Abstract
Cellular and molecular mechanisms of wound healing, tissue repair and fibrogenesis are established in different organs and are essential for the maintenance of function and tissue integrity after cell injury. These mechanisms are also involved in a plethora of fibroproliferative diseases or organ-specific fibrotic disorders, all of which are associated with the excessive deposition of extracellular matrix components. Fibroblasts, which are key cells in tissue repair and fibrogenesis, rely on communicative cellular networks to ensure efficient control of these processes and to prevent abnormal accumulation of extracellular matrix into the tissue. Despite the significant impact on human health, and thus the epidemiologic relevance, there is still no effective treatment for most fibrosis-related diseases. This paper provides an overview of current concepts and mechanisms involved in the participation of cellular communication via connexin-based pores as well as pannexin-based channels in the processes of tissue repair and fibrogenesis in chronic diseases. Understanding these mechanisms may contribute to the development of new therapeutic strategies to clinically manage fibroproliferative diseases and organ-specific fibrotic disorders.
Keywords: tissue repair and wound healing, cellular communication, fibrotic diseases
1. Introduction
Tissue repair is a fundamental biological process that allows the orderly replacement of damaged or dead cells after injury caused by acute or chronic stimuli, including infections, autoimmune reactions and mechanical injuries (Huang & Ogawa, 2012; Wynn, 2007). In addition, this process may be triggered by metabolic, immunological, genetic or iatrogenic factors (Wynn & Ramalingam, 2012). Tissue repair typically consists of two phases, namely (i) the regeneration phase, in which injured cells are replaced by cells of the same type, and (ii) the fibroplasia phase, in which normal tissue is replaced by connective tissue. Fibrosis is usually the outcome of abnormal tissue healing that follows continued aggressive stimulus, which results in the deposition of excessive amounts of extracellular matrix (ECM) components and the formation of permanent scars (Wynn, 2007).
Despite the diverse etiologies, fibroproliferative diseases, including idiopathic pulmonary fibrosis, systemic sclerosis, liver cirrhosis, rheumatoid arthritis, ulcerative colitis, myocardial infarction, macular degeneration, progressive renal disease, myelofibrosis, systemic lupus erythematosus, hypertrophic and keloid cutaneous scars share many cellular and molecular characteristics (Wynn, 2007; Wynn & Ramalingam, 2012). These diseases are associated with high morbidity and mortality rates. It has been estimated that 45% of all deaths recorded in developed countries are attributable to chronic fibroproliferative pathologies (Wenzke et al., 2012; Wynn, 2008). Despite the enormous impact on public health, there is still no specific and effective clinical treatment available for reducing tissue fibrosis (Wynn & Ramalingam, 2012). The most important cells that mediate fibrosis are fibroblasts that, when activated, transform into myofibroblasts, which are the primary sources of collagen and other ECM molecules (Wynn, 2007; Wynn, 2008). Myofibroblasts originate from several sources, including resident fibroblasts, endothelial and epithelial cells after undergoing a process known as the epithelial to mesenchymal transition (EMT). Myofibroblasts may also derive from circulating fibrocytes in bone marrow-related stem cells. The involvement of fibroblasts in fibrogenesis is a coordinated effort that relies on the sequential modulation of diverse cellular events, such as transdifferentiation into myofibroblasts, migration, proliferation, ECM synthesis, tissue remodeling and apoptosis (Wynn & Ramalingam, 2012). Upon fibrogenesis, fibroblasts are activated by various stimuli and require a complex system of signals to be received and transmitted.
In this context, intercellular communication through gap junctions plays an important role in the regulation of tissue homeostasis, wound healing and tissue repair, angiogenesis, control of cellular differentiation and proliferation (Chanson et al., 2005; Evert et al., 2002; Mesnil et al., 2005). Gap junctions, connecting adjacent cells, are composed of juxtaposed hemichannels, each is formed by six proteins named connexins. In 2000, a set of connexin-like proteins was demonstrated in vertebrates and named pannexins (Panchin et al., 2000). Pannexins are related to innexins, which form gap junctions in invertebrates (Baranova et al., 2004). In contrast to their connexin counterparts, pannexins only gather in a hemichannel configuration and, as such, do not form gap junctions (Dahl & Keane, 2012).
At present, one of the greatest challenges in clinical practice relates to the control and regulation of fibrogenic processes with several therapies already proposed for the reversion of tissue fibrosis. Although present in very different organs, fibrogenesis seems to be driven by a limited set of signaling pathways, including those involving transforming growth factor beta (TGF-β), mitogen-activated protein kinase (MAPK) and platelet-derived growth factor (PDGF) pathways as well as cascades that trigger metalloproteinases, EMT, mechanical stress, oxidative stress and inflammation (Wenzke et al., 2012). These intracellular signaling pathways are typically affected by cellular communication networks, including those mediated by connexin-based and pannexin-based channels. The present paper reviews current knowledge about these particular channels in wound healing and tissue repair in different organs and also exemplifies its clinical significance.
2. Connexin-based and pannexin-based channels: structure and functions
2.1. Connexin hemichannels and gap junctions
Most cells present in multicellular organisms have the capacity to communicate by different mechanisms, which is a de facto prerequisite for the control of tissue homeostasis. Thus, through extracellular, intracellular and intercellular signaling cascades, cells regulate and maintain their physiological and metabolic functions (Vinken et al., 2008). Direct intercellular communication is only mediated by gap junctions that are present in nearly all vertebrate cell types, except in red blood cells, mature skeletal muscle fibers, some neurons and sperm cells (Mesnil et al., 2005). Gap junctions are specialized regions of the plasma membrane that form juxtaposed connexons or hemichannels between adjacent cells. Hemichannels are hexameric structures composed of proteins named connexins in vertebrates (Goodenough, Goliger & Paul, 1996) that are members of a multigene family. In human, there are 21 different types of connexins. Analysis of the connexin cDNA revealed regions of high homology as well as areas with little or no homology and allowed classification according to their molecular weight; thus the nomenclature commonly used to designate the different connexin species refers to their predicted molecular weight expressed in kilodaltons preceded by the abbreviation Cx (i.e. Cx26, Cx32, Cx43, etc.). Connexins can interact with each other yielding homomeric connexons (i.e. formed by six equal connexins) or heteromeric connexons (i.e. formed by different connexins) (Fig. 1). In turn, connexons can also interact with each other, generating homotypic channels (i.e. formed by equal connexons) or heterotypic channels (i.e. formed by different connexons) (Yamasaki & Naus, 1996). The connexins isotypes are distributed among the tissues most in a tissue-specific way but some are present in more than one tissue type. Gap junctions allow the intercellular diffusion of small and hydrophilic molecules, such as cyclic adenosine monophosphate (cAMP) and inositol triphosphate (IP3), and ions (Bruzzone, White & Paul, 1996; King & Bertram, 2005). This flux is called gap junctional intercellular communication (GJIC) and is controlled by many mechanisms, including phosphorylation of connexins, calcium concentration, pH, etc. Because of the nature of the substances that can diffuse from one cell to another, gap junctions play a critical role in regulating tissue homeostasis and different processes responsible for the recovery of the homeostatic balance triggered as a result of damage, such as in the case of wound healing and tissue repair, angiogenesis and carcinogenesis (Chanson et al., 2005; Evert et al., 2002; Yamasaki & Naus, 1996). In the last decade, it has become clear that hemichannels in non-junctional areas at the cell plasma membrane surface can also function as transmembrane channels. In fact, connexons foresee a pathway for communication between the intracellular compartment and the extracellular environment. The messengers that diffuse through hemichannels are quite similar to those implied in GJIC, including adenosine triphosphate (ATP), nicotinamide adenine dinucleotide, glutamate, glutathione and prostaglandins (Fig. 2). However, in contrast to gap junctions, hemichannels are believed to specifically open up in pathological conditions, rather than maintaining tissue homeostasis (Vinken, 2011; Vinken et al., 2012; Wang et al., 2013b).
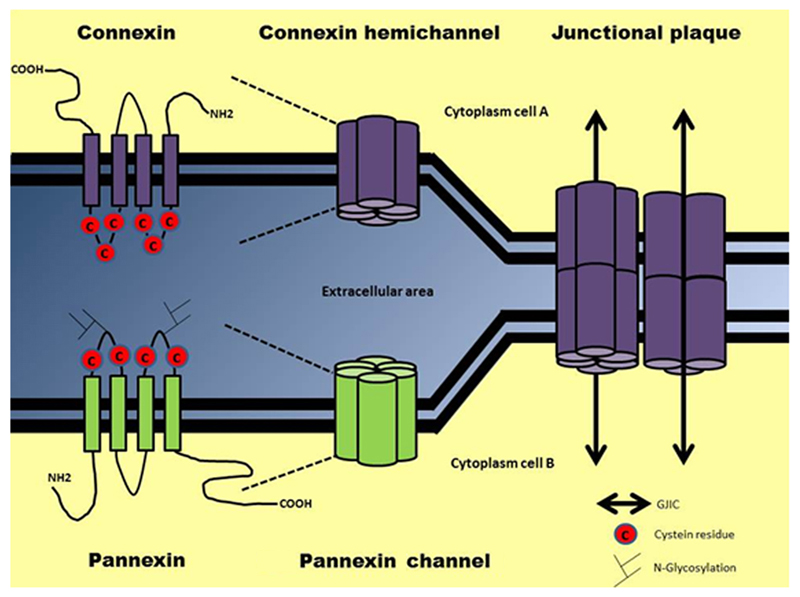
Fig. 1. Molecular architecture of connexin and pannexin (hemi)channels and gap junctions.
Connexins and pannexins consist of four membrane-spanning domains, two extracellular loops, one cytoplasmic loop, and cytoplasmic N-terminal tail and C-terminal tail. Connexins contain three cysteine residues in their extracellular loops, while pannexins only have two. Unlike connexins, pannexins may be N-glycosylated on their extracellular loops. 6 connexin proteins are able to form a hemichannel, while pannexins form a connexin-hemichannel-like channel, composed of six or eight pannexin proteins. Gap junctions are specialized regions of the plasma membrane that form juxtaposed connexons or hemichannels, composed only of connexins, between two adjacent cells. Gap junctions allow the intercellular diffusion of small molecules, metabolites and secondary messengers, a flux called GJIC.
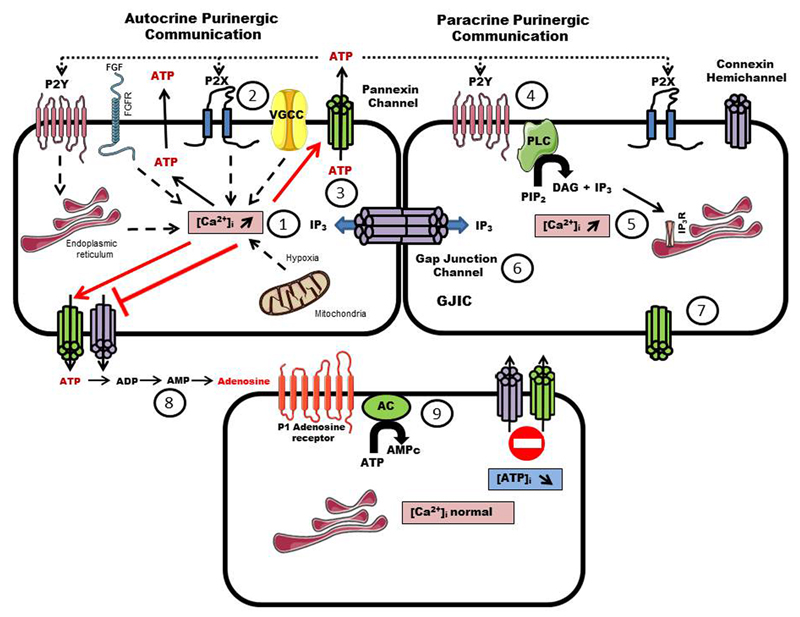
Fig. 2. Involvement of connexins and pannexins (hemi)channels and gap junctions in purinergic signaling.
Signals from purinergic P2Y metabotropic and/or purinergic P2X ionotropic responses, tyrosine kinase receptor activation, influx of calcium from voltage gated calcium channels (VGCC) and/or induction of calcium release from mitochondria during ischemia cause an increase of intracellular calcium concentrations (1) that will promote ATP release by a vesicular exocytosis way. ATP acts mainly as an autocrine/paracrine signal, modulating a variety of cellular functions by activating ionotropic P2X or metabotropic P2Y receptors (2). The increase of intracellular calcium concentrations also triggers the opening of pannexin channels, which release ATP (3). The binding of ATP to P2X receptors, which are ATP-gated cation channels, enhances the depolarization and cell response through influx of sodium, calcium or potassium ions. ATP also binds to PY2 receptors (4), which are metabotropic G-protein coupled receptors, leading to phospholipase C (PLC) activation, generation of IP3 and release of cytosolic calcium from intracellular stores (5). IP3 is able to diffuse through gap junctions to synchronize the tissue response through calcium wave propagation (6). This autocrine and paracrine communication through ATP release triggers the intercellular diffusion of calcium and IP3-mobilizing second messengers. Connexin hemichannels can open and release ATP by a reduction in the extracellular calcium concentration or by mechanical stimulation and hypoxia (7). The opening of connexin hemichannels occurs in response to many physiological and pathological situations, including volume regulation, proliferation, calcium wave propagation by extracellular messengers and cell death during metabolic inhibition. Released ATP is enzymatically degraded within seconds by ectonucleotidases (8). Purinergic signaling molecules, such as adenosine, promote metabotropic P1 receptors and G-protein coupled receptors, which lead to the activation of adenylate cyclase (AC) that degrades ATP into cAMP (9).
2.2. Pannexin channels
Pannexins constitute a family of three vertebrate proteins (D'hondt et al., 2009; Panchin, 2005; Penuela et al., 2008). They are named according to their order of discovery using the prefix Panx (i.e. Panx1, Panx2 and Panx3). Pannexins share no sequence homology (Panchin, 2005), but display considerable structural similarities with connexins (Fushiki et al., 2010). Indeed, the topology of pannexins consists of four transmembrane domains, two extracellular loops, an intracellular loop and intracellular N-terminal and C-terminal tails (Boassa et al., 2007; Locovei, Bao & Dahl, 2006a; Penuela et al., 2007). There are, however, also some structural differences between both types of proteins. Thus, whereas connexins contain three cysteine residues in their extracellular loops, pannexins only have two (Fig. 1). The cytoplasmic N-terminal tail is highly conserved among different pannexins, while the C-terminal region and the intracellular loop vary between pannexins. Six to eight pannexins can oligomerize to form transmembranous channels similar to connexin hemichannels, the connexons, which are therefore called pannexons (Ambrosi et al., 2010). Unlike connexin hemichannels, pannexin channels are not opened by high extracellular calcium, but can be activated by intracellular calcium and extracellular ATP (Bruzzone et al., 2005; Locovei, Wang & Dahl, 2006b; Taylor, Wright & Mahaut-Smith, 2015). Moreover, the opening of Panx1-based pannexons may be induced by various experimental conditions, most of a pathological nature, including activation of purinergic receptors, mechanical stress, high intracellular calcium concentrations and membrane depolarization (Locovei et al., 2006b; Pelegrin & Surprenant, 2006) (Fig. 2). The expression of Panx1, but not Panx2 or Panx3, in paired Xenopus laevis oocytes was reported to lead to intercellular channels, only with excessively long pairing times of 24 to 48 hours (Barbe, Monyer & Bruzzone, 2006; Bruzzone et al., 2003). Unlike connexins, pannexins are regulated by N-glycosylation at the single asparagine residue on the surface of their second extracellular loop (Fig. 1) (Boassa et al., 2007; Evans & Martin, 2002; Gehi, Shao & Laird, 2011; Penuela et al., 2007; Rahman, Carlile & Evans, 1993). This posttranslational modification appears to regulate cellular localization and may act as a barrier for the alignment and docking of two adjacent pannexin channels and, thus, to block the formation of gap junctions in vivo (Penuela et al., 2009; Penuela, Simek & Thompson, 2014). This notion is further substantiated by the fact that plasma membrane-localized pannexin channels have a different appearance in comparison with gap junctions in immunofluorescence and ultrastructural studies. In this respect, Panx1 and Panx3 are detectable at the plasma membrane not in cell-cell contact areas, but at the apical pole of polarized cells. Pannexins are widely distributed in several tissues and cell types (Penuela et al., 2008; Swayne, Sorbara & Bennett, 2010). Panx1 is, indeed, ubiquitously expressed in the central nervous system, eyes, prostate, thyroid, heart, skeletal muscles, gonads, liver and kidneys (Baranova et al., 2004; Bruzzone et al., 2003). Panx2 has been described mainly in the central nervous system (CNS) including hippocampus, neocortex, cerebellum, thalamus and hypothalamus (MacVicar & Thompson, 2010; Ray et al., 2005; Vogt, Hormuzdi & Monyer, 2005). However, recent research showed that the Panx2 expression is not exclusive for the CNS (Le Vasseur et al., 2014). Panx3 is detectable in skin, osteoblasts and chondrocytes (Barbe et al., 2006; Bruzzone et al., 2003). Pannexin channels are permeable to substances smaller than 1 kilodalton, including ions, IP3, ATP, amino acids, arachidonic acid and derivatives (Iglesias et al., 2009; Wang et al., 2013b). Hence, pannexons are currently believed to underlie autocrine and paracrine communication by controlling the extracellular release of ATP in a variety of cell types (Fig. 2), including neurons (Kawamura, Ruskin & Masino, 2010), astrocytes (Iglesias et al., 2009; Kim & Kang, 2011; Silverman et al., 2009), taste bud cells (Dando & Roper, 2009; Huang & Ogawa, 2012; Huang & Roper, 2010), T-cells (Schenk et al., 2008; Woehrle et al., 2010), erythrocytes (Locovei et al., 2006b; Sridharan et al., 2010), airway epithelial cells (Ransford et al., 2009; Seminario-Vidal et al., 2011), endothelial cells (Gödecke et al., 2012), skeletal and smooth muscle cells (Buvinic et al., 2009), pituitary cells (Li, Tomić & Stojilkovic, 2011) and liver cells (Xiao et al., 2012). In particular, Panx1-based channels have been characterized as a mechanosensitive ATP release channel involved in many cellular events, such as calcium-dependent cell death after ischemia involved neurodegeneration (Bargiotas et al., 2011), release of ATP and uridine triphosphate 'find-me' signals from apoptotic cells (Chekeni et al., 2010; Elliott et al., 2009), tumor suppression in glioma cells (Lai, Bechberger & Naus, 2009; Lai et al., 2007) and keratinocyte differentiation (Celetti et al., 2010). Panx1 was also reported to trigger the Toll-like receptor-independent inflammasome based on recognition of bacterial molecules passing from endosomes to cytosol (Silverman et al., 2009).
3. Connexins and pannexins in skin
3.1. Expression in keratinocytes and dermal fibroblasts
A wide repertoire of connexins is expressed in mammalian skin, including Cx26, Cx30, Cx30.3, Cx31, Cx31.1, Cx32, Cx37, Cx40, Cx43 and Cx45. Keratinocytes mainly produce Cx26 and Cx43 (Richard, 2000), while human dermal fibroblasts predominantly express Cx43 and Cx45 and lower quantities of Cx40. Moyer and Ehrlich (2003) demonstrated that human dermal fibroblasts treated with hyaluronic acid show a higher density of Cx43 in the plasma membrane and pronounced GJIC activity. Hyaluronic acid is a structural component of connective tissue and acts as a mediator of several cellular functions, including GJIC (Nagy et al., 1996). Furthermore, hyaluronic acid is important for the organization of de novo synthesized collagen fibrils by fibroblasts during tissue granulation (Iocono et al., 1998). Therefore, more efficient GJIC can improve structuring of collagen fibrils deposited on the skin wound, leading to aesthetically successful healing, in casu stimulated by hyaluronic acid. Skin wounds in rodents treated with Cx43-specific antisense oligodeoxynucleotides exhibit enhanced secretion of TGF-β1 from dermal fibroblasts, associated with increased proliferation and migration capacities (Mori et al., 2006; Qiu et al., 2003). Kretz et al. (2004) evaluated skin wound healing in the tail of Cx43-deficient mice and observed early closing of the skin wound, which was linked with increased mobilization and proliferation of keratinocytes. Moreover, experimental quenching of Cx43 production in keratinocytes showed an increase in proliferation and differentiation during wound healing (Pollok et al., 2011). Moreover, inhibitors of connexin hemichannels and gap junction improve cell migration rate in dermal fibroblasts in vitro, suggesting that a decrease in connexin-based GJIC capacity may be required for efficient skin regeneration during wound healing (Cogliati et al., 2011; Wright et al., 2013; Wright et al., 2009). Cx26 also seems to participate in wound healing, since a high level of Cx26 in keratinocytes is accompanied by a state of hyperproliferation and wound remodeling (Djalilian et al., 2006).
Panx1 is detectable in the stratum granulosum and stratum spinosum layers in human facial skin. While Panx1 is present at the plasma membrane surface, Panx3 resides in the cytosol of keratinocytes (Penuela et al., 2007). Panx1 and Panx3 are indispensable for maintaining keratinocyte homeostasis and for the control of differentiation and regeneration of the epidermis. Panx1 is expressed in the suprabasal layer of newborn skin and throughout all layers of thin epidermis. By contrast, Panx1 is absent in thick skin and is hardly detectable in sebaceous glands. On the other hand, Panx3 is produced in all thin and thick epidermal layers, yet located intracellularly in the basal layer and sebaceous glands. Celetti et al. (2010) reported that exogenous expression of Panx1 in rat epithelial keratinocytes cells disrupts the architecture of the epidermis and markedly increases cytokeratin 14 expression. On the other hand, Panx3 production in rat epithelial keratinocytes does not affect cytokeratin 14 abundance, thus supporting the notion that Panx3 is not involved in directing the differentiation of these cells. In human, western blotting experiments confirm Panx1 expression in subcutaneous fibroblasts (Pinheiro et al., 2013). Most of the epidermis is comprised of keratinocytes, but melanocytes are also interspersed, and although not explicitly observed in situ, a melanocyte cell line has been shown to express Panx1 (Penuela et al., 2012).
3.2. Skin fibrotic diseases
It is well known that connexin gene mutations are linked with several dermatological pathologies, such as hystrix-like ichthyosis with deafness, keratitis-ichthyosis-deafness syndrome, Vohwinkel syndrome erythrokeratoderma variabilis and oculodentodigital dysplasia (Brandner et al., 2004). Among those, oculodentodigital dysplasia is characterized by a fusion of the digits and malformations of the bones, eyes and teeth caused by mutant Cx43 expression (Paznekas et al., 2009). Following changes in Cx43 expression in animal models of wound healing, several authors have reported the same observations in human diseases related to poor skin healing, such as hypertrophic scars and keloids (Lu et al., 2007) or in wounds of diabetic patients (Abdullah et al., 1999; Bajpai et al., 2009; Wang et al., 2007). Fibroblasts derived from keloid or hypertrophic scars indeed have much smaller amounts of Cx43 in comparison with normal skin. This indicates that GJIC is important for controlling the balance between proliferation and apoptosis of fibroblasts in the skin as well as for managing the production of ECM. Diabetic patients typically cope with issues related to skin wound healing. These so-called diabetic ulcers are typified by delayed wound contraction, less migration of fibroblasts, ECM deposition and collagen synthesis (Bajpai et al., 2009). Abdullah et al. (1999) evaluated fibroblasts from diabetic patients and noticed downregulated gap junction activity, yet unaltered Cx43 expression. However, diabetic rats exhibit abnormal expression of dermal and epidermal connexins, with postponed reepithelialization of wounds and aberrant Cx43 production in the epidermis of wound edges (Abdullah et al., 1999). Multicenter randomized clinical trials have been showed that the topical administration of a Cx43-based peptide mimetic, ACT1, accelerates the healing of chronic venous leg ulcers and chronic diabetic foot ulcers in humans (Ghatnekar et al., 2015; Grek et al., 2015). This could point to a function for Cx43 in cutaneous tissue repair.
4. Connexins and pannexins in liver
4.1. Expression in hepatocytes and non-parenchymal liver cells
Cx32 and Cx26 are the main gap junction building blocks in the liver, located in the plasma membrane of hepatocytes (Stutenkemper et al., 1992; Vinken et al., 2008). Cx32 was the first connexin species to be cloned (Kumar & Gilula, 1986; Paul, 1986). As such, Cx32 is ten times more abundant than Cx26 in the liver of rats and humans, and occupies about 3% of the plasma membrane (Nicholson et al., 1987; Vinken et al., 2008). Cx32 is uniformly distributed in all regions of the liver, while Cx26 is preferably expressed in the acinar periportal region (Kojima et al., 1994). In contrast, most non-parenchymal liver cells, including stellate cells, Kupffer cells and sinusoidal endothelial cells, mainly harbour Cx43, while liver vascular cells predominantly produce Cx37 and Cx40 (Eugenín et al., 2007; Fischer et al., 2005; González et al., 2002; Hernández-Guerra et al., 2014). Fisher et al. (2005) demonstrated the occurrence of Cx43-based GJIC between hepatic stellate cells, which is of relevance for the development of liver fibrosis. When activated into myofibroblasts, these cells are responsible for the production and degradation of ECM components located in perisinusoidal regions or fibrous septa (Friedman, 2008). Quiescent hepatic stellate cells not only express Cx43, but also small amounts of Cx26. Upon transdifferentiation into myofibroblasts, Cx43 and Cx26 steady-state levels increase, with preferential location at the cell plasma surface and perinuclear area, respectively. The resulting GJIC activity is thought to contribute to liver fibrogenesis (Fischer et al., 2005). Portal fibroblasts can also transdifferentiate into myofibroblasts and are involved in the establishment of periportal fibrosis (Guyot et al., 2006). Suppression of GJIC has been reported to counteract hepatic stellate cell activation. In addition, DNA synthesis was downregulated and lower levels of type I collagen were obtained in these conditions (Uyama et al., 2003). In a similar study, cultured hepatic stellate cells were treated with TGF-β1, a cytokine with important profibrogenic action, and decreased Cx43 production and GJIC were observed. In addition, hepatic stellate cells in which Cx43 expression was epigenetically silenced showed reduced cellular proliferation (Lim, Maubach & Zhuo, 2009). A couple of reports demonstrated Panx1 expression in liver tissue, in particular produced by hepatocytes (Bruzzone et al., 2003; Csak et al., 2011; Ganz et al., 2011; Kim, Kim & Lee, 2015; Xiao et al., 2012) and Kupffer cells (Sáez et al., 2014). Two studies showed the presence of Panx2 in mouse liver (Le Vasseur et al., 2014) and rat hepatocytes (Li et al., 2008).
4.2. Liver fibrosis and cirrhosis
Studies carried out in patients suffering from alcoholic liver cirrhosis have demonstrated that liver expression of Cx32 gradually decreases upon progression of this disease. Likewise, progressively downregulated hepatic Cx32 levels have been observed in patients with chronic viral hepatitis and hepatocellular carcinoma. Furthermore, Cx32 also frequently shows subcellular localization in liver disease, a phenomenon called internalization (Nakashima et al., 2004). The downregulation of Cx32 in human liver during chronic disease can be reproduced in experimental models. In this light, induction of liver fibrosis in rodents with carbon tetrachloride or dimethylnitrosamine is accompanied by a drastic decrease in Cx32 expression in hepatocytes, whether or not associated with its cytosolic relocalization. It has been suggested that diminished Cx32 expression in chronic liver diseases may be related to the process of regeneration, which usually occurs in conjunction with liver injury (Mangnall, Bird & Majeed, 2003). Oloris et al. (2007) studied the development of hepatic granulomas induced by Schistosoma mansoni in Cx43-deficient mice. These animals showed greater deposits of collagen fibers around granulomas when compared to wild-type animals. In addition, our group demonstrated that Cx43-deficient mice exhibit excessive fibrosis after chronic administration of carbon tetrachloride. The fibrotic animals also showed less necroinflammatory lesions in liver parenchyma, reduced hepatocellular proliferation and lower serum levels of alanine aminotransferase and aspartate aminotransferase (Cogliati et al., 2011). These results suggest the involvement of Cx43 in the control of liver fibrogenesis. Recently, hepatic Cx43 expression was found to be increased in human acute-on-chronic liver failure (Balasubramaniyan et al., 2013) and gap junctions may contribute to modulating portal pressure and intrahepatic vascular relaxation in cirrhosis (Hernández-Guerra et al., 2014). Inflammation induced by lipopolysaccharide in animals as well as in isolated perfused livers showed an increase of Cx32 mRNA degradation in mice and rats (De Maio et al., 2000; Gingalewski et al., 1996; Theodorakis & De Maio, 1999). Cx26 also seemed to be downregulated under these circumstances, albeit independently of mRNA degradation (De Maio et al., 2000; Temme et al., 1997). Rat cholestasis models manifest a reversible decrease of hepatic gap junctions (De Vos & Desmet, 1978; Fallon et al., 1995). Indeed, it has been found that upon bile duct ligation, both Cx32 mRNA and protein levels drop, which is mediated by p38 MAPK (Kojima et al., 2003). Other studies indicated that Cx26 decreases after bile duct ligation (Cogliati et al., 2011). In contrast, Cx43 protein is positively affected in this model (Fallon et al., 1995). Teixeira et al. (2007) reported that livers of heterozygous Cx43 knock-out mice that had their bile ducts obliterated display less hepatic vein angiogenesis, while other parameters, such as biliary duct hyperplasia, remain unchanged.
5. Connexins and pannexins in heart
5.1. Expression in cardiomyocytes and cardiac fibroblasts
Cx43, Cx40 and Cx45 are the three predominant connexins expressed by the cardiomyocytes (Delmar & Makita, 2012). Most of the cardiac gap junctions composed of Cx43 are located at intercalated discs, often with larger junctional plaques at the disc periphery, and are rarely distributed to the sides of the cardiomyocytes. This specific localization of Cx43 in the heart is essential for rapid propagation of action potentials (Agullo-Pascual & Delmar, 2012). Cx43 knock out embryos die at birth as a result of a failure in pulmonar gas exchange caused by a swelling and blockage of the right ventricular outflow tract from the heart. This finding suggests that Cx43 plays an essential role in heart development (Reaume et al., 1995). In addition to GJIC, Cx43-based hemichannels between the cytosol and the extracellular area of cardiomyocytes are involved in cellular signaling to control intracellular volume, cell survival and cardioprotection (Goodenough & Paul, 2003; Plotkin, Manolagas & Bellido, 2002). Kienitz et al. (2011) have shown that Panx1 constitutes the molecular equivalent of a channel called the large conductance cation channel. This channel is activated upon calcium release from the sarcoplasmic reticulum following caffeine stimulation in cardiomyocytes, which potentially promotes arrhythmogenic activities. Moreover, ATP released by Panx1-based channels can bind to adenosine receptors at the plasma membrane of cardiomyocytes to participate in cardioprotection. These pores also contribute to cardioprotection during ischemic preconditioning by the release of endogenous cardioprotectants. Cardiac fibroblasts and myofibroblasts communicate with each other in three ways, namely (i) by a paracrine mechanism through secretion of various growth factors and cytokines, such as interleukin 1β, interleukin 6, tumor necrosis factor-α and TGF-β, (ii) in an indirect manner with the ECM, and (iii) directly by cell-cell interactions, including gap junctions. Collectively, this communicative network affects the function of the heart, cardiac myocyte proliferation, cardiac myocyte apoptosis, ECM composition, cell migration and electrical conductivity (Kakkar & Lee, 2010). After myocardial infarction, cardiac fibroblasts, which express Cx43 and Cx45, turn into myofibroblasts and are responsible for the healing of the affected tissue. These myofibroblasts communicate with cardiomyocytes, which may impact some functions of the former, such as scar contraction and collagen formation (Chilton, Giles & Smith, 2007). Experiments performed with Cx43-deficient mice pointed out that cardiac fibroblasts display more cell proliferation compared with normal fibroblasts and vice versa, whilst fibroblasts overexpressing Cx43 manifest decreased cell proliferation. These results confirm the involvement of Cx43-based signaling in the maintenance of homeostasis and functionality of cardiac fibroblasts. In addition to its antiproliferative action, Cx43-related GJIC also controls the differentiation of cardiac fibroblasts into myofibroblasts and thus wound healing and tissue repair in the heart (Zhang et al., 2008). Indeed, suppression of Cx43 expression in isolated murine fibroblasts is associated with inhibition of α-smooth muscle actin production, a marker of cell differentiation into myofibroblasts (Asazuma-Nakamura et al., 2009). Connexin hemichannels have been implicated in the extracellular release of ATP from cardiac fibroblasts. This was found to promote the activation of purinergic P2Y receptors to mediate profibrotic responses with an increase of migration, proliferation and expression of α-smooth muscle actin as well as several profibrotic markers. Although expressed in cardiac fibroblasts, Panx1-based channels seem no major players in the regulation of ATP release. Thus, ATP levels were not altered following downregulation of Panx1 expression, suggesting that hemichannels consisting of Cx43 and Cx45, but not of Panx1, control ATP release induced by hypotonic stimulation and cell swelling of rat cardiac fibroblasts (Lu et al., 2012).
5.2. Myocardial fibrosis
Acute ischemic heart diseases as well as arrhythmogenesis and myocardial infarction have been associated with defective GJIC as a result of connexin remodeling. During cardiac ischemia, Cx43 becomes phosphorylated, which destabilizes gap junctions resulting in the decrease of Cx43 amounts at the intercalary disks and an increase of Cx43 presence at lateral areas of cardiomyocytes (Lampe & Lau, 2004). These changes lead to disturbances in current flow from Purkinje fibers to the ventricular myocytes, which compromises the transfer current anisotropically down the longitudinal aspect of the cells. The mechanisms behind this Cx43 lateralization process are not known, but may involve acetylation of Cx43 (Colussi et al., 2011). Johansen et al. (2011) demonstrated that gap junctions close, while connexin hemichannels open up in cardiac ischemia. In vitro and in vivo studies using Gap19, a mimetic peptide which selected inhibit Cx43 hemichannels, showed a reduction in the cardiomyocytes injuries during ischemia/reperfusion while preserving electrical and metabolic cell–cell communication (Wang et al., 2013c). In cardiac fibrosis, fibroblasts are activated, causing them to transdifferentiate into myofibroblasts. This facilitates profibrotic responses, such as the production of ECM proteins, collagen and cytokines contributing to heart failure and the impairment of cardiac function (Baum & Duffy, 2011). Recently, Panx1-based signaling has been reported to be involved in the early stages of cardiac fibrosis. Dolmatova et al. (2012) showed that both expression and N-glycosylation of Panx1 are increased in these conditions. Consequently, the presence of Panx1 at the plasma membrane is promoted, where it interacts with the scaffolding protein synapse-associated protein 97 in early ischemia. ATP released by Panx1-based channels in cardiomyocytes during cellular stress activates cardiac fibroblasts via activation of the MAPK and p53 pathways, which, in turn, mediate cardiac fibrosis development (Nishida et al., 2008).
6. Connexins and pannexins in lung
6.1. Expression in airway epithelial cells and lung fibroblasts
Normal alveolar epithelium expresses Cx26, Cx32, Cx43 and Cx46 (Koval, 2002). Their hemichannels as well as Panx1 channels are involved in both surfactant secretion and apoptosis by mediating ATP release and subsequent activation of P2Y purinergic receptors. Furthermore, Panx1-based channels control mucociliary clearance and maintain proper airway epithelial function during stress (Lieb et al., 2002). In this respect, intercellular propagation of calcium waves between airway type I cells and type II cells underlies ciliary beating (Boitano, Dirksen & Evans, 1998), inflammatory cytokine production (Martin & Prince, 2008), surfactant production (Ichimura et al., 2006; Patel et al., 2005) and host defense (Homolya, Steinberg & Boucher, 2000). Seminario-Vidal et al. (2011) recently reported that hypotonic stress increases ATP release from Panx1-based channels in airway epithelial cells in vitro and ex vivo (Seminario-Vidal et al., 2011). It has been suggested that RhoA/Rho kinase signaling serves as a trigger for this process. Rho signaling promotes membrane-cytoskeletal rearrangements, which facilitates the insertion of Panx1 in the plasma membrane (Okada et al., 2013; Seminario-Vidal et al., 2009; Seminario-Vidal et al., 2011).
6.2. Cystic fibrosis and idiopathic pulmonary fibrosis
Both connexins and pannexins are able to modulate chronic inflammatory processes in the lung. Moreover, GJIC between pulmonary epithelial cells has been shown to be defective in cystic fibrosis, a chronic infectious and inflammatory disease caused by mutations of the cystic fibrosis transmembrane conductance regulator gene (Chanson et al., 2001). In addition, the activation and proliferation of lung fibroblasts forming focal aggregates is crucial in the development of idiopathic pulmonary fibrosis, leading to excessive deposits of ECM components. Studies with pulmonary fibroblasts derived from idiopathic pulmonary fibrosis patients indicated lower Cx43 expression and reduced gap junction activity (Trovato-Salinaro et al., 2006). Furthermore, double knock-out mice for Cx43 and Cx40 proteins die prematurely, correlating with severe spontaneous lung abnormalities, including increased fibrosis and alveolar wall thickening (Koval et al., 2011). These results thus show that aberrant GJIC may favor disordered proliferation of pulmonary fibroblasts, thereby promoting ECM synthesis.
7. Connexins and pannexins in kidney
7.1. Expression in kidney epithelial cells and fibroblasts
Wagner & Kurtz (2013) described the importance of GJIC for the functional control of renin-secreting cells in the kidney. Indeed, Cx40 turned out to have a profound impact on the regulation of renin secretion as well as on the intrarenal position of renin-expressing cells (Krattinger et al., 2007; Kurtz et al., 2007; Wagner et al., 2010; Wagner et al., 2009), while Cx37 and Cx45 appear to be less relevant in this regard (Wagner et al., 2009). However, Gerl et al. (2014) recently reported that Cx43 is not essential for the control of renin synthesis and secretion. Confirmed immunofluorescence for Cx37, Cx40 and Cx43 were found in the endothelial cells of interstitial microvessels. Cx40 was localized in glomerular mesangial cells as well as in smooth muscle cells of the juxtaglomerular area (Piao et al., 2011). Gap junctions and connexin hemichannels affect kidney function by facilitating tubular purinergic signaling and vascular conduction. Treatment of normal rat kidney epithelial cells with lipopolysaccharide leads to decreased cell growth through downregulation of Cx43 expression (Gerl et al., 2014). The role of pannexin channels in the kidney tissue and renal physiology is still limited; Panx1 expression was recently detected in renal tubules and in the endothelial of renal arteries and to a lesser extent in smooth muscle cells (Hanner et al., 2012).
7.2. Renal fibrosis
During tubulointerstitial fibrosis, a chronic kidney disease frequently diagnosed in diabetes patients and characterized by an increase of ECM deposits, fibrotic scar formation and deteriorating renal function, tubular epithelial cells are able to differentiate into myofibroblasts cells through an EMT-related mechanism (Hills & Squires, 2010; Okada et al., 1997). In fact, high concentrations of glucose increase TGF-β1 secretion from human proximal tubule cells, thereby inducing the EMT transition by downregulating epithelial markers as such as E-cadherin and by promoting the appearance of mesenchymal markers expression, including vimentin and N-cadherin. Moreover, TGF-β1 disrupts GJIC between kidney cells. Activation of Cx43-based hemichannels plays a role in the pathogenesis of renal ischemic lesions. In fact, moderate ATP depletion activates these pores in human renal proximal tubule cells, which increases cell death incidence (Vergara et al., 2003a; Vergara et al., 2003b).
8. Conclusions and perspectives
Tissue homeostasis is driven by entangled extracellular, intracellular and intercellular signaling mechanisms. The latter is mainly controlled by gap junctions, while both connexin hemichannels and pannexin channels mediate extracellular communication. The impairment of these cellular communicative networks typically burgeons into disease. The fibroproliferative pathologies are triggered by tissue insults and, in particular, by subsequent repair events. Tissue repair is accompanied by a plethora of changes in connexin and pannexin expression as well as in their channel opening. Vice versa, tissue repair can be modulated by interfering with connexin production and functionality. The role of connexin-based channels in fibrogenesis is dependent on a number of parameters, such as the tissue type, identity of the connexin isoform and the experimental setting (Table 1). Nevertheless, in some tissues, such as skin and cornea, inhibition of connexin-based channel activity by chemical modulators or genetic approaches generally enhances wound healing. These observations may open new perspectives for the clinical treatment of a multitude of diseases, especially chronic pathologies. In this respect, there is an urgent need for inhibitors that distinguish between pannexin and connexin signaling on the one hand and between connexin hemichannel communication and GJIC on the other hand. Given the opposite roles of gap junctions and connexin hemichannels in the (dys)regulation of the homeostatic balance, such specific inhibitors are indispensable for a targeted and efficient clinical outcome. While GJIC suppressors were described decades ago (For review Bodendiek & Raman, 2010), specific and in vivo-applicable connexin hemichannel (Abudara et al., 2014; Davidson et al., 2012; Iyyathurai et al., 2013; Wang et al., 2013a; Wang et al., 2013c) and pannexin channel inhibitors have only recently become available. Future efforts should be focused on the further development of such tools as well as on the testing of their clinical applicability, in casu for the clinical management of fibroproliferative diseases.
Table 1. Modulation of connexins expression in vitro and in vivo models related to fibrogenesis and fibrotic diseases.
| Tissue | Experimental Model | Treatment | Effects on Connexins | Observations | Reference |
|---|---|---|---|---|---|
| Skin | Wound healing in mice and culture of Swiss 3T3 fibroblasts | Cx43-specific antisense ODNs | ↓ Cx43 protein | ↑ wound closure ↑ fibroblast proliferation and migration ↑ hydroxyproline content ↑ collagen α1(I) and TGFβ-1 mRNA |
(Qiu et al., 2003 ; Mori et al., 2006) |
| Skin | Wound healing in diabetic rats induced by streptozotocin | No treatment | ↑ Cx43 protein ↑ GJIC |
↓ re-epithelialization ↓ wound closure |
(Wang et al., 2007) |
| Liver | Primary HSCs isolated from rats | Carbenoxolone | ↓ GJIC | ↓activation of myofibloblasts (↓ α-SMA) ↓ DNA synthesis (↓ cyclins D1 and D2) ↓ collagen α1(I) mRNA |
(Uyama et al., 2003) |
| Liver | HSC-2 (rat stellate cell line) | Cx43-specific siRNA | ↓ Cx43 mRNA/protein | ↓ cell proliferation | (Lim, Maubach & Zhuo, 2009) |
| Liver | Fibrosis induced by CCl4 in Cx43-deficient mice | No treatment | ↓ Cx43 mRNA/protein | ↑ fibrosis | (Cogliati et al., 2011) |
| Liver | Granulomas induced by S. mansoni eggs in Cx43-deficient mice | No treatment | ↓ Cx43 mRNA/protein | ↓ cell proliferation ↑ fibrosis |
(Oloris et al., 2005) |
| Heart | Primary fibroblasts isolated from Cx43-deficient mice | No treatment | ↓ Cx43 protein ↓ GJIC |
↑ cell proliferation | (Kakkar & Lee, 2010) |
| Heart | Cultured neonatal rat cardiac fibroblasts | Cx43-specific antisense ODNs | ↓ Cx43 protein ↓ GJIC |
↓differentiation ↓ αSMA protein |
(Asazuma-Nakamura, 2009) |
(α-SMA, alpha-smooth muscle actin; CCl4, carbon tetrachloride; HSC, hepatic stellate cells; ODNs, oligodeoxynucleotides; siRNA, small interfering RNA).
Acknowledgments
This work was financially supported by the grants of the São Paulo Research Foundation (FAPESP grants 05/59583-9 and 13/50420-6), the University of São Paulo-Brazil, the Agency for Innovation by Science and Technology in Flanders-Belgium (IWT), the University Hospital of the Vrije Universiteit Brussel-Belgium (“Willy Gepts Fonds” UZ-VUB), the Fund for Scientific Research Flanders-Belgium (FWO grants G009514N and G010214N) and the European Research Council (ERC Starting Grant 335476).
Abbreviations
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- Cx
connexin
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- GJIC
gap junctional intercellular communication
- IP3
inositol triphosphate
- MAPK
mitogen-activated protein kinase
- Panx
pannexin
- TGF-β(1)
transforming growth factor beta (1)
References
- Abdullah KM, Luthra G, Bilski JJ, Abdullah SA, Reynolds LP, Redmer DA, Grazul-Bilska AT. Cell-to-cell communication and expression of gap junctional proteins in human diabetic and nondiabetic skin fibroblasts: effects of basic fibroblast growth factor. Endocrine. 1999;10:35–41. doi: 10.1385/ENDO:10:1:35. [DOI] [PubMed] [Google Scholar]
- Abudara V, Bechberger J, Freitas-Andrade M, De Bock M, Wang N, Bultynck G, Naus CC, Leybaert L, Giaume C. The connexin43 mimetic peptide Gap19 inhibits hemichannels without altering gap junctional communication in astrocytes. Front Cell Neurosci. 2014;8:306. doi: 10.3389/fncel.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agullo-Pascual E, Delmar M. The noncanonical functions of Cx43 in the heart. J Membr Biol. 2012;245:477–82. doi: 10.1007/s00232-012-9466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi C, Gassmann O, Pranskevich JN, Boassa D, Smock A, Wang J, Dahl G, Steinem C, Sosinsky GE. Pannexin1 and Pannexin2 channels show quaternary similarities to connexons and different oligomerization numbers from each other. J Biol Chem. 2010;285:24420–31. doi: 10.1074/jbc.M110.115444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asazuma-Nakamura Y, Dai P, Harada Y, Jiang Y, Hamaoka K, Takamatsu T. Cx43 contributes to TGF-beta signaling to regulate differentiation of cardiac fibroblasts into myofibroblasts. Exp Cell Res. 2009;315:1190–9. doi: 10.1016/j.yexcr.2008.12.021. [DOI] [PubMed] [Google Scholar]
- Bajpai S, Shukla VK, Tripathi K, Srikrishna S, Singh RK. Targeting connexin 43 in diabetic wound healing: future perspectives. J Postgrad Med. 2009;55:143–9. doi: 10.4103/0022-3859.48786. [DOI] [PubMed] [Google Scholar]
- Balasubramaniyan V, Dhar DK, Warner AE, Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker DL, Jalan R. Importance of Connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J Hepatol. 2013;58:1194–200. doi: 10.1016/j.jhep.2013.01.023. [DOI] [PubMed] [Google Scholar]
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, Tiunova A, et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics. 2004;83:706–16. doi: 10.1016/j.ygeno.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology (Bethesda) 2006;21:103–14. doi: 10.1152/physiol.00048.2005. [DOI] [PubMed] [Google Scholar]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, Penuela S, von Engelhardt J, Monyer H, Schwaninger M. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci U S A. 2011;108:20772–7. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–9. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boassa D, Ambrosi C, Qiu F, Dahl G, Gaietta G, Sosinsky G. Pannexin1 channels contain a glycosylation site that targets the hexamer to the plasma membrane. J Biol Chem. 2007;282:31733–43. doi: 10.1074/jbc.M702422200. [DOI] [PubMed] [Google Scholar]
- Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem. 2010;17:4191–230. doi: 10.2174/092986710793348563. [DOI] [PubMed] [Google Scholar]
- Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium. 1998;23:1–9. doi: 10.1016/s0143-4160(98)90069-0. [DOI] [PubMed] [Google Scholar]
- Brandner JM, Houdek P, Hüsing B, Kaiser C, Moll I. Connexins 26, 30, and 43: differences among spontaneous, chronic, and accelerated human wound healing. J Invest Dermatol. 2004;122:1310–20. doi: 10.1111/j.0022-202X.2004.22529.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–43. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238:1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Almarza G, Bustamante M, Casas M, López J, Riquelme M, Sáez JC, Huidobro-Toro JP, Jaimovich E. ATP released by electrical stimuli elicits calcium transients and gene expression in skeletal muscle. J Biol Chem. 2009;284:34490–505. doi: 10.1074/jbc.M109.057315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celetti SJ, Cowan KN, Penuela S, Shao Q, Churko J, Laird DW. Implications of pannexin 1 and pannexin 3 for keratinocyte differentiation. J Cell Sci. 2010;123:1363–72. doi: 10.1242/jcs.056093. [DOI] [PubMed] [Google Scholar]
- Chanson M, Berclaz PY, Scerri I, Dudez T, Wernke-Dollries K, Pizurki L, Pavirani A, Fiedler MA, Suter S. Regulation of gap junctional communication by a pro-inflammatory cytokine in cystic fibrosis transmembrane conductance regulator-expressing but not cystic fibrosis airway cells. Am J Pathol. 2001;158:1775–84. doi: 10.1016/S0002-9440(10)64133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson M, Derouette JP, Roth I, Foglia B, Scerri I, Dudez T, Kwak BR. Gap junctional communication in tissue inflammation and repair. Biochim Biophys Acta. 2005;1711:197–207. doi: 10.1016/j.bbamem.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, et al. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863–7. doi: 10.1038/nature09413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–36. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati B, Da Silva TC, Aloia TP, Chaible LM, Real-Lima MA, Sanches DS, Matsuzaki P, Hernandez-Blazquez FJ, Dagli ML. Morphological and molecular pathology of CCL4-induced hepatic fibrosis in connexin43-deficient mice. Microsc Res Tech. 2011;74:421–9. doi: 10.1002/jemt.20926. [DOI] [PubMed] [Google Scholar]
- Colussi C, Rosati J, Straino S, Spallotta F, Berni R, Stilli D, Rossi S, Musso E, Macchi E, Mai A, Sbardella G, et al. Nε-lysine acetylation determines dissociation from GAP junctions and lateralization of connexin 43 in normal and dystrophic heart. Proc Natl Acad Sci U S A. 2011;108:2795–800. doi: 10.1073/pnas.1013124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology. 2011;54:133–44. doi: 10.1002/hep.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'hondt C, Ponsaerts R, De Smedt H, Bultynck G, Himpens B. Pannexins, distant relatives of the connexin family with specific cellular functions? Bioessays. 2009;31:953–74. doi: 10.1002/bies.200800236. [DOI] [PubMed] [Google Scholar]
- Dahl G, Keane RW. Pannexin: from discovery to bedside in 11±4 years? Brain Res. 2012;1487:150–9. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. J Physiol. 2009;587:5899–906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JO, Green CR, Nicholson LF, O'Carroll SJ, Fraser M, Bennet L, Gunn AJ. Connexin hemichannel blockade improves outcomes in a model of fetal ischemia. Ann Neurol. 2012;71:121–32. doi: 10.1002/ana.22654. [DOI] [PubMed] [Google Scholar]
- De Maio A, Gingalewski C, Theodorakis NG, Clemens MG. Interruption of hepatic gap junctional communication in the rat during inflammation induced by bacterial lipopolysaccharide. Shock. 2000;14:53–9. doi: 10.1097/00024382-200014010-00010. [DOI] [PubMed] [Google Scholar]
- De Vos R, Desmet VJ. Morphologic changes of the junctional complex of the hepatocytes in rat liver after bile duct ligation. Br J Exp Pathol. 1978;59:220–7. [PMC free article] [PubMed] [Google Scholar]
- Delmar M, Makita N. Cardiac connexins, mutations and arrhythmias. Curr Opin Cardiol. 2012;27:236–41. doi: 10.1097/HCO.0b013e328352220e. [DOI] [PubMed] [Google Scholar]
- Djalilian AR, McGaughey D, Patel S, Seo EY, Yang C, Cheng J, Tomic M, Sinha S, Ishida-Yamamoto A, Segre JA. Connexin 26 regulates epidermal barrier and wound remodeling and promotes psoriasiform response. J Clin Invest. 2006;116:1243–53. doi: 10.1172/JCI27186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmatova E, Spagnol G, Boassa D, Baum JR, Keith K, Ambrosi C, Kontaridis MI, Sorgen PL, Sosinsky GE, Duffy HS. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303:H1208–18. doi: 10.1152/ajpheart.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–6. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenín EA, González HE, Sánchez HA, Brañes MC, Sáez JC. Inflammatory conditions induce gap junctional communication between rat Kupffer cells both in vivo and in vitro. Cell Immunol. 2007;247:103–10. doi: 10.1016/j.cellimm.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19:121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- Evert M, Ott T, Temme A, Willecke K, Dombrowski F. Morphology and morphometric investigation of hepatocellular preneoplastic lesions and neoplasms in connexin32-deficient mice. Carcinogenesis. 2002;23:697–703. doi: 10.1093/carcin/23.5.697. [DOI] [PubMed] [Google Scholar]
- Fallon MB, Nathanson MH, Mennone A, Sáez JC, Burgstahler AD, Anderson JM. Altered expression and function of hepatocyte gap junctions after common bile duct ligation in the rat. Am J Physiol. 1995;268:C1186–94. doi: 10.1152/ajpcell.1995.268.5.C1186. [DOI] [PubMed] [Google Scholar]
- Fischer R, Reinehr R, Lu TP, Schönicke A, Warskulat U, Dienes HP, Häussinger D. Intercellular communication via gap junctions in activated rat hepatic stellate cells. Gastroenterology. 2005;128:433–48. doi: 10.1053/j.gastro.2004.11.065. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–72. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fushiki D, Hamada Y, Yoshimura R, Endo Y. Phylogenetic and bioinformatic analysis of gap junction-related proteins, innexins, pannexins and connexins. Biomed Res. 2010;31:133–42. doi: 10.2220/biomedres.31.133. [DOI] [PubMed] [Google Scholar]
- Ganz M, Csak T, Nath B, Szabo G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J Gastroenterol. 2011;17:4772–8. doi: 10.3748/wjg.v17.i43.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehi R, Shao Q, Laird DW. Pathways regulating the trafficking and turnover of pannexin1 protein and the role of the C-terminal domain. J Biol Chem. 2011;286:27639–53. doi: 10.1074/jbc.M111.260711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl M, Kurt B, Kurtz A, Wagner C. Connexin 43 is not essential for the control of renin synthesis and secretion. Pflugers Arch. 2014;466:1003–9. doi: 10.1007/s00424-013-1349-2. [DOI] [PubMed] [Google Scholar]
- Ghatnekar GS, Grek CL, Armstrong DG, Desai SC, Gourdie RG. The effect of a connexin43-based Peptide on the healing of chronic venous leg ulcers: a multicenter, randomized trial. J Invest Dermatol. 2015;135:289–98. doi: 10.1038/jid.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingalewski C, Wang K, Clemens MG, De Maio A. Posttranscriptional regulation of connexin 32 expression in liver during acute inflammation. J Cell Physiol. 1996;166:461–7. doi: 10.1002/(SICI)1097-4652(199602)166:2<461::AID-JCP25>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- González HE, Eugenín EA, Garcés G, Solís N, Pizarro M, Accatino L, Sáez JC. Regulation of hepatic connexins in cholestasis: possible involvement of Kupffer cells and inflammatory mediators. Am J Physiol Gastrointest Liver Physiol. 2002;282:G991–G1001. doi: 10.1152/ajpgi.00298.2001. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Grek CL, Prasad GM, Viswanathan V, Armstrong DG, Gourdie RG, Ghatnekar GS. Topical administration of a connexin43-based peptide augments healing of chronic neuropathic diabetic foot ulcers: A multicenter, randomized trial. Wound Repair Regen. 2015;23:203–12. doi: 10.1111/wrr.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyot C, Lepreux S, Combe C, Doudnikoff E, Bioulac-Sage P, Balabaud C, Desmoulière A. Hepatic fibrosis and cirrhosis: the (myo)fibroblastic cell subpopulations involved. Int J Biochem Cell Biol. 2006;38:135–51. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Gödecke S, Roderigo C, Rose CR, Rauch BH, Gödecke A, Schrader J. Thrombin-induced ATP release from human umbilical vein endothelial cells. Am J Physiol Cell Physiol. 2012;302:C915–23. doi: 10.1152/ajpcell.00283.2010. [DOI] [PubMed] [Google Scholar]
- Hanner F, Lam L, Nguyen MT, Yu A, Peti-Peterdi J. Intrarenal localization of the plasma membrane ATP channel pannexin1. Am J Physiol Renal Physiol. 2012;303:F1454–9. doi: 10.1152/ajprenal.00206.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Guerra M, González-Méndez Y, de Ganzo ZA, Salido E, García-Pagán JC, Abrante B, Malagón AM, Bosch J, Quintero E. Role of gap junctions modulating hepatic vascular tone in cirrhosis. Liver Int. 2014;34:859–68. doi: 10.1111/liv.12446. [DOI] [PubMed] [Google Scholar]
- Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31:68–74. doi: 10.1159/000256659. [DOI] [PubMed] [Google Scholar]
- Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–60. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res. 2012;53:187–96. doi: 10.3109/03008207.2011.642035. [DOI] [PubMed] [Google Scholar]
- Huang YA, Roper SD. Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–50. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol. 2006;291:L596–601. doi: 10.1152/ajplung.00036.2006. [DOI] [PubMed] [Google Scholar]
- Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J Neurosci. 2009;29:7092–7. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iocono JA, Krummel TM, Keefer KA, Allison GM, Paul H. Repeated additions of hyaluronan alters granulation tissue deposition in sponge implants in mice. Wound Repair Regen. 1998;6:442–8. doi: 10.1046/j.1524-475x.1998.60506.x. [DOI] [PubMed] [Google Scholar]
- Iyyathurai J, D'hondt C, Wang N, De Bock M, Himpens B, Retamal MA, Stehberg J, Leybaert L, Bultynck G. Peptides and peptide-derived molecules targeting the intracellular domains of Cx43: gap junctions versus hemichannels. Neuropharmacology. 2013;75:491–505. doi: 10.1016/j.neuropharm.2013.04.050. [DOI] [PubMed] [Google Scholar]
- Johansen D, Cruciani V, Sundset R, Ytrehus K, Mikalsen SO. Ischemia induces closure of gap junctional channels and opening of hemichannels in heart-derived cells and tissue. Cell Physiol Biochem. 2011;28:103–14. doi: 10.1159/000331719. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Lee RT. Intramyocardial fibroblast myocyte communication. Circ Res. 2010;106:47–57. doi: 10.1161/CIRCRESAHA.109.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura M, Ruskin DN, Masino SA. Metabolic autocrine regulation of neurons involves cooperation among pannexin hemichannels, adenosine receptors, and KATP channels. J Neurosci. 2010;30:3886–95. doi: 10.1523/JNEUROSCI.0055-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz MC, Bender K, Dermietzel R, Pott L, Zoidl G. Pannexin 1 constitutes the large conductance cation channel of cardiac myocytes. J Biol Chem. 2011;286:290–8. doi: 10.1074/jbc.M110.163477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Kim SJ, Lee SM. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 2015;282:259–70. doi: 10.1111/febs.13123. [DOI] [PubMed] [Google Scholar]
- Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121:2037–47. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King TJ, Bertram JS. Connexins as targets for cancer chemoprevention and chemotherapy. Biochim Biophys Acta. 2005;1719:146–60. doi: 10.1016/j.bbamem.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Kojima T, Sawada N, Zhong Y, Oyamada M, Mori M. Sequential changes in intercellular junctions between hepatocytes during the course of acute liver injury and restoration after thioacetamide treatment. Virchows Arch. 1994;425:407–12. doi: 10.1007/BF00189579. [DOI] [PubMed] [Google Scholar]
- Kojima T, Yamamoto T, Murata M, Lan M, Takano K, Go M, Ichimiya S, Chiba H, Sawada N. Role of the p38 MAP-kinase signaling pathway for Cx32 and claudin-1 in the rat liver. Cell Commun Adhes. 2003;10:437–43. doi: 10.1080/cac.10.4-6.437.443. [DOI] [PubMed] [Google Scholar]
- Koval M. Sharing signals: connecting lung epithelial cells with gap junction channels. Am J Physiol Lung Cell Mol Physiol. 2002;283:L875–93. doi: 10.1152/ajplung.00078.2002. [DOI] [PubMed] [Google Scholar]
- Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE. Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol. 2011;178:2536–46. doi: 10.1016/j.ajpath.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, Waeber G, Meda P, Haefliger JA. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–22. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- Kretz M, Maass K, Willecke K. Expression and function of connexins in the epidermis, analyzed with transgenic mouse mutants. Eur J Cell Biol. 2004;83:647–54. doi: 10.1078/0171-9335-00422. [DOI] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol. 1986;103:767–76. doi: 10.1083/jcb.103.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, Sauter A, Kurtz A, Wagner C. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–11. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- Lai CP, Bechberger JF, Naus CC. Pannexin2 as a novel growth regulator in C6 glioma cells. Oncogene. 2009;28:4402–8. doi: 10.1038/onc.2009.283. [DOI] [PubMed] [Google Scholar]
- Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumor-suppressive effects of pannexin 1 in C6 glioma cells. Cancer Res. 2007;67:1545–54. doi: 10.1158/0008-5472.CAN-06-1396. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–86. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vasseur M, Lelowski J, Bechberger JF, Sin WC, Naus CC. Pannexin 2 protein expression is not restricted to the CNS. Front Cell Neurosci. 2014;8:392. doi: 10.3389/fncel.2014.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Tomić M, Stojilkovic SS. Characterization of novel Pannexin 1 isoforms from rat pituitary cells and their association with ATP-gated P2X channels. Gen Comp Endocrinol. 2011;174:202–10. doi: 10.1016/j.ygcen.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Cao J, Jin Q, Xie C, He Q, Cao R, Xiong J, Chen P, Wang X, Liang S. A proteomic study reveals the diversified distribution of plasma membrane-associated proteins in rat hepatocytes. J Cell Biochem. 2008;104:965–84. doi: 10.1002/jcb.21680. [DOI] [PubMed] [Google Scholar]
- Lieb T, Frei CW, Frohock JI, Bookman RJ, Salathe M. Prolonged increase in ciliary beat frequency after short-term purinergic stimulation in human airway epithelial cells. J Physiol. 2002;538:633–46. doi: 10.1113/jphysiol.2001.013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MC, Maubach G, Zhuo L. TGF-beta1 down-regulates connexin 43 expression and gap junction intercellular communication in rat hepatic stellate cells. Eur J Cell Biol. 2009;88:719–30. doi: 10.1016/j.ejcb.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A. 2006a;103:7655–9. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006b;580:239–44. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB J. 2012;26:2580–91. doi: 10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Gao J, Ogawa R, Hyakusoku H. Variations in gap junctional intercellular communication and connexin expression in fibroblasts derived from keloid and hypertrophic scars. Plast Reconstr Surg. 2007;119:844–51. doi: 10.1097/01.prs.0000255539.99698.f4. [DOI] [PubMed] [Google Scholar]
- MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Mangnall D, Bird NC, Majeed AW. The molecular physiology of liver regeneration following partial hepatectomy. Liver Int. 2003;23:124–38. doi: 10.1034/j.1600-0676.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- Martin FJ, Prince AS. TLR2 regulates gap junction intercellular communication in airway cells. J Immunol. 2008;180:4986–93. doi: 10.4049/jimmunol.180.7.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–45. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Mori R, Power KT, Wang CM, Martin P, Becker DL. Acute downregulation of connexin43 at wound sites leads to a reduced inflammatory response, enhanced keratinocyte proliferation and wound fibroblast migration. J Cell Sci. 2006;119:5193–203. doi: 10.1242/jcs.03320. [DOI] [PubMed] [Google Scholar]
- Moyer KE, Ehrlich HP. Modulation of human fibroblast gap junction intercellular communication by hyaluronan. J Cell Physiol. 2003;196:165–70. doi: 10.1002/jcp.10288. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Hossain MZ, Lynn BD, Curpen GE, Yang S, Turley EA. Increased connexin-43 and gap junctional communication correlate with altered phenotypic characteristics of cells overexpressing the receptor for hyaluronic acid-mediated motility. Cell Growth Differ. 1996;7:745–51. [PubMed] [Google Scholar]
- Nakashima Y, Ono T, Yamanoi A, El-Assal ON, Kohno H, Nagasue N. Expression of gap junction protein connexin32 in chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. J Gastroenterol. 2004;39:763–8. doi: 10.1007/s00535-003-1386-2. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Dermietzel R, Teplow D, Traub O, Willecke K, Revel JP. Two homologous protein components of hepatic gap junctions. Nature. 1987;329:732–4. doi: 10.1038/329732a0. [DOI] [PubMed] [Google Scholar]
- Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27:3104–15. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–74. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Okada SF, Ribeiro CM, Sesma JI, Seminario-Vidal L, Abdullah LH, van Heusden C, Lazarowski ER, Boucher RC. Inflammation promotes airway epithelial ATP release via calcium-dependent vesicular pathways. Am J Respir Cell Mol Biol. 2013;49:814–20. doi: 10.1165/rcmb.2012-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oloris SC, Mesnil M, Reis VN, Sakai M, Matsuzaki P, Fonseca Ee S, da Silva TC, Avanzo JL, Sinhorini IL, Guerra JL, Costa-Pinto FA, et al. Hepatic granulomas induced by Schistosoma mansoni in mice deficient for connexin 43 present lower cell proliferation and higher collagen content. Life Sci. 2007;80:1228–35. doi: 10.1016/j.lfs.2006.12.030. [DOI] [PubMed] [Google Scholar]
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–4. doi: 10.1016/s0960-9822(00)00576-5. [DOI] [PubMed] [Google Scholar]
- Panchin YV. Evolution of gap junction proteins--the pannexin alternative. J Exp Biol. 2005;208:1415–9. doi: 10.1242/jeb.01547. [DOI] [PubMed] [Google Scholar]
- Patel AS, Reigada D, Mitchell CH, Bates SR, Margulies SS, Koval M. Paracrine stimulation of surfactant secretion by extracellular ATP in response to mechanical deformation. Am J Physiol Lung Cell Mol Physiol. 2005;289:L489–96. doi: 10.1152/ajplung.00074.2005. [DOI] [PubMed] [Google Scholar]
- Paul DL. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986;103:123–34. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW. GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat. 2009;30:724–33. doi: 10.1002/humu.20958. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, Bai D, Shao Q, Laird DW. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120:3772–83. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- Penuela S, Bhalla R, Nag K, Laird DW. Glycosylation regulates pannexin intermixing and cellular localization. Mol Biol Cell. 2009;20:4313–23. doi: 10.1091/mbc.E09-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Celetti SJ, Bhalla R, Shao Q, Laird DW. Diverse subcellular distribution profiles of pannexin 1 and pannexin 3. Cell Commun Adhes. 2008;15:133–42. doi: 10.1080/15419060802014115. [DOI] [PubMed] [Google Scholar]
- Penuela S, Gyenis L, Ablack A, Churko JM, Berger AC, Litchfield DW, Lewis JD, Laird DW. Loss of pannexin 1 attenuates melanoma progression by reversion to a melanocytic phenotype. J Biol Chem. 2012;287:29184–93. doi: 10.1074/jbc.M112.377176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Simek J, Thompson RJ. Regulation of pannexin channels by post-translational modifications. FEBS Lett. 2014;588:1411–5. doi: 10.1016/j.febslet.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Piao H, Sato A, Nozawa Y, Sun W, Morioka T, Oite T. Effects of connexin-mimetic peptides on perfusion pressure in response to phenylephrine in isolated, perfused rat kidneys. Clin Exp Nephrol. 2011;15:203–11. doi: 10.1007/s10157-010-0382-0. [DOI] [PubMed] [Google Scholar]
- Pinheiro AR, Paramos-de-Carvalho D, Certal M, Costa MA, Costa C, Magalhães-Cardoso MT, Ferreirinha F, Sévigny J, Correia-de-Sá P. Histamine induces ATP release from human subcutaneous fibroblasts, via pannexin-1 hemichannels, leading to Ca2+ mobilization and cell proliferation. J Biol Chem. 2013;288:27571–83. doi: 10.1074/jbc.M113.460865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–57. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- Pollok S, Pfeiffer AC, Lobmann R, Wright CS, Moll I, Martin PE, Brandner JM. Connexin 43 mimetic peptide Gap27 reveals potential differences in the role of Cx43 in wound repair between diabetic and non-diabetic cells. J Cell Mol Med. 2011;15:861–73. doi: 10.1111/j.1582-4934.2010.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Coutinho P, Frank S, Franke S, Law LY, Martin P, Green CR, Becker DL. Targeting connexin43 expression accelerates the rate of wound repair. Curr Biol. 2003;13:1697–703. doi: 10.1016/j.cub.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Rahman S, Carlile G, Evans WH. Assembly of hepatic gap junctions. Topography and distribution of connexin 32 in intracellular and plasma membranes determined using sequence-specific antibodies. J Biol Chem. 1993;268:1260–5. [PubMed] [Google Scholar]
- Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–34. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–90. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–4. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Richard G. Connexins: a connection with the skin. Exp Dermatol. 2000;9:77–96. doi: 10.1034/j.1600-0625.2000.009002077.x. [DOI] [PubMed] [Google Scholar]
- Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, Fumagalli M, Verderio C, Buer J, Scanziani E, Grassi F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1:ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, Boucher RC, Lazarowski ER. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284:20638–48. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminario-Vidal L, Okada SF, Sesma JI, Kreda SM, van Heusden CA, Zhu Y, Jones LC, O'Neal WK, Penuela S, Laird DW, Boucher RC, et al. Rho signaling regulates pannexin 1-mediated ATP release from airway epithelia. J Biol Chem. 2011;286:26277–86. doi: 10.1074/jbc.M111.260562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–51. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol. 2010;299:H1146–52. doi: 10.1152/ajpheart.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutenkemper R, Geisse S, Schwarz HJ, Look J, Traub O, Nicholson BJ, Willecke K. The hepatocyte-specific phenotype of murine liver cells correlates with high expression of connexin32 and connexin26 but very low expression of connexin43. Exp Cell Res. 1992;201:43–54. doi: 10.1016/0014-4827(92)90346-a. [DOI] [PubMed] [Google Scholar]
- Swayne LA, Sorbara CD, Bennett SA. Pannexin 2 is expressed by postnatal hippocampal neural progenitors and modulates neuronal commitment. J Biol Chem. 2010;285:24977–86. doi: 10.1074/jbc.M110.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez PJ, Shoji KF, Aguirre A, Sáez JC. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediators Inflamm. 2014;2014:742734. doi: 10.1155/2014/742734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Wright JR, Mahaut-Smith MP. Regulation of Pannexin-1 channel activity. Biochem Soc Trans. 2015;43:502–7. doi: 10.1042/BST20150042. [DOI] [PubMed] [Google Scholar]
- Teixeira TF, da Silva TC, Fukumasu H, de Lima CE, Lúcia Zaidan Dagli M, Guerra JL. Histological alterations in the livers of Cx43-deficient mice submitted to a cholestasis model. Life Sci. 2007;81:380–4. doi: 10.1016/j.lfs.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Temme A, Buchmann A, Gabriel HD, Nelles E, Schwarz M, Willecke K. High incidence of spontaneous and chemically induced liver tumors in mice deficient for connexin32. Curr Biol. 1997;7:713–6. doi: 10.1016/s0960-9822(06)00302-2. [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, De Maio A. Cx32 mRNA in rat liver: effects of inflammation on poly(A) tail distribution and mRNA degradation. Am J Physiol. 1999;276:R1249–57. doi: 10.1152/ajpregu.1999.276.5.R1249. [DOI] [PubMed] [Google Scholar]
- Trovato-Salinaro A, Trovato-Salinaro E, Failla M, Mastruzzo C, Tomaselli V, Gili E, Crimi N, Condorelli DF, Vancheri C. Altered intercellular communication in lung fibroblast cultures from patients with idiopathic pulmonary fibrosis. Respir Res. 2006;7:122. doi: 10.1186/1465-9921-7-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyama N, Shimahara Y, Okuyama H, Kawada N, Kamo S, Ikeda K, Yamaoka Y. Carbenoxolone inhibits DNA synthesis and collagen gene expression in rat hepatic stellate cells in culture. J Hepatol. 2003;39:749–55. doi: 10.1016/s0168-8278(03)00375-1. [DOI] [PubMed] [Google Scholar]
- Vergara L, Bao X, Bello-Reuss E, Reuss L. Do connexin 43 gap-junctional hemichannels activate and cause cell damage during ATP depletion of renal-tubule cells? Acta Physiol Scand. 2003a;179:33–8. doi: 10.1046/j.1365-201X.2003.01198.x. [DOI] [PubMed] [Google Scholar]
- Vergara L, Bao X, Cooper M, Bello-Reuss E, Reuss L. Gap-junctional hemichannels are activated by ATP depletion in human renal proximal tubule cells. J Membr Biol. 2003b;196:173–84. doi: 10.1007/s00232-003-0636-9. [DOI] [PubMed] [Google Scholar]
- Vinken M. Role of connexin-related signalling in hepatic homeostasis and its relevance for liver-based in vitro modelling. World J Gastrointest Pathophysiol. 2011;2:82–7. doi: 10.4291/wjgp.v2.i5.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinken M, Decrock E, Vanhaecke T, Leybaert L, Rogiers V. Connexin43 signaling contributes to spontaneous apoptosis in cultures of primary hepatocytes. Toxicol Sci. 2012;125:175–86. doi: 10.1093/toxsci/kfr277. [DOI] [PubMed] [Google Scholar]
- Vinken M, Henkens T, De Rop E, Fraczek J, Vanhaecke T, Rogiers V. Biology and pathobiology of gap junctional channels in hepatocytes. Hepatology. 2008;47:1077–88. doi: 10.1002/hep.22049. [DOI] [PubMed] [Google Scholar]
- Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–20. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, Gomez RA, van Veen TA, de Wit C, Kurtz A. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associated with arterial hypertension. Kidney Int. 2010;78:762–8. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner C, Kurtz A. Distribution and functional relevance of connexins in renin-producing cells. Pflugers Arch. 2013;465:71–7. doi: 10.1007/s00424-012-1134-7. [DOI] [PubMed] [Google Scholar]
- Wagner C, Kurtz L, Schweda F, Simon AM, Kurtz A. Connexin 37 is dispensable for the control of the renin system and for positioning of renin-producing cells in the kidney. Pflugers Arch. 2009;459:151–8. doi: 10.1007/s00424-009-0707-6. [DOI] [PubMed] [Google Scholar]
- Wang CM, Lincoln J, Cook JE, Becker DL. Abnormal connexin expression underlies delayed wound healing in diabetic skin. Diabetes. 2007;56:2809–17. doi: 10.2337/db07-0613. [DOI] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Bultynck G, Leybaert L. Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening. Neuropharmacology. 2013a;75:506–16. doi: 10.1016/j.neuropharm.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Wang N, De Bock M, Decrock E, Bol M, Gadicherla A, Vinken M, Rogiers V, Bukauskas FF, Bultynck G, Leybaert L. Paracrine signaling through plasma membrane hemichannels. Biochim Biophys Acta. 2013b;1828:35–50. doi: 10.1016/j.bbamem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, De Vuyst E, Ponsaerts R, Boengler K, Palacios-Prado N, Wauman J, Lai CP, De Bock M, Decrock E, Bol M, Vinken M, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013c;108:309. doi: 10.1007/s00395-012-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzke KE, Cantemir-Stone C, Zhang J, Marsh CB, Huang K. Identifying common genes and networks in multi-organ fibrosis. AMIA Jt Summits Transl Sci Proc. 2012;2012:106–15. [PMC free article] [PubMed] [Google Scholar]
- Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475–84. doi: 10.1182/blood-2010-04-277707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CS, Berends RF, Flint DJ, Martin PE. Cell motility in models of wounded human skin is improved by Gap27 despite raised glucose, insulin and IGFBP-5. Exp Cell Res. 2013;319:390–401. doi: 10.1016/j.yexcr.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Wright CS, van Steensel MA, Hodgins MB, Martin PE. Connexin mimetic peptides improve cell migration rates of human epidermal keratinocytes and dermal fibroblasts in vitro. Wound Repair Regen. 2009;17:240–9. doi: 10.1111/j.1524-475X.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117:524–9. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Waldrop SL, Khimji AK, Kilic G. Pannexin1 contributes to pathophysiological ATP release in lipoapoptosis induced by saturated free fatty acids in liver cells. Am J Physiol Cell Physiol. 2012;303:C1034–44. doi: 10.1152/ajpcell.00175.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Naus CC. Role of connexin genes in growth control. Carcinogenesis. 1996;17:1199–213. doi: 10.1093/carcin/17.6.1199. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Kanter EM, Laing JG, Aprhys C, Johns DC, Kardami E, Yamada KA. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]