Abstract
An increasing evidence base suggests that low bone mineral density (BMD) and fractures are associated with cardiovascular disease (CVD). We conducted a systematic review and meta-analysis summarizing the evidence of low BMD and fractures as risk factors for future CVD. Two independent authors searched major databases from inception to 1st August 2016 for longitudinal studies reporting data on CVD incidence (overall and specific CVD) and BMD status and fractures. The association between low BMD, fractures and CVD across longitudinal studies was explored by calculating pooled adjusted hazard ratios (HRs)±95% confidence intervals (CIs) with a random-effects meta-analysis. Twenty-eight studies (18 regarding BMD and 10 fractures) followed-up a total of 1,107,885 participants for a median of 5 years. Taking those with higher BMD as the reference, people with low BMD were at increased risk of developing CVD during follow-up (11 studies; HR=1.33; 95%CI: 1.27-1.38; I2=53%), after adjusting for a median of 8 confounders. This finding was confirmed using a decrease in one standard deviation of baseline BMD (9 studies; HR=1.16; 95%CI: 1.09-1.24; I2=69%). The presence of fractures at baseline was associated with an increased risk of developing CVD (HR=1.20; 95%CI: 1.06-1.37; I2=91%). Regarding specific CVD, low BMD was associated with an increased risk of developing coronary artery disease, cerebrovascular conditions, and CVD associated death. Fractures at baseline was associated with an increased risk of cerebrovascular conditions and death due to CVD. In conclusion, low BMD and fractures are associated with a small, but significant increased risk of CVD risk and possibly death.
Keywords: osteoporosis, bone mineral density, cardiovascular disease, meta-analysis
Introduction
Low bone mineral density (BMD) and osteoporosis are common public health concerns, particularly among women, and greatly predispose an individual at increased risk of experiencing a fracture.(1) It has been estimated that about 30% of postmenopausal women in developed countries have osteoporosis and at least 40% of women and 15–30% of men with osteoporosis will sustain a fracture.(1) Improving bone health and in particular preventing fractures are key to reduce the burden on the individual and healthcare systems.(2)
For over than 30 years, attention has considered the potential for people with low BMD being at increased risk of developing cardiovascular disease (CVD), a leading cause of mortality in the Western world.(3) A number of potential reasons might explain this possible relationship between poor bone health and CVD. This includes the notion that low BMD and CVD share some common pathways(4,5), that people with low BMD have higher prevalence of vascular calcifications than people with normal BMD (6–9), and that some common conditions present in people with low BMD (such as low physical activity and vascular calcifications) have been identified as a key predictors of CVD. (4,5,10–14) Given the fact that CVD is a leading cause of premature mortality, understanding if low BMD is a potential CVD risk factor is of high importance. However, to date, individual studies have lacked clarity and to date no comprehensive meta-analysis exists(4), except for a previous meta-analysis including 4 studies that suggested that low BMD was associated with a higher risk of CVD death, but not death due to stroke.(15)
There is some evidence to suggest that people who have experienced fractures are at increased risk of CVD.(16) Whilst it is acknowledged that CVD increases the risk of fractures (17), less is known whether such relationship is reciprocal.
Given the potential relationship between poor bone health (population) and incident CVD (outcome), the aim of the current systematic review and meta-analysis was to compare the incidence of CVD in longitudinal studies (study design), adjusted for potential confounders, between people with low BMD or fractures with people with higher BMD values or no fractures. We hypothesized that low BMD and fractures would be associated with a significant increased risk of developing CVD and associated mortality independent of pertinent confounders.
Methods
This systematic review was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology [STROBE] criteria(18) and the recommendations in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [PRISMA] statement.(19)
Search strategy
Two independent authors (BS, MS) searched for longitudinal studies considering BMD, osteoporosis, fractures, and CVD. Major databases (PubMed, EMBASE, SCOPUS) were searched from inception until 01st August 2016, without language restrictions. The search strategy used in Pubmed is reported in Supplementary Table 1. An identical search strategy was conducted in the other databases.
Eligibility criteria and study selection
Articles were eligible that: (1) included people with low BMD (defined using any validated tool for the assessment of BMD, such as Dual-energy X-ray absorptiometry or DXA, quantitative ultrasounds or QUS, or through medical/insurance records) or fractures (at any skeletal site); (2) had a longitudinal design; (3) included a control group (e.g. with higher BMD values or without fractures); (4) reported the incidence of any type of CVD (reported via medical records, self-report or ICD codes) assessed through a Cox’s regression analysis. This method allows for adjustment for potential confounders and competing risks.(20)
Studies were excluded if they: (1) included a control group consisting of people with a condition known to affect BMD (e.g. osteopenia or bone metastasis); (2) considered non-osteoporotic fractures; (3) reported only sub-clinical estimates of CVD; (4) investigated the CVD risk due to the use of a medication (e.g. bisphosphonates) in people with low BMD/osteoporosis. If two studies were reported from the same cohort, the largest and/or most recent study was included. Two authors (BS, MS) developed a list of included studies independently and third author (NV) was available for mediation. References of included articles included were hand-searched to identify additional, potentially relevant publications. Conference abstracts were also considered in our databases searches, and in such instances, we contacted the corresponding authors to acquire the data to enable inclusion.
Data extraction
To be included in the quantitative synthesis, studies had to provide data on risk estimates for any-type or specific CVD via Cox’s regression analysis, i.e. as hazard ratios (HRs), together with 95% confidence interval [95%CI]. When data were reported in another format rather than HRs (e.g. incidence rate, odds ratios), the corresponding author was contacted twice in a month period with a request for the HRs capturing the association between BMD/fractures with incident CVD.
Two authors (BS, MS) independently recorded data extracted from the selected studies into a standardized Microsoft Excel spreadsheet. Any disagreement was resolved by consensus or, if required, discussion with a third author (NV). The following information was extracted for each study: i) study characteristics (e.g. sample size, demographics, country in which the study was performed); ii) study setting; iii) follow-up (in years); iv) number and type of covariates used in the multivariate analyses; v) use of matching criteria; vi) methods of assessment of the exposure and outcome variables of interest (defined below).
When two groups were compared in each study (e.g. low vs. higher BMD or fractures vs. no fractures), the differences in prevalence of known CVD risk factors (diabetes, hypertension, obesity, smoking, previous CVD) were also extracted.
Outcomes
The primary outcome was the risk of any-type of CVD according to BMD status (evaluated in groups or as decrease in one standard deviation, SD) or presence of fractures (versus no fracture) at the baseline, assessed through HRs. Secondary outcomes included the risk for specific CVD, including: coronary heart disease (CHD), i.e. unstable angina and myocardial infarction; cerebrovascular disease (stroke and transient ischemic attack, TIA); heart failure, peripheral vascular disease (PVD); death due to CVD. Co-secondary outcomes were the pooled HRs by each site of fractures or to site of BMD assessment.
Assessment of study quality
The Newcastle-Ottawa Scale (NOS) (21) was used to evaluate the quality of included studies. The NOS assigns a maximum of 9 points to studies of highest quality according to three quality parameters: selection, comparability, and outcome.
Statistical analysis
A random effects meta-analysis was undertaken using Comprehensive Meta-Analysis (CMA) version 3 to account for the anticipated heterogeneity.(22) In primary analyses, pooled HRs and 95% CI were calculated to synthesize data from longitudinal studies. For each study, we included the HR adjusted for the highest number of covariates available. The summary HR with its 95% CI was calculated for the highest versus the lowest category of BMD values and for fractures vs. no fractures group. In secondary analyses, the same procedure was applied using specific CVD as outcome and by BMD/fracture site.
Study heterogeneity was assessed using the chi-squared and I-squared statistics, assuming that a p<0.05 for the former and a value ≥50% for the latter indicated a significant heterogeneity.(23) Whenever significant heterogeneity existed and ≥4 studies were available, a meta-regression analysis was performed examining the following pre-specified moderators: continent (Europe, Asia vs. others), setting (community-dwelling vs. others), use of a matching criterion between low and higher BMD or fracture versus no fracture (yes vs. no), assessment of BMD (categorized as DXA, QUS vs. other techniques) or fractures (self-reported vs. medical records), number of adjustments, follow-up period, mean age and percentage of women in the sample as whole. Where possible, differences in prevalence of possible CVD at the baseline were assessed as potential moderators.
Publication bias was assessed by visually inspection of funnel plots and using the Egger bias test.(24) When ≥3 studies were available, we used the Duval and Tweedie nonparametric trim-and-fill method to account for potential publication bias. Based on the assumption that the effect sizes of all the studies are normally distributed around the center of a funnel plot, in the event of asymmetries, this procedure adjusts for the potential effect of unpublished (trimmed) studies.(24)
Results
The search identified 5,642 non-duplicated, potentially eligible studies. After excluding 5,598 papers on the grounds of a review of their titles and abstracts, 45 full-text articles were examined. Of the full texts, 17 were excluded (mainly because they were reviews, not having meta-analyzable data or were duplicated study reports) (see Supplementary Table 2 for full details) and 28 articles (25–52) were finally included in our meta-analysis (Supplementary Figure 1).
Study and patient characteristics
As reported in Supplementary Table 3, the 28 studies (25–52) followed-up 1,107,885 participants for a median follow-up period of 5 (range: 2.25-22) years. The mean age of the participants was 65.3±8.0 years and they were predominantly women (64.4%). The median number of adjustments in the multivariate analyses was 8 (range: 0-13). In total, 18 studies included a matching criterion (mainly for sex and age) between cases and controls at the baseline.
The studies were mainly done in Asia (n=11), followed by Europe (n=9) and North America (n=5). Twenty-one studies were performed among community-dwellers.
Eighteen studies investigated BMD as exposure variable (participants = 127,031), whilst the other 10 the presence of fractures (participants = 980,854). BMD was analyzed through DXA in 12 studies, followed by QUS (n=2), insurance records (n=2), radioactive iodine source (n=1) and radiographic absorptiometry of the left hand (n=1). Regarding the site of fractures, hip fracture was the most commonly investigated (n=7), followed by vertebral fractures (n=2), and one study (46) explored numerous skeletal sites (any site, hip and vertebral fracture). Three studies reported the presence of fracture via self-report, three employed medical/insurance records, two used radiological data and, finally, two others recorded fracture following hospitalization due to the fracture (Supplementary Table 3).
None of the studies investigated the onset of CVD reported via self-report information. The most common CVD reported outcomes were CVD related death (n=13) followed by CHD (n=12) and cerebrovascular conditions (n=12).
Potential sources of bias and methodological quality
The median NOS score was 7, with a range between 4 and 9, indicating a sufficient quality of the studies included. The most common source of bias was the absence of sufficient information regarding the people lost during follow-up.
Findings regarding bone mineral density in groups and cardiovascular disease
The analyses regarding BMD were divided in studies reporting HRs for groups and those reporting estimates for a decrease in one SD in BMD.
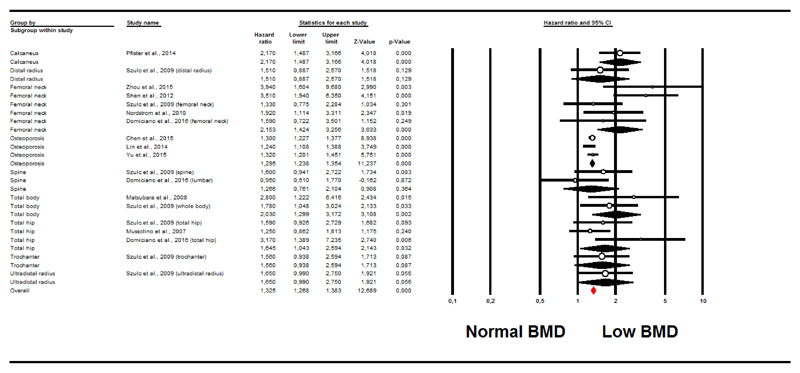
As reported in Figure 1, taking people with higher BMD as the reference group, people with lower BMD had an increased risk of developing CVD during follow-up (11 studies (28,31,35,36,38–40,42,43,51,52); HR=1.33; 95%CI: 1.27-1.38; p<0.0001; I2=53%). The trim and fill analysis reported an HR of 1.32 (95%CI: 1.18-1.48), after adjusting for 8 studies to the left of the mean.
Figure 1. Forrest plot of the association between low bone mineral density at baseline and incident cardiovascular diseases.
a. Subgroup analysis of bone site and cardiovascular disease
The association between low BMD and CVD incidence was significantly increased for studies investigating BMD at the calcaneus, femoral neck, total hip, total body and using a diagnosis of osteoporosis for defining low BMD, whilst no significant association emerged for the other sites included (p for interaction=0.02).
b. Subgroup analysis regarding bone mineral density and specific cardiovascular disease and mortality
Low BMD was associated with a higher risk of CHD (3 studies (28,42,51); HR=1.31; 95%CI: 1.25-1.38; p<0.0001; I2=82%), cerebrovascular conditions (5 studies (35,39,42,51,52); HR= 1.22; 95%CI: 1.12-1.34; p<0.0001; I2=83%) and death due to CVD reasons (3 studies (31,36,38); HR=1.52; 95%CI: 1.04-2.22; p=0.03; I2=52%). However, the trim and fill analysis nullified the association between low BMD with cerebrovascular diseases and death due to CVD, suggesting that publication bias may influence these results (Table 1). Finally, one study (51) reported that low BMD was associated with a significant higher risk of heart failure of 26% (95%CI: 1.10-1.43, p=0.001).
Table 1. Adjusted hazard ratios taking low bone mineral density as exposure and specific cardiovascular disease as outcome with publication bias assessment.
| Analysis | Number of studies | Meta-analysis | Heterogeneity | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | I2 | Egger bias & p-value | Adjusted risk estimates* (95% CI) [trimmed] | |||
| CHD | ||||||||
| Femoral neck | 1 | 3.51 | 1.94 | 6.35 | <0.0001 | - | ||
| Osteoporosis | 2 | 1.31 | 1.24 | 1.37 | <0.0001 | - | ||
| Overall | 3 | 1.31 | 1.25 | 1.38 | <0.0001 | 82 | 3.33; 0.18 | Unchanged |
| Stroke + TIA | ||||||||
| Femoral neck | 3 | 2.79 | 1.77 | 4.41 | <0.0001 | 31 | ||
| Osteoporosis | 2 | 1.18 | 1.08 | 1.29 | <0.0001 | 28 | ||
| Overall | 5 | 1.22 | 1.12 | 1.34 | <0.0001 | 83 | 3.22; 0.02 | 1.26 (0.95-1.71) [2] |
| Death for CVD | ||||||||
| Femoral neck | 1 | 1.59 | 0.72 | 3.50 | 0.25 | - | ||
| Spine | 1 | 0.95 | 0.51 | 1.77 | 0.87 | - | ||
| Total body | 1 | 2.80 | 1.22 | 6.42 | 0.02 | - | ||
| Total hip | 2 | 1.85 | 0.75 | 4.54 | 0.18 | 76 | ||
| Overall | 3 | 1.52 | 1.04 | 2.22 | 0.03 | 52 | 2.57; 0.22 | 1.22 (0.76-1.94) [2] |
Bold values represent significant results, as p-value <0.05
Abbreviations: CHD: coronary heart disease; CI: confidence intervals; CVD, cardiovascular diseases; HR, hazard ratio; TIA, transient ischemic attack.
Test for publication bias: Duval and Tweedie nonparametric trim-and-fill procedure.
Decrease in bone mineral density and cardiovascular disease
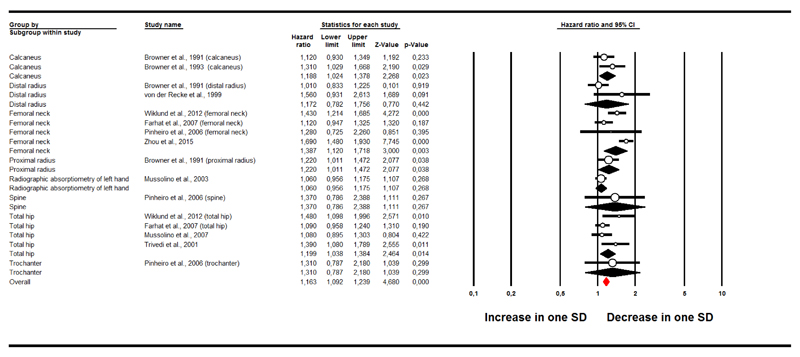
Figure 2 shows the association between a decrease in one SD in BMD at baseline and incident CVD. Each decrease in one SD of BMD corresponded to an increased risk of CVD at follow-up of 16% (9 studies, participants = 29,764 (32,38,41,44,47,49,52–54); HR=1.16; 95%CI: 1.09-1.24; p<0.0001; I2=69%). After adjusting for publication bias and trimming 7 studies to the left of the mean, the adjusted HR was 1.11 (95%CI: 1.00-1.25).
Figure 2. Forrest plot of the association between one decrease in standard deviation in bone mineral density at baseline and incident cardiovascular diseases.
a. Subgroup analysis of bone site and cardiovascular disease
The association with incident CVD was significant for BMD assessed at calcaneus, femoral neck and total hip. Table 2 shows the association between a decrease in one SD in BMD at baseline and the onset of specific CVD at follow-up.
Table 2. Adjusted hazard ratios taking one decrease in standard deviation in bone mineral density as exposure and specific cardiovascular disease as outcome with publication bias assessment.
| Analysis | Number of studies | Meta-analysis | Heterogeneity | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | I2 | Egger bias & p-value | Adjusted risk estimates* (95% CI) [trimmed] | |||
| CHD | ||||||||
| Femoral neck | 1 | 1.43 | 1.21 | 1.69 | <0.0001 | - | ||
| Total hip | 1 | 1.48 | 1.10 | 2.00 | <0.0001 | - | ||
| Overall | 1 | 1.44 | 1.25 | 1.66 | <0.0001 | 0 | Only two studies available | |
| Stroke + TIA | ||||||||
| Calcaneus | 1 | 1.31 | 1.03 | 1.67 | 0.03 | - | ||
| Femoral neck | 1 | 1.69 | 1.48 | 1.93 | <0.0001 | - | ||
| Radiographic | 1 | 1.03 | 0.92 | 1.16 | 0.62 | - | ||
| Overall | 3 | 1.28 | 1.18 | 1.39 | <0.0001 | 93 | 2.80; 0.85 | Unchanged |
| Death for CVD | ||||||||
| Calcaneus | 1 | 0.99 | 0.78 | 1.26 | 0.94 | - | ||
| Distal radius | 2 | 1.15 | 0.71 | 1.87 | 0.58 | 66 | ||
| Femoral neck | 2 | 1.67 | 1.46 | 1.90 | <0.0001 | 0 | ||
| Proximal radius | 1 | 1.17 | 0.91 | 1.50 | 0.21 | - | ||
| Radiographic | 1 | 1.09 | 0.99 | 1.20 | 0.08 | - | ||
| Spine | 1 | 1.37 | 0.79 | 2.39 | 0.27 | - | ||
| Total hip | 1 | 1.08 | 0.90 | 1.30 | 0.30 | - | ||
| Trochanter | 1 | 1.31 | 0.79 | 2.18 | 0.30 | - | ||
| Overall | 7 | 1.22 | 1.14 | 1.30 | <0.0001 | 77 | 0.08; 0.95 | 1.07 (0.90-1.29) [4] |
Bold values represent significant results, as p-value <0.05
Abbreviations: CHD: coronary heart disease; CI: confidence intervals; CVD, cardiovascular diseases; HR, hazard ratio; TIA, transient ischemic attack.
Test for publication bias: Duval and Tweedie nonparametric trim-and-fill procedure
b. Subgroup analysis regarding reduction in bone mineral density and specific cardiovascular disease and mortality
Each decrease in one SD of BMD at baseline corresponded to an increase risk of CHD at follow-up of 44% (1 study with two estimates (49); HR=1.44; 95%CI: 1.25-1.66; p<0.0001; I2=0%), of stroke and TIA of 28% (3 studies (25,37,52); HR=1.28; 95%CI: 1.18-1.39; p<0.0001; I2=93%) and death for CVD reasons of 22% (6 studies (25,37,38,41,47,52); HR=1.22; 95%CI: 1.14-1.30; p<0.0001; I2=77%). However, the trim and fill analysis nullified this last association (Table 2).
Meta-analysis of the relationship between fractures and cardiovascular disease
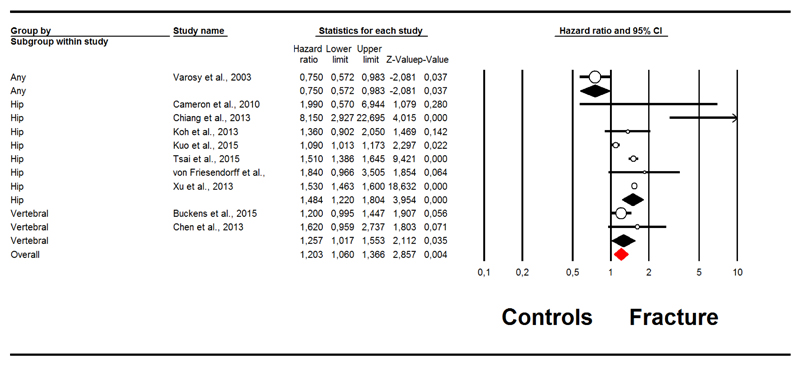
Ten studies (26,27,29,30,33,45,46,48,50,55) reported the association between fractures and the onset of CVD at follow-up. As shown in Figure 3, the presence of fractures at baseline was associated with an increased risk of CVD (HR=1.20; 95%CI: 1.06-1.37; p=0.004; I2=91%). After trimming 3 studies at the left of the mean, the adjusted HR was 1.25 (95%CI: 1.05-1.48). In a sensitivity analysis, after removing a study including the majority of the people of this meta-analysis (50), the HR was 1.33 (95%CI: 1.08-1.64) with an I2=87%. This outcome was not affected by publication bias and the updated trim and fill analysis did not modify these findings.
Figure 3. Forrest plot of the association between fractures at baseline and incident cardiovascular diseases.
a. Site of fractures and cardiovascular disease
The presence of hip (HR=1.48; 95%CI: 1.22-1.80; p<0.0001; I2=85%) and vertebral fractures (HR=1.26; 95%CI: 1.02-1.55; p=0.04; I2=65%) significantly increased the risk of CVD, whilst one study (46) reported that the presence of any fracture reduced the risk of incident CVD in people hospitalized for CHD.
b. Fractures and type of cardiovascular disease
As reported in Table 3, the presence of fractures increased the risk of both cerebrovascular conditions (4 studies (27,33,50,55); HR=1.47; 95%CI: 1.42-1.54; p<0.0001; I2=93%) and death for CVD (3 studies (29,30,48); HR=1.78; 95%CI: 1.09-2.91; p=0.02; I2=75%), but not of CHD. Publication bias did alter these findings. Finally, one study reported that people with fractures had a similar chance to develop PVD in one study (HR=1.06; 95%CI: 0.91-1.23, p=0.44).(34)
Table 3. Adjusted hazard ratios taking fracture as exposure and specific cardiovascular disease as outcome with publication bias assessment.
| Analysis | Number of studies | Meta-analysis | Heterogeneity | Publication bias | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | I2 | Egger bias & p-value | Adjusted risk estimates* (95% CI) [trimmed] | |||
| CHD | ||||||||
| Hip | 4 | 1.20 | 0.97 | 1.48 | 0.10 | 95 | ||
| Any | 1 | 0.75 | 0.57 | 0.98 | 0.037 | - | ||
| Overall | 5 | 1.00 | 0.85 | 1.19 | 0.97 | 94 | 0.35; 0.94 | Unchanged |
| Stroke + TIA | ||||||||
| Hip | 4 | 1.47 | 1.42 | 1.54 | <0.0001 | 97 | ||
| Overall | 4 | 1.47 | 1.42 | 1.54 | <0.0001 | 97 | -1.81; 0.77 | 1.47 (1.41-1.53) [1] |
| Death for CVD | ||||||||
| Hip | 2 | 3.66 | 0.86 | 15.69 | 0.08 | 83 | ||
| Vertebral | 1 | 1.62 | 0.96 | 2.74 | 0.07 | - | ||
| Overall | 3 | 1.78 | 1.09 | 2.91 | 0.02 | 75 | 6.27; 1.47 | Unchanged |
Bold values represent significant results, as p-value <0.05
Abbreviations: CHD: coronary heart disease; CI: confidence intervals; CVD, cardiovascular diseases; HR, hazard ratio; TIA, transient ischemic attack.
Test for publication bias: Duval and Tweedie nonparametric trim-and-fill procedure.
Meta-regression and sensitivity analyses
The meta regression analyses are reported in Supplementary Table 5 for moderators treated as continuous variables. We were not able to identify any significant moderator of the heterogeneity for the BMD analyses. However, when considering fractures as the exposure, the number of adjustments in the multivariate analyses was negatively associated with the incidence of CVD (beta: -0.07; 95%CI: -0.13 to -0.01), although this parameter did not explain any heterogeneity (R2=0.00) (Supplementary Table 5).
Supplementary Table 6 shows a stratification of our analyses for potential categorical moderators. Regarding BMD, studies made in Europe and in Asia reported a stronger association with incident CVD than studies made outside with a concomitant reduction in heterogeneity for the European researches (I2=0 for BMD treated as groups or SD). A similar finding was evident for studies using a matching criterion between participants with low and higher BMD (p for interaction=0.002) and for studies assessing BMD with DXA (p=0.001 for groups and =0.04 for decrease in SD). On the contrary, none of these moderators was able to explain the heterogeneity of the findings regarding fractures (Supplementary Table 6).
Discussion
In this meta-analysis including a total of 28 longitudinal studies and 1,107,885 participants, we found evidence that low BMD and fractures were associated with a modest increased risk of CVD. Specifically, low BMD (particularly of the lower limbs) were associated with a little increased risk of developing CHD and cerebrovascular conditions and the presence of fractures at the baseline limitedly increased the risk of cerebrovascular conditions. Moreover, some publication bias was evident (which we adjusted for) and as anticipated from pooling of observational data, we encountered some heterogeneity which we were only able to partially explain with meta regression. In addition, whilst the pooled weight of all studies demonstrated that poor bone health and fracture are typically associated with an increased risk of CVD, some studies did not find such a relationship (25–27,29,31–33,37,41,43,47,48) and one found that osteoporotic fractures protect against the onset of CVD (46) .
Whilst the precise mechanisms that may underpin the relationship between low BMD and CVD are yet to be fully elucidated, this relationship may potentially be explained by some common pathways, although these are at this stage speculative. For instance, alterations in signaling pathways that are common to normal bone remodeling and arterial calcifications, such as bone morphogenetic proteins, osteocalcin and matrix Gla proteins may play a role. (4,5) However, these factors have only been illustrated to be important in animal and in vitro models, thus clearly future research is needed to explore this theory in human beings. Another factor that may account for the increased risk of CVD among people with low BMD might be low-grade inflammation that seems to play a role in the development of low BMD (56) and could contribute to the higher CVD risk observed in these people. (57) Some literature suggests that the presence of vascular calcifications may be the most important factor explaining the association between low BMD and CVD. Specifically, subjects with low BMD have a higher prevalence of vascular calcifications and so of sub-clinical atherosclerosis. (6–9) Vascular calcifications are the result of an active and complex process involving numerous pathways leading to the calcium depositions in arterial walls.(58) These deposition may lead to increase in arterial stiffness and in pulse wave velocity, that seem to be relevant in the development of clinical and sub-clinical cardiovascular events since the calcification of vessels reduces their elasticity and negatively affect the hemodynamic parameters of the cardiovascular system.(59) Other risk factors common to both low BMD and CVD (such as low estrogens levels, low physical activity and obesity) may play an additional role in explaining our findings. (5) Another potential hypothesis that may explain our results is that people with poor bone health may have been more frail. Unfortunately there is insufficient data to explore this hypothesis and future research may wish to consider the impact of frailty status on CVD risk.
The relationship between fractures and CVD may be explained by the mechanisms that were elaborated as potential mechanisms due to low BMD. In addition, a reduction in physical activity after a fracture (particularly at the hip) is common (60) and is a key risk factor for CVD. (61–64) Whether or not increasing physical activity can potentially reduce the risk of CVD in people with fractures is a potentially important future research question. In addition, a recent meta-analysis demonstrated a potential dose response relationship between physical activity and reduced fracture risk.(65) Therefore, another important future research question is to explore whether increasing physical activity among people with low BMD may reduce the risk of fractures and subsequent CVD. Another potential factor could be the possible relationship between analgesic medications which have unfavorable cardiovascular profile. (66) Finally, a recent study (currently in abstract form) of almost 500,000 individuals in the UK Biobank cohort has suggested sex-specific relationships between a history of fracture and incident cardiovascular disease.(67) Thus, the hazard ratio for hospital admission with CHD following a history of prior osteoporotic fracture was 1.75 (95%CI: 1.23, 2.49) in men even after full adjustment. Although an association was present for women in the crude models, it was not robust after adjustment for a range of covariates. The reasons underlying these apparent sex differences remain, however, to be elucidated.
Regarding specific CVD outcomes, our meta-analysis suggests that low BMD and fractures are associated with a higher incidence of CHD, cerebrovascular diseases and death due to CVD. Our knowledge regarding these topics is limited to a previous meta-analysis regarding reporting that low BMD was associated with a higher risk of CVD death, but not due to stroke.(15) We can hypothesize that the few number of studies included in their meta-analysis regarding stroke mortality (only four) could limit this association, whilst in our meta-analysis we included eight studies. On the contrary, no other meta-analyses were available regarding fractures. Altogether our findings suggest that both low BMD and fractures promote the onset of cardiovascular atherosclerotic diseases, even if regarding the specific CVD outcomes further research is needed.
Our data support the concept that low BMD and fractures are CVD risk factors, independent of other known conditions. Since both low BMD and fractures are common among older people and in particular women, our meta-analysis suggests that people with low BMD/fractures may benefit from screening and interventions to minimize the risk of CVD. It is known that after menopause CVD risk increases in women, being comparable to men. For example in a large meta-analysis diabetic women had a greater risk of stroke than men.(68) These differences may be explained by potential differences in diagnosis and treatment of CVD, since it is known in some countries men at higher risk of CVD were diagnosed earlier and more often treated pharmacologically than women.(69) Therefore, our work reinforces again the concept to screen and treat older women from a cardiovascular point of view, since after menopause they probably mature some other CVD risk factors, differently from men. Given the larger literature considering the benefits of physical activity for fracture prevention and cardiovascular disease (70), our data adds to the growing importance of promoting active lifestyles. However, for some people with low BMD who may be at risk of falls, a more structured exercise program overseen by an exercise professional such as a physiotherapist may minimize future falls and fracture risk. (70)
Unfortunately, although as expected when combining observational data (71), many of the outcomes included reported a high degree of heterogeneity. In order to investigate what may have accounted for this heterogeneity, we conducted meta regression analyses and we were not able to identify any variable or characteristic that may have influence the outcomes involving BMD. However, the meta regression for the outcome of fractures revealed that studies adjusting for a higher number of covariates seemed to be important, although this clinical importance is limited. In this context, the studies conducted in Europe reported a low degree of heterogeneity. The reasons for this finding are not known, but it is possible that the homogeneity of populations included may play a role.
The findings of our meta-analysis must be considered within its limitations. First, some secondary outcomes appear to be affected by a publication bias. Even though we included papers written in languages other than English and conference abstracts in our search, the trim and fill analysis (a method of adjusting for effect size to account for potential missing studies, i.e. publication bias) suggested that publication bias could be considered a relevant issue for our secondary outcomes. Second, we were not able to explore some important moderators in our analysis, particularly the use of anti-resorptive medications, which could play a role in the association between low BMD/fractures and CVD. (72) Third, the large majority of the participants were represented in one study.(50) Fourth, some studies were excluded due to the lack of meta-analyzable data and the authors did not respond to our requests. Even if this could create a further bias in our findings, the direction of this is hard to determine. Finally, only one study was available for heart failure and peripheral vascular disease, respectively, even if they are very common diseases in middle-aged and older populations.
In conclusion, low BMD and fractures appear to be associated with a higher risk (albeit of small-modest effect size) of future CVD. Whilst the data suggests a relationship, one should note there were some sources of potential bias in the literature. Nonetheless, since CVD events could accelerate the transition of people with bone diseases to greater disability and mortality, our meta-analysis suggests it may be important to consider the cardiovascular health of people with osteoporosis and fractures. Future studies are required to evaluate if addressing low BMD and fractures could positively influence CV outcomes. Such studies should ensure matching between cases and controls and attempt to disentangle underlying pathophysiological and behavioral (e.g. physical activity, nutrition) mechanisms, which could potentially be targets for future preventative interventions.
Supplementary Material
Additional Supporting Information may be found in the online version of this article.
Acknowledgements
Funding
No direct funding was available for this article.
Footnotes
Authorship statement: All the authors 1) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2) participated in drafting the manuscript or revising it critically for important intellectual content; 3) approved the final version of the submitted manuscript, and 4) agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Specific roles of the authors: Conception and design: Veronese, Stubbs. Analysis and interpretation of the data: Solmi, Veronese, Stubbs, Crepaldi. Draft of the manuscript: Veronese, Cooper, Harvey, Reginster. Critical revision: Stubbs, Rizzoli, Civitelli, Schofield, Maggi, Lamb. All the authors approved the final version of the submitted manuscript.
This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as doi: [10.1002/jbmr.3089]
Conflict of interest
None to declare from any author.
References
- 1.Reginster JY, Burlet N. Osteoporosis: A still increasing prevalence. Bone. 2006;38 doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 2.Boonen S, Dejaeger E, Vanderschueren D, Venken K, Bogaerts A, Verschueren S, et al. Osteoporosis and osteoporotic fracture occurrence and prevention in the elderly: a geriatric perspective. Best Practice and Research: Clinical Endocrinology and Metabolism. 2008;22:765–85. doi: 10.1016/j.beem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Correction Naghavi M, Wang H, Lozano R, Davis A, Liang X, Zhou M, et al. Lancet. 9963. Vol. 385. Elsevier Ltd; 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013; pp. 117–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lello S, Capozzi A, Scambia G. Osteoporosis and cardiovascular disease: an update. Gynecol Endocrinol. 2015;31(8):590–4. doi: 10.3109/09513590.2015.1041908. Available from: http://www.tandfonline.com/doi/full/10.3109/09513590.2015.1041908. [DOI] [PubMed] [Google Scholar]
- 5.Lampropoulos CE, Papaioannou I, David P, Cruz D. Nat Rev Rheumatol. 10. Vol. 8. Nature Publishing Group; 2012. Osteoporosis — a risk factor for cardiovascular; pp. 587–98. Available from: http://dx.doi.org/10.1038/nrrheum.2012.120. [DOI] [PubMed] [Google Scholar]
- 6.Adragao T, Herberth J, Monier-Faugere M-C, Branscum AJ, Ferreira A, Frazao JM, et al. Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):450–5. doi: 10.2215/CJN.01870408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SISH, An JH, Lim S, Koo BK, Park SE, Chang HJ, et al. Lower bone mineral density is associated with higher coronary calcification and coronary plaque burdens by multidetector row coronary computed tomography in pre- and postmenopausal women. Clin Endocrinol (Oxf) 2009;71(5):644–51. doi: 10.1111/j.1365-2265.2009.03535.x. [DOI] [PubMed] [Google Scholar]
- 8.Hyder JA, Allison MA, Barrett-Connor E, Detrano R, Wong ND, Sirlin C, et al. Bone mineral density and atherosclerosis: The Multi-Ethnic Study of Atherosclerosis, Abdominal Aortic Calcium Study. Atherosclerosis. 2010;209(1):283–9. doi: 10.1016/j.atherosclerosis.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fusaro M, Crepaldi G, Maggi S, Galli F, D’Angelo A, Calò L, et al. Vitamin K, bone fractures, and vascular calcifications in chronic kidney disease: An important but poorly studied relationship. Journal of Endocrinological Investigation. 2011;34:317–23. doi: 10.1007/BF03347093. [DOI] [PubMed] [Google Scholar]
- 10.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15(12):2959–64. doi: 10.1097/01.ASN.0000145894.57533.C4. Available from: http://jasn.asnjournals.org/content/15/12/2959.full.pdf. [DOI] [PubMed] [Google Scholar]
- 11.Dalmeijer GW, Van Der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WMM, Boer JMA, et al. Matrix gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36(11):3766–71. doi: 10.2337/dc13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyce BF, Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5(3):98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Song Y, Manson JE, Pilz S, März W, Michaëlsson K, et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circulation. 2012;5(6):819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ballegooijen AJ, Reinders I, Visser M, Brouwer IA. Parathyroid hormone and cardiovascular disease events: A systematic review and meta-analysis of prospective studies. [cited 2015 Oct 4];Am Heart J. 2013 May;165(5):655–64. 664–5. doi: 10.1016/j.ahj.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Qu X, Huang X, Jin F, Wang H, Hao Y, Tang T, et al. Int J Cardiol. 2. Vol. 166. Elsevier Ireland Ltd; 2013. Bone mineral density and all-cause, cardiovascular and stroke mortality: A meta-analysis of prospective cohort studies; pp. 385–93. [DOI] [PubMed] [Google Scholar]
- 16.Fisher A, Srikusalanukul W, Davis M, Smith P. Cardiovascular diseases in older patients with osteoporotic hip fracture: Prevalence, disturbances in mineral and bone metabolism, and bidirectional links. Clin Interv Aging. 2013;8:239–56. doi: 10.2147/CIA.S38856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sennerby U, Melhus H, Gedeborg R, et al. CArdiovascular diseases and risk of hip fracture. JAMA. 2009 Oct 21;302(15):1666–73. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guildelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkwood B, Sterne J. Medical statistics. Med Stat. 2003;513 Available from: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Medical+statistics#2. [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012;2012 (Available from URL http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp) [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997 Sep;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Study of Osteoporotic Fractures Research Group. Lancet. 1991;338(8763):355–8. doi: 10.1016/0140-6736(91)90489-c. [DOI] [PubMed] [Google Scholar]
- 26.Buckens CF, de Jong PA, Verkooijen HM, Verhaar HJ, Mali WP, van der Graaf Y. Vertebral fractures on routine chest computed tomography: relation with arterial calcifications and future cardiovascular events. Int J Cardiovasc Imaging. 2015;31(2):437–45. doi: 10.1007/s10554-014-0567-9. [DOI] [PubMed] [Google Scholar]
- 27.Cameron ID, Chen JS, March LM, Simpson JM, Cumming RG, Seibel MJ, et al. Hip Fracture Causes Excess Mortality Due to Cardiovascular and Infectious Disease in Institutionalized Older People: A Prospective Five-Year Study. J Bone Miner Res. 2009;25(4) doi: 10.1359/jbmr.091029. 091019190442039–22. [DOI] [PubMed] [Google Scholar]
- 28.Chen S-J, Lin C-S, Lin C-L, Kao C-H. Osteoporosis Is Associated With High Risk for Coronary Heart Disease: A Population-Based Cohort Study. Medicine (Baltimore) 2015;94(27):e1146. doi: 10.1097/MD.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y-C, Wu J-C, Liu L, Huang W-C, Cheng H, Chen T-J, et al. Hospitalized osteoporotic vertebral fracture increases the risk of stroke: A population-based cohort study. J Bone Miner Res. 2013;28(3):516–23. doi: 10.1002/jbmr.1722. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C-H, Liu C-J, Chen P-J, Huang C-C, Hsu C-Y, Chen Z-Y, et al. Hip fracture and risk of acute myocardial infarction: a nationwide study. J Bone Miner Res. 2013;28(2):404–11. doi: 10.1002/jbmr.1714. [DOI] [PubMed] [Google Scholar]
- 31.Domiciano DS, Machado LG, Lopes JB, Figueiredo CP, Caparbo VF, Oliveira RM, et al. Bone Mineral Density and Parathyroid Hormone as Independent Risk Factors for Mortality in Community-Dwelling Older Adults: A Population-Based Prospective Cohort Study in Brazil. The São Paulo Ageing & Health (SPAH) Study. J Bone Miner Res. 2016;31(6):1146–57. doi: 10.1002/jbmr.2795. [DOI] [PubMed] [Google Scholar]
- 32.Farhat GN, Newman AB, Sutton-Tyrrell K, Matthews KA, Boudreau R, Schwartz AV, et al. The association of bone mineral density measures with incident cardiovascular disease in older adults. Osteoporos Int. 2007;18(7):999–1008. doi: 10.1007/s00198-007-0338-8. [DOI] [PubMed] [Google Scholar]
- 33.Koh GCH, Tai BC, Ang LW, Heng D, Yuan JM, Koh WP. All-cause and cause-specific mortality after hip fracture among Chinese women and men: The Singapore Chinese Health Study. Osteoporos Int. 2013;24(7):1981–9. doi: 10.1007/s00198-012-2183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo CH, Hsieh TC, Wang CH, Chou CL, Lai YH, Chen YY, et al. Increased risks of mortality and atherosclerotic complications in incident hemodialysis patients subsequently with bone fractures: A nationwide case-matched cohort study. PLoS One. 2015;10(4):1–13. doi: 10.1371/journal.pone.0121705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin CH, Chang WC, Kuo CN, Yu HC, Yang CC, Lin YW, et al. Bone. Vol. 72. Elsevier Inc; 2015. A population-based five-year study on the risk of stroke in patients with osteoporosis in Taiwan; pp. 9–13. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara K, Suliman ME, Qureshi AR, Axelsson J, Martola L, Heimbürger O, et al. Bone mineral density in end-stage renal disease patients: Association with wasting, cardiovascular disease and mortality. Blood Purif. 2008;26(3):284–90. doi: 10.1159/000126925. [DOI] [PubMed] [Google Scholar]
- 37.Mussolino ME, Madans JH, Gillum RF. Bone mineral density and stroke. Stroke. 2003;34(5):E20–2. doi: 10.1161/01.STR.0000065826.23815.A5. [DOI] [PubMed] [Google Scholar]
- 38.Mussolino ME, Armenian HK. Low Bone Mineral Density, Coronary Heart Disease, and Stroke Mortality in Men and Women: The Third National Health and Nutrition Examination Survey. Ann Epidemiol. 2007;17(11):841–6. doi: 10.1016/j.annepidem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Nordström A, Eriksson M, Stegmayr B, Gustafson Y, Nordström P. Low Bone Mineral Density Is an Independent Risk Factor for Stroke and Death. Cerebrovasc Dis. 2010;29:130–6. doi: 10.1159/000262308. Available from: www.karger.com. [DOI] [PubMed] [Google Scholar]
- 40.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw KT. Low Bone Mineral Density Predicts Incident Heart Failure in Men and Women. The EPIC (European Prospective Investigation Into Cancer and Nutrition)-Norfolk Prospective Study. JACC Hear Fail. 2014;2(4):380–9. doi: 10.1016/j.jchf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Pinheiro MM, Castro CM, Szejnfeld VL. Low femoral bone mineral density and quantitative ultrasound are risk factors for new osteoporotic fracture and total and cardiovascular mortality: A 5-year population-based study of Brazilian elderly women. Journals Gerontol Ser a-Biological Sci Med Sci. 2006;61(2):196–203. doi: 10.1093/gerona/61.2.196. [DOI] [PubMed] [Google Scholar]
- 42.Shen C, Deng J, Zhou R, Chen J, Fan S, Li Z, et al. Relation between bone mineral density, bone loss and the risk of cardiovascular disease in a Chinese cohort. Am J Cardiol. 2012;110(8):1138–42. doi: 10.1016/j.amjcard.2012.05.053. Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=22762713. [DOI] [PubMed] [Google Scholar]
- 43.Szulc P, Samelson EJ, Kiel DP, Delmas PD. Increased bone resorption is associated with increased risk of cardiovascular events in men: the MINOS study. J Bone Miner Res. 2009;24(12):2023–31. doi: 10.1359/JBMR.090531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trivedi DP, Khaw KT. Bone mineral density at the hip predicts mortality in elderly men. Osteoporos Int. 2001;12(4):259–65. doi: 10.1007/s001980170114. [DOI] [PubMed] [Google Scholar]
- 45.Tsai C-H, Lin C-L, Hsu H-C, Chung W-S. Increased risk of coronary heart disease in patients with hip fracture: a nationwide cohort study. Osteoporos Int. 2015;26(6):1849–55. doi: 10.1007/s00198-015-3097-y. [DOI] [PubMed] [Google Scholar]
- 46.Varosy PD, Shlipak MG, Vittinghoff E, Black DM, Herrington D, Hulley SB, et al. Fracture and the risk of coronary events in women with heart disease. Am J Med. 2003;115(3):196–202. doi: 10.1016/s0002-9343(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 47.von der Recke P, Hansen MA, Hassager C. The association between low bone mass at the menopause and cardiovascular mortality. Am J Med. 1999;106(3):273–8. doi: 10.1016/s0002-9343(99)00028-5. Available from: http://www.sciencedirect.com/science/article/pii/S0002934399000285. [DOI] [PubMed] [Google Scholar]
- 48.von Friesendorff M, McGuigan FE, Wizert A, Rogmark C, Holmberg AH, Woolf AD, et al. Osteoporos Int. Osteoporosis International; 2016. Hip fracture, mortality risk, and cause of death over two decades. [DOI] [PubMed] [Google Scholar]
- 49.Wiklund P, Nordström a, Jansson J-H, Weinehall L, Nordström P. Low bone mineral density is associated with increased risk for myocardial infarction in men and women. Osteoporos Int. 2012;23(3):963–70. doi: 10.1007/s00198-011-1631-0. [DOI] [PubMed] [Google Scholar]
- 50.Xu B, Han L, Liu H, Wang J, Bao XY, Xi HX, et al. Cardiovascular disease and hip fracture among older inpatients in Beijing, China. Biomed Res Int. 2013;2013 doi: 10.1155/2013/493696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu T-M, Lin C-L, Shu K-H, Liu Y-L, Chen C-H, Huang S-T, et al. Increased risk of cardiovascular events in end-stage renal disease patients with osteoporosis: a nationwide population-based cohort study. Osteoporos Int. 2014;26(2):785–93. doi: 10.1007/s00198-014-2982-0. [DOI] [PubMed] [Google Scholar]
- 52.Zhou R, Liu D, Li R, Zhou S, Cui M, Chen L, et al. Cell Biochem Biophys. 3. Vol. 71. Springer US; 2015. Low Bone Mass is Associated with Stroke in Chinese Postmenopausal Women: The Chongqing Osteoporosis Study; pp. 1695–701. [DOI] [PubMed] [Google Scholar]
- 53.Browner WS, Pressman AR, Nevitt MC, Cauley JA, Cummings SR. Association between low bone density and stroke in elderly women. The study of osteoporotic fractures. Stroke. 1993;24(7):940–6. doi: 10.1161/01.str.24.7.940. [DOI] [PubMed] [Google Scholar]
- 54.Mussolino ME, Looker AC, Madans JH, Langlois JA, Orwoll ES. Risk factors for hip fracture in white men: the NHANES I Epidemiologic Follow-up Study. J Bone Miner Res. 1998 Jun;13(6):918–24. doi: 10.1359/jbmr.1998.13.6.918. [DOI] [PubMed] [Google Scholar]
- 55.Liu L-K, Lee W-J, Chen L-Y, Hwang A-C, Lin M-H, Peng L-N, et al. Association between Frailty, Osteoporosis, Falls and Hip Fractures among Community-Dwelling People Aged 50 Years and Older in Taiwan: Results from I-Lan Longitudinal Aging Study. PLoS One. 2015;10(9):e0136968. doi: 10.1371/journal.pone.0136968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ilich JZ, Kelly OJ, Kim Y, Spicer MT. Low-grade chronic inflammation perpetuated by modern diet as a promoter of obesity and osteoporosis. Arch Ind Hyg Toxicol. 2014;65(2):139–48. doi: 10.2478/10004-1254-65-2014-2541. [DOI] [PubMed] [Google Scholar]
- 57.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321(7255):199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. 2012;18(1):RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Research. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O’Halloran PD, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil. 2014;28(12):1159–71. doi: 10.1177/0269215514536210. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=a9h&AN=99360044&site=ehost-live%5Cnhttp://cre.sagepub.com/content/28/12/1159.full.pdf. [DOI] [PubMed] [Google Scholar]
- 61.Dumurgier J, Elbaz A, Ducimetière P, Tavernier B, Alpérovitch A, Tzourio C. Slow walking speed and cardiovascular death in well functioning older adults: prospective cohort study. [cited 2015 Oct 7];BMJ. 2009 Jan;339:b4460. doi: 10.1136/bmj.b4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sergi G, Veronese N, Fontana L, De Rui M, Bolzetta F, Zambon S, et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: the pro.v.a. study. [cited 2015 Sep 25];J Am Coll Cardiol. 2015 Mar 17;65(10):976–83. doi: 10.1016/j.jacc.2014.12.040. United States. [DOI] [PubMed] [Google Scholar]
- 63.Cheng S-J, Yu H-K, Chen Y-C, Chen C-Y, Lien W-C, Yang P-Y, et al. Physical Activity and Risk of Cardiovascular Disease Among Older Adults. Int J Gerontol. 2013;7(3):133–6. Available from: http://www.sciencedirect.com/science/article/pii/S1873959813000379. [Google Scholar]
- 64.Shiroma EJ, Lee I-M. Physical Activity and Cardiovascular Health. Circulation. 2010;122(7) doi: 10.1161/CIRCULATIONAHA.109.914721. [DOI] [PubMed] [Google Scholar]
- 65.Rong K, Liu X, Wu X, Li X, Xia Q, Chen J, et al. Increasing Level of Leisure Physical Activity Could Reduce the Risk of Hip Fracture in Older Women: A Dose-Response Meta-analysis of Prospective Cohort Studies. [cited 2016 Sep 25];Medicine (Baltimore) 2016 Mar;95(11):e2984. doi: 10.1097/MD.0000000000002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. doi: 10.1136/bmj.c7086. jan11_1, Available from: http://www.bmj.com/content/342/bmj.c7086.full.pdf+html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paccou Julien, D’Angelo Stefania, Edwards Mark, Cooper Cyrus, Petersen Steffen E, Harvey NC. A fragility fracture increases the risk of incident cardiovascular events in men: Findings from UK Biobank. Osteoporosis International. 2016:S59–60. doi: 10.1007/s00198-018-4426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. [cited 2015 Sep 7];Lancet (London, England) 2014 Jun 7;383(9933):1973–80. doi: 10.1016/S0140-6736(14)60040-4. [DOI] [PubMed] [Google Scholar]
- 69.Krämer HU, Raum E, Rüter G, Schöttker B, Rothenbacher D, Rosemann T, et al. Gender disparities in diabetes and coronary heart disease medication among patients with type 2 diabetes: results from the DIANA study. [cited 2015 Oct 9];Cardiovasc Diabetol. 2012 Jan;11:88. doi: 10.1186/1475-2840-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stubbs B, Brefka S, Denkinger MD. What Works to Prevent Falls in Community-Dwelling Older Adults? Umbrella Review of Meta-analyses of Randomized Controlled Trials. [cited 2016 Sep 25];Phys Ther. 2015 Aug;95(8):1095–110. doi: 10.2522/ptj.20140461. [DOI] [PubMed] [Google Scholar]
- 71.Stroup DF, Berlin Ja, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 72.Kim DH, Rogers JR, Fulchino LA, Kim CA, Solomon DH, Kim SC. Bisphosphonates and Risk of Cardiovascular Events: A Meta-Analysis. PLoS One. 2015 Apr 17;10(4):e0122646. doi: 10.1371/journal.pone.0122646. Public Library of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.