Abstract
E6-associated protein (E6-AP), which was originally identified as an ubiquitin-protein ligase, also functions as a coactivator of estrogen (ER-α) and progesterone (PR) receptors. To investigate the in vivo role of E6-AP in mammary gland development, we generated transgenic mouse lines that either overexpress wild-type (WT) human E6-AP (E6-APWT) or ubiquitin-protein ligase-defective E6-AP (E6-APC833S) in the mammary gland. Here we show that overexpression of E6-APWT results in impaired mammary gland development. In contrast, overexpression of E6-APC833S or loss of E6-AP (E6-APKO) increases lateral branching and alveolus-like protuberances in the mammary gland. We also show that the mammary phenotypes observed in the E6-AP transgenic and knockout mice are due, in large part, to the alteration of PR-B protein levels. We also observed alteration in ER-α protein level, which might contribute to the observed mammary phenotype by regulating PR expression. Furthermore, E6-AP regulates PR-B protein levels via the ubiquitin-proteasome pathway. Additionally, we also show that E6-AP impairs progesterone-induced Wnt-4 expression by decreasing the steady state level of PR-B in both mice and in human breast cancer cells. In conclusion, we present the novel observation that E6-AP controls mammary gland development by regulating PR-B protein turnover via the ubiquitin proteasome pathway. For the first time, we show that the E3-ligase activity rather than the coactivation function of E6-AP plays an important role in the mammary gland development, and the ubiquitin-dependent PR-B degradation is not required for its transactivation functions. This mechanism appears to regulate normal mammogenesis, and dysregulation of this process may be an important contributor to mammary cancer development and progression.
E6-AP regulates mammary gland development by modulating the ubiquitin proteasome-mediated turnover of progesterone receptor-B protein.
The steroid hormones, estrogens and progesterones, are important regulators of cell growth and are essential for the maintenance of postnatal developmental plasticity of the mammary gland (1, 2, 3, 4, 5). Estrogens and progesterones exert their biological effects on target tissues through intracellular receptor proteins, estrogen receptor (ER-α) and progesterone receptor (PR), respectively. These receptors are members of a large class of nuclear transcription factors known as the nuclear hormone receptors (NHRs) (6, 7, 8, 9, 10, 11). When bound by hormone, these receptors dimerize and associate with the promoter or enhancer regions of target genes and recruit coactivators that facilitate chromatin remodeling and assembly of the transcription complex (12, 13, 14, 15).
Coactivators are molecules recruited by hormone-bound activated receptors that fine tune the expression of target genes (16, 17). Coactivators are structurally and functionally diverse: they mediate the transcriptional process through a wide array of enzymatic activities such as acetylation, methylation, ubiquitination, and phosphorylation or as chromatin remodelers (18, 19, 20, 21, 22). This specialized group of proteins is a key integrator of signals from steroid hormones. Because coactivators exist as multiprotein complexes on the promoter, active disruption, rearrangement, and clearing of these coactivators is required for transcription to proceed properly (18, 20, 23, 24, 25, 26, 27).
Some coactivator proteins have been found to be enzymes of the ubiquitin-proteasome pathway. Protein degradation via the proteasome appears to be linked to transcriptions of certain genes (20, 28, 29). Protein degradation mediated by the ubiquitin-proteasome system is a highly regulated and specific process, which may selectively remove coactivator proteins after they have fulfilled their roles in transcription, thereby clearing the way for subsequent coactivator complex association with the promoter for the next round of transcription (30, 31). Because coactivators are essential effectors of the biological activities of NHRs and their ligands, coactivator protein level critically determines the activity of the receptor in target tissues. Moreover, variations in hormone responsiveness between different cells or tissues may be due to differences in coactivator levels.
We previously reported the functional characterization of an E3 ubiquitin-protein ligase, E6-associated protein (E6-AP) as a steroid hormone receptor coactivator (32). E6-AP enhances the hormone-dependent transcriptional activity of steroid hormone receptors including PR, ER-α, androgen receptor, and glucocorticoid receptors. E6-AP is a member of the hect (homologous to the E6-AP carboxy terminus) domain containing E3 ubiquitin-protein ligases (33, 34). The carboxyl-terminal 350 amino acids of E6-AP constitute a hect domain, which is conserved among all the hect domain E3 ubiquitin-protein ligases (35). E3 enzymes play a major role in defining substrate specificity of the ubiquitin system. The extreme carboxyl-terminal 100 amino acids contain the region of E6-AP that catalyzes the transfer of ubiquitin to substrate protein targeted for degradation (35). This catalytic domain of E6-AP contains a conserved cysteine residue (at position 833) that forms a thioester bond with ubiquitin and is necessary for the transfer of ubiquitin to its substrate proteins. Mutation of the catalytic cysteine (C) 833 to alanine (A) or serine (S) results in the loss of the E3 ligase activity of E6-AP (34, 35). We have shown that E6-AP bearing a C-to-S mutation at the critical amino acid 833 can still act as a coactivator of NHRs in in vitro luciferase reporter gene assays. These data suggest that the coactivation and ubiquitin-protein ligase activities of E6-AP are separable (32). The present study investigated the dual function of E6-AP in ER and PR signaling in the mammary tissue.
The physiological role of ER-α, ER-β, and PR isoforms has been examined by generation of isoform-specific knockout (KO) mice of these receptors. Interestingly these studies suggest that ER-α and PR-B are the key mediators of estrogen and progesterone action in the normal mammary gland. A large amount of evidence suggests that mammary gland development involves coactivators of steroid hormone receptors, especially those of ER-α and PR-B (23, 36, 37, 38). Recent studies have shown that isoform-specific activation of target genes in the mammary gland by PR is due to differential coactivator recruitment. Levels of a number of ER-α and PR coactivators are elevated in human mammary tumors (39, 40, 41, 42, 43, 44, 45). Studies using peptide aldehydes as proteasome inhibitors, such as MG132 and lactacystine, have provided evidence that ER-α is degraded through the 26S proteasome pathway (20, 46, 47). PR is also subject to proteasomal degradation (48), but the biological significance of this and the mechanism regulating PR ubiquitination and degradation are not fully understood. It is well established that ER-α and PR-B are the key players in mammary gland development (49, 50, 51). Because E6-AP is a coactivator for both ER-α and PR, we have investigated the potential role of E6-AP in mammary gland development. The dual function of E6-AP as a coactivator and an E3 ligase suggests that the physiological roles of E6-AP may involve a delicate balance between coactivation and degradation of the SHRs with which it interacts.
Here, we report the generation and analysis of transgenic mouse lines that express either wild-type (WT) human E6-AP (E6-APWT) or an E6-AP mutant that retains coactivation function but is defective for ubiquitin-protein ligase activity in the mammary gland using the mouse mammary tumor virus (MMTV) promoter. E6-AP null mouse (E6-APKO) line was also analyzed to assess the effect of E6-AP loss on mammary development (52). Mammary glands of nulliparous E6-APKO and E6-APC833S mice show increased lateral branching and undergo extensive alveologenesis, a phenotype observed normally in glands of pregnant WT mouse. In contrast, E6-APWT overexpression significantly reduced mammary ductal side branching. The increased mammary gland morphogenesis in E6-APKO mice and E6-APC833S mice was due, in part, to elevated ER-α and PR-B protein levels. The increased levels of PR-B observed in E6-AP-deficient mammary glands prompted us to investigate whether E6-AP could function as an E3-ligase to govern PR-B protein stability. Here, we present evidence that E6-AP interacts with PR-B in vivo and specifically regulates PR-B degradation in breast cancer cells. Inhibition of E6-AP expression increased the PR-B half-life, altered PR-B target-gene expression, and increased progesterone-mediated cell growth in breast cancer cells. Taken together, these observations indicate that E6-AP-mediated regulation of PR-B protein stability significantly regulates mammary gland morphogenesis. Furthermore, our experiments indicate that E6-AP functions as an E3-ligase in the mammary gland rather then acting as a coactivator.
Results
Generation of mammary gland-specific E6-AP transgenic mice
To elucidate the role of E6-AP in mammary gland development, two different E6-AP transgenic mice lines were generated to overexpress either E6-APWT or a mutant E6-AP defective for ubiquitin protein ligase function (E6-APC833S) driven by the MMTV promoter. The transgenic constructs used in this study are depicted in Fig. 1A. These constructs also contain the requisite splice acceptor and donor sites for maximal transgene expression. To distinguish the expression of transgenes from endogenous murine E6-AP, a FLAG tag was incorporated at the amino termini of WT E6-AP and the mutant E6-AP vectors. These constructs were transiently transfected into HeLa cells, and Western blotting demonstrated expression of full length E6-APWT and E6-APC833S protein using anti-FLAG tag-specific antibodies (data not shown).
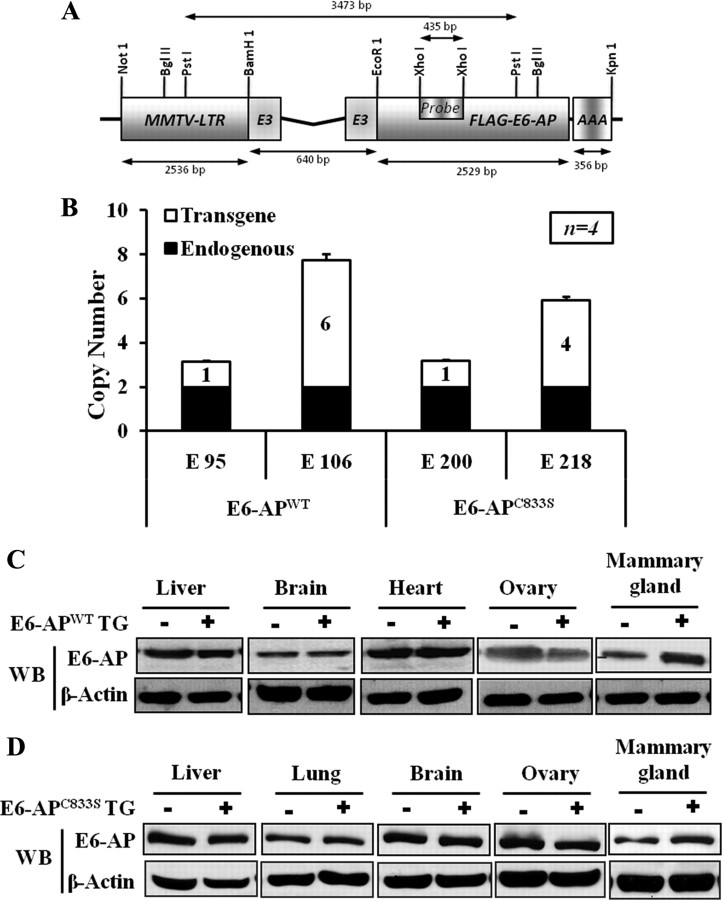
Fig. 1.
Generation and characterization of the MMTV-E6-AP transgenic mice. A, Schematic representation of E6-APWT and ubiquitin-ligase-defective mutant E6-AP (E6-APC833S) transgenic constructs. The E6-APWT and E6-APC833S transgenic constructs contain the MMTV-LTR and the full-length human E6-AP cDNA, linked to the polyadenylation signal (rBG PolyA) derived from the rabbit β-globin gene. This construct contains the requisite splice acceptors and donors to maximize transgene expression. B, Histogram showing the transgenic copy number in different E6-AP transgenic lines; black bar represents the endogenous E6-AP gene, and the white bar represents E6-AP transgene. Southern blot analysis was performed with PstI-digested genomic DNA (10 μg) from founder mice and hybridized with the 435-bp (XhoI-XhoI) long fragment of E6-AP Exon 3 as a probe as shown in A. The probe also detects the endogenous 2 copy of E6-AP, which was used to quantify the transgenic copy number. C and D, Western blot analysis of endogenous E6-AP and transgenic E6-AP expression in various mouse tissues in E6-APWT and E6-APC833S transgenic mice with their WT littermates, respectively. LTR, Long terminal repeat; TG, transgene; WB, Western blot.
We showed previously that E6-AP acts as a coactivator of PR and ER-α (32). To determine whether the transgenic E6-AP constructs were functional, the coactivation functions of E6-AP on PR was tested using reporter assays. E6-AP transgenic vectors, PR expression vector, and PRE-driven luciferase reporter plasmid were cotransfected into HeLa cells. Both E6-AP constructs enhanced luciferase activity in the presence of 10 nm progesterone confirming the expression and activities of the E6-APWT and E6-APC833S in mammalian cells.
The intact and biologically functional transgenes were then released from the transgenic expression constructs by digestion with NotI and KpnI restriction enzymes. After purification, the transgenes were microinjected into mouse zygotes from an inbred FVB colony. Six founder mice (three for E6-APWT named E37, E95, and E106 and three for E6-APC833S named E200, E218, and E220) were obtained whose progeny inherited E6-AP in a Mendelian manner. The founder mice were identified by PCR and Southern blot (Fig. 1B). Founders were bred with WT imprint control region mice to generate female mice that were used in the subsequent experiments.
Expression of the E6-APWT and E6-APC833S transgenes was compared with that of nontransgenic mice in liver, lung, brain, heart, ovary, and mammary gland tissues by Western blot. Figure 1, C and D, shows that E6-APWT and E6-APC833S transgene were overexpressed in the mammary gland but not in other tissues, confirming mammary-specific overexpression.
E6-AP alters mammary gland development in nulliparous mice
The mammary gland develops in discrete stages, and most of the growth occurs after birth (53). In newborn mice, the mammary gland comprises several ducts. Rapid elongation and branching of the mammary ducts occur during puberty due to the influence of the ovarian hormones, estrogen and progesterone. During pregnancy, additional ductal branching occurs, and extensive lobular-alveolar proliferation gradually results in the complete filling of the fat pad at parturition (11). To investigate the physiological role of E6-AP in mammary gland development, mammary gland morphogenesis was compared in E6-APWT, E6-APC833S, and E6-APKO mice by whole-mount staining at different stages of development: 1) virgin (8 wk and 12 wk of age); 2) pregnant; 3) lactating; and 4) involution. The whole-mount analysis of 8-wk-old (Fig. 2A) and 12-wk-old (data not shown) virgin mammary glands from MMTV-E6-APWT animals showed impaired mammary gland development compared with their nontransgenic littermates. The E6-APWT transgenic mammary glands failed to invade the entire fat pad (Fig. 2, A and B). In contrast, E6-APC833S female mice displayed highly branched mammary glands with increased side branching and alveolar buds compared with their WT littermates (Fig. 2, C and D). The invasion of fat pads was complete in E6-APC833S transgenic mammary glands.
Fig. 2.
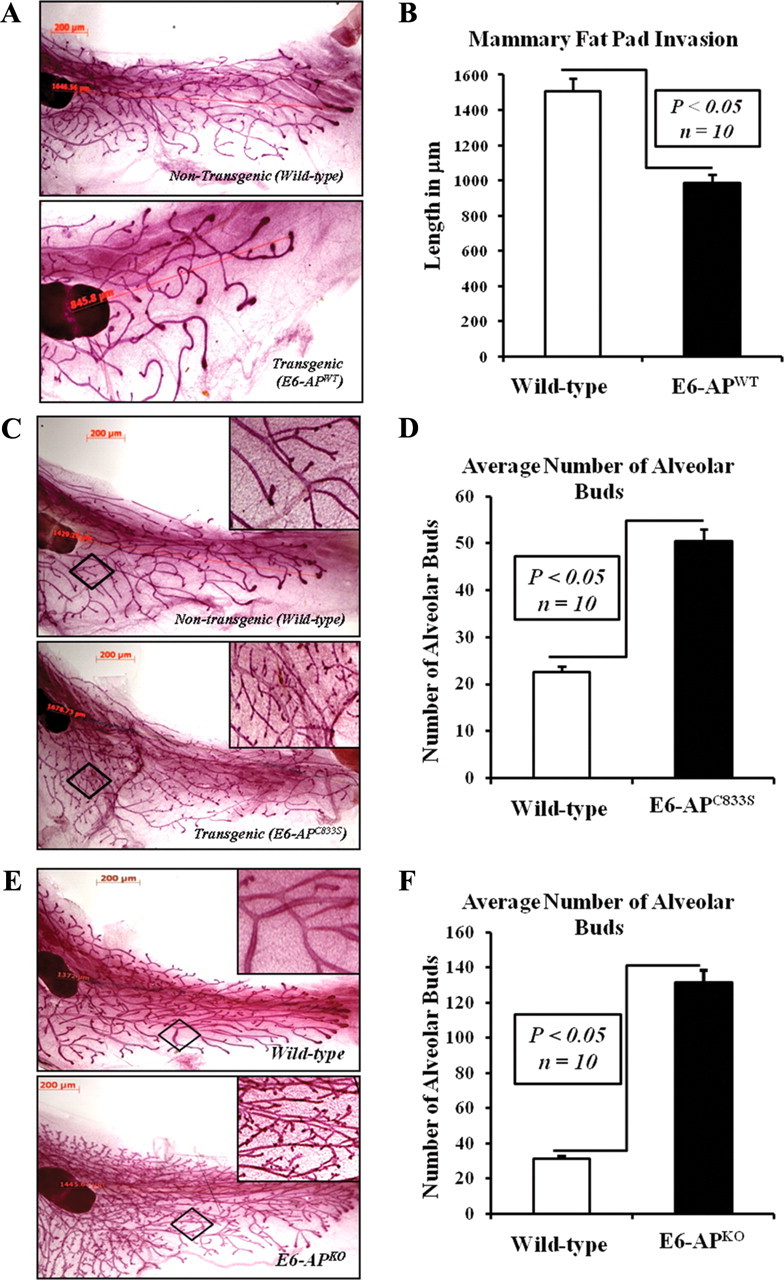
E6-AP is involved in mammary gland development. A, Overexpression of E6-APWT results in impaired mammary gland development. Comparison of mammary gland morphology between E6-APWT transgenic mice and their WT nontransgenic littermates using whole-mount analysis. Mammary glands with representative morphology are presented. B, Histogram comparing the length of mammary glands in E6-APWT transgenic mice and their WT nontransgenic littermate. Ten mice were examined for each group, and the data are presented as mean ± sd; P < 0.05. C, Overexpression of ligase-defective mutant E6-AP (E6-APC833S) results in increased side branching and alveolar buds. D, Histogram comparing the total number of alveolar buds in the mammary glands of E6-APC833S transgenic mice and their WT littermates. Ten mice were examined for each group, and the data are presented as mean ± sd; P < 0.05. E, Loss of E6-AP expression (KO) results in increased side branching and alveolar buds. F, Histogram comparing the total number of alveolar buds in the mammary glands of E6-APKO mice and their WT littermates. Ten mice were examined for each group, and the data are presented as mean ± sd; P < 0.05.
To evaluate the effect of E6-AP KO on mammary gland morphogenesis, we compare the mammary gland of E6-APKO mice and their WT littermate at different stages of development in virgin mice. Similar to the E6-APC833S mammary gland, E6-APKO female mice also showed increased ductal side branching and significantly higher number of lobuloalveolar buds compared with their WT littermates (Fig. 2, E and F). These results demonstrate that E6-AP modulates mammary ductal branching. Thus, E6-AP loss increases and E6-AP overexpression decreases primary ductal outgrowth. The phenotype of E6-APC833S transgenic mice was similar to that of KO.
Because mammary gland development is influenced by ovarian estrogen and progesterone, we tested the circulating estrogen and progesterone levels in the transgenic mice, KO mice, and their age-matched WT littermates. The 17β-estradiol and progesterone concentrations in sera of 12-wk-old mice in the proestrous phase of estrous cycle were similar in transgenic, KO, and WT mice (data not shown). Thus difference in mammary gland morphology observed in the E6-AP transgenic mice and E6-AP KO mice cannot be attributed to the varying levels of estrogen and progesterone in the system.
E6-AP has no significant effect on mammary morphogenesis during pregnancy, lactation, and involution
To assess the effect of E6-AP on mammary gland morphogenesis during pregnancy and lactation, we compared the mammary gland morphologies of pregnant E6-APWT, E6-APC833S, and E6-APKO mice and their WT littermates at d 15 postcoitum and d 10 lactation. Whole-mount analysis showed that the mammary gland phenotype was identical during pregnancy and lactation in transgenic, knockout, and WT mice. All the mammary glands exhibited identical lobular-alveolar formations during pregnancy. Thus, E6-APWT overexpression did not impair mammary gland and E6-AP knock out or transgenic expression of E6-APC833S did not augment pregnancy-mediated mammary gland morphogenesis (Supplemental Figs. 1–3 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Similarly, E6-APWT overexpression did not impair, and E6-AP knock out or transgenic expression of E6-APC833S did not alter, the phenotype of the lactating glands (Supplemental Figs. 1–3). The possible explanation for observing no differences in the mammary gland phenotype during pregnancy and lactation is that during pregnancy, proliferation and lobuloalveolar structure formation in the mammary gland are also mediated by prolactin receptor (5). It is possible that prolactin receptor may compensate for the loss of PR-mediated proliferation and lobuloalveolar structure formation.
Considering the increased ductal branching and alveolar morphogenesis in E6-APC833S transgenic mice and E6-APKO mice, we next assayed whether the mammary glands properly reverted to its prepregnancy status during involution stage. At involution (8 wk) the mammary glands of both transgenic and KO mice exhibited no significant differences (Supplemental Figs. 1–3). Taken together, these observations suggest that E6-AP levels do not affect pregnancy-induced mammary ductal increase, lactating gland morphology, or the reversion process of mammary gland during involution.
E6-AP modulates ER-α and PR-B levels in the mammary gland
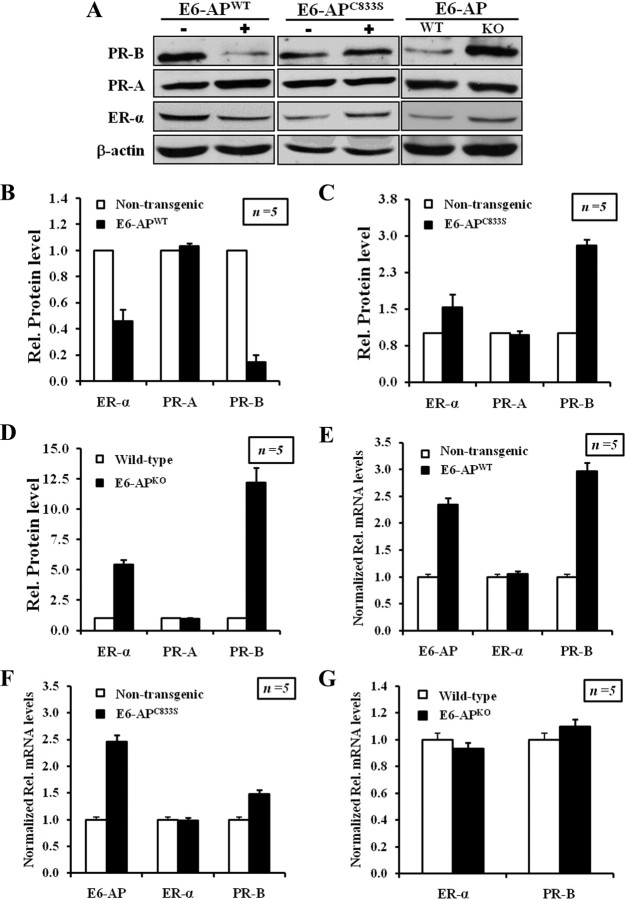
Estrogen and progesterone signaling pathways play an important role in mammary gland development; ER-α and PR-B protein expression was assayed in mammary tissue of E6-AP transgenic and E6-APKO mice by Western blot and immunohistochemistry. The ER-α protein was moderately reduced in the E6-APWT transgenic mice compared with that of a nontransgenic littermate (Fig. 3, A and B, and Supplemental Fig. 4) and was elevated in E6-APKO mice (Fig. 3, A and E, and Supplemental Fig. 6). A moderate increase in ER-α protein level was observed in E6-APC833S transgenic mammary gland (Fig. 3, A and C, and Supplemental Fig. 5). Thus E6-AP appears to negatively regulate ER-α protein levels in the mammary gland.
Fig. 3.
E6-AP alters the protein level of ER-α and PR-B protein in the mammary gland. A, Western blot analysis showing relative levels of endogenous ER-α, PR-B, and PR-A in E6-APWT, E6-APC833S, and E6-APKO mice with their WT littermate controls. B–D, The graphs represent the quantification of ER-α, PR-B, and PR-A protein levels from Western blot, expressed as fold increase in E6-APWT, E6-APC833S transgenic, and E6-APKO mice compared with their WT nontransgenic littermates, respectively (mean ± sd, five separate experiments). The differences in ERα and PR-B protein levels between E6-APWT or E6-APC833S or E6-APKO mice and their WT littermates were statistically significant according to Student’s t test (P < 0.05). Relative protein levels were quantitated by NIH ImageJ software. E–G, E6-AP-mediated regulation of ER-α and PR-B protein levels are not controlled at the transcriptional level. Total RNA extracted from mammary epithelial cells of E6-APWT, E6-APC833s, and E6-APKO mice were used in RT-PCR analysis using primers specific for E6-AP, ER-α, and PR-B. Results from three different experiments were averaged and plotted as relative mRNA levels. Error bars refer to mean ± sd of the average quantitated results. E–G, Relative mRNA levels in E6-APWT, E6-APC833S, and E6-APKO, respectively.
In mammary tissue, PR transcription is regulated by ER-α. PR-B is an essential regulator of mammary gland side branching and lobuloalveolar development. Therefore, we analyzed the protein levels of PR-A and PR-B in the mammary glands of our E6-AP transgenics and E6-APKO mice. PR-B protein was significantly reduced in the E6-APWT transgenic mammary gland (Fig. 3A, left panel, Fig. 3B, and Supplemental Fig. 4). In contrast, PR-B protein level was increased in the mammary glands of E6-APKO and E6-APC833S transgenic mice compared with nontransgenic animals (Fig. 3, A, C, and D, and Supplemental Figs. 5 and 6). PR-A protein levels in the mammary glands of E6-AP transgenics and E6-APKO mice did not change significantly from that of nontransgenic mammary glands. The reduced PR-B and ER-α protein level in E6-APWT transgenic mice could be due to accelerated proteolysis of both these receptors by E6-AP-mediated ubiquitination. The decrease in ER-α protein levels in E6-APWT transgenic mice would, in turn, lead to the decrease of its transcriptional target PR-B. Thus, PR-B expression in mouse mammary tissues is likely to be regulated both transcriptionally and posttranslationally by E6-AP.
To test whether E6-AP down-regulates the expression of ER-α and PR-B at the transcriptional or posttranslational level, relative mRNA level of ER-α, PR-B, and E6-AP in mammary epithelial cells from 12-wk-old transgenic mice (E6-APWT and E6-APC833S), E6-APKO mice, and their respective WT littermates were assayed by quantitative real time PCR. ER-α mRNA levels were similar in all samples assayed (Fig. 3, E, F, and G). Despite the reduced PR-B protein levels observed, there was a 3-fold increase in the PR-B mRNA in E6-APWT transgenic mammary gland compared with WT littermates. E6-APKO and E6-APC833S transgenic mammary glands showed no change in PR-B transcript levels. Primers used in these assays are listed in Supplemental Table 1. These results indicated that E6-AP regulates PR-B and ER-α level via a posttranslational mechanism.
E6-AP regulates PR-B protein stability in human breast cancer cells
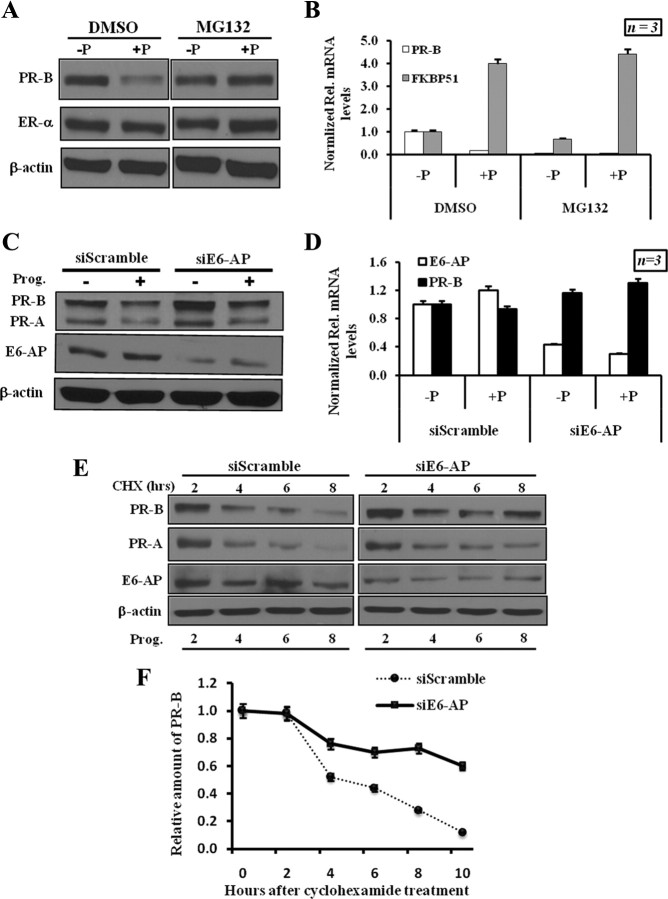
To further investigate E6-AP action on PR-B protein levels, we used T47D breast cancer cells that endogenously overexpress PR-B. PR is known to be ubiquitinated upon exposure to progesterone and is subsequently degraded by the 26S proteasome (48). Recent studies have suggested that the transcriptional activation of hormone receptors may be linked to receptor proteolysis (46). Hence, we assayed the effects of the proteasome inhibitor, MG132, on PR-B levels and transcriptional activity. Addition of progesterone to hormone-depleted cells led to a reduction in PR-B protein. Addition of MG132 together with progesterone blocked the hormone-dependent degradation of PR-B (Fig. 4A). To test whether receptor proteolysis is coupled to PR-B transcriptional activity, the expression of PR-B target gene FKBP51, was analyzed by quantitative PCR under similar treatment conditions (30). As illustrated in Fig. 4B, progesterone treatment increased FKBP51 transcripts both in the presence and absence of MG132. These data indicate that ligand-activated PR-B proteolysis of PR-B is not required for PR-B transcriptional activity at this target gene.
Fig. 4.
E6-AP regulates PR-B protein stability in human breast cancer cells. A, Effect of proteasome inhibitor on PR-B degradation. T47D cells were treated with vehicle (−P) or progesterone (+P). MG132 was added as indicated for 6 h before sample collection. Endogenous level of PR-B, ER-α, and β-actin was detected by Western blot. B, Proteasome inhibitor does not affect the hormone-dependent transcription of PR-B target genes. RT-PCR was performed with specific primers for PR-B and FKBP52. The results were normalized against calnexin transcript. C, RNA interference of E6-AP results in elevated PR-B protein levels. Endogenous E6-AP, PR-A, PR-B, and β-actin were detected by Western blotting. These experiments were repeated at least four times. D, E6-AP-mediated regulation of PR-B protein is not controlled at transcriptional level. Total RNA extracted from T47D cells treated with siE6-AP or siScrambled was used in RT-PCR analysis using primers specific for PR-B and E6-AP. Results from three different experiments were averaged and plotted as relative mRNA levels. Error bars represent 5% of the average quantitated results. E, Depletion of E6-AP increases the half-life of PR-B. T47D cells were transfected with siE6-AP or siScrambled; 48 h after transfection, cells were stimulated with progesterone and treated with 200 μg/ml of cycloheximide for 0, 2, 4, 6, 8, and 10 h. Cell lysates were subjected to Western blot analysis using PR-B antibody. F, The quantification of PR-B protein level was performed after normalization against β-actin (n = 4). CHX, Cycloheximide; DMSO, dimethylsulfoxide; Prog., progesterone.
To validate the observation that E6-AP functions as an E3-ligase in E6-AP transgenic and KO animals, we used E6-AP-specific small interfering RNAs (siRNAs) to knock down the expression of E6-AP in T47D cells. E6-AP Knock-down significantly increased PR-B protein levels compared with siRNA-treated control cells (Fig. 4C). No significant difference in PR-B mRNA levels was seen after treatment of cells with siE6-AP (Fig. 4D). To further investigate the effects E6-AP on PR-B protein stability, we analyzed the half-life of endogenous PR-B in T47D cells treated with either siScrambled or siE6-AP. At 2 d after siRNA transfection the cells were induced with progesterone and treated with cycloheximide at the same time to inhibit cellular protein synthesis. The half-life of PR-B protein was 6 h in progesterone-induced, siScrambled-treated cells consistent with previously published reports (Fig. 4E). However, progesterone-stimulated PR-B proteolysis was reduced in cells with siE6-AP, consistent with a role for E6-AP in progesterone-induced PR-B turnover (Fig. 4, E and F). Taken together, our results strongly suggest that E6-AP is a critical mediator of PR-B proteolysis.
E6-AP regulates PR-B proteolysis/turnover by targeting it for ubiquitination
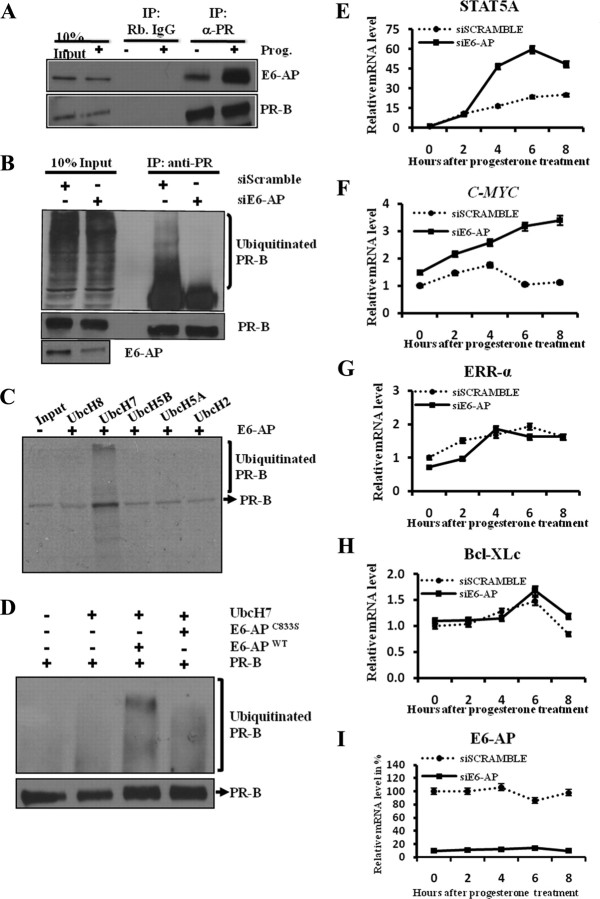
We next tested whether E6-AP can bind PR-B in cells and whether it can act as an E3 ligase to promote PR-B proteolysis in vitro. Cellular E6-AP coprecipitated with PR-B in the presence of progesterone, indicating that PR-B forms a complex with E6-AP in cells (Fig. 5A). We next tested the effect of E6-AP down-regulation on progesterone-induced PR-B ubiquitination. Cells were treated with either siE6-AP or control siScrambled and then treated with progesterone. Cell lysates were immunoprecipitated with anti-PR-B antibody, and the complexes were resolved, blotted, and probed with antiubiquitin antibody for PR-B ubiquitination. siRNA-mediated down-regulation of E6-AP reduced progesterone-stimulated PR-B ubiquitination compared with controls (Fig. 5B), consistent with the notion that E6-AP progesterone induced PR-B degradation.
Fig. 5.
E6-AP is involved in PR-B turnover by targeting it for ubiquitination. A, Interaction of endogenous E6-AP and PR-B in breast cancer cells. Cell lysates from T47D cells treated with progesterone (+P) or vehicle (−P) were immunoprecipitated with anti-PR-B or control IgG and detected by Western blotting using E6-AP- and PR-B-specific antibody. B, Knockdown of E6-AP reduces PR-B ubiquitination in a hormone-dependent manner. T47D cells were transfected with siE6-AP or siScrambled. Cells were induced 48 h after transfection with progesterone for 12 h. Cell lysates were immunoprecipitated with anti-PR-B antibody or normal IgG, and Western blot analysis was performed for ubiquitin and PR-B. C, Ubiquitin-conjugating enzyme UbcH7 is involved in E6-AP-mediated PR-B ubiquitination. 35S-labeled PR-B protein was subjected to in vitro ubiquitin assay with different ubiquitin-conjugating enzymes (UbcH2, UbcH5A, UbcH5B, UbcH7, and UbcH8). Reactions were analyzed by SDS-PAGE and autoradiography. Experiments were repeated at least three times. D, In vitro ubiquitination assay was performed with purified PR-B as mentioned in Materials and Methods, and Western blot analysis was performed to detect ubiquitinated PR-B with ubiquitin-specific antibody. E–I, Down-regulation of E6-AP results in the up-regulation of PR-B-specific target genes. T47D cells treated with siE6-AP or siScramble for 48 h followed by progesterone treatment for 2, 4, 6 and 8 h. Total RNA extracted at different progesterone treatment time points was used in RT-PCR analysis using primers specific for PR-B target genes (STAT5A and c-MYC), PR-A specific genes (ERR-α and Bcl-XLc), and E6-AP. Results from three different experiments were averaged and plotted as relative mRNA levels. Error bars represent 5% of the average quantitated results. IP, Immunoprecipitation; Prog., progesterone.
To further substantiate our in vivo ubiquitination data, we examined the ability of E6-AP to act as an E3 ligase for PR-B in a cell-free system, using a series of E2-ubiquitin-conjugating enzymes. As shown in Fig. 5C, only the ubiquitin-conjugating enzyme UbcH7, together with E6-AP, promoted the ubiquitination of PR-B in vitro, whereas the other E2 enzymes UbcH2, UbcH5A, UbcH5B, and UbcH8 failed to do so. These in vitro assays demonstrated that, E6-AP could act as an E3 ligase for PR-B ubiquitination and that the E2 enzyme UbcH7 is also needed for this E6-AP-mediated PR-B ubiquitination.
Because the level of PR-B protein was higher in the E6-APC833S transgenic mice, we examined the ability of this mutant E6-AP to ubiquitinate PR-B in our in vitro cell-free ubiquitination assay. We found that WT E6-AP ubiquitinated PR-B robustly, whereas the ubiquitin ligase-defective mutant E6-APC833S showed reduced PR-B ubiquitination (Fig. 5D). These results corroborate with our data from mouse models showing that E6-AP regulates PR-B protein stability.
To assess whether E6-AP specifically functions as a coactivator or an E3-ligase for PR-B, E6-AP was knocked down using siRNA in T47D cells, and the transcriptional activity of PR-A and PR-B was measured as a function of coactivator. As measured by quantitative real time PCR, there was significant increase in the expression of PR-B-specific target genes, STAT5A (Fig. 5E) and cMYC (Fig. 5F) (54). Similarly there was a significant up-regulation of FKBP51 and HSDIIβ2, which are regulated by both PR-A and PR-B isoforms (Supplemental Figs. 7 and 8) (54). In contrast, there was no change in the expression of PR-A-specific target genes (ERR-α and Bcl-XLc) (54) with knockdown of E6-AP (Fig. 5, G and H). These data suggest that E6-AP specifically functions as an E3-ligase for PR-B in the mammary gland rather than acting as a coactivator.
E6-AP negatively regulates PR-B-dependent transcription of Wnt-4
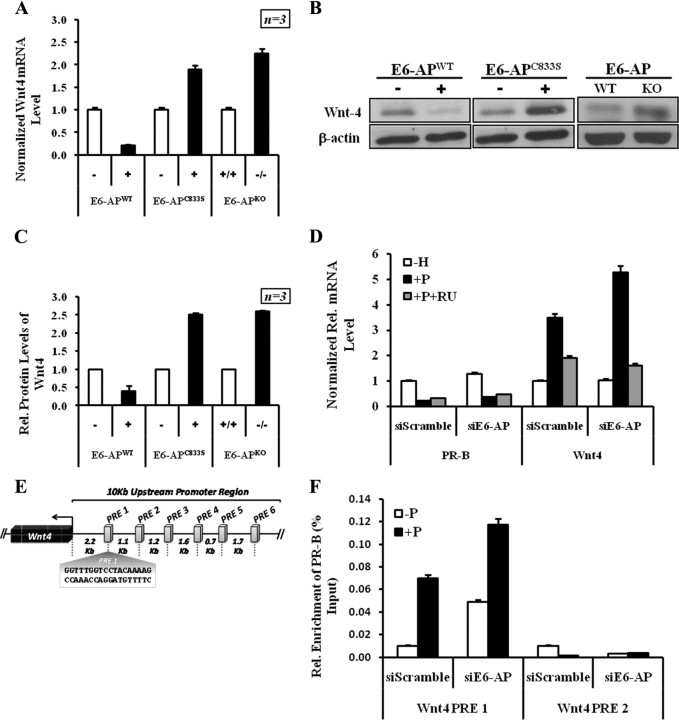
Previous findings indicate that Wnt-4 is a paracrine factor that functions downstream of PR (55). Wnt-4 is also centrally important for progesterone-induced side branching of the mammary ductal epithelium (56). Therefore we analyzed the expression of Wnt-4 as a potential downstream mediator of PR-B-induced mammary ductal branching. Wnt-4 protein level was decreased in our E6-APWT transgenic mice, whereas the E6-APKO and E6-APC833S transgenic murine mammary tissue showed a significant increase in Wnt-4 protein (Fig. 6, A and B). Wnt-4 mRNA expression in E6-APWT transgenic mammary was significantly reduced, in keeping with the reduced level of PR-B in these tissues, whereas there was a significant enhancement of Wnt-4 mRNA in E6-APKO and E6-APC833S transgenic mammary glands (Fig. 6C). Thus, E6-AP appears to regulate Wnt-dependent signaling by regulating the protein level of PR-B in the mammary gland. To further confirm our in vivo data, we next tested whether E6-AP depletion affects the expression of the progesterone-responsive Wnt-4 gene in T47D cells. Cells transfected with siE6-AP showed a progesterone-dependent increase in Wnt-4 expression (Fig. 6D and Supplemental Fig. 9). The decrease in Wnt-4 mRNA with RU486 treatment in E6-AP knockdown and control confirms that Wnt-4 transcription is regulated by PR-B. This PR-B-dependent 2-fold increase in Wnt-4 mRNA is consistent with our observations in the E6-APKO mice model and supports the hypothesis that E6-AP regulates PR-B turnover, thereby modulating PR-B-dependent transactivation of Wnt-4.
Fig. 6.
E6-AP affects PR-B-dependent transcription of Wnt-4. A, Quantification of Wnt-4 mRNA expression by RT-PCR in mammary epithelial cells (MEC) derived from E6-APWT transgenic mice, E6-APC833S transgenic mice, and E6-APKO mice along with their WT littermates. Wnt-4 mRNA was quantified using Wnt-4-specific primers in three independent littermates. PCRs were performed in triplicate, the results were normalized against calnexin transcripts, and the relative amount of mRNA is shown with standard deviation. B, Western blot analysis for Wnt-4 protein in E6-APWT, E6-APC833S, E6-APKO, and their WT littermates normalized to the β-actin levels. C, The graph represents the quantification of Wnt-4 protein levels from Western blot expressed as fold increase in E6-APWT transgenic mice, E6-APC833S transgenic mice, and E6-APKO along with their WT littermates (mean ± sd; three separate experiments). D, E6-AP influences PR-B-induced Wnt-4 transcription in human breast cancer cells. Results from three different experiments were averaged and plotted as relative mRNA levels. Error bars represent 5% error of the average quantitated results. E, Schematic representation of the 10-kb upstream region of Wnt4 showing the putative PREs (PRE 1–6). F, Recruitment of PR-B at the Wnt4 PRE 1 and 2 regions under control (siScramble) and E6-AP (siE6-AP) knockdown conditions in T47D CAT0 cells. PR-B recruitment to the Wnt4 PRE 1 and 2 regions in siScramble- and vehicle (−P)-treated cells is taken as 1-fold, and all the bars are scaled accordingly; data are presented as percent input. Rel., Relative.
PR-B is recruited to Wnt-4 promoter in a hormone-dependent manner
Although Wnt-4 is a major mediator of progesterone-driven side branching and alveologenesis in the mammary glands, it is not known whether Wnt4 is a direct target of PR-B. To test this, we asked whether PR-B is recruited to the Wnt-4 promoter. The 10-kb upstream region of the Wnt4 gene contained six possible PREs (PRE 1-6) with varying homology to consensus PRE (66–71%). We designed real-time PCR primers encompassing each of the six putative Wnt-4 PREs (Supplemental Table 2). Initially, sheared protein-DNA complexes from T47D-CAT0 cells were precleared with normal rabbit IgG followed by immunoprecipitation with anti-PR-B antibody to pull down PR-B-bound immunocomplexes. DNA from these immunocomplexes was analyzed by quantitative real-time PCR with Wnt4 PRE 1-6-specific primers as shown in Fig. 6E. As shown in Fig. 6F, our ChIP assays shows that, in cells treated with nonspecific siRNA (siScramble), PR-B is only recruited to the PRE 1 (−2.2 kb) and not to PRE 2 (−3.3 kb) at the Wnt-4 upstream region. In cell treated with E6-AP-specific siRNA (siE6-AP), recruitment of PR-B to Wnt-4 PRE 1 region is significantly increased; however, the Wnt-4 PRE 2 region shows no difference (Fig. 6F). The increased recruitment of PR-B to the Wnt-4 upstream region under E6-AP knockdown conditions can be explained by higher steady-state levels of PR-B in the cells. We did not observe any significant recruitment of PR-B to the other putative PREs (PRE 3-6) in the 10-kb upstream region of the Wnt4 gene (data not shown).
E6-AP modulates PR-B-mediated cell proliferation
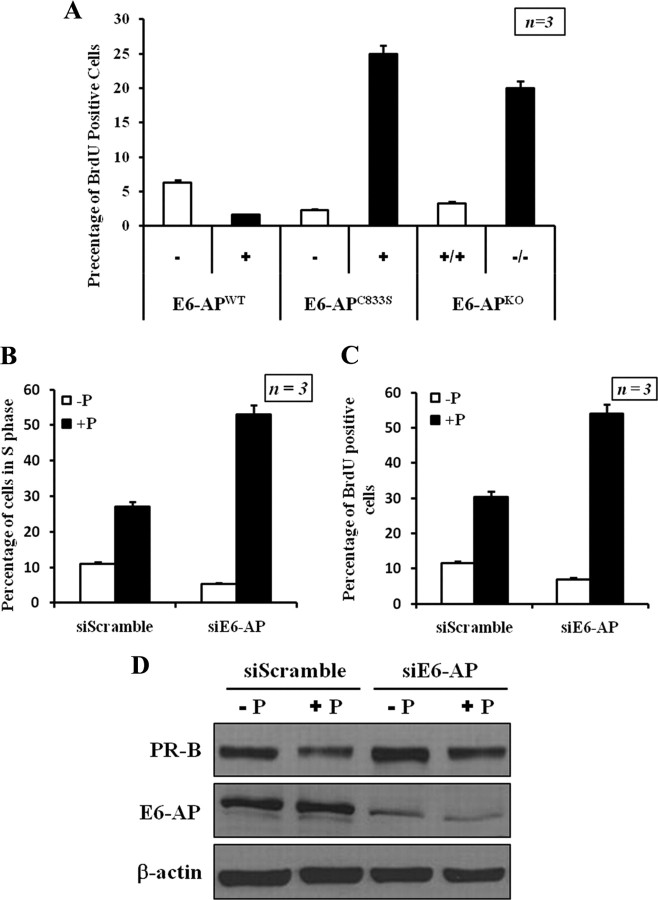
In vivo and in vitro data indicate that progesterone is required for epithelial proliferation in mammary glands of adult mice (53, 57). The present data suggest that E6-AP plays a central role in the regulation of PR-B stability. We therefore investigated whether alteration of E6-AP expression affects progesterone-dependent epithelial cell proliferation in our mouse models. As a measure of the proliferation rate of mammary epithelial cells in WT E6-AP transgenic, ubiquitin-protein ligase-defective E6-AP transgenic and E6-APKO mammary glands, bromodeoxyuridine (BrdU) incorporation studies were performed. BrdU incorporation in mammary epithelial cells in the E6-APWT transgenic was lower compared with that of nontransgenic mammary epithelial cells (Fig. 7A and Supplemental Fig. 10), whereas that in the E6-APC833S transgenic and E6-APKO mammary epithelial cells was higher compared with their WT controls (Fig. 7A and Supplemental Fig. 10). These results demonstrate that E6-AP might play an important role in epithelial cell proliferation by modulating the protein levels of PR-B in mammary epithelial cells. The high levels of PR-B in E6-APKO mice and E6-APC833S transgenic mice may explain the increase in ductal side branching observed in the mammary glands of these animals.
Fig. 7.
E6-AP influences cell proliferation through regulation of the steady-state level of PR-B. A, E6-AP alters the proliferation of mammary epithelial cells. Immunohistochemistry was performed to detect BrdU incorporation in mammary epithelial cells from E6-APWT transgenic mice, E6-APKC833S transgenic mice, and E6-APKO mice. B, Knockdown of E6-AP up-regulates cell cycle progression. Propidium iodide staining/FAC Scan analysis of T47D cells treated with control siRNA and siE6-AP. The graph represents the summarized percentage of cells in S phase from three independent experiments (mean ± sd; three separate experiments). C, BrdU incorporation in T47D cells treated with control siRNA and siE6-AP. The data are represented as mean ± sd. D, Western blot to demonstrate the efficient knockdown of E6-AP protein.
It is possible that the observed mammary gland developmental differences may also be due to differences in mammary epithelial cell migration and apoptosis. To test this possibility, we also examine the apoptosis in our E6-APWT, E6-APC833S, E6-APKO, and WT mice by terminal deoxynucleotide transferase-mediated dUTP nick end labeling assays. Our data show that there was no significant difference in mammary epithelial cell migration between the transgenic lines, E6-APKO, and WT mammary glands. Mammary gland organization in the transgenic, E6-APKO, and WT mice were intact. The apoptotic index in the transgenic, E6-APKO and WT mammary glands was uniform (∼ 1%), which was determined by terminal deoxynucleotide transferase-mediated dUTP nick end labeling assay on mammary tissue (Supplemental Fig. 11).
To further substantiate these findings, we next tested whether E6-AP affects cell proliferation via its effect on PR-B stability. The cell cycle profile of T47D cells was assayed after E6-AP knockdown. At 48 h after transfection of siE6-AP, cells were stimulated with progesterone for 24 h after which cell cycle profiles were assayed by flow cytometry. Progesterone stimulated an increase in the percent S phase population and decreased percent G0/G1 in both siE6-AP and siScramble conditions; however, the stimulation observed was greater in siE6-AP than in controls. A minimal increase in G2/M population was observed in siE6-AP-treated cells compared with control cells (Fig. 7B). The cell cycle changes observed in the cells treated with siE6-AP suggests that depletion of endogenous E6-AP likely resulted in elevated levels of mitogenic PR-B (Fig. 7D). In the same experiments, we also measured BrdU incorporation after siRNA treatment. Unlike the control siRNA-treated cells, knockdown of E6-AP increased BrdU incorporation by about 12% (Fig. 7C). We further confirmed that the changes in cell cycle and proliferation were mediated via PR, by blocking the functions of PR with antiprogestin, RU486 (Supplemental Fig. 12). Our results suggest that depletion of E6-AP results in the up-regulation of PR-B, which in turn results in mitogenic activation and deregulation of cell cycle checkpoints. These changes ultimately lead to increased proliferation as demonstrated by elevated BrdU incorporation in these assays. Taken together, our results show that E6-AP can influence cell growth through regulation of the steady-state levels of ER-α and PR-B in the mammary gland.
Discussion
Mammary gland development involves a complex interaction of several hormones (53). Normal mammary gland development requires both estrogen and progesterone acting through their cognate receptors, ER and PR, in developing mammary glands (4, 57, 58). In vivo studies using transgenic mice have shown that ER-α and PR-B are the primary proliferative stimuli in the mammary gland during development (7, 10). E6-AP is a coactivator that enhances the hormone-dependent transactivation functions of steroid hormone receptors ER-α and PR (32). Both ER-α and PR-B protein levels are tightly regulated and are subjected to ubiquitin-mediated proteolysis (48, 59). Because E6-AP is a characterized coactivator of ER-α and PR, and can also function as an E3 ubiquitin-protein ligase (32), we investigated its role in mammary gland development using MMTV transgenic mice that overexpress E6-APWT protein or E6-APC833S protein in the mammary gland. To study the consequences of loss of function of E6-AP on the normal mammary gland development, mammary tissues from a previously established E6-APKO mouse line (52) were also systematically analyzed.
Steroid receptor coactivators have been shown to be essential for normal mammary gland development (38). Because coactivators enhance the transactivation functions of steroid hormone receptors, which in turn induce proliferation in the mammary gland, overexpression of a coactivator would normally be expected to enhance or accelerate mammary gland development. Overexpression of coactivator proteins, including steroid receptor coactivator 3, steroid receptor RNA activator, and Cdc25B, result in pronounced hyper-proliferation and developmental abnormalities in the mammary gland (23, 60). Conversely, targeted deletion of a number of coactivators had been shown to impair development of the mammary gland (38, 61). Disruption of the steroid receptor coactivator-1 and 3 loci in mice retards mammary gland development (61). Thus it appeared paradoxical that targeted overexpression of the steroid receptor coactivator E6-APWT in mammary glands should result in underdeveloped rather than a hyperproliferative phenotype. Moreover, loss of E6-AP in KO animals resulted in an overly developed mammary gland. Previously, it was reported that loss of E6-AP had no significant mammary phenotype (62). However, the same study also mentioned that some E6-APKO animals exhibited highly branched mammary glands, consistent with our observations. The reduced penetrance of this mammary phenotype in this prior report may have resulted from KO of E6-AP in a mixed genetic background (C57BL/6 and 129/SvEv), whereas our experiments were performed in a pure C57BL/6 genetic background. As observed for E6-APKO animals, the ubiquitin ligase-defective E6-APC833S transgenic lines also showed overly developed mammary phenotype. These data suggest that the increase in the number of alveolar buds and increased ductal side branching in E6-AP null mice result from the loss of E6-AP ubiquitin-protein ligase activity.
Targeted deletion of ER-α and PR-B has demonstrated that both receptors are required for normal mammogenesis (9, 51, 63). Furthermore, overexpression of these receptors in transgenic mice indicates that receptor expression levels are critically regulated during normal mammary gland development (58, 64). Because E6-AP is an E3 ubiquitin-protein ligase, it is possible that E6-AP may promote ubiquitin-dependent degradation of ER-α and PR-B proteins in the mammary gland. Consistent with this, ER-α and PR-B protein levels were both decreased in E6-APWT transgenic mammary gland compared with nontransgenic mammary gland. Moreover, ER-α and PR-B protein levels were higher in both E6-APKO and in E6-APC833S transgenic mammary glands compared with WT animals. These observations suggest that E6-AP regulates mammary gland development by modulating the protein levels of ER-α and PR-B. These results are consistent with our previous report showing that E6-AP protein is decreased in invasive breast carcinoma (65), and this reduction in E6-AP protein level correlated with increased expression of ER-α in the epithelial cells (65).
The increased ductal branching and alveolar buds (similar to 5–10 d pregnant mice) observed in E6-AP null mice and the mutant E6-APC833S transgenic mice provide strong evidence that the phenotype is predominantly driven by alterations in the protein levels of PR-B and to a lesser extent, by ER-α protein level. PR-B levels were more notably reduced than that of ER-α in the mammary tissue of these mice. Studies from PR-B null mice have shown that PR-B expression is required for mammary ductal branching and alveologenesis (66). Because E6-APWT transgenic mice lines exhibit ductal elongation defect like the PR-B null mice, we investigated whether E6-AP regulates PR-B levels through 26S proteasome-mediated protein turnover.
The reduction in PR-B and ER-α protein levels observed in E6-APWT transgenic mammary glands was not due to decreased transcription of these genes, pointing to posttranscriptional regulation of these proteins by E6-AP. Indeed PR-B mRNA was modestly increased in these animals, in keeping with increased activity of its major transactivator ER-α. However, this enhancement in the transcriptional activity of ER-α was not observed in the E6-APC833S transgenic mouse mammary gland. The dual function of E6-AP as a coactivator and an E3 ligase suggests that the physiological roles of E6-AP may involve a delicate balance between coactivation and degradation of the SHRs with which it interacts. This raises the question of how the effect of E6-AP on both coactivation of ER-α and turnover of ER-α contributes to the overall transcriptional activation of ER-α. Ample evidence indicates that rapid protein turnover of ER-α may also directly contribute to enhanced transcriptional activation (46). Cyclic turnover of ER-α has been linked to its normal transcriptional activities (47). Here we suggest that E6-AP-mediated degradation of ER-α is essential for the transcriptional activation of ER-α.
In the current study we have dissected the two different functions of E6-AP in the context of PR signaling. Based on our observations we propose that E6-AP-mediated PR-B turnover is highly efficient and that this rate of degradation is sufficient to overcome the transcriptional stimulation of PR-B mRNA by ER-α. This is evident from our observation that although there is a significant increase in PR-B mRNA level, the PR-B protein level is drastically reduced in E6-APWT transgenic mammary gland. To better understand the functional consequences of the isoform-specific proteolytic turnover of PR-B by E6-AP, we studied six different target genes that are transcriptionally regulated by either PR-A, PR-B, or both. We demonstrate that with knockdown of E6-AP only PR-B protein level is stabilized and, consequently, only PR-B isoform-specific target genes are up-regulated. Interestingly there was no down-regulation of both PR-A and PR-B target genes with knockdown of E6-AP, which shows that the coactivation function is not active. Data presented here suggest that E6-AP exclusively functions as an E3-ligase and negatively regulates PR-B. We have also shown that ubiquitin-dependent proteasome-mediated degradation of PR-B is not required for the transcriptional activity of PR-B.
PR-regulated paracrine factors include the Wnt family members (55). Wnt-4 has been shown to act downstream of PR-B to induce ductal side branching during normal mammary gland development. Wnt-4 transgenic mice can rescue impaired side branching in the mammary glands of PR KO mice (56). The increase in PR-B levels in E6-AP knockdown cells was correlated with enhanced expression of PR-B target genes. We have also demonstrated that Wnt4 is a direct transcriptional target of PR-B. Overexpression of E6-AP in the mammary gland decreases PR-B stability and protein levels, which in turn results in decreased expression of its transcriptional target Wnt-4. In contrast to observations for thyroid hormone receptor and ER-α at certain promotes (47), the transcriptional activity of PR-B at the wnt-4 promoter does not require active PR-B proteolysis for its transactivation functions. This is similar to the findings with the glucocorticoid receptor, which showed that transcriptional activity of this receptor was not coupled to its proteolysis (67). Thus, this observation also corroborates our general conclusion that PR-B proteolysis is not required for its transcriptional activities.
In conclusion, we have provided genetic and molecular evidence that the E3 ubiquitin-ligaseç E6-AP, regulates PR-B turnover in vivo and in vitro. Because PR-B is a mitogenic signaling molecule involved in mammary morphogenesis, tight regulation of PR-B expression is important for normal mammogenesis and tissue homeostasis. In addition to its role in the transcriptional regulation of PR-B, we demonstrate that E6-AP also regulates PR-B ubiquitination and its proteasome-mediated degradation. Because this mechanism is typically involved in maintaining the normal physiological proliferation rates in the mammary gland, its disruption may lead to developmental abnormalities. In this respect, E6-AP may act as a growth suppressor by negatively regulating PR-B protein levels in breast tissue. Deregulation of PR-B levels through aberrant suppression of E6-AP expression or activity may play a role in the development and progression of breast cancer. This is supported by our prior observation that E6-AP is down-regulated in invasive human breast cancers compared with their adjacent normal tissues. This study allowed us to dissect the two distinct functions of E6-AP and indicates that E6-AP exclusively functions as an E3-ligase during mammary gland development. This provides further support for the physiological linkage of PR-B levels and how it is maintained by an E-3 ligase in the mammary tissue. In summary, for the first time; we show that E6-AP functions as an isoform-specific negative regulator of PR-B, and the ubiquitin proteasome-mediated degradation of PR-B is not essential for its transactivation function. This study also highlights the fact that this mechanism is typically involved in maintaining the normal physiology of the mammary gland, and dysregulation of this mechanism may lead to development and progression of cancer.
Materials and Methods
Cell lines and reagents
T47D cell line was routinely cultured in RPMI medium supplemented with 10% (vol/vol) fetal calf serum and 100 μg/ml penicillin/streptomycin. T47D (CAT0) cell line was maintained in 0.2 mg/ml geneticin (Invitrogen, Carlsbad, CA) containing medium in a 37 C incubator with 5% CO2. Cycloheximide solution was prepared in ethanol. The Western lighting chemiluminescence reagent plus was purchased from PerkinElmer Life Sciences (Boston, MA). All other chemicals were from Sigma Chemical Co. (St. Louis, MO). Antihuman progesterone antibody (1294) was kindly provided by Dr. Dean P. Edwards (Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX). The anti-FLAG and β-actin monoclonal antibodies were purchased from Sigma. The biotinylated goat secondary antibodies and Vectastain avidin biotinylated enzyme complex and 3,3′-diaminobenzidine kits were purchased from Vector Laboratories (Burlingame, CA). The anti-ER antibody (MC-20), anti-PR antibody (C-19), and anti-Wnt-4 antibody were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). E6-AP antibodies were purchased from Bethyl Laboratories (Montgomery, TX).
Animals
All animals were maintained in accordance with the National Institute of Health directives for the Care and Use of Laboratory Animals, and the University of Miami Animal Care and Use Committee approved all experimental procedures. E6-AP-null mice were developed as described previously (52) and were maintained in a C57BL/6 background. The progeny of mating between two heterozygous parents were screened for the desired genotype using PCR as described previously (52).
RNA extraction and real-time quantitative PCR
For RNA isolation from mammary tissue, the epithelial cells were isolated from the mammary tissue as described previously (68). Total RNA was isolated from mammary epithelial cells or T47D cells using RNeasy kit (QIAGEN, Hilden, Germany). Total RNA (1 μg) was used to synthesize cDNA with the Quantitect Reverse Transcription kit (QIAGEN). Relative mRNA levels were determined by real-time PCR incorporating SYBR green on an iCycler real-time PCR machine (Bio-Rad Laboratories, Inc., Hercules, CA). Transcripts were normalized against Calnexin mRNA and are expressed relative to the message levels in control samples. Values represent the average and sem of four independent experiments.
RNA interference
All siRNA duplexes were purchased from Dharmacon (Lafayette, CO). For each RNA interference experiment, cells were prepared at 70% confluence 1 d before transfection. Quantities of each siRNA (60 nm) were transfected into cells with RNAiMAX transfection reagent from Invitrogen following the manufacturer’s instructions. After 48 h cells were induced with 10 nm progesterone for 6 h and harvested for further analysis.
Cycloheximide chase
T47D cells were transfected with E6-AP siRNA or control scrambled siRNA in six-well plates as above. Cells were treated 2 d after transfection with 10−8 m progesterone and 200 μg/ml cycloheximide. The cells were lysed for Western blot analysis at the indicated time points after cycloheximide addition. Signal intensities derived from two separate experiments were quantified using Image J software and expressed relative to β-actin level.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed with T47D CAT0 cells as described previously (30). The ChIP assay was performed using real-time quantitative PCR with primers encompassing each of the six Wnt-4 PREs within the 10-kb upstream region (Supplemental Table 2).
In vitro ubiquitination using purified PR-B
Recombinant PR-B protein was produced from baculovirus-infected Sf9 cells. The protein was purified by HisTrap FF Column (GE Healthcare, Piscataway, NJ) chromatography using the N-terminal His6 tag. In vitro ubiquitination was performed with 20 ng of purified PR-B, 10 ng E1 (ubiquitin activating enzyme), 100 ng E2 (ubiquitin-conjugation enzyme) (Boston Biochem, Boston, MA), 100 ng of purified E6-AP, and 4 μg of ubiquitin in a 30-μl reaction volume for 6 h at 30 C. The reaction mixture was analyzed by Western blot using PR-B and ubiquitin (Novocastra Laboratories Ltd, Newcastle, UK)-specific antibodies.
Acknowledgments
We thank Drs. John P. Lydon, Jianming Xu (Baylor College of Medicine, Houston, TX), and Joyce Slingerland (University of Miami Miller School of Medicine, Miami, FL) for critical reading of the manuscript. We are grateful to Dr. Sophia Tsai (Baylor College of Medicine) for providing the MMTVkBpA expression vector and to Dr. Dean P. Edwards (Baylor College of Medicine) for the PR antibody (1294), respectively.
NURSA Molecule Pages:
Coregulators: E6AP;
Ligands: Progesterone | RU486;
Nuclear Receptors: ERα | PR.
Footnotes
This work was supported by grants from the Department of Defense breast cancer grants (DAMD-17-99-1-9075 and DAMD-17-00-1-0142) (to Z.N.) and funds provided by the University of Miami/Sylvester’s Braman Family Breast Cancer Institute.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 9, 2010
Abbreviations: BrdU, Bromodeoxyuridine; ChIP, chromatin immunoprecipitation; E6-AP, E6-associated protein; ER, estrogen receptor; hect, homologous to the E6-AP carboxy terminus; KO, knockout; MMTV, mouse mammary tumor virus; NHR, nuclear hormone receptor; PR, progesterone receptor; PRE, progesterone response element; siRNA, small interfering RNA; WT, wild type.
References
- 1.Daniel CW, Silberstein GB, Strickland P1987. Direct action of 17 β-estradiol on mouse mammary ducts analyzed by sustained release implants and steroid autoradiography. Cancer Res 47:6052–6057 [PubMed] [Google Scholar]
- 2.Fendrick JL, Raafat AM, Haslam SZ1998. Mammary gland growth and development from the postnatal period to postmenopause: ovarian steroid receptor ontogeny and regulation in the mouse. J Mammary Gland Biol Neoplasia 3:7–22 [DOI] [PubMed] [Google Scholar]
- 3.Hennighausen L, Robinson GW1998. Think globally, act locally: the making of a mouse mammary gland. Genes Dev 12:449–455 [DOI] [PubMed] [Google Scholar]
- 4.Silberstein GB, Van Horn K, Shyamala G, Daniel CW1994. Essential role of endogenous estrogen in directly stimulating mammary growth demonstrated by implants containing pure antiestrogens. Endocrinology 134:84–90 [DOI] [PubMed] [Google Scholar]
- 5.Topper YJ, Freeman CS1980. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev 60:1049–1106 [DOI] [PubMed] [Google Scholar]
- 6.Brisken C2002. Hormonal control of alveolar development and its implications for breast carcinogenesis. J Mammary Gland Biol Neoplasia 7:39–48 [DOI] [PubMed] [Google Scholar]
- 7.Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA1998. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci USA 95:5076–5081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, Eddy EM, Migliaccio S, Snedeker SM, Lubahn DB, Schomberg DW, Smith EP1996. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res 51:159–186; discussion 186–188 [PubMed] [Google Scholar]
- 9.Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery Jr CA, Shyamala G, Conneely OM, O'Malley BW1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 10.Mueller SO, Clark JA, Myers PH, Korach KS2002. Mammary gland development in adult mice requires epithelial and stromal estrogen receptor α. Endocrinology 143:2357–2365 [DOI] [PubMed] [Google Scholar]
- 11.Neville MC, McFadden TB, Forsyth I2002. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia 7:49–66 [DOI] [PubMed] [Google Scholar]
- 12.Beato M, Herrlich P, Schütz G1995. Steroid hormone receptors: many actors in search of a plot. Cell 83:851–857 [DOI] [PubMed] [Google Scholar]
- 13.Kinyamu HK, Archer TK2004. Modifying chromatin to permit steroid hormone receptor-dependent transcription. Biochim Biophys Acta 1677:30–45 [DOI] [PubMed] [Google Scholar]
- 14.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM1995. The nuclear receptor superfamily: the second decade. Cell 83:835–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai MJ, O'Malley BW1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- 16.Horwitz KB, Jackson TA, Bain DL, Richer JK, Takimoto GS, Tung L1996. Nuclear receptor coactivators and corepressors. Mol Endocrinol 10:1167–1177 [DOI] [PubMed] [Google Scholar]
- 17.McKenna NJ, O'Malley BW2002. Minireview: nuclear receptor coactivators–an update. Endocrinology 143:2461–2465 [DOI] [PubMed] [Google Scholar]
- 18.Chen JD2000. Steroid/nuclear receptor coactivators. Vitam Horm 58:391–448 [DOI] [PubMed] [Google Scholar]
- 19.Lonard DM, O'Malley BW2007. Nuclear receptor coregulators: judges, juries, and executioners of cellular regulation. Mol Cell 27:691–700 [DOI] [PubMed] [Google Scholar]
- 20.McKenna NJ, Xu J, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW1999. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J Steroid Biochem Mol Biol 69:3–12 [DOI] [PubMed] [Google Scholar]
- 21.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953–959 [DOI] [PubMed] [Google Scholar]
- 22.Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194–198 [DOI] [PubMed] [Google Scholar]
- 23.Freedman LP1999. Increasing the complexity of coactivation in nuclear receptor signaling. Cell 97:5–8 [DOI] [PubMed] [Google Scholar]
- 24.McKenna NJ, Nawaz Z, Tsai SY, Tsai MJ, O'Malley BW1998. Distinct steady-state nuclear receptor coregulator complexes exist in vivo. Proc Natl Acad Sci USA 95:11697–11702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset D, Chambon P1989. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell 57:433–442 [DOI] [PubMed] [Google Scholar]
- 26.Poukka H, Aarnisalo P, Karvonen U, Palvimo JJ, Jänne OA1999. Ubc9 interacts with the androgen receptor and activates receptor-dependent transcription. J Biol Chem 274:19441–19446 [DOI] [PubMed] [Google Scholar]
- 27.Shemshedini L, Ji JW, Brou C, Chambon P, Gronemeyer H1992. In vitro activity of the transcription activation functions of the progesterone receptor. Evidence for intermediary factors. J Biol Chem 267:1834–1839 [PubMed] [Google Scholar]
- 28.Alarid ET2006. Lives and times of nuclear receptors. Mol Endocrinol 20:1972–1981 [DOI] [PubMed] [Google Scholar]
- 29.Verma S, Ismail A, Gao X, Fu G, Li X, O'Malley BW, Nawaz Z2004. The ubiquitin-conjugating enzyme UBCH7 acts as a coactivator for steroid hormone receptors. Mol Cell Biol 24:8716–8726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis AP, Lonard DM, Nawaz Z, O'Malley BW2005. Inhibition of the 26S proteasome blocks progesterone receptor-dependent transcription through failed recruitment of RNA polymerase II. J Steroid Biochem Mol Biol 94:337–346 [DOI] [PubMed] [Google Scholar]
- 31.Rochette-Egly C2005. Dynamic combinatorial networks in nuclear receptor-mediated transcription. J Biol Chem 280:32565–32568 [DOI] [PubMed] [Google Scholar]
- 32.Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, O'Malley BW1999. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol 19:1182–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huibregtse JM, Scheffner M, Howley PM1991. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J 10:4129–4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huibregtse JM, Scheffner M, Howley PM1993. Localization of the E6-AP regions that direct human papillomavirus E6 binding, association with p53, and ubiquitination of associated proteins. Mol Cell Biol 13:4918–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huibregtse JM, Scheffner M, Beaudenon S, Howley PM1995. A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA 92:5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molenda HA, Kilts CP, Allen RL, Tetel MJ2003. Nuclear receptor coactivator function in reproductive physiology and behavior. Biol Reprod 69:1449–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi C, Kashireddy P, Zhu YT, Rao SM, Zhu YJ2004. Null mutation of peroxisome proliferator-activated receptor-interacting protein in mammary glands causes defective mammopoiesis. J Biol Chem 279:33696–33701 [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X, Loggie BW, Nawaz Z2002. The roles of sex steroid receptor coregulators in cancer. Mol Cancer 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudelist G, Czerwenka K, Kubista E, Marton E, Pischinger K, Singer CF2003. Expression of sex steroid receptors and their co-factors in normal and malignant breast tissue: AIB1 is a carcinoma-specific co-activator. Breast Cancer Res Treat 78:193–204 [DOI] [PubMed] [Google Scholar]
- 41.Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J2004. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res 64:1875–1885 [DOI] [PubMed] [Google Scholar]
- 42.Leygue E, Dotzlaw H, Watson PH, Murphy LC1999. Expression of the steroid receptor RNA activator in human breast tumors. Cancer Res 59:4190–4193 [PubMed] [Google Scholar]
- 43.Murphy LC, Watson P2002. Steroid receptors in human breast tumorigenesis and breast cancer progression. Biomed Pharmacother 56:65–77 [DOI] [PubMed] [Google Scholar]
- 44.Wang N, Kudryavtseva E, Ch'en IL, McCormick J, Sugihara TM, Ruiz R, Andersen B2004. Expression of an engrailed-LMO4 fusion protein in mammary epithelial cells inhibits mammary gland development in mice. Oncogene 23:1507–1513 [DOI] [PubMed] [Google Scholar]
- 45.Watkins G, Douglas-Jones A, Mansel RE, Jiang WG2004. The localisation and reduction of nuclear staining of PPARγ and PGC-1 in human breast cancer. Oncol Rep 12:483–488 [PubMed] [Google Scholar]
- 46.Lonard DM, Nawaz Z, Smith CL, O'Malley BW2000. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- 47.Reid G, Hübner MR, Métivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F2003. Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling. Mol Cell 11:695–707 [DOI] [PubMed] [Google Scholar]
- 48.Poole AJ, Li Y, Kim Y, Lin SC, Lee WH, Lee EY2006. Prevention of Brca1-mediated mammary tumorigenesis in mice by a progesterone antagonist. Science 314:1467–1470 [DOI] [PubMed] [Google Scholar]
- 49.Aupperlee MD, Haslam SZ2007. Differential hormonal regulation and function of progesterone receptor isoforms in normal adult mouse mammary gland. Endocrinology 148:2290–2300 [DOI] [PubMed] [Google Scholar]
- 50.Aupperlee MD, Smith KT, Kariagina A, Haslam SZ2005. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577–3588 [DOI] [PubMed] [Google Scholar]
- 51.Bocchinfuso WP, Korach KS1997. Mammary gland development and tumorigenesis in estrogen receptor knockout mice. J Mammary Gland Biol Neoplasia 2:323–334 [DOI] [PubMed] [Google Scholar]
- 52.Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL1998. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 21:799–811 [DOI] [PubMed] [Google Scholar]
- 53.Silberstein GB2001. Postnatal mammary gland morphogenesis. Microsc Res Tech 52:155–162 [DOI] [PubMed] [Google Scholar]
- 54.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem 277:5209–5218 [DOI] [PubMed] [Google Scholar]
- 55.Brisken C, Heineman A, Chavarria T, Elenbaas B, Tan J, Dey SK, McMahon JA, McMahon AP, Weinberg RA2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev 14:650–654 [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson GW, Hennighausen L, Johnson PF2000. Side-branching in the mammary gland: the progesterone-Wnt connection. Genes Dev 14:889–894 [PubMed] [Google Scholar]
- 57.Shyamala G, Yang X, Cardiff RD, Dale E2000. Impact of progesterone receptor on cell-fate decisions during mammary gland development. Proc Natl Acad Sci USA 97:3044–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E1998. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci USA 95:696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW1999. Proteasome-dependent degradation of the human estrogen receptor. Proc Natl Acad Sci USA 96:1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanz RB, Chua SS, Barron N, Söder BM, DeMayo F, O'Malley BW2003. Steroid receptor RNA activator stimulates proliferation as well as apoptosis in vivo. Mol Cell Biol 23:7163–7176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- 62.Smith CL, DeVera DG, Lamb DJ, Nawaz Z, Jiang YH, Beaudet AL, O'Malley BW2002. Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol 22:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lydon JP, DeMayo FJ, Conneely OM, O'Malley BW1996. Reproductive phenotpes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol 56:67–77 [DOI] [PubMed] [Google Scholar]
- 64.Tilli MT, Frech MS, Steed ME, Hruska KS, Johnson MD, Flaws JA, Furth PA2003. Introduction of estrogen receptor-α into the tTA/TAg conditional mouse model precipitates the development of estrogen-responsive mammary adenocarcinoma. Am J Pathol 163:1713–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z2005. Decreased expression of E6-associated protein in breast and prostate carcinomas. Endocrinology 146:1707–1712 [DOI] [PubMed] [Google Scholar]
- 66.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc Natl Acad Sci USA 100:9744–9749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deroo BJ, Rentsch C, Sampath S, Young J, DeFranco DB, Archer TK2002. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol Cell Biol 22:4113–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pullan S, Wilson J, Metcalfe A, Edwards GM, Goberdhan N, Tilly J, Hickman JA, Dive C, Streuli CH1996. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci 109:631–642 [DOI] [PubMed] [Google Scholar]