Abstract
Distal hereditary motor neuropathies represent a group of rare genetic disorders characterized by progressive distal motor weakness without sensory loss. Their genetic heterogeneity is high and thus eligible for diagnostic whole exome sequencing. The authors report successful application of whole exome sequencing in diagnosing a second consanguineous family with distal hereditary motor neuropathy due to a homozygous c.151+1G>T variant in SIGMAR1. This variant was recently proposed as causal for the same condition in a consanguineous Chinese family. Compared to this family, the Afghan ethnic origin of our patient is distinct, yet the features are identical, validating the SIGMAR1 deficiency phenotype: progressive muscle wasting/weakness in lower and upper limbs without sensory loss. Rapid disease progression during adolescent growth is similar and may be due to SIGMAR1’s role in regulating axon elongation and tau phosphorylation. Finally, the authors conclude that SIGMAR1 deficiency should be added to the differential diagnosis of distal hereditary motor neuropathies.
Keywords: SIGMAR1, sigma-1 receptor, distal hereditary motor neuropathy, dHMN, whole exome sequencing
Distal hereditary motor neuropathies represent a group of rare genetic disorders commonly characterized by progressive distal motor weakness without sensory loss.1 Genetic etiology of distal hereditary motor neuropathies is diverse, thus far, at least 19 causal genes and loci have been reported, exhibiting various modes of inheritance: autosomal dominant, autosomal recessive, and X-linked recessive.2 This genetic heterogeneity is expected to enrich over time, as more than 80% of distal hereditary motor neuropathy cases are estimated to be caused by variants in yet unreported genes.1
Increasingly in clinical practice, whole exome sequencing is applied as an effective diagnostic alternative to often unsuccessful single-gene analysis, especially for conditions with a high degree of genetic heterogeneity.3,4
Here, the authors report a successful application of whole exome sequencing in the diagnosis of a consanguineous Afghan family with distal hereditary motor neuropathy due to a homozygous c.151+1G>T variant in SIGMAR1 (MIM# 601978). This variant was recently reported and proposed by Li et al as a novel cause of distal hereditary motor neuropathy in 2 members of a consanguineous Chinese family.2 Their in vitro experiments confirmed the deleterious nature of this variant, which causes an alternative splicing event generating a premature truncation of exon 1 (c.92_151del), leading to a prediction of an internally shortened protein (p.Gly31_Ala50del).2 The authors validate the neurologic phenotype of SIGMAR1 deficiency with this report of the second unrelated family and propose a hypothesis for the disease course with stabilization in early adulthood.
Case Summary
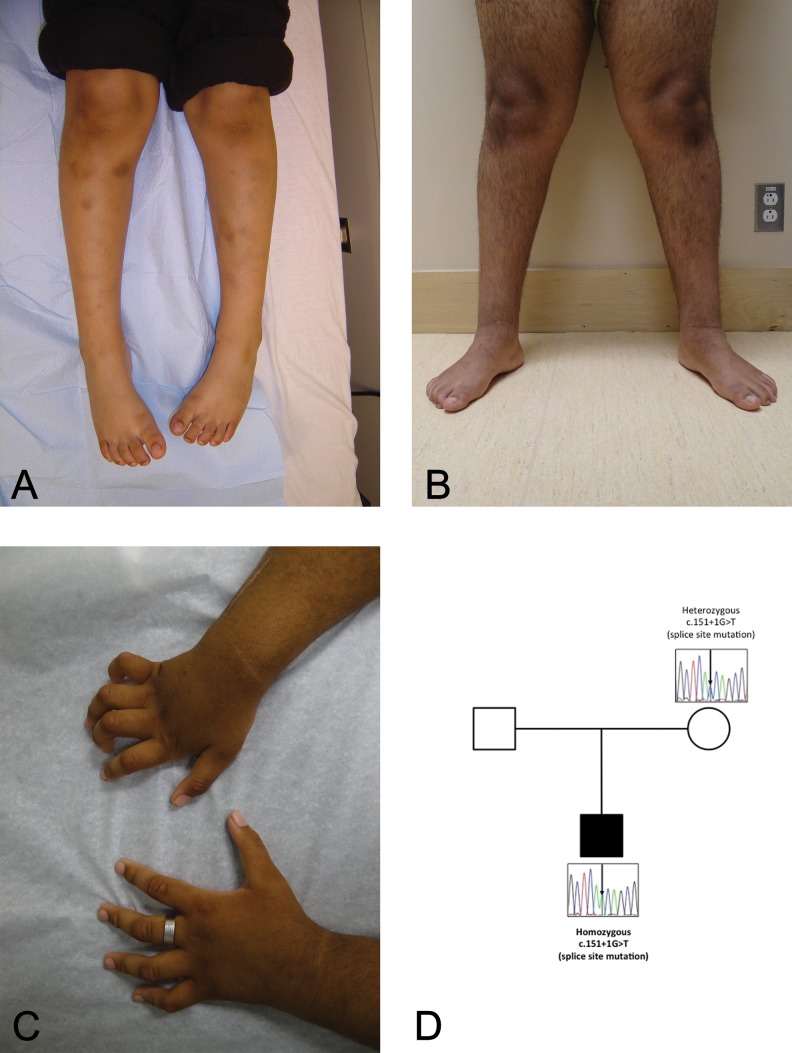
The now 17-year-old right-handed male proband was referred for neurological evaluation at age 13 years, with a 3-year history of slowly progressive distal symmetric upper and lower limb weakness, distal intrinsic hand and foot muscle wasting, and distal joint deformities. He was born to first cousin parents (maternal grandfather and paternal grandmother are siblings) who emigrated from Afghanistan to Canada during pregnancy, which was otherwise unremarkable. The mother is in good health, the father’s history is unavailable to us, and the proband has a healthy half-sister born to a different mother. Delivery was spontaneously at term, with good Apgar scores and normal growth parameters; tone was unremarkable, and orthopedic manifestations were absent. Investigation of excessive bruising at age 4 years led to the diagnosis of type 3 von Willebrand disease (homozygous c.5557C>T/p.Arg1853X variant in VWF gene), requiring intermittent medical treatment. There was no history of intracranial bleeding nor significant trauma to the head, neck, or spine. Aside from mild asthma, requiring occasional use of inhalers, he was in good health. Early neurodevelopmental milestones were normal: he crawled and sat without support at 6 months, walked independently at age 1 year, and spoke in 2-word phrases by age 2 years. His school performance was age appropriate; he was able to write, run, and play sports. However, by age 10 years, he was often noted to trip and fall while running. By age 13 years, he was no longer able to run; his hands and feet were weak, with claw deformities of fingers and toes (Figure 1B and C) and with impeding skills such as writing and using utensils. There was no history of cognitive regression, seizures, impairment of vision/hearing, diplopia, facial weakness/numbness, dysphagia/dysarthria, upper/lower limb paresthesias or sensory loss, significant cervical, thoracic, lumbosacral, or limb pain, or impairment of bowel/bladder sphincter control.
Figure 1.
A, Pictures of proband’s extremities at age 7 years. B and C, Pictures of proband’s extremities at age 13 years. D, Family pedigree and Sanger resequencing results. The mother is a carrier, and the index is homozygous for the variant in SIGMAR1.
Physical examination at age 13 years (Figure 1B and C) revealed normal mental status and normal cranial nerves. Tone was normal in upper limbs and slightly increased in lower limbs. Triceps reflexes were present, but biceps and brachioradialis reflexes were absent in both upper limbs; patellar and ankle reflexes were diminished bilaterally, without ankle clonus. Plantar reflexes were both downgoing. Proximal upper and lower limb bulk and power was normal in all the 4 limbs. However, there was marked symmetric weakness of distal small intrinsic muscles of both hands and feet, with wasting of thenar and hypothenar eminences bilaterally; no fasciculations were noted. At rest, the thumbs were held adducted and all the 4 fingers were clawed. His toes were also clawed bilaterally, with pes planus of both feet. Coordination was normal; Romberg sign was absent. Gait was wide based; he was unable to walk on his heels or toes. Sensory examination (light touch, vibration, proprioception, temperature, and pinprick) was normal, proximally and distally, in all the 4 limbs.
At age 13 and 16 years, motor nerve conduction studies (Table 1) showed diffusely and markedly reduced amplitudes, with mild slowing of conduction velocities and normal distal latencies. Sensory nerve conduction studies were normal. Needle electromyography studies (right extensor digitorum communis, right first dorsal interosseous, right tibialis posterior, and right vastus lateralis) showed markedly large amplitudes of motor unit action potentials, most pronounced in the right tibialis anterior where motor unit action potentials measured up to 10 mV. These findings were interpreted as consistent with anterior horn cell disease.
Table 1.
Comparison of Conduction Study Results of Our Patient to Those Reported by Li et al.2
| Our Patient | Patient Reported by Li et ala | ||
|---|---|---|---|
| Sex | Male | Male | |
| Age at examination, years | 13b | 16b | 30 |
| Motor nerve conduction velocities/compound muscle action potentials, m/s, mV (O–P) | |||
| Right median | 37.5/0.81 | 40.4/0.16 | 43.4/1.2 |
| Right ulnar | 45.9/0.53 | 52.0/0.48 | Not available |
| Right tibial anterior | 101/1.73 | 36.3/0.049 | Not available |
| Right deep peroneal | 35.2/1.02 | 52.9/0.51 | Not available |
| Right peroneus | Not done | Not done | 32.5/0.1 |
| Left peroneus | Not done | Not done | 34.9/0.2 |
| Sensory nerve conduction velocities/sensory nerve action potentials, m/s, µV | |||
| Right median | 60.4/13.2 | 62.2/12.2 | 58/45 |
| Right ulnar | 59.5/8.1 | 57.7/4.7 | Not available |
| Right radial | 61.7/30.3 | Not done | Not available |
| Right sural | 52.9/8.5 | 46.1/7.8 | Not available |
| Left sural | Not done | Not done | 55.5/39 |
| Right peroneus | Not done | Not done | 63.3/41 |
| Left peroneus | Not done | Not done | 61.2/34 |
aConduction studies were done only for 1 patient in the study of Li et al.2
bOur patient was examined twice, at different ages.
Because his inability to move his right thumb or extend his fingers compromised his ability to write, he underwent right-hand tendon transfer surgery at age 14 years. Postoperatively, he noted not only modest improvement in right thumb motion but also intermittent right hand pain and paresthesias.
At age 17.5 years, he underwent repeat neurological evaluation, which was unchanged. He continued to attend a regular school program, but he was falling behind academically most likely due to his physical disabilities. No regression or signs of an evolving cognitive have been observed; formal neurocognitive testing has not been performed due to logistical issues. He complained of ongoing intermittent cramping and pain in his right hand, provoked by prolonged handwriting with postsurgical paresthesias in dorsum and ulnar side of the hand; his left hand had normal sensation and was pain free. He noted intermittent pain in feet and ankles after prolonged walking or standing but denied paresthesias or impairment of sensation in lower limbs. Strength was unchanged compared to the previous examination.
Magnetic resonance imaging of the spine showed mild to moderate lumbar spinal stenosis at age 14 years, with anterior–posterior dimension of the spinal canal measuring 9 mm at L4; the stenosis is deemed unrelated to the SIGMAR1 deficiency. There was no evidence of Chiari malformation, syrinx, or spinal cord pathology; the spinal cord ends with the inferior border at L1.
Detailed biochemical investigations based on a published algorithm5 did not yield a diagnosis. Creatine kinase levels were within normal range. Molecular analysis of SMN and PMP22 revealed no pathogenic variants, deletions, or duplications. Chromosome microarray analysis (CytoScan HD; Affymetrix, Santa Barbara, CA) showed large regions of homozygosity (22 identified regions using a threshold of homozygosity regions >4 MB) but was otherwise unremarkable.
After enrollment of the family within the TIDEX gene discovery project (University of British Columbia Institutional Review Board approval H12-00067), whole exome sequencing was performed for the proband and his unaffected mother using the Agilent SureSelect kit and Illumina HiSeq 2000 (Perkin-Elmer, Santa Clara, CA). The sequencing reads were mapped to the hg19 human reference genome. Rare variants (those with minor allele frequency <0.01) were assessed for predicted impact on protein function and screened under multiple inheritance models. Given the consanguinity, the variants were prioritized for homozygous-recessive inheritance and filtered to focus on regions of homozygosity. Further details on the bioinformatics processing are available in Supplemental Material. Whole exome sequencing revealed 49 recessive candidate variants (4 hemizygous, 33 homozygous, and 12 compound heterozygous). Of those, the homozygous c.151+1 G>T variant in SIGMAR1 (located in a 8.5 MB long region of homozygosity spanning chromosome 9p21.1-p13.2) was considered a functional candidate based on the (1) report by Li et al2 of the same recessive SIGMAR1 variant in a family with highly overlapping phenotypes; (2) rarity of the variant—aside from the recent report,2 the variant has not been observed in dbSNP (release 142), Exome Aggregation Consortium (v.0.3), National Heart, Lung, and Blood Institute Exome Sequencing Project (v.0.0.30), or our in-house database of more than 350 whole exome/whole genome sequencing results; and (3) high deleteriousness (26.7) predicted by Combined Annotation-Dependent Depletion. Sanger resequencing confirmed that the proband was homozygous for the variant, and the mother was heterozygous for the same variant (Figure 1D); the father’s DNA was unavailable.
Discussion
In summary, this report further validates causality of the c.151+1G>T SIGMAR1 variant for a distal hereditary motor axonal neuropathy affecting the upper and lower extremities; the phenotypic presentation is highly similar to the patients first reported by Li et al.2 Interestingly, the first reported patients (in their 30 seconds at the time of the report) and our patient present a similar pattern of disease progression: onset of symptoms at age 9 to 13 years, subsequent progression during growth spurt, and stabilization by early adulthood (age 16-20 years). The authors speculate a mechanistic disease pathophysiology: the ability of motor axons to remain viable was limited while being required to elongate. This hypothesis is supported by a recent report by Tsai et al,6 which describes the role of SIGMAR1 protein (sigma-1 receptor) in regulating axon elongation and tau phosphorylation. The authors propose that the longest anterior horn cell motor axons are most vulnerable, explaining the neurodegenerative symptoms during the growth spurt.
In addition to distal hereditary motor neuropathy, SIGMAR1 has been tied to other neurodegenerative phenotypes, such as juvenile amyotrophic lateral sclerosis.7 A recent report of the SIGMAR1 knockout mice is consistent with the human phenotypes described: the mice displayed motor function defects, decreased muscle strength, progressive denervation of neuromuscular junctions in fast-twitch tibialis anterior muscle, and small progressive motor neuron death in the lumbar spinal cord.8
Another notable finding in our case is the presence of 2 different genetic defects (in VWF and SIGMAR1), accounting for the proband’s hematological and neurological features. The chance of dual genetic diagnoses increases with consanguinity, but such diagnoses have also been reported in offspring of unrelated parents,9 emphasizing the importance of thorough bioinformatics interpretations of whole exome sequencing data in the context of the patients’ phenotype.
The authors conclude that the SIGMAR1 deficiency should be added to the differential diagnosis of distal hereditary motor neuropathies. Further research including identification of other affected individuals with follow-up into adulthood—with repeat electrophysiological studies, as planned for our patient—is needed to confirm the favorable prognosis (stabilization during early adulthood) of the c.151+1G>T variant, including the contribution of other SIGMAR1 variants to distal hereditary motor neuropathy-related phenotypes.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the family for their participation in this study, Mrs X. Han for Sanger sequencing, Mr B. Sayson for consenting and data management, and Mrs M. Higginson for DNA extraction, sample handling, and technical data (University of British Columbia, Vancouver, British Columbia, Canada).
Author Contributions: CDMvK and JKW contributed equally to the work. JJYL performed bioinformatics analysis and interpretation, co-drafted, and edited the manuscript. CDMvK designed the study, identified the candidate gene, co-drafted, and edited the manuscript. BD performed the Sanger resequencing of the candidate variant, designed the pedigree figure, and contributed to the manuscript draft and revisions. CS contributed to the bioinformatics analysis and contributed to the manuscript draft and revisions. MT-G contributed to the bioinformatics analysis and interpretation and edited the manuscript. PE performed the chromosome microarray analysis to identify regions of homozygosity and contributed to the manuscript draft and revisions. CJR performed the Sanger resequencing and contributed to the manuscript draft and revisions. WWW supervised the bioinformatics analysis and contributed to the manuscript draft and revisions. BB conducted neurological phenotyping of the patient, interpreted the nerve conduction data, and contributed to the manuscript draft and revisions. JKW conducted hematological phenotyping of the patient, supervised, and contributed to the manuscript drafting and revisions.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the BC Children’s Hospital Foundation as “1st Collaborative Area of Innovation” (www.tidebc.org), Genome BC (SOF-195 grant), and the Canadian Institutes of Health Research (#301221 grant). Informatics infrastructure was supported by Genome BC and Genome Canada (ABC4DE Project). Clara D. M. van Karnebeek is recipient of the Michael Smith Foundation for Health Research Scholar Award. Jessica J. Y. Lee is supported by the Jan M. Friedman Studentship from BC Children’s Hospital Foundation.
Ethical Approval: The authors declare that the experiments comply with the current laws of Canada, the country in which they were performed. The patient’s family was enrolled within the TIDEX gene discovery project with the approval of the institutional review board of University of British Columbia (H12-00067). Informed consent was obtained from the patient and family for publication of case report and photographs.
Supplemental Material: The supplement material is available at http://cno.sagepub.com/supplemental.
References
- 1. Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. J Neurol Neurosurg Psychiatry. 2012;83(1):6–14. [DOI] [PubMed] [Google Scholar]
- 2. Li X, Hu Z, Liu L, et al. A SIGMAR1 splice-site mutation causes distal hereditary motor neuropathy. Neurology. 2015;84(24):2430–2437. [DOI] [PubMed] [Google Scholar]
- 3. Drew AP, Zhu D, Kidambi A, et al. Improved inherited peripheral neuropathy genetic diagnosis by whole-exome sequencing. Mol Genet Genomic Med. 2015;3(2):143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boycott K, Hartley T, Adam S, et al. ; Canadian College of Medical Geneticists. The clinical application of genome-wide sequencing for monogenic diseases in Canada: Position Statement of the Canadian College of Medical Geneticists. J Med Genet. 2015;52(7):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Karnebeek CDM, Shevell MI, Zschocke J, Moeschler JB, Stockler S. The metabolic evaluation of the child with an intellectual developmental disorder: diagnostic algorithm for identification of treatable causes and new digital resource. Mol Genet Metab. 2014;111(4):428–438. [DOI] [PubMed] [Google Scholar]
- 6. Tsai SY, Pokrass MJ, Klauer NR, Nohara H, Su TP. Sigma-1 receptor regulates tau phosphorylation and axon extension by shaping p35 turnover via myristic acid. Proc Natl Acad Sci U S A. 2015;112(21):6742–6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70(6):913–919. [DOI] [PubMed] [Google Scholar]
- 8. Bernard-Marissal N, Médard JJ, Azzedine H, Chrast R. Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain. 2015;138(pt 4):875–890. [DOI] [PubMed] [Google Scholar]
- 9. Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312(18):1870–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.