Abstract
Several lines of evidence indicate that endometriosis could be partially due to selective epigenetic deregulations. Promoter hypermethylation of some key genes, such as progesterone receptor and aromatase, has been associated with the silencing of these genes and might contribute to the disease. However, it is unknown whether global alterations in DNA methylation patterns occur in endometriosis and to what extent they are involved in its pathogenesis. We conducted a whole-genome scanning of methylation status in more than 25,000 promoters, using methylated DNA immunoprecipitation with hybridization to promoter microarrays. We detailed the methylation profiles for each subtype of the disease (superficial endometriosis, endometriomas, and deep infiltrating endometriosis) and compared them with the profile obtained for the eutopic endometrium. In line with the current theory of the endometrial origin of endometriosis, the overall methylation profile was highly similar between the endometrium and the lesions. It showed promoter regions consistently hypomethylated or hypermethylated (more than 1.5-times, as compared with endometrium) and others specific to one given subtype. Albeit there was no systematic correlation between promoter methylation and expression of nearby genes, 35 genes had both methylation and expressional alterations in the lesions. These genes, reported here for the first time, might be of interest in the development of endometriosis. In addition, hypermethylated regions were located at the ends of the chromosomes, whereas hypomethylated regions were randomly distributed all along the chromosomes. We postulated that this original observation might participate to the chromosomal stability and protect the endometriotic lesion against malignancy.
Extensive methylation alterations do not systematically influence gene expression modifications in endometriosis.
Endometriosis is a growing public health concern, as regards its high prevalence (up to 10% of women of childbearing age), its impact on the physical and mental well being, and its socioeconomic toll (1). Despite that, endometriosis remains a very perplexing disease, and its pathogenesis is still unclear (2, 3).
Endometriosis is characterized by the presence of endometrial tissue outside of the uterus, forming multiple nodules. The endometriotic lesions may rest on the peritoneal surface [superficial endometriosis (SUP)], give rise to ovarian cysts [endometriomas (OMA)] or sometimes invade the surrounding organs beneath the peritoneum, such as the bladder or the rectum [deeply infiltrating endometriosis (DIE)] (4). From this point of view, endometriosis could be considered as a benign metastasizing disease, resembling cancer. The nodules are responsible for chronic painful symptoms, occurring generally during the menses (5). Endometriosis also affects fertility to varying degrees, through inflammation and/or alteration of the endometrial receptivity and the ovarian reserve (6). Retrograde menstruations, shedding endometrial cells throughout the Fallopian tubes into the peritoneal cavity, are generally thought to be at the origin of the lesions (7). Because retrograde menstruations occur in most women, it is likely that the combined effects of environmental factors and predisposing genetic backgrounds may contribute to the onset of the disease. Endometriosis is thus regarded as a complex trait, showing familial aggregation and multifactorial inheritance (8). Recently, concordant massive transcriptional alterations in the endometriotic foci have been reported (9, 10). Multiple biological pathways are univocally deregulated, such as cell adhesion, steroid hormone metabolism, or cell cycle, indicating a well-defined molecular signature of the endometriosis phenotype (10). However, the search for the genes predisposing to endometriosis still remains unsuccessful, although many association and linkage studies have been carried out (11).
The concept of epigenetics refers to heritable changes in chromatin structure and/or gene expression that do not involve changes in the underlying DNA sequence. One fundamental function of epigenetics is to establish a dialogue between the genome and the environment. In that, alterations in the epigenome can trigger, or be associated with, human diseases and especially the development of cancers (12, 13). The epigenetic marks, essential for cell differentiation and maintenance, are stably maintained during cell divisions and sometimes between generations of individuals, showing in these cases a trans-generational inheritance (14). DNA methylation is the best known and most studied epigenetic molecular mechanism (15). DNA methylation occurs on cytosines that are immediately followed by a guanine (CpG), especially in regions in which CpGs are recurrent (referred to as “CpG islands”). CpG islands are found in the promoters of approximately 40% of human genes, mostly housekeeping genes. In general, methylation of CpG islands in the promoter region has been associated with gene silencing (15). In mammals, global cytosine methylation patterns seem to be established by a complex interplay of at least three independently encoded DNA methyltransferases (DNMTs): DNMT1, DNMT3a, and DNMT3b. DNMT1 is responsible for the maintenance of the tissue-specific methylation patterns during DNA replication by copying the DNA methylation of the original DNA strand onto the newly synthesized one (16). DNMT3a and DNMT3b are responsible for de novo methylation because they are able to target unmethylated CpG sites (17). They are expressed mainly in early embryo development or in pathological conditions, such as cancer, and are therefore susceptible to be influenced by environmental conditions (18).
Some authors argued that epigenetic deregulations could participate in endometriosis phenotypic heterogeneity and familial aggregation (19). Some recent works have been devoted to the demonstration of aberrant promoter methylation of HOX-A10 (20, 21, 22), steroid hormone receptors (23, 24, 25), aromatase (26), and DNMTs (27) in women with endometriosis. In these studies, methylation level in the promoter of these genes was related to their expression level and proposed as a possible mechanism for gene expression alterations in endometriosis.
Because accumulating lines of evidence tend to indicate that epigenetics may play a role in endometriosis, it is necessary to investigate, in a more global way, the contribution of DNA methylation in the pathogenesis of this disease and especially, to what extent methylation can be held responsible for the altered gene expression profile in endometriotic lesions. The recent introduction of methylated DNA immunoprecipitation (MeDIP) technology, in combination with comprehensive gene promoter arrays (28), prompted us to analyze the promoter methylation status in endometriosis using this new epigenomic tool.
We carried out the first genome-wide analysis of promoter methylation in endometriosis using DNA extracted from ectopic endometrium (SUP, OMA, and DIE) and eutopic endometrium (EE) taken as control. Samples were isolated from 15 patients, suffering from SUP, OMA, or DIE (five patients of each), in the same phase of the menstrual cycle (luteal phase), and submitted to MeDIP, followed by hybridization to a 25,500 promoters microarray. Ten differentially methylated regions were also individually tested on five patients (different from the initial set), by McrBC digestion assay and bisulfite sequencing. We described specific methylation patterns for each subtype of the disease (SUP, OMA, and DIE) and correlated these patterns with expressional data that have been largely validated by RT-PCR experiments by us and by others (9, 10). Finally, we revealed a nonrandom, subtelomeric localization of hypermethylated regions as an epigenomic signature of endometriosis and discussed this last point with regard to the chromosomal stability and the risk of malignant degeneration.
Results
MeDIP array analysis of promoters in endometriosis
We analyzed the methylation profile of 25,500 human promoters by immunoprecipitation of genomic DNA with an anti-5-methylcytosine antibody and hybridization to Affymetrix GeneChip Human Promoter 1.0R Arrays. Genomic DNA was extracted from EE and ectopic endometrium of patients suffering from SUP, OMA, and DIE. Clinical characteristics of the patients are supplied in Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org. We used five-patient DNA pools to minimize interindividual variability and to emphasize alterations caused by the disease and not by individual genetic background, as previously published (10). The complete dataset for these arrays has been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo) under accession number GSE16079, as recommended by Minimum Information About a Microarray Experiment standards (29).
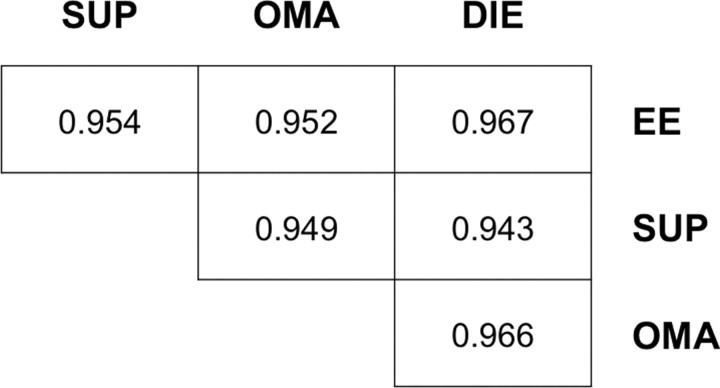
We first compared the global methylation patterns of SUP, OMA, and DIE lesions and EE. To make these comparisons, the normalized mean intensity values from the MeDIP arrays were used to generate the correlation coefficients between different pairs of samples (Fig. 1). This clearly showed that the global promoter methylation patterns of EE, SUP, OMA, and DIE correlated well with each other. The tight correlation of SUP, OMA, and DIE was expected given that all these subtypes form part of the same disease and can be present in association (30, 31, 32). The correlation between SUP, OMA, DIE, on the one hand, and EE, on the other hand, suggested that the endometriotic lesions do not differ from EE in terms of global methylation pattern but may vary only at specific positions. Our observation of a common epigenetic signature in ectopic and eutopic endometrium is compatible with the idea that they share a common tissular origin (7, 33).
Fig. 1.
Global comparison of promoter methylation patterns between SUP, OMA, DIE, and EE. Pair-wise correlation comparisons were made between all groups to establish the similarity of promoter methylation. R values were compared for significant correlation both within endometriotic subtypes (SUP, OMA, DIE) and between ectopic endometrium and EE.
Therefore, we focused on the differences that may exist at specific location between ectopic and eutopic endometrium. We initiated a comparative approach and a functional cluster analysis to identify genes that significantly differed in their promoter methylation levels in the different group (SUP, OMA, DIE, as compared with EE). We calculated methylation ratios for all promoter regions spotted in the array between each disease subtype and EE (i.e. SUP/EE, OMA/EE, and DIE/EE). When the ratio was above 1.50, the region was denoted as “hypermethylated.” When the ratio was below 0.66, the region was denoted as “hypomethylated.”
Differentially methylated promoter regions according to the subtype of endometriosis
Using the thresholds defined above, we detected 229, 161, and 108 differentially methylated regions in SUP, OMA, and DIE, respectively, as compared with EE. Their exact chromosomal location and their context in annotated genes and other genomic features are listed as supplemental data (Supplemental Table 2 for SUP vs. EE, Supplemental Table 3 for OMA vs. EE, and Supplemental Table 4 for DIE vs. EE). Nonsupervised gene functional clustering was performed for each category using DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov/home.jsp). DAVID was able to group these genes as “transcription factors” and “transmembrane proteins,” whatever the subtype. For example, the cluster of transcription factors, which was found for the OMA category, is detailed in Table 1.
Table 1.
Transcription factors located close to differentially methylated regions in OMA, as compared to EE
| Gene symbol | Gene name | Methylation status | Induction ratio | Ratio OMA/EE | Consistent methylation/ expression |
|---|---|---|---|---|---|
| C20orf20 | Chromosome 20 open reading frame 20 | Hypomethylated | 1.66 | 0.545 | |
| MAFB | v-maf Musculoaponeurotic fibrosarcoma oncogene homolog b (avian) | Hypomethylated | 1.21 | 0.584 | |
| E2F3 | e2f Transcription factor 3 | Hypomethylated | 0.47 | 0.593 | No |
| ZNF79 | Zinc finger protein 79 (pt7) | Hypomethylated | 1.15 | 0.611 | |
| RXRA | Retinoid x receptor, α | Hypomethylated | 2.38 | 0.648 | Yes |
| RING1 | Ring finger protein 1 | Hypermethylated | 1.04 | 1.511 | |
| RXRB | Retinoid x receptor, β | Hypermethylated | 2.44 | 1.511 | No |
| HOXD10 | Homeobox d10 | Hypermethylated | 0.27 | 1.525 | Yes |
| HOXD11 | Homeobox d11 | Hypermethylated | 0.43 | 1.525 | Yes |
| BBX | Bobby sox homolog (Drosophila) | Hypermethylated | 0.60 | 1.586 | |
| TFDP3 | Transcription factor dp family, member 3 | Hypermethylated | 7.45 | 1.641 | No |
| PCGF3 | Polycomb group ring finger 3 | Hypermethylated | 0.49 | 1.649 | Yes |
| MAFF | v-maf Musculoaponeurotic fibrosarcoma oncogene homolog f (avian) | Hypermethylated | 1.10 | 1.829 | |
| ZNF707 | Zinc finger protein 707 | Hypermethylated | 0.94 | 1.884 | |
| HSFX1 | lw-1 | Hypermethylated | 1.83 | 1.907 | |
| ZNF12 | Zinc finger protein 12 | Hypermethylated | 1.09 | 2.009 | |
| TCFL5 | Transcription factor-like 5 (basic helix-loop-helix) | Hypermethylated | 1.52 | 2.107 | |
| ZNF22 | Zinc finger protein 22 (kox 15) | Hypermethylated | 0.79 | 2.188 | |
| ZNF426 | Zinc finger protein 426 | Hypermethylated | 2.25 | 2.418 | No |
| CBFA2T3 | Core-binding factor, runt domain, α subunit 2; translocated to, 3 | Hypermethylated | 1.14 | 2.689 |
This gene list results from the DAVID gene functional clustering for the 161 regions found differentially methylated in OMA. Induction ratio denotes the gene expression level in OMA vs. EE; when above 2.0, the gene is up-regulated; when below 0.5, the gene is down-regulated. Ratio OMA/EE denotes the promoter methylation level in OMA vs. EE; when above 1.5, the promoter is hypermethylated: when below 0.66, the promoter is hypomethylated. The eight genes, the expression of which is consistent with promoter methylation status, are marked in bold. Expressional data result from Borgheseet al. (10 ).
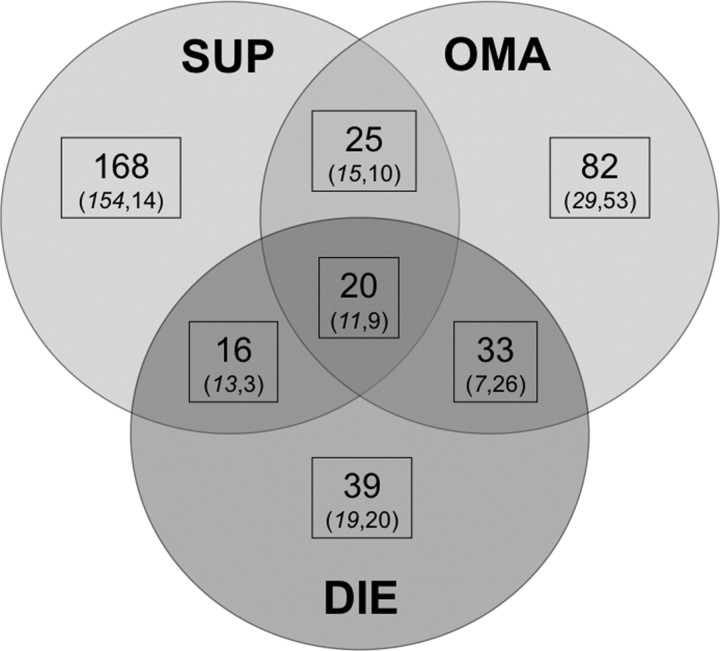
Common and specific promoter regions between the different conditions (SUP, OMA, and DIE) are summarized in Fig. 2. Twenty regions were common to all subtypes of endometriosis (hypomethylated, 11; hypermethylated, 9). These regions are detailed in Table 2. Twenty-five regions were common to SUP and OMA (hypomethylated, 15; hypermethylated, 10), 16 to SUP and DIE (hypomethylated, 13; hypermethylated, 3), and 33 to DIE and OMA (hypomethylated, 7; hypermethylated, 26), listed as supplemental data (Supplemental Table 5). One hundred sixty eight regions were specific to SUP (hypomethylated, 154; hypermethylated, 14), 83 to OMA (hypomethylated, 30; hypermethylated, 53), and 39 to DIE (hypomethylated, 19; hypermethylated, 20), listed as supplemental data (Supplemental Table 6).
Fig. 2.
Promoter methylation profiles in SUP, OMA, and DIE. The Venn diagram compares the number of active regions (in bold) identified as hypomethylated (in italic characters) or hypermethylated (in roman characters) in the three subtypes.
Table 2.
Genomic regions significantly and consistently hypermethylated or hypomethylated in SUP, OMA, and DIE, as compared with EE
| Chromosome | No. active region | Start | Length | Ratio SUP/EE | Ratio OMA/EE | Ratio DIE/EE | Gene symbol | Gene name |
|---|---|---|---|---|---|---|---|---|
| 2 | 1466 | 242661047 | 1477 | 0.52 | 0.29 | 0.59 | FLJ38379 | Hypothetical protein FLJ38379 |
| 2 | 1467 | 242662876 | 1138 | 0.62 | 0.40 | 0.39 | FLJ38379 | Hypothetical protein FLJ38379 |
| 2 | 1470 | 131249876 | 565 | 0.43 | 0.36 | 0.41 | DEFB125 | Defensin, β 125 |
| 3 | 1728 | 122953778 | 587 | 0.60 | 0.59 | 0.60 | GOLGB1 | Golgin B1, Golgi integral membrane protein |
| 5 | 1896 | 763481 | 1315 | 0.37 | 0.65 | 0.60 | ||
| 5 | 1911 | 1669770 | 393 | 0.55 | 0.35 | 0.52 | ||
| 5 | 1999 | 170219671 | 206 | 0.44 | 0.62 | 0.59 | ||
| 6 | 2091 | 138477515 | 243 | 0.66 | 0.60 | 0.57 | PERP | TP53 apoptosis effector |
| 7 | 2186 | 56207807 | 8821 | 1.54 | 1.61 | 1.91 | ||
| 7 | 2263 | 156232815 | 1524 | 3.10 | 3.06 | 2.16 | NOM1 | Nucleolar protein with MIF4G domain 1 |
| 9 | 2396 | 17261249 | 762 | 0.64 | 0.65 | 0.60 | CNTLN | Centlein, centrosomal protein |
| 10 | 224 | 44815659 | 252 | 2.05 | 2.19 | 1.82 | RASSF4 | ras Association (RalGDS/AF-6) domain family member 4 |
| 10 | 224 | 44815659 | 252 | 2.05 | 2.19 | 1.82 | C10orf25 | Chromosome 10 open reading frame 25 |
| 10 | 224 | 44815659 | 252 | 2.05 | 2.19 | 1.82 | ZNF22 | Zinc finger protein 22 (KOX 15) |
| 11 | 310 | 520194 | 1229 | 1.69 | 2.58 | 1.83 | HRAS | v-Ha-ras Harvey rat sarcoma viral oncogene homolog |
| 11 | 310 | 520194 | 1229 | 1.69 | 2.58 | 1.83 | LRRC56 | Leucine-rich repeat containing 56 |
| 13 | 558 | 112809520 | 1027 | 1.83 | 2.47 | 2.03 | F7 | Coagulation factor VII (serum prothrombin conversion accelerator) |
| 13 | 561 | 113114806 | 556 | 2.30 | 2.44 | 1.52 | DKFZp451A211 | DKFZp451A211 protein |
| 13 | 561 | 113114806 | 556 | 2.30 | 2.44 | 1.52 | ADPRHL1 | ADP-ribosylhydrolase like 1 |
| 16 | 735 | 1250431 | 492 | 1.57 | 1.59 | 1.67 | TPSD1 | tryptase δ 1 |
| 16 | 815 | 31142886 | 513 | 1.63 | 1.71 | 1.64 | PYDC1 | PYD (pyrin domain) containing 1 |
| 17 | 986 | 54115047 | 229 | 0.51 | 0.56 | 0.66 | TEX14 | Testis expressed 14 |
| 17 | 986 | 54115047 | 229 | 0.51 | 0.56 | 0.66 | RAD51C | RAD51 homolog C (S. cerevisiae) |
| 19 | 1136 | 617852 | 1086 | 2.38 | 1.85 | 1.58 | RNF126 | Ring finger protein 126 |
| 19 | 1136 | 617852 | 1086 | 2.38 | 1.85 | 1.58 | FSTL3 | Follistatin-like 3 (secreted glycoprotein) |
| X | 2538 | 37114623 | 597 | 0.53 | 0.55 | 0.58 | FTHL19 | Ferritin, heavy polypeptide-like 19 |
Regions with a ratio above 1.50 or below 0.66 were taken into consideration.
Validation of MeDIP on chip results by McrBC digestion and bisulfite sequencing for ten differentially methylated regions
To validate the MeDIP on chip data, we studied the methylation status of ten among the most differentially methylated regions by quantitative PCR after DNA digestion by the McrBC methylation-sensitive restriction enzyme in ectopic vs. eutopic endometrium (Fig. 3). These regions were also chosen because they were abnormally methylated in at least two of three subtypes of the disease. Five patients with OMA, different from the initial set, were used for this experiment. McrBC is a methylation-specific endonuclease, which cleaves DNA containing 5′-methylcytosine residues but will not act on unmethylated DNA. Thus, a lesser PCR product recovery is indicative of hypermethylation. DNA isolated from OMA presented a higher PCR product recovery for FLJ38379, DEFB125, and LOC285634 regions, indicative of hypomethylation, and a lower PCR product recovery for C7orf3, FAM38A, HRAS, ADPRHL1, MAP2K1, LAMA5, and THEG, indicative of hypermethylation (Fig. 3). These results corroborate the finding provided by the MeDIP on chip experiment.
Fig. 3.
Quantitative RT-PCR results obtained for McrBC assay. McrBC is a methylation-specific endonuclease, which cleaves DNA containing 5′-methylcytosine residues but will not act on unmethylated DNA. Thus, a lesser PCR product recovery is indicative of hypermethylation. The black horizontal line represents the reference level and corresponds to EE; above this line, the region is hypermethylated; below, the region is hypomethylated. * and ** denote a significant difference in methylation level between OMA and EE (P < 0.05 and P < 0.01, respectively).
Then, we checked the promoter region of the LAMA5 gene, which contains six CpGs, using bisulfite treatment followed by PCR and direct sequencing (Fig. 4). The difference in methylation level was highly significant when the six CpGs analyzed were taken together (P = 0.001). Four CpGs of six were also significant when analyzed one by one (Fig. 4A). The ratio of hypermethylation was on average around 2-fold, close to the MeDIP on chip results (Fig. 4B).
Fig. 4.
Methylation analysis of the LAMA5 region by bisulfite treatment and direct sequencing. The peak heights in the sequencing chromatogram were visually evaluated to quantify the respective amount of methylated C vs. unmethylated C at each CpG position. A, Average values of methylation at each of the six analyzed CpGs. B, Ratios of methylation between ectopic and eutopic endometrium at each CpG position. *, P < 0.05; **, P < 0.01; ****, P < 0.001.
Impact of methylation on gene expression in endometriosis
We correlated these results with the expressional data available for the OMA (10). These expressional data, provided by two previously published studies, conducted by two independent teams on different sets of patients, are considered to be very reliable because they have been largely validated by RT-PCR and by a 81% concordance rate (9, 10). For convenience, we used only the data provided by our team, the largest one to date (5605 differentially expressed genes), which encompasses the results reported by the other group.
General correlation with transcriptional alterations in OMA failed to systematically link the methylation level in promoter regions and the expression level of nearby genes (χ2 test and correlation coefficients, data not shown). However, detailed examination of specific functional clusters provided different conclusions. For example, when considering the above-mentioned transcription factors cluster, the promoters of which are differentially methylated in OMA, eight genes of 20 (40%) were also modified at the expressional level (Table 1). This proportion is highly superior to the number of genes deregulated in endometriosis vs. EE (17%, according to Ref. 10) as tested by a χ2 test (P = 0.001). Although differentially methylated regions are generally located close to genes the expression of which is modified (induced or repressed), we were unable to link hypermethylation with gene extinction, and vice versa (Tables 1 and 3). In some cases, abnormal methylation status in a regulatory region was associated with a modification of the gene expression level, which is listed in Table 3. These 35 genes have never been highlighted in endometriosis, except the scavenger receptor class B1 (SCARB1) (34). SCARB1 is a receptor that mediates cholesterol transfer from plasma high-density lipoprotein to the steroidogenic pathway. Higher levels of SCARB1 mRNA (6.5-fold induction) and protein have been reported in ectopic endometrium, as compared with EE (10, 34). Up-regulation of SCARB1, possibly related to its promoter hypomethylation, might enhance the local estrogen production in the ectopic endometrium, in addition to aromatase (35), and thus contribute to endometriosis development. In all, these 35 genes might have an important role in the physiopathology of the disease through epigenetic deregulation of their expression and should be examined more in depth.
Table 3.
List of active regions and nearby genes, which share alterations both in methylation and expressional patterns
| Gene symbol | Gene name | Methylation status | Induction ratio | No. active region | Chromosome | Ratio OMA/EE | Ratio SUP/EE | Ratio DIE/EE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PYDC1 | PYD (pyrin domain) containing 1 | Hypermethylated | 2.52 | 815 | 16 | 1.71 | 1.63 | 1.64 | ||||||||
| FSTL3 | Follistatin-like 3 (secreted glycoprotein) | Hypermethylated | 2.07 | 1136 | 19 | 1.85 | 2.38 | 1.58 | ||||||||
| FLJ38379 | Hypothetical protein FLJ38379 | Hypomethylated | 0.41 | 1466 | 2 | 0.29 | 0.52 | 0.59 | ||||||||
| FLJ38379 | Hypothetical protein FLJ38379 | Hypomethylated | 0.41 | 1467 | 2 | 0.40 | 0.62 | 0.39 | ||||||||
| PERP | TP53 apoptosis effector | Hypomethylated | 0.08 | 2091 | 6 | 0.60 | 0.66 | 0.57 | ||||||||
| EDARADD | EDAR-associated death domain | Hypermethylated | 2.32 | 178 | 1 | 1.68 | NS | 1.53 | ||||||||
| LOC440993 | Hypothetical gene supported by AK128346 | Hypermethylated | 2.18 | 1773 | 3 | 2.24 | NS | 1.50 | ||||||||
| ADAP1 | ArfGAP with dual PH domains 1 | Hypermethylated | 0.37 | 2127 | 7 | 1.55 | NS | 1.55 | ||||||||
| JAKMIP3 | Janus kinase and microtubule interacting protein 3 | Hypermethylated | 3.65 | 275 | 10 | 1.81 | NS | 1.50 | ||||||||
| MPP3 | Membrane protein, palmitoylated 3 (MAGUK p55 subfamily member 3) | Hypermethylated | 2.12 | 963 | 17 | 1.91 | NS | 1.77 | ||||||||
| SLC16A3 | Solute carrier family 16, member 3 (monocarboxylic acid transporter 4) | Hypermethylated | 0.42 | 1043 | 17 | 1.60 | NS | 1.53 | ||||||||
| TFDP3 | Transcription factor Dp family, member 3 | Hypermethylated | 3.08 | 2614 | X | 1.64 | NS | 1.53 | ||||||||
| SLC16A3 | Solute carrier family 16, member 3 (monocarboxylic acid transporter 4) | Hypermethylated | 0.42 | 1042 | 17 | 1.51 | 1.51 | NS | ||||||||
| FAIM3 | Fas apoptotic inhibitory molecule 3 | Hypomethylated | 4.44 | 148 | 1 | 0.65 | 0.64 | NS | ||||||||
| PIGR | polymeric immunoglobulin receptor | Hypomethylated | 0.24 | 148 | 1 | 0.65 | 0.64 | NS | ||||||||
| EPCAM | Epithelial cell adhesion molecule | Hypomethylated | 0.11 | 1357 | 2 | 0.62 | 0.64 | NS | ||||||||
| PRKCQ | Protein kinase C, θ | Hypomethylated | 0.25 | 200 | 10 | 0.62 | 0.51 | NS | ||||||||
| PLD2 | Phospholipase D2 | Hypomethylated | 2.01 | 910 | 17 | 0.56 | 0.57 | NS | ||||||||
| CBS | Cystathionine-β-synthase | Hypomethylated | 4.07 | 1571 | 21 | 0.51 | 0.58 | NS | ||||||||
| TNNI3K | TNNI3-interacting kinase | Hypermethylated | 3.17 | 95 | 1 | 1.77 | NS | NS | ||||||||
| (Continued) | ||||||||||||||||
Table 3A.
Continued
| Gene symbol | Gene name | Methylation status | Induction ratio | No. active region | Chromosome | Ratio OMA/EE | Ratio SUP/EE | Ratio DIE/EE |
|---|---|---|---|---|---|---|---|---|
| CCDC76 | Coiled-coil domain containing 76 | Hypermethylated | 0.49 | 106 | 1 | 1.77 | NS | NS |
| HOXD11 | Homeobox D11 | Hypermethylated | 0.43 | 1423 | 2 | 1.53 | NS | NS |
| HOXD10 | Homeobox D10 | Hypermethylated | 0.30 | 1423 | 2 | 1.53 | NS | NS |
| CDC2L6 | Cell division cycle 2-like 6 (CDK8-like) | Hypermethylated | 0.35 | 2077 | 6 | 1.63 | NS | NS |
| TBC1D2 | TBC1 domain family, member 2 | Hypermethylated | 0.50 | 2433 | 9 | 1.71 | NS | NS |
| PIK3AP1 | Phosphoinositide-3-kinase adaptor protein 1 | Hypermethylated | 2.64 | 248 | 10 | 1.65 | NS | NS |
| DRD4 | Dopamine receptor D4 | Hypermethylated | 2.06 | 314 | 11 | 1.64 | NS | NS |
| ATP11A | ATPase, class VI, type 11A | Hypermethylated | 0.28 | 548 | 13 | 1.70 | NS | NS |
| LOC388022 | Hypothetical gene supported by AK131040 | Hypermethylated | 2.44 | 631 | 14 | 1.60 | NS | NS |
| DTNA | Dystrobrevin, α | Hypermethylated | 4.04 | 1078 | 18 | 1.54 | NS | NS |
| FXYD3 | FXYD domain containing ion transport regulator 3 | Hypermethylated | 0.09 | 1238 | 19 | 1.98 | NS | NS |
| AGPAT3 | 1-Acylglycerol-3-phosphate O-acyltransferase 3 | Hypermethylated | 0.50 | 1580 | 21 | 2.03 | NS | NS |
| PRKAG2 | Protein kinase, AMP-activated, γ 2 noncatalytic subunit | Hypomethylated | 0.40 | 2255 | 7 | 0.55 | NS | NS |
| PKP3 | Plakophilin 3 | Hypomethylated | 0.19 | 309 | 11 | 0.66 | NS | NS |
| ANO9 | Anoctamin 9 | Hypomethylated | 0.22 | 309 | 11 | 0.66 | NS | NS |
| ANO1 | Anoctamin 1, calcium activated chloride channel | Hypomethylated | 0.10 | 379 | 11 | 0.64 | NS | NS |
| SCARB1 | Scavenger receptor class B, member 1 | Hypomethylated | 6.64 | 473 | 12 | 0.59 | NS | NS |
Induction ratios listed in the table were for the comparison between OMA and eutopic endometrium and collected from Borgheseet al. (10 ). An induction ratio above 2.0 denotes an up-regulation of the corresponding gene (>2-fold) whereas an induction ratio below 0.5 denotes a down-regulation of the gene (>2-fold). Genes figured in bold denote genes with consistent methylation and expression pattern (hypomethylated and up-regulated or hypermethylated and down-regulated). NS, Nonsignificant (means that the methylation ratio is comprised between 0.66 and 1.50).
Chromosomal distribution of differentially methylated promoter regions in endometriosis
Localization of hypermethylated and hypomethylated regions on each chromosome is presented in Fig. 5. Analysis of chromosomal location of methylation alterations showed a nonrandom distribution. In endometriosis, whatever the subtype is taken into consideration and whatever the threshold is selected (10%, 5%, or 2% from the end of the chromosome), DNA hypermethylation occurred significantly more frequently at the chromosomal ends whereas hypomethylation was uniformly distributed along the chromosome (Table 4). In other words, hypomethylation appeared to be a random event, whereas hypermethylation is probably an active event accompanying the pathogenesis, being itself a molecular characteristic of the endometriotic phenotype. When comparing each type of endometriosis separately, this appeared to be mainly due to OMA and DIE, which are the most severe forms of endometriosis. When considering each chromosome separately, this systematic distribution of hypermethylation in subtelomeric regions appeared to be very strong and demonstrative, especially for chromosomes 1, 2, 3, 4, 7, 8, 14, 17, 19, 20, and X (Fig. 5). However, it can be noticed that for specific regions, this statistical rule may not be observed, such as for the telomeric end of 1q and 2q where only one hypomethylated region is observed. This global observation should also be nuanced by the fact that active regions are not covered in centromeric regions in acrocentric chromosomes (chromosomes13, 15, 21 and 22).
Fig. 5.

Chromosomal distribution of DNA methylation in endometriosis. Plotted is the methylation level (Log2 ratio) relative to chromosomal position for SUP (in green), OMA (in red), and DIE (in blue). Regions of significant differences are above 0.4 or below −0.4.
Table 4.
Chromosomal distribution of hypermethylated and hypomethylated promoter regions
| Threshold (% of chromosomal ends) | OMA | SUP | DIE | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypomethylated | Hypermethylated | Hypomethylated | Hypermethylated | Hypomethylated | Hypermethylated | ||||
| 10% | NS | <0.01 | NS | NS | NS | <0.01 | |||
| 5% | NS | <0.01 | 0.05 | <0.01 | NS | <0.01 | |||
| 2% | NS | <0.01 | NS | <0.01 | NS | <0.01 | |||
P values for each given threshold (2%, 5%, or 10% of the ends of the chromosome) were computed using the χ2 test. P < 0.05 was considered significant. NS, Nonsignificant.
Discussion
This study reports on the mapping of DNA methylation for 25,500 promoters in endometriosis by the way of methylated DNA immunoprecipitation in combination with microarray analysis (MeDIP on chip). This is the first description of a genome-wide DNA methylation profile in endometriosis to date. It also provides some new insights into endometriosis pathogenesis.
First, global methylation patterns for each endometriosis subtypes (SUP, OMA, and DIE) do not differ from each other and from EE, as shown by correlation analysis. However, when looking in more detail, specific regions are consistently hypermethylated (or hypomethylated) in the three subtypes. Also, other specific regions are strictly altered in one type of endometriosis only. This can be due either to various cellular make-ups in the different subtypes, or to actual differences in the methylation of active regions. Of course, both issues can contribute to the differences, although the existence of regions consistently modified in more than one disease subtype militates in favor of the second hypothesis, at least for 94 regions (25+20+33+16) (Fig. 2). Overall, promoter methylation levels for some specific genes might be regarded as an epigenetic signature of endometriosis and might help to delineate SUP, OMA, or DIE.
Second, there is no evident correlation between methylation and expression in endometriosis, except for a few genes that might be implicated in the pathogenesis of the disease, and in which epigenetic deregulation could be important. This is in apparent contradiction with the current dogma that methylated CpGs are associated with repression of expression. However, the use of pangenomic approaches, together with the analysis of specific gene promoters, indicates that the expected correlation is far from being a rule. In a recent series of pangenomic experiments in cancer cells, the results were very similar to ours (Töst J., personal communication). Also, in the IGF-2/H19 complex, methylation of the differentially methylated region, separating the two genes, leads to transcriptional activation of IGF-2 and to transcriptional repression of H19 (36). Consistently, the cullin (CUL)-7 promoter appears to be hypomethylated in human intrauterine growth restriction placenta and hypermethylated in normal placenta, whereas the gene is up-regulated in both conditions (37). Therefore, the vision of a strict correlation between promoter hypomethylation, on the one hand, and gene up-regulation and promoter hypermethylation and gene down-regulation, on the other hand, is clearly a simplification. Mechanistically, the MeDIP on chip selects regions with a high density of CpGs and immunoprecipitates those that are hypermethylated CpG islands. The recent literature has shown that several transcriptional activators are recruited through the action of specific Methyl-CpG binding proteins such as MeCP2, the major protein in this family (38, 39, 40). Such mechanisms are involved in gene reactivation in cancer cells, because there is a Methyl-CpG targeted recruitment of p300, which reactivates tumor suppressor genes in human cancer cells (40). In addition, recent reports show that MeCP2 is associated with chromatin remodeling-inducing gene repression as well as gene activation, which is totally congruent with our own results (41, 42). Also, several studies indicate that DNA methylation is involved in the maintenance rather than the initiation of gene silencing (15, 43). Thus, our results might be consistent with the fact that DNA methylation is not required to silence the gene but is required to stably prevent an increase of its expression level (44, 45).
The genes that we have identified, do not overlap with those that have been previously reported to be influenced by methylation level in endometriosis, even though they are modified at the expression level (9, 10): HOX-A10 (20, 21, 22), PR-B (23, 25), CYP19A1 (aromatase) (26), ESR2 (24), DNMT1, DNMT3A, DNMT3B (27), and SF-1 (24). In our opinion, these findings are not conflicting, because whereas MeDIP on chip is highly specific, its capability to detect low CpG-containing regions, which may be meaningful in biological terms, is probably relatively limited, as suggested by some studies which pointed this out as its main drawback (46). The screening provided by the MeDIP on chip assay, as comprehensive as it is, probably fails in detecting all differentially methylated regions, in particular when they are GC poor. However, it is likely that the detected regions are scientifically relevant and robust. The genome-wide approach is now recognized as being particularly reliable and of proven efficiency for detecting the most distinctive abnormalities of methylation profile in human cells (47). Therefore these 35 new genes, modified at the expression level (whatever the direction of the expression alteration), as well as in their nearby methylation profile (Table 3), are very good candidates and should be further investigated for their implication in endometriosis. The example of active region 148 is very demonstrative. This region is located in a DNA region that can be held as a promoter for two genes encoded by opposite DNA strands: FAIM3 and PIGR. Whereas active region 148 is significantly hypomethylated, FAIM3 appears to be induced and PIGR repressed, both more than 4-fold (Table 3).
Finally, the most striking and intriguing result of our study is the localization of hypermethylated regions at the ends of the chromosomes whereas hypomethylation seems to be a random event. It is the first example of such an asymmetry in a human disease. The observation is consistent with the known expressional data for methyltransferases in endometriosis (9, 10). DNMT1 and DNMT2 (referred to as “maintenance” methyltransferases) were significantly down-regulated in ectopic endometrium compared with EE (induction ratios: 0.49 and 0.41, respectively). This could limit their efficiency and induce a defective methylation during cell divisions accompanying the lesion development. This is consistent with a random hypomethylation. DNMT3A and DNMT3B (referred to as “de novo” methyltransferases), on the other hand, are not significantly modified (induction ratios: 0.85 and 0.74, respectively). It would be interesting to conceive the mechanisms that target DNA methylation to the chromosome telomeric ends. First, there might be a phenomenon of under-regionalization, fostering the tropism of de novo DNMTs to the telomeres. This is likely to occur during interphase, during which chromosomes are regionalized (48). Then, recruitment of sequence-specific transcription factors might specifically target DNA methylation activity to a subset of subtelomeric promoters. This function has been suggested for MYC (49) and SPI1 (PU.1) (50). A possible mechanism targeting methylation modifications at the chromosome end has been very recently suggested for variants of DNMT3L, known to act in combination with DNMT3A for de novo methylation (51).
This result raises also the question of the cellular and clinical consequences of such a distribution. One hypothesis might be an enhanced stability of the chromosome and the prevention of telomeric fusions and chromosomal rearrangements. Indeed, mouse ES cells, deficient for DNMT3A/3B, have elongated telomeres and an increased frequency of telomere recombination (52). This condition might provide a certain protection against malignancy, because many cancer cells display global hypomethylation of their genome, which has been causally linked to increased chromosomal instability and tumor progression (53, 54). Thus, the observed asymmetry in hypermethylation/hypomethylation could be regarded as an additional mechanism “invented” by the endometriotic cells to preserve themselves from the risk of malignant transformation and guarantee to endometriosis the oxymoron status of a benign metastatic disease (10).
To conclude, we believe that this study, dealing with endometriosis, a very frequent and debilitating gynecological disease, is a first step toward understanding epigenetic mechanisms, which emerge as a very promising and stimulating field of future research. Therefore, generalizing our observations to a larger sample of individuals would be interesting, although the pooling strategy used suggests that only robust modifications have been found. It could be very appealing to establish specific epigenomic profiles that could be used as markers for diagnosing endometriosis, monitoring its progression, and quantifying its severity. In addition, some drugs have been reported to induce reversal in methylation profile (55), making it possible to consider their use as therapeutic agents in endometriosis.
Materials and Methods
Biological samples
Tissue samples were isolated from 20 patients afflicted with painful endometriosis and operated in luteal phase for complete surgical exeresis of the lesions. All samples were obtained from surgical biopsies. The material consisted of approximately 1 cm3 of the ovarian cyst wall in the case of OMA and of the endometriotic nodule for SUP and DIE. Immediately after surgical removal, each sample was frozen in liquid nitrogen for at least 15 min and kept frozen at −80 C until use. A fraction of the collection was also histologically examined to confirm the diagnosis of endometriosis and the luteal phase of the menstrual cycle. Categorization into SUP, OMA, and DIE was established in accordance with the widely accepted surgical classification proposed by Chapron et al. (30). Overall, five samples of SUP, 10 samples of OMA, five samples of DIE, and the 20 corresponding endometrial biopsies (EE) were collected. Because aging is known to affect the global DNA methylation in healthy individuals (56), patients included in each subtype were selected according to their age, which does not significantly differ between the three groups (average ages were 32.8 ± 1.2 yr, 32.2 ± 1.6 and 33.1 ± 1.1 in SUP, OMA, and DIE, respectively). All patients recruited for the study provided written informed consent. The local Institutional Review Board (‘Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale’ of Paris-Cochin) approved the study protocol.
Genomic DNA extraction
DNA was isolated from tissue samples using the FastDNA SPIN Kit and the FastPrep Instrument (Qbiogene, Carlsbad, CA) according to the manufacturer’s recommendations. DNA was purified by phenol chloroform extraction followed by ethanol precipitation and subsequently dissolved in water overnight. The DNA concentration and quality were determined by measuring the absorbance at 260 nm and 280 nm in a spectrophotometer (NanoDrop 1000; Thermo Scientific, Wilmington, DE).
MeDIP-on-Chip/promoter assay
Three DNA pools of five samples each were constituted, using precisely 5 μg of DNA per sample: SUP, OMA, and DIE. A fourth DNA pool, named EE, was composed with the 15 DNA samples extracted from endometrial biopsies of all the patients and used as a control. This was done intentionally as a means to limit interindividual variations and to focus on what is really characteristic of the disease. Each DNA pool was purified by phenol chloroform extraction followed by ethanol precipitation. DNA concentration and quality were verified several times on a spectrophotometer. DNA pools were shipped to Genpathway (San Diego, CA) for Methylated DNA IP-on-Chip/Promoter Assay, making it possible to combine DNA immunoprecipitation and hybridization to Affymetrix (Santa Clara, CA) promoter microarrays. Briefly, DNA samples were immunoprecipitated with an antibody against 5-methyl-cytosine using GenPathway’s protocols, adapted from the method first described by Weber et al. (28). The quality of the immunoprecipitation was determined by analyzing the enrichment of four regions with known methylation patterns, before and after amplification using an unbiased approach (untr12, JDP2, GEMIN4, KIAA0853). Before proceeding, it was checked that the immunoprecipitation profile was highly similar before and after amplification. Immunoprecipitated DNA samples were amplified, labeled, and hybridized to Affymetrix GeneChip Human Promoter 1.0R Arrays. These arrays contain more than 4.6 million probes of 25 nucleotides separated by 10-bp gap and tiled through more than 25,500 promoter regions. Each promoter region covers approximately 7.5 kb upstream through 2.45 kb downstream of 5′-transcription start sites. The array contains probes for approximately 59 percent of CpG islands annotated by the University of California Santa Cruz in the National Center for Biotechnology Information human genome assembly. Arrays were washed and scanned according to Affymetrix standard procedures.
Raw data from the array scans were analyzed using Affymetrix’ Tiling Analysis Software. This analysis generates files that contain the intensities for all probes on the arrays, which are expressed either as signals (estimating the fold enrichment in Log2 scale) or as P values (probabilities that measure the significance of the enrichment). For this analysis, we used a moderate-low threshold setting (2.0) and identified active regions that are genomic regions containing one or more genomic segments where signals or P values are above the threshold. For comparison between the samples, we calculated the ratio between the intensity for SUP, OMA, and DIE, at a given active region, and the matched intensity for EE. Ratios above 1.50, denoting a hypermethylated region in SUP, OMA, or DIE as compared with EE, and below 0.66, denoting a hypomethylated region, were taken into consideration for subsequent analysis.
Methylation analysis by McrBC digestion and quantitative PCR
Methylation status of 10 active regions (FLJ38379, DEFB125, LOC285634, C7orf3, FAM38A, HRAS, ADPRHL1, MAP2K1, LAMA5, and THEG) was determined for five patients with OMA, by methylation-sensitive McrBC-PCR assay, as previously described (57, 58). Genomic DNA was extracted from ectopic endometrium (OMA) and EE of the same patient, taken as her own control. DNA (1μg) was treated either with 25 U of McrBC endonuclease (New England Biolabs, Beverly, MA) or mock treated with an equivalent volume of water in a 50-μl reaction mixture containing 1× NEB2 buffer, 0.1 mg/ml BSA, and 2 mm GTP. Treated and mock-treated reactions were incubated at 37 C during 6 h. Then, the enzyme was inactivated (65 C, 20 min), and the samples were precipitated, washed, and resuspended in 30 μl of H2O. After the McrBC treatment, subsequent fluorescence-based quantitative PCR was used, starting from 2 μl of resuspended DNAs. Quantitative PCR was carried out using the amplification kit Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA) complemented with MgCl2 (4 mmol/liter final concentration), BSA (0.05 g/liter), and primers (10−3 mmol/liter) to a final volume of 17 μl per point. The reaction was performed in a Light Cycler Thermocycler (Roche, Indianapolis, IN). Ten pairs of locus-specific PCR primers were used and detailed as supplemental data (Supplemental Table 7). We used the following PCR program: 50 C, 120 sec; 95 C 120 sec; 35 cycles of three temperature steps (94 C, 5 sec; 58 C, 10 sec; 72 C, 15 sec). Finally, samples were submitted to a progressive temperature elevation (from 65–99 C at 0.1 C/sec), resulting in a fusion curve, enabling us to check the PCR products homogeneity. In addition, the products were systematically controlled by agarose gel electrophoresis. The Ct (threshold cycle number) values were collected with the LightCycler software (Roche) in the exponential phase of the PCR. Cleavage of methylated DNA by McrBC induces DNA strand breaks and abrogates PCR amplification. Conversely, the presence of unmethylated cytosines in DNA prevents enzyme cleavage and can be detected by PCR amplification. Mock-treated DNA (undigested) served as a control and was considered as the maximal PCR product recovery. Methylated DNA has decreased amounts of PCR product after McrBC digestion. Thus, the Ct values of treated samples were normalized by the Ct values of the mock-treated DNA (ΔCt). The results are presented as fold induction of PCR product recovery after the digestion of DNA with McrBC relative to undigested DNA (computed by 2−ΔCt).
Methylation analysis by bisulfite treatment and direct sequencing
Validation of the LAMA5 region (no. 1517) methylation status was determined by direct sequencing of bisulfite-treated genomic DNA extracted from ectopic endometrium (OMA) and EE according to a previously described protocol (59). For bisulfite conversion, 700 ng of genomic DNA were treated using the EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA) according to manufacturer’s instructions. One tenth of the column elution product was used as a template for direct PCR amplification of differentially methylated target regions. When necessary, a subsequent hemi-nested amplification was performed using 1/50th of the initial PCR as secondary template to obtain a perfect specific product. Amplimers were column purified before direct sequencing. The primers used for amplification after bisulfite treatment were GG-F TTTGGGGGTTGGTGTTATTAT and GG-R CTACCTAATAACTCTAATACC. The resulting amplification product of 746 bp was further reamplified with the nested primer GG-INTF TAGGAGTTGGTTTAGATTTTGT, and with the same reverse primer (hemi-nested PCR). This second PCR yielded a 697-bp genomic DNA fragment that was directly sequenced for each DNA sample. The amplifications were carried out in standard PCR conditions (59).
Gene expression profile for ectopic vs. eutopic endometrium
Expressional data were provided by our previously published work that reported the gene expression alterations in OMA, as compared with EE (10). Using long oligonucleotide arrays and validated by quantitative RT-PCR experiments and others (9), this work may be considered as very reliable and is the largest to date. The data are available under the National Center for Biotechnology Information accession nos. GSE5108 and GSE12768.
Statistics
For global comparison of promoter methylation patterns between SUP, OMA, DIE, and EE, pair-wise correlation comparisons were made between all groups. R values were compared for significant correlation between SUP, OMA, DIE, and EE. For analyzing the distribution of differences in methylation levels along the chromosomes, the complete set of active regions was analyzed with regard to their position along the chromosomes using three different thresholds: the last 10%, 5%, or 2% of the chromosome. Predicted numbers of hypermethylated and hypomethylated regions were calculated using the distribution of the complete set of active regions as a reference, and used to calculate probabilities of random distributions using a χ2 test. P < 0.05 was considered to be significant. All statistics were performed on Excel 2008 (Microsoft, Redmond, WA).
Acknowledgments
We thank Sonia Chelbi and Ludivine Doridot (Institut Cochin) for their technical help in McrBC digestion experiments.
Footnotes
This work was supported by Institut National de la santé et de la Recherche Médicale; Centre National de la Recherche Scientifique; Université Paris Descartes; and Assistance Publique-Hôpitaux de Paris.
Disclosure Summary: All authors have nothing to declare.
First Published Online August 4, 2010
C.C. and D.V. contributed equally to the direction of the work.
Abbreviations: DIE, Deeply infiltrating endometriosis; DNMT, DNA methyltransferases; EE, eutopic endometrium; MeDIP, methylated DNA immunoprecipitation; MeDIP on chip, MeDIP in combination with microarray hybridization; OMA, endometrioma (endometriotic ovarian cyst); SUP, superficial endometriosis.
References
- 1.Simoens S, Hummelshoj L, D'Hooghe T2007. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 13:395–404 [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC2004. Endometriosis. Lancet 364:1789–1799 [DOI] [PubMed] [Google Scholar]
- 3.Bulun SE2009. Endometriosis. N Engl J Med 360:268–279 [DOI] [PubMed] [Google Scholar]
- 4.Chapron C, Bourret A, Chopin N, Dousset B, Leconte M, Amsellem-Ouazana D, de Ziegler D, Borghese B2010. Surgery for bladder endometriosis: long-term results and concomitant management of associated posterior deep lesions. Hum Reprod 25:884–889 [DOI] [PubMed] [Google Scholar]
- 5.Fauconnier A, Chapron C2005. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update 11:595–606 [DOI] [PubMed] [Google Scholar]
- 6.de Ziegler D, Borghese B, Chapron C2010. Endometriosis and infertility: pathophysiology and management. Lancet 376:730–738 [DOI] [PubMed] [Google Scholar]
- 7.Sampson J1927. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 14:422–469 [Google Scholar]
- 8.Stefansson H, Geirsson RT, Steinthorsdottir V, Jonsson H, Manolescu A, Kong A, Ingadottir G, Gulcher J, Stefansson K2002. Genetic factors contribute to the risk of developing endometriosis. Hum Reprod 17:555–559 [DOI] [PubMed] [Google Scholar]
- 9.Eyster KM, Klinkova O, Kennedy V, Hansen KA2007. Whole genome deoxyribonucleic acid microarray analysis of gene expression in ectopic versus eutopic endometrium. Fertil Steril 88:1505–1533 [DOI] [PubMed] [Google Scholar]
- 10.Borghese B, Mondon F, Noël JC, Fayt I, Mignot TM, Vaiman D, Chapron C2008. Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Mol Endocrinol 22:2557–2562 [DOI] [PubMed] [Google Scholar]
- 11.Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, Kennedy SH, Zondervan KT2008. The search for genes contributing to endometriosis risk. Hum Reprod Update 14:447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones PA, Baylin SB2007. The epigenomics of cancer. Cell 128:683–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esteller M2008. Epigenetics in cancer. N Engl J Med 358:1148–1159 [DOI] [PubMed] [Google Scholar]
- 14.Richards EJ2006. Inherited epigenetic variation—revisiting soft inheritance. Nat Rev Genet 7:395–401 [DOI] [PubMed] [Google Scholar]
- 15.Weber M, Schübeler D2007. Genomic patterns of DNA methylation: targets and function of an epigenetic mark. Curr Opin Cell Biol 19:273–280 [DOI] [PubMed] [Google Scholar]
- 16.Leonhardt H, Page AW, Weier HU, Bestor TH1992. A targeting sequence directs DNA methyltransferase to sites of DNA replication in mammalian nuclei. Cell 71:865–873 [DOI] [PubMed] [Google Scholar]
- 17.Okano M, Bell DW, Haber DA, Li E1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247–257 [DOI] [PubMed] [Google Scholar]
- 18.Esteller M2007. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 8:286–298 [DOI] [PubMed] [Google Scholar]
- 19.Di W, Guo SW2007. The search for genetic variants predisposing women to endometriosis. Curr Opin Obstet Gynecol 19:395–401 [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW2005. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol 193:371–380 [DOI] [PubMed] [Google Scholar]
- 21.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT2007. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod 13:323–332 [DOI] [PubMed] [Google Scholar]
- 22.Lee B, Du H, Taylor HS2009. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod 80:79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW2006. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 1:106–111 [DOI] [PubMed] [Google Scholar]
- 24.Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE2007. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod 77:681–687 [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Starzinski-Powitz A, Guo SW2008. Prolonged stimulation with tumor necrosis factor-α induced partial methylation at PR-B promoter in immortalized epithelial-like endometriotic cells. Fertil Steril 90:234–237 [DOI] [PubMed] [Google Scholar]
- 26.Izawa M, Harada T, Taniguchi F, Ohama Y, Takenaka Y, Terakawa N2008. An epigenetic disorder may cause aberrant expression of aromatase gene in endometriotic stromal cells. Fertil Steril 89:1390–1396 [DOI] [PubMed] [Google Scholar]
- 27.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW2007. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril 87:24–32 [DOI] [PubMed] [Google Scholar]
- 28.Weber M, Davies JJ, Wittig D, Oakeley EJ, Haase M, Lam WL, Schübeler D2005. Chromosome-wide and promoter-specific analyses identify sites of differential DNA methylation in normal and transformed human cells. Nat Genet 37:853–862 [DOI] [PubMed] [Google Scholar]
- 29.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, Aach J, Ansorge W, Ball CA, Causton HC, Gaasterland T, Glenisson P, Holstege FC, Kim IF, Markowitz V, Matese JC, Parkinson H, Robinson A, Sarkans U, Schulze-Kremer S, Stewart J, Taylor R, Vilo J, Vingron M2001. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nat Genet 29:365–371 [DOI] [PubMed] [Google Scholar]
- 30.Chapron C, Fauconnier A, Vieira M, Barakat H, Dousset B, Pansini V, Vacher-Lavenu MC, Dubuisson JB2003. Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum Reprod 18:157–161 [DOI] [PubMed] [Google Scholar]
- 31.Somigliana E, Infantino M, Candiani M, Vignali M, Chiodini A, Busacca M, Vignali M2004. Association rate between deep peritoneal endometriosis and other forms of the disease: pathogenetic implications. Hum Reprod 19:168–171 [DOI] [PubMed] [Google Scholar]
- 32.Chapron C, Chopin N, Borghese B, Foulot H, Dousset B, Vacher-Lavenu MC, Vieira M, Hasan W, Bricou A2006. Deeply infiltrating endometriosis: pathogenetic implications of the anatomical distribution. Hum Reprod 21:1839–1845 [DOI] [PubMed] [Google Scholar]
- 33.Signorile PG, Baldi F, Bussani R, D'Armiento M, De Falco M, Baldi A2009. A Ectopic endometrium in human foetuses is a common event and sustains the theory of mullerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res 28:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramachandran S, Song M, Murphy AA, Parthasarathy S2001. Expression of scavenger receptor class B1 in endometrium and endometriosis. J Clin Endocrinol Metab 86:3924–3928 [DOI] [PubMed] [Google Scholar]
- 35.Noble LS, Simpson ER, Johns A, Bulun SE1996. Aromatase expression in endometriosis. J Clin Endocrinol Metab 81:174–179 [DOI] [PubMed] [Google Scholar]
- 36.Lewis A, Murrell A2004. Genomic imprinting: CTCF protects the boundaries. Curr Biol 14:R284–R286 [DOI] [PubMed]
- 37.Gascoin-Lachambre G, Buffat C, Rebourcet R, Chelbi ST, Rigourd V, Mondon F, Mignot TM, Legras E, Simeoni U, Vaiman D, Barbaux S2010. Cullins in human intra-uterine growth restriction: expressional and epigenetic alterations. Placenta 31:151–157 [DOI] [PubMed] [Google Scholar]
- 38.Rietveld LE, Caldenhoven E, Stunnenberg HG2002. In vivo repression of an erythroid-specific gene by distinct corepressor complexes. EMBO J 21:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukushige S, Kondo E, Horii A2008. Methyl-CpG targeted transcriptional activation allows re-expression of tumor suppressor genes in human cancer cells. Biochem Biophys Res Commun 377:600–605 [DOI] [PubMed] [Google Scholar]
- 40.Fukushige S, Kondo E, Horii A2009. Methyl-CpG targeted recruitment of p300 reactivates tumor suppressor genes in human cancer cells. Biochem Biophys Res Commun 379:1021–1026 [DOI] [PubMed] [Google Scholar]
- 41.Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY2008. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320:1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhasarathy A, Wade PA2008. The MBD protein family-reading an epigenetic mark? Mutat Res 647:39–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walsh CP, Bestor TH1999. Cytosine methylation and mammalian development. Genes Dev 13:26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman N, Gerson A, Fang J, Li E, Zhang Y, Shinkai Y, Cedar H, Bergman Y2006. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol 8:188–194 [DOI] [PubMed] [Google Scholar]
- 45.Feng YQ, Desprat R, Fu H, Olivier E, Lin CM, Lobell A, Gowda SN, Aladjem MI, Bouhassira EE2006. DNA methylation supports intrinsic epigenetic memory in mammalian cells. PLoS Genet 2:e65 [DOI] [PMC free article] [PubMed]
- 46.Irizarry RA, Ladd-Acosta C, Carvalho B, Wu H, Brandenburg SA, Jeddeloh JA, Wen B, Feinberg AP2008. Comprehensive high-throughput arrays for relative methylation (CHARM). Genome Res 18:780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schones DE, Zhao K2008. Genome-wide approaches to studying chromatin modifications. Nat Rev Genet 9:179–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokota H, Singer MJ, van den Engh GJ, Trask BJ1997. Regional differences in the compaction of chromatin in human G0/G1 interphase nuclei. Chromosome Res 5:157–166 [DOI] [PubMed] [Google Scholar]
- 49.Brenner C, Deplus R, Didelot C, Loriot A, Viré E, De Smet C, Gutierrez A, Danovi D, Bernard D, Boon T, Pelicci PG, Amati B, Kouzarides T, de Launoit Y, Di Croce L, Fuks F2005. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J 24:336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Yamada T, Kihara-Negishi F, Sakurai T, Hara E, Tenen DG, Hozumi N, Oikawa T2006. Site-specific DNA methylation by a complex of PU. 1 and Dnmt3a/b. Oncogene 25:2477–2488 [DOI] [PubMed] [Google Scholar]
- 51.El-Maarri O, Kareta MS, Mikeska T, Becker T, Diaz-Lacava A, Junen J, Nüsgen N, Behne F, Wienker T, Waha A, Oldenburg J, Chédin F2009. A systematic search for DNA methyltransferase polymorphisms reveals a rare DNMT3L variant associated with subtelomeric hypomethylation. Hum Mol Genet 18:1755–1768 [DOI] [PubMed] [Google Scholar]
- 52.Gonzalo S, Jaco I, Fraga MF, Chen T, Li E, Esteller M, Blasco MA2006. DNA methyltransferases control telomere length and telomere recombination in mammalian cells. Nat Cell Biol 8:416–424 [DOI] [PubMed] [Google Scholar]
- 53.Eden A, Gaudet F, Waghmare A, Jaenisch R2003. Chromosomal instability and tumors promoted by DNA hypomethylation. Science 300:455. [DOI] [PubMed] [Google Scholar]
- 54.Vera E, Canela A, Fraga MF, Esteller M, Blasco MA2008. Epigenetic regulation of telomeres in human cancer. Oncogene 27:6817–6833 [DOI] [PubMed] [Google Scholar]
- 55.Pereira MA, Tao L, Liu Y, Li L, Steele VE, Lubet RA2007. Modulation by budesonide of DNA methylation and mRNA expression in mouse lung tumors. Int J Cancer 120:1150–1153 [DOI] [PubMed] [Google Scholar]
- 56.Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, Miyazaki T, Ogura C, Okazaki Y, Jinno Y2004. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet 68:196–204 [DOI] [PubMed] [Google Scholar]
- 57.Ordway JM, Bedell JA, Citek RW, Nunberg A, Garrido A, Kendall R, Stevens JR, Cao D, Doerge RW, Korshunova Y, Holemon H, McPherson JD, Lakey N, Leon J, Martienssen RA, Jeddeloh JA2006. Comprehensive DNA methylation profiling in a human cancer genome identifies novel epigenetic targets. Carcinogenesis 27:2409–2423 [DOI] [PubMed] [Google Scholar]
- 58.Holemon H, Korshunova Y, Ordway JM, Bedell JA, Citek RW, Lakey N, Leon J, Finney M, McPherson JD, Jeddeloh JA2007. MethylScreen: DNA methylation density monitoring using quantitative PCR. Biotechniques 43:683–693 [DOI] [PubMed] [Google Scholar]
- 59.Fauque P, Jouannet P, Lesaffre C, Ripoche MA, Dandolo L, Vaiman D, Jammes H2007. Assisted reproductive technology affects developmental kinetics, H19 imprinting control region methylation and H19 gene expression in individual mouse embryos. BMC Dev Biol 7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]