nanoparticles can be used to elicit enhanced immune responses against a variety of diseases. The mannose receptor, a lectin expressed on the surface of macrophages and dendritic cells, can be easily targeted by introducing its ligand as a surface functional group, which can help to facilitate the process of antigen presentation [37–39]. Furthermore, some carbohydrate structures themselves, such as galactose-alpha-1,3-galactose, are capable of stimulating immune responses in humans [40]. In a recent study, a generic nanoparticle vaccination platform employing this carbohydrate was shown to induce antibody titers and antigen-specific CD4+ T cell proliferation [41]. For anticancer therapy, a mannosylated vaccine nanoformulation loaded with an immunological adjuvant and melanoma-associated antigen peptide achieved impressive in vivo efficacy in mouse model of melanoma [42]. In another example, plasmid DNA encoding murine IL12, a potent cytokine with antitumor properties, was delivered via mannosylated chitosan nanoparticles to dendritic cells and enabled tumor regression in a murine colon cancer model [43]. Other platforms have sought to further improve targeting specificity, such as a recently reported mannose-displaying nanoparticle functionalized with a pH-sensitive polyethylene glycol layer [44]. This strategy enabled more specific delivery to macrophages within the tumor microenvironment, where the relatively low pH resulted in exposure of the mannose for targeting purposes. Regarding immunosuppression, a sialic acid variant that binds to Siglec-E on macrophages and neutrophils was conjugated onto biodegradable polymeric nanoparticles and could reduce the detrimental effects of systemic inflammation due to bacterial infection or pulmonary injury [45].
2.1.3 Sensing and detection
The wide range of binding interactions that carbohydrates have with different biological substrates has enabled the design of biomimetic nanoparticles platforms for diagnostic applications. Carbohydrate-lectin binding has been explored, one such pair being mannose and concanavalin A. The positive correlation between concanavalin A binding efficiency and the mannose density on a nanoparticle surface has been demonstrated [46]. Gold nanoparticles functionalized with a mannose coating have been employed in sandwich-based sensing constructs designed for the detection of lectin analytes. By employing either microgravimetric piezoelectric or surface plasmon resonance transducers, high sensitivity concanavalin detection has been reported down to concentrations under 20 nM [47,48]. In a different colorimetric sensing scheme, concanavalin A in suspension could be detected in less than a minute based on ultraviolet-visible absorption changes [49]. The same method was also adopted to detect influenza virus using sialic acid stabilized gold nanoparticles [50]. Further, similar designs that employ other monosaccharide-lectin pairs or other nanomaterials like carbon nanotubes have also been explored [51–53]. Beyond pure sensing applications, magnetic nanoparticle-based platforms can help to further isolate target analytes or cells. In one example, magnetic nanoparticles coated with mannose or galactose derivatives could rapidly detect and remove E. coli from culture medium [54]. As a follow-up work, nanoparticles coated with different tumor-associated carbohydrates successfully distinguished different cancer cell lines that had unique carbohydrate receptor profiles [55]. Additionally, the binding of sugar-functionalized nanoparticles to pathogens can serve other purposes, a notable one being the inhibition of infection potency [56,57].
2.2 Peptides
Peptides represent one of the most widely used class of ligands for nanoparticle functionalization. They are facile to synthesize, and have applications ranging from targeted delivery to immune modulation. There are two major ways in which peptides can be identified, which is either as derivatives of naturally occurring proteins or via established protocols such as phage display [9]. Despite their widespread use, there are surprisingly few examples of peptides that have their origins in the cell membrane. Regardless of this fact, the ones that have been reported represent examples of how cell membrane-derived peptides can be useful in nanomedicine (Fig. 3).
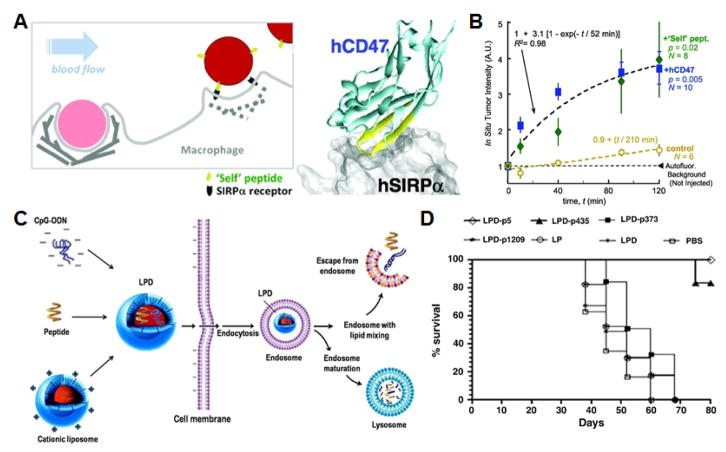
Fig. 3. Peptide-functionalized nanoparticles.
A) Schematic of particle functionalization with CD47-derived “self” peptide for suppressing phagocytosis. B) Total fluorescence quantification by in situ tumor imaging demonstrates increased uptake over time of the peptide-functionalized nanoformulation. C) Schematic of liposome-polycation-DNA nanoparticles incorporated with immunological adjuvant and the HER2/neu-derived peptide being taken up across the cell membrane for anticancer vaccination. D) Survival of mice over 80 days after tumor challenge demonstrates prophylactic efficacy of different nanoformulations. A, B adapted with permission from [58]. Copyright American Association for the Advancement of Science, 2013. C, D adapted with permission from [75]. Copyright Elsevier, 2012.
2.2.1 Suppression of uptake
One of the most notable examples of a cell membrane-derived peptide is that from the immunomodulatory CD47 “self” marker [58]. CD47 is a transmembrane protein expressed on many cell types, including RBCs and interacts with its receptor, the signal regulatory protein alpha, on macrophages to inhibit phagocytosis [59]. The self-peptide, a 21 amino acid peptide derived from the receptor binding site on human CD47, was identified and incorporated onto polystyrene nanobeads via biotin-streptavidin binding. The functionalized nanobeads demonstrated suppressed phagocytic uptake, longer circulation time in vivo, and significantly enhanced accumulation at tumor sites due to an increased ability to take advantage of the enhanced permeation and retention effect. With the peptide functionality, nanobeads loaded with paclitaxel showed the ability to control tumor growth in a subcutaneous in vivo model. Such biomimetic strategies for enhancing nanoparticle circulation serve as a potential alternative to the use of polyethylene glycol, which more recently has been demonstrated to elicit an antibody response that can impact performance over time [60]. Much still needs to be studied regarding this biomimetic approach, and factors such as ligand density and affinity are all important factors to consider [61].
2.2.2 Immune activation
The use of peptides in vaccination can have many advantages over the use of whole proteins, including the ability to easily synthesize the antigen as well as the ability to limit response to the desired epitopic target. By employing peptides found on the cell membrane, it is possible to further focus the immune response to important targets of interest. An example is a bacterial peptide derived from the virulent M protein on the surface of group A streptococcus (GAS). The M protein has a hypervariable N-terminus and is conserved towards the C-terminus. As such, several epitopes derived from the conserved region have been employed in vaccines to induce specific antibody titers and inhibit GAS colonization [62–64]. Nanoparticles can serve as an ideal delivery platform for more enhancing vaccine efficacy [3]. One such platform against GAS has been reported, consisting of a self-adjuvanting polyacrylate core and peripheral layer of J14 peptides derived from the M protein [65]. In a follow-up work, a dual-peptide delivery system was proposed using both J14 and a peptide derived from GAS fibronectin-binding protein, a surface-localized adhesin that helps the bacteria recognize and adhere to host cells [66]. For Staphylococcus aureus, a 30 amino acid peptide of the D2 domain from its fibronectin-binding protein B was genetically expressed on viral nanoparticles to generate higher antibody titers [67]. This resulted in reduced bacterial counts in an intravenous infection model. Further, a similar strategy has been employed towards malaria [68]. The circumsporozoite protein is a surface protein from the sporozoite stage of malaria parasites, and polypeptide nanoparticles displaying the B cell epitope from the protein elicited a high avidity antibody response and provided long term immunity up to 15 months against live sporozoite challenge.
For anticancer vaccines, one of the greatest challenges in their design is identifying the appropriate antigenic material against which to train the immune system. Modulated membrane composition can play a large role in tumor pathogenesis [69], and many peptides derived from membrane proteins have been explored for use in vaccine formulations. This includes the transmembrane tumor-associated antigen MUC1, which was recently designated as a priority target for translational research by the National Cancer Institute [70]. In some preliminary investigations, a MUC1 peptide and the adjuvant monophosphoryl lipid A were encapsulated into PLGA macrospheres or nanoparticles, and these formulations were able to induce dendritic cell maturation, primary T cell proliferation, and IFNγ cytokine production in vitro and in vivo [71,72]. Researchers have also incorporated multiple different components, including MUC1 peptides, tumor-associated carbohydrates, immunostimulating T cell peptide epitopes, and adjuvants, to make multifunctional vaccines. In more recent studies, MUC1 glycopeptides were linked to T cell peptide epitopes and either coated onto gold nanoparticles or incorporated into adjuvant-containing nanogels [73,74]. The antisera from the mice immunized with these nanoformulations showed significant increase in antibody titers and could recognize cancer cell lines ex vivo. In addition to MUC1, peptides derived from other tumor-related membrane proteins have also been used for nanoparticle vaccine design, including HER2/neu for breast cancer [75], as well as gp100, MelanA, and MART1 for melanomas [76,77].
2.2.3 Immune tolerance
In contrast to immune activation, cell membrane peptides have also been exploited for inducing immune tolerance in autoimmune disease treatment. Researchers have used polystyrene beads coupled with an encephalitogenic myelin peptide to prevent disease symptoms in an experimental model of autoimmune encephalomyelitis [78]. Compared with a previous strategy of delivering antigens using splenic leukocytes, a particulate formulation can be advantageous given its ease of manufacturing and storage. Mice immunized intravenously with the tolerizing nanoparticles a week before challenge showed impressive amelioration of symptoms. In a follow-up work, tolerogenic biodegradable particles conjugated with the same peptide likewise showed efficacy, and it was further shown that the tolerizing effect was exclusive to the intravenous route of delivery [79]. With this route of administration, the particles localized at the splenic marginal zone to induce T cell anergy and increased Treg activity.
3. Membrane-bound biomacromolecules
Cell membranes are approximately 50% protein by mass, and they also contain many complex glycan structures based on the simple sugars discussed in the previous section [80]. In the move towards biomimetic systems, the natural specificities and functions of these biomacromolecules can be deliberately leveraged for the development of highly sensitive therapeutics and diagnostics. While promising, examples of platforms that employ whole membrane-derived biomacromolecules are relatively few, which is likely due to the difficulty in deriving or replicating these structures. Future study along these lines will benefit from new methods of isolation as well as advancements in glycochemistry.
3.1 Carbohydrate chains
The most common carbohydrate chains present on cell membranes are glycans, embedded in the membrane through protein or lipid anchors. Due to their biological function in cell-cell recognition and as enzymatic triggers, glycans have important implications for improving the biocompatibility and specificity of nanomedicines as well as in biosensing [81]. Cell membrane-derived glycans are of particular interest because, unlike proteins, glycans are not encoded by the genome; their structures are dictated instead by cellular status, metabolism, and signal transduction [82]. Despite the difficulty in deriving or replicating glycan structures, their use in the design of biomimetic nanoparticles can be highly advantageous (Fig. 4).
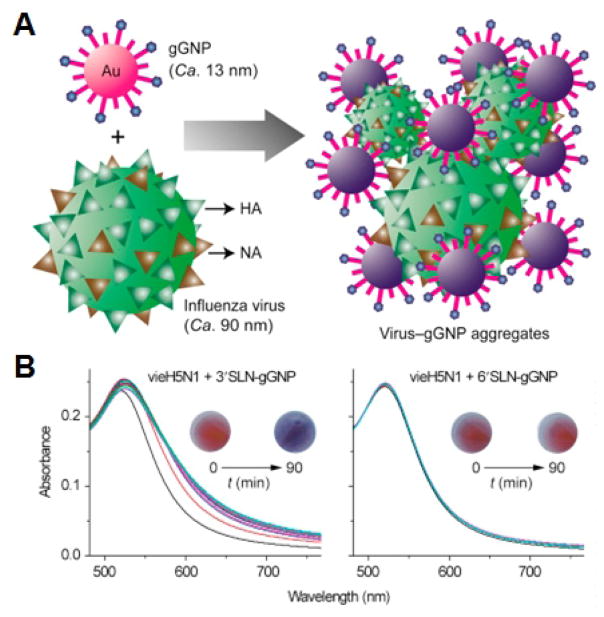
Fig. 4. Glycan-functionalized nanoparticles.
A) Schematic of glycan-functionalized gold nanoparticles for the colorimetric detection of viruses. B) Colorimetric response after incubation of viruses with the nanoformulations can be used to distinguish each strain based on its reactions with the different glycans. Adapted with permission from [95]. Copyright Elsevier, 2017.
3.1.1 Delivery
The specificity of some cellular receptors for glycan structures can be exploited to modulate localization for enhanced delivery. This has been demonstrated on a cellular level, as the engineering of CD44 receptors from native to synthetic glycan expression has been used to improve osteotropism of mesenchymal stem cells [83]. Sialyl Lewis X is a tetrasaccharide that is expressed on bloodborne leukocyte cell surfaces and is one of the key carbohydrates interacting with selectins. This membrane-derived molecule can be used as a biomimetic targeting ligand to endow nanoparticles with the ability to mimic the rolling behavior of leukocytes [84,85]. It is also an attractive candidate to study inflammation and has been used to target E-selectin and P-selectin for identifying early cerebral endothelial activation using iron oxide nanoparticles [86,87]. The dendritic cell marker DC-SIGN can be targeted by Lewis X, and its efficacy has been compared to that of pathogen-derived carbohydrates, such as the mannosylated glycolipid lipoarabinomannan from tuberculosis bacteria and the glycoprotein gp120 from HIV [88]. The glycan components on the latter two are both considered virulence factors for their respective diseases. Coated onto polymeric nanoparticles via biotin-streptavidin interactions, these carbohydrate ligands were able to enhance the specific binding to and activation of dendritic cells. This study also compared the performance of these carbohydrates to DC-SIGN-specific antibodies; gp120 was able to target the DCs in mixed cell cultures as effectively as the antibodies, and all three carbohydrates were shown to activate both CD8+ T cells and stimulate cytokine production.
3.1.2 Immune modulation
Carbohydrate-lectin binding is a key step to immune activation. As a result, ligands such as those based on sialic acid have shown the potential to address issues of autoimmunity, graft-versus-host disease, inflammation, pathogen and tumor evasion, and age-related diseases [89]. For immune activation, Lewis X, with its dendritic cell-targeting properties, has been used with liposomal platforms to formulate vaccines capable of boosting T cell responses [90]. When loaded with the MART1 tumor antigen along with an immunological adjuvant, the platform was able to elicit a high number of antigen-specific effector cells. In addition to the use of naturally occurring glycans, it should be noted that there are a few emerging examples of synthetic glycan structures that have been employed in biomimetic nanoparticle design. This includes a high affinity Lewis X-based glycomimetic ligand that was capable of targeting nanoparticles to the dendritic cell marker DC-SIGN for various applications [91]. It is also possible to induce tolerance and suppress immune responses, which was the case for a CD22-engaging glycan that could be used to promote antigen-specific tolerance for the treatment of autoimmunity [92]. In a different example, synthetic mannose-dendrimers inspired by mannosylated lipoarabinomannan were shown to inhibit neutrophil recruitment at sites of inflammation in the lung [93].
3.2.3 Biosensing
Used for biosensing, Lewis X has been conjugated onto fluorescent ferrite magnetic silica glyconanoparticles to probe mammalian and pathogenic cells in an attempt to understand the roles of specific surface lectins, and the platform also has further applications in diagnosing disease [94]. Recently, sialyl Lewis X, sialic acid, sialyllactose, sialyllactosamine and sulfated sialyllactoseamine were used to functionalize gold nanoparticles to develop a rapid diagnostic for distinguishing influenza strains [95]. The aggregation of the particles mediated by different glycan binding specificities for each influenza viral strain resulted in distinct colorimetric profiles.
3.2.4 Binding decoys
An interesting application enabled by glycan-based functionality is the use of nanoparticles displaying the ligands as decoys for binding. Lewis X-conjugated gold nanoparticles have been reported, and they have been further studied as an agent for anti-adhesion therapy, where they can potentially be used as inhibitors of cancer metastases [96,97]. The ligand has also been conjugated onto dendrimers to compete for DC-SIGN binding with the HIV gp120 protein, thus preventing infection via DC-SIGN and langerin [98]. In the same manner, glycans are also considered a candidate for the prevention of microbial adhesion, with functionalized nanoparticles competing for glycan binding sites essential for binding and biofilm formation [99]. Finally, glycan structures have been used as toxin decoys, although currently no studies employing naturally derived ligands for this function have been reported [100].
3.2 Proteins
Cell membrane proteins also play key roles in transport, cell-cell recognition, and other critical interactions. Many of the same carbohydrate-protein interactions as mentioned above can be leveraged conversely by introducing protein functionalized nanoparticles to biological systems. The specific use of membrane-derived proteins has not been commonly reported, and this is likely due to the fact that these ligands are hard to purify. Regardless, there exist some interesting examples in which their use is justified and cannot be easily replicated by other means (Fig. 5).
Fig. 5. Protein-functionalized nanoparticles.
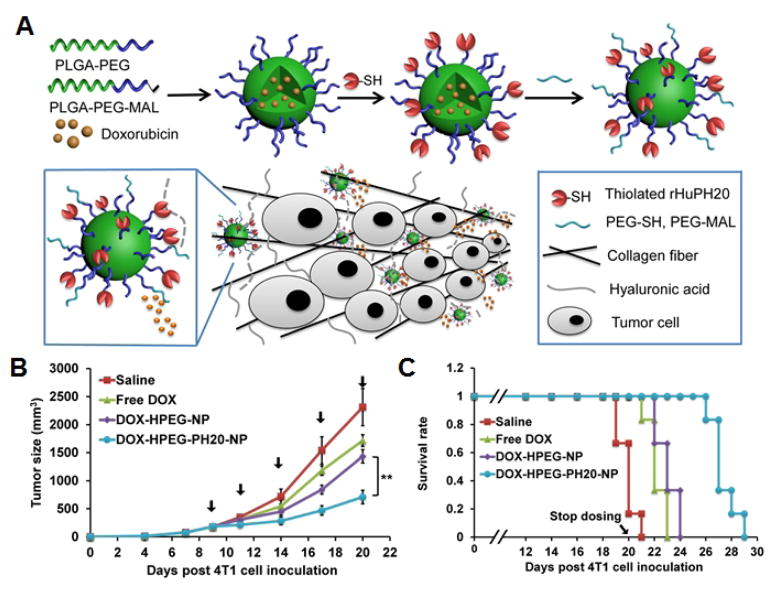
A) Schematic of hyaluronidase-functionalized drug loaded nanoparticles for enhanced tumor drug delivery. B) Tumor growth kinetics for 20 days after inoculation demonstrates antitumor activity of the nanoformulations. C) Survival curve demonstrates therapeutic efficacy of the nanoformulations over time. Adapted with permission from [114]. Copyright American Chemical Society, 2016.
3.2.1 Delivery
By employing the specificities exhibited by certain membrane proteins, it is possible to design naturally targeted nanoformulations. One useful family of proteins are lectins, which are membrane-anchored proteins that interact with carbohydrates at cell surfaces for cell-cell recognition and adhesion. Peanut and wheat germ lectins have been conjugated to the surface of drug-loaded nanoparticles, demonstrating bioadhesion to inflamed tissues and enhancing efficacy in an animal model of colitis [101]. Further, magnetic nanoparticles have been coated with peanut agglutinin and used to bind sperm as a means of improving their fertility [102]. Another protein, glycoprotein Ib plays a role in platelet adhesion to the vascular wall by interacting with P-selectin on damaged endothelial cells and with the von Willebrand factor on subendothelium. It was coated onto drug-loaded polymeric nanoparticles via carbodiimide chemistry, and the resulting formulation was used for targeted and controlled delivery of dexamethasone [103]. Even under the flow conditions at a site of vascular injury, particles were able to bind to substrates, although the adhesion was found to be inversely related to shear stress.
3.2.2 Immune modulation
The major histocompatibility complex (MHC) proteins play an integral role in adaptive immunity, enabling the process of antigen presentation. Recently, there has been a significant amount of research into using nanoparticles displaying peptide-loaded MHCs for artificial antigen presentation. While traditionally accomplished with microparticles, the first nanoparticle-based system for artificial antigen presentation combined MHCs with either CD80 or anti-CD28 as a co-stimulatory signal onto a single magnetic nanoparticle [104]. These nanoparticles were able to induce T cell proliferation in vitro and, when injected in vivo, were also able to inhibit tumor growth. Subsequent studies have used the same platform to enhance T cell proliferation under magnetic fields as well as for purification of antigen-specific T cell populations [105,106]. Further, it has been demonstrated that ellipsoidal nanoparticles are more effective than their spherical counterparts when used for artificial antigen presentation, likely due to an enhanced ability to interface with T cells [107]. Finally this strategy has also demonstrated utility for addressing autoimmunity, helping to resolve type 1 diabetes in a murine model of the disease [108]. It was determined that peptide-loaded MHC class II nanoparticles were ultimately able to promote the differentiation of cognate B cells into disease-suppressing regulatory B cells, thus addressing the autoimmune condition without compromising systemic immunity.
3.2.3 Enzymatic therapies
Hyaluronidase is a membrane protein that degrades hyaluronic acid, a glycosaminoglycan distributed in extracellular matrix tissue throughout the body. Some skin cancers can overexpress hyaluronic acid, which results in a thick gel-like matrix, reducing penetration of traditional therapeutics. Thus, a clinically approved strategy for enhancing treatment of such tumors is their pretreatment with free-form enzyme via localized injection [109–111]. To simplify the treatment, a layer-by-layer self-assembly technique was used to construct hyaluronidase-displaying silica nanoparticles [112]. These functionalized particles improved the retention of the enzyme at tumor sites due to a reduced diffusion coefficient of the bigger particle along with multivalent interactions. When the particles were loaded with the small molecule therapeutic carboplatin, drug infiltration of the tumor was improved due to reduction of the extracellular matrix. Membrane-coated polymeric nanoparticles have been further enhanced by adding hyaluronidase to the nanoparticle surface [113,114]. Rather than localized injection, systemic tail vein injections resulted in preferential diffusion to tumor sites. The enzyme was attributed for targeting the particle to the extracellular matrix, as well as helping to improve tumor penetration.
4. Cell membrane-based nanostructures
Instead of using individual components derived from the cell membrane, researchers have also been exploring the direct use of cell membrane itself as a material for enhancing nanoparticle functionality. This approach carries many advantages, including the ability to present multiple functional moieties in their natural context without the need for complicated methods to identify, synthesize, and conjugate individual ligands. There are two main types of platforms that follow this approach, including naturally derived vesicles and membrane-coated nanoparticles that combine synthetic and natural elements. These biomimetic platforms have been the subject of intense research and have the potential to overcome many of the limitations facing traditional nanoparticle platforms.
4.1 Vesicular structures
Cell membrane-derived vesicles (Fig. 6) are a widely studied category of nanostructures that are the natural analog of liposomes, which have been used in drug delivery for several decades. They can be fashioned from larger membrane structures, or they can be naturally occurring. A prime example is exosomes, vesicles that are secreted by most mammalian cells as a means of intercellular communication and biomaterial transfer [115]. They are heavily implicated in tumor biology and play a large role in tumorigenesis [116–118]. As autologous biologics, exosomes currently represent one of the few membrane-derived platforms that have been subject to a good deal of clinical investigation. Bacteria employ analogous structures known as outer membrane vesicles (OMVs) as tools to enhance their survival within a host [119,120]. A major benefit of using naturally occurring nanovesicles for nanomedicine applications is that they are inherently adept at performing certain biological functions, whether it be for targeted delivery or immune modulation [121].
Fig. 6. Natural membrane vesicles.
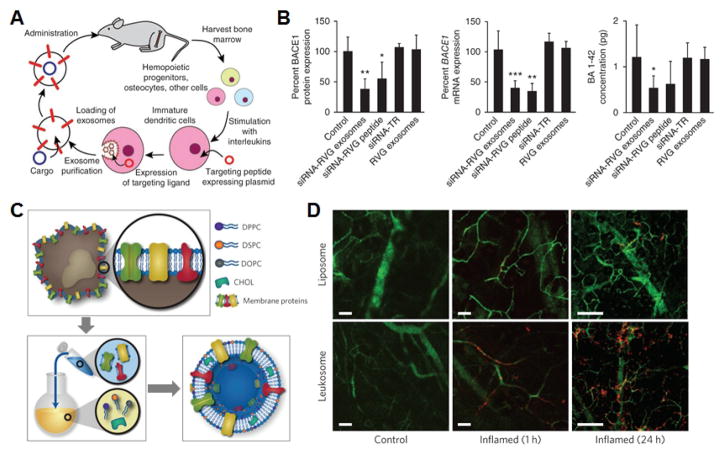
A) Schematic demonstrating workflow for the fabrication and subsequent administration of siRNA-loaded exosomes for brain-targeted delivery. B) Quantification of BACE1 protein and mRNA expression as well as β-amyloid concentration demonstrates significant knockdown efficacy for the nanoformulation. C) Schematic demonstrating the fabrication of leukosomes via the isolation of membrane proteins followed by reconstitution with synthetic lipids. D) Intravital microscopy images demonstrate preferential accumulation at the site of inflamed tissue over time. Scale bars = 50 μm. A, B adapted with permission from [131]. Copyright Nature Publishing Group, 2011. C, D adapted with permission from [150]. Copyright Nature Publishing Group, 2016.
4.1.1 Delivery
One of the most explored applications for naturally derived vesicular structures has been drug delivery. Depending on their origin, exosomes can be inherently biocompatible and lowly immunogenic, making them well-suited for the purpose [122–124]. Employing plasma-derived exosomes, researchers have demonstrated siRNA delivery to monocytes and lymphocytes [125]. Mesenchymal stem cells have been shown to efficiently produce these natural vesicles, providing another possible source of autologous material [126]. Depending on their expression of different surface markers such as integrins, exosomes can be targeted to specific regions within the body [127]. Modulated delivery can also be achieved by introducing targeting peptides via fusion with membrane-bound proteins [128]. The protein Lamp2b is a good candidate for introducing additional functionality, as it is highly localized to the exosomal compartment [129]. Notably, exosomes have been employed for delivery across the blood-brain barrier, which traditionally is impermeable to almost all systemically administered therapies. This has been accomplished through the use of brain endothelial cell-derived exosomes [130] or by engineering exosomes that specifically target brain receptors [131]. Intranasal administration of exosomes can also be used for brain localization, and researchers have employed this strategy to administer experimental therapies against Parkinson’s disease [132]. With regards to the cargo, a variety of different payloads have been incorporated into exosomes, including both hydrophobic and hydrophilic cancer drugs [129,133]. Encapsulation of cargoes within exosomes can improve their bioavailability, boosting potency compared with free drug formulations [134]. One interesting way in which paclitaxel, an antineoplastic, has been incorporated involves feeding the drug to live cells, allowing them to naturally package the drug into their exosomes [133]. Perhaps one of the most studied applications of exosomes has been for delivery of RNA interference agents. In one notable example, exosomes derived from dendritic cells and modified to target brain cells were used to deliver siRNA capable of knocking down a therapeutic target for Alzheimer’s disease [131]. Finally, given their biological role, exosomes absent of any cargo have also demonstrated significant therapeutic potential. To this end, mesenchymal stem cell-derived exosomes have been administered to improve functional recovery in rats after a stroke or traumatic brain injury [135,136].
Other than exosomes, another major class of natural vesicle-based nanoparticles are those fashioned from the plasma membrane of mammalian cells. They can be more plentiful than exosomes, and this is often cited as one of their major advantages [137]. These vesicles mirror the membrane composition of the source cells, which likewise makes them suitable for biological use. The membrane proteins present on these nanovesicles are essential for their function, as it has been shown that their absence abrogates their desirable functions [137]. The first such structures were derived from RBCs by extrusion of membrane ghosts and were termed nanoerythrosomes [138–141]. In addition to their cancer drug delivery roots, nanoerythrosomes can be used for the delivery of photoactivatable agents for imaging and photothermal therapy [142], antihypertensive drugs [143], and perfluorocarbons for acoustic responsiveness [144]. Nanoerythrosomes have also been bestowed additional targeting functionality via the conjugation of antibodies to a polymeric tether [145]. Beyond RBCs, stem cells are a popular source of membrane material due to their tumor targeting ability. In one such work, mesenchymal stem cell-derived nanoghosts were loaded with a proapoptotic protein cargo and were used to treat a mouse xenograft model of human prostate cancer, significantly outperforming the unencapsulated form of the protein [146]. A follow-up work later used the same platform for gene delivery [147]. Other than stem cells, endothelial cells and white blood cells have also been employed to create nanodelivery vesicles capable of unique functions [148,149]. In a recent example, the proteins derived from the plasma membrane of leukocytes were mixed with synthetic lipids, and the resulting leukosomes were able to deliver dexamethasone to treat inflamed vasculature [150].
While less commonly used due to their inherent immunogenicity, bacterial OMVs have also been explored as nanodelivery vehicles. One way to address potential issues of toxicity is to engineer bacterial strains with modified lipopolysaccharides [151]. With endotoxicity at acceptable levels, the facile ability to genetically modify bacteria makes them suitable for generating targeted constructs. One prime example is the use of ClyA fusion proteins in E. coli, which helps to target a protein of interest specifically to OMVs [152]. This strategy has been used to incorporate a HER2-targeting affibody onto the OMV surface, enabling delivery of an siRNA cargo for potential cancer therapies [153]. OMVs also carry the potential for enzyme and protein delivery, protecting such cargoes from harsh biological conditions [154]. In a proof-of-concept study, encapsulation within bacterial vesicles significantly protected phosphotriesterase under a variety of different test conditions, and this may one day lead to improved methods of detoxification [154].
4.1.2 Immune modulation
Besides delivery, immune modulation is another application towards which natural nanovesicles are well suited. Exosomes have been explored extensively for use in the design of anticancer vaccines [155,156]. Compared with tumor cell lysates, it has been shown that tumor exosomes can elicit better antitumor immunity [157]. While tumor-derived exosomes are generally used with the intent of producing a strong immune response, it is also known that, by themselves, these natural vesicles can be highly immunosuppressive [158]. Thus, the context in which they are presented is of high importance. Many different strategies, from using heat-shocked tumors cells [159,160] to having the cells express markers such as Hsp70 [161,162], CD40L [163], and Rab27a [164] have been proposed for improving immunogenicity. Instead of direct vaccination, tumor exosomes have been used as the antigenic material for pulsing dendritic cells to promote downstream immunity [165]. In a different strategy, exosomes derived from dendritic cells pulsed with a model antigen were shown to be more effective at promoting CD8+ T cell responses compared with exosomes derived from antigen-expressing tumor cells [166]. Interestingly, it has been shown that such vesicles may be able to transfer loaded MHCs to dendritic cells in vivo to prime T cell responses [167,168]. Beyond anticancer vaccination, use of antigen-pulsed dendritic cells can also be expanded towards generation of anti-pathogen immunity [169]. Finally, it has been suggested that the potency of dendritic cell-derived exosomal vaccine formulations can be further improved by directly loading properly restricted peptides onto their MHCs [170].
At the other end of the spectrum, there has also been some studies on the use of mammalian exosomes for immune suppression, which can also be highly useful in the case of autoimmunity [171]. In one interesting example, it was demonstrated that exosomes from immature bone marrow-derived dendritic cells could promote a tolerogenic effect for intestinal transplantation in a rat model [172]. Another study employed vesicles derived from IL10-treated dendritic cells to help reduce inflammation for potential use in addressing arthritis [173]. Others have focused on modifying dendritic cells to express tolerogenic cytokines such as TGF-β1 or IL4, and the exosomes derived from these genetically engineered cells have shown utility for addressing inflammatory conditions [174,175].
Just like tumor exosomes have proven useful for anticancer vaccine design, bacteria-derived OMVs have been extensively explored for generating effective vaccines against a variety of pathogens [176–180]. One of the most studied OMV-based vaccines are those against serogroup B meningococcal disease, for which the strategy has shown considerable protective efficacy [181,182]. To improve vaccine potency, researchers have sought to engineer the overexpression of specific markers, including GNA1870, FetA, fFbp [183–185]. Further, attenuating endotoxin can help to reduce toxicity and improve the safety of bacteria-derived vaccines [185,186]. Other pathogens from which OMVs have been derived for vaccination purposes include Vibrio cholerae [187,188], Bordetella pertussis [189], Haemophilus parasuis [190], and Burkholderia pseudomallei [191]. In a different strategy, E. coli OMVs were engineered to exogenously express HtrA from Chlamydia muridarum and were able to elicit protective antibodies against the antigen [192]. Additionally, bacterial OMVs have been explored as immunological adjuvants for use in vaccination against non-bacterial pathogens, including malaria [193] and HIV [194]. More recently, bacterial protoplast vesicles, which are actually free of outer membrane material and their toxic components, have been used as an alternative to OMVs and have also demonstrated utility for protecting against bacterial sepsis [195].
4.1.3 Biosensing
Within the broad scope of biosensing applications, cell-derived membrane vesicles have found use as the recognition element in some special cases. Specifically, they have been employed in bioelectronic nose and tongue applications [196–198]. In general, these biosensors employ protein-based taste and smell receptors coupled with transducers such as microelectrode arrays, quartz microbalances, optical detectors, or field effect transistors. Binding of the target molecule to the receptor results in a signal change, and enables detection at extremely low concentrations. While these devices originally employed purified proteins, it was later discovered that employing cell-derived vesicles expressing the receptor enabled the preservation of calcium influx mechanisms, which helped to further amplify the signal and lower detection limits [199]. Since then, this strategy has been used for detecting different odorants as well as sweet and umami tastants [200–202]. Vesicle-based biosensors have also demonstrated utility for other applications beyond taste and smell, including the detection of lung cancer biomarkers [203], neurotransmitters [204], and fungal contaminants [205].
4.2 Membrane-coated nanoparticles
In the past few years, a new class of biomimetic nanoparticle has been reported that combines the advantages of natural, cell membrane-derived vesicles with more traditional synthetic nanoparticulate platforms. These particles generally employ a core-shell design, with a layer of cell membrane coated around a preformed nanoparticle core. Initially, membrane-coated nanoparticles were fabricated using a combination of RBC membrane and poly(lactic-co-glycolic acid), a biodegradable polymer, via a co-extrusion approach [206]. These particles exhibited significantly enhanced circulation, which was shown to result from the preservation of RBC self-markers such as CD47 on the nanoparticle surface in a right-side-out orientation [207–209]. Afterwards, a plethora of different platforms have been reported given the flexibility of choosing different membrane materials and different nanoparticle cores [210–213]. These have been employed for a variety of applications, some of which uniquely take advantage of their biomimetic properties (Fig. 7).
Fig. 7. Cell membrane-coated nanoparticles.
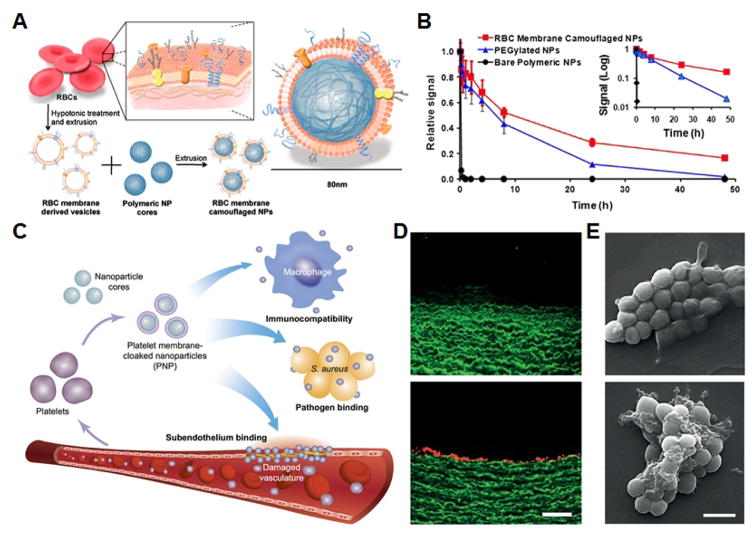
A) Schematic of RBC membrane-coated nanoparticle fabrication via an extrusion method. B) Monitoring of fluorescent nanoparticle signal over time demonstrates enhanced circulation for the membrane-coated nanoparticles. C) Schematic of the different applications towards which platelet membrane-coated nanoparticles can be applied, including targeting of damaged vasculature and pathogen binding. D) Platelet membrane-coated nanoparticles do not target intact endothelium (top), but show significant binding when the endothelial layer is removed (bottom). Scale bar = 200 μm. E) Methicillin-resistant Staphylococcus aureus (top) shows significant binding when incubated with platelet membrane-coated nanoparticles (bottom). Scale bar = 1 μm. A,B adapted with permission from [206]. National Academy of Sciences, 2011. C–E adapted with permission from [229]. Nature Publishing Group, 2015.
4.2.1 Delivery
After the first RBC membrane-coated nanoparticles were reported, additional studies were carried out to demonstrate their utility for cancer drug delivery. It was shown that doxorubicin could be incorporated into the polymeric cores, and these drug-loaded nanoparticles demonstrated considerable efficacy in a mouse model of lymphoma [214,215]. Further, polymer tethers can be used introduce elements onto the outer membrane surface that can provide additional functionality such as tumor targeting and penetration [113,216,217]. Other strategies have been developed that can enable on-demand, triggered release of cancer drugs, helping to further improve nanoparticle efficacy and minimize systemic toxicity [218–221]. One major advantage of the RBC membrane coating is that it enables extended circulation after repeated administration due to its biocompatible and nonimmunogenic nature [222]. In addition to RBCs, many other cell types have been used to source the membrane for coating, and each carries with it unique benefits. Cancer cell membrane is lowly immunogenic and can take advantage of the homotypic targeting effect in which the particles have high affinity towards the same cells from which the membrane was derived [219,223,224]. White blood cell membrane can bestow cancer targeting properties and additionally has been shown to help particles traverse endothelium [225–227]. Stem cell membrane can likewise promote improved localization to tumor regions [228]. Of particular interest, recently reported platelet membrane-coated nanoparticles have proven to be adept in a variety of disease targeting applications [229]. As the sentinels of the body, platelets play major roles in hemostasis as well as pathogen clearance. When loaded with a cytotoxic drug, the nanoparticles were able prevent restenosis in a rat model of arterial denudation. Additionally, an antibiotic cargo enabled them to effectively combat methicillin-resistant S. aureus infection. In later works, platelet membrane-coated nanoparticles have demonstrated utility for cancer drug delivery as well as thrombus inhibition [230,231].
4.2.2 Imaging and phototherapy
The flexibility of choosing different core materials for cell membrane-coated nanoparticles has enabled the incorporation of powerful platforms for both imaging and phototherapy-based applications. One novel way in which the membrane coating has been introduced is through the biogenic release of nanoparticles after uptake by live cells [232]. This approach can be used for encapsulation of metallic and inorganic nanoparticles of different geometries. Iron oxide-based nanoparticle cores are strong candidates for use in magnetic resonance imaging, and both stem cell membrane-coated and RBC membrane-coated versions have been reported [233,234]. Upconversion nanoparticles, which have high utility for in vivo applications due to their ability to take advantages of tissue-penetrating infrared light, have been coated with macrophage membrane or cancer cell membrane, and they can be effectively targeted to tumors [235,236]. Small molecules such as indocyanine green have also been loaded inside polymeric cores, and cancer cell membrane-coated versions of these nanoparticles have likewise demonstrated utility for imaging applications [237].
Many of the same platforms that are used for imaging can also be applied towards light-based treatments, including photodynamic and photothermal therapies. Gold nanoparticles coated with RBC membrane or macrophage membrane have long blood circulation times and are taken up efficiently by tumors, enabling efficient photothermal conversion for treatment [238,239]. By combining upconversion nanoparticles with photosensitizers, it is possible to fabricate photodynamic platforms capable being triggered by higher wavelength infrared light, which is very useful for in vivo application. This has been accomplished both with RBC membrane-coated upconversion nanoparticles with a layer of photosensitizer in between them [240], as well as for stem cell membrane-coated upconversion nanoparticles encapsulated within a mesoporous silica shell that serves as a compartment for loading multiple sensitizers [241]. Further, a cancer cell membrane-coated porous zeolitic imidazolate framework has been shown capable of tumor targeted delivery and oxygen-independent photodynamic therapy, helping to overcome the inherent hypoxia present deep within tumors [242].
4.2.3 Detoxification
Due to the preservation of cell-specific markers on the surface of cell membrane-coated nanoparticles, they have demonstrated natural affinity to many different kinds of harmful toxins [4]. This has enabled the development of nanosponges, which are cell membrane-coated nanoparticles capable of binding to and neutralizing toxic moieties. The first such platform was based on RBC membrane-coated nanoparticles for neutralizing α-hemolysin secreted by S. aureus [243]. It was shown that RBC-based nanosponges could completely prevent toxin-mediated hemolysis of healthy RBCs in vitro, and the nanoparticles were able to significantly improve survival in an in vivo model of lethal toxin challenge. A later study demonstrated the ability of a hydrogel loaded with nanosponges to address local bacterial infections and minimize the appearance of skin lesions [244]. This platform has also been used to neutralize streptolysin-O, which is secreted by Streptococcus pyogenes, a common cause of bacterial infection across the world [245]. More recently, acoustic propulsion-based nanomotors have been successfully coated with RBC membrane and applied towards accelerated neutralization of toxins [246]. Other than traditional bacterial toxins, the nanosponge strategy has also been applied towards the removal of pathological autoimmune antibodies [247,248] as well as small molecule intoxicants [249,250].
4.2.4 Immune modulation
Like their natural vesicle counterparts, cell membrane-coated nanoparticles have also been used for modulating the immune system. Currently, most studies have revolved around using these nanoparticle for vaccine development. A notable example involves the use of nanosponges to first neutralize bacterial toxins, enabling subsequent administration with the resulting nanoparticle-toxin complex, termed a nanotoxoid, as a safe and effective means of antivirulence vaccination [251]. This strategy has demonstrated the ability to elicit potent humoral responses against bacterial toxins, protecting mice when infected with the secreting pathogen [252]. For cancers, cell membrane-coated nanoparticles have been proposed as a platform for co-delivering multivalent tumor antigen material along with immunological adjuvants, helping to overcome some of the fundamental issues currently facing anticancer vaccine development [223]. Functionalization of RBC membrane-coated nanoparticles with tumor antigens has also been proposed for a similar purpose [253]. Finally, bacterial OMV-coated nanoparticles have been fabricated and represent a strong candidate for vaccinating against bacterial infection [254].
5. Conclusions
Biomimetic design strategies have the potential to greatly improve upon and enhance the functionality of current nanoparticle platforms. The composition of cell membranes is largely responsible for many of the unique functions exhibited by different cell types. Using the cell membrane as the inspiration for nanoparticle design, several different strategies have been discussed. These include the use of protein and glycans, along with their derivatives, as well as employing natural cell membranes either directly as nanocarriers or as the coating material for synthetic nanoparticulate cores. These approaches are incredibly powerful, and they leverage natural interactions and specificities that have evolved over millions of years, circumventing the need for exhaustive discovery and validation processes often required for their synthetic counterparts.
Bioinspired nanomedicine is still in its infancy, and there are ways in which each of the discussed strategies for cell membrane-derived functionalization can be further leveraged in the future. Regarding small subunit derivatives, especially for peptides, the introduction of purposeful workflows for finding relevant sequences from proteins, based on desired functions, can lead to a larger library of useful ligands that are facile to fabricate. Biomacromolecules such as proteins can benefit from improved techniques for production and purification, especially for those integrated into the membrane, and advances in glycochemistry may eventually streamline the use of glycans. Finally, for vesicular structures and cell membrane-coated nanoparticles, using membranes of specialized cells or combining functions from different membrane types may help to further enhance their utility. Commercialization and clinical investigation of nanoparticle-based medical technologies has accelerated in recent years [255]; while membrane-derived systems are still relatively new, it can be foreseen that they will soon be subject to significant translational efforts. To accommodate this, a shift in focus from discovery to process development will need to occur, as methods and workflows for reliable scale-up of fabrication will become increasingly important. Overall, as researchers continue to explore biomimetic design strategies, new and exciting platforms will be developed with the potential to drastically alter the landscape of nanomedicine.
Acknowledgments
This work is supported by the National Institutes of Health under Award Number R01CA200574 and the National Science Foundation Grant DMR-1505699.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fang RH, Zhang L. Nanoparticle-based modulation of the immune system. Annu Rev Chem Biomol Eng. 2016;7:305–326. doi: 10.1146/annurev-chembioeng-080615-034446. [DOI] [PubMed] [Google Scholar]
- 2.Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- 3.Fang RH, Kroll AV, Zhang L. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11(41):5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang RH, Luk BT, Hu C-MJ, Zhang L. Engineered nanoparticles mimicking cell membranes for toxin neutralization. Adv Drug Deliv Rev. 2015;90:69–80. doi: 10.1016/j.addr.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dehaini D, Fang RH, Zhang L. Biomimetic strategies for targeted nanoparticle delivery. Bioeng Transl Med. 2016;1(1):30–46. doi: 10.1002/btm2.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu C-MJ, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthc Mater. 2012;1(5):537–547. doi: 10.1002/adhm.201200138. [DOI] [PubMed] [Google Scholar]
- 8.Fang RH, Hu C-MJ, Zhang L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin Biol Ther. 2012;12(4):385–389. doi: 10.1517/14712598.2012.661710. [DOI] [PubMed] [Google Scholar]
- 9.Molek P, Strukelj B, Bratkovic T. Peptide phage display as a tool for drug discovery: Targeting membrane receptors. Molecules. 2011;16(1):857–887. doi: 10.3390/molecules16010857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan Y, Wang H. The structure and function of cell membranes examined by atomic force microscopy and single-molecule force spectroscopy. Chem Soc Rev. 2015;44(11):3617–3638. doi: 10.1039/c4cs00508b. [DOI] [PubMed] [Google Scholar]
- 11.Berg JM, Tymoczko JL, Stryer L. Carbohydrates Can Be Attached to Proteins to Form Glycoproteins. W H Freeman; 2002. [Google Scholar]
- 12.Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiess M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry. 1990;29(43):10009–10018. doi: 10.1021/bi00495a001. [DOI] [PubMed] [Google Scholar]
- 14.Cho CS, Cho KY, Park IK, Kim SH, Sasagawa T, Uchiyama M, Akaike T. Receptor-mediated delivery of all trans-retinoic acid to hepatocyte using poly(L-lactic acid) nanoparticles coated with galactose-carrying polystyrene. J Control Release. 2001;77(1–2):7–15. doi: 10.1016/s0168-3659(01)00390-x. [DOI] [PubMed] [Google Scholar]
- 15.Lai C-H, Lin C-Y, Wu H-T, Chan H-S, Chuang Y-J, Chen C-T, Lin C-C. Galactose encapsulated multifunctional nanoparticle for HepG2 cell internalization. Adv Funct Mater. 2010;20(22):3948–3958. [Google Scholar]
- 16.Kawakami S, Wong J, Sato A, Hattori Y, Yamashita F, Hashida M. Biodistribution characteristics of mannosylated, fucosylated, and galactosylated liposomes in mice. Biochim Biophys Acta. 2000;1524(2–3):258–265. doi: 10.1016/s0304-4165(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 17.Bergen JM, von Recum HA, Goodman TT, Massey AP, Pun SH. Gold nanoparticles as a versatile platform for optimizing physicochemical parameters for targeted drug delivery. Macromol Biosci. 2006;6(7):506–516. doi: 10.1002/mabi.200600075. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Garrison JC, Poluektova LY, Bronich TK, Osna NA. Liver-targeted antiviral peptide nanocomplexes as potential anti-HCV therapeutics. Biomaterials. 2015;70:37–47. doi: 10.1016/j.biomaterials.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TH, Park IK, Nah JW, Choi YJ, Cho CS. Galactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrier. Biomaterials. 2004;25(17):3783–3792. doi: 10.1016/j.biomaterials.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 20.Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials. 2007;28(7):1434–1442. doi: 10.1016/j.biomaterials.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 22.Jeong Y-I, Seo S-J, Park I-K, Lee H-C, Kang I-C, Akaike T, Cho C-S. Cellular recognition of paclitaxel-loaded polymeric nanoparticles composed of poly(γ-benzyl l-glutamate) and poly(ethylene glycol) diblock copolymer endcapped with galactose moiety. Int J Pharm. 2005;296(1–2):151–161. doi: 10.1016/j.ijpharm.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Quan S, Wang Y, Zhou A, Kumar P, Narain R. Galactose-based thermosensitive nanogels for targeted drug delivery of iodoazomycin arabinofuranoside (IAZA) for theranostic management of hypoxic hepatocellular carcinoma. Biomacromolecules. 2015;16(7):1978–1986. doi: 10.1021/acs.biomac.5b00576. [DOI] [PubMed] [Google Scholar]
- 24.Babiuch K, Dag A, Zhao J, Lu H, Stenzel MH. Carbohydrate-specific uptake of fucosylated polymeric micelles by different cancer cell lines. Biomacromolecules. 2015;16(7):1948–1957. doi: 10.1021/acs.biomac.5b00299. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida M, Takimoto R, Murase K, Sato Y, Hirakawa M, Tamura F, Sato T, Iyama S, Osuga T, Miyanishi K, Takada K, Hayashi T, Kobune M, Kato J. Targeting anticancer drug delivery to pancreatic cancer cells using a fucose-bound nanoparticle approach. PLoS One. 2012;7(7):e39545. doi: 10.1371/journal.pone.0039545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh Y-C, Kim ST, Tang R, Yan B, Rotello VM. Insulin-based regulation of glucose-functionalized nanoparticle uptake in muscle cells. J Mater Chem B Mater Biol Med. 2014;2:4610–4614. doi: 10.1039/C4TB00608A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gromnicova R, Davies HA, Sreekanthreddy P, Romero IA, Lund T, Roitt IM, Phillips JB, Male DK. Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro. PLoS One. 2013;8(12):e81043. doi: 10.1371/journal.pone.0081043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tosi G, Vergoni AV, Ruozi B, Bondioli L, Badiali L, Rivasi F, Costantino L, Forni F, Vandelli MA. Sialic acid and glycopeptides conjugated PLGA nanoparticles for central nervous system targeting: In vivo pharmacological evidence and biodistribution. J Control Release. 2010;145(1):49–57. doi: 10.1016/j.jconrel.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Marradi M, Martín-Lomas M, Penadés S. Glyconanoparticles: Polyvalent tools to study carbohydrate-based interactions. In: Horton Derek., editor. Advances in Carbohydrate Chemistry and Biochemistry. Academic Press; 2010. pp. 211–290. [DOI] [PubMed] [Google Scholar]
- 30.Valero E, Tambalo S, Marzola P, Ortega-Muñoz M, López-Jaramillo FJ, Santoyo-González F, de Dios López J, Delgado JJ, Calvino JJ, Cuesta R, Domínguez-Vera JM, Gálvez N. Magnetic nanoparticles-templated assembly of protein subunits: A new platform for carbohydrate-based MRI nanoprobes. J Am Chem Soc. 2011;133(13):4889–4895. doi: 10.1021/ja110014p. [DOI] [PubMed] [Google Scholar]
- 31.Horák D, Babiè M, Jendelová P, Herynek V, Trchová M, Pientka Z, Pollert E, Hájek M, Syková E. d-Mannose-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2007;18(3):635–644. doi: 10.1021/bc060186c. [DOI] [PubMed] [Google Scholar]
- 32.Yoo MK, Kim IY, Kim EM, Jeong H-J, Lee C-M, Jeong YY, Akaike T, Cho CS. Superparamagnetic iron oxide nanoparticles coated with galactose-carrying polymer for hepatocyte targeting. J Biomed Biotechnol. 2007;2007(10):94740. doi: 10.1155/2007/94740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H, Mortensen LJ, DeLouise LA. Thiol antioxidant-functionalized CdSe/ZnS quantum dots: Synthesis, characterization, cytotoxicity. J Biomed Nanotechnol. 2013;9(3):382–392. doi: 10.1166/jbn.2013.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohyanagi T, Nagahori N, Shimawaki K, Hinou H, Yamashita T, Sasaki A, Jin T, Iwanaga T, Kinjo M, Nishimura S-I. Importance of sialic acid residues illuminated by live animal imaging using phosphorylcholine self-assembled monolayer-coated quantum dots. J Am Chem Soc. 2011;133(32):12507–12517. doi: 10.1021/ja111201c. [DOI] [PubMed] [Google Scholar]
- 35.Higuchi Y, Oka M, Kawakami S, Hashida M. Mannosylated semiconductor quantum dots for the labeling of macrophages. J Control Release. 2008;125(2):131–136. doi: 10.1016/j.jconrel.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Kikkeri R, Lepenies B, Adibekian A, Laurino P, Seeberger PH. In vitro imaging and in vivo liver targeting with carbohydrate capped quantum dots. J Am Chem Soc. 2009;131(6):2110–2112. doi: 10.1021/ja807711w. [DOI] [PubMed] [Google Scholar]
- 37.Irache JM, Salman HH, Gamazo C, Espuelas S. Mannose-targeted systems for the delivery of therapeutics. Expert Opin Drug Deliv. 2008;5(6):703–724. doi: 10.1517/17425247.5.6.703. [DOI] [PubMed] [Google Scholar]
- 38.Linehan SA, Martínez-Pomares L, Stahl PD, Gordon S. Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: In situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med. 1999;189(12):1961–1972. doi: 10.1084/jem.189.12.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J Exp Med. 1995;182(2):389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Galα1→3Gal epitope in primates. Proc Natl Acad Sci U S A. 1987;84(5):1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Phanse Y, Carrillo-Conde BR, Ramer-Tait AE, Broderick S, Kong CS, Rajan K, Flick R, Mandell RB, Narasimhan B, Wannemuehler MJ. A systems approach to designing next generation vaccines: Combining α-galactose modified antigens with nanoparticle platforms. Sci Rep. 2014;4:3775. doi: 10.1038/srep03775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Z, Ramishetti S, Tseng Y-C, Guo S, Wang Y, Huang L. Multifunctional nanoparticles co-delivering Trp2 peptide and CpG adjuvant induce potent cytotoxic T-lymphocyte response against melanoma and its lung metastasis. J Control Release. 2013;172(1):259–265. doi: 10.1016/j.jconrel.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Kim TH, Jin H, Kim HW, Cho MH, Cho CS. Mannosylated chitosan nanoparticle-based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther. 2006;5(7):1723–1732. doi: 10.1158/1535-7163.MCT-05-0540. [DOI] [PubMed] [Google Scholar]
- 44.Zhu S, Niu M, O’Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm. 2013;10(9):3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spence S, Greene MK, Fay F, Hams E, Saunders SP, Hamid U, Fitzgerald M, Beck J, Bains BK, Smyth P, Themistou E, Small DM, Schmid D, O’Kane CM, Fitzgerald DC, Abdelghany SM, Johnston JA, Fallon PG, Burrows JF, McAuley DF, Kissenpfennig A, Scott CJ. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7(303):303ra140. doi: 10.1126/scitranslmed.aab3459. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Ramström O, Yan M. Quantitative analysis of multivalent ligand presentation on gold glyconanoparticles and the impact on lectin binding. Anal Chem. 2010;82(21):9082–9089. doi: 10.1021/ac102114z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lyu Y-K, Lim K-R, Lee BY, Kim KS, Lee W-Y. Microgravimetric lectinbiosensor based on signal amplification using carbohydrate-stabilized gold nanoparticles. Chem Commun. 2008;(39):4771–4773. doi: 10.1039/b807438k. [DOI] [PubMed] [Google Scholar]
- 48.Bellapadrona G, Tesler AB, Grünstein D, Hossain LH, Kikkeri R, Seeberger PH, Vaskevich A, Rubinstein I. Optimization of localized surface plasmon resonance transducers for studying carbohydrate–protein interactions. Anal Chem. 2012;84(1):232–240. doi: 10.1021/ac202363t. [DOI] [PubMed] [Google Scholar]
- 49.Hone DC, Haines AH, Russell DA. Rapid, quantitative colorimetric detection of a lectin using mannose-stabilized gold nanoparticles. Langmuir. 2003;19(17):7141–7144. [Google Scholar]
- 50.Lee C, Gaston MA, Weiss AA, Zhang P. Colorimetric viral detection based on sialic acid stabilized goldnanoparticles. Biosens Bioelectron. 2013;42:236–241. doi: 10.1016/j.bios.2012.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Otsuka H, Akiyama Y, Nagasaki Y, Kataoka K. Quantitative and reversible lectin-induced association of gold nanoparticles modified with α-lactosyl-ω-mercapto-poly(ethylene glycol) J Am Chem Soc. 2001;123(34):8226–8230. doi: 10.1021/ja010437m. [DOI] [PubMed] [Google Scholar]
- 52.Vedala H, Chen Y, Cecioni S, Imberty A, Vidal S, Star A. Nanoelectronic detection of lectin–carbohydrate interactions using carbon nanotubes. Nano Lett. 2011;11(1):170–175. doi: 10.1021/nl103286k. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Vedala H, Kotchey GP, Audfray A, Cecioni S, Imberty A, Vidal S, Star A. Electronic detection of lectins using carbohydrate-functionalized nanostructures: Graphene versus carbon nanotubes. ACS Nano. 2012;6(1):760–770. doi: 10.1021/nn2042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El-Boubbou K, Gruden C, Huang X. Magnetic glyco-nanoparticles: A unique tool for rapid pathogen detection, decontamination, and strain differentiation. J Am Chem Soc. 2007;129(44):13392–13393. doi: 10.1021/ja076086e. [DOI] [PubMed] [Google Scholar]
- 55.El-Boubbou K, Zhu DC, Vasileiou C, Borhan B, Prosperi D, Li W, Huang X. Magnetic glyco-nanoparticles: A tool to detect, differentiate, and unlock the glyco-codes of cancer via magnetic resonance imaging. J Am Chem Soc. 2010;132(12):4490–4499. doi: 10.1021/ja100455c. [DOI] [PubMed] [Google Scholar]
- 56.Lin Y-H, Tsai S-C, Lai C-H, Lee C-H, He ZS, Tseng G-C. Genipin-cross-linked fucose–chitosan/heparin nanoparticles for the eradication of Helicobacter pylori. Biomaterials. 2013;34(18):4466–4479. doi: 10.1016/j.biomaterials.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 57.Papp I, Sieben C, Ludwig K, Roskamp M, Böttcher C, Schlecht S, Herrmann A, Haag R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small. 2010;6(24):2900–2906. doi: 10.1002/smll.201001349. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Minimal “self” peptides that inhibit phagocytic clearance and enhance delivery of nanoparticles. Science. 2013;339(6122):971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 60.Yang Q, Lai SK. Anti-PEG immunity: Emergence, characteristics, and unaddressed questions. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7(5):655–677. doi: 10.1002/wnan.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsai RK, Rodriguez PL, Discher DE. Self inhibition of phagocytosis: The affinity of “marker of self” CD47 for SIRPα dictates potency of inhibition but only at low expression levels. Blood Cells Mol Dis. 2010;45(1):67–74. doi: 10.1016/j.bcmd.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Olive C, Clair T, Yarwood P, Good MF. Protection of mice from group A streptococcal infection by intranasal immunisation with a peptide vaccine that contains a conserved M protein B cell epitope and lacks a T cell autoepitope. Vaccine. 2002;20(21–22):2816–2825. doi: 10.1016/s0264-410x(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 63.Olive C, Batzloff MR, Horváth A, Wong A, Clair T, Yarwood P, Toth I, Good MF. A lipid core peptide construct containing a conserved region determinant of the group A streptococcal M protein elicits heterologous opsonic antibodies. Infect Immun. 2002;70(5):2734–2738. doi: 10.1128/IAI.70.5.2734-2738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hayman WA, Brandt ER, Relf WA, Cooper J, Saul A, Good MF. Mapping the minimal murine T cell and B cell epitopes within a peptide vaccine candidate from the conserved region of the M protein of group A streptococcus. Int Immunol. 1997;9(11):1723–1733. doi: 10.1093/intimm/9.11.1723. [DOI] [PubMed] [Google Scholar]
- 65.Skwarczynski M, Zaman M, Urbani CN, Lin I-C, Jia Z, Batzloff MR, Good MF, Monteiro MJ, Toth I. Polyacrylate dendrimer nanoparticles: A self-adjuvanting vaccine delivery system. Angew Chem Int Ed Engl. 2010;49(33):5742–5745. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- 66.Skwarczynski M, Parhiz BH, Soltani F, Srinivasan S, Kamaruzaman KA, Lin I-C, Toth I. Lipid peptide core nanoparticles as multivalent vaccine candidates against Streptococcus pyogenes. Aust J Chem. 2012;65(1):35–39. [Google Scholar]
- 67.Rennermalm A, Li YH, Bohaufs L, Jarstrand C, Brauner A, Brennan FR, Flock JI. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine. 2001;19(25–26):3376–3383. doi: 10.1016/s0264-410x(01)00080-9. [DOI] [PubMed] [Google Scholar]
- 68.Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, Aebi U, Burkhard P, Lanar DE. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183(11):7268–7277. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kampen KR. Membrane proteins: The key players of a cancer cell. J Membr Biol. 2011;242(2):69–74. doi: 10.1007/s00232-011-9381-7. [DOI] [PubMed] [Google Scholar]
- 70.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. The prioritization of cancer antigens: A national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newman KD, Sosnowski DL, Kwon GS, Samuel J. Delivery of MUC1 mucin peptide by poly(d,l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci. 1998;87(11):1421–1427. doi: 10.1021/js980070s. [DOI] [PubMed] [Google Scholar]
- 72.Diwan M, Elamanchili P, Lane H, Gainer A, Samuel J. Biodegradable nanoparticle mediated antigen delivery to human cord blood derived dendritic cells for induction of primary T cell responses. J Drug Target. 2003;11(8–10):495–507. doi: 10.1080/10611860410001670026. [DOI] [PubMed] [Google Scholar]
- 73.Cai H, Degliangeli F, Palitzsch B, Gerlitzki B, Kunz H, Schmitt E, Fiammengo R, Westerlind U. Glycopeptide-functionalized gold nanoparticles for antibody induction against the tumor associated mucin-1 glycoprotein. Bioorg Med Chem. 2016;24(5):1132–1135. doi: 10.1016/j.bmc.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 74.Hartmann S, Nuhn L, Palitzsch B, Glaffig M, Stergiou N, Gerlitzki B, Schmitt E, Kunz H, Zentel R. CpG-loaded multifunctional cationic nanohydrogel particles as self-adjuvanting glycopeptide antitumor vaccines. Adv Healthc Mater. 2015;4(4):522–527. doi: 10.1002/adhm.201400460. [DOI] [PubMed] [Google Scholar]
- 75.Jalali SA, Sankian M, Tavakkol-Afshari J, Jaafari MR. Induction of tumor-specific immunity by multi-epitope rat HER2/neu-derived peptides encapsulated in LPD nanoparticles. Nanomedicine. 2012;8(5):692–701. doi: 10.1016/j.nano.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 76.Lin AY, Lunsford J, Bear AS, Young JK, Eckels P, Luo L, Foster AE, Drezek RA. High-density sub-100-nm peptide–gold nanoparticle complexes improve vaccine presentation by dendritic cells in vitro. Nanoscale Res Lett. 2013;8(1):72. doi: 10.1186/1556-276X-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, Willers J, Geldhof C, Prior JO, Kündig TM, Michielin O, Bachmann MF, Speiser DE. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8+ T-cell responses in melanoma patients. Eur J Immunol. 2012;42(11):3049–3061. doi: 10.1002/eji.201142361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Getts DR, Martin AJ, McCarthy DP, Terry RL, Hunter ZN, Yap WT, Getts MT, Pleiss M, Luo X, King NJC, Shea LD, Miller SD. Microparticles bearing encephalitogenic peptides induce T-cell tolerance and ameliorate experimental autoimmune encephalomyelitis. Nat Biotechnol. 2012;30(12):1217–1224. doi: 10.1038/nbt.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hunter Z, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, Miller SD. A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease. ACS Nano. 2014;8(3):2148–2160. doi: 10.1021/nn405033r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Q, Smith C. Molecular Biology of the Cell (Fifth Edition) Bioscience Education. 2008;11(1):1–2. [Google Scholar]
- 81.Jebali A, Nayeri EK, Roohana S, Aghaei S, Ghaffari M, Daliri K, Fuente G. Nano-carbohydrates: Synthesis and application in genetics, biotechnology, and medicine. Adv Colloid Interface Sci. 2016 doi: 10.1016/j.cis.2016.11.002.. [DOI] [PubMed] [Google Scholar]
- 82.Johannssen T, Lepenies B. Glycan-based cell targeting to modulate immune responses. Trends Biotechnol. 2016 doi: 10.1016/j.tibtech.2016.10.002.. [DOI] [PubMed] [Google Scholar]
- 83.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14(2):181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 84.Eniola AO, Rodgers SD, Hammer DA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials. 2002;23(10):2167–2177. doi: 10.1016/s0142-9612(01)00349-0. [DOI] [PubMed] [Google Scholar]
- 85.Eniola AOAO, Hammer DADA. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes II: Effect of degradation on targeting activity. Biomaterials. 2005;26(6):661–670. doi: 10.1016/j.biomaterials.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 86.van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci U S A. 2009;106(1):18–23. doi: 10.1073/pnas.0806787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farr TD, Lai C-H, Grünstein D, Orts-Gil G, Wang C-C, Boehm-Sturm P, Seeberger PH, Harms C. Imaging early endothelial inflammation following stroke by core shell silica superparamagnetic glyconanoparticles that target selectin. Nano Lett. 2014;14(4):2130–2134. doi: 10.1021/nl500388h. [DOI] [PubMed] [Google Scholar]
- 88.Cruz LJ, Tacken PJ, Pots JM, Torensma R, Buschow SI, Figdor CG. Comparison of antibodies and carbohydrates to target vaccines to human dendritic cells via DC-SIGN. Biomaterials. 2012;33(16):4229–4239. doi: 10.1016/j.biomaterials.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 89.Büll C, Heise T, Adema GJ, Boltje TJ. Sialic acid mimetics to target the sialic acid–Siglec axis. Trends Biochem Sci. 2016;41(6):519–531. doi: 10.1016/j.tibs.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 90.Unger WWJ, van Beelen AJ, Bruijns SC, Joshi M, Fehres CM, van Bloois L, Verstege MI, Ambrosini M, Kalay H, Nazmi K, Bolscher JG, Hooijberg E, de Gruijl TD, Storm G, van Kooyk Y. Glycan-modified liposomes boost CD4+ and CD8+ T-cell responses by targeting DC-SIGN on dendritic cells. J Control Release. 2012;160(1):88–95. doi: 10.1016/j.jconrel.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Arosio D, Chiodo F, Reina JJ, Marelli M, Penadés S, van Kooyk Y, Garcia-Vallejo JJ, Bernardi A. Effective targeting of DC-SIGN by α-fucosylamide functionalized gold nanoparticles. Bioconjug Chem. 2014;25(12):2244–2251. doi: 10.1021/bc500467u. [DOI] [PubMed] [Google Scholar]
- 92.Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, Paulson JC. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123(7):3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blattes E, Vercellone A, Eutamène H, Turrin C-O, Théodorou V, Majoral J-P, Caminade A-M, Prandi J, Nigou J, Puzo G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc Natl Acad Sci U S A. 2013;110(22):8795–8800. doi: 10.1073/pnas.1221708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Park S, Kim G-H, Park S-H, Pai J, Rathwell D, Park J-Y, Kang Y-S, Shin I. Probing cell-surface carbohydrate binding proteins with dual-modal glycan-conjugated nanoparticles. J Am Chem Soc. 2015;137(18):5961–5968. doi: 10.1021/jacs.5b00592. [DOI] [PubMed] [Google Scholar]
- 95.Zheng L, Wei J, Lv X, Bi Y, Wu P, Zhang Z, Wang P, Liu R, Jiang J, Cong H, Liang J, Chen W, Cao H, Liu W, Gao GF, Du Y, Jiang X, Li X. Detection and differentiation of influenza viruses with glycan-functionalized gold nanoparticles. Biosens Bioelectron. 2016;91:46–52. doi: 10.1016/j.bios.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 96.de la Fuente JM, Barrientos AG, Rojas TC, Rojo J, Cañada J, Fernández A, Penadés S. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew Chem Int Ed Engl. 2001;113(12):2317–2321. doi: 10.1002/1521-3773(20010618)40:12<2257::AID-ANIE2257>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 97.Rojo J, Díaz V, de la Fuente JM, Segura I, Barrientos AG, Riese HH, Bernad A, Penadés S. Gold glyconanoparticles as new tools in antiadhesive therapy. Chembiochem. 2004;5(3):291–297. doi: 10.1002/cbic.200300726. [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Vallejo JJ, Koning N, Ambrosini M, Kalay H, Vuist I, Sarrami-Forooshani R, Geijtenbeek TBH, van Kooyk Y. Glycodendrimers prevent HIV transmission via DC-SIGN on dendritic cells. Int Immunol. 2013;25(4):221–233. doi: 10.1093/intimm/dxs115. [DOI] [PubMed] [Google Scholar]
- 99.Sattin S, Bernardi A. Glycoconjugates and glycomimetics as microbial anti-adhesives. Trends Biotechnol. 2016;34(6):483–495. doi: 10.1016/j.tibtech.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Kulkarni AA, Fuller C, Korman H, Weiss AA, Iyer SS. Glycan encapsulated gold nanoparticles selectively inhibit shiga toxins 1 and 2. Bioconjug Chem. 2010;21(8):1486–1493. doi: 10.1021/bc100095w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moulari B, Béduneau A, Pellequer Y, Lamprecht A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J Control Release. 2014;188:9–17. doi: 10.1016/j.jconrel.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 102.Feugang J, Liao S, Crenshaw M, Clemente H, Willard S, et al. Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod Med Genet. 2015;3(145):17–19. [Google Scholar]
- 103.Kona S, Dong J-F, Liu Y, Tan J, Nguyen KT. Biodegradable nanoparticles mimicking platelet binding as a targeted and controlled drug delivery system. Int J Pharm. 2012;423(2):516–524. doi: 10.1016/j.ijpharm.2011.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Perica K, De León Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, Niemöller M, Assenmacher M, Richter A, Edidin M, Oelke M, Schneck J. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine. 2014;10(1):119–129. doi: 10.1016/j.nano.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic field-induced T cell receptor clustering by nanoparticles enhances T cell activation and stimulates antitumor activity. ACS Nano. 2014;8(3):2252–2260. doi: 10.1021/nn405520d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perica K, Bieler JG, Schütz C, Varela JC, Douglass J, Skora A, Chiu YL, Oelke M, Kinzler K, Zhou S, Vogelstein B, Schneck JP. Enrichment and expansion with nanoscale artificial antigen presenting cells for adoptive immunotherapy. ACS Nano. 2015;9(7):6861–6871. doi: 10.1021/acsnano.5b02829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11(13):1519–1525. doi: 10.1002/smll.201402369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clemente-Casares X, Blanco J, Ambalavanan P, Yamanouchi J, Singha S, Fandos C, Tsai S, Wang J, Garabatos N, Izquierdo C, Agrawal S, Keough MB, Yong VW, James E, Moore A, Yang Y, Stratmann T, Serra P, Santamaria P. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature. 2016;530(7591):434–440. doi: 10.1038/nature16962. [DOI] [PubMed] [Google Scholar]
- 109.Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller G-A, Lim JE, Edgington TS, Shepard HM, Patton JS, Frost GI. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114(2):230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 110.Eikenes L, Tari M, Tufto I, Bruland OS, de Lange Davies C. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer. 2005;93(1):81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muckenschnabel I, Bernhardt G, Spruss T, Buschauer A. Hyaluronidase pretreatment produces selective melphalan enrichment in malignant melanoma implanted in nude mice. Cancer Chemother Pharmacol. 1996;38(1):88–94. doi: 10.1007/s002800050452. [DOI] [PubMed] [Google Scholar]
- 112.Scodeller P, Catalano PN, Salguero N, Duran H, Wolosiuk A, Soler-Illia GJAA. Hyaluronan degrading silica nanoparticles for skin cancer therapy. Nanoscale. 2013;5(20):9690–9698. doi: 10.1039/c3nr02787b. [DOI] [PubMed] [Google Scholar]
- 113.Zhou H, Fan Z, Lemons PK, Cheng H. A facile approach to functionalize cell membrane-coated nanoparticles. Theranostics. 2016;6(7):1012–1022. doi: 10.7150/thno.15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB, Cheng H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016;16(5):3268–3277. doi: 10.1021/acs.nanolett.6b00820. [DOI] [PubMed] [Google Scholar]
- 115.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 116.Xu W, Yang Z, Lu N. From pathogenesis to clinical application: Insights into exosomes as transfer vectors in cancer. J Exp Clin Cancer Res. 2016;35(1):156. doi: 10.1186/s13046-016-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dorayappan KDP, Wallbillich JJ, Cohn DE, Selvendiran K. The biological significance and clinical applications of exosomes in ovarian cancer. Gynecol Oncol. 2016;142(1):199–205. doi: 10.1016/j.ygyno.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang X, Pei Z, Chen J, Ji C, Xu J, Zhang X, Wang J. Exosomes for immunoregulation and therapeutic intervention in cancer. J Cancer. 2016;7(9):1081–1087. doi: 10.7150/jca.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Olsen I, Amano A. Outer membrane vesicles - Offensive weapons or good Samaritans? J Oral Microbiol. 2015;7:27468. doi: 10.3402/jom.v7.27468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: Biogenesis and functions. Nat Rev Microbiol. 2015;13(10):605–619. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Andaloussi SEL, Mäger I, Breakefield XO, Wood MJA. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 122.Ha D, Yang N, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: Current perspectives and future challenges. Acta Pharm Sin B. 2016;6(4):287–296. doi: 10.1016/j.apsb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnsen KB, Gudbergsson JM, Skov MN, Pilgaard L, Moos T, Duroux M. A comprehensive overview of exosomes as drug delivery vehicles — Endogenous nanocarriers for targeted cancer therapy. BBA Rev Cancer. 2014;1846(1):75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Ren J, He W, Zheng L, Duan H. From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomater Sci. 2016;4(6):910–921. doi: 10.1039/c5bm00583c. [DOI] [PubMed] [Google Scholar]
- 125.Wahlgren J, De L Karlson T, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40(17):e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yeo RWY, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, Lim SK. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336–341. doi: 10.1016/j.addr.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 127.Hoshino A, Costa-Silva B, Shen T-L, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Labori KJ, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ohno S-I, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, Fujita K, Mizutani T, Ohgi T, Ochiya T, Gotoh N, Kuroda M. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, Wei J, Nie G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 130.Yang T, Martin P, Fogarty B, Brown A, Schurman K, Phipps R, Yin VP, Lockman P, Bai S. Exosome delivered anticancer drugs across the blood–brain barrier for brain cancer therapy in Danio rerio. Pharm Res. 2015;32(6):2003–2014. doi: 10.1007/s11095-014-1593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 132.Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: A new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 134.Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang H-G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. 2010;18(9):1606–1614. doi: 10.1038/mt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–1715. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, Mahmood A, Xiong Y. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856–867. doi: 10.3171/2014.11.JNS14770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jang SC, Kim OY, Yoon CM, Choi D-S, Roh T-Y, Park J, Nilsson J, Lötvall J, Kim Y-K, Gho YS. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7(9):7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 138.Lejeune A, Moorjani M, Gicquaud C, Lacroix J, Poyet P, Gaudreault R. Nanoerythrosome, a new derivative of erythrocyte ghost: Preparation and antineoplastic potential as drug carrier for daunorubicin. Anticancer Res. 1994;14(3A):915–919. [PubMed] [Google Scholar]
- 139.Lejeune A, Poyet P, Gaudreault RC, Gicquaud C. Nanoerythrosomes, a new derivative of erythrocyte ghost: III. Is phagocytosis involved in the mechanism of action? Anticancer Res. 1997;17(5A):3599–3603. [PubMed] [Google Scholar]
- 140.Moorjani M, Lejeune A, Gicquaud C, Lacroix J, Poyet P, Gaudreault RC. Nanoerythrosomes, a new derivative of erythrocyte ghost: II. Identification of the mechanism of action. Anticancer Res. 1996;16(5A):2831–2836. [PubMed] [Google Scholar]
- 141.Désilets J, Lejeune A, Mercer J, Gicquaud C. Nanoerythrosomes, a new derivative of erythrocyte ghost: IV. Fate of reinjected nanoerythrosomes. Anticancer Res. 2001;21(3B):1741–1747. [PubMed] [Google Scholar]
- 142.Bahmani B, Bacon D, Anvari B. Erythrocyte-derived photo-theranostic agents: Hybrid nano-vesicles containing indocyanine green for near infrared imaging and therapeutic applications. Sci Rep. 2013;3:2180. doi: 10.1038/srep02180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gupta N, Patel B, Ahsan F. Nano-engineered erythrocyte ghosts as inhalational carriers for delivery of fasudil: Preparation and characterization. Pharm Res. 2014;31(6):1553–1565. doi: 10.1007/s11095-013-1261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hsieh C-C, Kang S-T, Lin Y-H, Ho Y-J, Wang C-H, Yeh C-K, Chang C-W. Biomimetic acoustically-responsive vesicles for theranostic applications. Theranostics. 2015;5(11):1264–1274. doi: 10.7150/thno.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mac JT, Nuñez V, Burns JM, Guerrero YA, Vullev VI, Anvari B. Erythrocyte-derived nano-probes functionalized with antibodies for targeted near infrared fluorescence imaging of cancer cells. Biomed Opt Express. 2016;7(4):1311–1322. doi: 10.1364/BOE.7.001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Toledano Furman NE, Lupu-Haber Y, Bronshtein T, Kaneti L, Letko N, Weinstein E, Baruch L, Machluf M. Reconstructed stem cell nanoghosts: A natural tumor targeting platform. Nano Lett. 2013;13(7):3248–3255. doi: 10.1021/nl401376w. [DOI] [PubMed] [Google Scholar]
- 147.Kaneti L, Bronshtein T, Malkah Dayan N, Kovregina I, Letko Khait N, Lupu-Haber Y, Fliman M, Schoen BW, Kaneti G, Machluf M. Nanoghosts as a novel natural nonviral gene delivery platform safely targeting multiple cancers. Nano Lett. 2016;16(3):1574–1582. doi: 10.1021/acs.nanolett.5b04237. [DOI] [PubMed] [Google Scholar]
- 148.Gao J, Chu D, Wang Z. Cell membrane-formed nanovesicles for disease-targeted delivery. J Control Release. 2016;224:208–216. doi: 10.1016/j.jconrel.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Peng L-H, Zhang Y-H, Han L-J, Zhang C-Z, Wu J-H, Wang X-R, Gao J-Q, Mao Z-W. Cell membrane capsules for encapsulation of chemotherapeutic and cancer cell targeting in vivo. ACS Appl Mater Interfaces. 2015;7(33):18628–18637. doi: 10.1021/acsami.5b05065. [DOI] [PubMed] [Google Scholar]
- 150.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E. Biomimetic proteolipid vesicles for targeting inflamed tissues. Nat Mater. 2016;15(9):1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim S-H, Kim K-S, Lee S-R, Kim E, Kim M-S, Lee E-Y, Gho YS, Kim J-W, Bishop RE, Chang K-T. Structural modifications of outer membrane vesicles to refine them as vaccine delivery vehicles. Biochim Biophys Acta. 2009;1788(10):2150–2159. doi: 10.1016/j.bbamem.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim J-Y, Doody AM, Chen DJ, Cremona GH, Shuler ML, Putnam D, DeLisa MP. Engineered bacterial outer membrane vesicles with enhanced functionality. J Mol Biol. 2008;380(1):51–66. doi: 10.1016/j.jmb.2008.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC, Jon S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano. 2014;8(2):1525–1537. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 154.Alves NJ, Turner KB, Medintz IL, Walper SA. Emerging therapeutic delivery capabilities and challenges utilizing enzyme/protein packaged bacterial vesicles. Ther Deliv. 2015;6(7):873–887. doi: 10.4155/tde.15.40. [DOI] [PubMed] [Google Scholar]
- 155.Hao S, Moyana T, Xiang J. Review: Cancer immunotherapy by exosome-based vaccines. Cancer Biother Radiopharm. 2007;22(5):692–703. doi: 10.1089/cbr.2007.368-R. [DOI] [PubMed] [Google Scholar]
- 156.Tan A, De La Peña H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int J Nanomedicine. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136(4):E74–84. doi: 10.1002/ijc.29100. [DOI] [PubMed] [Google Scholar]
- 158.Kunigelis KE, Graner MW. The dichotomy of tumor exosomes (TEX) in cancer immunity: Is it all in the conTEXt? Vaccines (Basel) 2015;3(4):1019–1051. doi: 10.3390/vaccines3041019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen W, Wang J, Shao C, Liu S, Yu Y, Wang Q, Cao X. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol. 2006;36(6):1598–1607. doi: 10.1002/eji.200535501. [DOI] [PubMed] [Google Scholar]
- 160.Dai S, Wan T, Wang B, Zhou X, Xiu F, Chen T, Wu Y, Cao X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin Cancer Res. 2005;11(20):7554–7563. doi: 10.1158/1078-0432.CCR-05-0810. [DOI] [PubMed] [Google Scholar]
- 161.Cho J-A, Lee Y-S, Kim S-H, Ko J-K, Kim C-W. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275(2):256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 162.Xie Y, Bai O, Zhang H, Yuan J, Zong S, Chibbar R, Slattery K, Qureshi M, Wei Y, Deng Y, Xiang J. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell Mol Med. 2010;14(11):2655–2666. doi: 10.1111/j.1582-4934.2009.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang J, Wang L, Lin Z, Tao L, Chen M. More efficient induction of antitumor T cell immunity by exosomes from CD40L gene-modified lung tumor cells. Mol Med Rep. 2014;9(1):125–131. doi: 10.3892/mmr.2013.1759. [DOI] [PubMed] [Google Scholar]
- 164.Li W, Mu D, Tian F, Hu Y, Jiang T, Han Y, Chen J, Han G, Li X. Exosomes derived from Rab27a-overexpressing tumor cells elicit efficient induction of antitumor immunity. Mol Med Rep. 2013;8(6):1876–1882. doi: 10.3892/mmr.2013.1738. [DOI] [PubMed] [Google Scholar]
- 165.Hao S, Bai O, Li F, Yuan J, Laferte S, Xiang J. Mature dendritic cells pulsed with exosomes stimulate efficient cytotoxic T-lymphocyte responses and antitumour immunity. Immunology. 2007;120(1):90–102. doi: 10.1111/j.1365-2567.2006.02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hao S, Bai O, Yuan J, Qureshi M, Xiang J. Dendritic cell-derived exosomes stimulate stronger CD8+ CTL responses and antitumor immunity than tumor cell-derived exosomes. Cell Mol Immunol. 2006;3(3):205–211. [PubMed] [Google Scholar]
- 167.André F, Chaput N, Schartz NEC, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu D-H, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172(4):2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 168.Chaput N, Schartz NEC, André F, Taïeb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172(4):2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 169.Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I. A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice. Microbes Infect. 2007;9(14–15):1614–1622. doi: 10.1016/j.micinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 170.Hsu D-H, Paz P, Villaflor G, Rivas A, Mehta-Damani A, Angevin E, Zitvogel L, Le Pecq J-B. Exosomes as a tumor vaccine: Enhancing potency through direct loading of antigenic peptides. J Immunother. 2003;26(5):440–450. doi: 10.1097/00002371-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 171.Yin W, Ouyang S, Li Y, Xiao B, Yang H. Immature dendritic cell-derived exosomes: A promise subcellular vaccine for autoimmunity. Inflammation. 2013;36(1):232–240. doi: 10.1007/s10753-012-9539-1. [DOI] [PubMed] [Google Scholar]
- 172.Yang X, Meng S, Jiang H, Zhu C, Wu W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J Surg Res. 2011;171(2):826–832. doi: 10.1016/j.jss.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 173.Kim S-H, Lechman ER, Bianco N, Menon R, Keravala A, Nash J, Mi Z, Watkins SC, Gambotto A, Robbins PD. Exosomes derived from IL-10-treated dendritic cells can suppress inflammation and collagen-induced arthritis. J Immunol. 2005;174(10):6440–6448. doi: 10.4049/jimmunol.174.10.6440. [DOI] [PubMed] [Google Scholar]
- 174.Cai Z, Zhang W, Yang F, Yu L, Yu Z, Pan J, Wang L, Cao X, Wang J. Immunosuppressive exosomes from TGF-β1 gene-modified dendritic cells attenuate Th17-mediated inflammatory autoimmune disease by inducing regulatory T cells. Cell Res. 2012;22(3):607–610. doi: 10.1038/cr.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol. 2007;179(4):2242–2249. doi: 10.4049/jimmunol.179.4.2242. [DOI] [PubMed] [Google Scholar]
- 176.Acevedo R, Fernández S, Zayas C, Acosta A, Sarmiento ME, Ferro VA, Rosenqvist E, Campa C, Cardoso D, Garcia L, Perez JL. Bacterial outer membrane vesicles and vaccine applications. Front Immunol. 2014;5:121. doi: 10.3389/fimmu.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Price NL, Goyette-Desjardins G, Nothaft H, Valguarnera E, Szymanski CM, Segura M, Feldman MF. Glycoengineered outer membrane vesicles: A novel platform for bacterial vaccines. Sci Rep. 2016;6:24931. doi: 10.1038/srep24931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–387. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 179.van der Pol L, Stork M, van der Ley P. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10(11):1689–1706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discov Med. 2011;12(62):7–15. [PubMed] [Google Scholar]
- 181.Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O’Hallahan J, Rosenqvist E, Black S. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9(6):1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338(8775):1093–1096. doi: 10.1016/0140-6736(91)91961-s. [DOI] [PubMed] [Google Scholar]
- 183.Koeberling O, Welsch JA, Granoff DM. Improved immunogenicity of a H44/76 group B outer membrane vesicle vaccine with over-expressed genome-derived Neisserial antigen 1870. Vaccine. 2007;25(10):1912–1920. doi: 10.1016/j.vaccine.2006.03.092. [DOI] [PubMed] [Google Scholar]
- 184.Sanders H, Norheim G, Chan H, Dold C, Vipond C, Derrick JP, Pollard AJ, Maiden MCJ, Feavers IM. FetA antibodies induced by an outer membrane vesicle vaccine derived from a serogroup B meningococcal isolate with constitutive FetA expression. PLoS One. 2015;10(10):e0140345. doi: 10.1371/journal.pone.0140345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Koeberling O, Seubert A, Granoff DM. Bactericidal antibody responses elicited by a meningococcal outer membrane vesicle vaccine with overexpressed factor H-binding protein and genetically attenuated endotoxin. J Infect Dis. 2008;198(2):262–270. doi: 10.1086/589308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koeberling O, Seubert A, Santos G, Colaprico A, Ugozzoli M, Donnelly J, Granoff DM. Immunogenicity of a meningococcal native outer membrane vesicle vaccine with attenuated endotoxin and over-expressed factor H binding protein in infant rhesus monkeys. Vaccine. 2011;29(29–30):4728–4734. doi: 10.1016/j.vaccine.2011.04.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77(1):472–484. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008;76(10):4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Raeven RHM, van der Maas L, Tilstra W, Uittenbogaard JP, Bindels THE, Kuipers B, van der Ark A, Pennings JLA, van Riet E, Jiskoot W, Kersten GFA, Metz B. Immunoproteomic profiling of Bordetella pertussis outer membrane vesicle vaccine reveals broad and balanced humoral immunogenicity. J Proteome Res. 2015;14(7):2929–2942. doi: 10.1021/acs.jproteome.5b00258. [DOI] [PubMed] [Google Scholar]
- 190.McCaig WD, Loving CL, Hughes HR, Brockmeier SL. Characterization and vaccine potential of outer membrane vesicles produced by Haemophilus parasuis. PLoS One. 2016;11(3):e0149132. doi: 10.1371/journal.pone.0149132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, Morici LA. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine. 2011;29(46):8381–8389. doi: 10.1016/j.vaccine.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bartolini E, Ianni E, Frigimelica E, Petracca R, Galli G, Berlanda Scorza F, Norais N, Laera D, Giusti F, Pierleoni A, Donati M, Cevenini R, Finco O, Grandi G, Grifantini R. Recombinant outer membrane vesicles carrying Chlamydia muridarum HtrA induce antibodies that neutralize chlamydial infection in vitro. J Extracell Vesicles. 2013;2:20181. doi: 10.3402/jev.v2i0.20181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pritsch M, Ben-Khaled N, Chaloupka M, Kobold S, Berens-Riha N, Peter A, Liegl G, Schubert S, Hoelscher M, Löscher T, Wieser A. Comparison of intranasal outer membrane vesicles with cholera toxin and injected MF59C.1 as adjuvants for malaria transmission blocking antigens AnAPN1 and Pfs48/45. J Immunol Res. 2016;2016:3576028. doi: 10.1155/2016/3576028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reza Aghasadeghi M, Sharifat Salmani A, Mehdi Sadat S, Javadi F, Memarnejadian A, Vahabpour R, Zabihollahi R, Moshiri A, Davar Siadat S. Application of outer membrane vesicle of Neisseria meningitidis serogroup B as a new adjuvant to induce strongly Th1-oriented responses against HIV-1. Curr HIV Res. 2011;9(8):630–635. doi: 10.2174/157016211798998772. [DOI] [PubMed] [Google Scholar]
- 195.Kim OY, Choi SJ, Jang SC, Park K-S, Kim SR, Choi JP, Lim JH, Lee S-W, Park J, Di Vizio D, Lötvall J, Kim Y-K, Gho YS. Bacterial protoplast-derived nanovesicles as vaccine delivery system against bacterial infection. Nano Lett. 2015;15(1):266–274. doi: 10.1021/nl503508h. [DOI] [PubMed] [Google Scholar]
- 196.Wasilewski T, Gębicki J, Kamysz W. Bioelectronic nose: Current status and perspectives. Biosens Bioelectron. 2017;87:480–494. doi: 10.1016/j.bios.2016.08.080. [DOI] [PubMed] [Google Scholar]
- 197.Lee SH, Park TH. Recent advances in the development of bioelectronic nose. Biotechnol Bioprocess Eng. 2010;15(1):22–29. [Google Scholar]
- 198.Oh EH, Song HS, Park TH. Recent advances in electronic and bioelectronic noses and their biomedical applications. Enzyme Microb Technol. 2011;48(6–7):427–437. doi: 10.1016/j.enzmictec.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 199.Park J, Lim JH, Jin HJ, Namgung S, Lee SH, Park TH, Hong S. A bioelectronic sensor based on canine olfactory nanovesicle–carbon nanotube hybrid structures for the fast assessment of food quality. Analyst. 2012;137(14):3249–3254. doi: 10.1039/c2an16274a. [DOI] [PubMed] [Google Scholar]
- 200.Song HS, Jin HJ, Ahn SR, Kim D, Lee SH, Kim U-K, Simons CT, Hong S, Park TH. Bioelectronic tongue using heterodimeric human taste receptor for the discrimination of sweeteners with human-like performance. ACS Nano. 2014;8(10):9781–9789. doi: 10.1021/nn502926x. [DOI] [PubMed] [Google Scholar]
- 201.Ahn SR, An JH, Song HS, Park JW, Lee SH, Kim JH, Jang J, Park TH. Duplex bioelectronic tongue for sensing umami and sweet tastes based on human taste receptor nanovesicles. ACS Nano. 2016;10(8):7287–7296. doi: 10.1021/acsnano.6b02547. [DOI] [PubMed] [Google Scholar]
- 202.Jin HJ, Lee SH, Kim TH, Park J, Song HS, Park TH, Hong S. Nanovesicle-based bioelectronic nose platform mimicking human olfactory signal transduction. Biosens Bioelectron. 2012;35(1):335–341. doi: 10.1016/j.bios.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 203.Lim JH, Park J, Oh EH, Ko HJ, Hong S, Park TH. Nanovesicle-based bioelectronic nose for the diagnosis of lung cancer from human blood. Adv Healthc Mater. 2014;3(3):360–366. doi: 10.1002/adhm.201300174. [DOI] [PubMed] [Google Scholar]
- 204.Park SJ, Song HS, Kwon OS, Chung JH, Lee SH, An JH, Ahn SR, Lee JE, Yoon H, Park TH, Jang J. Human dopamine receptor nanovesicles for gate-potential modulators in high-performance field-effect transistor biosensors. Sci Rep. 2014;4:4342. doi: 10.1038/srep04342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ahn JH, Lim JH, Park J, Oh EH, Son M, Hong S, Park TH. Screening of target-specific olfactory receptor and development of olfactory biosensor for the assessment of fungal contamination in grain. Sens Actuators B Chem. 2015;210:9–16. [Google Scholar]
- 206.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci U S A. 2011;108(27):10980–10985. doi: 10.1073/pnas.1106634108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hu C-MJ, Fang RH, Luk BT, Chen KNH, Carpenter C, Gao W, Zhang K, Zhang L. “Marker-of-self” functionalization of nanoscale particles through a top-down cellular membrane coating approach. Nanoscale. 2013;5(7):2664–2668. doi: 10.1039/c3nr00015j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luk BT, Hu C-MJ, Fang RH, Dehaini D, Carpenter C, Gao W, Zhang L. Interfacial interactions between natural RBC membranes and synthetic polymeric nanoparticles. Nanoscale. 2014;6(5):2730–2737. doi: 10.1039/c3nr06371b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fan Z, Zhou H, Li PY, Speer JE, Cheng H. Structural elucidation of cell membrane-derived nanoparticles using molecular probes. J Mater Chem B Mater Biol Med. 2014;2(46):8231–8238. doi: 10.1039/c4tb00980k. [DOI] [PubMed] [Google Scholar]
- 210.Gao W, Hu C-MJ, Fang RH, Luk BT, Su J, Zhang L. Surface functionalization of gold nanoparticles with red blood cell membranes. Adv Mater. 2013;25(26):3549–3553. doi: 10.1002/adma.201300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang J, Gao W, Fang RH, Dong A, Zhang L. Synthesis of nanogels via cell membrane-templated polymerization. Small. 2015;11(34):4309–4313. doi: 10.1002/smll.201500987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fu Q, Lv P, Chen Z, Ni D, Zhang L, Yue H, Yue Z, Wei W, Ma G. Programmed co-delivery of paclitaxel and doxorubicin boosted by camouflaging with erythrocyte membrane. Nanoscale. 2015;7(9):4020–4030. doi: 10.1039/c4nr07027e. [DOI] [PubMed] [Google Scholar]
- 213.Kroll A, Fang RH, Zhang L. Biointerfacing and applications of cell membrane-coated nanoparticles. Bioconjug Chem. 2016;28(1):23–32. doi: 10.1021/acs.bioconjchem.6b00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Aryal S, Hu C-MJ, Fang RH, Dehaini D, Carpenter C, Zhang D-E, Zhang L. Erythrocyte membrane-cloaked polymeric nanoparticles for controlled drug loading and release. Nanomedicine (Lond) 2013;8(8):1271–1280. doi: 10.2217/nnm.12.153. [DOI] [PubMed] [Google Scholar]
- 215.Luk BT, Fang RH, Hu C-MJ, Copp JA, Thamphiwatana S, Dehaini D, Gao W, Zhang K, Li S, Zhang L. Safe and immunocompatible nanocarriers cloaked in RBC membranes for drug delivery to treat solid tumors. Theranostics. 2016;6(7):1004–1011. doi: 10.7150/thno.14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fang RH, Hu C-MJ, Chen KNH, Luk BT, Carpenter CW, Gao W, Li S, Zhang D-E, Lu W, Zhang L. Lipid-insertion enables targeting functionalization of erythrocyte membrane-cloaked nanoparticles. Nanoscale. 2013;5(19):8884–8888. doi: 10.1039/c3nr03064d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Su J, Sun H, Meng Q, Yin Q, Tang S, Zhang P, Chen Y, Zhang Z, Yu H, Li Y. Long circulation red-blood-cell-mimetic nanoparticles with peptide-enhanced tumor penetration for simultaneously inhibiting growth and lung metastasis of breast cancer. Adv Funct Mater. 2016;26(8):1243–1252. [Google Scholar]
- 218.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang Z, Yu H, Zhang P, Wang S, Li Y. Cancer cell membrane-coated gold nanocages with hyperthermia-triggered drug release and homotypic target inhibit growth and metastasis of breast cancer. Adv Funct Mater. 2016;27(3):1604300. [Google Scholar]
- 219.Zhu J-Y, Zheng D-W, Zhang M-K, Yu W-Y, Qiu W-X, Hu J-J, Feng J, Zhang X-Z. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 2016;16(9):5895–5901. doi: 10.1021/acs.nanolett.6b02786. [DOI] [PubMed] [Google Scholar]
- 220.Rao L, Meng Q-F, Huang Q, Liu P, Bu L-L, Kondamareddy KK, Guo S-S, Liu W, Zhao X-Z. Photocatalytic degradation of cell membrane coatings for controlled drug release. Adv Healthc Mater. 2016;5(12):1420–1427. doi: 10.1002/adhm.201600303. [DOI] [PubMed] [Google Scholar]
- 221.Su J, Sun H, Meng Q, Yin Q, Zhang P, Zhang Z, Yu H, Li Y. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv Funct Mater. 2016;26(41):7495–7506. [Google Scholar]
- 222.Rao L, Bu L-L, Xu J-H, Cai B, Yu G-T, Yu X, He Z, Huang Q, Li A, Guo S-S, Zhang W-F, Liu W, Sun Z-J, Wang H, Wang T-H, Zhao X-Z. Red blood cell membrane as a biomimetic nanocoating for prolonged circulation time and reduced accelerated blood clearance. Small. 2015;11(46):6225–6236. doi: 10.1002/smll.201502388. [DOI] [PubMed] [Google Scholar]
- 223.Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014;14(4):2181–2188. doi: 10.1021/nl500618u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sun H, Su J, Meng Q, Yin Q, Chen L, Gu W, Zhang P, Zhang Z, Yu H, Wang S, Li Y. Cancer-cell-biomimetic nanoparticles for targeted therapy of homotypic tumors. Adv Mater. 2016;28(43):9581–9588. doi: 10.1002/adma.201602173. [DOI] [PubMed] [Google Scholar]
- 225.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions. Nat Nanotechnol. 2013;8(1):61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Krishnamurthy S, Gnanasammandhan MK, Xie C, Huang K, Cui MY, Chan JM. Monocyte cell membrane-derived nanoghosts for targeted cancer therapy. Nanoscale. 2016;8(13):6981–6985. doi: 10.1039/c5nr07588b. [DOI] [PubMed] [Google Scholar]
- 227.Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4(11):1645–1652. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 228.Gao C, Lin Z, Jurado-Sánchez B, Lin X, Wu Z, He Q. Stem cell membrane-coated nanogels for highly efficient in vivo tumor targeted drug delivery. Small. 2016;12(30):4056–4062. doi: 10.1002/smll.201600624. [DOI] [PubMed] [Google Scholar]
- 229.Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L. Nanoparticle biointerfacing by platelet membrane cloaking. Nature. 2015;526(7571):118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hu Q, Sun W, Qian C, Wang C, Bomba HN, Gu Z. Anticancer platelet-mimicking nanovehicles. Adv Mater. 2015;27(44):7043–7050. doi: 10.1002/adma.201503323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hu Q, Qian C, Sun W, Wang J, Chen Z, Bomba HN, Xin H, Shen Q, Gu Z. Engineered nanoplatelets for enhanced treatment of multiple myeloma and thrombus. Adv Mater. 2016;28(43):9573–9580. doi: 10.1002/adma.201603463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Silva AKA, Di Corato R, Pellegrino T, Chat S, Pugliese G, Luciani N, Gazeau F, Wilhelm C. Cell-derived vesicles as a bioplatform for the encapsulation of theranostic nanomaterials. Nanoscale. 2013;5(23):11374–11384. doi: 10.1039/c3nr01541f. [DOI] [PubMed] [Google Scholar]
- 233.Lai P-Y, Huang R-Y, Lin S-Y, Lin Y-H, Chang C-W. Biomimetic stem cell membrane-camouflaged iron oxide nanoparticles for theranostic applications. RSC Adv. 2015;5(119):98222–98230. [Google Scholar]
- 234.Ren X, Zheng R, Fang X, Wang X, Zhang X, Yang W, Sha X. Red blood cell membrane camouflaged magnetic nanoclusters for imaging-guided photothermal therapy. Biomaterials. 2016;92:13–24. doi: 10.1016/j.biomaterials.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 235.Rao L, He Z, Meng Q-F, Zhou Z, Bu L-L, Guo S-S, Liu W, Zhao X-Z. Effective cancer targeting and imaging using macrophage membrane-camouflaged upconversion nanoparticles. J Biomed Mater Res A. 2017;105(2):521–530. doi: 10.1002/jbm.a.35927. [DOI] [PubMed] [Google Scholar]
- 236.Rao L, Bu L-L, Cai B, Xu J-H, Li A, Zhang W-F, Sun Z-J, Guo S-S, Liu W, Wang T-H, Zhao X-Z. Cancer cell membrane-coated upconversion nanoprobes for highly specific tumor imaging. Adv Mater. 2016;28(18):3460–3466. doi: 10.1002/adma.201506086. [DOI] [PubMed] [Google Scholar]
- 237.Chen Z, Zhao P, Luo Z, Zheng M, Tian H, Gong P, Gao G, Pan H, Liu L, Ma A, Cui H, Ma Y, Cai L. Cancer cell membrane-biomimetic nanoparticles for homologous-targeting dual-modal imaging and photothermal therapy. ACS Nano. 2016;10(11):10049–10057. doi: 10.1021/acsnano.6b04695. [DOI] [PubMed] [Google Scholar]
- 238.Piao J-G, Wang L, Gao F, You Y-Z, Xiong Y, Yang L. Erythrocyte membrane is an alternative coating to polyethylene glycol for prolonging the circulation lifetime of gold nanocages for photothermal therapy. ACS Nano. 2014;8(10):10414–10425. doi: 10.1021/nn503779d. [DOI] [PubMed] [Google Scholar]
- 239.Xuan M, Shao J, Dai L, Li J, He Q. Macrophage cell membrane camouflaged Au nanoshells for in vivo prolonged circulation life and enhanced cancer photothermal therapy. ACS Appl Mater Interfaces. 2016;8(15):9610–9618. doi: 10.1021/acsami.6b00853. [DOI] [PubMed] [Google Scholar]
- 240.Ding H, Lv Y, Ni D, Wang J, Tian Z, Wei W, Ma G. Erythrocyte membrane-coated NIR-triggered biomimetic nanovectors with programmed delivery for photodynamic therapy of cancer. Nanoscale. 2015;7(21):9806–9815. doi: 10.1039/c5nr02470f. [DOI] [PubMed] [Google Scholar]
- 241.Gao C, Lin Z, Wu Z, Lin X, He Q. Stem-cell-membrane camouflaging on near-infrared photoactivated upconversion nanoarchitectures for in vivo remote-controlled photodynamic therapy. ACS Appl Mater Interfaces. 2016;8(50):34252–34260. doi: 10.1021/acsami.6b12865. [DOI] [PubMed] [Google Scholar]
- 242.Cheng H, Zhu J-Y, Li S-Y, Zeng J-Y, Lei Q, Chen K-W, Zhang C, Zhang X-Z. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv Funct Mater. 2016;26(43):7847–7860. [Google Scholar]
- 243.Hu C-MJ, Fang RH, Copp J, Luk BT, Zhang L. A biomimetic nanosponge that absorbs pore-forming toxins. Nat Nanotechnol. 2013;8(5):336–340. doi: 10.1038/nnano.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang Q, Hu C-MJ, Fang RH, Copp JA, Pornpattananangkul D, Lu W, Zhang L. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv Mater. 2015;27(22):3437–3443. doi: 10.1002/adma.201501071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Chhabria V, Beeton S. Development of nanosponges from erythrocyte ghosts for removal of streptolysin-O from mammalian blood. Nanomedicine (Lond) 2016;11(21):2797–2807. doi: 10.2217/nnm-2016-0180. [DOI] [PubMed] [Google Scholar]
- 246.Wu Z, Li T, Gao W, Xu T, Jurado-Sánchez B, Li J, Gao W, He Q, Zhang L, Wang J. Cell-membrane-coated synthetic nanomotors for effective biodetoxification. Adv Funct Mater. 2015;25(25):3881–3887. [Google Scholar]
- 247.Copp JA, Fang RH, Luk BT, Hu C-MJ, Gao W, Zhang K, Zhang L. Clearance of pathological antibodies using biomimetic nanoparticles. Proc Natl Acad Sci U S A. 2014;111(37):13481–13486. doi: 10.1073/pnas.1412420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wei X, Gao J, Fang RH, Luk BT, Kroll AV, Dehaini D, Zhou J, Kim HW, Gao W, Lu W, Zhang L. Nanoparticles camouflaged in platelet membrane coating as an antibody decoy for the treatment of immune thrombocytopenia. Biomaterials. 2016;111:116–123. doi: 10.1016/j.biomaterials.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pang Z, Hu C-MJ, Fang RH, Luk BT, Gao W, Wang F, Chuluun E, Angsantikul P, Thamphiwatana S, Lu W, Jiang X, Zhang L. Detoxification of organophosphate poisoning using nanoparticle bioscavengers. ACS Nano. 2015;9(6):6450–6458. doi: 10.1021/acsnano.5b02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Nguyen TDT, Pitchaimani A, Koirala MB, Muhammad F, Aryal S. Engineered biomimetic nanoabsorbent for cellular detoxification of chemotherapeutics. RSC Adv. 2016;6(39):33003–33008. [Google Scholar]
- 251.Hu C-MJ, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8(12):933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wang F, Fang RH, Luk BT, Hu C-MJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, Lu W, Zhang L. Nanoparticle-based antivirulence vaccine for the management of methicillin-resistant Staphylococcus aureus skin infection. Adv Funct Mater. 2016;26(10):1628–1635. doi: 10.1002/adfm.201505231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, Kong M, Qi Y, Tan S, Zhang Z. Erythrocyte membrane-enveloped polymeric nanoparticles as nanovaccine for induction of antitumor immunity against melanoma. ACS Nano. 2015;9(7):6918–6933. doi: 10.1021/acsnano.5b01042. [DOI] [PubMed] [Google Scholar]
- 254.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu C-MJ, Zhang L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15(2):1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]