Abstract
In ovarian cancer, it has been shown that E-cadherin is down-regulated by epidermal growth factor (EGF) receptor (EGFR) activation, and that cells with low E-cadherin expression are particularly invasive. Although it is generally believed that reactive oxygen species play important roles in intracellular signal transduction, the role of reactive oxygen species in EGF-mediated reductions in E-cadherin remains to be elucidated. In this study, we show that EGF treatment down-regulated E-cadherin by up-regulating its transcriptional repressors, Snail and Slug, in human ovarian cancer cells. Using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester staining, we found that intracellular hydrogen peroxide (H2O2) production was increased in EGF-treated cells and could be inhibited by treatment with an EGFR inhibitor, AG1478, or an H2O2 scavenger, polyethylene glycol (PEG)-catalase. In addition, PEG-catalase diminished EGF-induced p38 MAPK, but not ERK1/2 or c-Jun N-terminal kinase, phosphorylation. PEG-catalase and the p38 MAPK inhibitor SB203580 abolished EGF-induced Snail, but not Slug, expression and E-cadherin down-regulation. Furthermore, the involvement of p38 MAPK in the down-regulation of E-cadherin was confirmed using specific p38α MAPK small interfering RNA. Finally, we also show that EGF-induced cell invasion was abolished by treatment with PEG-catalase and SB203580, as well as p38α MAPK small interfering RNA, and that forced expression of E-cadherin diminished intrinsic invasiveness as well as EGF-induced cell invasion. This study demonstrates a novel mechanism in which EGF down-regulates E-cadherin expression through production of H2O2, activation of p38 MAPK, and up-regulation of Snail in human ovarian cancer cells.
EGF increases ovarian cancer cell invasion by downregulating E-cadherin expression through production of H2O2, activation of p38 MAPK and upregulation of Snail.
Epithelial ovarian cancer is the fifth leading cause of cancer-related deaths among women in developed countries. Most deaths from ovarian cancer are due to metastases that are resistant to conventional therapies. Although ovarian cancer has been recognized to metastasize primarily by exfoliation followed by peritoneal implantation, approximately 40% of patients with advanced ovarian cancer show lymph node metastasis and/or extraabdominal metastasis. Accumulating evidence suggests a role for epidermal growth factor receptor (EGFR) in tumor metastasis (1). Overexpression of EGFR is found in many types of cancer, including ovarian cancer, and is associated with more aggressive clinical behavior and poor prognosis (2, 3, 4). EGFR signaling is known to regulate cell adhesion, motility, proliferation, and invasion in many types of cancer (5, 6).
Reactive oxygen species (ROS) such as superoxide (O2−), hydroxyl radical (·OH) and hydrogen peroxide (H2O2) are constantly generated in aerobic organisms during intracellular metabolism and in response to environmental stimuli. The generation of ROS by ligand-receptor interactions results in the activation/inhibition of signaling molecules, and therefore ROS can be considered as second messengers in signal transduction. Indeed, accumulating evidence suggests that ROS play important roles in intracellular signal transduction, thereby regulating a variety of cellular functions including cell cycle progression, apoptosis, and motility (7, 8, 9). Recently, the involvement of ROS in tumor progression has been highlighted (10). The predominant ROS produced upon EGF stimulation appears to be H2O2 (11, 12), and the elimination of H2O2 by incorporation of catalase has been shown to inhibit EGF-induced tyrosine phosphorylation of various signaling proteins, including the EGF receptor itself (11).
Development of malignant tumors, in particular the transition from benign lesions to invasive metastatic cancer, is characterized by the ability of tumor cells to overcome cell-cell adhesion and invade surrounding tissue. E-cadherin, the prototypical member of the classical cadherin family, is localized to the surface of epithelial cells in regions of cell-cell contact known as “adherens junctions” and maintains cell polarity and normal epithelial structure (13, 14). Aberrant epithelial differentiation is an early event in epithelial ovarian carcinogenesis; thus, in contrast to most carcinomas that lose E-cadherin expression with progression, E-cadherin is abundant in primary differentiated ovarian carcinomas. Although complete loss of E-cadherin expression is uncommon, reduced E-cadherin staining is often detected in late-stage ovarian cancer and in ascites-derived tumor cells (15). Down-regulation of E-cadherin expression can be achieved by transcriptional suppression mediated by members of the basic helix-loop-helix family, in particular by Snail, Slug, and Twist (16). Ovarian cancer cells with low E-cadherin expression are more invasive (17), and the absence of E-cadherin expression in ovarian cancers predicts poor patient survival as compared with ovarian tumors that express E-cadherin (18). Several studies have demonstrated that reestablishing the expression of E-cadherin results in a reversion from an invasive to a benign epithelial tumor cell phenotype (19, 20).
In addition to the effects of EGFR on tumor cell growth and survival, EGFR also influences tumor metastasis in ovarian cancer (21, 22). Although it has been reported that manipulation of EGFR can regulate E-cadherin protein expression and complex formation in ovarian cancer cells, controversy exists with regard to the exact nature of this relationship (23, 24). Some studies have shown that EGF treatment down-regulates E-cadherin expression in ovarian cancer cells (25); however, the underlying mechanisms mediating this response are not well understood. In the current study we tested the hypothesis that H2O2 mediates EGF-induced suppression of E-cadherin expression in ovarian cancer cells. Our results indicate that EGF treatment down-regulates E-cadherin expression and increases invasiveness in SKOV3 ovarian cancer cells. Furthermore, the effects of EGF on E-cadherin are mediated by H2O2 through activation of p38 MAPK and increased Snail expression.
Results
EGF treatment down-regulates E-cadherin expression in SKOV3 cells
We have previously shown that treatment with EGF increases cell invasion in two ovarian cancer cell lines, SKOV3 and OVCAR3 (26). As shown in Fig. 1A, both ovarian cancer cell lines express EGFR, although SKOV3 cells have a higher expression level. To characterize the effect of EGF on cell morphology, we treated cells with EGF (100 ng/ml) for 24 or 48 h. Treatment with EGF induced a morphological change in SKOV3 cells, from a cobblestone-like morphology to fibroblastic-spindle shape, whereas EGF did not alter cell morphology in OVCAR3 cells (Fig. 1B). Western blot analysis performed on total cell lysates collected from cells treated with EGF for 24 and 48 h showed that EGF down-regulated total E-cadherin protein levels in SKOV3 cells but not in OVCAR3 cells (Fig. 1C). These findings were confirmed by immunofluorescent staining of E-cadherin (Fig. 1D). In addition to its proinvasive effects in OVCAR3 cells (26), EGF also induces EGFR phosphorylation and MAPK activation (data not shown), which suggests that the EGFR in OVCAR3 cells is functional. To further investigate the molecular mechanism by which EGF down-regulates E-cadherin expression, we chose SKOV3 cells as an in vitro model. Figure 2 shows that EGF treatment down-regulated E-cadherin mRNA (Fig. 2A) and protein (Fig. 2B) levels in a dose- and time-dependent manner. Similar results have been obtained in another ovarian cancer cell line, OVCAR5 (our unpublished data). Moreover, the inhibitory effect of EGF on E-cadherin mRNA and protein levels was eliminated by treatment with an EGFR inhibitor, AG1478 (Fig. 2C).
Fig. 1.
EGF induces morphological changes and down-regulates E-cadherin in SKOV3 ovarian cancer cells. A, Endogenous levels of EGFR were examined in SKOV3 and OVCAR3 cells by Western blot. B, Cells were treated without (control; Ctrl) or with 100 ng/ml EGF for 24 or 48 h, and the resultant morphology was microscopically examined. Scale bar, 200 μm. C, Cells were treated with 100 ng/ml EGF for 24 or 48 h, and total cellular levels of E-cadherin were analyzed by Western blot. D, Cells were treated with 100 ng/ml EGF for 48 h and fixed in cold methanol, and E-cadherin was examined by immunofluorescence staining. Scale bar, 50 μm.
Fig. 2.

EGF down-regulates E-cadherin in a dose- and time-dependent manner in SKOV3 cells. SKOV3 cells were treated with increasing doses of EGF for 24 h or with 100 ng/ml EGF for different durations. A and B, E-cadherin mRNA (A) and protein (B) levels were analyzed by RT-qPCR and Western blot, respectively. C, Cells were treated with EGFR inhibitor, AG1478 (AG, 10 μm), in the presence or absence of 100 ng/ml EGF for 24 h, and the levels of E-cadherin mRNA (top panel) and protein (bottom panel) were analyzed. Results are expressed as mean ± sem of at least three independent experiments. *, P < 0.05 compared with time-matched control (Ctrl). #, P < 0.05 compared with EGF.
EGF induces intracellular H2O2 production in SKOV3 cells
The H2O2-specific fluorescent dye, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) was used to examine the effect of EGF treatment on intracellular ROS production. EGF treatment induced H2O2 production in a time-dependent manner in SKOV3 cells, whereas it had little effect on H2O2 production in OVCAR3 cells (Fig. 3A). To confirm the specificity of H2O2 production, cells were treated with the EGFR inhibitor AG1478 or catalase, a specific H2O2 scavenger. EGF-stimulated H2O2 production was eliminated by treatment with AG1478 (Fig. 3B). In addition, treatment with cell-permeable polyethylene glycol (PEG)-catalase abolished EGF-induced H2O2 production (Fig. 3B). These results suggest that EGF-EGFR signaling is coupled to H2O2 production in SKOV3 cells.
Fig. 3.

EGF induces intracellular H2O2 production. A, SKOV3 and OVCAR3 cells were serum starved for 24 h and treated with or without 100 ng/ml EGF. At the indicated time points, cells were washed twice with PBS and fixed with 10% formaldehyde. Intracellular H2O2 levels were measured by staining with CM-H2DCFDA (5 μm), and images were captured using a fluorescence microscope (top panel). The corresponding phase-contrast micrographs are shown in the bottom panel. B, SKOV3 cells were pretreated with 10 μm AG1478 (AG) or the H2O2 scavenger PEG-catalase (Cat) (1000 U/ml) for 30 min, followed by treatment with 100 ng/ml EGF for 30 min. Intracellular H2O2 levels were measured as described above. Scale bar, 200 μm.
H2O2 production is required for EGF-induced down-regulation of E-cadherin
To determine whether H2O2 production is involved in the suppression of E-cadherin expression by EGF, cells were pretreated with PEG-catalase (500 and 1000 U/ml) followed by treatment with EGF for 24 h. As shown in Fig. 4A, PEG-catalase inhibited the morphological effects of EGF in SKOV3 cells. Reverse transcription quantitative real-time PCR (RT-qPCR) and Western blot results showed that PEG-catalase treatment diminished the inhibitory effects of EGF on E-cadherin mRNA (Fig. 4B) and protein levels (Fig. 4C). Treatment with PEG-catalase alone did not alter E-cadherin mRNA or protein levels. To further confirm the effect of H2O2 on E-cadherin expression, SKOV3 and OVCAR3 cells were treated with exogenous H2O2. Interestingly, H2O2 treatment down-regulated E-cadherin protein levels in a dose-dependent manner in both cell lines (Fig. 4D). These results suggest that the lack of effect of EGF on E-cadherin in OVCAR3 cells reflects an uncoupling of EGFR activation from H2O2 production rather than a lack of effect of H2O2 on E-cadherin.
Fig. 4.

EGF down-regulates E-cadherin expression via H2O2 production. A, SKOV3 cells were treated for 24 h with PEG-catalase (cat) (500 and 1000 U/ml) in the presence or absence of 100 ng/ml EGF, and the resultant morphology was microscopically assessed. Scale bar, 200 μm. B and C, E-cadherin mRNA (B) and protein (C) levels were analyzed by RT-qPCR and Western blot, respectively. D, SKOV3 and OVCAR3 cells were treated with different doses of H2O2 for 24 h, and E-cadherin protein levels were examined by Western blot. Results are expressed as mean ± sem of at least three independent experiments. *, P < 0.05 compared with control (Ctrl). #, P < 0.05 compared with EGF.
Snail expression is up-regulated by EGF-induced H2O2 production
To investigate whether EGF down-regulates E-cadherin expression by modulating the transcriptional regulation of E-cadherin, we used RT-qPCR to examine the mRNA levels of E-cadherin transcriptional repressors, Snail and Slug. Treatment with EGF significantly increased Snail and Slug mRNA levels in a time-dependent manner (Fig. 5A). Treatment with the EGFR inhibitor AG1478 abolished the effects of EGF on Snail and Slug mRNA levels (Fig. 5B). To determine whether H2O2 production is involved in EGF-induced increases in Snail and Slug mRNA, cells were treated with PEG-catalase in the presence or absence of EGF. As shown in Fig. 5C, PEG-catalase treatment diminished EGF-stimulated Snail mRNA levels, whereas increases in Slug mRNA levels were not affected. Interestingly, exogenous H2O2 increased both Snail and Slug mRNA levels; however the degree and pattern of elevation were different from that induced by EGF (Fig. 5D).
Fig. 5.
EGF induces Snail expression at the transcriptional level through H2O2 production. A, SKOV3 cells were treated with 100 ng/ml EGF for various times, and the mRNA levels of Snail (left panel) and Slug (right panel) were analyzed by RT-qPCR. B, SKOV3 cells were treated with AG1478 (AG) (10 μm) in the presence or absence of 100 ng/ml EGF for 3 h (Snail) or 6 h (Slug), and mRNA levels were analyzed by RT-qPCR. C, SKOV3 cells were treated with PEG-catalase (Cat) (1000 U/ml) in the presence or absence of 100 ng/ml EGF for 3 h (Snail) or 6 h (Slug), and mRNA levels were analyzed by RT-qPCR. Note that PEG-catalase inhibits only EGF-induced Snail mRNA levels. D, SKOV3 cells were treated with H2O2 for different durations, and mRNA levels of Snail and Slug were examined by RT-qPCR. Results are expressed as mean ± sem of at least three independent experiments. *, P < 0.05 compared with control (Ctrl). #, P < 0.05 compared with EGF.
H2O2 production and p38 MAPK activation are involved in EGF-induced down-regulation of E-cadherin
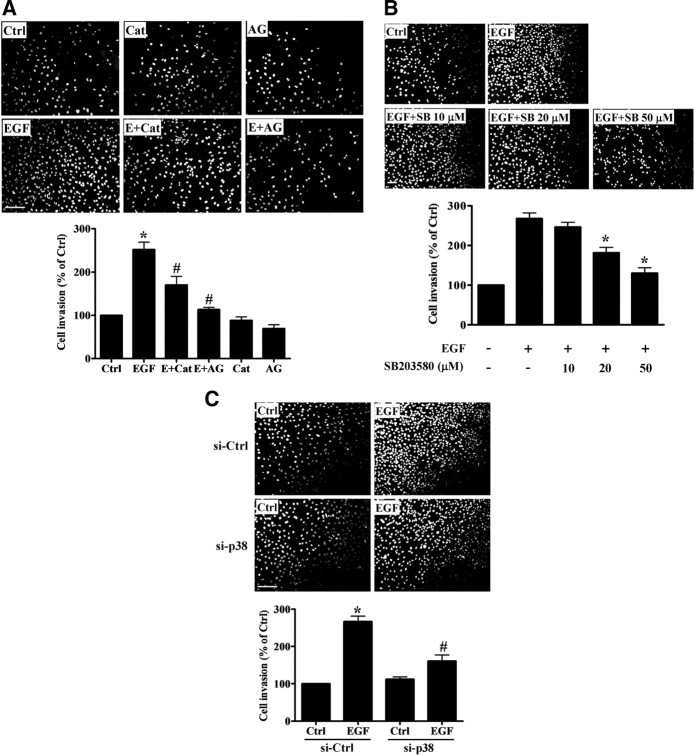
MAPK pathways, including ERK, p38, and c-Jun N-terminal kinase (JNK), are important downstream signaling cascades involved in tumor invasion (7). EGF treatment induced the phosphorylation of ERK1/2, p38, and JNK in a time-dependent manner in SKOV3 cells (Fig. 6A). These effects of EGF could be blocked by the EGFR inhibitor AG1478 (Fig. 6B). To assess whether EGF-induced H2O2 production is involved in MAPK activation, cells were treated with both EGF and PEG-catalase. Interestingly, we found that PEG-catalase diminished EGF-induced p38 MAPK phosphorylation but did not affect EGF-induced phosphorylation of ERK1/2 or JNK (Fig. 6B). Furthermore, treatment with the p38 MAPK inhibitor SB203580 diminished the inhibitory effects of EGF on E-cadherin mRNA and protein levels (Fig. 7A), as well as EGF-stimulated Snail mRNA levels (Fig. 7B).
Fig. 6.

EGF activates p38 MAPK through H2O2 production. A, SKOV-3 cells were treated with 100 ng/ml EGF for the indicated durations. Phosphorylation of ERK1/2, p38, and JNK were determined by Western blot using antibodies specific for phosphorylated, activated forms of ERK1/2 (p-ERK1/2), p38 (p-p38), and JNK (p-JNK). Membranes were stripped and reprobed with antibodies to total ERK1/2, p38, and JNK. B, SKOV3 cells were treated with AG1478 (AG) (10 μm), PEG-catalase (Cat) (1000 U/ml), or selective MAPK pathway inhibitors, PD98059 (PD) (50 μm), SB203580 (SB) (50 μm), and SP600125 (SP) (50 μm) in the presence or absence of 100 ng/ml EGF for 30 min. Phosphorylation of ERK1/2, p38, and JNK were analyzed by Western blot. Ctrl., Control.
Fig. 7.
EGF down-regulates E-cadherin expression through p38 MAPK activation. A, SKOV3 cells were treated for 24 h with SB203580 (SB) (50 μm) in the presence or absence of 100 ng/ml EGF. E-cadherin mRNA (top panel) and protein (bottom panel) levels were analyzed by RT-qPCR and Western blot, respectively. B, Cells were treated for 3 h with SB203580 (50 μm) in the presence or absence of 100 ng/ml EGF, and Snail mRNA levels were analyzed by RT-qPCR. C, Cells were transfected with p38α MAPK siRNA for 48 h and then treated with 100 ng/ml EGF for 24 h. E-cadherin and p38 MAPK protein levels were analyzed by Western blot. Results are expressed as mean ± sem of at least three independent experiments. *, †, P < 0.05 compared with control (Ctrl); #, P < 0.05 compared with EGF.
There are four genes that encode p38 MAPKs including MAPK14 (which encodes p38α), MAPK11 (which encodes p38β), MAPK12 (which encodes p38γ), and MAPK13 (which encodes p38δ). Among them, p38α is highly abundant in most cell types whereas p38β seems to be expressed at very low levels, and its contribution to p38 MAPK signaling is not clear. p38γ and p38δ have more restricted expression patterns and are likely to have specialized functions (27). Thus, to avoid the nonspecific effects of pharmacological inhibition, we used specific small interfering RNA (siRNA) to knock down the endogenous expression of p38α MAPK, which is likely to be the most abundant, SB203580-sensitive isoform of p38 MAPK in our cells. As shown in Fig. 7C, treatment with p38α MAPK siRNA significantly reduced total p38 MAPK levels and abolished the EGF-induced down-regulation of E-cadherin. These results demonstrate that H2O2 production and p38α MAPK activation are involved in EGF-induced down-regulation of E-cadherin expression in SKOV3 cells.
Increased H2O2 production, activation of p38α MAPK, and loss of E-cadherin are involved in EGF-induced cell invasion
To investigate whether H2O2 production, p38 MAPK activation, and the subsequent loss of E-cadherin are involved in EGF-induced cell invasion, we performed an invasion assay with Boyden chambers coated with Matrigel. Consistent with previous studies, EGF treatment induced a significant increase in cell invasion. EGF-induced cell invasion was blocked by AG1478 treatment and diminished by treatment with PEG-catalase (Fig. 8A). The p38 MAPK inhibitor SB203508 reduced EGF-induced cell invasion in a dose-dependent manner (Fig. 8B). Similarly, EGF-induced cell invasion was diminished in cells treated with p38α MAPK siRNA (Fig. 8C). To confirm the requirement for E-cadherin down-regulation in EGF-stimulated cell invasion, E-cadherin was transiently overexpressed in SKOV3 cells, and cell invasiveness was examined (Fig. 9). Having demonstrated that EGF down-regulates endogenous E-cadherin, a mouse E-cadherin construct lacking regulatory elements was employed to avoid these effects. Western blot analysis showed that cells transfected with E-cadherin expressed more E-cadherin whereas cells transfected with empty vector did not (Fig. 9A). Importantly, EGF treatment reduced E-cadherin protein levels in control cells and cells transfected with empty vector, whereas no effect was detected in cells overexpressing E-cadherin (Fig. 9A). The invasion assay showed that basal invasiveness was decreased and EGF-induced cell invasion was reduced, but still detectable, in the transfected cells overexpressing E-cadherin (Fig. 9B). Taken together, these results indicated that endogenous E-cadherin plays an important regulatory role in cell invasion, and that EGF-induced cell invasion is mediated, at least in part, by H2O2 production, p38α MAPK activation, and the down-regulation of E-cadherin expression.
Fig. 8.
EGF-induced cell invasion involves H2O2 production and p38α MAPK expression. A, SKOV3 cells were treated for 1 h with AG1478 (AG) (10 μm) or PEG-catalase (Cat) (1000 U/ml) in the presence or absence of 100 ng/mL EGF. B, Cells were treated with 100 ng/ml EGF in combination with different doses of SB203580 (SB). C, Cells were transfected with p38α MAPK siRNA for 48 h and then treated with 100 ng/ml EGF. After treatment, cells were seeded into Matrigel-coated transwell inserts and cultured for 24 h. Noninvading cells were wiped from the upper side of the filter, and the nuclei of invading cells were stained with Hoechst 33258. Top panels show representative photos of the invasion assay. Scale bar, 200 μm. Bottom panels show summarized quantitative results, which are expressed as mean ± sem of at least three independent experiments. *, P < 0.05 compared with control (Ctrl); #, P < 0.05 compared with EGF.
Fig. 9.
Enhanced expression of E-cadherin diminishes EGF-induced cell invasion. A, SKOV3 cells were transfected for 48 h with control vector (pIRES) or vector containing full-length E-cadherin cDNA (pIRES-EC). After transfection, cells were treated with 100 ng/ml EGF for 24 h, and E-cadherin protein levels were analyzed by Western blot. B, After 48 h of transfection, cells were treated with 100 ng/ml EGF, seeded into Matrigel-coated transwell inserts, and cultured for 24 h. Noninvading cells were wiped from the upper side of the filter, and the nuclei of invading cells were stained with Hoechst 33258. Top panel shows representative photos of the invasion assay. Scale bar, 200 μm. Bottom panel shows summarized quantitative results, which are expressed as mean ± sem of at least three independent experiments. *, P < 0.05 compared with control (Ctrl); #, P < 0.05 compared with EGF. †, P < 0.05 compared with pIRES-EC Ctrl.
Discussion
Metastasis is a major obstacle for cancer therapy and is a primary cause of mortality in many cancers, including that of the ovary. Most deaths from ovarian cancer are due to metastasis characterized by widespread ip dissemination. EGF is known to play a predominant role in ovarian cancer by directly stimulating invasiveness and enhancing the metastatic capability of cancer cells (21, 22). EGF has been shown to down-regulate E-cadherin expression in human cancer cells, resulting in tumorigenesis and metastasis (25, 28). Moreover, studies show that ROS are essential mediators of EGF-induced cell migration and invasion (29, 30). However, the exact role of H2O2 in the down-regulation of E-cadherin expression caused by EGF signaling remains to be elucidated. The current study was thus undertaken to examine the regulatory relationships between EGF, H2O2 , and E-cadherin in ovarian cancer cells. We report that H2O2 mediates EGF-induced down-regulation of E-cadherin expression and is involved in EGF-induced cell invasion. In addition, our studies suggest that H2O2 exerts its effects via the activation of p38α MAPK and increased expression of Snail.
To date, several mechanisms have been reported with respect to transient or sustained loss of E-cadherin function, including E-cadherin cleavage and shedding (31, 32), epigenetic silencing (33), and defective E-cadherin recycling and trafficking (34). However, loss of E-cadherin gene expression, which is the hallmark of epithelial-mesenchymal transition (EMT), is mainly due to an up-regulation of Snail, Slug, and other transcription factors that directly repress E-cadherin expression (35). Indeed, ectopic expression of Snail or Slug in SKOV3 cells results in EMT-associated enhanced motility, invasiveness, and tumorigenicity (36). In the present study, we found that the EGFR inhibitor AG1478 could totally block EGF-induced H2O2 production and subsequent p38 MAPK activation, Snail and Slug expression, E-cadherin down-regulation, and cell invasion. In contrast, treatment with PEG-catalase totally blocked H2O2 production but only partially abolished the other EGF-induced events and, importantly, did not inhibit EGF-induced Slug expression. Thus, our results indicate that Slug, and the pathway(s) leading to its induction, may constitute an H2O2-independent mechanism mediating EGF-induced E-cadherin down-regulation. It has been shown that Slug expression can be regulated by Akt/β-catenin signaling, which can also be activated by EGF treatment (37, 38). In addition, alternative mechanisms of EGF-induced E-cadherin down-regulation have been described including E-cadherin cleavage by matrix metalloproteinase 9 (25) and internalization by caveolin-1 (28). In this context, our study characterizes a novel mechanism in which H2O2 production contributes to EGF-induced E-cadherin down-regulation via the activation of p38 MAPK and the enhancement of Snail expression. This is consistent with a link between ROS signaling and Snail expression that was found in a recent study showing that matrix metalloproteinase 3 stimulates Snail expression via a Rac1-dependent ROS generation mechanism, resulting in EMT (39). In addition, our results provide evidence that, within a single cell line, EGF utilizes additional mechanisms to down-regulate E-cadherin, which is consistent with the diversity of mechanisms described to date.
Ovarian cancer cells with low E-cadherin expression are more invasive, and the absence of E-cadherin expression predicts poor patient survival (18). Thus, E-cadherin likely functions as a suppressor of invasiveness in ovarian cancer. Indeed, down-regulation of E-cadherin by siRNA in ovarian cancer cells results in an up-regulation of α5-integrin mRNA and protein levels, thus permitting cells to attach and invade more efficiently (40). In addition, forced expression of a dominant-negative E-cadherin mutant in ovarian carcinoma cells disrupts adherens junctions, increases mesenchymal cell migration, and prevents spheroidal morphogenesis (41). Our data show that EGF increases cell invasiveness by down-regulating E-cadherin, and that overexpression of E-cadherin inhibits basal invasiveness and diminishes EGF-induced invasion. Interestingly, EGF-induced cell invasion was not completely abolished in cells overexpressing E-cadherin. It has been shown that various intracellular signaling pathways including phospholipase C-γ, phosphatidylinositol 3-kinase, and MAPK are involved in EGF-induced cell invasion (42). Moreover, additional mechanisms such as enhanced protease activity/secretion, changes in actin cytoskeleton, and enhanced motility have also been described (43, 44). Taken together, these results demonstrate that E-cadherin acts as an important suppressor of invasiveness in ovarian cancer cells and that, along with other described mechanisms, E-cadherin down-regulation plays an important role in EGF-induced cell invasion.
Our finding that EGF is able to induce H2O2 production in ovarian cancer cells is consistent with prior studies (11) and with the hypothesis that ROS signaling may mediate some of the downstream effects of EGF-EGFR activation. In addition, we provide evidence that H2O2 production can be uncoupled from EGFR activation and for a high degree of complexity and specificity in ROS signaling. Treatment of SKOV3 cells with EGF induced H2O2 production, decreased levels of E-cadherin, and produced a significant change in morphology. In contrast, no such changes were observed in OVCAR3 cells treated with EGF. Such a difference may be due to the reduced expression of EGFR in OVCAR3 cells. However, because treatment with exogenous H2O2 was able to reduce E-cadherin expression in both OVCAR3 and SKOV3 cells, and we have confirmed that EGF treatment can activate EGFR, MAPK and induce cell invasion in OVCAR3 cells (data not shown and Ref. 26) our data suggest that the lack of effect of EGF on E-cadherin in OVCAR3 cells likely reflects an uncoupling of EGFR activation from H2O2 production rather than a lack of effect of H2O2 on E-cadherin. Moreover, because EGFR is functional and EGF treatment did not affect E-cadherin expression in OVCAR3 cells, it suggests that the production of H2O2 may be a critical mediator of EGF-induced E-cadherin down-regulation. Whereas PEG-catalase abolished EGF-induced Snail, but not Slug, expression in SKOV3 cells, treatment with exogenous H2O2 increased both Snail and Slug. Moreover, the time course of exogenous H2O2-induced Slug expression differed significantly from that of EGF. These results indicate that the functional consequences of EGF-induced, endogenous H2O2 signaling differ from those of exogenous H2O2 treatment. In mouse mammary gland epithelial cells, prolonged exposure to H2O2 affects the subcellular distribution of E-cadherin without changing total protein levels. Most importantly, the epithelial cells exposed to oxidative conditions eventually become invasive, providing substantial evidence in support of a direct role for ROS signaling in the malignant transformation of epithelial cells (45). Given that cancer cells are exposed to both intracellular and extracellular ROS, future studies will be required to clarify their individual and combined contributions to tumor progression.
MAPK cascades are known to be major signaling pathways that drive tumor cell metastasis and are regulated by PKC, TGF-β/Smad, and integrin-mediated signaling (7). MAPK signaling cascades are also known to be regulated by ROS signaling; however, how MAPKs are activated by ROS is not clear. One potential target of ROS signaling are protein tyrosine phosphatases (PTPs), known negative regulators of growth factor receptor tyrosine kinase signaling. ROS can rapidly oxidize an essential cysteine in the active site of PTPs, effectively blocking their enzymatic activity (46, 47, 48). Thus, inactivation of multiple PTPs by ROS may enhance tyrosine phosphorylation-dependent signaling and contribute to tumor progression (49, 50). In this study, EGF treatment significantly increased ERK1/2, p38, and JNK activation in ovarian cancer cells. However, only EGF-induced activation of p38 MAPK was inhibited in cells treated with PEG-catalase. Using the p38 MAPK inhibitor SB203580 and specific siRNA for p38α MAPK, we show that p38α MAPK, the most abundant isoform of p38 MAPK in these cells, is required for EGF-induced E-cadherin down-regulation and cell invasion. These results are consistent with a previous study demonstrating a requirement for p38 MAPK signaling in EGF-induced invasion in ovarian cancer cells (22). In embryonic development, it has been reported that p38 MAPK and a p38-interacting protein are critically required for the down-regulation of E-cadherin during gastrulation (51). Taken together, our results indicate that H2O2-dependent p38α MAPK activation is involved in EGF-induced E-cadherin down-regulation and cell invasion in ovarian cancer cells.
In summary, this study demonstrates that H2O2 is a mediator of EGF-induced down-regulation of E-cadherin expression and is involved in EGF-induced cell invasion in ovarian cancer cells. Moreover, our data suggest that H2O2 signaling contributes to EGF-induced p38α MAPK activation and subsequent Snail expression. These results suggest that targeting ROS could be a useful therapeutic strategy for the prevention of tumor metastasis.
Materials and Methods
Cell culture
The human epithelial ovarian cancer cell lines SKOV3 and OVCAR3 were obtained from American Type Culture Collection (Manassas, VA). Cells were grown in a 1:1 (vol/vol) mixture of M199/MCDB105 medium (Sigma-Aldrich, Oakville, Ontario, Canada) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Inc., Logan, UT). Cultures were maintained at 37 C in a humidified atmosphere of 5% CO2 in air.
Antibodies and reagents
Polyclonal anti-EGFR and anti-β-actin antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The monoclonal anti-E-cadherin antibody was obtained from BD Biosciences (Mississauga, Ontario, Canada). Monoclonal anti-phospho-ERK1/2 (Thr202/Tyr204) antibody and polyclonal anti-ERK1/2, anti-phospho-p38 MAPK (Thr180/Tyr182), anti-p38 MAPK (which can detect endogenous levels of p38α, -β, and -γ MAPKs), anti-phospho-JNK (Thr183/Tyr185), and anti-JNK antibodies were obtained from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase-conjugated goat antimouse IgG and goat antirabbit IgG were obtained from Bio-Rad Laboratories, Inc. (Hercules, CA). Horseradish peroxidase-conjugated donkey antigoat IgG was obtained from Santa Cruz Biotechnology. Human EGF, AG1478, and PEG-catalase were obtained from Sigma. CM-H2DCFDA) was obtained from Invitrogen (Burlington, Ontario, Canada). PD98059, SB203580, and SP600125 were obtained from Calbiochem (San Diego, CA).
Western blots
Cells were lysed in buffer containing 20 mm Tris, pH 7.4, 2 mm EGTA, 2 mm Na2VO3, 2 mm Na4P2O7, 2% Triton X-100, 2% sodium dodecyl sulfate, 1 μm aprotinin, 1 μm leupeptin, and 1 mm phenylmethylsulfonylfluoride. Protein concentrations were determined using a DC protein assay kit with BSA as the standard (Bio-Rad Laboratories). Equal amounts of protein were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. After blocking with Tris-buffered saline containing 5% nonfat dry milk for 1 h, membranes were incubated overnight at 4 C with primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibody. Immunoreactive bands were detected with enhanced chemiluminescent substrate. Membranes were stripped with stripping buffer (62.5 mm Tris, 10 mm dithiothreitol, 2% sodium dodecyl sulfate, pH 6.7) at 50 C for 30 min and reprobed with anti-β-actin as a loading control.
Immunofluorescence staining
Cells were cultured on coverslips, fixed in methanol at −20 C for 20 min, postfixed in cold methanol-acetone (1:1) for 5 min, and dried. After rehydration in PBS, the coverslips were blocked with Dako Protein Block (DAKO, Mississauga, Ontario, Canada) for 1 h and incubated with antibody to E-cadherin diluted in Dako Protein Block. Alexa 594-labeled goat antimouse was used as a secondary antibody. Cells were counterstained with Hoechst 33258 (Sigma), rinsed with PBS, mounted with Gelvatol, and examined using a Zeiss Axiophot epifluorescence microscope equipped with a digital camera (Q Imaging, Burnaby, British Columbia, Canada).
Intracellular H2O2 detection
CM-H2DCFDA is a cell-permeable fluorescent dye specific for H2O2 and is used to detect intracellular H2O2 levels. Cells were seeded on 12-well plates and cultured in serum-free medium for 24 h and then treated with 100 ng/ml EGF for different periods of time. At the designated time points, cells were washed twice with PBS and fixed with 10% formaldehyde for 10 min. Intracellular H2O2 levels were assessed by staining with CM2-2DCFDA (5 μm) for 30 min at 37 C. Fluorescent images were captured using a inverted fluorescence microscope (Nikon Eclipse TE300; Nikon, Melville, NY).
Reverse transcription quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. Reverse transcription was performed with 3 μg RNA, random primers, and M-MLV reverse transcriptase (Promega Corp., Madison, WI). The primers used for SYBR Green reverse transcription-qPCR (RT-qPCR) were as follows: E-cadherin, 5′-ACA GCC CCG CCT TAT GAT T-3′ (sense) and 5′-TCG GAA CCG CTT CCT TCA-3′ (antisense); Snail, 5′-CCC CAA TCG GAA GCC TAA CT-3′ (sense) and 5′-GCT GGA AGG TAA ACT CTG GAT TAG A-3′ (antisense); Slug, 5′-TTC GGA CCC ACA CAT TAC CT-3′ (sense) and 5′-GCA GTG AGG GCA AGA AAA AG-3′ (antisense); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), 5′-GAG TCA ACG GAT TTG GTC GT-3′ (sense) and 5′-GAC AAG CTT CCC GTT CTC AG-3′ (antisense). Quantitative real-time PCR was performed on an Applied Biosystems 7300 Real-Time PCR System (PerkinElmer Corp., Wellesley, MA) equipped with a 96-well optical reaction plate. All RT-qPCR experiments were run in triplicate, and a mean value was used for the determination of mRNA levels. Relative quantification of the mRNA levels was performed using the comparative Ct method with GAPDH as the reference gene and with the formula 2−ΔΔCt.
Invasion assay
Invasion assays were performed in Boyden chambers with minor modifications (52). Transwell filters (8-μm pore size, 24 wells, BD Biosciences) were coated with 1 mg/ml growth factor-reduced Matrigel (BD Biosciences). Cells in M199/MCDB105 medium supplemented with 0.1% FBS were incubated for 24 h against a gradient of 5% FBS. Cells that penetrated the membrane were fixed with cold methanol, and cell nuclei were stained with Hoechst 33258 and counted by epifluorescence microscopy with Northern Eclipse 6.0 software (Empix Imaging, Mississauga, Ontario, Canada). Triplicate inserts were used for each individual experiment, and five microscopic fields were counted per insert.
E-cadherin overexpression and siRNA transfection
Cells were seeded in six-well plates and transfected for 48 h with Lipofectamine 2000 (Invitrogen) and 3 μg empty pIRES vector or vector encoding a full-length wild type mouse E-cadherin (provided by Dr. C. Sasaki, National Institute on Aging, Baltimore, MD). To knock down endogenous p38α MAPK, cells were transfected with 20 nm ON-TARGETplus SMARTpool p38α MAPK (MAPK14) siRNA (Dharmacon, Lafayette, CO) using Lipofectamine RNAiMAX (Invitrogen) for 48 h.
Statistical analysis
Results are presented as mean ± SEM of at least three independent experiments. Results were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Significant differences were defined as P < 0.05.
Footnotes
This work was supported by an operating grant from the Canadian Institutes of Health Research (to P.C.K.L.). P.C.K.L. is the recipient of a Child & Family Research Institute Senior Investigator Award. J.C.C. is the recipient of a Graduate Studentship Award from Interdisciplinary Women’s Reproductive Health Research Training Program, University of British Columbia.
Disclosure Summary: The authors have nothing to disclose.
First Published Online July 7, 2010
Abbreviations: CM-H2DCFDA, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester; EGF, epidermal growth factor; EGFR, EGF receptor; EMT, epithelial-mesenchymal transition; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; JNK, c-Jun N-terminal kinase; PEG, polyethylene glycol; PTP, protein tyrosine phosphatase; ROS, reactive oxygen species; RT-qPCR, reverse transcription quantitative real-time PCR; siRNA, small interfering RNA.
References
- 1.Barr S, Thomson S, Buck E, Russo S, Petti F, Sujka-Kwok I, Eyzaguirre A, Rosenfeld-Franklin M, Gibson NW, Miglarese M, Epstein D, Iwata KK, Haley JD2008. Bypassing cellular EGF receptor dependence through epithelial-to-mesenchymal-like transitions. Clin Exp Metastasis 25:685–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett JM, Langdon SP, Simpson BJ, Stewart M, Katsaros D, Sismondi P, Love S, Scott WN, Williams AR, Lessells AM, Macleod KG, Smyth JF, Miller WR1996. The prognostic value of epidermal growth factor receptor mRNA expression in primary ovarian cancer. Br J Cancer 73:301–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niikura H, Sasano H, Sato S, Yajima A1997. Expression of epidermal growth factor-related proteins and epidermal growth factor receptor in common epithelial ovarian tumors. Int J Gynecol Pathol 16:60–68 [DOI] [PubMed] [Google Scholar]
- 4.Mendelsohn J, Baselga J2003. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21:2787–2799 [DOI] [PubMed] [Google Scholar]
- 5.El-Rayes BF, LoRusso PM2004. Targeting the epidermal growth factor receptor. Br J Cancer 91:418–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marmor MD, Skaria KB, Yarden Y2004. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys 58:903–913 [DOI] [PubMed] [Google Scholar]
- 7.Wu WS2006. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 25:695–705 [DOI] [PubMed] [Google Scholar]
- 8.Wu WS, Wu JR, Hu CT2008. Signal cross talks for sustained MAPK activation and cell migration: the potential role of reactive oxygen species. Cancer Metastasis Rev 27:303–314 [DOI] [PubMed] [Google Scholar]
- 9.Boonstra J, Post JA2004. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene 337:1–13 [DOI] [PubMed] [Google Scholar]
- 10.Storz P2005. Reactive oxygen species in tumor progression. Front Biosci 10:1881–1896 [DOI] [PubMed] [Google Scholar]
- 11.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG1997. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272:217–221 [PubMed] [Google Scholar]
- 12.Peus D, Meves A, Vasa RA, Beyerle A, O'Brien T, Pittelkow MR1999. H2O2 is required for UVB-induced EGF receptor and downstream signaling pathway activation. Free Radic Biol Med 27: 1197–1202 [DOI] [PubMed]
- 13.Cavallaro U, Christofori G2004. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer 4:118–132 [DOI] [PubMed] [Google Scholar]
- 14.Pećina-Slaus N2003. Tumor suppressor gene E-cadherin and its role in normal and malignant cells. Cancer Cell Int 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hudson LG, Zeineldin R, Stack MS2008. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis 25:643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peinado H, Olmeda D, Cano A2007. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428 [DOI] [PubMed] [Google Scholar]
- 17.Veatch AL, Carson LF, Ramakrishnan S1994. Differential expression of the cell-cell adhesion molecule E-cadherin in ascites and solid human ovarian tumor cells. Int J Cancer 58:393–399 [DOI] [PubMed] [Google Scholar]
- 18.Daraï E, Scoazec JY, Walker-Combrouze F, Mlika-Cabanne N, Feldmann G, Madelenat P, Potet F1997. Expression of cadherins in benign, borderline, and malignant ovarian epithelial tumors: a clinicopathologic study of 60 cases. Hum Pathol 28:922–928 [DOI] [PubMed] [Google Scholar]
- 19.Birchmeier W, Behrens J1994. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta 1198:11–26 [DOI] [PubMed] [Google Scholar]
- 20.Vleminckx K, Vakaet Jr L, Mareel M, Fiers W, van Roy F1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107–119 [DOI] [PubMed] [Google Scholar]
- 21.Colomiere M, Findlay J, Ackland L, Ahmed N2008. Epidermal growth factor-induced ovarian carcinoma cell migration is associated with JAK2/STAT3 signals and changes in the abundance and localization of α6β1 integrin. Int J Biochem Cell Biol 41:1034–1045 [DOI] [PubMed] [Google Scholar]
- 22.Zhou HY, Pon YL, Wong AS2007. Synergistic effects of epidermal growth factor and hepatocyte growth factor on human ovarian cancer cell invasion and migration: role of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase. Endocrinology 148:5195–5208 [DOI] [PubMed] [Google Scholar]
- 23.Zeineldin R, Rosenberg M, Ortega D, Buhr C, Chavez MG, Stack MS, Kusewitt DF, Hudson LG2006. Mesenchymal transformation in epithelial ovarian tumor cells expressing epidermal growth factor receptor variant III. Mol Carcinog 45:851–860 [DOI] [PubMed] [Google Scholar]
- 24.Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS2000. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer 88:566–574 [DOI] [PubMed] [Google Scholar]
- 25.Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, Stack MS, Hudson LG2008. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res 68:4606–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon SL, Hammond GT, Leung PC2009. Epidermal growth factor-induced GnRH-II synthesis contributes to ovarian cancer cell invasion. Mol Endocrinol 23:1646–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner EF, Nebreda AR2009. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 9:537–549 [DOI] [PubMed] [Google Scholar]
- 28.Lu Z, Ghosh S, Wang Z, Hunter T2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499–515 [DOI] [PubMed] [Google Scholar]
- 29.Huo Y, Qiu WY, Pan Q, Yao YF, Xing K, Lou MF2009. Reactive oxygen species (ROS) are essential mediators in epidermal growth factor (EGF)-stimulated corneal epithelial cell proliferation, adhesion, migration, and wound healing. Exp Eye Res 89:876–886 [DOI] [PubMed] [Google Scholar]
- 30.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI2009. EGF promotes invasion by PANC-1 cells through Rac1/ROS-dependent secretion and activation of MMP-2. Biochem Biophys Res Commun 379:445–450 [DOI] [PubMed] [Google Scholar]
- 31.Covington MD, Burghardt RC, Parrish AR2006. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14). Am J Physiol Renal Physiol 290:F43–F51 [DOI] [PubMed]
- 32.Symowicz J, Adley BP, Gleason KJ, Johnson JJ, Ghosh S, Fishman DA, Hudson LG, Stack MS2007. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res 67:2030–2039 [DOI] [PubMed] [Google Scholar]
- 33.Makarla PB, Saboorian MH, Ashfaq R, Toyooka KO, Toyooka S, Minna JD, Gazdar AF, Schorge JO2005. Promoter hypermethylation profile of ovarian epithelial neoplasms. Clin Cancer Res 11:5365–5369 [DOI] [PubMed] [Google Scholar]
- 34.Bryant DM, Stow JL2004. The ins and outs of E-cadherin trafficking. Trends Cell Biol 14:427–434 [DOI] [PubMed] [Google Scholar]
- 35.Elloul S, Silins I, Tropé CG, Benshushan A, Davidson B, Reich R2006. Expression of E-cadherin transcriptional regulators in ovarian carcinoma. Virchows Arch 449:520–528 [DOI] [PubMed] [Google Scholar]
- 36.Kurrey NK, K A, Bapat SA2005. Snail and Slug are major determinants of ovarian cancer invasiveness at the transcription level. Gynecol Oncol 97:155–165 [DOI] [PubMed] [Google Scholar]
- 37.Saegusa M, Hashimura M, Kuwata T, Okayasu I2009. Requirement of the Akt/β-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of slug. Am J Pathol 174:2107–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CH, Hung HW, Hung PH, Shieh YS2010. Epidermal growth factor receptor regulates β-catenin location, stability, and transcriptional activity in oral cancer. Mol Cancer 9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ2005. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawada K, Mitra AK, Radjabi AR, Bhaskar V, Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A, Kenny HA, Peter ME, Ramakrishnan V, Yamada SD, Lengyel E2008. Loss of E-cadherin promotes ovarian cancer metastasis via α 5-integrin, which is a therapeutic target. Cancer Res 68:2329–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu C, Cipollone J, Maines-Bandiera S, Tan C, Karsan A, Auersperg N, Roskelley CD2008. The morphogenic function of E-cadherin-mediated adherens junctions in epithelial ovarian carcinoma formation and progression. Differentiation 76:193–205 [DOI] [PubMed] [Google Scholar]
- 42.Wells A2000. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res 78:31–101 [DOI] [PubMed] [Google Scholar]
- 43.Kassis J, Lauffenburger DA, Turner T, Wells A2001. Tumor invasion as dysregulated cell motility. Semin Cancer Biol 11:105–117 [DOI] [PubMed] [Google Scholar]
- 44.Ellerbroek SM, Halbleib JM, Benavidez M, Warmka JK, Wattenberg EV, Stack MS, Hudson LG2001. Phosphatidylinositol 3-kinase activity in epidermal growth factor-stimulated matrix metalloproteinase-9 production and cell surface association. Cancer Res 61:1855–1861 [PubMed] [Google Scholar]
- 45.Mori K, Shibanuma M, Nose K2004. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res 64:7464–7472 [DOI] [PubMed] [Google Scholar]
- 46.Meng TC, Fukada T, Tonks NK2002. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9:387–399 [DOI] [PubMed] [Google Scholar]
- 47.Juarez JC, Manuia M, Burnett ME, Betancourt O, Boivin B, Shaw DE, Tonks NK, Mazar AP, Doñate F2008. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA 105:7147–7152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiarugi P2003. Reactive oxygen species as mediators of cell adhesion. Ital J Biochem 52:28–32 [PubMed] [Google Scholar]
- 49.Chiarugi P2005. PTPs versus PTKs: the redox side of the coin. Free Radic Res 39:353–364 [DOI] [PubMed] [Google Scholar]
- 50.Lee K, Esselman WJ2002. Inhibition of PTPs by H2o2 regulates the activation of distinct MAPK pathways. Free Radic Biol Med 33:1121–1132 [DOI] [PubMed] [Google Scholar]
- 51.Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L2006. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125:957–969 [DOI] [PubMed] [Google Scholar]
- 52.Woo MM, Salamanca CM, Minor A, Auersperg N2007. An improved assay to quantitate the invasiveness of cells in modified Boyden chambers. In Vitro Cell Dev Biol Anim 43:7–9 [DOI] [PubMed] [Google Scholar]