Abstract
Background
Leukoencephalopathy is observed in some children undergoing chemotherapy for acute lymphoblastic leukemia (ALL), though its impact on long-term outcomes is unknown. This study examines associations between acute leukoencephalopathy, and neurobehavioral, neurocognitive and brain white matter imaging outcomes in long-term survivors of ALL treated with chemotherapy without cranial radiation.
Methods
During active therapy, 190 survivors (mean[SD] age 13·7[4·4] years, 7·7[1·7] years post-diagnosis) had brain magnetic resonance imaging (MRI’s), which were systematically coded for leukoencephalopathy using Common Terminology Criteria for Adverse Event v4. At ≥5 years post-diagnosis, survivors completed neurocognitive testing, another brain MRI, and their parents completed neurobehavioral ratings. Follow-up MRI included diffusion tensor imaging to assess white matter integrity, with indices of fractional anisotropy (FA), axial diffusivity (AD) and radial diffusivity (RD) from frontal and parietal lobes, and within the frontostriatal tract, an axonal fiber bundle associated with executive function.
Findings
Compared to population norms, survivors were reported to demonstrate more problems with Working Memory, Organization, Initiation and Planning (all p’s<0·001). The 51/190 survivors (27%) with a history of acute leukoencephalopathy displayed more problems than survivors with no history of leukoencephalopathy on Organization and Initiation. Survivors with acute leukoencephalopathy also had reduced white matter integrity within the frontostriatal tract at follow-up: lower FA (p=0·069), higher AD (p=0·020) and higher RD (p=0·0077). A one-unit change in the RD index corresponded to a 15·0, 30·3 and 28·0 increase in raw score points on Initiation, Planning and Working Memory, respectively (all p’s<0·050).
Interpretation
Acute leukoencephalopathy during chemotherapy treatment without cranial radiation for childhood ALL predicted higher risk for long-term neurobehavioral problems and reduced white matter integrity in frontal brain regions. Survivors of ALL may benefit from preventative cognitive and/or behavioral interventions, particularly those who develop acute leukoencephalopathy.
Cranial radiation therapy (CRT) has been associated with serious adverse effects, including cognitive impairment and behavioral abnormalities that can lead to poor educational attainment and unemployment in long-term survivors of pediatric acute lymphoblastic leukemia (ALL).1 Contemporary protocols have now replaced CRT with systemic and intrathecal chemotherapy2, 3, which has reduced the degree of survivors’ cognitive impairment.4 Among a large cohort of adults who underwent neurocognitive assessment at 26 years post-diagnosis, the risk for impaired executive function in survivors treated with chemotherapy only was six times less than those treated with 24-Gy CRT.1 Non-irradiated survivors also had better academic achievement than those who received CRT.
Despite lower prevalence of neurocognitive deficits with the omission of CRT, ALL survivors treated with chemotherapy only are still at risk for neurotoxicity that can occur during the course of treatment and persist into long-term survivorship.4–6 Contemporary chemotherapy protocols now employ high-dose intravenous methotrexate (MTX), intrathecal MTX and corticosteroids. In recent literature, these neurotoxic agents are associated with long-term problems in memory, attention, processing speed and executive function.1, 6–8 These cognitive deficits may manifest as functional impairment in survivors’ behavioral regulation and metacognitive abilities. 7,9 The criteria for early identification of those at risk for long-term problems have not been established.
Leukoencephalopathy, seizures and cerebrovascular injury are additional neurologic events that occur in ALL patients treated with chemotherapy protocols.5, 10 We recently reported that 21% of children treated on an institutional protocol that eliminated CRT developed asymptomatic leukoencephalopathy while on therapy, and that 69% of them had abnormal findings on magnetic resonance imaging at the end of therapy.5 In a small cross-sectional study (n=66) of survivors treated with chemotherapy only and evaluated at more than 2 years post-treatment, the rate of leukoencephalopathy was 68% and these survivors presented with marginally more attention problems than those who did not have leukoencephalopathy.8 However, there is a paucity of literature that elucidate the impact of neurotoxicity during active treatment on brain function and integrity in long-term ALL survivors. Although these on-therapy radiological changes in brain structure may be transient, little is known about whether these acute white matter alterations may affect normal brain maturation and development during the survivorship phase, leading to long-term deficits.
In lieu of these research gaps, the current study examined the association between leukoencephalopathy during active treatment (referred to as “acute leukoencephalopathy” hereafter) with neurobehavioral and neurocognitive function, and white matter integrity during long-term follow-up in a large cohort of ALL survivors treated with contemporary risk-adapted chemotherapy without CRT. We hypothesized that survivors who demonstrated acute leukoencephalopathy would be more likely to display neurocognitive and parent-reported neurobehavioral problems, and reduced white matter integrity at long-term follow-up compared to survivors with no history of acute leukoencephalopathy.
Method
Design and patients
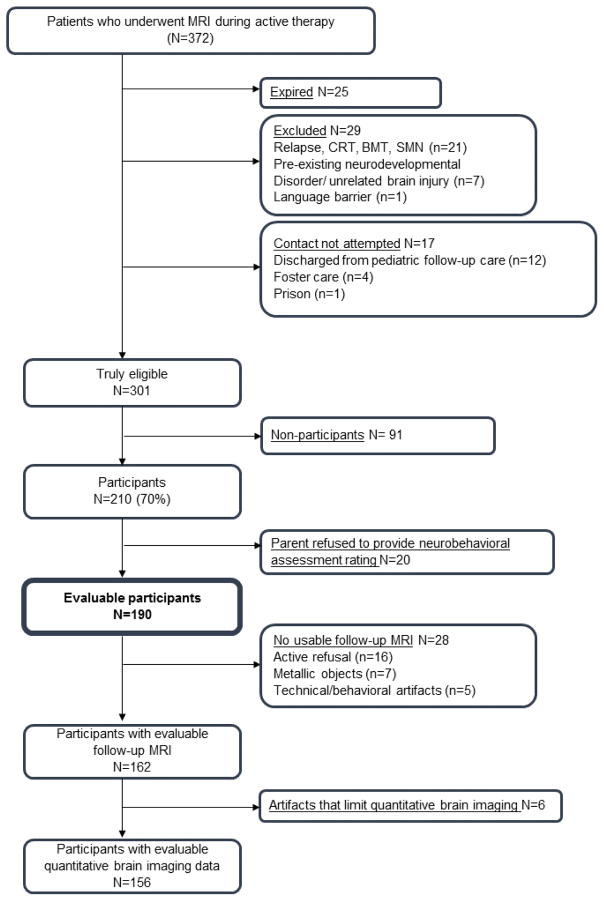
From June 2000 to October 2010, 408 children with ALL were treated at St. Jude Children’s Research Hospital on an institution-based chemotherapy protocol (Total Therapy Study XV, ClinicalTrials.gov, NCT00137111) that omitted CRT in all patients.3 Eligible patients were diagnosed with non-B-cell ALL by immunephenotyping, as determined by flow cytometry, morphology and cytochemical staining properties.3 During the active treatment phase, 372 patients received prospective brain MRIs to monitor the occurrence of acute leukoencephalopathy.5 To be eligible for this long-term follow-up study, survivors had to be at least 5 years post-diagnosis and 8 years of age. Survivors were excluded if they had relapsed and received CRT or hematopoietic stem cell transplantation, had secondary cancer, were not proficient in English, or had an unrelated neurological disorder associated with cognitive impairment. Among survivors who met the eligibility criteria, 210/310 (70%) participated in outcome studies between February 18, 2010 and October 22, 2014 (Figure 1: Consort diagram and Table 1). The study was approved by the institutional review board and written informed consent/assent was obtained from all participants. This study is registered as an observational study (ClinicalTrials.gov, number NCT01014195).
Figure 1. Consort diagram.
Consort Note: Potentially eligible survivors were treated according to the Total Therapy XV protocol and underwent on-therapy magnetic resonance imaging (MRI). Survivors were excluded from follow-up analyses if they had relapsed and were treated with cranial radiation therapy (CRT) or bone marrow transplantation (BMT), had a second malignant neoplasm (SMN) requiring additional treatment, had a neurodevelopmental disorder (e.g. prematurity with intra-ventricular hemorrhage), had a genetic disorder associated with neurocognitive impairment (e.g. Down Syndrome), had an unrelated brain injury (e.g. traumatic brain injury) or were non-fluent in English. Recruitment was not attempted for survivors who were discharged from pediatric follow-up care (i.e. >18 years old and >10 years from diagnosis), were in Foster care, or were in prison. Some survivors actively refused testing or brain imaging. Survivors were not assessable for brain imaging data if they had metallic objects in their body (e.g. braces). There are technical (program malfunction) or behavioral (participant movement during task performance) artifacts that prevented analysis of the imaging data. The subset of survivors with usable brain imaging data did not differ from the larger cohort on neurobehavioral or neurocognitive outcomes. Survivors with evaluable brain imaging data were older, due to behavioral limitations (eg. anxiety, unable to keep still during the brain scan) seen in survivors of younger age.
Table 1.
Demographic and Treatment Characteristics of Survivors
| Survivors with acute leukoencephalopathy (N=51) | Survivors without acute leukoencephalopathy (N=139) | P | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No (%) | Mean (SD) | Median (IQR) | No (%) | Mean (SD) | Median (IQR) | ||
|
| |||||||
| Demographics | |||||||
|
| |||||||
| Gender | |||||||
| Male | 30 (59) | 66 (47) | 0·17 | ||||
| Female | 21 (41) | 73 (52) | |||||
| Race/ethnicity | |||||||
| White | 39 (76) | 102 (73) | 0·84 | ||||
| Asian | 1 (2) | 2 (2) | |||||
| Black | 5 (10) | 19 (14) | |||||
| Hispanics | 5 (10) | 10 (7) | |||||
| Others | 1 (2) | 6 (4) | |||||
| Current age (years) | 14·8 (4·5) | 14·4 (11·3 – 18·4) | 13·3 (4·3) | 12·3 (10·0 – 15·4) | 0·024 | ||
| Survivor education | 8·1 (3·9) | 8·0 (5·0 – 12·0) | 6·7 (3·7) | 6·0 (4·0 – 9·0) | 0·023 | ||
| Highest maternal education | 14·1 (2·4) | 14·0 (12·0 – 16·0) | 13·7 (2·6) | 13·5 (12·0 – 16·0) | 0·39 | ||
| Highest paternal education | 14·3 (2·8) | 12·5 (12·0 – 17·0) | 13·6 (3·1) | 13·0 (12·0 – 16·0) | 0·38 | ||
|
| |||||||
| Treatment characteristics | |||||||
|
| |||||||
| Age at diagnosis (years) | 7·1 (4·7) | 5·3 (3·3 – 10·8) | 5·7 (3·8) | 4·5 (2·9 – 7·2) | 0·19 | ||
| Time since diagnosis (years) | 7·8 (1·9) | 7·2 (6·2 – 9·3) | 7·6 (1·6) | 7·5 (6·3 – 8·8) | 0·69 | ||
| Risk | |||||||
| Low | 27 (53) | 88 (63) | 0·20 | ||||
| High/standard | 24 (47) | 51 (37) | |||||
| Oral dexamethasone (g/m2) | 1·0 (0·3) | 1·1 (0·9 – 1·2) | 1·1 (0·3) | 1·1 (1·0 – 1·2) | 0·28 | ||
| IV asparaginase (1000 units/m2) | |||||||
| Erwinia-asparaginase | 547·0 (328·7) | 634·8 (231·5 – 858·2) | 294·7 (201·4) | 244·8 (178·9 – 291·0) | 0·056 | ||
| L-asparaginase | 267·3 (176·9) | 243·6 (111·8 – 464·0) | 271·4 (178·1) | 238·5 (115·6 – 464·5) | 0·85 | ||
| Pegylated asparaginase | 9·1 (8·9) | 7·5 (2·5 – 10·3) | 11·9 (9·8) | 10·11 (7·5 – 12·5) | 0·14 | ||
| IV cytarabine (g/m2) | |||||||
| Standard-dose | 2·6 (2·5) | 0·7 (0·6 – 4·3) | 1·9 (1·9) | 0·6 (0·6 – 4·3) | 0·43 | ||
| High-dose | 7·8 (0·8) | 8·0 (8·0 – 8·0) | 8·9 (3·9) | 8·0 (8·0 – 8·0) | 0·18 | ||
| IV cyclophosphamide (g/m2) | 2·8 (1·9) | 1·0 (1·0 – 4·7) | 2·3 (1·8) | 1·0 (1·0 – 4·6) | 0·49 | ||
| IV daunorubicin (mg/m2) | 47·9 (6·8) | 50·0 (49·0 – 50·0) | 50·4 (15·2) | 50·0 (49·3 – 50·9) | 0·051 | ||
| IV doxorubicin (mg/m2) | 115·1 (58·2) | 78·5 (61·0 – 180·7) | 102·7 (54·8) | 62·3 (60·8 – 173·6) | 0·16 | ||
| IV leucovorin (mg/m2) | 345·5 (160·8) | 310·0 (220·0 – 390·0) | 331·9 (191·4) | 282·5 (200·0 – 395·0) | 0·15 | ||
| Methotrexate + | |||||||
| IV standard-dose (g/m2) | 3·5 (0·9) | 3·6 (3·0 – 4·1) | 4·1 (2·5) | 3·8 (3·3 – 4·6) | 0·069 | ||
| IV high-dose (g/m2) | 15·3 (4·5) | 14·8 (11·1 – 18·5) | 15·6 (7·3) | 14·1 (11·4 – 19·1) | 1·00 | ||
| IT (ml)^ | 171·5 (58·0) | 166·0 (144·0 – 204·0) | 165·1 (52·4) | 144·0 (132·0 – 180·0) | 0·11 | ||
| IV vincristine (mg/m2) | 57·1 (13·9) | 59·3 (45·4 – 67·1) | 61·2 (10·7) | 64·5 (57·9 – 68·0) | 0·028 | ||
| IT MHA chemotherapy^ | |||||||
| No. of IT injections | 15·1 (4·1) | 15·0 (12·0 – 17·0) | 14·0 (3·9) | 12·0 (11·0 – 16·0) | 0·065 | ||
Abbreviations: HD, high-dose; IQR, inter-quartile range; IT MHA, intrathecal injection of MTX plus hydrocortisone plus cytarabine; IV, intravenous; SD, standard deviation
Standard and HD IV methotrexate was calculated separately. HD IV methotrexate was defined as more than 1g/m2 of IV methotrexate.
IT methotrexate is administered, where 1 mL contains methotrexate 1 mg, hydrocortisone 2 mg, and cytarabine 3 mg
Treatment
Children in the low-risk arm received 13 to 18 intrathecal (IT) treatments with MTX, hydrocortisone, and cytarabine, and high dose intravenous MTX at 2·5 gm/m2 per dose with leucovorin rescue for four doses and oral dexamethasone pulses at 8 mg/m2 per day for 5 days, in addition to other chemotherapeutic agents.2, 3 Children in the standard-/high-risk arm received 16 to 25 intrathecal injections, and IV high-dose MTX at 5·0 gm/m2 per dose with leucovorin rescue for four doses, and oral dexamethasone pulses at 12 mg/m2 per day for 5 days. Prophylactic CRT was not administered to any patient, regardless of presenting features, including the presence of central nervous system (CNS) leukemia at diagnosis.
Neurobehavioral outcomes
At follow-up, parents of 190 survivors rated their children’s neurobehavioral functioning using the Behavior Rating Inventory of Executive Function (BRIEF).11 The BRIEF is a questionnaire used to evaluate everyday executive functions within the domains of behavioral regulation and metacognition. The Behavioral Regulation Index (BRI) consists of Inhibition- the ability to resist impulses; Shifting- the ability to transition and shift attention; and Emotional control- the regulation of emotions. The Metacognition Index consists of Initiation- the ability to get started on activities; Working memory- the capacity to manipulate information in one’s mind; Planning and Organization- the ability to plan and organize information to achieve a goal; Organization of Materials- the ability to organize one’s environment; and Monitoring - the ability to attend to one’s success or failure and modify strategy appropriately.
Neurocognitive outcomes
Survivors also completed neurocognitive testing with certified examiners under the supervision of a board-certified clinical neuropsychologist. Testing procedures followed standard clinical guidelines, with fixed test order and schedules controlled to reduce effects of interference and fatigue. Executive function was measured by the Delis-Kaplan Executive Function System12 (DKEFS), which assessed cognitive flexibility, cognitive fluency, working memory, organization and problem solving abilities. Survivors’ intelligence13 (IQ), processing speed14, 15, attention16, memory17 and fine motor dexterity18 were also evaluated. Details of the neurocognitive tests are provided in Appendix, Page 1.
Brain Imaging
Survivors completed brain MRIs during active therapy at four time points: post-induction (days 33–46), post-consolidation (week 1 of reinduction), continuation (week 48) and upon the completion of therapy (week 120).5 MRIs were also obtained in a subset of 162/190 (85%) survivors during long-term follow-up, collected during the same visit as the neurobehavioral and neurocognitive assessments. Abnormal MRIs were identified by a board-certified neuroradiologist and graded for the extent of leukoencephalopathy according to the radiographic criteria of Common Terminology Criteria for Adverse Events (version 4·03). Axial T2-weighted and axial T2 FLAIR images were used for grading. The neuroradiologist was blinded to both the risk arm on which the survivors were treated and the neurobehavioral and neurocognitive outcomes. To estimate rater reliability, a second board-certified neuroradiologist independently graded images from 30 survivors. A weighted kappa statistic of 0.75 (95% confidence interval 0.64 – 0.85) was obtained, indicating substantial agreement.
The MRI at long-term follow-up also included diffusion tensor imaging (DTI) to assess white matter integrity, with usable data obtained in a subset of 156/190 (82%) survivors. DTI is a MRI technique that is used to evaluate white matter integrity based on diffusion of water in the brain, and provides several objective indices. Fractional anisotropy (FA) is a measure of the unidirectionality of water diffusion on a scale of 0 to 1, with 0 representing no limitation on direction (isotropic diffusion) and 1 indicating diffusion confined to one direction. Axial diffusivity (AD) is a coefficient of the rate of diffusion along the primary axis, while radial diffusivity (RD) measures the diffusion perpendicular to the primary axis.19 Injury to a white matter tract causes alterations in anisotropy that leads to reduced FA and increased AD and RD compared to healthy white matter.20, 21
Measures of FA, AD and RD were obtained from voxels in the frontal and parietal lobes, and within the frontostriatal tract.6 These regions and tract were selected a priori as they are associated with executive function, and a previous study by our group has shown brain activation in the frontal and parietal regions to be positively correlated with treatment measures in long-term survivors of ALL. A 1·5 T high-resolution 3D T1 scan (approximately 1mm3) was performed. DTI was acquired using a double spin echo EPI pulse sequence (TR/TE=10000/100ms, 3X1·8X1·8mm) with 4 acquisitions and 12 gradient directions. Voxel-wise tensor calculations were performed with SPM8 (fil.ion.ucl.ac.uk/spm/).
Statistical analysis
Chi-square (for categorical variables) and Mann-Whitney U (for continuous variables) tests were conducted to examine differences in demographic and treatment characteristics between survivors with versus without acute leukoencephalopathy. The primary outcomes are neurobehavioral function and neurocognitive performance, as measured by the parent-rated BRIEF and direct neurocognitive tests, respectively. For the BRIEF, raw scores for each individual domain, as well as global indices, were transformed into age- and gender- adjusted T-scores based on nationally representative normative data provided by the BRIEF manual. Effort was taken by examiners to ensure survivors’ parents completed every item on the BRIEF. Missing responses within the BRIEF were handled according to the test manual: missing responses for one or two items that contribute to a scale raw score were assigned a score of 1 when calculating the scale raw score. Neurocognitive scores were transformed into the respective standard scores in accordance to directions provided by the test manuals. Comparison of neurobehavioral and neurocognitive measures between survivors and the normative sample was conducted using one-sample t-test, adjusted for false discovery rate (FDR). Generalized linear modeling (GLM) was employed to examine the association between leukoencephalopathy and survivors’ neurobehavioral and neurocognitive standard scores. Statistical models were adjusted for age of diagnosis and parent’s highest education, as these variables were known to be associated with functional outcomes.1, 22, 23 Parent’s education was not available for 4/190 (2%) survivors. Imputation of missing data was not conducted and these survivors were excluded pair-wise from the GLM. Comparison of DTI measures (FA, AD and RD) between survivors with versus without leukoencephalopathy was conducted using GLM, adjusting for age at evaluation. Associations between DTI measures and neurobehavioral outcomes were also assessed using GLM, adjusting for age at evaluation; unadjusted BRIEF raw scores were employed to avoid problems of multicollinearity as T-scores are age-adjusted. All models that involve DTI measures as the outcome have been corrected for FDR. The potential effect of treatment risk group (low risk versus standard-high risk) was assessed through sensitivity analysis by adding risk to GLMs to determine whether significant associations were obtained. Statistical significance was defined as a P value of less than 0·050 and all statistical tests were two-sided. All analyses were conducted in SAS (SAS 9.3, SAS Institute, Cary NC).
Role of the funding source
The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Parent-reported neurobehavioral ratings were below population norms for Initiation, Organization of Materials, Planning and Organizing, Working Memory and the overall Metacognition Index (all p’s<0·05, adjusted for FDR; Appendix, Page 2). As compared to the general population with an expected 10% rate of impairment, survivors were 1.6 to 3.2 times more likely to demonstrate problems across all neurobehavioral domains on the BRIEF (Appendix, Page 2). Neither age nor gender were related to BRIEF T-scores (Appendix, Page 3). Survivors performed worse than the general population on direct measures of memory span (Digit-forward test), processing speed (Digit-symbol and DKEFS number sequencing tests) and executive function (DKEFS number-letter switch and letter fluency tests) (all p’s<0·050, adjusted for FDR; Appendix, Page 4). Digit-symbol and DKEFS number-letter switch were higher in females compared to males (females mean[SD] = 9·6[2·8], males = 7·8[2·6], p=0·00030; females = 8·9[3·6], males = 7·7[3·4], p=0·043; respectively). Age was positively correlated with Digit-symbol (rho=0·21, p=0·017), CPT Omissions (rho=0·33, p=0·0013) and Grooved pegboard (rho=0·23, p=0·010).
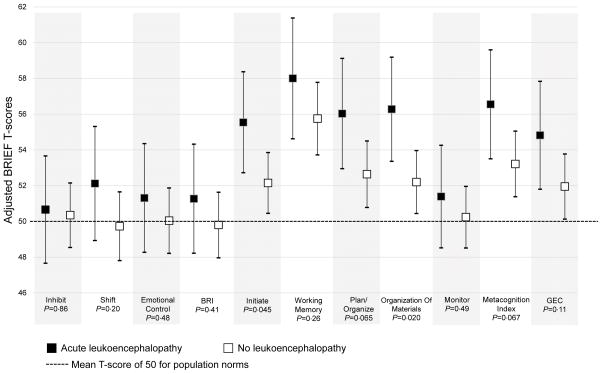
A total of 51/190 (27%) of the survivors had a history of leukoencephalopathy during active therapy, of which brain MRIs were performed on 46/51 (90%) of these survivors during long-term follow-up. Results showed that 36/46 (78%) continued to have leukoencephalopathy at follow-up. Gender, treatment risk group, and leucovorin rescue were not associated with acute leukoencephalopathy. Survivors with a history of acute leukoencephalopathy demonstrated more neurobehavioral problems than those without on scales of Initiation (adjusted T-score[95% CI] 55·5[52·7–58·3] v. 52·1[50·4–53·8]; p=0·045) and Organization of Materials (56·2[53·3–59·1] v. 52·2[50·4–53·9]; p=0·020; Figure 2). There was no difference in Working Memory between groups, even though its frequency of impairment was the highest amongst all scales when compared with population norms. Neurocognitive performance did not differ between those with and those without a history of acute leukoencephalopathy (Appendix, Page 4).
Figure 2. Acute Leukoencephalopathy and Neurobehavioral Outcomes.
Figure 2 shows the association between acute leukoencephalopathy and neurobehavioral outcomes. Group P compares mean standardized T-scores (μ=50, σ=10), between survivors with versus without acute leukoencephalopathy for the specific BRIEF domains and overall scales, using general linear modeling, adjusted for age at diagnosis and parent’s education. A higher T-score is indicative of more neurobehavioral problems. Survivors who developed acute leukoencephalopathy demonstrated more parent-rated neurobehavioral problems than those who did not, on Initiation and Organization of Materials, and marginally significant for Planning and the overall Metacognition Index.
Abbreviation: BRI, Behavioral Regulation Index; BRIEF, Behavior Rating Inventory of Executive Function; GEC, Global Executive Composite
Survivors with a history of acute leukoencephalopathy had abnormal measures of white matter integrity in the frontostriatal tract at long-term follow-up (higher AD [p=0·020] and RD [p=0·0077], adjusted for FDR; Figure 3). Survivors with acute leukoencephalopathy also had reduced white matter integrity at follow-up in the frontal (lower FA [p=0·0018], higher AD [p=0·013] and RD [p=0·00090]) and parietal (lower FA [p=0·028], higher AD [p=0·015] and RD [p=0·0030]) lobes. Survivors with persistent leukoencephalopathy at follow-up also had reduced white matter integrity compared to survivors without leukoencephalopathy (Appendix, Page 5).
Figure 3. Acute Leukoencephalopathy and Diffusion Tensor Imaging of White Matter Integrity at Follow-up.
Figure 3 shows the association between acute leukoencephalopathy and white matter integrity at follow-up within the frontostriatal tract, frontal and parietal lobes. The P-values compare the DTI measures (FA, AD and RD) between survivors with and without acute leukoencephalopathy using general linear modeling, adjusted for current age. All models have been adjusted for false discovery rate. Survivors who developed acute leukoencephalopathy had reduced white matter integrity, demonstrated by lower FA and higher AD and RD indices.
Abbreviation: AD, axial diffusivity; FA, fractional anisotropy; RD, radial diffusivity
DTI measures within the frontostriatal tract were associated with neurobehavioral outcomes, especially domains within the Metacognition index (Table 2). Higher FA was associated with fewer problems on the neurobehavioral domains (all p’s<0·050), while higher RD was associated with more problems (all p’s<0·050). The association of neurobehavioral problems with white matter integrity was weaker within frontal and parietal lobes, compared to the frontostriatal tract.
Table 2.
Diffusion Tensor Imaging of White Matter Integrity and Neurobehavioral Outcomes
| Frontostriatal tract | Frontal lobes | Parietal lobes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | AD | RD | FA | AD | RD | FA | AD | RD | ||||||||||
| BRIEF domains | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* | Est. (SE) | P* |
| Inhibit | −16·1 (10·9) | 0·15 | 6·2 (7·7) | 0·44 | 13·5 (7·1) | 0·059 | 3·4 (13·6) | 0·80 | 17·4 (6·7) | 0·027 | 13·1 (7·7) | 0·11 | 12·1 (10·3) | 0·98 | 15·5 (7·6) | 0·076 | 1·14 (8·1) | 0·89 |
| Shift | −18·0 (8·5) | 0·054 | 4·6 (6·0) | 0·44 | 13·5 (5·5) | 0·018 | −4·8 (10·5) | 0·80 | 9·6 (5·2) | 0·086 | 8·0 (6·0) | 0·18 | 3·1 (8·1) | 0·91 | 13·6 (5·9) | 0·050 | 6·6 (6·3) | 0·43 |
| Emotional control | −12·9 (11·8) | 0·27 | 15·4 (8·2) | 0·43 | 15·6 (7·6) | 0·046 | −4·2 (14·6) | 0·80 | 20·5 (7·1) | 0·018 | 16·1 (8·2) | 0·098 | −17·6 (11·1) | 0·98 | 14·6 (8·2) | 0·086 | 9·8 (8·7) | 0·43 |
| BRI | −48·9 (28·4) | 0·10 | 35·3 (19·9) | 0·43 | 48·0 (18·4) | 0·016 | −14·5 (35·3) | 0·80 | 64·0 (17·0) | 0·0022 | 51·9 (19·6) | 0·093 | 0·51 (26·9) | 0·98 | 47·7 (19·7) | 0·050 | 29·7 (20·9) | 0·43 |
| Initiate | −18·3 (8·9) | 0·058 | 9·2 (6·3) | 0·43 | 15·0 (5·8) | 0·016 | −9·1 (11·1) | 0·75 | 7·7 (5·6) | 0·16 | 8·9 (6·3) | 0·17 | 1·4 (8·5) | 0·95 | 14·1 (6·2) | 0·050 | 7·7 (6·6) | 0·43 |
| Working memory | −40·3 (13·2) | 0·023 | 10·7 (9·5) | 0·44 | 28·0 (8·6) | 0·0070 | −16·4 (16·6) | 0·75 | 18·1 (8·3) | 0·054 | 19·1 (9·4) | 0·098 | 6·0 (12·7) | 0·97 | 18·1 (9·4) | 0·076 | 6·4 (9·9) | 0·57 |
| Plan/Organize | −43·4 (15·6) | 0·023 | 9·3 (11·2) | 0·44 | 30·3 (10·2) | 0·0099 | −13·6 (19·8) | 0·77 | 18·3 (9·8) | 0·086 | 18·7 (11·2) | 0·11 | 3·4 (15·1) | 0·95 | 26·3 (11·1) | 0·050 | 11·9 (11·8) | 0·43 |
| Organization of Materials | −25·4 (9·8) | 0·023 | 5·4 (7·0) | 0·44 | 16·3 (6·4) | 0·016 | 15·8 (12·2) | 0·75 | 14·6 (6·0) | 0·038 | 15·6 (6·9) | 0·093 | −6·8 (9·4) | 0·98 | 12·8 (6·9) | 0·080 | 12·8 (7·3) | 0·43 |
| Monitor | −20·6 (9·0) | 0·044 | 5·4 (6·4) | 0·44 | 15·6 (5·9) | 0·016 | −9·9 (11·3) | 0·75 | 8·1 (5·6) | 0·16 | 10·9 (6·4) | 0·11 | 2·4 (8·6) | 0·95 | 8·9 (6·4) | 0·16 | 4·8 (6·8) | 0·57 |
| Metacognition Index | −145·5 (49·7) | 0·023 | 38·2 (35·6) | 0·44 | 102·9 (32·4) | 0·0070 | −60·6 (62·6) | 0·75 | 63·8 (31·1) | 0·068 | 69·3 (35·3) | 0·098 | 9·0 (48·0) | 0·95 | 79·1 (35·2) | 0·050 | 41·9 (37·4) | 0·43 |
| GEC | −194·4 (72·7) | 0·023 | 73·5 (51·8) | 0·43 | 150·9 (47·2) | 0·0070 | −75·2 (91·3) | 0·75 | 127·7 (44·8) | 0·018 | 121·1 (51·1) | 0·093 | 8·2 (69·9) | 0·96 | 126·8 (51·1) | 0·050 | 71·6 (54·4) | 0·43 |
P values for the association between neurobehavioral outcomes (raw scores of the BRIEF domains, as well as the overall BRI and Metacognition indices) and white matter integrity at follow-up (FA, AD and RD measures) within the frontostriatal tract and the frontal and parietal lobes. General linear modeling was used to examine the associations, adjusting for age at evaluation. All models have been adjusted for false discovery rate. A higher raw score on the BRIEF is indicative of more neurobehavioral problems.
Within the frontostriatal tract, higher FA was associated with fewer problems on all of the metacognitive domains while higher RD was associated with more problems. However, the association of neurobehavioral problems with white matter integrity was weaker within the frontal and parietal lobes, as compared to the frontostriatal tract.
Abbreviations: AD, axial diffusivity; BRI, Behavioral Regulation Index; BRIEF, Behavior Rating Inventory of Executive Function; Est, estimate; FA, fractional anisotropy; GEC, Global Executive Composite; RD, radial diffusivity; SE, standard error
Sensitivity analyses revealed that treatment risk group was not associated with the occurrence of leukoencephalopathy (Table 1), neurobehavioral (with the exception of the Shift domain) or neurocognitive outcomes, or DTI measures (Appendix, Page 7).
Discussion
At more than 5 years post-diagnosis, survivors of childhood ALL displayed more neurocognitive and parent-rated neurobehavioral problems than population norms. Survivors who developed acute leukoencephalopathy during therapy demonstrated more neurobehavioral problems than those who did not, specifically on the metacognitive scales of Initiation and Organization of Materials. Moreover, these survivors had reduced white matter integrity at follow-up, as reflected by lower FA, and higher AD and RD in the frontal and parietal regions, and within the frontostriatal tract. DTI measures within the frontostriatal tract were correlated with neurobehavioral ratings in the metacognition scales. No associations were observed between acute leukoencephalopathy and neurocognitive measures.
As a group, long-term survivors of ALL treated without CRT demonstrated more parent-rated neurobehavioral problems compared to population norms. The survivors who develop acute leukoencephalopathy appear to be at increased risk for long-term functional and neuroanatomical consequences. Our results suggest that these mild changes during active therapy may disrupt healthy brain development, resulting in reduced white matter integrity more than two years following completion of therapy. These microstructural changes in the brain can be subtle but may be sufficient to manifest as long-term neurobehavioral problems in survivors. While our results suggest that all survivors may require some form of early intervention to support healthy brain development, patients who experience acute leukoencephalopathy may be at greatest need. Our finding suggests that survivors who developed on-therapy leukoencephalopathy may benefit from closer monitoring of their neurodevelopment, such as their neurocognitive, behavioral, emotional and psychosocial functioning. There is now an increasing amount of research in the literature that evaluates the use of pharmacological treatment options (eg. methylphenidate for attention problems), as well as cognitive rehabilitation and educational intervention in childhood cancer survivors.24–26
Acute leukoencephalopathy was not associated with poorer neurocognitive outcomes, though as a group survivors performed worse than the general population on multiple neurocognitive measures. Our previous report demonstrated associations between higher exposures to MTX and post-HDMTX homocysteine and executive dysfunction in long-term survivors.6 Associations of neurocognitive measures with cortical thickness and cortical activation (as reflected through functional MRI) were observed. A threshold effect may exist, such that differences in neurobehavioral problems do not become apparent until white matter damage to the brain is extensive enough to result in clinically detected leukoencephalopathy, while neurocognitive problems begin with less extensive damage that is only detectable through DTI. Alternatively, the BRIEF domains that are associated with acute leukoencephalopathy may not be adequately measured by the neurocognitive tests selected for the current study.
Neurobehavioral domains were more closely associated with white matter integrity in the frontostriatal tract, compared to global frontal and parietal lobes. The frontostriatal tract is a region integral to executive function. This localization suggests impact on coordinated activation of distributed neuronal populations in basal ganglia and dorsolateral frontal brain regions. The association between disruption in this neural pathway and neurobehavioral abnormalities is supported by existing findings from other neurodegenerative and behavioral disorders in young adults.27, 28 Studies have shown that adolescents with attention-deficit/hyperactivity disorders have lower FA compared to healthy controls within the frontostriatal circuit.28 Longitudinal follow-up studies in ALL survivors are warranted to determine whether white matter continues to be disrupted and whether this disruption further impacts survivors’ functional outcomes. Future work also includes identifying genes that regulate white matter microstructural changes, neuronal plasticity and axonal growth to unveil the biological pathways behind these neurotoxic phenotypes with large inter-patient variations.
Acute leukoencephalopathy was observed in a quarter of the survivors but clinically important differences in demographic factors were not identified. Gender is not associated with leukoencephalopathy in our results. Although female gender is often identified as a risk factor for CNS outcomes such as neurocognitive deficits29, these findings are primary identified in patients that were treated with CRT, and are not consistently reported in survivors treated without CRT. We did not expect a gender difference in the rate of leukoencephalopathy in these survivors who were treated with chemotherapy only. One study also reported higher rates of leukoencephalopathy in ALL patients who were treated on a protocol that involved more frequent intrathecal and/or intravenous MTX administration, as compared to another protocol that had less intensive administration and additional leucovorin rescue.8 However, we did not observe association between treatment risk group and the occurrence of on-therapy leukoencephalopthy. This discrepancy may be attributed to a threshold effect, such that beyond a certain level of dose intensity, the effect of the treatment on neurologic outcomes is as detrimental regardless of the treatment risk group. Notably, all survivors in this current study were treated with 12 to 17 doses of intrathecal methotrexate, a cumulative average of 15g/m2 of intravenous high-dose MTX and 1g/m2 of oral dexamethasone. There is consistent robust data that supports ongoing concerns that even with the omission of CRT, intensive MTX exposure is a major contributor to CNS outcomes. Other than treatment risk, the interaction between treatment agents and physiological responses to the treatment (eg. biomarkers of oxidative stress and inflammation) may also determine the severity of white matter changes.
Several limitations should be considered in the interpretation of these results. Parent-reported neurobehavioral outcomes are utilized in this study, and parents may have been influenced by recall of child symptoms during active therapy. However, the majority (>80%) of the children who developed acute leukoencephalopathy were asymptomatic and rated as Grade 1 based on MRI findings5, hence it is reasonable to assume that parents were not influenced by this asymptomatic event. Differences in neurobehavioral outcomes between survivors with and without acute leukoencephalopathy were only found in the Initiation and Organization of Materials scales, as well as trending differences observed on the planning scale and overall Metacognition Index. This is by itself relatively weak evidence of impact; considering the current sample size and rate of leukoencephalopathy, our study may be slightly underpowered, yet we observe obvious trends of worse neurobehavioral outcomes in the leukoencephalopathy group. Furthermore, there are substantial correlations between the metacognitive scales and FA/RD indices within the frontostriatal tract. These associations across multiple scales and imaging parameters make this finding more convincing. Another limitation is that neurobehavioral measures were not collected during therapy. These problems might have already existed before treatment and the occurrence of leukoencephalopathy. However, since the majority of the survivors were below 10 years of age at the time of diagnosis, their higher order cognitive skills were likely still under development, hence ascertaining pre-existing neurobehavioral problems is difficult. Lastly, given the gap of 2 to 3 years between the last brain MRI during active therapy and assessment at follow-up, multiple factors during this time period could have affected the functional and brain imaging outcomes, such as lifestyle, coping strategies and health behaviors.
Conclusion
Long-term survivors of ALL treated with a chemotherapy-only protocol featuring intravenous and intrathecal MTX and dexamethasone demonstrate more parent-rated neurobehavioral problems and neurocognitive impairment compared to population norms. Acute leukoencephalopathy during active treatment is associated with further increased risk for neurobehavioral problems with initiation and organization of materials. Neurobehavioral outcomes are also associated with persistent neuroanatomical changes in white matter integrity. These findings warrant attention from the clinical, radiological, psychological and neuroscience community. Even with the omission of CRT, survivors of ALL who received solely chemotherapy may benefit from preventive cognitive rehabilitation. Children who develop acute neurotoxicity during the active treatment phase may have an even greater need for early behavioral intervention.
Supplementary Material
Acknowledgments
Funding:
This study is supported by the National Institute of Mental Health (Grant No. MH085849 to K.R.K.), the National Cancer Institute (Grant No. CA195547 to M.M.H. and L.L.R.), and by the American Lebanese Syrian Associated Charities.
The authors would like to acknowledge Dr. Cara Kimberg, Ms. Cynthia Jones, Ms. Deborah Stewart and Ms. Adrienne Studaway for administering the neurocognitive tests; Ms. Joycelynn Butler for extracting and cleaning the data.
Footnotes
Declaration of interests
We declare no competing interests.
Contributors
Dr. Krull had full access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Cheung and Sabin served as co–first authors, each with equal contribution to this manuscript. The following authors have made substantial contributions to the intellectual content of the paper, including conception/design (KRK, YTC, NDS, WER, DS, CHP, LLR, MMH), acquisition, analysis, or interpretation of data (KRK, YTC, WL, WER, JOG, NDS, SNH, TMB, DS, DB, CHP, LLR, MMH), drafting of the manuscript (KRK, YTC) and critical revision of the manuscript for important intellectual content (KRK, YTC, WL, WER, JOG, NDS, SNH, TMB, DS, DB, CHP, LLR, MMH). Contributions also include statistical analysis (YTC, WL, DS), obtaining funding (KRK, LLR, MMH). All authors have approved the final version of this manuscript. KRK had final responsibility for the decision to submit for publication.
References
- 1.Krull KR, Brinkman TM, Li C, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(35):4407–15. doi: 10.1200/JCO.2012.48.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pui CH. Toward optimal central nervous system-directed treatment in childhood acute lymphoblastic leukemia. J Clin Oncol. 2003;21(2):179–81. doi: 10.1200/JCO.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–41. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung YT, Krull KR. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–20. doi: 10.1016/j.neubiorev.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):949–59. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krull KR, Cheung YT, Liu W, et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2016 doi: 10.1200/JCO.2015.65.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conklin HM, Krull KR, Reddick WE, Pei D, Cheng C, Pui CH. Cognitive outcomes following contemporary treatment without cranial irradiation for childhood acute lymphoblastic leukemia. J Natl Cancer Inst. 2012;104(18):1386–95. doi: 10.1093/jnci/djs344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duffner PK, Armstrong FD, Chen L, et al. Neurocognitive and neuroradiologic central nervous system late effects in children treated on pediatric oncology group (POG) P9605 (Standard Risk) and P9201 (Lesser Risk) acute lymphoblastic leukemia protocols (ACCL0131): A methotrexate consequence? A report from the children’s oncology group. J Pediatr Hematol Oncol. 2014;36(1):8–15. doi: 10.1097/MPH.0000000000000000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krull KR, Khan RB, Ness KK, et al. Symptoms of attention-deficit/hyperactivity disorder in long-term survivors of childhood leukemia. Pediatr Blood Cancer. 2011;57(7):1191–6. doi: 10.1002/pbc.22994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26(5):1263–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Gioia G, Isquith P, Guy S, Kenworthy L. BRIEF – Behavior Rating Inventory of Executive Function, Professional Manual. Odessa, FL: Psychological Assessment Resources; 2000. [Google Scholar]
- 12.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: Psychological Corporation; 2001. [Google Scholar]
- 13.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 14.Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition. San Antonio, TX: Harcourt Assessment, Inc; 2003. [Google Scholar]
- 15.Wechsler D. Wechsler Adult Intelligence Scale - Fourth Edition. San Antonio: Pearson; 2008. [Google Scholar]
- 16.Conners CK. Conners’ Continuous Performance Performance Test II. North Tonawanda, NY: Multi-Health Systems; 2001. [Google Scholar]
- 17.Meyers JE, Meyers KR. Rey Complex Figure and Recognition Trial. Odessa, FL: Psychological Assessment Resources; 1996. [Google Scholar]
- 18.Strauss E, EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3. New York: Oxford University Press; 2006. [Google Scholar]
- 19.Choudhri AF, Chin EM, Blitz AM, Gandhi D. Diffusion Tensor Imaging of Cerebral White Matter. Technique, Anatomy, and Pathologic Patterns. Radiol Clin North Am. 2014;52(2):413–25. doi: 10.1016/j.rcl.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Edelmann MN, Krull KR, Liu W, et al. Diffusion tensor imaging and neurocognition in survivors of childhood acute lymphoblastic leukaemia. Brain. 2014;137(11):2973–83. doi: 10.1093/brain/awu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elalfy M, Ragab I, Azab I, Amin S, Abdel-Maguid M. Neurocognitive outcome and white matter anisotropy in childhood acute lymphoblastic leukemia survivors treated with different protocols. Pediatr Hematol Oncol. 2014;31(2):194–204. doi: 10.3109/08880018.2013.871763. [DOI] [PubMed] [Google Scholar]
- 22.Noble KG, Houston SM, Brito NH, et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18(5):773–8. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;102(12):881–93. doi: 10.1093/jnci/djq156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox LE, Ashford JM, Clark KN, et al. Feasibility and acceptability of a remotely administered computerized intervention to address cognitive late effects among childhood cancer survivors. Neuro Oncol Pract. 2015;2(2):78–87. doi: 10.1093/nop/npu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore Ki IM, Hockenberry MJ, Anhalt C, McCarthy K, Krull KR. Mathematics intervention for prevention of neurocognitive deficits in childhood leukemia. Pediatr Blood Cancer. 2012;59(2):278–84. doi: 10.1002/pbc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conklin HM, Reddick WE, Ashford J, et al. Long-term efficacy of methylphenidate in enhancing attention regulation, social skills, and academic abilities of childhood cancer survivors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(29):4465–72. doi: 10.1200/JCO.2010.28.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li TQ, Mathews VP, Wang Y, Dunn D, Kronenberger W. Adolescents with disruptive behavior disorder investigated using an optimized MR diffusion tensor imaging protocol. Ann N Y Acad Sci. 2005;1064:184–92. doi: 10.1196/annals.1340.034. [DOI] [PubMed] [Google Scholar]
- 28.van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, Oosterlaan J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2012;36(4):1093–106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong GT, Sklar CA, Hudson MM, Robison LL. Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;25(28):4477–89. doi: 10.1200/JCO.2007.11.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.