Abstract
This study examines the effects of the dietary supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) (LA) on productive performances, incidence of foot pad dermatitis and caecum microbioma in broiler chickens. A total of 1,100 one-day old male Ross 308 chicks were divided into 2 groups of 16 replicates with 25 birds each and reared from 1–41 d. One group was fed a basal diet (CON) and the other group the same diet supplemented with LA. Caecum contents were collected from 4 selected birds at day one and 5 selected birds at the end of the rearing period. Then, they were submitted to DNA extraction and whole DNA shotgun metagenomic sequencing. Overall, the LA supplementation produced a significant beneficial effect on body weight gain between 15–28 d and improved feed conversion rate in the overall period. On the contrary, litter moisture, pH and incidence of the foot pad lesions were not affected by LA. Birds treated with LA showed a lower occurrence of pasty vent at both 14 and 28 d. At the end of the rearing period, Lachanospiraceae were significantly higher in LA birds in comparison to CON (17.07 vs 14.39%; P = 0.036). Moreover, Ruminococcus obeum, Clostridium clostridioforme, Roseburia intestinalis, Lachnospiraceae bacterium 14-2T and Coprococcus eutactus were significantly higher in LA birds in comparison to CON. The relative abundance of Lactobacillus acidophilus was comparable between LA and CON groups. However, a positive effect was observed in relation to the metabolic functions in the treated group, with particular reference to the higher abundance of β-glucosidase. In conclusion, the LA supplementation improved broiler productive performances and metabolic functions promoting animal health.
Introduction
The intestinal microbiota of homoeothermic animals constitutes a complex ecosystem composed by a large variety of microorganisms. It plays an important role in maintaining the host normal gut functions and health, and its imbalance, or dysbiosis, can produce negative effects on gut physiology [1]. Clinical signs of dysbiosis in broilers are thinning of the small intestine, increased water content and presence of indigested residues in the faces [2]. Autochthonous Lactobacillus species, such as L. acidophilus, can be identified in the gastro-intestinal (GI) tract of broilers raised under commercial conditions. Lactobacilli become established in the chicken GI tract soon after hatching, and their metabolic activity lowers the digesta pH, which, in turn, inhibits the proliferation of enterobacteria and other unwanted bacteria [3, 4]. However, the microbiota composition changes with ageing until a labile homeostasis is reached [5–7]. Furthermore, due to intensive rearing systems, farm animals are very susceptible to enteric dysbiosis [8].
Probiotics, or direct-fed microbials, have been defined as “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host” [9]. Modes of action of probiotic Lactic Acid Bacteria (LAB) that have been proposed include: competitive exclusion toward harmful bacteria, alteration of microbial and host metabolism, stimulation of immunity [2, 6, 8, 10–12]. Lactobacillus strains have been described as beneficial additives because of their effects in promoting poultry production performance [12–14]. Some authors highlight the role of probiotics as a sound alternative to antibiotic growth promoters [2, 15, 16]. However, kind of probiotic strain [17], dosage (i.e., colony forming unit (cfu)/bird/day), which should be modulated according to the flock health status and/or the farm hygienic conditions, as well as treatment duration, are among the critical factors influencing a probiotic efficacy. Other important variables are probiotic conservation and distribution technology, feed composition, also in terms of presence of antimicrobial agents and probiotic carriers (i.e., feed or drinking water) [18].
In the past researchers investigated the impact of the administration of probiotics on broiler GI tract by testing those microorganisms that could be recovered on growth media. However, they represent less that 20% of bacterial taxa inhabiting the poultry GI tract [19]. Within the last decade, the development of high-throughput sequencing technologies, targeting the whole set of genes within a system, gained a relatively unbiased view of both GI community structure (i.e., bacterial species richness and distribution) and functional (metabolic) potential [20].
The aim of this study was to evaluate the effects of the supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) in broiler chicken diets on productive performances, foot pad dermatitis and caecum microbioma, in terms of bacteria population and metabolic functions, by whole DNA shotgun metagenomic sequencing.
Materials and methods
Animals and treatments
The experiment was approved by the Ethical Committee of the University of Bologna on 17/3/2014 (ID: 10/79/2014). A total of 1,100 one-day old male Ross 308 chicks, obtained from the same breeder flock and hatching session, were used. Birds were vaccinated against infectious bronchitis virus, Marek’s disease virus, Newcastle and Gumboro diseases and coccidiosis at the hatchery. Before housing, chicks were individually weighed and divided in the following 5 classes according to their live weight: <42 g, 42–44 g, 45–47 g, 48–50 g, >50 g. The first and the last groups were discarded, while the remaining were distributed in 32 pens (2.5 m2 each) at the stocking density of 10 chicks/m2 (25 birds/pen), while maintaining the same class distribution of live-weight of the population. Pens were equipped with pan feeders, to assure at least 2 cm/bird of front space, and an independent drinking system with 1 nipple/5 birds. Feeders were of identical manufacture, type, size, color, and other notable physical features. Each pen was equipped with an individual bin, clearly labeled as reservoir for the experimental feeds. On a daily basis, the experimental feeds were manually transferred from the bin to the feeder. Any change in the diet was made uniformly for all animals. Feed and water were provided for ad libitum consumption. At each diet switch, feeders were emptied, orts were weighed back and the feeders were filled with the diets described below. Twice daily observations were recorded for general flock condition, temperature, lighting, water, feed, litter condition and mortality. The experiment lasted 41 days when birds reached the slaughter weight of about 2.8 kg of live weight. Photoperiod and temperature programs were set up according to the European welfare regulation 43/2007 [21].
The chicks were divided into 2 groups of 16 replicates with 25 birds each, fed with the basal diet (control group, CON) (Table 1) or the basal diet supplemented with L. acidophilus D2/CSL (bacterial concentration of 5.0 x 1010 cfu g–1) at the dosage of 20 g ton-1 feed (LA group). The probiotic strain L. acidophilus D2/CSL has been isolated from the GI tract of a healthy adult chicken [22] and supplied by Centro Sperimentale del Latte S.r.l. (Lodi, Italy).
Table 1. Basal diets composition.
| Starter (0–14 d) |
Grower (15–28 d) |
Finisher (29–41 d) |
|
|---|---|---|---|
| Ingredients, g/100 g | |||
| Corn | 42.17 | 34.96 | 12.73 |
| White corn | 0.00 | 0.00 | 15.00 |
| Wheat | 10.00 | 20.00 | 25.01 |
| Sorghum | 0.00 | 0.00 | 5.00 |
| Soybean meal | 23.11 | 20.63 | 17.60 |
| Expanded soybean | 10.00 | 10.00 | 13.00 |
| Sunflower | 3.00 | 3.00 | 3.00 |
| Corn gluten meal | 4.00 | 3.00 | 0.00 |
| Soybean oil | 3.08 | 4.43 | 5.48 |
| Dicalcium phosphate | 1.52 | 1.20 | 0.57 |
| Calcium carbonate | 0.91 | 0.65 | 0.52 |
| Sodium bicarbonate | 0.15 | 0.10 | 0.15 |
| Salt | 0.27 | 0.27 | 0.25 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Lysine sulphate | 0.59 | 0.55 | 0.46 |
| Dl-methionine | 0.27 | 0.29 | 0.30 |
| Threonine | 0.15 | 0.14 | 0.14 |
| Xylanase | 0.08 | 0.08 | 0.08 |
| Phytase | 0.10 | 0.10 | 0.10 |
| Vitamin-mineral premix1 | 0.50 | 0.50 | 0.50 |
| Proximate composition, g/100g | |||
| Dry matter | 88.57 | 88.65 | 88.64 |
| Protein | 22.70 | 21.49 | 19.74 |
| Lipid | 7.06 | 8.24 | 9.74 |
| Fiber | 3.08 | 3.04 | 3.07 |
| Ash | 5.85 | 5.17 | 4.49 |
| Lys | 1.38 | 1.29 | 1.21 |
| Ca | 0.91 | 0.80 | 0.59 |
| P | 0.63 | 0.57 | 0.46 |
| ME (kcal/kg) | 3,076 | 3,168 | 3,264 |
1 Provided the following per kg of diet: vitamin A (retinyl acetate), 13,000 IU; vitamin D3 (cholecalciferol), 4,000 IU; vitamin E (DL-α_tocopheryl acetate), 80 IU; vitamin K (menadione sodium bisulfite), 3 mg; riboflavin, 6.0 mg; pantothenic acid, 6.0 mg; niacin, 20 mg; pyridoxine, 2 mg; folic acid, 0.5 mg; biotin, 0.10 mg; thiamine, 2.5 mg; vitamin B12 20 μg; Mn, 100 mg; Zn, 85 mg; Fe, 30 mg; Cu, 10 mg; I, 1.5 mg; Se, 0.2 mg; ethoxyquin, 100 mg.
The experimental diets were weekly produced by adding the LA to the common basal diet. The feeding program included three feeding phases: Starter (0–14 d), Grower (15–28 d) and Finisher (29–41 d). The basal diet composition is given in Table 1.
Productive performance and slaughtering traits
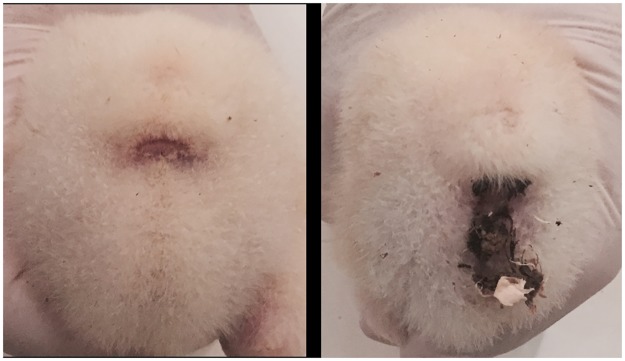
At housing, chicks individually weighed as previously described, in order to maintain the same class distribution of live-weight of the population within each pen, were counted and weighed on a pen basis, representing the experimental unit for productive performance measurements. Chickens and feed were weighed pen wise at 14, 28 and 41 days. Daily weight gain (DWG), feed intake (FI) and feed conversion ratio (FCR) were calculated for each feeding phase. Mortality was recorded and the above mentioned measures were adjusted for mortality. Litter moisture and pH were determined at the end of the experiment on a representative sample collected in two different areas within each pen, away from feeders and drinkers [23]. The incidence of pasty vent on a bird basis was recorded at 14 and 28 days by visual examination, based on presence or absence of sticky feces in the vent area (Fig 1).
Fig 1. Bird showing normal vent (on the left) or pasty vent (on the right).
All birds were processed at a commercial slaughterhouse and carcass yields were measured (breast, wings and legs) according to the standard procedures. Prior slaughter, the birds were subjected to a total feed withdrawal of 12 h, including the holding time of 2 h at the processing plant and processed under commercial conditions using electrical stunning (120 V, 200 Hz). Carcasses were obtained by removing head, neck, shanks and abdominal fat from bled, plucked and eviscerated birds. The incidence of foot pad dermatitis was assessed on all the birds, by collecting one foot/bird, and evaluated on a replicate basis. The feet were macroscopically examined and scored according to the available classification [24] in three classes of foot pad dermatitis (FPD): 0 = no lesions, 1 = mild lesions, and 2 = severe lesions.
Sample collection for metagenomics
To characterize the impact of the investigated diets on the caecum microbioma, representing both the microbial populations and the genes related to their metabolic functions, four chickens were randomly selected and humanely euthanized at day 1, before starting the dietary treatment. Moreover, five chickens were randomly selected from both CON and LA groups and humanely euthanized at 41 days. The entire gastrointestinal (GI) tract of the 14 individual selected birds was dissected out and a small sample (i.e., 0.5 to 2 g) of cecum content was collected into 15-ml sterile plastic tubes. The samples collected were then stored at -80°C until further testing.
DNA Extraction
The DNA was extracted from each caecum content using a bead-beating procedure [25]. Briefly, 0.25 g of cecal content were suspended in 1 ml lysis buffer (500 mM NaCl, 50 mM Tris-Cl, pH 8.0, 50 mM EDTA, 4% SDS) with MagNA Lyser Green Beads (Roche, Milan, Italy) and homogenized on the MagNA Lyser (Roche) for 25 sec at 6500 rpm. The samples were then heated at 70°C for 15 min, followed by centrifugation to separate the DNA from bacterial cellular debris. This process was repeated with a second 300 μl aliquot of lysis buffer. The samples were then subjected to 10 M v/v ammonium acetate (Sigma, Milan, Italy) precipitation, followed by isopropanol (Sigma) precipitation, 70% ethanol (Carlo Erba, Milan, Italy) washing and suspension in 100 ul 1X Tris-EDTA (Sigma). All samples were treated with DNase-free RNase (Roche) and incubated overnight at 4°C, before being processed through the QIAmp® DNA Stool Mini Kit (Qiagen, Milan, Italy) according to manufacturer’s directions with some modifications. DNA quantity and quality were measured on a BioSpectrometer® (Eppendorf, Milan, Italy).
Metagenomic sequencing
Total DNA from each of the 14 samples was fragmented and tagged with sequencing adapters using the Nextera XT DNA Library Preparation Kit (Illumina, San Diego, CA). Whole genome sequencing was performed using the HiScanSQ sequencer (Illumina) at 100 bp in paired-end mode. Metagenomic sequencing yielded an average of 6.841 million mapped reads/sample, with a Phread quality score always higher than 30. Following sequencing, all reads were assessed for quality parameters and the paired end merged. The MG-RAST pipeline [26] (metagenomics.anl.gov) was used to identify the relative abundances of bacterial taxa performing a BLAST similarity search for the longest cluster representative against the M5rna database, integrating SILVA [27], Greengenes [28] and RDP [29]. Moreover, the sequenced reads were assigned to functional groups using the Kyoto Encyclopedia of Genes and Genome (KEGG) database (www.genome.jp/kegg/) [30] and the percentage of abundance was calculated.
Statistical analysis
Data regarding productive traits were analyzed using the General Lineal Model (GLM) procedure of SAS [31]. One-way ANOVA with significance level of P < 0.05 was used to test the effect of LA supplementation on performance parameters. Data, expressed as percentage, were transformed to arc-sin before the analysis to homogenize the variance. Data regarding carcass yield and incidence of meat abnormalities were analyzed using the chi square test. The results concerning the relative abundances of bacterial taxa and functional groups were compared through the White’s non-parametric t-test, using Statistical Analysis of Metagenomic profile Software v 2.0.9 (STAMP) [32].
Results
Productive performances
Productive performances recorded during the trial (Table 2) were consistent with those reported by the Ross 308 performance objectives. At 14 days of age birds receiving LA resulted significantly heavier than CON (464 vs 453 g, P<0.05) and consequently DWG resulted higher (29.9 vs 29.0 g/bird/d; P<0.01). LA chicks consumed more feed than CON ones (36.3 vs 35.7 g/bird/d, P = 0.06). Mortality resulted in general low and similar in both groups, whereas pasty vent incidence was higher in CON (25.7 vs 10.7%, P<0.01) (Table 2).
Table 2. Productive performances and incidence of pasty vent of chickens untreated (CON) and treated with L. acidophilus (LA) separated by feeding phase.
| CON | LA | SE | P-value | |
|---|---|---|---|---|
| n. | 16 | 16 | ||
| -----------------------------------0–14 d-------------------------------- | ||||
| Chick body weight (g) | 46.05 | 46.02 | 0.05 | 0.76 |
| Body weight (g) | 453 b | 464 a | 3.38 | 0.03 |
| Daily weight gain (g/bird/d)* | 29.0 b | 29.9 a | 0.25 | 0.03 |
| Daily feed intake (g/bird/d)* | 35.7 | 36.3 | 0.22 | 0.06 |
| Feed conversion rate* | 1.229 | 1.217 | 0.008 | 0.28 |
| Mortality (%) | 0.50 | 1.50 | 0.02 | 0.11 |
| Pasty vent (%) | 25.7 A | 10.7 B | 0.03 | < 0.01 |
| -----------------------------------15–28 d-------------------------------- | ||||
| Body weight (g/bird) | 1,498 b | 1,531 a | 9.87 | 0.03 |
| Daily weight gain (g/bird/d)* | 74.6 | 76.1 | 0.63 | 0.09 |
| Daily feed intake (g/bird/d)* | 111 | 112 | 0.74 | 0.39 |
| Feed conversion rate* | 1.492 | 1.473 | 0.008 | 0.09 |
| Mortality (%) | 0.75 | 0.50 | 0.02 | 0.66 |
| Pasty vent (%) | 52.2 A | 37.8 B | 0.04 | < 0.01 |
| -----------------------------------29–41 d-------------------------------- | ||||
| Body weight (g/bird) | 2,757 | 2,784 | 13.64 | 0.17 |
| Daily weight gain (g/bird/d)* | 96.5 | 95.7 | 0.86 | 0.52 |
| Daily feed intake (g/bird/d)* | 178 a | 174 b | 1.29 | 0.03 |
| Feed conversion rate* | 1.842 | 1.815 | 0.02 | 0.26 |
| Mortality (%) | 0.75 | 0.75 | 0.02 | 0.82 |
| -----------------------------------0–41 d-------------------------------- | ||||
| Body weight (g/bird) | 2,757 | 2,784 | 13.64 | 0.17 |
| Daily weight gain (g/bird/d)* | 65.83 | 66.31 | 0.37 | 0.37 |
| Daily feed intake (g/bird/d)* | 106.2 | 105.3 | 0.46 | 0.30 |
| Feed conversion rate* | 1.613 a | 1.588 b | 0.008 | 0.03 |
| Mortality (%) | 2.00 | 2.75 | 0.03 | 0.25 |
* corrected for mortality
a, b: P<0.05;
A, B: P<0.01.
At 28 days of age, birds receiving LA weighed 33 g more than CON (1,531 vs 1,498 g, P<0.05). FCR resulted slightly, but not significantly, improved in LA birds (1.473 vs 1.492, P = 0.09). Mortality resulted similar in both groups and incidence of pasty vent was significantly lower in LA group (37.8 vs 52.2%; P<0.01) (Table 2).
At the end of trial (41 d), LA birds showed a slightly higher body weight than CON but the difference was not statistically significant (2,784 vs 2,757, P = 0.17). Daily feed intake from 29 to 41 days was significantly lower in LA birds (173 vs 178 g/bird/d; P<0.05) (Table 2).
Overall the productive performance from 0 to 41 days (Table 2) showed that LA supplementation significantly improved feed conversion rate (1.588 vs 1.613; P<0.03). Mortality presented low values in both groups ranging from 2 (CON) to 2.75% (LA).
Carcass and cut up yields at slaughter were not affected by LA supplementation (Table 3). Litter moisture and pH resulted similar in the two groups (35.4 vs 34.8% and 8.68 vs 8.63 for CON and LA, respectively; data not shown). Similarly, the incidence of foot pad lesions was not affected by the dietary treatment (Table 4).
Table 3. Carcass evaluation at slaughtering of chickens untreated (CON) and treated with L. acidophilus (LA).
| CON | LA | |
|---|---|---|
| n. | 386 | 384 |
| Eviscerated yield (%) | 70.0 | 70.6 |
| Breast* (%) | 30.3 | 30.7 |
| Legs*(%) | 44.4 | 44.6 |
| Unseparated wings*(%) | 19.3 | 19.2 |
| Chi2, (P-value) | 0.998 | |
*calculated as a percentage of eviscerated carcass weight
Table 4. Incidence of foot pad lesions of chickens untreated (CON) and treated with L. acidophilus (LA).
| CON | LA | |
|---|---|---|
| Bird scored, n. | 386 | 384 |
| Score 0 (no lesions) (%) | 97.1 | 99.5 |
| Score 1 (mild lesions) (%) | 2.9 | 0.5 |
| Score 2 (severe lesions) (%) | 0.0 | 0.0 |
| Chi2, (P-value) | 0.401 | |
Metagenomic results
The metagenomes of the 14 samples included in this study are public available from MG RAST (http://metagenomics.anl.gov/linkin.cgi?project=13081). The metagenome ID mgm 4624898.3, 4625263.3, 4625261.3 and 4625265.3 refer to samples collected at day one. The mgm 4625297.3, 4625262.3, 4625269.3, 4625304.3 and 4625316.3 refer to samples collected from the control group at 41 days. The mgm 4625288.3, 4625285.3, 4625287.3, 4625273.3 and 4625272.3 refer to samples collected from the treated group at 41 days.
Caecum microbiota composition
The microbiota composition of one-day old chicks is summarized in Table 5. More than 95% of bacterial population was represented by Firmicutes (85.5%) and Proteobacteria (9.61%). Both these Phyla were largely represented also in the caeca of CON and LA birds at 41 days (Table 5). The relative frequency of abundance of Firmicutes in one-day old chicks was significantly lower than that observed in both groups at 41 days (P = 0.01), whereas Proteobacteria were significantly higher (P = 0.0067).
Table 5. Mean relative frequency of abundance (%) of phyla, classes and families of caecum bacteria in one-day old and 41-day old chickens untreated (CON) and treated with L. acidophilus (LA).
| Phylum | Class | Family | One-day | CON | LA | P value CON vs LA |
P value One-day vs CON |
P value One-day vs LA |
|---|---|---|---|---|---|---|---|---|
| Firmicutes | 85.85 | 93.93 | 92.14 | 0.121 | 0.056 | 0.098 | ||
| Bacilli | 43.55 | 20.72 | 18.91 | 0.703 | 0.038 | 0.029 | ||
| Bacillaceae | 1.17 | 1.12 | 0.81 | 0.331 | 0.949 | 0.636 | ||
| Paenibacillaceae | 0.18 | 0.49 | 0.95 | 0.278 | 0.196 | 0.104 | ||
| Staphylococcaceae | 0.93 | 0.22 | 0.18 | 0.616 | 0.392 | 0.369 | ||
| Enterococcaceae | 3.72 | 0.50 | 0.37 | 0.488 | 0.132 | 0.123 | ||
| Lactobacillaceae | 33.45 | 17.22 | 15.62 | 0.752 | 0.069 | 0.053 | ||
| Streptococcaceae | 1.79 | 0.44 | 0.32 | 0.678 | 0.030 | 0.029 | ||
| Clostridia | 41.92 | 70.51 | 70.79 | 0.950 | 0.048 | 0.043 | ||
| Clostridiaceae | 12.28 | 11.89 | 14.30 | 0.070 | 0.939 | 0.697 | ||
| Eubacteriaceae | 3.47 | 3.87 | 3.73 | 0.828 | 0.839 | 0.893 | ||
| Lachnospiraceae | 13.25 | 14.39 | 17.07 | 0.036 | 0.621 | 0.160 | ||
| Peptococcaceae | 0.18 | 0.60 | 0.43 | 0.306 | 0.110 | 0.281 | ||
| Peptostreptococcaceae | 1.18 | 3.89 | 3.39 | 0.724 | 0.119 | 0.181 | ||
| Ruminococcaceae | 6.80 | 29.53 | 26.27 | 0.423 | < 0.0001 | 0.001 | ||
| Erysipelotrichi | 0.37 | 2.21 | 1.83 | 0.424 | 0.002 | 0.008 | ||
| Negativicutes | 0\ | 0.47 | 0.60 | 0.456 | 0.001 | 0.014 | ||
| Proteobacteria | 9.61 | 1.74 | 2.10 | 0.389 | 0.050 | 0.057 | ||
| Alphaproteobacteria | 0.49 | 0.57 | 0.66 | 0.689 | 0.809 | 0.657 | ||
| Betaproteobacteria | 0.18 | 0.10 | 0.10 | 0.993 | 0.683 | 0.692 | ||
| Deltaproteobacteria | 0.18 | 0.27 | 0.48 | 0.213 | 0.666 | 0.249 | ||
| Gammaproteobacteria | 8.74 | 0.77 | 0.84 | 0.873 | 0.075 | 0.076 | ||
| Enterobacteriaceae | 7.63 | 0.62 | 0.70 | 0.851 | 0.069 | 0.070 | ||
| Actinobacteria | 1.60 | 0.92 | 1.43 | 0.397 | 0.466 | 0.850 | ||
| Bacteroidetes | 0.18 | 0.35 | 0.29 | 0.627 | 0.456 | 0.614 | ||
| Tenericutes | 1.42 | 1.18 | 1.47 | 0.612 | 0.447 | 0.924 |
Within Firmicutes, in one-day old chicks Bacilli was the most abundant class, followed by Clostridia. On the contrary, at 41 days, Clostridia represented the most abundant class in both LA and CON groups (70.8 and 70.5% respectively), followed by Bacilli, presenting a relative frequency of abundance of 20.7 and 18.9%, respectively. The mean relative abundances of Clostridia and Bacilli in the birds at the end of the rearing period were significantly higher (P = 0.0086) and lower (P = 0.0094) respectively, in comparison to those of one-day old chicks (Table 5). In one-day old chicks, as well as LA and CON groups, Gammaproteobacteria was the most representative class of Proteobacteria. This class was the only one significantly higher in one-day old chicks in comparison to CON and LA groups at 41 days of age (P = 0.015 and P = 0.017 respectively). Moreover, in one-day old chicks Enterobacteriaceae was the most represented family (7.63%) in comparison to the birds at 41 days, where the relative abundances of the same family were as low as 0.77 and 0.84% in CON and LA, respectively (Table 5). Within Bacilli class, the most represented family in one-day old chicks was Lactobacillaceae (33.5%), followed by Enterococcaceae (3.72%), Streptococcaceae (1.79%) and Bacillaceae (1.17%) (Table 5). This distribution was similar in CON and LA birds at 41 days, except for Bacillaceae and Paenibacillaceae, representing the second most abundant families in CON and LA birds at 41 days, respectively.
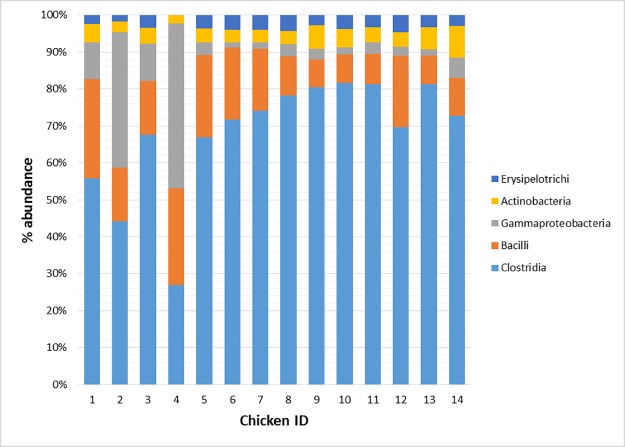
Chickens identified as 1 and 3 showed a percentage of abundance of Gammaproteobacteria lower in comparison to the other one-day old chicks (i.e., 9.91 and 10.03 vs 36.47 and 44.70%) (Fig 2). The decrease of Gammaproteobacteria corresponded to a higher abundance of Clostridia (55.81 and 67.70%, respectively). On the contrary, Chicken ID 4 showed the highest abundance of Gammaproteobacteria (44.70%) and the lowest abundance of Clostridia (26.88%) in comparison with the other one-day old chicks. t 41 days the percentage of abundance of the top five classes was quite similar among chickens belonging to CON and LA groups. However, chicken ID 12 showed a higher percentage of Bacilli abundance and a lower abundance of Clostridia in comparison to the other LA chickens (Fig 2).
Fig 2. Mean relative frequency of abundance (% abundance) of most represented bacterial classes in each of the 14 chickens tested (Day 1: Chicken ID 1–4; Control 41 days: Chicken ID 5–9; Treated 41 days: Chicken ID 10–14).
In comparison to one-day old chicks, Lactobacillaceae, Enterococcaceae and Streptococcaceae decreased significantly in both CON (i.e., P = 0.023, P = 0.042 and P = 0.01, respectively) and LA (i.e. P = 0.031, P = 0.013, P = 0.002, respectively) at 41 d. Lachanospiraceae was the most represented family identified within the Clostridia class in one-day old chicks (13.25%). On the contrary, in CON and LA at 41 days Ruminococcaceae was the most represented family (29.53 and 26.27%, respectively) and showed a relative frequency of abundance significantly higher than in one-day old chicks (P = 0.00044 and P = 0.0107 for CON and LA at 41 days, respectively). At the end of the rearing period Lachanospiraceae was significantly higher in LA birds in comparison to CON (17.07 vs 14.39; P = 0.036) and the same trend was observed for Clostridiaceae (14.30 vs 11.89%; P = 0.074) (Table 5).
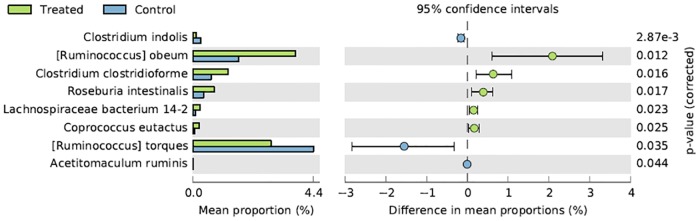
Overall, among the first 30 bacterial species identified in one-day old chicks the most represented species were Lactobacillus johnsonii, Lactobacillus crispatus, Escherichia coli, Ruminococcus torques, Lactobacillus helveticus, Lactobacillus gasseri, Ruminococcus obeum, Ruminococcaceae bacterium D16, Clostrifium hylemonae and Eubacterium limosum (Table 6). At 41 days, the most represented species in CON were Faecalibacterium prausnitzii, Lactobacillus crispatus, Ruminococcus torques, Subdoligranulum variabile, Ruminococcaceae bacterium D16, Lactobacillus johnsonii, Pseudoflavonifractor capillosus, Ruminococcus obeum, Clostridium difficile and Blautia hydrogenotrophica, whereas in LA they were Faecalibacterium prausnitzii, Lactobacillus johnsonii, Ruminococcus obeum, Subdoligranulum variabile, Ruminococcus torques, Ruminococcaceae bacterium D16, Lactobacillus reuteri, Lactobacillus crispatus, Blautia hydrogenotrophica and Clostrium leptum (Table 6). In relation to the bacterial species significantly different among the tested groups (Table 7), the relative frequency of abundance of Lactobacillus crispatus was significantly higher in one-day old chicks in comparison to LA, whereas Ruminococcus lactaris was significantly higher in one-day old chicks in comparison to CON. The species Faecalibacterium prausnitzii and Subdoligranulum variabile showed a significantly higher relative frequency of abundance in LA and CON birds in comparison to one-day old chicks. On the contrary, all other species were significantly higher in one-day old chicks in comparison to both LA and CON groups (Table 7). Overall, among all the bacterial species identified in CON and LA groups at 41 days, those showing a significantly higher relative frequency of abundance in LA birds were Ruminococcus obeum, Clostridium clostridioforme, Roseburia intestinalis, Lachnospiraceae bacterium 14-2T and Coprococcus eutactus. On the contrary, Clostridium indolis and Ruminococcus torques were significantly higher in CON (Fig 3).
Table 6. Mean relative frequency of abundance (%) of the 30 most representative species (MRS) of caecum bacteria in one-day old and 41-day old chickens untreated (CON) and treated with L. acidophilus (LA).
| One-day | CON | LA | ||||
|---|---|---|---|---|---|---|
| MRS1 | Species | Mean | Species | Mean | Species | Mean |
| 1 | Lactobacillus johnsonii | 11.36 | Faecalibacterium prausnitzii | 17.35 | Faecalibacterium prausnitzii | 14.00 |
| 2 | Lactobacillus crispatus | 6.14 | Lactobacillus crispatus | 5.62 | Lactobacillus johnsonii | 4.17 |
| 3 | Escherichia coli | 4.80 | Ruminococcus torques | 4.41 | Ruminococcus obeum | 3.76 |
| 4 | Ruminococcus torques | 3.80 | Subdoligranulum variabile | 3.26 | Subdoligranulum variabile | 2.99 |
| 5 | Lactobacillus helveticus | 2.94 | Ruminococcaceae bacterium D16 | 3.10 | Ruminococcus torques | 2.86 |
| 6 | Lactobacillus gasseri | 2.73 | Lactobacillus johnsonii | 2.44 | Ruminococcaceae bacterium D16 | 2.73 |
| 7 | Ruminococcus obeum | 1.98 | Pseudoflavonifractor capillosus | 2.05 | Lactobacillus reuteri | 2.44 |
| 8 | Ruminococcaceae bacterium D16 | 1.98 | Ruminococcus obeum | 1.68 | Lactobacillus crispatus | 2.12 |
| 9 | Clostridium hylemonae | 1.85 | Clostridium difficile | 1.59 | Blautia hydrogenotrophica | 1.62 |
| 10 | Eubacterium limosum | 1.80 | Blautia hydrogenotrophica | 1.38 | Clostridium leptum | 1.62 |
| 11 | Clostridium bolteae | 1.74 | butyrate-producing bacterium SM4/1 | 1.30 | Pseudoflavonifractor capillosus | 1.54 |
| 12 | Lactobacillus vaginalis | 1.69 | Clostridium leptum | 1.27 | Blautia sp. Ser8 | 1.31 |
| 13 | Lactobacillus reuteri | 1.68 | Lactobacillus reuteri | 1.23 | Ruminococcus bromii | 1.29 |
| 14 | Enterococcus faecalis | 1.48 | Lactobacillus acidophilus | 1.18 | Clostridium difficile | 1.29 |
| 15 | Shigella boydii | 1.43 | Ruminococcus bromii | 1.11 | Clostridium clostridioforme | 1.29 |
| 16 | Enterococcus faecium | 1.32 | Ruminococcus albus | 1.06 | Clostridium bolteae | 1.16 |
| 17 | Clostridium asparagiforme | 1.30 | Lactobacillus vaginalis | 1.05 | butyrate-producing bacterium SM4/1 | 1.03 |
| 18 | Lactobacillus delbrueckii | 1.30 | Lactobacillus helveticus | 1.02 | Ruminococcus flavefaciens | 0.94 |
| 19 | Ruminococcus lactaris | 1.18 | Ruminococcus flavefaciens | 0.90 | Lactobacillus helveticus | 0.92 |
| 20 | butyrate-producing bacterium SL7/1 | 1.11 | Lactobacillus agilis | 0.87 | Lactobacillus agilis | 0.90 |
| 21 | Granulicatella adiacens | 0.93 | butyrate-producing bacterium SL7/1 | 0.84 | Ruminococcus gnavus | 0.90 |
| 22 | Blautia hydrogenotrophica | 0.93 | Clostridium bartlettii | 0.84 | Clostridium scindens | 0.89 |
| 23 | Clostridium sphenoides | 0.93 | Ruminococcus gnavus | 0.83 | butyrate-producing bacterium SL7/1 | 0.88 |
| 24 | Enterococcus pseudoavium | 0.92 | Anaerotruncus colihominis | 0.81 | Ruminococcus albus | 0.86 |
| 25 | Lactobacillus acidophilus | 0.87 | Butyricicoccus pullicaecorum | 0.81 | Clostridium saccharolyticum | 0.84 |
| 26 | Faecalibacterium prausnitzii | 0.87 | Clostridium bolteae | 0.80 | Lactobacillus vaginalis | 0.82 |
| 27 | Pseudoflavonifractor capillosus | 0.87 | Eubacterium hallii | 0.76 | Lactobacillus acidophilus | 0.78 |
| 28 | Lactobacillus plantarum | 0.87 | Blautia sp. Ser8 | 0.74 | Roseburia intestinalis | 0.77 |
| 29 | Clostridium scindens | 0.80 | Dorea formicigenerans | 0.68 | Anaerotruncus colihominis | 0.70 |
| 30 | butyrate-producing bacterium SM4/1 | 0.80 | Clostridium clostridioforme | 0.66 | Blautia hansenii | 0.65 |
1MRS: most represented species
Table 7. Statistically significant differences between means of relative frequency of abundance (%) of caecum bacterial species in one-day old and 41-day old chickens untreated (CON) and treated with L. acidophilus (LA).
| One-day | CON | LA | One-day vs CON | One-day vs LA | CON vs LA | |
|---|---|---|---|---|---|---|
| Species | Mean | P-values | ||||
| Lactobacillus johnsonii | 11.36 | 2.44 | 4.17 | 0.009 | 0.004 | 0.410 |
| Lactobacillus crispatus | 6.14 | 5.61 | 2.12 | 0.005 | 0.005 | 0.192 |
| Escherichia coli | 4.80 | 0.40 | 0.50 | 0.016 | 0.022 | 0.751 |
| Lactobacillus gasseri | 2.73 | 0.37 | 0.45 | 0.006 | 0.008 | 0.676 |
| Clostridium bolteae | 1.74 | 0.80 | 1.16 | 0.003 | 0.039 | 0.130 |
| Shigella boydii | 1.43 | 0.03 | 0.03 | 0.0006 | 0.0009 | 0.794 |
| Lactobacillus delbrueckii | 1.30 | 0.23 | 0.28 | 0.019 | 0.028 | 0.609 |
| Ruminococcus lactaris | 1.18 | 0.17 | 0.30 | 0.046 | 0.106 | 0.451 |
| Faecalibacterium prausnitzii | 0.87 | 17.35 | 14.00 | < 0.0001 | 0.0005 | 0.331 |
| Subdoligranulum variabile | 0.62 | 3.26 | 2.99 | 0.002 | 0.003 | 0.665 |
Fig 3. Bacterial species resulting significantly different in chickens treated with L. acidophilus (Treated) in comparison to the untreated birds (Control) at 41 days.
Caecum metabolic genes composition
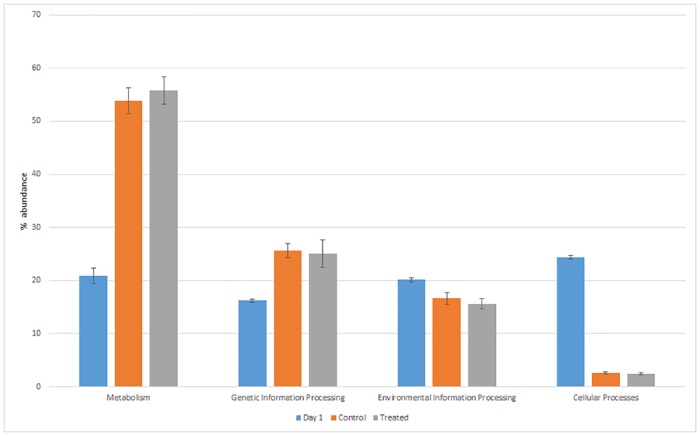
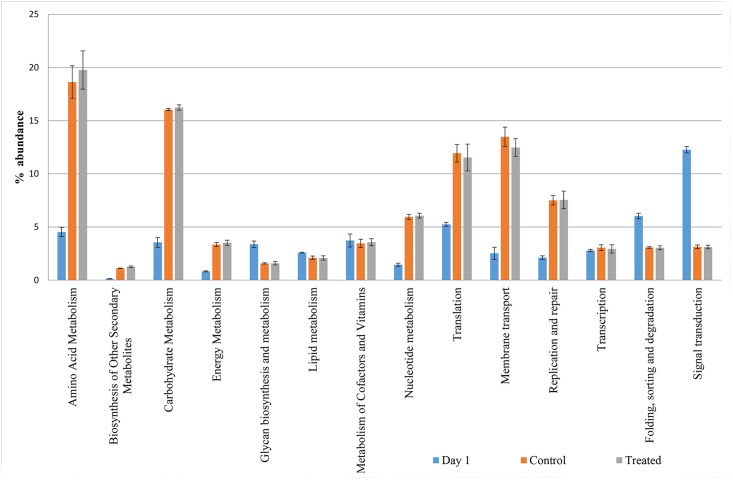
The mean relative abundance of the KEGG pathways related to metabolism and genetic information processing in one-day old chicks corresponded to 20.9 and 16.2%, respectively. These values were significantly lower than those detected at 41 days in both CON and LA groups (53.8 vs 55.8% and 25.6 vs 25.0%, respectively) (Fig 4). On the contrary, the environmental information processing and cellular processes pathways were significantly higher in one-day old chicks. In relation to specific metabolism pathways, one-day old chickens showed relative frequencies of abundance of aminoacid and carbohydrate metabolisms significantly lower (4.54 and 3.55%, P<0.001) than those detected in both LA and CON (19.8 and 18.6%; 16.2 and 16.1%, respectively) (Fig 5). The biosynthesis of other secondary metabolites was the only metabolism pathway significantly higher in LA than CON birds (1.27 vs 1.12% respectively; P<0.012) (Fig 5).
Fig 4. Mean relative frequency of abundance (% abundance) of the KEGG pathways in caeca of Day 1 chickens (Day 1) and in ceca of chickens treated with L. acidophilus (Treated) in comparison to the untreated birds (Control) at 41 days.
Fig 5. Mean relative frequency of abundance (% abundance) of the KEGG in caeca of day 1 chickens (Day 1) and in ceca of chickens treated with L. acidophilus (Treated) in comparison to the untreated birds (Control) at 41 days.
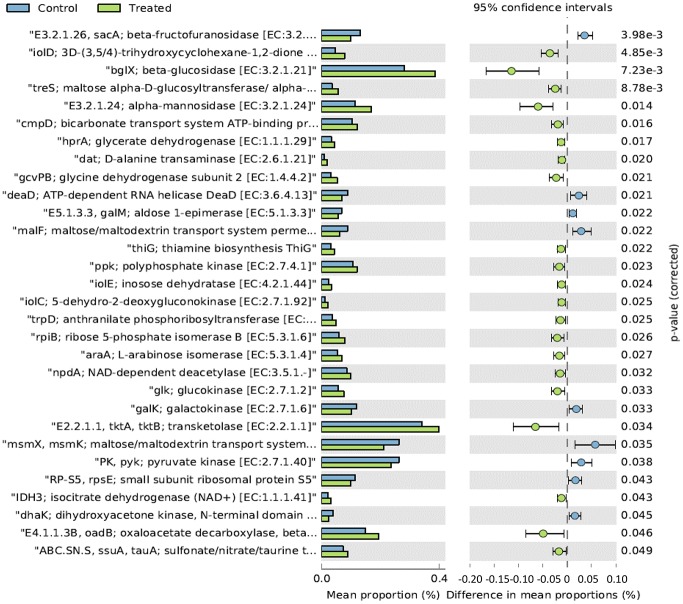
Overall, in terms of mean relative frequency of abundance, the top 20 metabolic functions identified using the KEGG database are reported in Table 8. At the end of the rearing period the following functions resulted significantly higher in LA in comparison to CON (Fig 6): bglX beta-glucosidase (EC:3.2.1.21), tkatkb transketolase EC:2.2.1.1, alpha-mannosidase (EC:3.2.1.24), ppk polyphosphate kinase (EC:2.7.4.1), oadB oxaloacetate decarboxylase beta subunit (EC: 4.1.1.3), glk glucokinase (EC:2.7.1.2), rpiB ribose 5-phosphate isomerase B (EC: 5.3.1.6), araA L.arabinose isomerase (EC:5.3.1.4) and npdA NAD-dependent deacetylase (EC:3.5.1.-). On the contrary sacA beta-fructofuranosidase (EC:3.3.1.26), malF:maltose/maltodestrin transport system permease, msmX, msmK; maltose/maltodestrine transport system ATP-binding protein, pyk; pyruvate kinase (EC:2.7.1.40) were higher in CON in comparison to LA (Fig 6). The distribution of the top 20 metabolic functions among the 14 tested chickens showed slight differences only in one-day old chicks (Fig 7).
Table 8. Mean relative frequency of abundance (%) of the top 20 metabolic functions identified in caeca of one-day old and 41-day old chickens untreated (CON) and treated with L. acidophilus (LA) by using KEGG database.
| Metabolic functions | One-day | CON | LA | CON vs LA | One-day vs CON | One-day vs LA | |||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P value | |||
| uvrA; excinuclease ABC subunit A | 0.06 | 0.020 | 0.95 | 0.09 | 1.06 | 0.08 | 0.129 | <0.0001 | <0.0001 |
| gpmA. PGAM; 2.3-bisphosphoglycerate-dependent phosphoglycerate mutase [EC:5.4.2.1] | 0.04 | 0.010 | 0.49 | 0.03 | 0.48 | 0.06 | 0.799 | <0.0001 | <0.0001 |
| LARS. leuS; leucyl-tRNA synthetase [EC:6.1.1.4] | 0.10 | 0.020 | 0.48 | 0.05 | 0.48 | 0.09 | 0.967 | <0.0001 | <0.0001 |
| ABC-2.AB.A; antibiotic transport system ATP-binding protein | 0.03 | 0.010 | 0.54 | 0.08 | 0.49 | 0.06 | 0.418 | 0.0002 | 0.0001 |
| ppdK; pyruvate.orthophosphate dikinase [EC:2.7.9.1] | 0.03 | 0.007 | 0.50 | 0.04 | 0.49 | 0.06 | 0.910 | <0.0001 | <0.0001 |
| E2.3.1.54. pflD; formate C-acetyltransferase [EC:2.3.1.54] | 0.03 | 0.007 | 0.47 | 0.05 | 0.50 | 0.08 | 0.632 | <0.0001 | 0.0003 |
| glnA; glutamine synthetase [EC:6.3.1.2] | 0.06 | 0.020 | 0.51 | 0.03 | 0.53 | 0.06 | 0.492 | <0.0001 | <0.0001 |
| uvrB; excinuclease ABC subunit B | 0.02 | 0.010 | 0.52 | 0.03 | 0.53 | 0.06 | 0.631 | <0.0001 | <0.0001 |
| DPO3A1. dnaE; DNA polymerase III subunit alpha [EC:2.7.7.7] | 0.03 | 0.020 | 0.53 | 0.02 | 0.56 | 0.06 | 0.371 | <0.0001 | <0.0001 |
| VARS. valS; valyl-tRNA synthetase [EC:6.1.1.9] | 0.05 | 0.009 | 0.58 | 0.04 | 0.58 | 0.06 | 0.860 | <0.0001 | <0.0001 |
| DPO3A2. polC; DNA polymerase III subunit alpha. Gram-positive type [EC:2.7.7.7] | 0.03 | 0.010 | 0.60 | 0.07 | 0.58 | 0.13 | 0.880 | <0.0001 | 0.001 |
| dnaK; molecular chaperone DnaK | 0.06 | 0.020 | 0.62 | 0.04 | 0.64 | 0.05 | 0.615 | <0.0001 | <0.0001 |
| secA; preprotein translocase subunit SecA | 0.03 | 0.010 | 0.66 | 0.05 | 0.64 | 0.06 | 0.620 | <0.0001 | <0.0001 |
| cbiO; cobalt/nickel transport system ATP-binding protein | 0.07 | 0.030 | 0.70 | 0.09 | 0.65 | 0.08 | 0.499 | <0.0001 | <0.0001 |
| IARS. ileS; isoleucyl-tRNA synthetase [EC:6.1.1.5] | 0.07 | 0.010 | 0.66 | 0.02 | 0.66 | 0.11 | 0.946 | <0.0001 | 0.0004 |
| E6.3.5.3. purL; phosphoribosylformylglycinamidine synthase [EC:6.3.5.3] | 0.03 | 0.010 | 0.58 | 0.18 | 0.69 | 0.15 | 0.407 | 0.0039 | 0.0009 |
| nrdD; ribonucleoside-triphosphate reductase [EC:1.17.4.2] | 0.04 | 0.020 | 0.74 | 0.10 | 0.74 | 0.12 | 0.994 | <0.0001 | 0.0002 |
| carB. CPA2; carbamoyl-phosphate synthase large subunit [EC:6.3.5.5] | 0.04 | 0.010 | 0.78 | 0.03 | 0.82 | 0.09 | 0.453 | <0.0001 | <0.0001 |
| rpoC; DNA-directed RNA polymerase subunit beta' [EC:2.7.7.6] | 0.05 | 0.002 | 1.03 | 0.13 | 0.95 | 0.14 | 0.450 | 0.0001 | 0.0002 |
| rpoB; DNA-directed RNA polymerase subunit beta [EC:2.7.7.6] | 0.07 | 0.030 | 1.00 | 0.09 | 0.96 | 0.15 | 0.711 | <0.0001 | 0.0002 |
Fig 6. Mean relative frequency of abundance (% abundance) of the KEGG functions showing P < 0.05 between chickens treated with L. acidophilus (Treated) in comparison to the untreated birds (Control) at 41 days.
Fig 7. Mean relative frequency of abundance (% abundance) of the top 20 KEGG functions in each of the 14 chickens tested (Day 1: Chicken ID 1–4; Control 41 days: Chicken ID 5–9; Treated 41 days: Chicken ID 6–10).
Discussion
The administration of probiotic Lactobacillus has been demonstrated to stimulate immune responses [6, 33] and improve digestive health [34], as well as growth performance [35–37] in poultry. Lactobacillus administration has also been shown to reduce colonization by Campylobacter [38, 39], Clostridium [40], and Salmonella [38, 41], improving the microbial food safety of poultry meat. The ability of Lactobacillus species to adhere to epithelial tissues and colonize poultry gut has been reported [42–44]. However, the most important microbial factors implicated in Lactobacillus GI persistence in poultry are not well characterized. The lack of a species-specific cell culture model prevented investigations of Lactobacillus adhesion and its contribution to GI colonization.
In this study, the supplementation with L. acidophilus to growing chicken diet produced a significant beneficial effect, with particular regard to body weight gain up to 28 days of age (1,531 vs 1,498 g, respectively for LA and CON; P<0.05) and feed efficiency for the overall experimental period (1.588 vs 1.613 respectively for LA and CON; P<0.05). According to other authors [45–47], the results of this study show that the feed supplementation with Lactobacillus improves body weight gain and feed efficiency. These results can be attributed to the improvement of the intestinal microbial balance of the host through a competitive exclusion mechanism and antagonism [10]. Indeed it is well documented that the mode of action of Lactobacillus consists in competitive exclusion against harmful bacteria in the gut in favor of beneficial microbial populations, leading to improvement in nutrients utilization of feed [10, 46]. After studying the effect of Lactobacillus species supplementation in chickens, Award et al. [48] attributed the improvement of growth performance of broiler chickens to the greater development of small intestinal villi, particularly villus height:crypt depth ratio in both duodenum and ileum. In this study, treated birds showed a lower incidence of pasty vent, both at 14 and 28 days of age, probably due to both a better utilization of nutrients and prevalence of healthy conditions in the gut. In general, the reduction of pasty vent incidence is observed in birds with improvement nutrients digestibility, particularly crude fat and protein [49].
In line with previous studies [50], Firmicutes and Proteobacteria were the most common phyla identified in caeca tested in our research. At finer taxonomic resolutions scale, the majority of sequences belonged to various members of Clostridia class. In the one-day old chicks the most represented bacteria genera were Lactobacillus, Clostridium, Blautia, Escherichia, Enterococcus, Eubacterium and Ruminococcus. This trend was partially observed also in the chickens at 41 days. However, at the end of the rearing period the most representative genera were Faecalibacterium, Subdonigranulum, Roseburia and Eubacterium. The presence of Clostridium-related species in the chicken caeca was observed by other authors [51–53]. Clostridium clusters IV (including Faecalibacterium prausnitzzi, Subdonigranulum variabile and Anaerotruncus colihominis) and XIVa (including Roseburia intestinalis and Ruminococcus torques) produce primarily butyrate [54]. Among these species, Faecalibacterium prausnitzzi and Subdonigranulum variabile were not significantly different between LA and CON (Table 7), whereas Roseburia intestinalis and Ruminococcus torques were significantly higher in LA (Fig 3). Differences in the abundance of Anaerotruncus colihominis between the two groups were not analyzed for its low abundance (Table 6). Faecalibacterium prausnitzzi has a requirement for acetate, and produces butyrate, formate and lactate [55]. Butyric acid has been shown to have an important function in protection against pathogens in poultry [56]. Furthermore, it is involved in several intestinal functions, being an energy source stimulating the epithelial cells proliferation and differentiation, other than exerting an antimicrobial effect by promoting the production of peptides and stimulating the production of tight junction protein [57]. Overall, the microbiological profiles identified in one-day old chicks, as well as CON and LA, partially confirm those reported by other authors, showing that the first days after hatching broiler caecum is colonized by facultative aerobes bacteria [58]. Oxygen consumption by those bacteria drives the lower gut environment to more reducing conditions, facilitating subsequent growth and colonization by extremely oxygen-sensitive obligate anaerobes [59–61].
The relative abundance of Lactobacillus acidophilus in the caeca of LA chickens was comparable with that of CON group. This result might be explained taking into account the colonization preference of the administered strain for the crop and the small intestine, even if this specific aspect was not investigated. Besides the lack of colonization of Lactobacillus acidophilus in broiler caeca, the results of this study suggest that the metabolic activity of supplemented Lactobacillus acidophilus positively affects the microbial species producing butyric acid by a cross feeding mechanism.
Ruminococcus torques was significantly higher in CON group in comparison to LA at 41 days. R. torques is known to degrade GI mucin [62], representing a carbon and energy source for intestinal microbiota. It has been estimated that 1% of colonic microbiota is able to degrade host mucin using enzymes (e.g. glycosidases and sulfatases) that can degrade the oligosaccharide chains [63, 64]. Moreover, degradation of mucin is regarded as a pathogenicity factor, since loss of the protective mucus layer may expose GI tract cells to pathogens [65]. Therefore, the higher abundance of R. torques in CON might be possibly related to the higher incidence of pasty vent in CON in comparison to LA group, but this hypothesis needs to be confirmed.
In relation to the metabolic functions, LA group showed a significantly higher level of β-glucosidase (Fig 6). This enzyme contributes to the hydrolysis of glucose monomers from non-starch polysaccharides (e.g., cellulose, β-glucans), playing an important role in the fermentation of undigested carbohydrates and, ultimately, in animal performance and health. In particular, β-glucosidase (β-glucoside glucohydrolase; EC3.2.1.21) hydrolyzes alkyl- and aryl-β-glucosides, as well as diglucosides and oligosaccharides, to release glucose and an aglycone [66]. It also hydrolyzes isoflavonal glycoside conjugates into isoflavone aglycones, such as genistein, daidzein, and glycitein. An increase of the concentrations of genistein and daidzein in soy milk has been reported by using strains of Streptococcus thermophilus, L. acidophilus, L. delbrueckii ssp. bulgaricus, L. casei, L. plantarum, L. fermentum and several Bifidobacterium species [67, 68]. These aglycones hydrolyzed by β-glucosidases from intestinal microorganisms are readily absorbed across the villi of the intestine [69], possess greater bioavailability than the corresponding glycoside conjugates [70] and a wide range of biological properties, such as antioxidant and anti-tumor activities [71, 72]. In broilers fed with 0.2% β-glucosidase, a significant increase in average daily weight gain (P<0.05) and feed conversion ratios (P<0.05) were observed in comparison to controls [73].
Conclusions
The supplementation with Lactobacillus acidophilus D2/CSL (CECT 4529) at the recommended dietary dosage of 1x109 cfu/kg feed in broiler chickens significantly improved body weight at 28 days (commercial weight of 1.5 kg) and feed conversion rate from 0 to 41 days. In addition, the incidence of pasty vent was reduced in LA birds. The relative abundance of Lactobacillus acidophilus in the caeca of LA chickens was comparable with that of CON group. However, a positive effect of the supplementation with Lactobacillus acidophilus was observed in relation to the metabolic functions in the treated group, with particular reference to the higher abundance of β-glucosidase, improving animal performances and health.
Acknowledgments
The authors express their appreciation to Dr. Stefano Pignata and Mr. Roberto Donatini of the Department of Agricultural and Food Sciences of the University of Bologna for their technical assistance.
Data Availability
The metagenomes of the 14 samples included in this study are publicly available from MG RAST (http://metagenomics.anl.gov/linkin.cgi?project=13081). IDs are included in the paper under the "Metagenomic results" section.
Funding Statement
The Centro Sperimentale del Latte S.r.l. provided support in the form of salary for Alberto Giardini, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dominguez-Bello MG, Blaser MJ. Do you have a probiotic in your future? Microbes and Infection. 2008;10(9):1072–6. 10.1016/j.micinf.2008.07.036. 10.1016/j.micinf.2008.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huyghebaert G, Ducatelle R, Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. The Veterinary Journal. 2011;187(2):182–8. 10.1016/j.tvjl.2010.03.003. 10.1016/j.tvjl.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 3.Tannock GW. A Special Fondness for Lactobacilli. Applied and Environmental Microbiology. 2004;70(6):3189–94. 10.1128/AEM.70.6.3189-3194.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R, Sun J, Mo H, Zhu Y. Analysis of functional properties of Lactobacillus acidophilus. World Journal of Microbiology and Biotechnology. 2007;23(2):195–200. [Google Scholar]
- 5.Tannock GW, Fuller R, Pedersen K. Lactobacillus succession in the piglet digestive tract demonstrated by plasmid profiling. Applied and Environmental Microbiology. 1990;56(5):1310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haghighi HR, Gong J, Gyles CL, Hayes MA, Sanei B, Parvizi P, et al. Modulation of antibody-mediated immune response by probiotics in chickens. Clinical and Diagnostic Laboratory Immunology. 2005;12(12):1387–92. 10.1128/CDLI.12.12.1387-1392.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souza MR, Moreira JL, Barbosa FHF, Cerqueira MMOP, Nunes ÁC, Nicoli JR. Influence of intensive and extensive breeding on lactic acid bacteria isolated from Gallus gallus domesticus ceca. Veterinary Microbiology. 2007;120(1–2):142–50. 10.1016/j.vetmic.2006.10.019. 10.1016/j.vetmic.2006.10.019 [DOI] [PubMed] [Google Scholar]
- 8.Nousiainen J, Javanainen P, Setälä J, Wright Av, Salminen S, von Wright A, et al. Lactic acid bacteria as animal probiotics In: Lactic acid bacteria: microbiology and functional aspects. 2004. Ed. 3; pp. 547–80. [Google Scholar]
- 9.Pineiro M, Stanton C. Probiotic bacteria: legislative framework—requirements to evidence basis. The Journal of nutrition. 2007;137(3):850S–3S. [DOI] [PubMed] [Google Scholar]
- 10.Fuller R. Probiotics in man and animals. Journal of Applied Bacteriology. 1989;66(5):365–78. [PubMed] [Google Scholar]
- 11.Patterson J, Burkholder K. Application of prebiotics and probiotics in poultry production. Poultry Science. 2003;82(4):627–31. [DOI] [PubMed] [Google Scholar]
- 12.Willis WL, Reid L. Investigating the effects of dietary probiotic feeding regimens on broiler chicken production and Campylobacter jejuni presence. Poultry Science. 2008;87(4):606–11. 10.3382/ps.2006-00458 [DOI] [PubMed] [Google Scholar]
- 13.Huang MK, Choi YJ, Houde R, Lee J-W, Lee B, Zhao X. Effects of Lactobacilli and an acidophilic fungus on the production performance and immune responses in broiler chickens. Poultry Science. 2004;83(5):788–95. [DOI] [PubMed] [Google Scholar]
- 14.Jin LZ, Ho YW, Abdullah N, Jalaludin S. Growth performance, intestinal microbial populations, and serum cholesterol of broilers fed diets containing Lactobacillus cultures. Poultry Science. 1998;77(9):1259–65. [DOI] [PubMed] [Google Scholar]
- 15.Angelakis E, Raoult D. The increase of lactobacillus species in the gut flora of newborn broiler chicks and ducks is associated with weight gain. PLoS ONE. 2010;5(5):e10463 10.1371/journal.pone.0010463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mountzouris KC, Tsirtsikos P, Kalamara E, Nitsch S, Schatzmayr G, Fegeros K. Evaluation of the efficacy of a probiotic containing Lactobacillus, Bifidobacterium, Enterococcus, and Pediococcus strains in promoting broiler performance and modulating cecal microflora composition and metabolic activities. Poultry Science. 2007;86(2):309–17. [DOI] [PubMed] [Google Scholar]
- 17.FAO/WHO. Working group report on drafting guidelines for the evaluation of probiotics in food. London, Ontario, Canada. 2002.
- 18.Gallazzi D, Giardini A, Mangiagalli GM, Marelli S, Ferrazzi V, Orsi C, et al. Effects of Lactobacillus acidophilus D2/CSL on laying hen performance. Italian Journal of Animal Science. 2008;7(1):27–37. [Google Scholar]
- 19.Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Animal Biotechnology. 2002;13(1):29–42. 10.1081/ABIO-120005768 [DOI] [PubMed] [Google Scholar]
- 20.Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J. Metagenomic pyrosequencing and microbial identification. Clinical chemistry. 2009;55(5):856–66. 10.1373/clinchem.2008.107565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Commission European. Council Directive 43/EC of 28 June 2007, laying down minimum rules for the protection of chickens kept for meat production. Official Journal of the European Union L. 2007;182:19–28. [Google Scholar]
- 22.Bianchi Salvadori B, Camaschella P, Lavezzari D. Les lactobacilles specifiques du poulet. Leur influence sur la microflore du tube digestif Microbiologie Aliment Nutrition. 1985;3:73–82. [Google Scholar]
- 23.Meluzzi A, Fabbri C, Folegatti E, Sirri F. Effect of less intensive rearing conditions on litter characteristics, growth performance, carcase injuries and meat quality of broilers. British Poultry Science. 2008;49(5):509–15. 10.1080/00071660802290424 [DOI] [PubMed] [Google Scholar]
- 24.Ekstrand C, Carpenter TE, Andersson I, Algers B. Prevalence and control of foot-pad dermatitis in broilers in Sweden. British Poultry Science. 1998;39(3):318–24. 10.1080/00071669888845 [DOI] [PubMed] [Google Scholar]
- 25.Danzeisen JL, Kim HB, Isaacson RE, Tu ZJ, Johnson TJ. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE. 2011;6(11):e27949 10.1371/journal.pone.0027949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer F, Paarmann D, D'Souza M, Olson R, Glass E, Kubal M, et al. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics. 2008;9(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35(21):7188–96. 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Applied and Environmental Microbiology. 2006;72(7):5069–72. 10.1128/AEM.03006-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, et al. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Research. 2003;31(1):442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M. The KEGG database. silico simulation of biological processes. 2002;247:91–103. [Google Scholar]
- 31.SAS Institute. SAS/STAT user's guide, release 6. SAS Institute Inc Cary, NC; 1988. [Google Scholar]
- 32.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–4. 10.1093/bioinformatics/btu494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brisbin JT, Gong J, Orouji S, Esufali J, Mallick AI, Parvizi P, et al. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clinical and Vaccine Immunology. 2011;18(9):1447–55. 10.1128/CVI.05100-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JS, Ingale SL, Kim YW, Kim KH, Sen S, Ryu MH, et al. Effect of supplementation of multi-microbe probiotic product on growth performance, apparent digestibility, cecal microbiota and small intestinal morphology of broilers. Journal of Animal Physiology and Animal Nutrition. 2012;96(4):618–26. 10.1111/j.1439-0396.2011.01187.x [DOI] [PubMed] [Google Scholar]
- 35.Askelson TE, Campasino A, Lee JT, Duong T. Evaluation of phytate-degrading Lactobacillus culture administration to broiler chickens. Applied and Environmental Microbiology. 2014;80(3):943–50. 10.1128/AEM.03155-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loh TC, Thanh NT, Foo HL, Hair-Bejo M, Azhar BK. Feeding of different levels of metabolite combinations produced by Lactobacillus plantarum on growth performance, fecal microflora, volatile fatty acids and villi height in broilers. Animal Science Journal. 2010;81(2):205–14. 10.1111/j.1740-0929.2009.00701.x [DOI] [PubMed] [Google Scholar]
- 37.Shim YH, Ingale SL, Kim JS, Kim KH, Seo DK, Lee SC, et al. A multi-microbe probiotic formulation processed at low and high drying temperatures: effects on growth performance, nutrient retention and caecal microbiology of broilers. British Poultry Science. 2012;53(4):482–90. 10.1080/00071668.2012.690508 [DOI] [PubMed] [Google Scholar]
- 38.Ghareeb K, Awad WA, Mohnl M, Porta R, Biarnés M, Böhm J, et al. Evaluating the efficacy of an avian-specific probiotic to reduce the colonization of Campylobacter jejuni in broiler chickens. Poultry Science. 2012;91(8):1825–32. 10.3382/ps.2012-02168 [DOI] [PubMed] [Google Scholar]
- 39.Neal-McKinney JM, Lu X, Duong T, Larson CL, Call DR, Shah DH, et al. Production of organic acids by probiotic lactobacilli can be used to reduce pathogen load in poultry. PLoS ONE. 2012;7(9):e43928 10.1371/journal.pone.0043928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.La Ragione RM, Narbad A, Gasson MJ, Woodward MJ. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Letters in Applied Microbiology. 2004;38(3):197–205. [DOI] [PubMed] [Google Scholar]
- 41.Chen C-Y, Tsen H-Y, Lin C-L, Yu B, Chen C-S. Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poultry Science. 2012;91(9):2139–47. 10.3382/ps.2012-02237 [DOI] [PubMed] [Google Scholar]
- 42.Bouzaine T, Dauphin RD, Thonart P, Urdaci MC, Hamdi M. Adherence and colonization properties of Lactobacillus rhamnosus TB1, a broiler chicken isolate. Letters in Applied Microbiology. 2005;40(5):391–6. 10.1111/j.1472-765X.2005.01684.x [DOI] [PubMed] [Google Scholar]
- 43.Edelman S, Westerlund-Wikström B, Leskelä S, Kettunen H, Rautonen N, Apajalahti J, et al. In vitro adhesion specificity of indigenous Lactobacilli within the avian intestinal tract. Applied and Environmental Microbiology. 2002;68(10):5155–9. 10.1128/AEM.68.10.5155-5159.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jin LZ, Ho YW, Ali MA, Abdullah N, Ong KB, Jalaludin S. Adhesion of Lactobacillus isolates to intestinal epithelial cells of chicken. Letters in Applied Microbiology. 1996;22(3):229–32. [DOI] [PubMed] [Google Scholar]
- 45.Kalavathy R, Abdullah N, Jalaludin S, Ho YW. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. British Poultry Science. 2003;44(1):139–44. 10.1080/0007166031000085445 [DOI] [PubMed] [Google Scholar]
- 46.Timmerman HM, Veldman A, van den Elsen E, Rombouts FM, Beynen AC. Mortality and growth performance of broilers given drinking water supplemented with chicken-specific probiotics. Poultry Science. 2006;85(8):1383–8. [DOI] [PubMed] [Google Scholar]
- 47.Zulkifli I, Abdullah N, Azrin NM, Ho YW. Growth performance and immune response of two commercial broiler strains fed diets containing Lactobacillus cultures and oxytetracycline under heat stress conditions. British Poultry Science. 2000;41(5):593–7. 10.1080/713654979 [DOI] [PubMed] [Google Scholar]
- 48.Awad WA, Ghareeb K, Abdel-Raheem S, Böhm J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poultry Science. 2009;88(1):49–56. 10.3382/ps.2008-00244 [DOI] [PubMed] [Google Scholar]
- 49.Roy A, Haldar S, Mondal S, Ghosh TK. Effects of supplemental exogenous emulsifier on performance, nutrient metabolism, and serum lipid profile in broiler chickens. Veterinary Medicine International. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oakley BB, Morales CA, Line J, Berrang ME, Meinersmann RJ, Tillman GE, et al. The poultry-associated microbiome: network analysis and farm-to-fork characterizations. PLoS ONE. 2013;8(2):e57190 10.1371/journal.pone.0057190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bjerrum L, Engberg RM, Leser TD, Jensen BB, Finster K, Pedersen K. Microbial community composition of the ileum and cecum of broiler chickens as revealed by molecular and culture-based techniques. Poultry Science. 2006;85(7):1151–64. [DOI] [PubMed] [Google Scholar]
- 52.Gong J, Si W, Forster RJ, Huang R, Yu H, Yin Y, et al. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: from crops to ceca. FEMS Microbiology Ecology. 2007;59(1):147–57. 10.1111/j.1574-6941.2006.00193.x [DOI] [PubMed] [Google Scholar]
- 53.Lund M, Bjerrum L, Pedersen K. Quantification of Faecalibacterium prausnitzii- and Subdoligranulum variabile-like bacteria in the cecum of chickens by real-time PCR. Poultry Science. 2010;89(6):1217–24. 10.3382/ps.2010-00653 [DOI] [PubMed] [Google Scholar]
- 54.Van den Abbeele P, Grootaert C, Marzorati M, Possemiers S, Verstraete W, Gérard P, et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium Cluster IX. Applied and Environmental Microbiology. 2010;76(15):5237–46. 10.1128/AEM.00759-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scupham AJ. Succession in the intestinal microbiota of preadolescent turkeys. FEMS Microbiology Ecology. 2007;60(1):136–47. 10.1111/j.1574-6941.2006.00245.x [DOI] [PubMed] [Google Scholar]
- 56.Fernández-Rubio C, Ordóñez C, Abad-González J, Garcia-Gallego A, Honrubia MP, Mallo JJ, et al. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poultry Science. 2009;88(5):943–8. 10.3382/ps.2008-00484 [DOI] [PubMed] [Google Scholar]
- 57.Dalmasso G, Nguyen HTT, Yan Y, Charrier-Hisamuddin L, Sitaraman SV, Merlin D. Butyrate transcriptionally enhances peptide transporter PepT1 expression and activity. PLoS ONE. 2008;3(6):e2476 10.1371/journal.pone.0002476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gong J, Yu H, Liu T, Gill JJ, Chambers JR, Wheatcroft R, et al. Effects of zinc bacitracin, bird age and access to range on bacterial microbiota in the ileum and caeca of broiler chickens. Journal of Applied Microbiology. 2008;104(5):1372–82. 10.1111/j.1365-2672.2007.03699.x [DOI] [PubMed] [Google Scholar]
- 59.Barnes EM, Mead GC, Barnuml DA, Harry EG. The intestinal flora of the chicken in the period 2 to 6 weeks of age, with particular reference to the anaerobic bacteria. British Poultry Science. 1972;13(3):311–26. 10.1080/00071667208415953 [DOI] [PubMed] [Google Scholar]
- 60.Gong J, Forster RJ, Yu H, Chambers JR, Sabour PM, Wheatcroft R, et al. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiology Letters. 2002;208(1):1–7. [DOI] [PubMed] [Google Scholar]
- 61.Wise MG, Siragusa GR. Quantitative analysis of the intestinal bacterial community in one- to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. Journal of Applied Microbiology. 2007;102(4):1138–49. 10.1111/j.1365-2672.2006.03153.x [DOI] [PubMed] [Google Scholar]
- 62.Kenneth HW, Ikeda JS, Rhonda BB. Phylogenetic placement of community members of human colonic biota. Clinical Infectious Diseases. 1997;25:S114–S6. [DOI] [PubMed] [Google Scholar]
- 63.Hoskins LC, Boulding ET. Mucin degradation in human colon ecosystems: evidence for the existence and role of bacterial subpopulations producing glycosidases as extracellular enzymes. Journal of Clinical Investigation. 1981;67(1):163–72. 10.1172/JCI110009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International Journal of Systematic and Evolutionary Microbiology. 2004;54(5):1469–76. [DOI] [PubMed] [Google Scholar]
- 65.Ruseler-van Embden JGH, van Lieshout LMC, Gosselink MJ, Marteau P. Inability of Lactobacillus casei Strain GG, L. acidophilus, and Bifidobacterium bifidum to degrade intestinal mucus glycoproteins. Scandinavian Journal of Gastroenterology. 1995;30(7):675–80. [DOI] [PubMed] [Google Scholar]
- 66.Reese ET. Degradation of polymeric carbohydrates by microbial enzymes In: Loewus FA, Runeckles VC, editors. The structure, biosynthesis, and degradation of wood. Boston, MA: Springer US; 1977. pp. 311–67. [Google Scholar]
- 67.Donkor ON, Shah NP. Production of β‐glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soymilk. Journal of Food Science. 2008;73(1):M15–M20. 10.1111/j.1750-3841.2007.00547.x [DOI] [PubMed] [Google Scholar]
- 68.Rekha CR, Vijayalakshmi G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. International Journal of Food Sciences and Nutrition. 2011;62(2):111–20. 10.3109/09637486.2010.513680 [DOI] [PubMed] [Google Scholar]
- 69.Ismail B, Hayes K. β-glycosidase activity toward different glycosidic forms of isoflavones. Journal of Agricultural and Food Chemistry. 2005;53(12):4918–24. 10.1021/jf0404694 [DOI] [PubMed] [Google Scholar]
- 70.Izumi T, Piskula MK, Osawa S, Obata A, Tobe K, Saito M, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. The Journal of Nutrition. 2000;130(7):1695–9. [DOI] [PubMed] [Google Scholar]
- 71.Brouns F. Soya isoflavones: a new and promising ingredient for the health foods sector. Food Research International. 2002;35(2–3):187–93. 10.1016/S0963-9969(01)00182-X. [DOI] [Google Scholar]
- 72.Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19(12):2151–8. [DOI] [PubMed] [Google Scholar]
- 73.Qian L, Sun J. Effect of β-glucosidase as a feed supplementary on the growth performance, digestive enzymes and physiology of broilers. Asian-Australasian Journal of Animal Sciences. 2009;22(2):260–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The metagenomes of the 14 samples included in this study are publicly available from MG RAST (http://metagenomics.anl.gov/linkin.cgi?project=13081). IDs are included in the paper under the "Metagenomic results" section.