Abstract
Retrovirus insertion-mediated random mutagenesis was applied in 3T3-L1 preadipocyte cells to better understand the molecular basis of obesity (the expansion of individual adipocytes). We found that tryptophan hydroxylase-1, a rate-limiting enzyme for the synthesis of serotonin (5-HT), is expressed in adipocytes and is required for their differentiation. A 5-HT type 2A receptor (5-HT2AR) antagonist, ketanserin, and a 5-HT2cR antagonist, SB-242084, inhibited adipocyte differentiation. Because 5-HT2cR mRNA levels are up-regulated during adipocyte differentiation and micro-RNA (miR)-448 is located in the fourth intron of Htr2c, we also studied the role of miR-448 in 3T3-L1 cells. Through a bioinformatics approach, Krüppel-like factor 5 (KLF5) was identified as a potential target of miR-448. Using a luciferase reporter assay, we confirmed that miR-448 targets the Klf5 3′-intranslated region. Overexpression of miR-448 reduced the expression of Klf5 and adipocyte differentiation, which was confirmed by the reduced expression of adipogenic genes and triglyceride accumulation. To examine the loss of miR-448 function, we constructed a decoy gene that had tandem complementary sequences for miR-448 in the 3′-untranslated region of a luciferase gene under the control of a cytomegalovirus promoter. When the miR-448 decoy gene was introduced into 3T3-L1 preadipocytes, KLF5 was up-regulated and triglyceride concentration was increased. In this study, we identified the regulation of adipocyte differentiation by 5-HT, 5-HT2AR, and 5-HT2CR. miR-448-mediated repression of KLF5 was identified as a negative regulator for adipocyte differentiation.
Serotonin (5-HT) is required for adipocyte differentiation, and micro RNA-448 which is encoded by an intron of 5-HT2CR inhibits adipocyte differentiation through targeting KLF5.
The key role of white adipose tissue is the storage of energy in the form of triglycerides. Recent findings have indicated that white adipocytes also function as a dynamic tissue, because these cells express and secrete many cytokines and other proteins to maintain the balance between energy intake and output (1, 2). The functions of these adipocytokines are disrupted in the obese state, which is associated with several important diseases, such as hypertension, atherosclerosis, and diabetes (3). Therefore, further insight into the molecular basis of obesity is needed to better understand obesity-associated diseases. Obesity is the result of an expansion and increase in the number of individual adipocytes. 3T3-L1 preadipocyte cells, which are fibroblastic cells determined to the adipocyte lineage, are widely used to investigate the differentiation mechanisms of adipocytes (4, 5). In vitro, adipocyte differentiation is initiated by 3T3-L1 preadipocytes in response to adipogenic inducers.
In this study, we used retrovirus insertion-mediated random mutagenesis to generate cell lines that cannot differentiate after the addition of adipogenic inducers to 3T3-L1 cells. After the isolation and expansion of each clone, we discovered that tryptophan hydroxylase-1 (TPH1), which is a rate-limiting enzyme for the synthesis of serotonin [5-hydroxytryptamine (5-HT)], is required for adipocyte differentiation in 3T3-L1cells. The need for TPH1 in adipocyte differentiation in this clone was confirmed by the restoration of potential to differentiate after the reconstitution of Tph1 gene expression using the Cre-loxP system.
5-HT is not only a neurotransmitter but also a hormone with various extraneuronal functions (6). It is a potent mitogen and modulates the remodeling of tissue (7). Thus, 5-HT is now considered to have pleiotropic effects, including effects on cell growth, inflammation, and hormone secretion (8, 9, 10). Several reports have shown that 5-HT has direct effects on peripheral tissues, which suggests that 5-HT may be involved in glucose metabolism (11, 12). The report about Htr2c knockout mice showed that the mice became obese as a result of abnormal control of feeding behavior and established that a role for this receptor is the serotonergic control of appetite in central nervous system (13). However, its peripheral function has not been tested.
We revealed that 5-HT accelerated adipocyte differentiation and 5-HT type 2A receptor (5-HT2AR) antagonist Ketanserin and the 5-HT2CR antagonist SB-242084 both reduced adipogenesis. Because Htr2c mRNA is up-regulated during adipocyte differentiation and microRNA (miR)-448 is located in the fourth intron of Htr2c, we also studied the role of miR-448 in 3T3-L1 cells.
MicroRNAs (miRNAs) are endogenous, noncoding RNAs generally 21–23 nucleotides in length that control gene expression at the posttranscriptional level by targeting mRNAs for degradation or translational repression or both (14, 15, 16). We determined that the expression of miR-448 was up-regulated during adipocyte differentiation in 3T3-L1 cells and a mouse stromal vascular fraction. The induction of miR-448 inhibited adipocyte differentiation by translational repression of Krüppel-like factor 5 (KLF5). KLF5 is a transcription factor that activates the peroxisome proliferator-activated receptor (PPAR)γ2 promoter and regulates adipocyte differentiation (17). Moreover, the suppression of endogenous miR-448 by decoy sequences increased KLF5 protein levels and enhanced adipocyte differentiation.
In this study, we identified a unique regulation of adipocyte differentiation by 5-HT, 5-HT2AR, and 5-HT2CR. The present results demonstrate that miR-448 plays a role as a negative regulator in adipogenesis through targeting KLF5.
Results
TPH1 was disrupted by the insertion of pDisrup-ΔEn-loxP in one of the differentiation-resistant cell lines
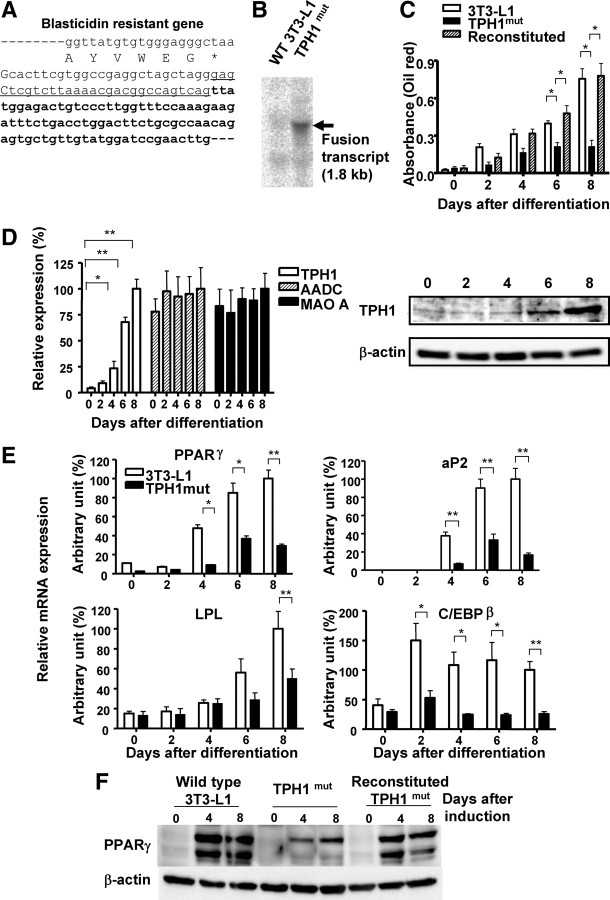
We used retrovirus insertion-mediated mutagenesis in 3T3-L1 preadipocytes coupled with adipocyte induction to select differentiation-resistant cell lines. Supplemental Fig. 1A, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org, shows the pDisrup-ΔEn-loxP gene-trap vector. The 5′-untranslated region (UTR) of the vector has a wild-type sequence, whereas the 3′-long terminal repeat lacks the transcriptional enhancer in its U3 portion. Because the nucleotide sequence of the 3′-UTR is accurately copied to the 5′-UTR during reverse transcription and integration, the 5′-UTR in infected cells also lacks the enhancer and possesses a loxP signal in the same orientation as that in the 3′-UTR (Supplemental Fig. 1B). Therefore, Cre-mediated excision can remove this viral insertion (Supplemental Fig. 1C). Because the retroviral vector was designed so that the blasticidin gene was fused to the sequence of the exon at the 3′-end of the viral insertion site, the disrupted genes in various differentiation-resistant cell lines were determined by 3′-rapid amplification of cDNA ends (RACE) of the fused blasticidin mRNA. The gene that was disrupted in one of the differentiation-resistant cell lines was identified as Tph1. A partial sequence of the fused gene product generated by retroviral insertion in this line (TPH1mut) is shown in Fig. 1A. Northern blotting analysis using a blasticidin probe indicated that there is a single viral insertion in the TPH1mut line (Fig. 1B). The size of the blasticidin Tph1 fusion mRNA was consistent with the predicted length.
Fig. 1.
TPH1 is involved in adipocyte differentiation in 3T3-L1 cells. A, The fused mRNA of blasticidin and an endogenous gene in a differentiation-resistant 3T3-L1 cell line was amplified by 3′-RACE. The junction sequence of the fused cDNA is shown, which revealed that viral insertion occurred at the 5′-end of exon 4 of the Tph1 gene. The amino acid sequence at the C terminus of blasticidin is shown beneath the mRNA sequence. The sequence introduced by the viral vector is shown in lowercase. B, Total RNA was prepared from TPHmut and wild-type 3T3-L1cells. Blasticidin mRNA levels were analyzed by Northern bloting using a 32P-labeled double-stranded blasticidin probe. A single blasticidin fusion mRNA was detected in TPH1mut cells, as indicated by the triangle. C, Wild-type 3T3-L1, TPH1mut, and reconstituted TPH1mut cells were treated with adipogenic inducers for various periods of time, as indicated. Adipogenesis was measured at baseline and at 2 d, 4 d, 6 d, and 8 d after the initiation of adipogenesis using the triglyceride-specific dye oil red O. Triglyceride accumulation was expressed in OD units. Values are the means ± se from at least three experiments. *, P < 0.05 vs. control 3T3-L1 cells or TPH1mut cells. D, The expression levels of the genes encoding Tph1, Aadc, and Maoa were measured by real-time PCR analysis in 3T3-L1 cells. The mean expression level at 8 d after differentiation was defined as 100%. The data shown are means ± se from four replicates. *, P < 0.05; **, P < 0.01 vs. 3T3-L1 cells on d 0. Protein expression of TPH1 was confirmed by Western blotting. E, Expression levels of adipogenic marker genes by real-time RT-PCR in TPH1mut and wild-type 3T3-L1cells. The mean expression level at 8 d after differentiation was defined as 100%. Values are the means ± se from three independent experiments. *, P < 0.05; **, P < 0.01 vs. control 3T3-L1 cells. F, Protein expression of PPARγ was assessed by Western blotting in wild-type 3T3-L1, TPH1mut, and reconstituted TPH1mut cells. LPL, Lipoprotein lipase.
Disruption of the TPH1 gene in 3T3-L1 cells conferred resistance to differentiation induction
We next removed the integrated proviruses by Cre-mediated homologous recombination and obtained a clone with phenotypic reversion after provirus excision (designated reconstituted). Western blotting analysis showed that there was a reduction in TPH1 immunoreactivity in the TPH1mut cell line compared with wild-type 3T3-L1 cells and with TPH1-reconstituted cells (Supplemental Fig. 2A). We assessed the resistance to differentiation induction in the TPH1mut line based on the concentration of triglyceride as measured by use of the triglyceride-specific dye oil red O. At 6 d and 8 d after the initiation of adipogenesis, triglyceride accumulation was also reduced in the TPH1mut cell line compared with those in the wild-type 3T3-L1 and reconstituted lines (Fig. 1C). TPH converts l-tryptophan to 5-hydroxy-tryptophan, which is metabolized to 5-HT by aromatic amine decarboxylase (AADC). The mitochondrial enzyme monoamine oxidase A (MAO-A) then metabolizes 5-HT. We measured the expression levels of the genes encoding Tph1, Aadc, and Maoa in 3T3-L1 cells by real-time RT-PCR analysis. Tph1 expression was markedly enhanced during adipocyte differentiation, whereas the expression levels of Aadc and Maoa were relatively constant (Fig. 1D). According to the results of Western blotting, the expression level of TPH1 protein increased gradually after adipogenic induction (Fig. 1D). Immunohistochemical analysis revealed that TPH1 is mainly expressed in differentiated 3T3-L1 cells (Supplemental Fig. 2B).
We determined the expression levels of adipogenic marker genes after the induction of differentiation by real-time RT-PCR in TPH1mut cells and wild-type 3T3-L1 cells. The expression levels of PPARγ, adipocyte fatty acid-binding protein (aP2), lipoprotein lipase, and CCAAT/enhancer binding protein (C/EBP)β after the induction of differentiation were lower in TPH1mut cells than in wild-type cells (Fig. 1E), indicating that the reduction in Tph1 expression inhibited adipogenesis of 3T3-L1 cells. The changes in differentiation levels in each cell line were also confirmed by Western blotting using PPARγ in wild-type 3T3-L1, TPH1mut, and reconstituted TPH1mut cells (Fig. 1F).
Serotonergic agents affected adipocyte differentiation
After treatment with differentiation-inducing agents, the concentration of 5-HT increased in wild-type 3T3-L1 cells and reconstituted lines but not in the TPH1mut cell line (Fig. 2A). This was consistent with the prediction that 5-HT peptide synthesis is reduced in TPH1mut cells. We also confirmed that the mutant phenotype is caused by the lack of 5-HT. We compared the differentiation efficiency among three lines of wild-type 3T3-L1, TPH1mut, and TPH1mut treated with 20 μm 5-HT. As shown in Fig. 2B, 5-HT clearly enhanced the staining of oil red O to the same level as wild-type 3T3-L1 cells 8 d after differentiation. The effects of 5-HT and serotonergic agents on adipocyte differentiation were studied in 3T3-L1 cells and a stromal vascular fraction (SVF) prepared from C57/BL6 mice. When 5-HT was added along with differentiation-inducing agents, triglyceride accumulation shown by oil red O staining was enhanced significantly in 3T3-L1 cells and in SVF (Fig. 2C). We also measured the effects of p-chlorophenylalanine (PCPA), which inhibits TPH activity, on adipogenesis. The stimulatory effect of 5-HT on adipogenesis was measured on d 8 using oil red O. Although 5-HT enhanced adipogenesis, PCPA inhibited adipogenesis in a concentration-dependent manner in 3T3-L1 cells (Fig. 2D) and in SVF (Fig. 2E).
Fig. 2.
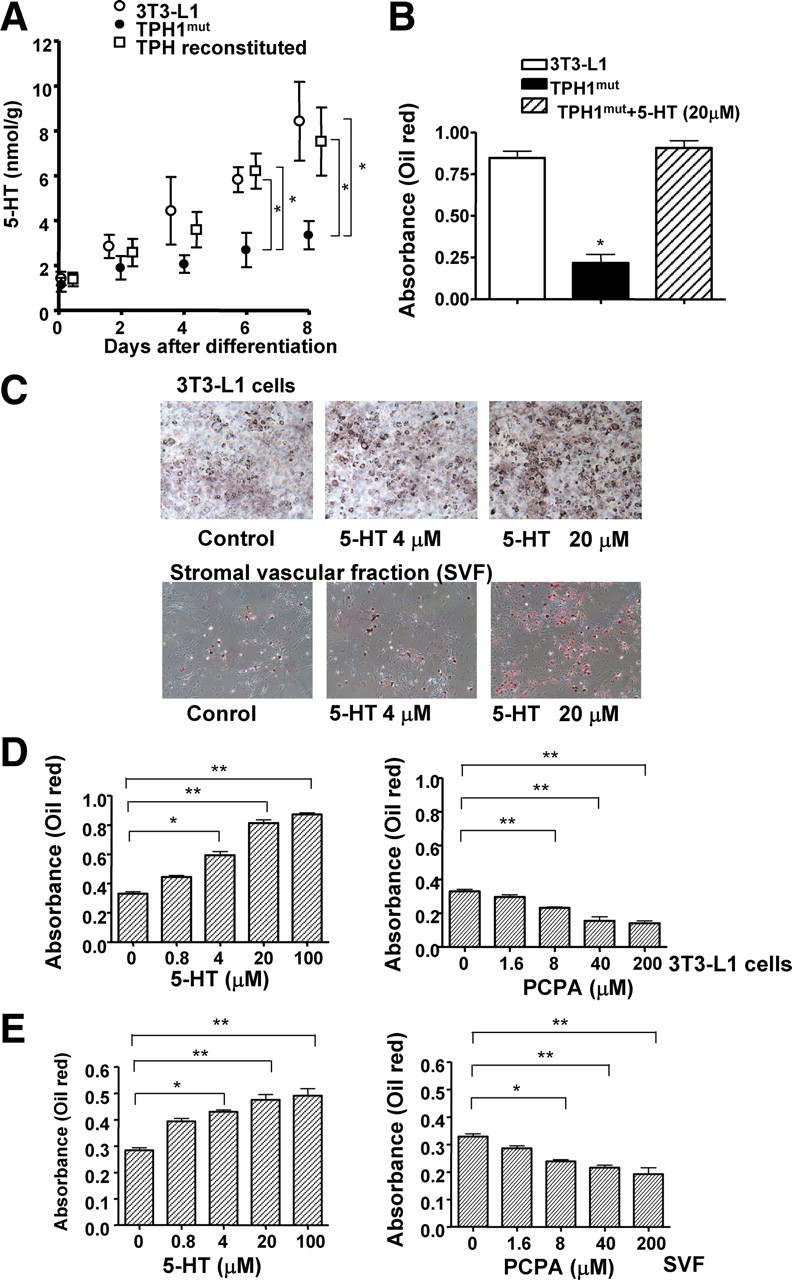
Serotonergic agents affect adipocyte differentiation. A, Stable cell lines were generated from TPH1mut cells by transduction with Cre recombinase. The concentration of 5-HT in these stable cell lines was measured by ELISA. Values are the means ± se from at least three experiments. *, P < 0.05 vs. TPH1mut cells. B, The efficiency of differentiation was measured using the accumulation of oil red O by densitometric analysis in wild-type 3T3-L1, TPH1mut, and TPH1mut treated with 5-HT (20 μm). *, P < 0.05. C, Photographs show oil red O staining of differentiated adipocytes at various concentrations of 5-HT in 3T3-L1 cells and SVF. D and E, The efficiency of differentiation was measured using the accumulation of oil red O by densitometric analysis. Effects of 5-HT or PCPA on adipocyte differentiation in 3T3-L1 cells (D) and SVF (E). The data shown are means ± se from six experiments.*, P < 0.05; **, P < 0.01 vs. control cells.
5-HT receptors (5-HTRs) and adipogenesis
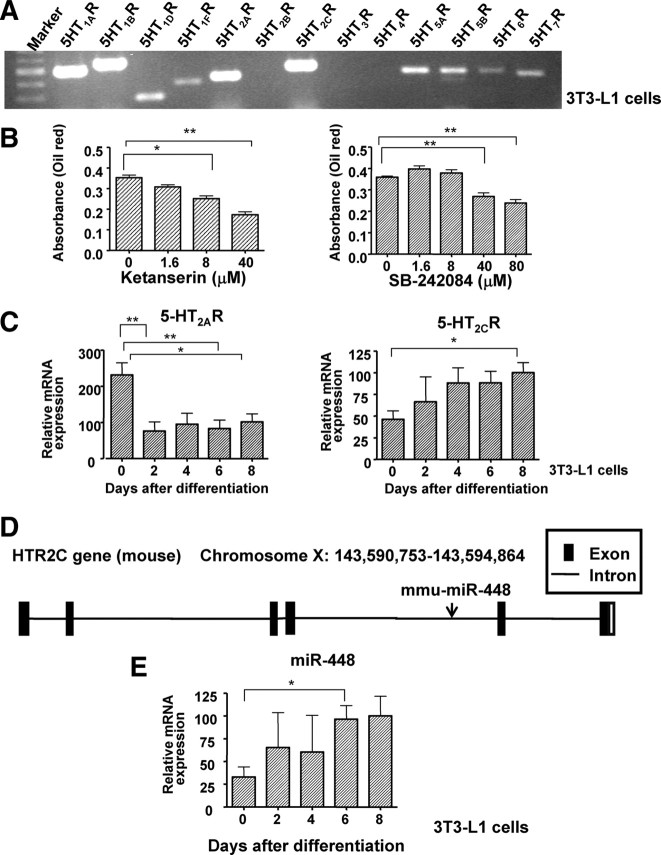
The effect of 5-HT on physiology and behavior are mediated by multiple 5-HTRs clustered into seven distinct families that are widely expressed in the central and/or peripheral nervous systems (18). The distribution of 5-HTR subtypes in white adipose tissue had not been determined in detail. We detected numerous kinds of 5-HTRs, including 5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1FR, 5-HT2AR, 5-HT2CR, 5-HT5AR, 5-HT5BR, 5-HT6R, and 5-HT7R (Fig. 3A). We have found that the 5-HT2AR antagonist ketanserin and the 5-HT2cR antagonist SB-242084 both inhibited adipocyte differentiation (Fig. 3B). The expression of Htr2c was increased in 3T3-L1 cells differentiated by treatment with differentiation-inducing agents. On the other hand, the expression of Htr2a was decreased during adipocyte differentiation (Fig. 3C).
Fig. 3.
5-HTRs are related to adipogenesis. A, The expression of 5-HT receptor subtypes in 3T3-L1 cells was measured by RT-PCR. 5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1FR, 5-HT2AR, 5-HT2CR, 5-HT5AR, 5-HT5BR, 5-HT6R, and 5-HT7R were detected in 3T3-L1 cells. B, Effects of 5-HT2AR antagonist (ketanserin) and 5-HT2CR antagonist (SB-242084) on adipocyte differentiation in 3T3-L1 cells. The graphs shows oil red O staining of differentiated adipocytes under various concentrations at 8 d after differentiation. The data shown are means ± se from six replicates. *, P < 0.05; **, P < 0.01 vs. control cells. C, The expression levels of 5-HT2AR and 5-HT2CR after induction of differentiation. D, mmu-miR-448 is located within the fourth intron of 5-HT2CR. E, Mature miR-448 expression during adipocyte differentiation was measured in 3T3-L1 cells by RT-PCR. U6 was used as an internal control for normalization. The mean expression level at 8 d after differentiation was defined as 100%. The data shown are means ± se. from three experiments. *, P < 0.05 vs. 3T3-L1 on d 0.
miR-448 inhibits 3T3-L1 cell differentiation
miR-448 is encoded by an intron of Htr2c (Fig. 3D). We measured the expression level of miR-448 during adipocyte differentiation. The miR-448 expression level was increased in 3T3-L1 cells differentiated by treatment with differentiation-inducing agents (Fig. 3E).
Through a bioinformatics approach (Targetscan database http://www.targetscan.org/), Krüppel-like factor 5 (KLF5) was identified as a potential target of miR-448 (Fig. 4A). Using a luciferase reporter assay, we confirmed that miR-448 targets the Klf5 3′-UTR. We cotransfected a construct containing a luciferase gene with the 3′-UTR of Klf5 cloned immediately downstream and the miR-448 expression plasmid into human embryonic kidney (HEK) 293T cells and we measured luciferase activity. Overexpression of miR-448 resulted in a reduction of luciferase activity (Fig. 4B and Supplemental Fig. 3A). To further confirm this specificity, we mutated the candidate miR-448 target sites (Fig. 4A), which resulted in the loss of miR-448-mediated repression (Fig. 4B). Overexpression of miR-448 reduced the protein level of KLF5, whereas it did not change the Klf5 mRNA level (Fig. 4C and Supplemental Fig. 3B). Transfection of miR-448 in 3T3-L1 cells before the addition of differentiation-inducing agents inhibited differentiation, which was confirmed by oil red O staining (Fig. 4D) or by direct measurement of intracellular triglyceride content (Fig. 4E). We determined the expression levels of adipogenic markers by real-time RT-PCR in cells overexpressing miR-448 (Fig. 4F). The expression of C/EBPα and PPARγ was decreased significantly in cells transfected with miR-448. Mature adipocyte marker aP2 was decreased on d 2, d 4, and d 8. Moreover, the expression level of perilipin, which is important in lipolysis, was lower in miR-448-overexpressing cells than in control-microRNA (c-miR) expressing cells. In contrast, C/EBPβ and C/EBPδ were not affected significantly by miR-448. We also confirmed the difference in differentiation by Western blotting for PPARγ. As shown in Fig. 4H, the PPARγ protein expression level was clearly reduced in miR-448-overexpressing cells.
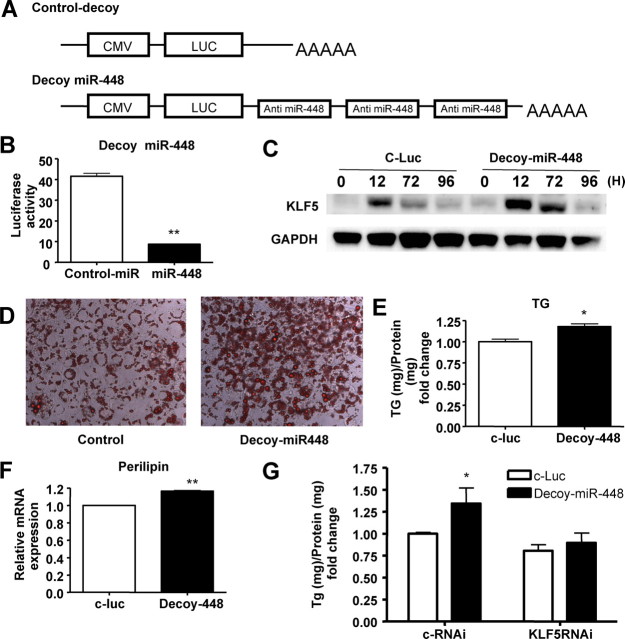
Fig. 4.
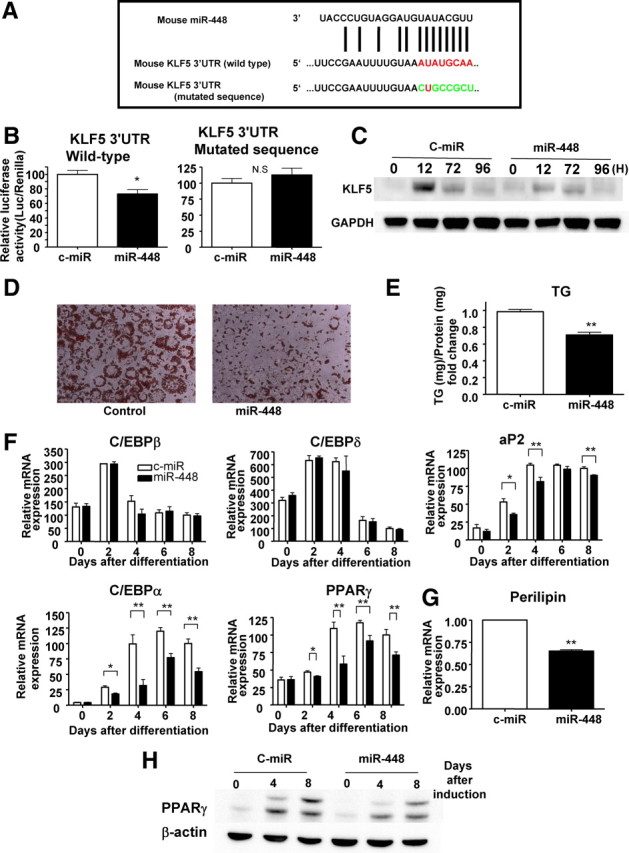
Effect of miR-448 on Klf5 expression levels and adipogenesis. A, Mature miR-448 sequence and the 3′-UTR of the Klf5 mRNA. Constructs with mutated seed sequences were used as mutated controls. B, Klf5 3′-UTR firefly luciferase activity in HEK 293T cells and normalized using Renilla luciferase. Left, Cotransfection of the constructs containing the luciferase gene with the 3′-UTR of Klf5 (wild type) and the miR-448 or control miR expression plasmids. Right, Cotransfection of constructs containing the luciferase gene with the 3′-UTR of Klf5 (mutated sequence) and the miR-448 or control-miR expression plasmids. Mean values of the miR-control were set at 100%. The data represent means ± se from at least three experiments. *, P < 0.05; **, P < 0.01 vs. control-miR cells. C, Western blotting analysis for the expression of Klf5 in 3T3-L1 cells transduced with miR-448 or control-miR during adipogenesis. D, Oil red O staining of 3T3-L1 cells transfected with miR-448 or control at 8 d after differentiation. E, The amounts of intracellular triglyceride (TG) of 3T3-L1 cells transfected with miR-448 or control were quantified at 8 d after differentiation. The data represent means ± se from at least three replicates. **, P < 0.01 vs. control 3T3-L1 cells. F, Expression levels of adipogeneic marker genes determined by real-time RT-PCR after normalization to Gapdh mRNA in 3T3-L1 cells transfected with miR-448 or control-miR. The mean expression level at 8 d after differentiation in control 3T3-L1 cells was defined as 100%. The data shown are means ± se from three independent experiments. *, P < 0.05; **, P < 0.01 vs. control 3T3-L1 cells. G, Relative amounts of perilipin were quantified at 8 d after differentiation. The data represent means ± se from at least three replicates. **, P < 0.01 vs. control 3T3-L1 cells. H, Protein expression of PPARγ by Western blotting in c-miR and miR-448-overexpressing cells.
Decoy-miR448 accelerates adipocyte differentiation
To assess the functional consequences of silencing endogenous miR-448 in vitro, HEK 293T cells or 3T3-L1 cells infected with a lentivirus vector were used, in which the 3′-UTR with three tandem sequences complementary to miR-448 was linked to a luciferase reporter gene (decoy-miR-448) (Fig. 5A). The complementary sequences acted as a decoy, sequestering endogenous miR-448 (19, 20, 21). To confirm the effect of the decoy, we transduced the same amount of c-miR or miR-448 with decoy-miR-448 into HEK 293T cells (Fig. 5B). The cotransfection of decoy-miR448 and miR-448 reduced luciferase activity compared with c-miR. Next, we examined the functions of endogenous miR-448 in adipocyte differentiation using decoy miR-448. The differentiation efficiency of 3T3-L1 cells transduced with decoy miR-448 was greater than those transfected with the control-luc gene. Protein levels of KLF5 were higher in decoy-miR-448-transduced cells than in control cells (Fig. 5C). The greater amount of intracellular lipid accumulation was confirmed by oil red O staining (Fig. 5D) and by direct measurement of the intracellular triglyceride content (Fig. 5E). The expression level of perilipin was elevated in decoy-miR-448 transduced cells compared with control cells (Fig. 5F). To further confirm that KLF5 is the target of miR-448, we examined the effect of decoy miR-448 with or without the depletion of KLF5. As shown in Fig. 5G, the effect of decoy-miR-448 was abolished after silencing KLF5. Therefore, this experiment also suggested that KLF5 is a main target of miR-448 in the differentiation of 3T3-L1. We also confirmed the functions of KLF5 in adipocyte differentiation. We constructed Klf5 small interfering RNA (siRNA) sequences and transduced them into 3T3-L1 cells before hormonal induction. Klf5 siRNA led to a reduction of Klf5 at the mRNA level (Supplemental Fig. 4A). Consequently, knockdown of KLF5 resulted in a remarkable reduction in adipogenesis (Supplemental Fig. 4, B and C).
Fig. 5.
Knockdown of endogenous miR-448 accelerates adipocyte differentiation. A, Decoy-miR-448 vector in which a 3′-UTR with three tandem sequences complementary to miR-448 was linked to the luciferase reporter gene. B, The graph shows luciferase activity. Cotransfection of decoy-miR448 and miR-448 was compared with decoy-miR-448 and control-miR. C, Western blotting analysis for the expression of KLF5 in 3T3-L1 cells transduced with control-luc or decoy-miR-448 during adipogenesis. D, Oil red O staining of 3T3-L1 cells transfected with control-luc or decoy-miR-448 at 8 d after differentiation. E, The amounts of intracellular triglyceride (TG) were quantified at 8 d after differentiation. The data represent means ± se from at least three experiments. F, Expression levels of perilipin by real-time RT-PCR after being normalized to GAPDH mRNA levels in 3T3-L1 cells transfected with decoy-miR-448 or control at 8 d after differentiation. The data shown are means ± se from three replicates. *, P < 0.05; **, P < 0.01 vs. control 3T3-L1 cells. G, The amounts of intracellular TG were quantified at 8 d after differentiation after transduction of decoy miR-448 with or without the depletion of KLF5. The data represent means ± se from at least three experiments. *, P < 0.05 vs. control. CMV, Cytomegalovirus; LUC, luciferase.
Discussion
The present study provides the first evidence that serotonin (5-HT) is a novel autocrine/paracrine factor that is required for adipocyte differentiation. The major findings of this study include; 1) TPH1 is expressed in adipocytes and is required for their differentiation; 2) 5-HT signaling is involved in adipocyte differentiation in culture, especially through 5-HT2A R and 5-HT2C R; and 3) miR-448, which is located in the fourth intron of 5-HT2C R, inhibited adipocyte differentiation through targeting KLF5.
It has been well documented that the transcription factors PPARγ, SREBP-1, and the C/EBP family function as master regulators of adipocyte differentiation (22). To obtain new insights into the mechanism of the regulation of adipocyte differentiation, we used a functional gene identification procedure based on the poly-A trap technique. The combination of a potentially strong splice acceptor and an effective polyadenylation signal assured complete disruption of the function of the trapped gene (23). Because loxP contains LTRs at both ends, the integrated proviruses can be removed from the genome of infected cells by Cre-mediated homologous recombination (24). This method enabled us to confirm that the resulting resistance to the adipogenic inducers was actually brought about by provirus insertion.
Although fat tissue is known to synthesize a variety of peptide growth factors and cytokines (25, 26, 27), before our studies there was no evidence of biogenic monoamine synthesis in adipocytes. The 5-HT biosynthetic system was demonstrated by the discovery of a Tph1 gene that was preferentially expressed in peripheral tissues as well as a gene for Tph2, a neuronal Tph isoform (28). We discovered that the peripheral Tph1 gene is expressed in adipocytes and is required for adipocyte differentiation. Treatment with 5-HT enhanced adipocyte differentiation in 3T3-L1 cells and in a stromal vascular fraction prepared from mice. Conversely, inactivation of the 5-HT pathway by a TPH inhibitor, a 5-HTR antagonist, or Tph1 gene deletion resulted in reduced differentiation potency in preadipocytes. These findings demonstrated that adipocytes constitute a serotonergic and autocrine-paracrine system that plays an important regulatory role in adipocyte differentiation.
Although detailed and extensive pharmacological studies with a variety of serotonergic agonists and antagonists will be needed to sort out the roles of each 5-HTR subtype according to their specific functions in fat tissue, the present results indicate that 5-HT2A R and 5-HT2C R signaling is involved in adipocyte differentiation. We did not show detailed mechanisms in this manuscript; however, both 5-HT2AR and 5-HT2CR are coupled with a Gq subunit, and signaling events initiated through these G protein-coupled receptors may affect adipocyte differentiation in vitro.
Because Htr2c is up-regulated during adipocyte differentiation and it has miR-448 in its fourth intron, we further evaluated its function during adipogenesis. WAY-161503 is a modestly selective 5-HT2CR agonist that possesses exceptional potency with 5-HT2C R (Ki = 3 nm; EC50 = 8 nm) (29, 30). WAY-161503 decreases food intake in several animal models of obesity and decreases body weight gain or body weight after repeated administration (29). Because WAY-161503 induced adipocyte differentiation (Supplemental Fig. 5), the present results are consistent with the improvement of insulin sensitivity by 5-HT2C R agonists, which act not only in the central nervous system but also in non-central nervous system sites, such as adipose tissues (31).
miRNAs are endogenous, noncoding RNAs, generally 21–23 nucleotides in length, that control gene expression at the posttranscriptional level by targeting mRNAs for degradation or translational repression or both (14, 15, 16). miRNAs control cell growth and differentiation and are involved in diseases such as cancer, neurological disorders, and other developmental diseases (32). Although miRNA expression profiles and functions have been investigated extensively in the hematopoietic system and neuronal and muscle tissues, little is known about the role of miRNAs in metabolic disorders. Several groups have examined the expression of miRNAs during adipocyte differentiation (33, 34). miR-143 is up-regulated after induction of differentiation in human preadipocytes and mouse 3T3-L1 cells, and its inhibition with antisense oligonucleotides blocked differentiation (35). Up-regulated expression of miR-143 is associated with obesity in adipose tissue of mice fed a high-fat diet (36). let-7 is up-regulated during 3T3-L1 adipogenesis, and ectopic introduction of let-7 inhibits clonal expansion as well as terminal differentiation by targeting HMGA2 (37). On the other hand, the miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor RB2/P130 (38).
In the present study, we focused on miR-448, which is encoded in an intronic sequence of the serotonin Htr2c. The miR-448-binding site in the 3′-UTR of Klf5 is highly conserved among mammals (TargetScan), and the luciferase assay indicated that miR-448 targets Klf5 3′-UTR. Both overexpression and knockdown of miR-448 did not alter the mRNA level of Klf5 but changed the protein level in 3T3-L1 cells. These findings were consistent with the translational repression by miRNAs. miR-448 expression levels were increased during adipogenesis especially in the late stage. On the other hand, the peak mRNA level of Klf5 was in the early differentiation stage, and expression was reduced drastically in the late stage. This reciprocal change indicated that KLF5 is the direct target of miR-448. Moreover, miR-448-transduced cells had lower expression levels of C/EBPα and PPARγ during adipocyte differentiation, which are the direct targets of KLF5, and also supported the notion that KLF5 is regulated by miR-448.
KLF5 is a basic transcription factor and mediates signaling functions in cell proliferation, cell cycle, apoptosis, migration, and differentiation by regulation of gene expression in response to environmental stimuli (39). KLF proteins have been shown to regulate both the early and, in the case of KLF15, also the later stages of adipogenesis. KLF2 inhibits expression of the PPARγ gene (40), whereas KLF5 and KLF15 drive it (17, 41). The present results, i.e. that Klf5 siRNAs decreased adipocyte differentiation, are in accordance with the report by Oishi et al. (17) about the role of KLF5 during adipogenesis.
The present study showed that 5-HT in adipose tissue is a novel autocrine factor that is required for adipocyte differentiation and a 5HT2CR agonist accelerated adipogenesis. In addition, we revealed that miR-448 inhibited adipocyte differentiation through targeting KLF5. We showed that 5-HT enhances adipocyte differentiation, and PPARγ, aP2, lipoprotein lipase, and C/EBPβ, are globally reduced in TPH1mut cells. Because KLF5 is known to be induced by C/EBPβ and -δ, 5-HT seems to affect adipocyte differentiation at an earlier stage than KLF5. There is a complex regulation of adipocyte differentiation by the serotonergic system. A previous study suggested that endogenous hypothalamic 5-HT is involved in within-meal satiation and postmeal satiety (42). Thus, the 5-HT system may play important roles in weight control at both neural and peripheral levels. However, Tph1 mRNA is not expressed in the brain (28), and 5-HT does not penetrate the blood-brain barrier (43). Therefore, our discovery may lead to new approaches to treat diet- induced obesity by specifically affecting peripheral 5-HT actions.
Materials and Methods
Cell lines, reagents, and antibodies
Mouse 3T3-L1 cells were obtained from the American Type Culture Collection (Manassas, VA) and from Human Science Research Resources Bank (JCRB9014, Osaka, Japan). The antibodies used were a goat polyclonal anti-TPH1 antibody (sc-15114: Santa Cruz Biotechnology Inc., Santa Cruz, CA), a mouse monoclonal anti-β-actin antibody (A5441: Sigma, St. Louis, MO), a rabbit polyclonal anti-BTEB2 (KLF5) antibody (sc-22797: Santa Cruz Biotechnology, Inc.), and an anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (catalog no. 2118; Cell Signaling Technology, Beverly, MA).
Cellular and tissue 5-HT concentrations were determined using a 5-HT enzyme immunoassay kit (DLD Diagnostika GmbH, Hamburg, Germany). PCPA, ketanserin, SB269970, and 5-HT were purchased from Sigma. WAY-161503 and SB-242084 were purchased from Tocris Bioscience (Ellisville, MO).
Cell culture and adipocyte differentiation
To prepare an SVF, inguinal and visceral adipose tissues were excised from mice and digested with collagenase in DMEM for 60 min at 37 C. After sequential filtration through 100-μm and 40-μm filters, isolated SVF was cultured in DMEM containing 10% fetal bovine serum (FBS) and penicillin/streptomycin. 3T3-L1 cells were cultured under the recommended conditions in DMEM containing 10% bovine serum and P/S. For the differentiation experiment, the medium was replaced with DMEM containing 10% FBS, 10 μg/ml insulin, 0.5 mm 3-isobutyl-1-methylxanthine, and 0.25 μm dexamethasone until 2 d after confluence. After another 2 d, the medium was changed to DMEM containing 10 μg/ml insulin and 10% FBS and replaced every 2 d. Differentiation-resistant cell lines derived from 3T3-L1 cells were established by retroviral infection.
Retroviral vector construction
pDisrup-ΔEn-loxP was made by the insertion of XbaI and ClaI sites of the 3′-LTR of pRET.IS.Ires-EGFP(N2) (provided by Dr. Philip Leder; Department of Genetics, Harvard Medical School, Boston, MA) into the BstZ17I and ClaI sites of pDisrup8 (23, 44). For pFB-NLS/Cre, the coding region of pMC1-Cre was PCR amplified to generate a Kozak consensus sequence for efficient translational initiation and transferred into the pFB retroviral vector. Reconstitution of viral insertion was achieved by the transfection of Cre recombinase by retroviral infection.
Isolation of mutant clones
After pDisrup retroviral infection, cells were treated with blasticidin S·HCl to select clones the genes of which had been disrupted. After isolation and expansion of each single clone, differentiation was induced by 3-isobutyl-1-methylxanthine, dexamethasone, and insulin. As expected, some of the blasticidin-resistant clones could not differentiate into mature adipocytes. The identities of the disrupted genes in various differentiation-resistant cell lines were determined by 3′-RACE of the fused blasticidin mRNA.
3′-RACE
A portion of the endogenous gene that was fused with the blasticidin gene was amplified by 3′-RACE, as described previously (23).
Plasmids
Expression vectors for the negative control and the microRNAs were generated using BLOCK-iT PolIImiR RNAi. Expression Vector Kits in accordance with the manufacturer’s protocol (Invitrogen, Carlsbad, CA). To create an anti-miR-448 (decoy) vector, the luciferase 3′-UTR was modified to include three tandem sequences complementary to miR-448 separated by three nucleotides spacers. All of these constructs were correctly inserted into a pLenti6/V5-D-TOPO vector (Invitrogen). The siRNAs for mouse were: KLF5 siRNA (sense 5′-AGCTCACCTGAGGACTCAT-3′). The siRNA constructs were made using a pSINsi-mU6 vector (Takara Bio. Inc., Shiga, Japan). siRNAs were introduced into the lentivirus vector plasmid pLenti6/V5-D-TOPO (Invitrogen). These plasmids were transfected into 3T3-L1 cells by lentiviral infection. The 3T3-L1 cells were subjected to adipocyte differentiation 3 d after transfection.
Lentivirus production and DNA transduction
As previously described, lentiviral stocks were produced in HEK 293T cells following the manufacturer’s protocol (Invitrogen). In brief, virus-containing medium was collected 48 h after transfection and filtered through a 0.45-μm filter. One round of lentiviral infection was performed by replacing the medium with virus-containing medium (containing 8 μg of Polybrene per ml), followed by centrifugation at 2500 rpm for 30 min at 32 C. Cells were used for analysis 3 d after DNA transduction.
Measurement of adipogenesis
Adipogenesis was assessed at baseline and at 2 d, 4 d, 6 d, and 8 d after the initiation of adipogenesis using the triglyceride-specific dye oil red O (Sigma-Aldrich) as described previously (45). Triglyceride accumulation was then expressed by measuring optical density (OD) units at 540 nm.
Triglyceride analysis
3T3-L1 cells were washed with PBS, and lipids were extracted using hexane-2-propanol (3:2, vol/vol). The amount of intracellular triglyceride was determined using a Triglyceride E test from Wako and normalized to the amount of total cellular protein in accordance with each manufacturer’s instructions.
Quantification of mRNA by real-time RT-PCR
Real-time RT-PCR was performed using a 7900 Sequence Detection System (Applied Biosystems). The primers used for the assay are listed in the supplemental methods.
miRNA quantitative RT-PCR
miRNA expression levels were analyzed by quantitative RT-PCR using a Taqman micro RNA Assays (Applied Biosystems, Foster City, CA) for miR-448 in accordance with the manufacturer’s instructions. miRNA expression was normalized to small nuclear RNA U6.
Western blotting
Cell lysates were prepared as described previously and subjected to SDS-PAGE, followed by standard Western blotting procedures.
Luciferase assay
A construct in which a fragment of the 3′-UTR of KLF5 mRNA containing the putative miR-448-binding sequence was cloned into a firefly luciferase reporter construct (PMIR-REPORT Luciferase) and transfected into HEK 293T cells with a plasmid expressing miR-448 or control-miR using Fugene 6 transfection reagent (Roche Applied Science, Indianapolis, IN). Constructs with mutated fragments of the 3′-UTR of KLF5 mRNA without putative miR-448-binding sequences were used as mutated controls (seed sequence AUAUGCAA was mutated to CUGCCGCU).
Statistics
Data are presented as means ± se. Statistical significance (P < 0.05) was determined between groups using an ANOVA followed by Tukey’s multiple comparison test for multiple groups or a Student’s t test for two groups.
Acknowledgments
We thank N. Sowa (Kyoto University, Kyoto, Japan) for providing technical assistance.
Footnotes
This work was supported by grants from the Ministry of Education, Culture, Science, Sports, and Technology of Japan (to T. Kita, K.H., and K.O.), and by grants from the Metabolic Syndrome Research Foundation, the ONO Medical Research Foundation, the Takeda Medical Research Foundation, the Takeda Science Foundation, the Sakakibara Memorial Foundation, the Japan Heart Foundation, and the Japan Society for the Promotion of Science (to K.O.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online August 18, 2010
Abbreviations: AADC, Aromatic amine decarboxylase; C/EBP, CCAAT/enhancer binding protein; c-miR, control-microRNA; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; 5-HT, serotonin; 5-HTR, 5-HT receptor; KLF5, Krüppel-like factor 5; LTR, long terminal repeat; MAO-A, monoamine oxidase A; miRNA, micro-RNA; miR-448, miRNA-448; PCPA, p-chlorophenylalanine; PPAR, peroxisome proliferator- activated receptor; RACE, 3′-rapid amplification of cDNA ends; siRNA, small interfering RNA; SVF, stromal vascular fraction; TPH1, tryptophan hydroxylase-1; UTR, untranslated region.
References
- 1.Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F1995. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T2001. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7:941–946 [DOI] [PubMed] [Google Scholar]
- 3.Matsuzawa Y, Funahashi T, Nakamura T1999. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann NY Acad Sci 892:146–154 [DOI] [PubMed] [Google Scholar]
- 4.Foti M, Porcheron G, Fournier M, Maeder C, Carpentier JL2007. The neck of caveolae is a distinct plasma membrane subdomain that concentrates insulin receptors in 3T3-L1 adipocytes. Proc Natl Acad Sci USA 104:1242–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed BC, Kaufmann SH, Mackall JC, Student AK, Lane MD1977. Alterations in insulin binding accompanying differentiation of 3T3–L1 preadipocytes. Proc Natl Acad Sci USA 74:4876–4880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veenstra-VanderWeele J, Anderson GM, Cook Jr EH2000. Pharmacogenetics and the serotonin system: initial studies and future directions. Eur J Pharmacol 410:165–181 [DOI] [PubMed] [Google Scholar]
- 7.Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, Bailey JP, Nieport KM, Walther DJ, Bader M, Horseman ND2004. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell 6:193–203 [DOI] [PubMed] [Google Scholar]
- 8.Kimball ES, Fisher MC1988. Potentiation of IL-1-induced BALB/3T3 fibroblast proliferation by neuropeptides. J Immunol 141: 4203–4208 [PubMed]
- 9.O'Connell PJ, Wang X, Leon-Ponte M, Griffiths C, Pingle SC, Ahern GP2006. A novel form of immune signaling revealed by transmission of the inflammatory mediator serotonin between dendritic cells and T cells. Blood 107:1010–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada K, Hu L, Mores N, Navarro CE, Fuda H, Krsmanovic LZ, Catt KJ2006. Serotonin (5-HT) receptor subtypes mediate specific modes of 5-HT-induced signaling and regulation of neurosecretion in gonadotropin-releasing hormone neurons. Mol Endocrinol 20:125–135 [DOI] [PubMed] [Google Scholar]
- 11.Hajduch E, Rencurel F, Balendran A, Batty IH, Downes CP, Hundal HS1999. Serotonin (5-hydroxytryptamine), a novel regulator of glucose transport in rat skeletal muscle. J Biol Chem 274:13563–13568 [DOI] [PubMed] [Google Scholar]
- 12.Moore MC, Kimura K, Shibata H, Honjoh T, Saito M, Everett CA, Smith MS, Cherrington AD2005. Portal 5-hydroxytryptophan infusion enhances glucose disposal in conscious dogs. Am J Physiol Endocrinol Metab 289:E225–E231 [DOI] [PMC free article] [PubMed]
- 13.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D1995. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374:542–546 [DOI] [PubMed] [Google Scholar]
- 14.Baek D, Villén J, Shin C, Camargo FD, Gygi SP, Bartel DP2008. The impact of microRNAs on protein output. Nature 455:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel DP2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297 [DOI] [PubMed] [Google Scholar]
- 16.Selbach M, Schwanhäusser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N2008. Widespread changes in protein synthesis induced by microRNAs. Nature 455:58–63 [DOI] [PubMed] [Google Scholar]
- 17.Oishi Y, Manabe I, Tobe K, Tsushima K, Shindo T, Fujiu K, Nishimura G, Maemura K, Yamauchi T, Kubota N, Suzuki R, Kitamura T, Akira S, Kadowaki T, Nagai R2005. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab 1:27–39 [DOI] [PubMed] [Google Scholar]
- 18.Barnes NM, Sharp T1999. A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152 [DOI] [PubMed] [Google Scholar]
- 19.Horie T, Ono K, Nishi H, Iwanaga Y, Nagao K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T, Kimura T2009. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem Biophys Res Commun 389:315–320 [DOI] [PubMed] [Google Scholar]
- 20.Brown BD, Naldini L2009. Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 10:578–585 [DOI] [PubMed] [Google Scholar]
- 21.Ebert MS, Neilson JR, Sharp PA2007. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen ED, Spiegelman BM2000. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–171 [DOI] [PubMed] [Google Scholar]
- 23.Ono K, Wang X, Han J2001. Resistance to tumor necrosis factor-induced cell death mediated by PMCA4 deficiency. Mol Cell Biol 21:8276–8288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishida Y, Leder P1999. RET: a poly A-trap retrovirus vector for reversible disruption and expression monitoring of genes in living cells. Nucleic Acids Res 27:e35 [DOI] [PMC free article] [PubMed]
- 25.Frederich RC, Löllmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS1995. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96:1658–1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu E, Liang P, Spiegelman BM1996. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703 [DOI] [PubMed] [Google Scholar]
- 27.Samad F, Yamamoto K, Loskutoff DJ1996. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-α and lipopolysaccharide. J Clin Invest 97:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walther DJ, Peter JU, Bashammakh S, Hörtnagl H, Voits M, Fink H, Bader M2003. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig-Lipson S, Zhang J, Mazandarani H, Harrison BL, Sabb A, Sabalski J, Stack G, Welmaker G, Barrett JE, Dunlop J2006. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res 1073–1074:240–251 [DOI] [PubMed]
- 30.Welmaker GS, Nelson JA, Sabalski JE, Sabb AL, Potoski JR, Graziano D, Kagan M, Coupet J, Dunlop J, Mazandarani H, Rosenzweig-Lipson S, Sukoff S, Zhang Y2000. Synthesis and 5-hydroxytryptamine (5-HT) activity of 2,3,4,4a-tetrahydro-1H-pyrazino[1,2-a]quinoxalin-5-(6H)ones and 2,3,4,4a,5,6-hexahydro-1H-pyrazino[1,2-a]quinoxalines. Bioorg Med Chem Lett 10:1991–1994 [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Sutton GM, Rochford JJ, Semple RK, Lam DD, Oksanen LJ, Thornton-Jones ZD, Clifton PG, Yueh CY, Evans ML, McCrimmon RJ, Elmquist JK, Butler AA, Heisler LK2007. Serotonin 2C receptor agonists improve type 2 diabetes via melanocortin-4 receptor signaling pathways. Cell Metab 6:398–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wienholds E, Plasterk RH2005. MicroRNA function in animal development. FEBS Lett 579:5911–5922 [DOI] [PubMed] [Google Scholar]
- 33.Kajimoto K, Naraba H, Iwai N2006. MicroRNA and 3T3–L1 pre-adipocyte differentiation. RNA 12:1626–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie H, Lim B, Lodish HF2009. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58:1050–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esau C, Kang X, Peralta E, Hanson E, Marcusson EG, Ravichandran LV, Sun Y, Koo S, Perera RJ, Jain R, Dean NM, Freier SM, Bennett CF, Lollo B, Griffey R2004. MicroRNA-143 regulates adipocyte differentiation. J Biol Chem 279:52361–52365 [DOI] [PubMed] [Google Scholar]
- 36.Takanabe R, Ono K, Abe Y, Takaya T, Horie T, Wada H, Kita T, Satoh N, Shimatsu A, Hasegawa K2008. Up-regulated expression of microRNA-143 in association with obesity in adipose tissue of mice fed high-fat diet. Biochem Biophys Res Commun 376:728–732 [DOI] [PubMed] [Google Scholar]
- 37.Sun T, Fu M, Bookout AL, Kliewer SA, Mangelsdorf DJ2009. MicroRNA let-7 regulates 3T3-L1 adipogenesis. Mol Endocrinol 23:925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X2008. miR-17–92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA 105:2889–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong JT, Chen C2009. Essential role of KLF5 transcription factor in cell proliferation and differentiation and its implications for human diseases. Cell Mol Life Sci 66:2691–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee SS, Feinberg MW, Watanabe M, Gray S, Haspel RL, Denkinger DJ, Kawahara R, Hauner H, Jain MK2003. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. J Biol Chem 278:2581–2584 [DOI] [PubMed] [Google Scholar]
- 41.Mori T, Sakaue H, Iguchi H, Gomi H, Okada Y, Takashima Y, Nakamura K, Nakamura T, Yamauchi T, Kubota N, Kadowaki T, Matsuki Y, Ogawa W, Hiramatsu R, Kasuga M2005. Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J Biol Chem 280:12867–12875 [DOI] [PubMed] [Google Scholar]
- 42.Miller KJ2005. Serotonin 5-ht2c receptor agonists: potential for the treatment of obesity. Mol Interv 5:282–291 [DOI] [PubMed] [Google Scholar]
- 43.Carley DW, Radulovacki M1999. Role of peripheral serotonin in the regulation of central sleep apneas in rats. Chest 115:1397–1401 [DOI] [PubMed] [Google Scholar]
- 44.Zarubin T, Jing Q, New L, Han J2005. Identification of eight genes that are potentially involved in tamoxifen sensitivity in breast cancer cells. Cell Res 15:439–446 [DOI] [PubMed] [Google Scholar]
- 45.Green H, Kehinde O1976. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 7:105–113 [DOI] [PubMed] [Google Scholar]