Abstract
Thyroid hormone is essential for brain development where it acts mainly through the thyroid hormone receptor α1 (TRα1) isoform. However, the potential for the hormone to act in adult neurons has remained undefined due to difficulties in reliably determining the expression pattern of TR proteins in vivo. We therefore created a mouse strain that expresses TRα1 and green fluorescent protein as a chimeric protein from the Thra locus, allowing examination of TRα1 expression during fetal and postnatal development and in the adult. Furthermore, the use of antibodies against other markers enabled identification of TRα1 expression in subtypes of neurons and during specific stages of their maturation. TRα1 expression was first detected in postmitotic cells of the cortical plate in the embryonic telencephalon and preceded the expression of the mature neuronal protein NeuN. In the cerebellum, TRα1 expression was absent in proliferating cells of the external granular layer, but switched on as the cells migrated towards the internal granular layer. In addition, TRα1 was expressed transiently in developing Purkinje cells, but not in mature cells. Glial expression was found in tanycytes in the hypothalamus and in the cerebellum. In the adult brain, TRα1 expression was detected in essentially all neurons. Our data demonstrate that thyroid hormone, unexpectedly, has the capacity to play an important role in virtually all developing and adult neurons. Because the role of TRα1 in most neuronal cell types in vivo is largely unknown, our findings suggest that novel functions for thyroid hormone remain to be identified in the brain.
Thyroid hormone receptor alpha1 protein is expressed in most post-mitotic mouse neurons and tanycytes, suggesting important roles in both maturation and maintenance of brain function.
Thyroid hormone is essential for fetal and postnatal nervous system development but is also known to have a role in the maintenance of adult brain function (1, 2, 3). Thyroid hormone mediates its effect through the thyroid hormone receptors (TRs) α and β, transcribed from different genes (4, 5), Thra and Thrb. In mammals, these give rise to the isoforms TRα1 and -2, and TRβ1 and -2. TRs are ligand-modulated transcription factors that bind to regulatory regions of target genes and either activate or repress their expression. Unlike the other TRs, TRα2 does not bind thyroid hormone, and its function is largely unclear (6, 7).
Even though the first TR was cloned more than 20 yr ago (4, 5), there is still a lack of reliable antibodies for immunohistochemical detection of TR isoforms in specific, identified cell types on natural tissue samples. The main obstacles have been the low expression levels of the receptors in vivo and potential cross-reaction between TR isoforms. This has greatly hampered the field and limited the understanding of thyroid hormone action, especially in the brain because of its great complexity. Many previous studies of the roles of TRα and -β in brain development and function have attempted to determine TR expression in different brain areas and cell types (reviewed by Ref. 8). Previous analyses employing techniques such as immunoprecipitation, Northern blot, or PCR have been performed on homogenized tissue and have yielded general but not cell-specific data on TR expression patterns (9, 10). In situ hybridization has demonstrated TR isoform mRNA expression patterns on tissue sections (11, 12, 13) although resolution at the single cell level has not been possible. Other studies on isolated cells grown in culture cannot be expected to fully reflect the in vivo situation (14, 15, 16, 17). Most of these previously employed techniques cannot identify specific cell types in vivo through double immunolabeling.
Our recent work has focused on thyroid hormone function in the brain, where TRα1 accounts for 70–80% of all TR expression (18). Several effects of hypothyroidism have been identified, such as delayed migration of cortical neurons (19, 20, 21), incorrect development of γ-aminobutyric acid (GABA)ergic cells (22, 23, 24, 25), defects in cerebellar development (26, 27, 28), and reduced adult neurogenesis (29, 30, 31). In addition, the role of thyroid hormone in the central regulation of metabolism from the hypothalamus has been studied (reviewed by Ref. 32). Through work performed on mice with mutations in TRα1, this receptor isoform, when unoccupied by ligand, has been proposed to be responsible for many of the deleterious effects of hypothyroidism on brain development and function (33, 34, 35, 36, 37, 38, 39, 40). To allow the study of the temporal and spatial expression of TRα1 at the cellular level in vivo, we generated mice expressing TRα1 fused to green fluorescent protein (GFP) as a chimeric protein from the endogenous Thra gene. This was done through gene targeting by fusing the reading frame of GFP 3′ to exon 9 of the Thra gene. As the chimeric protein is expressed under the native Thra promotor, the TRα1-GFP protein is expressed where and when the Thra gene is active.
In this study we describe the properties of this mouse line by investigating the transactivation properties of the chimeric protein as well as the basic development and physiology of the mice. Through double immunolabeling we found the earliest TRα1 expression in postmitotic neurons of the embryo. The expression persisted in the mature cells, and in the adult brain TRα1 was expressed in essentially all neurons. Expression of TRα1 in glial cells was restricted to specialized cells, e.g. tanycytes. The results suggest that TRα1 is involved during specific stages of neuronal cell development, and that it has the potential to influence the properties of virtually all cerebral and cerebellar neurons in the adult.
Results
Generation of TRα1-GFP mice
The sequence of enhanced GFP (hereafter referred to as GFP) was inserted in frame 3′ to exon 9 of the Thra1 gene (Fig. 1A). To determine the gene-regulatory functions of the chimeric protein, a plasmid construct expressing it was transfected into JEG-3 cells. Figure 1, G and H, shows that the ligand-activated TRα1-GFP protein activated to the same extent as the wild-type (wt) protein reporter genes carrying responsive elements of either the everted (F2T2) or the palindromic (PAL0) types. When cotransfected with increasing amounts of the corepressor NCoR (nuclear receptor corepressor), TRα1-GFP did not suppress transcription of the reporter gene to the same extent as the wt receptor, indicating a slightly impaired ability to repress target gene expression (Fig. 1I).
Fig. 1.
The knock-in TRα1-GFP allele. A, Map of the genomic locus encoding the TRα1 and -α2 isoforms and the rev-erbA gene, encoded by the opposing strand. Homologous recombination between the genomic locus and the targeting vector results in a targeted locus with GFP inserted in frame 3′ of exon 9 of the Thra1 gene. The resulting protein product is the chimeric TRα1-GFP protein. B, Correct homologous recombination was determined using Southern blot analyses of BamHI-cleaved genomic ES cell DNA hybridized with a 3′-probe that detect a sequence adjacent to but outside the targeting vector, or a GFP probe binding to the GFP sequence. The lane marked c is control DNA from heterozygote TRα1R384C mice. C, Southern blot analyses of BamHI-cleaved tail DNA, hybridized with a 5′-probe showing examples of all three genotypes after a heterozygote cross as well as heterozygote mice from which the neocassette had not been removed. D, Northern blot analyses of brain DNA from wt, homozygote, and heterozygote mice revealing that knock-in of GFP results in abolished expression from the Thra2 gene (2.6 kb). Mature TRα1-GFP RNA was 5.8 kb in size whereas the wt RNA was 5.2 kb. The insertion of GFP resulted in a novel 3.6-kb transcript in TRα1+/gfp and TRα1gfp/gfp mice and was detectable with all probes, indicating that it is a nonfunctional RNA. E, Quantitative real-time PCR showed the absence of TRα2 mRNA in homozygote mice and that expression was reduced to half in heterozygotes as compared with wt mice, whereas expression of TRα1 was increased in TRα1-GFP mice. F, Western blot of JEG-3 cell extracts after transfection with pCMV-TRα1, pCMV-TRα1-GFP; a nuclear extract from HeLa cells infected with a vaccinia virus vector expressing the chicken c-erbA/TRα protein is shown as a control. Lane c is a control with extract from untransfected cells. The membranes were hybridized with antibodies against TRα1 (left) or GFP and β-actin (right). TRα1: 46 kDa; TRα1-GFP: 75 kDa; β-actin: 42 kDa. G–I, Gene-regulatory functions of TRα1-GFP in transfected JEG-3 cells. Ligand-activated TRα1-GFP protein was equally potent as wt TRα1, as shown by detection of luciferase activity after cotransfection with 0, 50, or 150 ng of TRα1 and a reporter plasmid containing TRE-F2T2 (panel G) or TRE-Pal (H) response elements in front of a TK-luciferase cassette. The chimeric protein was not as effective in suppressing luciferase transcription as the wt protein, when cotransfected at 100 ng with increasing amounts of the corepressor NCoR in addition to the TRE-Pal reporter plasmid (I). All transfections were performed both in the presence and absence of 50 nm T3 in the medium and done in triplicate. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001, as compared with wt mice. DBD, DNA-binding domain; g, gfp; LBD, ligand-binding domain; UTR, untranslated region.
Extracts of cells transfected with Thra1-GFP contained a 75-kDa protein as demonstrated by Western blot using primary antibodies against TRα1 and GFP (Fig. 1F). This corresponds approximately to a theoretical value of the chimeric protein (TRα1, 46 kDa; enhanced GFP, 27 kDa).
In embryonal stem cell clones, the GFP-coding sequence was fused to exon 9 of the Thra1 locus by homologous recombination (7), as verified by Southern blot using both TRα1 (3′) and GFP-specific probes (Fig. 1B). Heterozygote offspring were intercrossed to generate mice homozygous for the Thra1-GFP chimeric gene or bred against C57Bl/6 for three generations and then intercrossed (Fig. 1C, using a 5′-TRα1-specific probe).
RNA expression analyses showed that the gene targeting caused an increase in both the size and amount of transcripts from the Thra locus (Fig. 1D, 5.2 and 6.4 kb for TRα1, and 5.8 and 7.0 kb for TRα1-GFP). Unexpectedly, the targeting also abrogated production of the TRα2 transcript (2.6 kb). This was confirmed using real-time PCR (Fig. 1E), showing an 80% increase of RNA encoding TRα1-GFP in the TRα1+/gfp mice and a 120% increase in the homozygotes as compared with expression of wt TRα1 in normal (TRα1+/+) animals (n = 3). Furthermore, TRα2 mRNA was reduced to half in TRα1+/gfp heterozygous mice as compared with wt controls and was completely absent in TRα1gfp/gfp homozygotes. TRα1+/gfp and TRα1gfp/gfp mice also express a novel 3.6-kb transcript. Because this transcript hybridized with all probes including that for TRα2, we assume that it represents a nonfunctional RNA. In summary, the targeted manipulation produced expression of a TRα1-GFP fusion mRNA as expected. Somewhat surprisingly, this manipulation also abolished expression of TRα2 mRNA from the same allele. The gene-targeting event did not perturb rev-erbα expression (Fig. 1D).
Physiological parameters of TRα1-GFP mice
To test whether the altered TRα1-GFP protein affects the physiology of the recombinant mice, we analyzed parameters known to be influenced by thyroid hormone status. All experiments were conducted on male and female wt, TRα1+/gfp, and TRα1gfp/gfp mice as well as on heterozygote mice in which the neocassette had not been removed (data not shown).
To determine postnatal increase in body weight, mice were weighed twice weekly during their first 6 wk of life. As shown in Fig. 2, A and B, only minor variation between genotypes were observed (n = 10). These differences were detected with males and females at 3 wk of age and for females again at 6 wk. However, these changes were seen in heterozygous but not homozygous animals, indicating that the alterations may be attributable to other factors such as litter size, time of weaning, etc and not to disturbances in postnatal development caused by the genotype. Furthermore, no difference in the time for eye opening was observed between the genotypes (data not shown). Figure 2, C and D, shows that the adult body weight of all groups were equal in both males and females (n = 6). Similarly, brain and heart weights did not differ between genotypes, although male TRα1gfp/gfp mice had significantly smaller livers as compared with wt controls (wt, 44.5 ± 1.6; TRα1gfp/gfp, 36.2 ± 1.3; grams/body weight ± sem; P ≤ 0.01; n = 6). The latter, however, was not observed in female mice.
Fig. 2.
Physiological parameters of TRα1-GFP mice. Weight development of males (A) and females (B) from 3 d after birth until 6 wk of age demonstrated minor aberrancies in heterozygote but not in homozygote mice. Body weights of adult males (C) and females (D) showed no significant difference between the genotypes. E, Organ weights (grams per body weight) of male and female adult mice showed no alterations in brain or heart weights, whereas male homozygotes had significantly smaller livers as compared with wt controls. F and G, Serum total T4 (F) and T3 (G) levels of adult mice. Only a small difference was detected between the T3 levels of wt and heterozygote male mice; all other samples were normal. H, Analyses of relative gene expression levels of pituitary TSHβ, liver-specific Dio1, and cardiac MyHCα showed no differences between genotypes, whereas an increased expression of cardiac MyHCβ was seen in hearts of homozygous mutants. *, #, ‡, P ≤ 0.05; ##, P ≤ 0.01. *, wt vs. heterozygote; #, wt vs. homozygote; ‡, heterozygote vs. homozygote. TT3, total T3; TT4, total T4.
We next measured thyroid hormone levels in the adult mice. As shown in Fig. 2, F and G, serum total T3 and T4 levels were normal, apart from a small increase in T3 levels that was only significant in male heterozygote mice (wt, 1.08 ± 0.04; TRα1+/gfp, 1.32 ± 0.08; TRα1gfp/gfp, 1.24 ± 0.07; nmol/liter ± sem; P ≤ 0.05, wt vs. TRα1+/gfp; n = 6). In addition, TSHβ RNA levels in the pituitary, as measured with real-time PCR, were unaltered in the TRα1-GFP mice (Fig. 2H, n = 5). Thyroid hormone regulates deiodinase 1 (Dio1) mRNA in the liver (41), and we therefore decided to measure Dio1, using real-time PCR, in the TRα1-GFP mice. As shown in Fig. 2H, no difference was found in liver Dio1 RNA levels in the knock-in mice as compared with wt mice (n = 5).
Thyroid hormone-responsive elements in the cardiac muscle myosin heavy chain (MyHC) genes have been shown to regulate MyHCα positively and MyHCβ negatively (42, 43, 44). To test whether the TRα1-GFP chimeric protein alters transcription from the MyHC genes, we performed real-time PCR analyses of heart RNA isolated from wt and knock-in mice using primers specific for the two isoforms. As shown in Fig. 2H, there was a significant increase in MyHCβ in TRα1gfp/gfp hearts (wt, 0.27 ± 0.05; TRα1gfp/gfp, 1.56 ± 0.33; relative gene expression ± sem; P ≤ 0.05; n = 5) whereas MyHCα showed no change upon genotype. This indicates a minor impairment in the transcriptional repression function of the chimeric receptor.
To examine potential irregularities in the brains of the TRα1-GFP mice, we measured gene expression in neonatal mice. In the striatum at postnatal d 7 (P7), in situ hybridization showed that there was no statistically significant difference between heterozygote and wt mice for the T3-regulated gene RC3 (wt, 78.7 ± 2.8; TRα1+/gfp, 70.5 ± 8.7; average grain density ± sem; n = 3). In addition, there was no difference in gene expression between genotypes, as shown with real-time PCR at P10, for the TR corepressors alien (wt, 1.21 ± 0.70; TRα1+/gfp, 1.09 ± 0.63; relative gene expression ± sem; n = 3) and NCoR (wt, 1.04 ± 0.60; TRα1+/gfp, 0.73 ± 0.42; relative gene expression ± sem; n = 3). The same result was obtained for the TR-responsive corepressor hairless (wt, 2.01 ± 1.16; TRα1+/gfp, 1.23 ± 0.71; relative gene expression ± sem; n = 3).
To test whether the Thra1-GFP gene conferred any major alterations in brain function, a SHIRPA primary screen for behavioral perturbations (45) was done with 10 male mice. The results (data not shown) revealed no aberrancies. We conclude that the TRα1-GFP mice exhibit only minor variations from the wt phenotype.
TRα1 is expressed in all adult cerebral neurons
The immunohistochemistry in Fig. 3, G–K, shows that the TRα1-GFP chimeric protein was expressed in a large number of cells in the adult brain. These include cells in the striatum (Fig. 3L), neocortex (Fig. 3M), hippocampus including the CA1–3 regions and the dentate gyrus (Fig. 3N), hypothalamus (Fig. 3O), and cerebellum (Fig. 3, D–F and K). Notably, GFP immunostaining was absent in the white matter. Also, the expression in neocortical layers II/III and VI was higher than that in layer IV (Fig. 3M). To verify that staining intensity can be translated to actual expression levels, we compared sections of homo and heterozygote mice. A comparison in Fig. 3, A–F, between wt, hetero, and homozygote mice showed that no GFP expression was found in the wt mice, that the expression was nuclear, and, as expected, higher in the TRα1gfp/gfp mice than in TRα1+/gfp mice.
Fig. 3.
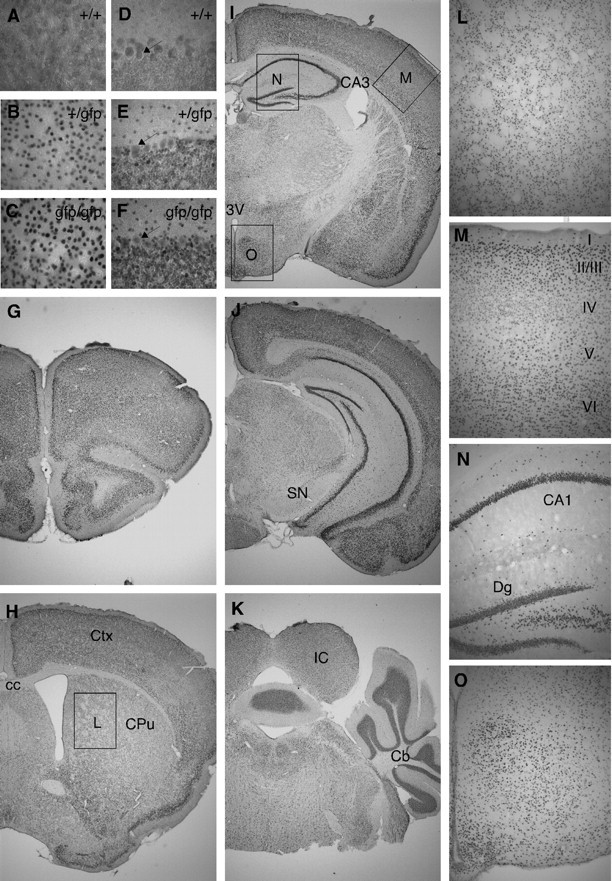
TRα1-GFP is expressed in gray matter areas of the adult brain. A–O, Micrographs of sections of the brain after immunohistochemistry for GFP to detect TRα1-GFP with DAB staining. Immunoreactivity was absent in wt mice (A and D) with higher expression in homozygotes (C and F) than in heterozygotes (B and H), as shown in the cortex (A–C) and in the cerebellum (D–F); magnification, ×400. The arrows in D–F indicate the Purkinje cells that were equally stained in all groups and thus only show background staining. G–K, ×20 magnification overviews. Boxes in H–J indicate ×100 magnifications in L–O. TRα1-GFP expression in the caudate putamen was structured and corresponds to gray matter areas (L). Immunoreactivity was detected in all cortical layers but was notably lower in layer IV (M). In the hippocampus TRα1-GFP was expressed in the stratum pyramidale and in the granular cell layer and hilar region of the dentate gyrus, as well as scattered in the stratum oriens and the stratum radiatum (N). In addition, a large number of cells in the hypothalamus expressed TRα1-GFP (O). cc, Corpus callosum; Cb, cerebellum; CPu, caudate putamen (striatum); Ctx, cortex; Dg, dentate gyrus; IC, inferior colliculus; SN, substantia nigra; 3V, third ventricle.
The absence of staining in white matter indicated that TRα1 is mainly expressed in neurons. To test this, we performed double immunohistochemistry for GFP and the neuronal marker neuronal nuclei (NeuN) on wt and TRα1+/gfp mouse brain sections. Remarkably, all examined neurons in the brain expressed TRα1. As shown in Fig. 4, A–D, all NeuN-positive (NeuN+) cells in the caudate putamen, somatosensory cortex, CA1, and dentate gyrus subfields of the hippocampus were TRα1-GFP positive. Note that in the dentate gyrus TRα1 expression was found in both the granular cell layer and the hilus. In addition, all NeuN+ cells in the thalamus and hypothalamus expressed TRα1 (data not shown).
Fig. 4.
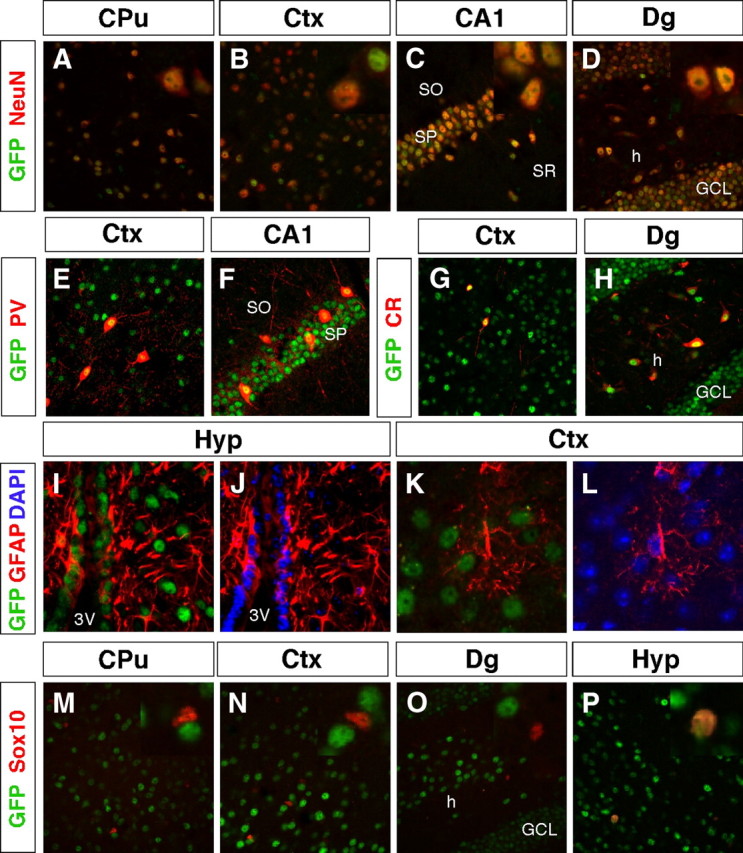
TRα1-GFP is expressed in virtually all neurons but in only a few specialized glial cells. A–P, Confocal imaging micrographs were taken of adult sections of the brain after immunohistochemistry against GFP to detect TRα1-GFP and markers for different cell types. Insets show high magnifications of the same region. A–D, TRα1-GFP expression was detected in all analyzed neurons (NeuN+ cells) in the adult brain, including in the caudate putamen (A), somatosensory cortex (B), CA1 region (C), and the dentate gyrus (D). E–H, Expression in GABAergic interneurons. TRα1-GFP was detected in PV+ interneurons in the cortex (E) and in the CA1 region of the hippocampus (F). TRα1-GFP and CR were coexpressed in interneurons of the cortex (G) and in interneurons and mossy cells in the hilus of the hippocampus (H). I–L, TRα1-GFP expression was detected in GFAP+ tanycytes lining the third ventricle of the hypothalamus (I and J), but not in astrocytes as shown in the cortex (K and L). Nuclei were counterstained with 4′6,-diamidino-2-phenylindole (DAPI). M–P, Double immunolabeling of oligodendrocytes with Sox10 show that TRα1-GFP was expressed only in adult oligodendrocytes of the hypothalamus (P), but not in the caudate putamen, cortex, or dentate gyrus (M–O). CPu, Caudate putamen; Ctx, cortex; GCL, granular cell layer; h, hilus; Hyp, hypothalamus; SO, stratum oriens; Sox, SRY related high-mobility group box; SP, stratum pyramidale; SR, stratum radiatum; 3V, third ventricle.
TRα1-GFP staining intensity is equal in excitatory and inhibitory neurons
We have shown previously that mice with a point mutation in TRα1 show a delayed appearance of inhibitory parvalbumin (PV)-positive (PV+) cells in the motor and somatosensory cortices as well as a reversible reduced expression of PV in the CA1 region of the hippocampus (35, 36). In addition to atypical PV expression, these mutant TRα1 mice show an increased expression of calretinin (CR) positive (CR+) cells in the adult neocortex and hippocampus (36, 46). To confirm that all neurons indeed expressed TRα1 and to determine whether there was a difference in the expression level of TRα1 between these cell types and excitatory cells, we performed double immunohistochemistry on GFP with PV and CR. As shown in Fig. 4, E–H, TRα1 is expressed at high levels in PV+ and CR+ cells of both the somatosensory cortex and the hippocampus. PV+ cells were found in both stratum pyramidale and stratum oriens of the CA1, and CR+ cells were found in interneurons and mossy cells of the dentate gyrus hilar region. The thalamus is an area where excitatory and inhibitory neurons can easily be distinguished because of the separated location of the cells. In this area no intensity differences were observed between excitatory glutamatergic and inhibitory GABAergic neurons (data not shown).
Glial expression of TRα1
TRα1 was absent in white matter, but because glial cells are also found dispersed among neurons, we used markers for astrocytes and oligodendrocytes for further analyses. Glial fibrillary acidic protein (GFAP) is expressed in mature astrocytes as well as in tanycytes lining the third ventricle. The latter cells are known to convert T4 to T3 using deiodinase 2 (Dio2) and then release it to neurons and oligodendrocytes (47, 48), although it has been unclear whether these cells express TR protein. Our careful examination of GFAP and GFP expression in the adult brain, however, showed that tanycytes expressed TRα1, as evidenced by strong GFP staining in GFAP-positive (GFAP+) cells lining the third ventricle (Fig. 4, I and J). In contrast, GFAP+ cells from other parts of the brain did not express GFP (see Fig. 6, K and L). We conclude that astrocytes of the adult cortex express, at most, very low levels of TRα1.
Fig. 6.
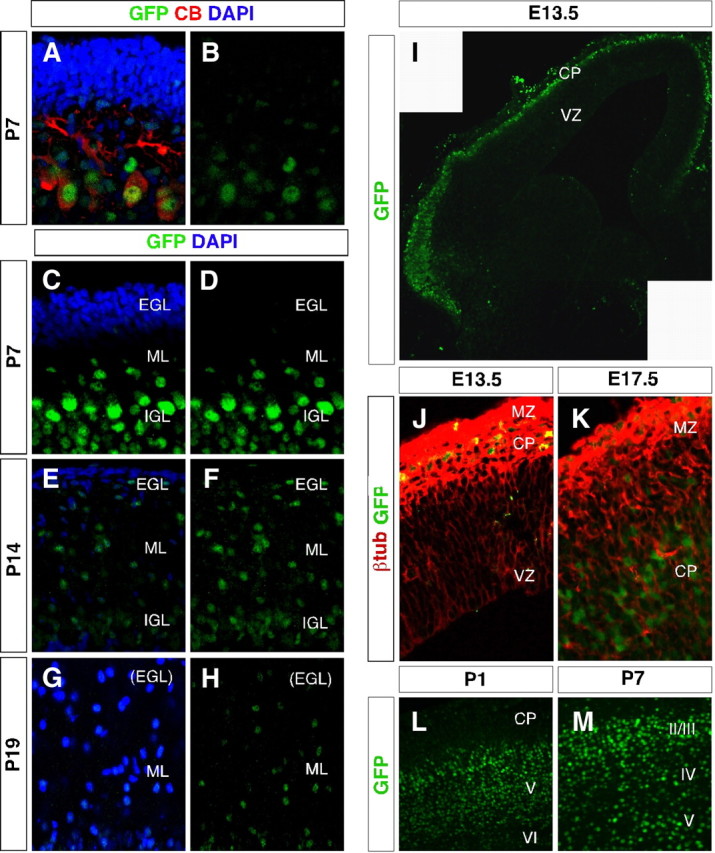
TRα1-GFP is expressed in postmitotic neurons in the developing brain. A–M, Confocal imaging micrographs showing TRα1-GFP expression (detected with GFP antibody) in the developing cerebellum and telencephalon. A–H, Expression in the cerebellum. In contrast to adults, calbindin (CB)-positive Purkinje cells coexpressed TRα1-GFP at P7 (A and B). No TRα1-GFP expression was detected in the external granular layer at P7, P14, or P19, but in migrating cells of the molecular layer and in the internal granular layer (A–F). Nuclei were counterstained with 4′6,-diamidino-2-phenylindole (DAPI) (A, C, E, and G). I–M, Expression in the telencephalon. In the E13.5 telencephalon, TRα1-GFP was expressed in the marginal zone and cortical plate, but not in the ventricular zone (I and J). The image (panel I) is a montage of two micrographs. The TRα1-GFP-positive cells expressed the postmitotic neuronal marker β-tubIII (J). At E17.5 TRα1-GFP expression was higher in the cortical plate than in the marginal zone (K), whereas at P1 the expression was highest in the deeper cortical layers V and VI where the oldest neurons are located (L). At P7, TRα1-GFP was equally expressed in all cortical layers (M). CP, cortical plate; IGL, intergranular layer; ML, molecular layer; MZ, marginal zone; VZ, ventricular zone.
Immunohistochemistry for SRY-related high-mobility group box 10 (Sox10), a marker for mature oligodendrocytes, revealed TRα1-GFP+ cells in the hypothalamus but not in the striatum, somatosensory cortex, or hippocampus (Fig. 4, M–P).
Cerebellar expression of TRα1
Several reports have identified TRα1 as a mediator of thyroid hormone signaling on cerebellar development, particularly in the postnatal proliferation and migration of external granular cells (26, 27, 33, 35, 37, 49). We decided to examine the cerebellar expression of TRα1 thoroughly using markers for cell types found in different layers. Double immunohistochemistry with calbindin and GFP failed to show TRα1 expression in Purkinje cells of the adult mouse (Fig. 3, D–F, and Fig. 5, A and B). In contrast, TRα1 was found in a number of smaller cells in the molecular layer adjacent to the Purkinje cells. Using the marker PV we identified these cells as stellate/basket cells (Fig. 5C). In addition, cells in the molecular layer coexpressed NeuN and TRα1 (Fig. 5D). As shown in Fig. 5, E and F, cells of the granular layer expressed TRα1 in addition to CR and NeuN, identifying them as granular cells. Colabeling with TRα1 and GFAP in Fig. 5, G and H, showed that TRα1 is expressed in glial cells of the cerebellum, in contrast to what we found in the cortex.
Fig. 5.
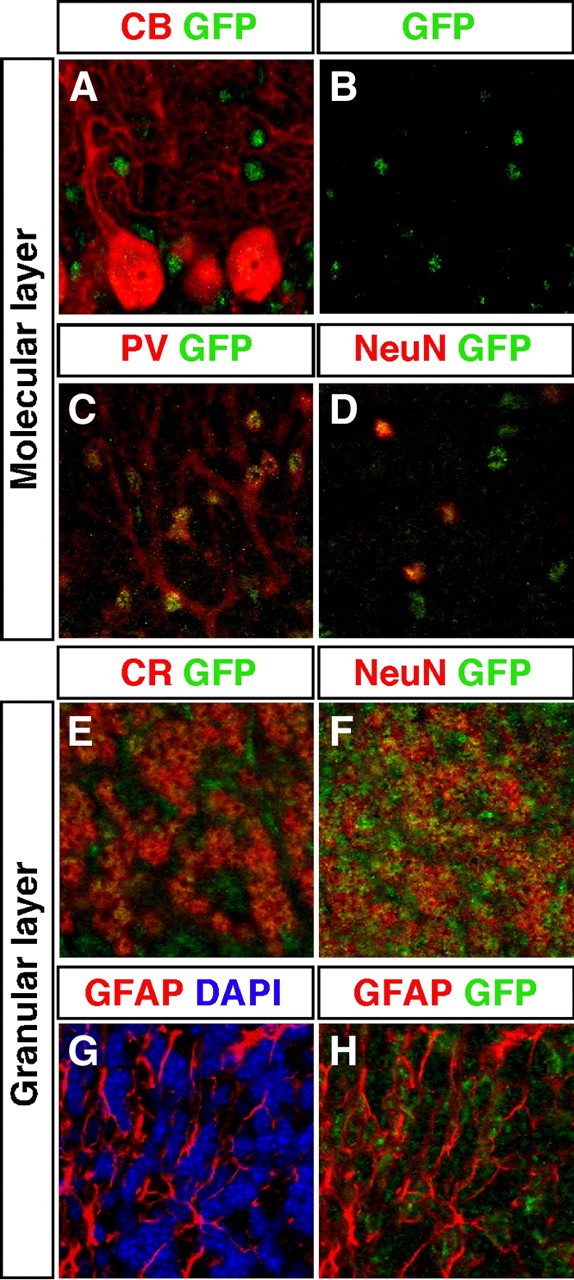
TRα1-GFP is expressed in specific neurons and glial cells in the cerebellum. A–H, Confocal imaging micrographs of adult sections showing expression of TRα1-GFP (detected with GFP antibody) and markers for different cell types. Examination of Purkinje cells marked with calbindin (CB) showed no colocalization with TRα1-GFP (A and B). In contrast, in the molecular layer, TRα1-GFP was expressed in PV+ stellate/basket cells (C) and in all neurons marked with NeuN (D). In the granular layer TRα1-GFP was expressed in granular cells as shown by immunostaining for both CR (E) and NeuN (F). TRα1-GFP was also expressed in GFAP+ glia cells (G and H). Nuclei were counterstained with 4′6,-diamidino-2-phenylindole (DAPI) in G.
The ligand-bound TRα1 has been suggested to have a permissive effect on migration of granular cells from the external granular layer (EGL) to the internal granular layer (in adults referred to as the granular layer) (35, 37). To investigate whether this is a cell-autonomous effect, we performed immunohistochemistry for GFP on cerebella from postnatal mice during the time when migration occurs, i.e. P7-19). Figure 6, A–F, shows no detectable TRα1 expression in the immature cells of the EGL at any time point. Instead, TRα1 expression was found in cells of the molecular layer. This staining was strong at P7 and increased as the cells approached the molecular layer. The data indicate that TRα1 may play a role in the migration and/or maturation of these cells. At P7 the Purkinje cells also expressed TRα1, in contrast to the adult situation (Fig. 6, G and H), suggesting that TRα1 may act only in the immature cells.
Neuronal development in the cortex
We had found that all neurons in the brain expressed TRα1 and therefore decided to examine when during neuronal development the expression is turned on. Neurons of the neocortex are generated in the embryo at the lateral ventricles. Immature neurons leave this proliferative zone and migrate through the cortical layers to reach the cortical plate. The oldest born neurons make up the deepest layer VI, and new neurons migrate past this layer to become layer V, etc. TRα1 was first expressed in the cortical plate and marginal zone at embryonic d 13.5 (E13.5) (Fig. 6I). In contrast, there were no TRα1+ cells at the ventricular zone (Fig. 6I), indicating that TRα1 is not expressed in proliferating cells. Also, no expression of TRα1 was found at an earlier time point investigated, i.e. E9.5 (not shown). As shown in Fig. 6J, immunohistochemistry for β-tubulin III (β-tubIII) identified the TRα1+ cells as immature neurons. TRα1 continued to be expressed in the cortical plate as it had increased in thickness at E17.5 (Fig. 6K). However, at P1 the expression was markedly higher in cortical layers V and VI than in the younger neurons of the cortical plate (Fig. 6L). As layering was completed at P7 TRα1 was equally expressed throughout the laminar structure of the cortex (Fig. 6M).
We conclude that TRα1 is found solely in postmitotic neurons subsequent to β-tubIII expression, and that it persists in adult NeuN+ neurons. Furthermore, our finding that TRα1 expression is increased in deeper cortical layers as compared with younger born cells of the cortical plate indicate a role for TRα1 not only during neuronal differentiation, but also in maintenance of mature function of these cells.
Discussion
Although known for a long time that TRα1 is the most abundantly expressed TR isoform in the brain (18) and that it is responsible for several of the neurological deficits observed during hypothyroidism (35, 36, 50), the information on which specific cell types and areas TRα1 is expressed in has been insufficient for understanding the types of processes that could be governed by thyroid hormone. In addition, the interplay between TRα1 and other transcription factors is presumably important for brain development but has not yet been unraveled. We therefore aimed to determine the temporal and spatial expression patterns of TRα1 in the mouse brain, by developing a novel mouse model that expresses TRα1 and GFP as a chimeric protein from the native Thra promoter in all cells in which TRα1 is expressed. The TRα1-GFP mice have the potential to be used not only for determining the in vivo expression of TRα1 in a wide variety of cells but also for microdissection of TRα1+ cells, electrophysiological measurements, and other in vitro applications. These TRα1-GFP mice can furthermore become a valuable tool for research on other organs in which TRα1 has a functional role.
Function of the TRα1-GFP gene
To mimic as closely as possible the wt TRα1 expression pattern, we designed a chimeric protein by inserting the GFP-coding sequence in frame 3′ to exon 9 of the Thra gene. The targeting vector subsequently constructed was designed to allow expression of the non-T3-binding TRα2 isoform. The reason for why TRα2 mRNA failed to be expressed is unclear, but the cause could be, for example, interference of the GFP cassette with normal splicing signals. Previously, we showed that both the TRα2+/− and TRα2−/− mice exhibit a prominent phenotype characteristic of a mixed hyper- and hypothyroidism, resulting from a 3- and 6-fold respective overexpression of the potent TRα1 isoform. Notably, no function could be ascribed with certainty to TRα2 (5). The TRα1+/gfp and TRα1gfp/gfp mice are both distinct from the aforementioned mice: only a very mild phenotype was detectable, e.g. failure to properly down-regulate cardiac MyHCβ and altered liver weight in males, which may reflect a mildly impaired ability of the TRα1-GFP protein to repress target gene expression as indicated by our NCoR interaction experiments. Also, the overexpression observed for the TRα1-GFP RNA was less than the 5- to 6-fold elevation reported for the TRα2−/− mice (7). The novel 3.6-kb transcript hybridized with all RNA probes and therefore contains sequences of both TRα1, TRα2, and GFP. This suggests that it is the result of an irregular splicing process downstream of GFP. It is highly unlikely that such a splicing event would yield an mRNA that produces a tripartite protein with TRα1, GFP, and TRα2 amino acid sequences. However, we cannot rule out that the 3.6-kb RNA does not code for a DNA-binding protein. Nonetheless, we conclude that our careful examination of the mice with regard to physiological, endocrine, and behavioral parameters, revealing only minor alterations as compared with wt mice, validates the use of this mouse line for determining expression patterns for the TRα1 isoform.
Cell type-specific expression of the TRα1-GFP allele
The expression levels in vivo of the TRα1-GFP protein were too low for easy detection of GFP fluorescence; however, the use of anti-GFP antibodies allowed a robust detection of the chimeric protein in cell nuclei. The immunohistochemical analyses of the TRα1-GFP mice subsequently showed that virtually all neurons in the brain express TRα1. Given previously published expression profiles and the effects reported for thyroid hormone on adult brain function, we had anticipated a more restricted expression of TRα1 in neurons, especially as many of these functions appeared to be specific to certain cell types and brain areas. However, the expression we found is in concordance with previously published TRα1 in situ hybridizations on the brain (11, 12, 13, 51).
The possibility that major variations in expression levels would occur in different neuronal cell types, thereby providing diversity in effect, is not supported by our studies on excitatory and inhibitory neurons. In the thalamus these two cell types can be easily distinguished based on their location; however, we failed to demonstrate significant differences in expression intensity. We also examined two subpopulations of inhibitory GABAergic interneurons, the PV and CR cells, which are known to be differently affected by thyroid hormone (36). However, no differences in TRα1-GFP expression were detected between these cell types. The data thus indicate that thyroid hormone may affect the function of a very wide variety of neuronal cells and that many of the effects assigned to subtypes of neurons still await identification. Such effects are likely to be dependent on the expression of transcriptional cofactors that are differentially expressed in neuronal subtypes. Corepressors such as NCoR, hairless (52), and alien (53) associate with TRs in the brain, and the two latter are known to be regulated by T3 in this tissue (54, 55). Moreover, the coactivator, SRC1 (steroid receptor coactivator 1), is expressed in developing neurons but not glial cells (56). The presence of the potent GFP epitope on the TRα1 molecule will allow unraveling the repertoire of interacting proteins in neuronal subtypes.
Hypothalamic expression of TRα1
Previous studies have shown that TR mRNAs or proteins are expressed in several hypothalamic regions, such as the arcuate nucleus, which governs anorexic or orexigenic processes and the paraventricular nucleus, which is an important integrator for the neuroendocrine axes (57, 58). Our data corroborate and extend these data, because TRα1 expression was found in all neurons studied in the hypothalamus. Furthermore, we found that TRα1 is expressed in cells lining the third ventricle, a region known to contain the astrocyte-like tanycytes. These cells reside at the interface of the blood-brain barrier and have previously been shown to express TRα1 mRNA (47). They can directly take up components from the cerebrospinal fluid including the thyroid hormone T4 and convert this to the active form T3 using Dio2, an enzyme the expression of which has been suggested to be suppressed by T3. As the basal processes of tanycytes contact the paraventricular nucleus and neuropeptide Y neurons (57), thyroid hormones have the potential to control, by regulation of Dio2 expression via TRα1, the known hypothalamic mechanisms that regulate metabolism.
TRα1 expression during development
We have previously demonstrated that many of the defects caused by a mutant TRα1 that acts as an aporeceptor during hypothyroidism can only be reversed by thyroid hormone treatment during specific stages of development (35, 36). When examining TRα1-GFP expression during development, we discovered that no TRα1 was detected in the ventricular zone, the area where neurons are born and proliferate. Instead, our data showed that TRα1 is expressed in immature neurons, preceding the expression of NeuN, and is increased as the cells reach their final destination and differentiate. That TRα1 acts at a later stage of neural development, i.e. after cell cycle exit, was also supported by our finding that in the cerebellum TRα1 was expressed in cells migrating from the EGL to the internal granular layer, but not in the EGL where cells are still mitotic. This is similar to the results we have on adult hippocampal neurogenesis (66), where we found that TRα1 regulated progenitor survival, and is further supported by the work of Munoz et al. (17), which showed that the TRα1 aporeceptor arrested neuronal progenitors at specific stages of development in a CNS-derived neuronal cell line.
We found oligodendrocytic TRα1 expression only in certain areas of the adult brain. This is in line with previous data, reporting that oligodendrocyte precursors differentiate upon T3 activation of a transiently expressed TRα1 (59). Thus, maturation of several cell types in the brain may be dependent on TRα1 being expressed only during specific windows during development. Taken together, our data encourage further study of the interplay between TRα1 and transcriptional cofactors known to control development, maturation, and maintenance of brain functions.
Materials and Methods
Introduction of the TRα1-GFP chimeric gene into the mouse genome and animal procedures
A cDNA encoding mouse TRα1 was amplified so as to introduce a BamHI site at the stop codon and subsequently cloned into pEGFP-N1 (Clontech, St-Germain-en-Laye, France), resulting in the following sequence at the junction (TRα1 and GFP sequences in bold, BamHI site in parentheses): 5′…GAA GTC C(CG GAT CC)A CCG GTC GCC ACC ATG GTG…3′. The fusion protein was analyzed by transfections into JEG-3 cells as described below. A fragment of the coding region including GFP as well as a neoR cassette was subsequently introduced into the targeting vector. The procedures used for generating chimeric mice from embryonic stem (ES)cells (129/Ola) have been described previously (60). Chimeric founder mice were bred against C57Bl/6NCrl (Charles River, Sulzfelt, Germany), and the offspring were subsequently crossed with EIIa-Cre mice (61) for removal of the Neo-cassette. Heterozygote offspring were intercrossed to generate mice homozygous for TRα1-GFP or backcrossed against C57Bl/6 for three generations and then intercrossed. The litters were of two to 10 pups. Experiments were performed on adult mice (3 months old) or as indicated. The mice were kept at 21 C on a 12-h light, 12-h dark cycle. Animal care procedures were in accordance with the guidelines set by the European Community Council Directives (86/609/EEC). Required animal permissions were obtained from the local ethical committees.
Transfection of JEG-3 cells
Human choriocarcinoma trophoblastic JEG-3 cells were transfected using FuGENE 6 transfection reagent (Roche, Bromma, Sweden) according to the manufacturer’s instructions. Briefly, the cells were transfected with or without 50 nm T3 present in the medium, with 0–150 ng of either pCMV-TRα1 or pCMV-TRα1-EGFP in addition to pCMV-F2T2-luc or pCMV-Pal-luc and pCMV-β-gal for internal control. The experiments were performed in DMEM with 10% fetal calf serum depleted of T3 and T4 by ion exchange resin (62). Empty pCMV-vector was added to equalize the amount of transfected DNA in all experiments. In a separate experiment the TRα1 plasmids were kept at 100 ng and were transfected with 0–150 ng pCMX-NCoR. Cells were lysed 24 h after transfection, and the lysates were assayed for luciferase and β-galactosidase expression in a Rosys Anthos lucy-1 luminometer. β-Galactosidase activity was assayed to assess transfection efficiency. Luciferase expression levels were expressed as light units relative to β-galactosidase expression.
Hormone assays
Animals were killed with CO2 and bled. Serum was prepared by centrifugation, and total T3 and T4 levels were determined with a RIA kit (Diagnostic Products Corp., Los Angeles, CA).
Histological analyses
Immunohistochemistry was performed on 25-μm free floating adult brain sections or 12-μm embryonic brain sections from heterozygote mice as described previously (36). Primary antibodies: mouse anti-β-tubIII (T8578, Sigma-Aldrich, Stockholm, Sweden), goat anti-CR 1:2500 (AB1550; Millipore, Nödinge, Sweden), mouse anticalbindin 1:2000 (no. 300, Swant, Bellinzona, Switzerland), rabbit anti-GFP 1:5000 [antibody (ab) 290; Abcam, Cambridge, UK], mouse anti-NeuN 1:500 (MAB377, Millipore), mouse anti-PV 1:2000 (no. 235; Swant, Bellinzona, Switzerland), goat-anti Sox10 1:500 (AF2864; R&D systems, Abingdon, UK). The secondary antibodies were donkey antirabbit, antigoat, antimouse alexa 488-, 555-, or 594-conjugated fluoropores (Invitrogen, Stockholm, Sweden). The sections were mounted with Vectashield mounting medium containing DAPI (Vector Laboratories, Peterborough, UK).
In situ hybridization was carried out on juvenile 25-μm brain sections as described elsewhere (63). Sense and antisense riboprobes were synthesized from the cDNA template of RC3 spanning nucleotides 253–486. Signals in the striatum were quantified with the National Institutes of Health Image J software.
Western blot and real-time PCR
Western blot assays on nuclear extracts from transfected JEG-3 cells were performed using standard procedures. An extract containing the chicken TRα expressed by a vaccinia virus vector served as control (64). Antibodies: rabbit anti-β-actin 1:5000 (ab 8227, Abcam), rabbit anti-GFP 1:1000 (ab 290, Abcam), chicken anti-TRα 1:500 [a kind gift from Dr Robert Eisenman, (65)].
PolyA+ RNA was isolated from adult whole brain and analyzed using Northern blot as described previously (7) or real-time PCR for quantification. Total RNA from juvenile whole brain and adult liver, heart, and pituitary was isolated and analyzed as described by Sjögren et al. (34). Primers: Alien forward (for), 5′-ATG AGG AGG ACT ACG ACC TGG-3′; reverse (rev), 5′-ACC TTC AAG CTC CAA AAC CTT T-3′; Dio1 for, 5′-GTT GTC AGG GGC GAA TCG G-3′; rev, 5′-GCT GAA GCG GCT TGT GAT ATT-3′; hairless for, 5′TCC AAG CCA GAC GAC GAT ATT-3′; rev, 5′-CAC ACT CTC CGC TCT TTA TCT TC-3′; MyHCα for, 5′-ATA GGG GAC CGT AGC AAG AAG-3′; rev, 5′-TCC TCT CAG CCT TTA GCT GGA-3′; MyHCβ for, 5′-TTC ATC CGA ATC CAT TTT GGG G-3′; rev, 5′-GCA TAA TCG TAG GGG TTG TTG G-3′; NCoR for, 5′-CCT CTT CAG CAG TTC CAA CC-3′; rev, 5′-CGC ACT CCT CAT GGT CTA CG-3′; TRα1 and -2 for, 5′-CAT CTT TGA ACT GGG CAA GT-3′, TRα1 rev, 5′-CTG AGG CTT TAG ACT TCC TGA TC-3′; TRα2 rev, 5′-GCA CTC GAC TTT CAT GTG GA-3′; TSHβ for, 5′-TCA ACA CCA CCA TCT GTG CT-3′; rev, 5′-TTG CCA CAC TTG CAG CTT AC-3′.
SHIRPA
The SHIRPA primary level screen for behavior was performed on four wt, four heterozygote, and two homozygote adult mice as described previously (36).
Acknowledgments
We thank the Karolinska Institute Transgene Facility KCCT for assistance in producing the TRα1-GFP mouse strain. We thank Dr. Christian Broberger for viewing and commenting on our immunohistochemistry data in the hypothalamus and Dr. Ola Hermanson for the pCMX-NCoR construct. We also thank Dr. Douglas Forrest for critical reading of the manuscript. M.v.H. constructed the targeting vector and screened ES clones, K.W. analyzed TRα1-GFP function, and screened ES cell clones. K.W., S.D. and K.N. performed animal breeding, genotyping, and phenotype analyses. All histological analyses were done by K.W. and S.D. K.W., S.D., and B.V. designed the experiments and wrote the manuscript. B.V. conceived the project.
NURSA Molecule Pages:
Ligands: Thyroid hormone;
Nuclear Receptors: TRα.
Footnotes
This work was supported by funds from The Swedish Cancer Society, the Swedish Research Council, Svenska medicinska sällskapet (to M.v.H.), the Karobio Foundation, the Wallenberg Foundations, Söderbergs Stiftelse, and the Karolinska Institute.
Disclosure summary: The authors have no conflict of interest to disclose.
First Published Online August 25, 2010
Abbreviations: ab, Antibody; CR, calretinin; Dio1, deiodinase 1; Dio2, deiodinase 2; E13.5, embryonic d 13.5; EGL, external granular layer; ES, embryonic stem; for, forward; GABA, γ-aminobutyric acid; GFAP, glial fibrillary acidic protein; GFP, green fluorescent protein; MyHC, muscle myosin heavy chain; NCoR, nuclear receptor corepressor; NeuN, neuronal nuclei; P7, postanatal d 7; PV, parvalbumin; β-tubIII, β-tubulin III; rev, reverse; TRα1, thyroid hormone receptor α1; wt, wild type.
References
- 1.Delange FM1996. Endemic cretinism. In: Braverman LE, Utiger RD eds. Werner and Ingbar’s the thyroid, 7th ed. Philadelphia: Lippincott-Raven Publishers; 756–767
- 2.DeLong GR1996. The neuromuscular system and brain in hypothyroidism. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s the thyroid, 7th ed. Philadelphia: Lippincott-Raven Publishers; 826–835
- 3.Morreale de Escobar G, Obregon MJ, Escobar del Rey F2004. Role of thyroid hormone during early brain development. Eur J Endocrinol 151(Suppl 3):U25–U37 [DOI] [PubMed]
- 4.Sap J, Muñoz A, Damm K, Goldberg Y, Ghysdael J, Leutz A, Beug H, Vennström B1986. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324:635–640 [DOI] [PubMed] [Google Scholar]
- 5.Weinberger C, Thompson CC, Ong ES, Lebo R, Gruol DJ, Evans RM1986. The c-erb-A gene encodes a thyroid hormone receptor. Nature 324:641–646 [DOI] [PubMed] [Google Scholar]
- 6.Mitsuhashi T, Tennyson GE, Nikodem VM1988. Alternative splicing generates messages encoding rat c-erbA proteins that do not bind thyroid hormone. Proc Natl Acad Sci USA 85:5804–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saltó C, Kindblom JM, Johansson C, Wang Z, Gullberg H, Nordström K, Mansén A, Ohlsson C, Thorén P, Forrest D, Vennström B2001. Ablation of TRα2 and a concomitant overexpression of α1 yields a mixed hypo- and hyperthyroid phenotype in mice. Mol Endocrinol 15:2115–2128 [DOI] [PubMed] [Google Scholar]
- 8.Sarliève LL, Rodriguez-Peña A, Langley K2004. Expression of thyroid hormone receptor isoforms in the oligodendrocyte lineage. Neurochem Res 29:903–922 [DOI] [PubMed] [Google Scholar]
- 9.Hodin RA, Lazar MA, Chin WW1990. Differential and tissue-specific regulation of the multiple rat c-erbA messenger RNA species by thyroid hormone. J Clin Invest 85:101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH1990. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem 265:10514–10521 [PubMed] [Google Scholar]
- 11.Mellström B, Naranjo JR, Santos A, Gonzalez AM, Bernal J1991. Independent expression of the α and β c-erbA genes in developing rat brain. Mol Endocrinol 5:1339–1350 [DOI] [PubMed] [Google Scholar]
- 12.Forrest D, Hallböök F, Persson H, Vennström B1991. Distinct functions for thyroid hormone receptors α and β in brain development indicated by differential expression of receptor genes. EMBO J 10:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley DJ, Towle HC, Young III WS1992. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β 2-subtype, in the developing mammalian nervous system. J Neurosci 12:2288–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bury F, Carré JL, Vega S, Ghandour MS, Rodriguez-Peña A, Langley K, Sarliève LL2002. Coexpression of thyroid hormone receptor isoforms in mouse oligodendrocytes. J Neurosci Res 67:106–113 [DOI] [PubMed] [Google Scholar]
- 15.Carlson DJ, Strait KA, Schwartz HL, Oppenheimer JH1996. Thyroid hormone receptor isoform content in cultured type 1 and type 2 astrocytes. Endocrinology 137:911–917 [DOI] [PubMed] [Google Scholar]
- 16.Baas D, Bourbeau D, Carre JL, Sarlieve LL, Dussault JH, Puymirat J1994. Expression of α and β thyroid receptors during oligodendrocyte differentiation. Neuroreport 5:1805–1808 [DOI] [PubMed] [Google Scholar]
- 17.Muñoz A, Wrighton C, Seliger B, Bernal J, Beug H1993. Thyroid hormone receptor/c-erbA: control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J Cell Biol 121:423–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz HL, Strait KA, Ling NC, Oppenheimer JH1992. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. J Biol Chem 267:11794–11799 [PubMed] [Google Scholar]
- 19.Martinez-Galan JR, Escobar del Rey F, Morreale de Escobar G, Santacana M, Ruiz-Marcos A2004. Hypothyroidism alters the development of radial glial cells in the term fetal and postnatal neocortex of the rat. Brain Res Dev Brain Res 153:109–114 [DOI] [PubMed] [Google Scholar]
- 20.Lavado-Autric R, Ausó E, Garcia-Velasco JV, Arufe Mdel C, Escobar del Rey F, Berbel P, Morreale de Escobar G2003. Early maternal hypothyroxinemia alters histogenesis and cerebral cortex cytoarchitecture of the progeny. J Clin Invest 111:1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ausó E, Lavado-Autric R, Cuevas E, Del Rey FE, Morreale De Escobar G, Berbel P2004. A moderate and transient deficiency of maternal thyroid function at the beginning of fetal neocorticogenesis alters neuronal migration. Endocrinology 145:4037–4047 [DOI] [PubMed] [Google Scholar]
- 22.Berbel P, Marco P, Cerezo JR, DeFelipe J1996. Distribution of parvalbumin immunoreactivity in the neocortex of hypothyroid adult rats. Neurosci Lett 204:65–68 [DOI] [PubMed] [Google Scholar]
- 23.Guadaño-Ferraz A, Benavides-Piccione R, Venero C, Lancha C, Vennström B, Sandi C, DeFelipe J, Bernal J2003. Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits. Mol Psychiatry 8:30–38 [DOI] [PubMed] [Google Scholar]
- 24.Gilbert ME, Sui L, Walker MJ, Anderson W, Thomas S, Smoller SN, Schon JP, Phani S, Goodman JH2007. Thyroid hormone insufficiency during brain development reduces parvalbumin immunoreactivity and inhibitory function in the hippocampus. Endocrinology 148:92–102 [DOI] [PubMed] [Google Scholar]
- 25.Cuevas E, Ausó E, Telefont M, Morreale de Escobar G, Sotelo C, Berbel P2005. Transient maternal hypothyroxinemia at onset of corticogenesis alters tangential migration of medial ganglionic eminence-derived neurons. Eur J Neurosci 22:541–551 [DOI] [PubMed] [Google Scholar]
- 26.Morte B, Manzano J, Scanlan TS, Vennström B, Bernal J2004. Aberrant maturation of astrocytes in thyroid hormone receptor α 1 knockout mice reveals an interplay between thyroid hormone receptor isoforms. Endocrinology 145:1386–1391 [DOI] [PubMed] [Google Scholar]
- 27.Heuer H, Mason CA2003. Thyroid hormone induces cerebellar Purkinje cell dendritic development via the thyroid hormone receptor α1. J Neurosci 23:10604–10612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manzano J, Bernal J, Morte B2007. Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int J Dev Neurosci 25:171–179 [DOI] [PubMed] [Google Scholar]
- 29.Lemkine GF, Raj A, Alfama G, Turque N, Hassani Z, Alegria-Prévot O, Samarut J, Levi G, Demeneix BA2005. Adult neural stem cell cycling in vivo requires thyroid hormone and its α receptor. FASEB J 19:863–865 [DOI] [PubMed] [Google Scholar]
- 30.Ambrogini P, Cuppini R, Ferri P, Mancini C, Ciaroni S, Voci A, Gerdoni E, Gallo G2005. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology 81:244–253 [DOI] [PubMed] [Google Scholar]
- 31.Desouza LA, Ladiwala U, Daniel SM, Agashe S, Vaidya RA, Vaidya VA2005. Thyroid hormone regulates hippocampal neurogenesis in the adult rat brain. Mol Cell Neurosci 29:414–426 [DOI] [PubMed] [Google Scholar]
- 32.Alkemade A, Visser TJ, Fliers E2008. Thyroid hormone signaling in the hypothalamus. Curr Opin Endocrinol Diabetes Obes 15:453–458 [DOI] [PubMed] [Google Scholar]
- 33.Tinnikov A, Nordström K, Thorén P, Kindblom JM, Malin S, Rozell B, Adams M, Rajanayagam O, Pettersson S, Ohlsson C, Chatterjee K, Vennström B2002. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J 21:5079–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sjögren M, Alkemade A, Mittag J, Nordström K, Katz A, Rozell B, Westerblad H, Arner A, Vennström B2007. Hypermetabolism in mice caused by the central action of an unliganded thyroid hormone receptor α1. EMBO J 26:4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venero C, Guadaño-Ferraz A, Herrero AI, Nordström K, Manzano J, de Escobar GM, Bernal J, Vennström B2005. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes Dev 19:2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis K, Sjögren M, van Hogerlinden M, Silberberg G, Fisahn A, Nordström K, Larsson L, Westerblad H, Morreale de Escobar G, Shupliakov O, Vennström B2008. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor α1. J Neurosci 28:1904–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morte B, Manzano J, Scanlan T, Vennström B, Bernal J2002. Deletion of the thyroid hormone receptor α 1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneshige M, Suzuki H, Kaneshige K, Cheng J, Wimbrow H, Barlow C, Willingham MC, Cheng S2001. A targeted dominant negative mutation of the thyroid hormone α 1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc Natl Acad Sci USA 98:15095–15100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YY, Schultz JJ, Brent GA2003. A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem 278:38913–38920 [DOI] [PubMed] [Google Scholar]
- 40.Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J2002. Congenital hypothyroid Pax8(−/−) mutant mice can be rescued by inactivating the TRα gene. Mol Endocrinol 16:24–32 [DOI] [PubMed] [Google Scholar]
- 41.Berry MJ, Kates AL, Larsen PR1990. Thyroid hormone regulates type I deiodinase messenger RNA in rat liver. Mol Endocrinol 4:743–748 [DOI] [PubMed] [Google Scholar]
- 42.Izumo S, Mahdavi V1988. Thyroid hormone receptor α isoforms generated by alternative splicing differentially activate myosin HC gene transcription. Nature 334:539–542 [DOI] [PubMed] [Google Scholar]
- 43.Edwards JG, Bahl JJ, Flink IL, Cheng SY, Morkin E1994. Thyroid hormone influences β myosin heavy chain (β MHC) expression. Biochem Biophys Res Commun 199:1482–1488 [DOI] [PubMed] [Google Scholar]
- 44.Wright CE, Haddad F, Qin AX, Bodell PW, Baldwin KM1999. In vivo regulation of β-MHC gene in rodent heart: role of T3 and evidence for an upstream enhancer. Am J Physiol 276:C883–C891 [DOI] [PubMed]
- 45.Rogers DC, Fisher EM, Brown SD, Peters J, Hunter AJ, Martin JE1997. Behavioral and functional analysis of mouse phenotype: SHIRPA, a proposed protocol for comprehensive phenotype assessment. Mamm Genome 8:711–713 [DOI] [PubMed] [Google Scholar]
- 46.Hadjab-Lallemend S, Wallis K, van Hogerlinden M, Dudazy S, Nordstrom K, Vennstrom B, Fisahn A2010. A mutant thyroid hormone receptor α1 alters hippocampal circuitry and reduces seizure susceptibility in mice. Neuropharmacology 58:1130–1139 [DOI] [PubMed] [Google Scholar]
- 47.Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J1997. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94:10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu HM, Kim SW, Salvatore D, Bartha T, Legradi G, Larsen PR, Lechan RM1997. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology 138:3359–3368 [DOI] [PubMed] [Google Scholar]
- 49.Manzano J, Cuadrado M, Morte B, Bernal J2007. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar γ-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology 148:5746–5751 [DOI] [PubMed] [Google Scholar]
- 50.Bernal J2007. Thyroid hormone receptors in brain development and function. Nat Clin Pract Endocrinol Metab 3:249–259 [DOI] [PubMed] [Google Scholar]
- 51.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, et al.2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445:168–176 [DOI] [PubMed] [Google Scholar]
- 52.Thompson CC, Bottcher MC1997. The product of a thyroid hormone-responsive gene interacts with thyroid hormone receptors. Proc Natl Acad Sci USA 94:8527–8532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dressel U, Thormeyer D, Altincicek B, Paululat A, Eggert M, Schneider S, Tenbaum SP, Renkawitz R, Baniahmad A1999. Alien, a highly conserved protein with characteristics of a corepressor for members of the nuclear hormone receptor superfamily. Mol Cell Biol 19:3383–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potter GB, Zarach JM, Sisk JM, Thompson CC2002. The thyroid hormone-regulated corepressor hairless associates with histone deacetylases in neonatal rat brain. Mol Endocrinol 16:2547–2560 [DOI] [PubMed] [Google Scholar]
- 55.Tenbaum SP, Juenemann S, Schlitt T, Bernal J, Renkawitz R, Muñoz A, Baniahmad A2003. Alien/CSN2 gene expression is regulated by thyroid hormone in rat brain. Dev Biol 254:149–160 [DOI] [PubMed] [Google Scholar]
- 56.Nishihara E, Moriya T, Shinohara K2007. Expression of steroid receptor coactivator-1 is elevated during neuronal differentiation of murine neural stem cells. Brain Res 1135:22–30 [DOI] [PubMed] [Google Scholar]
- 57.Herwig A, Ross AW, Nilaweera KN, Morgan PJ, Barrett P2008. Hypothalamic thyroid hormone in energy balance regulation. Obesity Facts 1:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lechan RM, Fekete C2007. Infundibular tanycytes as modulators of neuroendocrine function: hypothetical role in the regulation of the thyroid and gonadal axis. Acta Biomed 78(Suppl 1):84–98 [PubMed] [Google Scholar]
- 59.Billon N, Jolicoeur C, Tokumoto Y, Vennström B, Raff M2002. Normal timing of oligodendrocyte development depends on thyroid hormone receptor α 1 (TRα1). EMBO J 21:6452–6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α 1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H1996. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA 93:5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Samuels HH, Stanley F, Casanova J1979. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80–85 [DOI] [PubMed] [Google Scholar]
- 63.Vujovic M, Nordström K, Gauthier K, Flamant F, Visser TJ, Vennström B, Mittag J2009. Interference of a mutant thyroid hormone receptor α1 with hepatic glucose metabolism. Endocrinology 150:2940–2947 [DOI] [PubMed] [Google Scholar]
- 64.Sap J, de Magistris L, Stunnenberg H, Vennström B1990. A major thyroid hormone response element in the third intron of the rat growth hormone gene. EMBO J 9:887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bigler J, Eisenman RN1988. c-erbA encodes multiple proteins in chicken erythroid cells. Mol Cell Biol 8:4155–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapoor R, van Hogerlinden M, Wallis K, Ghosh H, Nordström K, Vennström B, Vaidya VA 13 August 2010. Unliganded thyroid hormone receptor α1 impairs adult hippocampal neurogenesis. FASEB J 10.1096/fj.10-161802 [DOI] [PMC free article] [PubMed]




