Abstract
The discovery of estrogen receptor-β (ERβ) in 1996 stimulated great interest in the physiological roles and molecular mechanisms of ERβ action. We now know that ERβ plays a major role in mediating estrogen action in several tissues and organ systems, including the ovary, cardiovascular system, brain, and the immune system, and that ERβ and ERα generally play distinct physiological roles in the body. Although significant progress has been made toward understanding the molecular mechanisms of ERβ action, particularly in vitro, there remains a large gap in our understanding of the mechanisms by which ERβ elicits its biological functions in a true physiological context.
This manuscript reviews the known molecular mechanisms that underlie the physiological functions of Estrogen Receptor-β, and find that a variety of such mechanisms contribute to its role.
In a 2007 minireview, Heather Harris (1) reviewed the state of knowledge of estrogen receptor ERβ’s physiological functions by examining data from transgenic and knockout mouse models and from the preclinical characterization of ERβ-selective agonists. In that review, Dr. Harris concluded that “the field is currently sorely lacking in mechanistic hypotheses” to explain how ERβ might exert its effects. The purpose of this minireview is to summarize the progress made to date toward developing mechanistic hypotheses and understanding the molecular mechanisms by which ERβ exerts these physiological effects.
Unique Aspects of ERβ
ERs are nuclear hormone receptors that act as transcription factors to regulate genes involved in homeostasis, development, and metabolism. In similar fashion to other ligand-inducible nuclear hormone receptors, the classic mechanism of ER action involves binding of the ER to ligand, resulting in receptor dimerization, interaction with consensus estrogen-response elements (EREs), and recruitment of transcriptional coregulators, resulting in the formation of a complex that modulates transcription of estrogen target genes. Two forms of the ER have been identified, ERα and ERβ. In mice, ERα is widely expressed, with the highest mRNA expression in the uterus, ovary, pituitary gland, male reproductive organs, white and brown adipose tissue, prostate, skin, skeletal muscle, aorta, kidney, gall bladder, and bone (2). In contrast, ERβ mRNA levels are highest in the ovary, lung, male reproductive organs, colon, brain, and kidney (2), and of these, ERβ protein has been demonstrated in all but the kidney (3, 4, 5, 6, 7, 8, 9, 10). Thus, ERα mediates most estrogen signaling in classic estrogen target tissues such as the uterus, mammary gland, and skeleton, whereas ERβ has a minor role in these tissues (1). However, ERβ regulates signaling in the ovary, the immune system, the prostate, gastrointestinal tract, and hypothalamus (1), and the two ERs have little functional overlap. Although much is known regarding ERα-regulated transcriptional mechanisms in vitro and in vivo, a great deal remains to be learned regarding the mechanisms of ERβ-mediated transcription, particularly in vivo.
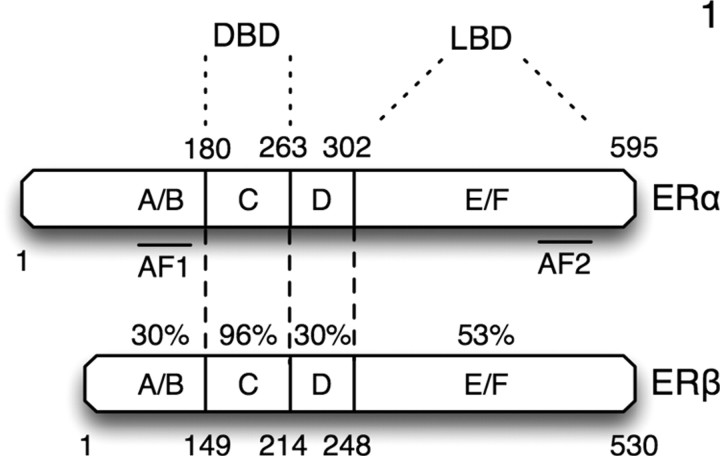
Although ERβ shares many transcriptional mechanisms with ERα, there are mechanisms unique to ERβ (11, 12). These similarities and differences can be ascribed, at least in part, to their protein sequences (Fig. 1). Both ERs contain a DNA-binding domain (DBD), a ligand-binding domain, and two activation function (AF) domains: AF-1 at the N terminus and AF-2 at the C terminus. The DBD and AF-2 domains possess the highest similarity in amino acid identity (96%) (13), whereas the AF-1 and N-terminal domains are the least conserved (30%) (14). Although both ERs have nearly identical DBDs, ERβ has a lower affinity for ERE half-sites than ERα (15, 16, 17). When bound to different ERE sequences, ERβ undergoes conformational changes specific to each ERE, resulting in differential coactivator recruitment (18). Thus, these differences in half-site binding and ERE-dependent conformational changes may all contribute to differences in transcriptional activity between ERα and ERβ.
Fig. 1.
Protein sequence identity comparison between human ERα and ERβ. LBD, Ligand-binding domain. DBD, DNA-binding domain.
Challenges to Studying ERβ Mechanisms of Action
Two major challenges to studying ERβ’s molecular mechanisms of action include 1) the lack of immortalized cell lines expressing endogenous ERβ and 2) the lack of highly specific, consistently available commercial antibodies to ERβ, particularly for detection of rodent ERβ.
Although the majority of ERβ mechanistic studies have been conducted using cell lines engineered to express ERβ, far fewer have used cell lines that express endogenous ERβ. This is due, in part, to confusion in the field regarding which cell lines express ERβ mRNA and, more importantly, which express endogenous ERβ protein. For example, ERβ expression in the human breast carcinoma cell line MCF-7 is particularly controversial. Although MCF-7 cells are generally considered ERβ negative, some reports indicate they are ERβ positive (19, 20). There are several cell lines that do appear to express ERβ, although relatively few laboratories use them for ERβ studies. For example, the prostate cancer cell line DU145 (20, 21) and the Cath.a cell line derived from mouse brainstem (22) reportedly express ERβ protein in the absence of ERα. ERβ protein has also been detected in the prostate cancer cell line LNCaP (20, 23), the immortalized human prostate benign epithelial cell line BPH-1 (20), the medulloblastoma cell lines D283Med and Daoy (24), and the mouse mammary epithelial cell line HC11 (25). Thus, the identification of cell lines widely accepted to express endogenous ERβ, preferably in the absence of ERα, remains a challenge that needs to be addressed.
A second issue that has hampered efforts to dissect ERβ mechanisms of action is the lack of a commercially available, highly specific ERβ antibody to detect endogenous rodent ERβ that could be used, for example, in chromatin immunoprecipitation (ChIP) assays with mouse tissue. This same problem appears to a lesser extent with antibodies to detect human ERβ (9), and it is not uncommon for investigators to use several human ERβ antibodies within one study to confirm their results (26). The main problem is that currently available antimouse or -human ERβ antibodies either detect many spurious bands or cross-react with ERα in a complex lysate such as that derived from whole cells or tissue. A highly specific antibody is essential for ChIP-chip and ChIP-Seq analysis, the former of which has been used since 2005 to detect ERα-binding regions within the genome (27, 28, 29). Recently, ChIP-chip has been used to identify ERβ-binding sites in the absence or presence of ERα using MCF-7 cells engineered to express ERβ (30); however, ChIP-chip studies in rodent tissues are hindered by the lack of a highly specific commercially available ERβ antibody. Nevertheless, ERβ-specific ligands are commercially available and have greatly facilitated the dissection of ERβ-specific mechanisms of action.
ERβ-Specific Ligands
ERβ and ERα bind to 17β-estradiol (E2) with comparable affinity (13, 31), and whereas the selective ER modulators (SERMs) tamoxifen and raloxifene also bind both ERs, their effects on transcription are often ER subtype specific. For example, although tamoxifen acts as an agonist through ERα, this is not the case for ERβ (32, 33). However, tamoxifen acts as an antagonist through both ERs (34). Interestingly, raloxifene is preferentially an ERβ-selective agonist (35, 36). ER subtype specificity with various agonists and antagonists has been observed on many synthetic promoters in various cell lines (32, 34, 37, 38). To facilitate the study of ERβ-specific mechanisms of action, several highly specific ERβ agonists and antagonists have been developed (39, 40, 41, 42, 43). These reagents have proved invaluable for the study of ERβ activity both in vitro and in vivo. The first ERβ-selective agonist, diarylpropionitrile (DPN), was reported in 2001 (44), followed by ERB-041 (WAY-202041), WAY-200070, and 8β-VE2 in 2004 (45, 46), WAY-202196 in 2005 (47), and the selective estrogen receptor β-agonist compounds (Eli Lilly, Indianapolis, IN) in 2006 (48, 49, 50). Since 2007, phytoestrogenic compounds have also been added to this list, including 1) MF101, an extract derived from 22 different herbs (51), and 2) liquiritigenin (LIQ) (52) and nyasol (NYA) (26). LIQ and NYA were both isolated from individual plants that constitute the MF101 extract.
Although these ERβ-specific agonists have been well characterized in vitro using traditional ligand-binding and reporter assays and, in some cases, gene expression arrays, their biological activities have not been equally tested in vivo. Although DPN is the most widely used ERβ agonist in rodent studies, comparative studies with other ERβ agonists suggest that each compound produces distinct biological effects in vivo. For example, although DPN, ERB-041, WAY-202196, and WAY-200070 do not significantly increase uterine weight in rodents at comparable doses, 8β-VE2 increases uterine weight at a dose 100-fold lower than the other agonists (1). MF101 and LIQ, however, do not induce uterine weight gain (51, 52). ERβ-selective compounds also differentially regulate vasomotor instability (hot flashes). DPN, but not ERB-041, alleviated hot flashes in rats, although two different models of vasomotor instability were used with each agonist (45, 53). In a 2009 phase II randomized placebo-controlled study, MF101 significantly reduced the frequency of hot flashes in postmenopausal women (54). These in vivo data suggest that each ERβ agonist induces unique biological effects by using distinct mechanisms to regulate gene expression.
Paruthiyil et al. (26) recently suggested a classification scheme that separates ERβ-selective agonists into three classes based on their ER-selective binding affinity and transcriptional activity as determined by in vitro studies. The first class, ERβ-selective binders, binds with vastly higher affinity (∼200-fold) to ERβ than to ERα and includes ERB-041 and 8β-VE2. The second class, ERβ-selective activators, binds with similar affinity to both ERs but activates genes only via ERβ and includes MF101, LIQ, and NYA (26). Finally, the third class, combined ERβ-selective binders and activators, binds with higher affinity (∼70-fold) to ERα than to ERβ and preferentially activates genes via ERβ and includes DPN, WAY-202196, and WAY-200070. To address the question of whether these three classes of agonists induce similar biological activities, these authors compared the global patterns of gene expression induced by E2, ERB-041, MF101, and LIQ in U2OS osteosarcoma cell lines stably transfected with either ERα (U2OS-α) or ERβ (U2OS-β) (26). Although all ERβ-selective agonists regulated comparable numbers of genes in the U2OS-β cell line, only DPN and NYA regulated a significant number of genes in the U2OS-α cell line (indicating cross-activation of the ERα receptor). Notably, although the agonists regulated similar numbers of genes, the identity of these genes was not identical between the agonists. Although all agonists regulated a common subset of genes, some genes were uniquely regulated by each agonist, indicating that these uniquely regulated genes might contribute to the differing biological effects observed in vivo. In addition, the authors also found a remarkable cell-type specificity in the gene expression response to MF101 and LIQ. Three different cell lines (Caco-2, HeLa, and Ishikawa) were engineered to express ERβ and then treated with MF101 and LIQ. Surprisingly, there was very little overlap in the genes regulated in each cell line, suggesting that cell (and tissue) type is an important parameter that determines the biological response to ERβ-specific agonists. More comparative studies such as this, both in vitro and in vivo, using a variety of models, will be necessary to evaluate these agonists for potential therapeutic use and to identify the variety of molecular mechanisms used by ERβ to regulate transcription.
Classic Mechanisms of ERβ Action
In the classic nuclear or genomic pathway of estrogen action, ligand binding to the ER induces a change in receptor conformation, dissociation of the ER-chaperone complex, dimerization, and binding of the ER to EREs located within the proximity of or within the gene. Additionally, ligand-bound ER can interact with other transacting factors, therefore regulating transcription but not through direct ER-DNA binding. A role for ERβ in this classic mechanism has been investigated using 1) synthetic reporter constructs transfected into immortalized cell lines and primary cells, 2) reporter constructs of natural estrogen-responsive promoters transfected into immortalized cell lines and primary cells, and 3) treatment of cells with estrogens and investigation of expression levels of endogenous genes. From these studies, it is clear that ERα and ERβ have both overlapping and distinct mechanisms of action (11, 12) due to many factors, including the ligand, promoter, and cell type (17, 32, 34, 37, 55, 56).
Insights from in vitro reporter studies
Many of the early studies investigating ERβ’s transcriptional mechanism of action relied on ERE-reporter constructs transiently expressed in immortalized cell lines. These studies indicated that ERβ’s activity was both promoter and cell type dependent. Even in these simple model systems, it was clear that ERβ transcriptional specificity and efficacy were not identical to ERα and that cell and tissue type are critical regulators of ERβ activity. These reconstitution studies paved the way for the study of ERβ activity on endogenous E2-regulated genes.
ERβ-specific activation of endogenous genes and cross talk with ERα
The creation of ER subtype-specific cell lines and ERβ-specific agonists and the advent of microarray technology greatly facilitated the ability to address the issue of ERβ’s mechanism of action in a more physiologically relevant context, namely, on endogenous genes. Many genes regulated by ERβ have been identified in immortalized cell lines and primary cells; in addition, the impact of coexpression of ERα on ERβ-mediated activation has also been investigated (11, 30, 57, 58, 59, 60, 61). However, in few cases has the mechanism of action been delineated by experiments such as in-depth promoter analysis or ChIP.
Although ERα is the predominant ER expressed in breast tumors, a majority also express ERβ. In addition, both ER subtypes are expressed in bone. This coexpression gives rise to the question of whether ERβ alters ERα-mediated gene expression. ERβ levels drop during the progression of many tumor types (62), and ERβ has recently been described as a tumor suppressor (63). In contrast, ERα is thought to promote proliferation, resulting in tumor growth and progression. Determining the mechanisms by which ERβ might act as a brake on ERα-driven tumor progression is currently a very active area of investigation. In transient transfection assays, ERβ antagonizes ERα activity on E2-responsive promoters (64). Recent global gene expression studies using breast cancer or osteosarcoma cell lines engineered to express one or both ERs also suggest that ERβ can regulate ERα activity and that the relative levels of ERα and ERβ may contribute to gene expression in response to estrogens. In U2OS osteosarcoma cells engineered to express either ERα or ERβ, each ER regulates both distinct and overlapping sets of genes in the presence of E2 (57, 59, 61). In U2OS cells expressing both ERα and ERβ (60), E2 regulates a unique set of genes compared with U2OS cell lines expressing either ERα or ERβ alone. ERα and ERβ also regulate distinct sets of genes in human breast cancer cells. By expressing ERβ in MCF-7 cells endogenously expressing ERα, the effects of ERβ on ERα global gene expression (11) and site-specific binding of each ER (30) have been examined. In this model, ERβ both enhances and inhibits ERα-mediated regulation, and in the absence of E2, ERβ activates or represses genes that are regulated by ERα in the presence of E2. In addition, ERβ regulates a unique group of genes that are not regulated by ERα. When coexpressed, each ERα restricts the binding site occupancy of ERβ, and vice versa (30). Finally, in a T47D (ERα-containing) human breast cancer cell line engineered to express ERβ, ERβ significantly lowers expression of the ERα target genes pS2 and PR (58). ChIP assays indicated reduced recruitment of activator protein 1 (AP1) to both promoters in the presence of ERβ, and p300 levels were reduced 3- to 4-fold at the PR promoter. Although these studies raise the intriguing possibility that ERβ can regulate ERα signaling, and vice versa, the biological or physiological relevance of these studies remains to be shown.
Until recently, evidence for the formation of ERα/ERβ heterodimers has been derived from transient transfection, mammalian two-hybrid, electrophoretic mobility shift, and fluorescence resonance energy transfer experiments (15, 60, 65, 66). However, whether the two ERs were present simultaneously on DNA remained controversial. Very recently, evidence for the in vivo existence of ERα/ERβ heterodimers was strengthened by Papoutsi et al. (67), who used re-ChIP assays to demonstrate the simultaneous presence of both ERs on endogenous E2-regulated promoters in a stable cell line that expresses endogenous ERα and an inducible ERβ. What remains to be determined is whether ERα/ERβ heterodimers are found on endogenous E2-regulated genes in tissues that express both ERs, such as mammary tumor tissue, using similar re-ChIP assays.
Recruitment of transcriptional coregulators by ERβ
Many coregulators that either enhance or inhibit ERα transactivation have been shown to be essential for ERα-mediated transcription both in vitro and in vivo; however, the same cannot be said for ERβ. Although a small subset of known ERα coregulators also regulates ERβ-driven transcription, the majority of these data are derived from studies with synthetic and natural estrogen-responsive reporter constructs and in vitro binding assays. Currently, the importance of these coregulators in mediating ERβ’s physiological effects remains largely unknown. Of these coactivators, members of the p160/steroid receptor coactivator/nuclear receptor coactivator (NCOA) family have been the best characterized with respect to ERβ transcription (56, 68, 69, 70, 71).
Both ERα and ERβ recruit members of the NCOA family to target promoters to regulate ligand-dependent and -independent transcription; however, these interactions are cell type and promoter specific. Although NCOA1 binds to both ERs in vitro and enhances their ligand-dependent activity in transient transfection assays (31, 56, 68, 72), some members of the NCOA family stimulate transcription from unliganded ERβ more readily than unliganded ERα (31, 56). In support of these ligand-independent ERβ-mediated effects, Bai and Giguère (66) used fluorescence resonance energy transfer assays with HEK293 cells expressing fluorescently tagged ERs to demonstrate that ERβ interacted with all three NCOA family members in the absence of ligand (66). Other ERα coregulators regulate ERβ-mediated transcription from transiently transfected promoters (Table 1) (20, 73, 74, 75, 76). However, whether NCOAs or other coregulators are important for ERβ in a physiological context is an area that clearly merits further study.
Table 1.
Coregulators that bind to and regulate the activity of both ERα and ERβ
| Gene symbol | Gene name | Role | Ref. |
|---|---|---|---|
| p160/SRC/NCOA family | Nuclear receptor coactivator | Coactivator | 56 68 69 70 71 |
| ARID5A/MRF1 | AT-rich interactive domain 5A/modulator recognition factor 1 | Corepressor | 73 |
| CCDC62 | Coiled-coil domain containing 62 | Coactivator | 20 |
| CREBBP/CBP | CREB-binding protein | Coactivator | 56 68 |
| MMS19 | MMS19 nucleotide excision repair homolog | Coactivator | 74 |
| NR0B1/DAX-1 | Nuclear receptor subfamily 0, group B, member 1 | Corepressor | 75 |
| NCOR1/2 | Nuclear receptor corepressor 1 | Corepressor | 76 |
| CARM1 | Coactivator-associated arginine methyltransferase 1 | Coactivator/corepressor | 56 |
| PRMT1 | Protein arginine N-methyltransferase 1 | Coactivator/corepressor | 56 |
Relatively few studies have demonstrated that ERβ recruits coactivators to endogenous genes. Using a U2OS stable cell line expressing ERβ, Cvoro et al. (77) showed that ERβ-specific agonists repress the TNFα-mediated activation of the TNFα gene by recruiting NCOA2 to the endogenous TNFα promoter. Using ChIP analysis, they demonstrated that LIQ recruits NCOA2 to endogenous E2-responsive promoters in U2OS-ERβ but not in U2OS-ERα cells (52). Similarly, unliganded ERβ recruits NCOA2, but not NCOA1 or NCOA3, to a known E2-induced gene in U2OS cells (78). However, because ERβ is not expressed in parental U2OS cells, it is unclear whether ERβ recruits NCOA2 to E2-regulated genes in bone tissue or primary bone cells.
ERβ is highly expressed in granulosa cells of the ovary, where it is critical for cellular differentiation in response to FSH (79). Gonadotropin-inducible transcription repressor-4 (GIOT-4) was recently identified as a novel ERβ coactivator in granulosa cells. GIOT-4 is a Cys2-His2 (C2H2)-type zinc finger protein (80) that is induced by pregnant mare’s serum gonadotropin (PMSG) treatment in the KGN granulosa tumor-derived cell line and in the ovaries of PMSG-treated mice. In KGN cells treated with PMSG, or in which GIOT-4 is overexpressed, GIOT-4 binds ERβ in a complex containing the mammalian SWI/SNF complex components Brahma-related gene-1 and BRG1-associated factor 57. In KGN cells overexpressing both ERβ and GIOT-4 and, remarkably, in ovarian tissue from PMSG-treated mice, ERβ, GIOT-4, and Brahma-related gene-1 are recruited to putative EREs in E2-regulated endogenous genes in the presence of ligand. This study is notable in that it is one of the first to carry out ERβ-ChIP using mouse tissue, to demonstrate ERβ targeting to a promoter in a physiological context, and to identify a putative molecular mechanism for ERβ’s actions within the ovary.
The N-terminal domain of ERβ
The N-terminal A/B domain is the domain with the lowest sequence identity (30%) between the two ERs and is thought to be a major contributor to the differential activities of ERα and ERβ. This region regulates differences in ligand specificity, ligand-independent activity, recruitment of coregulatory proteins, cross talk with other signaling pathways, and proteasomal degradation.
The N-terminal domain contributes to both ligand-dependent and -independent ERβ activity. As previously mentioned, tamoxifen can act as an agonist through ERα on ERE-driven reporters in vitro, but it has very little agonist activity through ERβ. The N-terminal domain is largely responsible for these differences (15, 32). In vitro, the AF-1 domain is constitutively active in ERα but contains a repressor function in ERβ, such that when the AF-1 domain is removed from ERβ, an increase in ERβ-mediated activity is observed (64). The N-terminal domain also contributes to the specificity of ligand-independent ERβ activity. Several studies have shown that unliganded ERβ binds members of the NCOA family more readily than unliganded ERα and that the AF-1 domain is required for NCOA1 to bind ERβ and to enhance basal (unliganded) ERβ-mediated transcription (81). In addition, phosphorylation of the AF-1 domain after growth factor stimulation increases recruitment of NCOA1 to ERβ in the absence of ligand.
The N-terminal region can also mediate the interaction between ERβ and other cellular pathways such as the Ras and proteasomal degradation pathways. The oncoprotein H-rasV12 (an active form of ras) enhances activation of ERβ in the presence of E2 (31), and mutagenesis of Ser60 (found within a Ras-Raf-1-MAPK kinase consensus phosphorylation site) in the N terminus abolishes this effect, indicating that AF-1 is required for cross talk with the Ras signaling pathway. The N-terminal region also regulates degradation of ERβ by the proteasome. In the presence of ligand, turnover of both ERα and ERβ is required for optimal ligand-dependent promoter activation. However, in the absence of ligand, turnover is not required for ERβ transcriptional activity, although it is required for ERα transcriptional activity (82). This difference was attributed to MAPK consensus sites in the AF-1 domain of ERβ. In summary, these studies suggest that the AF-1 domain regulates ERβ transcriptional activity through several mechanisms and predict that more roles for this domain in ERβ activity are likely to be uncovered in the future.
Cross Talk with Other Signaling Pathways
Cross talk with other transcription factors
One mechanism by which both ERs activate transcription is by binding to other DNA-bound transcription factors, and data from ChIP-chip studies strongly indicate that this tethering mechanism is a common mechanism of transcriptional regulation for both ERs (27, 28, 29, 30). A well-known target of ER tethering is AP1. Ligand-activated ERβ and ERα signal in opposite ways on AP1-driven promoters in the presence of E2 (36); ERα activates transcription, whereas ERβ inhibits transcription. ERβ tethering is also reported via the DNA-bound sterol regulatory element binding protein-1A, which can bind ERE half-sites (83). There is also evidence that in the presence of antiestrogens, ERβ binds to an electrophile/antioxidant response element (EpRE/ARE) in the proximal promoter of the endogenous NADH dehydrogenase, quinone 1 (NQO1) gene in MCF-7 cells (84, 85, 86). ERβ binds to the EpRE/ARE as part of a complex that includes hPMC2, and both ERβ and human prevention of mitotic catastrophe 2 are required to recruit coactivators to the EpRE/ARE of the endogenous promoter as determined by ChIP assays (87). Finally, a recent ChIP-chip study in MCF-7 cells engineered to express ERβ identified a set of novel transcription factor-binding motifs that were enriched near sites of ERβ-DNA binding (30), suggesting novel tethering targets for ERβ.
Negative cross talk of ERβ with other transcription factors can also occur. For example, ERβ inhibits nuclear factor κB (NFκB) transcriptional activity on NFκB consensus site-driven reporters in an E2-dependent manner (88, 89). This ERβ-NFκB cross talk is bidirectional, because NFκB can also inhibit ERβ-mediated signaling (88). Mutual antagonism between NFκB and ERα has also been described (89, 90). ERβ cross talk also occurs with the peroxisome proliferator-activated receptor (PPAR) pathway. PPARγ regulates adipocyte differentiation and has been implicated in the pathology of obesity, diabetes, atherosclerosis, and cancer (91). In transient transfection assays, E2 in the presence of ERβ inhibited PPARγ transcriptional activity (92), and expression of NCOA1 and NCOA2 blocked this inhibition. These results suggested that ERβ and PPARγ compete for these coactivators. In support of this hypothesis, NCOA1 and NCOA2 binding to the endogenous PPARγ-regulated adiponectin promoter was enhanced in gonadal fat from ERβ-null mice. These studies suggest a novel mechanism by which ERβ regulates adipocyte differentiation and may participate in the development or progression of the aforementioned diseases. Bidirectional cross talk of PPARγ with ERα has also been demonstrated (93, 94). Other transcription factors that engage in negative cross talk with ERβ include signal transducer and activator of transcription 5A (STAT5A) (95) and the orphan nuclear receptor, nuclear receptor subfamily 0, group B, member 2 (NR0B2/SHP) (96).
Transcription factors can also enhance ERβ activity. The aryl hydrocarbon receptor (AHR) is a transcription factor that is activated by planar aromatic hydrocarbons. AHR nuclear translocator (ARNT) dimerizes with AHR and can act as an ER coactivator. In a mammary epithelial cell line stably transfected with a 3xERE luciferase reporter and expressing both ERs, ERβ activity was reduced when ARNT availability was reduced (97), indicating that ARNT enhances ERβ-mediated transcription. The reduction in ERβ activity was greater than for ERα. ARNT interacted directly with ERβ and RNA polymerase II on the GREB1 and pS2 promoters in response to E2, and this association was reduced after depletion of ERβ. This study suggests that ARNT is an ERβ coactivator, which may have implications for cellular responses, particularly in the reproductive system, under conditions where both estrogens and planar aromatic hydrocarbons (such as dioxins) may be present.
Although these studies indicating cross talk between ERβ and other transcription factors are intriguing and suggest multiple modes of action for ERβ, the majority were conducted using in vitro binding assays and reporter assays; thus, whether these mechanisms play a role in the whole animal remains to be determined.
Cross talk with cholesterol metabolism
Oxysterols are cholesterol metabolites that are produced in peripheral tissues and contribute to the elimination of cholesterol. 27-Hydroxycholesterol (27HC), the most abundant circulating oxysterol, was recently identified as an endogenous SERM that is a competitive antagonist of both ERs. Interestingly, 27HC inhibited the activity of ERβ (90%) more than the activity of ERα (50%) (98). Both ERs bound to 27HC, and 27HC induces a conformational change in both ERs that is different from that induced by E2 or other SERMs (99). 27HC inhibited the E2-dependent interaction of ERβ with NCOA1, but the interaction with ERα was unaffected. 27HC also inhibited ER-dependent nitric oxide synthase (NOS) expression in vivo, resulting in reduced E2-induced vasorelaxation of rat aorta. This inhibition was lost in both ERα- and ERβ-null mice, indicating a role for both ERs in the biological effects of 27HC. These results strongly suggest that circulating cholesterol metabolites regulate cardiovascular function via an ERβ-mediated mechanism and provide an important and novel new mechanism for ERβ action.
Cross talk with the hypoxia-response pathway
ERβ also interacts with members of the hypoxia-response pathway, as does ERα (100). In immortalized human prostate cancer lines generated from patients with good or poor prognosis (101), both good- and poor-prognosis cell lines expressed ERβ but not ERα. The poor-prognosis cell line exhibited a constitutively hypoxic phenotype, which correlated with increased NO production. E2 treatment of this cell line increased the expression of the endogenous human telomerase (TERT) promoter, which is a direct target of both ER and hypoxia signaling. ChIP assays demonstrated that ERβ/endothelial NOS (eNOS), ERβ/hypoxia-inducible factor-1α, and ERβ/hypoxia-inducible factor-2α complexes were associated with the hTERT promoter under conditions of hypoxia. These studies provide a novel mechanism by which ERβ activity responds to changes in oxygen tension and provide an important new mechanism for ERβ’s predicted role in the regulation of tumor progression.
Alternative Mechanisms of ERβ Action
Extranuclear mechanisms
Much evidence for the nuclear/genomic model of estrogen action has resulted from decades of studies, and it is the most widely accepted model of estrogen action. However, accumulating evidence indicates that E2 induces nongenomic or extranuclear events via an ER localized to either the cytoplasm or the membrane or via other estrogen-binding proteins. These extranuclear events activate second messenger pathways so rapidly that their activation cannot be explained by the nuclear/genomic mechanism. Although the idea of a membrane-localized ER is gaining wider acceptance (102, 103), many researchers remain unconvinced. Much of the controversy stems from 1) the overexpression of ER in immortalized cell lines to demonstrate membrane localization, 2) concern that isolated membrane fractions are contaminated by nuclear or cytoplasmic ERs, and 3) the limited understanding of the mechanistic steps linking membrane-bound ERs to intracellular signaling pathways, either in vitro or in a physiological context. Extranuclear ERβ signaling has been primarily associated with actions in the vasculature and brain, although a role in other tissues is also emerging.
Regulation of nitric oxide
Both clinical and basic research indicates that estrogen signaling through both ERs is involved in the response to vascular injury and atherosclerosis (104). E2 induces nitric oxide (NO) release in human endothelial cells within minutes, resulting from increased activity of the endothelial isoform of NO synthase (eNOS) through both ERα and ERβ (105). In ovine endothelial cells, a small proportion of endogenous ERβ was localized to the plasma membrane as determined by immunoblot analysis of fractionated extracts. In addition, an ERβ antagonist blocked the rapid stimulation of eNOS activity by E2 in ovine endothelial cell plasma membranes (106). These studies suggest that ERβ localized to the plasma membrane may regulate vascular injury through the rapid, extranuclear activation of eNOS in endothelial cells. In addition to the vasculature, ERβ also regulates NO levels in the hypothalamus. DPN treatment rapidly increased phosphorylation of neuronal NOS and NO production in primary rat embryonic hypothalamic neurons via the phosphatidylinositol 3-kinase pathway, and Src kinase mediated the ERβ-induced phosphorylation of neuronal NOS (107). Thus, ERβ may regulate the NO pathway through the extranuclear actions of E2 in both the vasculature and hypothalamus.
Extranuclear actions in the brain and other tissues
E2 exerts a neuroprotective effect on hippocampal neurons, and both ERs have been implicated in this protection. ERβ regulates calcium (Ca2+) signaling in hippocampal neurons (108), and DPN attenuates the increase in intracellular Ca2+ that results after exposure of rat primary hippocampal neurons to excitotoxic levels of glutamate. DPN increased the levels of ERK phosphorylation, and pharmacological inhibition of voltage-gated L-type Ca2+ channels completely blocked the DPN-induced increase in ERK phosphorylation. This links activation of ERβ to a rapid influx of intracellular Ca2+ via the L-type channels through a rapid, extranuclear mechanism in hippocampal neurons. GnRH-producing neurons of the hypothalamus are also susceptible to extranuclear signaling through ERβ. Within 15 min, preovulatory (high) doses of E2 depolarize GnRH neurons, increase their firing rate, and increase neuron excitability in brain slices from mice (109). DPN mimics these effects, whereas an ERα-specific agonist does not. ERβ agonists increased the firing rate, GnRH secretion, and cAMP production in cultured fetal and adult rat hypothalamic neurons (110), implying that ligand-activated ERβ regulates hypothalamic GnRH neuronal activity. Extranuclear actions of ERβ have also been observed in human platelets (111) and in non-small-cell lung cancer cell lines (112).
Overlap of nuclear with extranuclear pathways
In MCF-7 cells, a subset of E2-regulated genes can also be regulated via extranuclear signaling (113), suggesting that estrogen-mediated extranuclear signaling may occur in mammary tumors. MCF-7 cells were treated with E2 or estrogen-dendrimer conjugates (EDCs), which are unable to enter the nucleus and thus can trigger only extranuclear signaling. Global gene expression analysis indicated that roughly 25% of E2-regulated genes were also regulated by EDCs. Interestingly, MAPK and c-Src kinase inhibitors suppressed both E2- and EDC-stimulated transcription, and ERα was not recruited to EDC-stimulated genes. Studies such as these in cell lines expressing ERβ (but not ERα) may identify other ER subtype-specific mechanisms of estrogen action.
ERβ signaling in mitochondria
Although the localization of ER to the plasma membrane remains a controversial issue, the possible localization of ERs to mitochondria is even more so (114, 115, 116, 117, 118, 119). Although accumulating evidence indicates that mitochondria are targets of estrogen action (114, 116, 118, 119), two major questions have emerged, namely: 1) are ERs present in the mitochondria, and if so, 2) do they directly regulate transcription of mitochondrial genes encoded by mitochondrial DNA?
Evidence for the presence of both ERs in mitochondria in various mammalian tissues and cell lines has been obtained from ligand-binding assays as well as antibody-based assays (114, 115, 116, 117, 118, 119). These studies indicate that ERβ is generally the predominant or sole ER found in mitochondria (115). In contrast to these studies, a recent analysis using protein mass spectrometry characterized mitochondrial proteins in 14 mouse tissues and did not identify either ER protein within the mitochondria (120). Thus, the presence of ERs in the mitochondria remains a highly contested issue, and rigorous investigation using multiple techniques and model systems will be required before this concept is widely accepted.
Concluding Remarks
One overarching question is why ERβ evolved with its particular pattern of tissue expression and its apparently unique role in normal physiology. Determining this role has been particularly challenging. For example, a 2003 study examined the gene expression profile of 10,000 genes in 13 tissues of E2-treated wild-type and ERβ-null mice and found essentially no ERβ-regulated genes (121). The improvements in global gene expression technology since 2003 may yet yield bona fide E2/ERβ-regulated genes in these mouse models. Another area in which evidence is lacking is the identity of the coactivators and corepressors targeted by ERβ to regulatory regions on endogenous genes. Although a few studies have addressed this question, given the number of ERβ-regulated genes identified in cell lines by global gene expression analysis, there is now a large number of potential targets to investigate for coregulator recruitment. The role of unliganded ERβ in cellular function also remains an intriguing question, as accumulating evidence indicates that unliganded ERβ can regulate gene expression in various models. The currently debated issues of whether ERβ localizes to the plasma membrane or to the mitochondria and contributes to ERβ’s physiological actions will require much further investigation. Finally, several ERβ mRNA isoforms have been isolated that result from alternative splicing, codon deletion, or expression of untranslated exons (122, 123). The functional significance of these isoforms in normal or disease conditions also remains unknown but is worthy of further study, given the widespread presence of these isoforms in tissues.
It has been 3 yr since the original minireview, “Estrogen Receptor-β: Recent Lessons from in Vivo Studies,” was published. In her concluding remarks, Dr. Harris commented that “we have a poor understanding of the mechanisms that underlie the in vivo observations described in this review.” Three years later, it can be said that we have a better understanding of these mechanisms, but to a large extent, the gap in our understanding that Dr. Harris identified remains. Although the availability of ERβ-specific agonists, global gene expression analyses, RNA interference, and chromatin-based (ChIP) assays have made significant progress toward narrowing this gap, mechanistic studies with rodent or human tissue or cells remain relatively rare. However, these studies are key to understanding ERβ’s contribution to normal estrogen action and estrogen’s action in disease and are necessary to complement the observations made in other, less physiologically relevant model systems. With the ongoing development of new and specific reagents, such as antibodies, adenoviral vectors that allow expression of ERβ within cells refractory to efficient transfection, and RNA interference in vivo, the next few years will undoubtedly see the generation of exciting new data and a further shrinking of this gap in our knowledge of the mechanisms of ERβ action that underlie its previously identified physiological roles.
Acknowledgments
We are grateful to Drs. Gabriel DiMattia, Andy Babwah, and Karina Rodriguez for critical reading of the manuscript and helpful discussions.
NURSA Molecule Pages:
Coregulators: AIB1 | GRIP1 | SRC-1;
Ligands: 17β-estradiol | 8β-VE2 | Diarylpropionitrile | Raloxifene | Tamoxifen;
Nuclear Receptors: ER-β.
Footnotes
Disclosure Summary: Both authors have nothing to declare.
First Published Online April 2, 2010
Abbreviations: AF, Activation function; AHR, aryl hydrocarbon receptor; AP1, activator protein 1; ARNT, AHR nuclear translocator; ChIP, chromatin immunoprecipitation; DBD, DNA-binding domain; DPN, diarylpropionitrile; E2, 17β-estradiol; EDC, estrogen-dendrimer conjugate; eNOS, endothelial NOS; EpRE/ARE, electrophile/antioxidant response element; ER, estrogen receptor; ERE, estrogen-response element; GIOT-4, gonadotropin-inducible transcription repressor-4; 27HC, 27-hydroxycholesterol; LIQ, liquiritigenin; NCOA, nuclear receptor coactivator; NFκB, nuclear factor κB; NYA, nyasol; PMSG, pregnant mare’s serum gonadotropin; PPAR, peroxisome proliferator-activated receptor; NOS, nitric oxide synthase; SERM, selective ER modulator.
References
- 1.Harris HA2007. Estrogen receptor-β: recent lessons from in vivo studies. Mol Endocrinol 21:1–13 [DOI] [PubMed] [Google Scholar]
- 2.Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ2006. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell 126:789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell-Thompson M, Lynch IJ, Bhardwaj B2001. Expression of estrogen receptor (ER) subtypes and ERβ isoforms in colon cancer. Cancer Res 61:632–640 [PubMed] [Google Scholar]
- 4.Dougherty SM, Mazhawidza W, Bohn AR, Robinson KA, Mattingly KA, Blankenship KA, Huff MO, McGregor WG, Klinge CM2006. Gender difference in the activity but not expression of estrogen receptors α and β in human lung adenocarcinoma cells. Endocr Relat Cancer 13:113–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley EF, Jazaeri AA, Shupnik MA, Jazaeri O, Rice LW2000. Selective loss of estrogen receptor β in malignant human colon. Cancer Res 60:245–248 [PubMed] [Google Scholar]
- 6.Hestiantoro A, Swaab DF2004. Changes in estrogen receptor-α and -β in the infundibular nucleus of the human hypothalamus are related to the occurrence of Alzheimer’s disease neuropathology. J Clin Endocrinol Metab 89:1912–1925 [DOI] [PubMed] [Google Scholar]
- 7.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mäkinen S, Mäkelä S, Weihua Z, Warner M, Rosenlund B, Salmi S, Hovatta O, Gustafsson JA2001. Localization of oestrogen receptors α and β in human testis. Mol Hum Reprod 7:497–503 [DOI] [PubMed] [Google Scholar]
- 9.Merchenthaler I, Lane MV, Numan S, Dellovade TL2004. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol 473:270–291 [DOI] [PubMed] [Google Scholar]
- 10.Saunders PT, Sharpe RM, Williams K, Macpherson S, Urquart H, Irvine DS, Millar MR2001. Differential expression of oestrogen receptor α and β proteins in the testes and male reproductive system of human and non-human primates. Mol Hum Reprod 7:227–236 [DOI] [PubMed] [Google Scholar]
- 11.Chang EC, Frasor J, Komm B, Katzenellenbogen BS2006. Impact of estrogen receptor β on gene networks regulated by estrogen receptor α in breast cancer cells. Endocrinology 147:4831–4842 [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Li X, Maguire CA, Hilf R, Bambara RA, Muyan M2005. Binding of estrogen receptor β to estrogen response element in situ is independent of estradiol and impaired by its amino terminus. Mol Endocrinol 19:2696–2712 [DOI] [PubMed] [Google Scholar]
- 13.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA1996. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 93:5925–5930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaunay F, Pettersson K, Tujague M, Gustafsson JA2000. Functional differences between the amino-terminal domains of estrogen receptors α and β. Mol Pharmacol 58:584–590 [DOI] [PubMed] [Google Scholar]
- 15.Cowley SM, Parker MG1999. A comparison of transcriptional activation by ER α and ER β. J Steroid Biochem Mol Biol 69:165–175 [DOI] [PubMed] [Google Scholar]
- 16.Hyder SM, Chiappetta C, Stancel GM1999. Interaction of human estrogen receptors α and β with the same naturally occurring estrogen response elements. Biochem Pharmacol 57:597–601 [DOI] [PubMed] [Google Scholar]
- 17.Loven MA, Wood JR, Nardulli AM2001. Interaction of estrogen receptors α and β with estrogen response elements. Mol Cell Endocrinol 181:151–163 [DOI] [PubMed] [Google Scholar]
- 18.Loven MA, Likhite VS, Choi I, Nardulli AM2001. Estrogen response elements alter coactivator recruitment through allosteric modulation of estrogen receptor β conformation. J Biol Chem 276:45282–45288 [DOI] [PubMed] [Google Scholar]
- 19.Bianco NR, Perry G, Smith MA, Templeton DJ, Montano MM2003. Functional implications of antiestrogen induction of quinone reductase: inhibition of estrogen-induced deoxyribonucleic acid damage. Mol Endocrinol 17:1344–1355 [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Ni J, Chang HC, Lin CY, Muyan M, Yeh S2009. CCDC62/ERAP75 functions as a coactivator to enhance estrogen receptor β-mediated transactivation and target gene expression in prostate cancer cells. Carcinogenesis 30:841–850 [DOI] [PubMed] [Google Scholar]
- 21.Pravettoni A, Mornati O, Martini PG, Marino M, Colciago A, Celotti F, Motta M, Negri-Cesi P2007. Estrogen receptor β (ERβ) and inhibition of prostate cancer cell proliferation: studies on the possible mechanism of action in DU145 cells. Mol Cell Endocrinol 263:46–54 [DOI] [PubMed] [Google Scholar]
- 22.Rincavage HL, McDonnell DP, Kuhn CM2003. Expression of functional estrogen receptor β in locus coeruleus-derived Cath.a cells. Endocrinology 144:2829–2835 [DOI] [PubMed] [Google Scholar]
- 23.Hurtado A, Pinós T, Barbosa-Desongles A, López-Avilés S, Barquinero J, Petriz J, Santamaria-Martínez A, Morote J, de Torres I, Bellmunt J, Reventós J, Munell F2008. Estrogen receptor β displays cell cycle-dependent expression and regulates the G1 phase through a non-genomic mechanism in prostate carcinoma cells. Cell Oncol 30:349–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belcher SM, Ma X, Le HH2009. Blockade of estrogen receptor signaling inhibits growth and migration of medulloblastoma. Endocrinology 150:1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faulds MH, Olsen H, Helguero LA, Gustafsson JA, Haldosén LA2004. Estrogen receptor functional activity changes during differentiation of mammary epithelial cells. Mol Endocrinol 18:412–421 [DOI] [PubMed] [Google Scholar]
- 26.Paruthiyil S, Cvoro A, Zhao X, Wu Z, Sui Y, Staub RE, Baggett S, Herber CB, Griffin C, Tagliaferri M, Harris HA, Cohen I, Bjeldanes LF, Speed TP, Schaufele F, Leitman DC2009. Drug and cell type-specific regulation of genes with different classes of estrogen receptor β-selective agonists. PLoS One 4:e6271 [DOI] [PMC free article] [PubMed]
- 27.Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M2005. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- 28.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M2006. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- 29.Lin CY, Vega VB, Thomsen JS, Zhang T, Kong SL, Xie M, Chiu KP, Lipovich L, Barnett DH, Stossi F, Yeo A, George J, Kuznetsov VA, Lee YK, Charn TH, Palanisamy N, Miller LD, Cheung E, Katzenellenbogen BS, Ruan Y, Bourque G, Wei CL, Liu ET2007. Whole-genome cartography of estrogen receptor α binding sites. PLoS Genet 3:e87 [DOI] [PMC free article] [PubMed]
- 30.Charn TH, Liu ET, Chang EC, Lee YK, Katzenellenbogen JA, Katzenellenbogen BS2010. Genome-wide dynamics of chromatin binding of estrogen receptors α and β: mutual restriction and competitive site selection. Mol Endocrinol 24:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, Giguère V1997. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor β. Mol Endocrinol 11:353–365 [DOI] [PubMed] [Google Scholar]
- 32.McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS1998. Transcription activation by the human estrogen receptor subtype β (ERβ) studied with ERβ and ERα receptor chimeras. Endocrinology 139:4513–4522 [DOI] [PubMed] [Google Scholar]
- 33.Waters KM, Rickard DJ, Riggs BL, Khosla S, Katzenellenbogen JA, Katzenellenbogen BS, Moore J, Spelsberg TC2001. Estrogen regulation of human osteoblast function is determined by the stage of differentiation and the estrogen receptor isoform. J Cell Biochem 83:448–462 [DOI] [PubMed] [Google Scholar]
- 34.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S1998. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol Pharmacol 54:105–112 [DOI] [PubMed] [Google Scholar]
- 35.Ball LJ, Levy N, Zhao X, Griffin C, Tagliaferri M, Cohen I, Ricke WA, Speed TP, Firestone GL, Leitman DC2009. Cell type- and estrogen receptor-subtype specific regulation of selective estrogen receptor modulator regulatory elements. Mol Cell Endocrinol 299:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paech K, Webb P, Kuiper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS1997. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277:1508–1510 [DOI] [PubMed] [Google Scholar]
- 37.Jones PS, Parrott E, White IN1999. Activation of transcription by estrogen receptor α and β is cell type- and promoter-dependent. J Biol Chem 274:32008–32014 [DOI] [PubMed] [Google Scholar]
- 38.McInerney EM, Katzenellenbogen BS1996. Different regions in activation function-1 of the human estrogen receptor required for antiestrogen- and estradiol-dependent transcription activation. J Biol Chem 271:24172–24178 [DOI] [PubMed] [Google Scholar]
- 39.De Angelis M, Stossi F, Carlson KA, Katzenellenbogen BS, Katzenellenbogen JA2005. Indazole estrogens: highly selective ligands for the estrogen receptor β. J Med Chem 48:1132–1144 [DOI] [PubMed] [Google Scholar]
- 40.Güngör T, Chen Y, Golla R, Ma Z, Corte JR, Northrop JP, Bin B, Dickson JK, Stouch T, Zhou R, Johnson SE, Seethala R, Feyen JH2006. Synthesis and characterization of 3-arylquinazolinone and 3-arylquinazolinethione derivatives as selective estrogen receptor β modulators. J Med Chem 49:2440–2455 [DOI] [PubMed] [Google Scholar]
- 41.Harris HA2006. Preclinical characterization of selective estrogen receptor β agonists: new insights into their therapeutic potential. Ernst Schering Found Symp Proc 2006:149–161 [DOI] [PubMed] [Google Scholar]
- 42.Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA 4 December 2009. Estrogen receptor β ligands: recent advances and biomedical applications. Med Res Rev 10.1002/med.20186 [DOI] [PubMed]
- 43.Wahlgren A, Svechnikov K, Strand ML, Jahnukainen K, Parvinen M, Gustafsson JA, Söder O2008. Estrogen receptor β selective ligand 5α-androstane-3β, 17β-diol stimulates spermatogonial deoxyribonucleic acid synthesis in rat seminiferous epithelium in vitro Endocrinology 149:2917–2922 [DOI] [PubMed] [Google Scholar]
- 44.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA2001. Estrogen receptor-β potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem 44:4230–4251 [DOI] [PubMed] [Google Scholar]
- 45.Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith Jr JC, Harris HA2004. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-β ligands. J Med Chem 47:5021–5040 [DOI] [PubMed] [Google Scholar]
- 46.Hillisch A, Peters O, Kosemund D, Müller G, Walter A, Schneider B, Reddersen G, Elger W, Fritzemeier KH2004. Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol Endocrinol 18:1599–1609 [DOI] [PubMed] [Google Scholar]
- 47.Mewshaw RE, Edsall Jr RJ, Yang C, Manas ES, Xu ZB, Henderson RA, Keith Jr JC, Harris HA2005. ERβ ligands. 3. Exploiting two binding orientations of the 2-phenylnaphthalene scaffold to achieve ERβ selectivity. J Med Chem 48:3953–3979 [DOI] [PubMed] [Google Scholar]
- 48.Norman BH, Dodge JA, Richardson TI, Borromeo PS, Lugar CW, Jones SA, Chen K, Wang Y, Durst GL, Barr RJ, Montrose-Rafizadeh C, Osborne HE, Amos RM, Guo S, Boodhoo A, Krishnan V2006. Benzopyrans are selective estrogen receptor β agonists with novel activity in models of benign prostatic hyperplasia. J Med Chem 49:6155–6157 [DOI] [PubMed] [Google Scholar]
- 49.Richardson TI, Dodge JA, Durst GL, Pfeifer LA, Shah J, Wang Y, Durbin JD, Krishnan V, Norman BH2007. Benzopyrans as selective estrogen receptor β agonists (SERBAs). Part 3: synthesis of cyclopentanone and cyclohexanone intermediates for C-ring modification. Bioorg Med Chem Lett 17:4824–4828 [DOI] [PubMed] [Google Scholar]
- 50.Norman BH, Richardson TI, Dodge JA, Pfeifer LA, Durst GL, Wang Y, Durbin JD, Krishnan V, Dinn SR, Liu S, Reilly JE, Ryter KT2007. Benzopyrans as selective estrogen receptor β agonists (SERBAs). Part 4: functionalization of the benzopyran A-ring. Bioorg Med Chem Lett 17:5082–5085 [DOI] [PubMed] [Google Scholar]
- 51.Cvoro A, Paruthiyil S, Jones JO, Tzagarakis-Foster C, Clegg NJ, Tatomer D, Medina RT, Tagliaferri M, Schaufele F, Scanlan TS, Diamond MI, Cohen I, Leitman DC2007. Selective activation of estrogen receptor-β transcriptional pathways by an herbal extract. Endocrinology 148:538–547 [DOI] [PubMed] [Google Scholar]
- 52.Mersereau JE, Levy N, Staub RE, Baggett S, Zogovic T, Zogric T, Chow S, Ricke WA, Tagliaferri M, Cohen I, Bjeldanes LF, Leitman DC2008. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Mol Cell Endocrinol 283:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bowe J, Li XF, Kinsey-Jones J, Heyerick A, Brain S, Milligan S, O’Byrne K2006. The hop phytoestrogen, 8-prenylnaringenin, reverses the ovariectomy-induced rise in skin temperature in an animal model of menopausal hot flushes. J Endocrinol 191:399–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grady D, Sawaya GF, Johnson KC, Koltun W, Hess R, Vittinghoff E, Kristof M, Tagliaferri M, Cohen I, Ensrud KE2009. MF101, a selective estrogen receptor β modulator for the treatment of menopausal hot flushes: a phase II clinical trial. Menopause 16:458–465 [DOI] [PubMed] [Google Scholar]
- 55.Hall JM, Korach KS2002. Analysis of the molecular mechanisms of human estrogen receptors α and β reveals differential specificity in target promoter regulation by xenoestrogens. J Biol Chem 277:44455–44461 [DOI] [PubMed] [Google Scholar]
- 56.Klinge CM, Jernigan SC, Mattingly KA, Risinger KE, Zhang J2004. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors α and β by coactivators and corepressors. J Mol Endocrinol 33:387–410 [DOI] [PubMed] [Google Scholar]
- 57.Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC2004. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell 15:1262–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matthews J, Wihlén B, Tujague M, Wan J, Ström A, Gustafsson JA2006. Estrogen receptor (ER) β modulates ERα-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol 20:534–543 [DOI] [PubMed] [Google Scholar]
- 59.Monroe DG, Getz BJ, Johnsen SA, Riggs BL, Khosla S, Spelsberg TC2003. Estrogen receptor isoform-specific regulation of endogenous gene expression in human osteoblastic cell lines expressing either ERα or ERβ. J Cell Biochem 90:315–326 [DOI] [PubMed] [Google Scholar]
- 60.Monroe DG, Secreto FJ, Subramaniam M, Getz BJ, Khosla S, Spelsberg TC2005. Estrogen receptor α and β heterodimers exert unique effects on estrogen- and tamoxifen-dependent gene expression in human U2OS osteosarcoma cells. Mol Endocrinol 19:1555–1568 [DOI] [PubMed] [Google Scholar]
- 61.Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS2004. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) α or ERβ in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology 145:3473–3486 [DOI] [PubMed] [Google Scholar]
- 62.Deroo BJ, Korach KS2006. Estrogen receptors and human disease. J Clin Invest 116:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lazennec G2006. Estrogen receptor β, a possible tumor suppressor involved in ovarian carcinogenesis. Cancer Lett 231:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall JM, McDonnell DP1999. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140:5566–5578 [DOI] [PubMed] [Google Scholar]
- 65.Pettersson K, Grandien K, Kuiper GG, Gustafsson JA1997. Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α. Mol Endocrinol 11:1486–1496 [DOI] [PubMed] [Google Scholar]
- 66.Bai Y, Giguère V2003. Isoform-selective interactions between estrogen receptors and steroid receptor coactivators promoted by estradiol and ErbB-2 signaling in living cells. Mol Endocrinol 17:589–599 [DOI] [PubMed] [Google Scholar]
- 67.Papoutsi Z, Zhao C, Putnik M, Gustafsson JA, Dahlman-Wright K2009. Binding of estrogen receptor α/β heterodimers to chromatin in MCF-7 cells. J Mol Endocrinol 43:65–72 [DOI] [PubMed] [Google Scholar]
- 68.Tremblay A, Giguère V2001. Contribution of steroid receptor coactivator-1 and CREB binding protein in ligand-independent activity of estrogen receptor β. J Steroid Biochem Mol Biol 77:19–27 [DOI] [PubMed] [Google Scholar]
- 69.Webb P, Nguyen P, Shinsako J, Anderson C, Feng W, Nguyen MP, Chen D, Huang SM, Subramanian S, McKinerney E, Katzenellenbogen BS, Stallcup MR, Kushner PJ1998. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol Endocrinol 12:1605–1618 [DOI] [PubMed] [Google Scholar]
- 70.Suen CS, Berrodin TJ, Mastroeni R, Cheskis BJ, Lyttle CR, Frail DE1998. A transcriptional coactivator, steroid receptor coactivator-3, selectively augments steroid receptor transcriptional activity. J Biol Chem 273:27645–27653 [DOI] [PubMed] [Google Scholar]
- 71.Ko L, Cardona GR, Iwasaki T, Bramlett KS, Burris TP, Chin WW2002. Ser-884 adjacent to the LXXLL motif of coactivator TRBP defines selectivity for ERs and TRs. Mol Endocrinol 16:128–140 [DOI] [PubMed] [Google Scholar]
- 72.Duong BN, Elliott S, Frigo DE, Melnik LI, Vanhoy L, Tomchuck S, Lebeau HP, David O, Beckman BS, Alam J, Bratton MR, McLachlan JA, Burow ME2006. AKT regulation of estrogen receptor β transcriptional activity in breast cancer. Cancer Res 66:8373–8381 [DOI] [PubMed] [Google Scholar]
- 73.Georgescu SP, Li JH, Lu Q, Karas RH, Brown M, Mendelsohn ME2005. Modulator recognition factor 1, an AT-rich interaction domain family member, is a novel corepressor for estrogen receptor α. Mol Endocrinol 19:2491–2501 [DOI] [PubMed] [Google Scholar]
- 74.Wu X, Li H, Chen JD2001. The human homologue of the yeast DNA repair and TFIIH regulator MMS19 is an AF-1-specific coactivator of estrogen receptor. J Biol Chem 276:23962–23968 [DOI] [PubMed] [Google Scholar]
- 75.Zhang H, Thomsen JS, Johansson L, Gustafsson JA, Treuter E2000. DAX-1 functions as an LXXLL-containing corepressor for activated estrogen receptors. J Biol Chem 275:39855–39859 [DOI] [PubMed] [Google Scholar]
- 76.Webb P, Valentine C, Nguyen P, Price Jr RH, Marimuthu A, West BL, Baxter JD, Kushner PJ2003. ERβ Binds N-CoR in the Presence of Estrogens via an LXXLL-Like Motif in the N-CoR C-terminus. Nucl Recept 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cvoro A, Tatomer D, Tee MK, Zogovic T, Harris HA, Leitman DC2008. Selective estrogen receptor-β agonists repress transcription of proinflammatory genes. J Immunol 180:630–636 [DOI] [PubMed] [Google Scholar]
- 78.Levy N, Paruthiyil S, Zhao X, Vivar OI, Saunier EF, Griffin C, Tagliaferri M, Cohen I, Speed TP, Leitman DC2010. Unliganded estrogen receptor-β regulation of genes is inhibited by tamoxifen. Mol Cell Endocrinol 315:201–207 [DOI] [PubMed] [Google Scholar]
- 79.Couse JF, Yates MM, Deroo BJ, Korach KS2005. Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- 80.Kouzu-Fujita M, Mezaki Y, Sawatsubashi S, Matsumoto T, Yamaoka I, Yano T, Taketani Y, Kitagawa H, Kato S2009. Coactivation of estrogen receptor β by gonadotropin-induced cofactor GIOT-4. Mol Cell Biol 29:83–92 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Tremblay A, Tremblay GB, Labrie F, Giguère V1999. Ligand-independent recruitment of SRC-1 to estrogen receptor β through phosphorylation of activation function AF-1. Mol Cell 3:513–519 [DOI] [PubMed] [Google Scholar]
- 82.Picard N, Charbonneau C, Sanchez M, Licznar A, Busson M, Lazennec G, Tremblay A2008. Phosphorylation of activation function-1 regulates proteasome-dependent nuclear mobility and E6-associated protein ubiquitin ligase recruitment to the estrogen receptor β. Mol Endocrinol 22:317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lopez D, Sanchez MD, Shea-Eaton W, McLean MP2002. Estrogen activates the high-density lipoprotein receptor gene via binding to estrogen response elements and interaction with sterol regulatory element binding protein-1A. Endocrinology 143:2155–2168 [DOI] [PubMed] [Google Scholar]
- 84.Montano MM, Katzenellenbogen BS1997. The quinone reductase gene: a unique estrogen receptor-regulated gene that is activated by antiestrogens. Proc Natl Acad Sci USA 94:2581–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montano MM, Jaiswal AK, Katzenellenbogen BS1998. Transcriptional regulation of the human quinone reductase gene by antiestrogen-liganded estrogen receptor-α and estrogen receptor-β. J Biol Chem 273:25443–25449 [DOI] [PubMed] [Google Scholar]
- 86.Montano MM, Wittmann BM, Bianco NR2000. Identification and characterization of a novel factor that regulates quinone reductase gene transcriptional activity. J Biol Chem 275:34306–34313 [DOI] [PubMed] [Google Scholar]
- 87.Sripathy SP, Chaplin LJ, Gaikwad NW, Rogan EG, Montano MM2008. hPMC2 is required for recruiting an ERβ coactivator complex to mediate transcriptional upregulation of NQO1 and protection against oxidative DNA damage by tamoxifen. Oncogene 27:6376–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chu S, Nishi Y, Yanase T, Nawata H, Fuller PJ2004. Transrepression of estrogen receptor β signaling by nuclear factor-κB in ovarian granulosa cells. Mol Endocrinol 18:1919–1928 [DOI] [PubMed] [Google Scholar]
- 89.Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG2000. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem 275:25322–25329 [DOI] [PubMed] [Google Scholar]
- 90.Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD2005. Crossroads of estrogen receptor and NF-κB signaling. Sci STKE 2005:pe27 [DOI] [PubMed]
- 91.Zieleniak A, Wójcik M, Woźniak LA2008. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ. Arch Immunol Ther Exp (Warsz) 56:331–345 [DOI] [PubMed] [Google Scholar]
- 92.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U2008. Metabolic actions of estrogen receptor β (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet 4:e1000108 [DOI] [PMC free article] [PubMed]
- 93.Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Andò S2005. Estrogen receptor α binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor γ signaling in breast cancer cells. Clin Cancer Res 11:6139–6147 [DOI] [PubMed] [Google Scholar]
- 94.Wang X, Kilgore MW2002. Signal cross-talk between estrogen receptor α and β and the peroxisome proliferator-activated receptor γ1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol 194:123–133 [DOI] [PubMed] [Google Scholar]
- 95.Faulds MH, Pettersson K, Gustafsson JA, Haldosén LA2001. Cross-talk between ERs and signal transducer and activator of transcription 5 is E2 dependent and involves two functionally separate mechanisms. Mol Endocrinol 15:1929–1940 [DOI] [PubMed] [Google Scholar]
- 96.Johansson L, Båvner A, Thomsen JS, Färnegårdh M, Gustafsson JA, Treuter E2000. The orphan nuclear receptor SHP utilizes conserved LXXLL-related motifs for interactions with ligand-activated estrogen receptors. Mol Cell Biol 20:1124–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rüegg J, Swedenborg E, Wahlström D, Escande A, Balaguer P, Pettersson K, Pongratz I2008. The transcription factor aryl hydrocarbon receptor nuclear translocator functions as an estrogen receptor-selective coactivator, and its recruitment to alternative pathways mediates antiestrogenic effects of dioxin. Mol Endocrinol 22:304–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ2007. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13:1185–1192 [DOI] [PubMed] [Google Scholar]
- 99.DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP2008. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol 22:65–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kazi AA, Jones JM, Koos RD2005. Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: estrogen-induced recruitment of both estrogen receptor α and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Mol Endocrinol 19:2006–2019 [DOI] [PubMed] [Google Scholar]
- 101.Nanni S, Benvenuti V, Grasselli A, Priolo C, Aiello A, Mattiussi S, Colussi C, Lirangi V, Illi B, D’Eletto M, Cianciulli AM, Gallucci M, De Carli P, Sentinelli S, Mottolese M, Carlini P, Strigari L, Finn S, Mueller E, Arcangeli G, Gaetano C, Capogrossi MC, Donnorso RP, Bacchetti S, Sacchi A, Pontecorvi A, Loda M, Farsetti A2009. Endothelial NOS, estrogen receptor β, and HIFs cooperate in the activation of a prognostic transcriptional pattern in aggressive human prostate cancer. J Clin Invest 119:1093–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levin ER2008. Rapid signaling by steroid receptors. Am J Physiol Regul Integr Comp Physiol 295:R1425–R1430 [DOI] [PMC free article] [PubMed]
- 103.Micevych P, Dominguez R2009. Membrane estradiol signaling in the brain. Front Neuroendocrinol 30:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Luksha L, Kublickiene K2009. The role of estrogen receptor subtypes for vascular maintenance. Gynecol Endocrinol 25:82–95 [DOI] [PubMed] [Google Scholar]
- 105.Chambliss KL, Shaul PW2002. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev 23:665–686 [DOI] [PubMed] [Google Scholar]
- 106.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW2002. ERβ has nongenomic action in caveolae. Mol Endocrinol 16:938–946 [DOI] [PubMed] [Google Scholar]
- 107.Gingerich S, Krukoff TL2008. Activation of ERβ increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology 55:878–885 [DOI] [PubMed] [Google Scholar]
- 108.Zhao L, Brinton RD2007. Estrogen receptor α and β differentially regulate intracellular Ca2+ dynamics leading to ERK phosphorylation and estrogen neuroprotection in hippocampal neurons. Brain Res 1172:48–59 [DOI] [PubMed] [Google Scholar]
- 109.Chu Z, Andrade J, Shupnik MA, Moenter SM2009. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci 29: 5616–5627 [DOI] [PMC free article] [PubMed]
- 110.Hu L, Gustofson RL, Feng H, Leung PK, Mores N, Krsmanovic LZ, Catt KJ2008. Converse regulatory functions of estrogen receptor-α and -β subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol 22:2250–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F2005. Nongenomic effects of 17β-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor β and Src kinase. Blood 105:115–121 [DOI] [PubMed] [Google Scholar]
- 112.Zhang G, Liu X, Farkas AM, Parwani AV, Lathrop KL, Lenzner D, Land SR, Srinivas H2009. Estrogen receptor β functions through nongenomic mechanisms in lung cancer cells. Mol Endocrinol 23:146–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Madak-Erdogan Z, Kieser KJ, Kim SH, Komm B, Katzenellenbogen JA, Katzenellenbogen BS2008. Nuclear and extranuclear pathway inputs in the regulation of global gene expression by estrogen receptors. Mol Endocrinol 22:2116–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Klinge CM2008. Estrogenic control of mitochondrial function and biogenesis. J Cell Biochem 105:1342–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Psarra AM, Sekeris CE2008. Steroid and thyroid hormone receptors in mitochondria. IUBMB Life 60:210–223 [DOI] [PubMed] [Google Scholar]
- 116.Simpkins JW, Yang SH, Sarkar SN, Pearce V2008. Estrogen actions on mitochondria: physiological and pathological implications. Mol Cell Endocrinol 290:51–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yager JD, Chen JQ2007. Mitochondrial estrogen receptors–new insights into specific functions. Trends Endocrinol Metab 18:89–91 [DOI] [PubMed] [Google Scholar]
- 118.Felty Q, Roy D2005. Estrogen, mitochondria, and growth of cancer and non-cancer cells. J Carcinog 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gavrilova-Jordan LP, Price TM2007. Actions of steroids in mitochondria. Semin Reprod Med 25:154–164 [DOI] [PubMed] [Google Scholar]
- 120.Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK2008. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134:112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jelinsky SA, Harris HA, Brown EL, Flanagan K, Zhang X, Tunkey C, Lai K, Lane MV, Simcoe DK, Evans MJ2003. Global transcription profiling of estrogen activity: estrogen receptor α regulates gene expression in the kidney. Endocrinology 144:701–710 [DOI] [PubMed] [Google Scholar]
- 122.Lewandowski S, Kalita K, Kaczmarek L2002. Estrogen receptor β. Potential functional significance of a variety of mRNA isoforms. FEBS Lett 524:1–5 [DOI] [PubMed] [Google Scholar]
- 123.Zhao C, Dahlman-Wright K, Gustafsson JA2008. Estrogen receptor β: an overview and update. Nucl Recept Signal 6:e003 [DOI] [PMC free article] [PubMed]