Abstract
The activation of G protein-coupled receptor 103 (GPR103) by its endogenous peptidic ligands, QRFPs, is involved in the central regulation of feeding by increasing food intake, body weight, and fat mass after intracerebroventricular injection in mice. However, the role of GPR103 in regulating peripheral metabolic pathways has not yet been explored. The present study aimed to investigate the role of GPR103 in adipogenesis and lipid metabolism using 3T3-L1 adipocyte cells. Our results show that differentiated 3T3-L1 cells expressed the GPR103b subtype mRNA and protein, as well as QRFP mRNA. QRFP-43 and -26 induced an increase in triglyceride accumulation of 50 and 41%, respectively, and elicited a dose-dependent increase in fatty acid uptake, by up to approximately 60% at the highest concentration, in 3T3-L1-differentiated cells. QRFP-43 and -26 inhibited isoproterenol (ISO)-induced lipolysis in a dose-dependent manner, with IC50s of 2.3 ± 1.2 and 1.1 ± 1.0 nm, respectively. The expression of genes involved in lipid uptake (FATP1, CD36, LPL, ACSL1, PPAR-γ, and C/EBP-α), was increased by 2- to 3-fold after treatment with QRFP. The effects of QRFP on ISO-induced lipolysis and fatty acid uptake were abolished when GPR103b was silenced. In a mouse model of diet-induced obesity, the expression of GPR103b in epididymal fat pads was elevated by 16-fold whereas that of QRFP was reduced by 46% compared to lean mice. Furthermore, QRFP was bioactive in omental adipocytes from obese individuals, inhibiting ISO-induced lipolysis in these cells. Our results suggest that GPR103b and QRFP work in an autocrine/paracrine manner to regulate adipogenesis.
GPR103b mediates QRFP-dependent peripheral modulation of adipogenesis including upregulation of transcription factors, increased fatty acids uptake and inhibition of isoproterenol-induced lipolysis in adipocytes.
The deorphanized G protein-coupled receptor 103 (GPR103) (1, 2) is known to be activated by its endogenous peptide agonists QRFPs (3, 4), a 43-aa pyroglutamylated RFamide peptide and its constitutive C-terminal part QRFP-26 (also known as 26RFa). In rodents, the activation of GPR103 by intracerebroventricular administration of QRFP peptides results in orexigenic activity (3, 5, 6). In addition to an increase in fat mass and body weight, long-term central administration of QRFP leads to reduced thermogenesis (6). Besides stimulating food intake, an effect which appears to be exacerbated when the animals are fed a high-fat diet (HFD) (6, 7), GPR103 receptors and QRFP have been shown to be involved in regulating behavioral arousal, blood pressure (5), bone formation (8), gonadotropic axis (9), aldosterone secretion (4), insulin secretion (10), and analgesia (11). However, whether GPR103 and QRFP may also exert metabolic effect at the periphery has not been investigated.
The orexigenic response of QRFP mediated through GPR103 signaling involves central neuropeptide Y (NPY) pathways (5, 12). The GPR103 receptor shares significant sequence identity with NPFF2, NPY-Y2, and galanin-R1 receptors (1). Two subtypes of GPR103 have been identified in rodents, mGPR103a and mGPR103b, while only one receptor has been identified in human, hGPR103 (5). Rodent subtypes feature differential expression: whereas mGPR103a is mainly distributed to the central nervous system, retina, thymus, liver, kidney, and bladder, mGPR103b is expressed in multiple tissues, predominantly in nuclei of the hypothalamus regulating appetite (13). QRFP peptides are expressed in multiple tissues, predominantly in brain (14), but also in peripheral tissues including white adipose tissue (WAT) (13). Interestingly, other members of the RFamide peptides family, including the neuropeptides FF, AF, and SF, were found to modulate adipose tissue differentiation (15), adipocyte metabolism (16), and to regulate food intake (17, 18). In the present study, we investigated whether GPR103b plays a regulatory role in adipogenesis after its activation by QRFP peptides.
To this aim, the expression of GPR103b and QRFP in 3T3-L1 adipocytes has been assayed by quantitative real-time PCR (qPCR) along with flow cytometry for GPR103b detection by immunofluorescence in differentiated 3T3-L1 adipocytes. Functional lipolysis assays and determination of triglyceride accumulation were performed, along with gene expression profiles of adipogenic transcription factors and fatty acid (FA) transporters. The involvement of GPR103b in these cellular responses has been confirmed by gene silencing using retrovirus infection. In vivo expression profiles of mGPR103b and QRFP were documented in mouse adipose tissues following a high-fat feeding regimen. In addition, the physiological relevance of GPR103b in human adipose tissue metabolism has been investigated with isoproterenol (ISO)-induced lipolysis experiments in human omental adipocytes. Our findings support that GPR103b and QRFP might regulate adipogenesis through an autocrine/paracrine mechanism.
Results
Expression profile of GPR103 receptors and QRFP in 3T3-L1 cells
The mRNA expression of GPR103 receptor subtypes was investigated in 3T3-L1 preadipocytes and differentiated adipocytes by RT-PCR. We found that the mRNA levels of GPR103b, and not those of GPR103a, are induced in a time-dependent manner after initiation of 3T3-L1 cell differentiation (Fig. 1, A and B). Increased mRNA levels correlated with increased GPR103b receptor expression as assessed by flow cytometry (Fig. 1C). Low levels of QRFP mRNA were detected in 3T3-L1 preadipocytes (Fig. 1A), which increased by 5-fold (P < 0.05) at d 4 after the induction of differentiation and appeared to plateau thereafter (Fig. 1D).
Fig. 1.
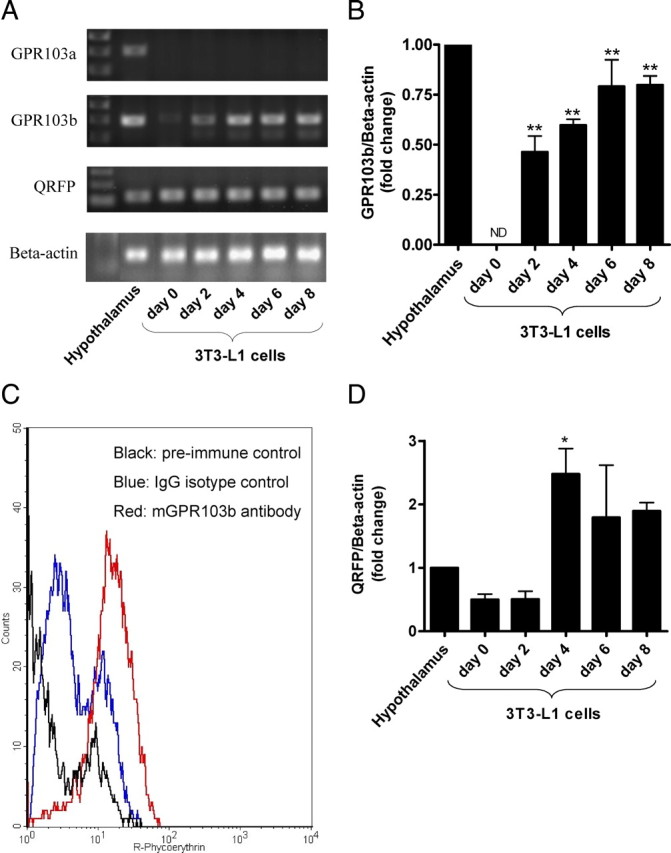
Expression of GPR103b and QRFP during 3T3-L1 differentiation. A, Ethidium bromide-stained agarose gel for visualization of RT-PCR amplification products derived from 3T3-L1 cells. 3T3-L1 cells were induced to differentiate at confluence (designated d 0) as described in Material and Methods. PCR amplification products of hypothalamus were used as positive controls. PCR products were resolved on 1.5% agarose gels. B, qPCR of GPR103b during 3T3-L1 cell differentiation. Data are presented as fold change relative to hypothalamic expression set to 1 (n = 3). C, Detection of GPR103b in differentiated 3T3-L1 cells by flow cytometry immunofluorescence. The histogram shows preimmune control fluorescence (black), IgG isotype control fluorescence (blue), and specific GPR103b fluorescence (red) (n = 4). D, qPCR of pre-QRFP during 3T3-L1 differentiation. Data are presented as fold change relative to hypothalamic expression set to 1 (n = 3). ND, Not detected; *, P < 0.05 and **, P < 0.01 vs. d 0.
Effects of GPR103 activation by QRFP peptides on adipogenesis and lipolysis in differentiated 3T3-L1
To assess the potential role of GRP103b on 3T3-L1 differentiation, cells were incubated in the presence of QRFP-43 or -26 (10 nm) during 4 d, from d 2 after the induction of differentiation. At the end of the incubation period, neutral lipids were stained with Oil Red O (ORO) and quantified by optical densitometry. Our results show that QRFP peptides increased intracellular triglyceride content, as shown by increased ORO staining (Fig. 2A) by 1.4-fold (QRFP-26) and 1.5-fold (QRFP-43) (P < 0.01), whereas 167 nm insulin induced a 1.2-fold (P < 0.05) elevation (Fig. 2B).
Fig. 2.
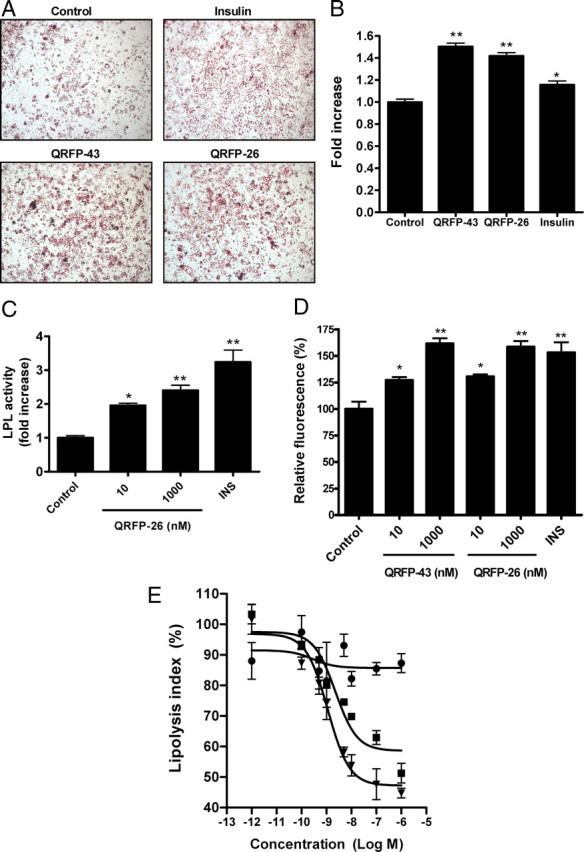
Effects of QRFPs peptides on triglyceride accumulation, LPL activity, FA uptake, and lipolysis. A, Triglyceride accumulation by ORO staining of 3T3-L1 adipocytes. At d 2 of differentiation, 3T3-L1 cells were treated or not with insulin (167 nm) or QRFP-43 or -26 (10 nm) for 4 d. B, Quantitative analysis of ORO staining. Stained Oil Red O was eluted with isopropanol and quantified by measuring the optical density at 510 nm (n = 3). Oil Red O data are expressed as fold increase (mean ± sem) over control (vehicle treated). C, Heparin-releasable activity in d 2 differentiated 3T3-L1 cells treated with insulin (INS, 167 nm) and QRFP-26 (10 and 1000 nm) for 2 more d (n = 3). D, Bar graphs in percent change (±sem) in mean fluorescence intensity over fluorescence in vehicle-treated cells set at 100%, as assessed by flow cytometry of C12-BODIPY-labeled FA uptake in 3T3-L1 adipocytes starved 1 h and then treated 2 h with insulin (INS, 167 nm) or QRFP peptides (10 and 1000 nm) (n = 4). E, Dose-response curve of inhibition of ISO-induced lipolysis by QRFP. ISO + QRFP-43 (square), ISO + QRFP-26 (triangle), and ISO + QRFP-28n (circle) in differentiated 3T3-L1 cells. QRFP-43 and -26 inhibited the lipolytic effect of ISO in a dose-dependent manner. Data are expressed as lipolysis index (sample free glycerol concentration − basal concentration)/(ISO-elicited free glycerol concentration − basal concentration) (n = 3). *, P < 0.05; **, P < 0.01 vs. vehicle.
To assess whether the observed increase in triglyceride content in QRFP-treated adipocytes was associated with increased lipid uptake, we investigated the effect of the peptides on FA uptake. We first determined lipoprotein lipase (LPL) activity, inasmuch as the latter is responsible for the generation of FA from circulating triglyceride-rich lipoproteins. Insulin (167 nm), used as a positive control (19), increased LPL activity by 3.2-fold (P < 0.01) compared with vehicle on d 2 following the induction of differentiation of 3T3-L1 adipocytes (Fig. 2C). In comparison, incubation with 10 and 1000 nm QRFP-26 resulted in a significant 2- (P < 0.05) and 2.4-fold (P < 0.01) increase in LPL activity, respectively (Fig. 2C). FA uptake was investigated using a C12-BODIPY FA. Incubation of differentiated adipocytes with insulin (167 nm) for 2 h resulted in a 53 ± 10% (P < 0.01) increase in FA uptake compared with vehicle (Fig. 2D), whereas QRFP-43 and -26 (10 nm) treatment resulted in a 27 ± 3 and 31 ± 2% (P < 0.05) increase in FA uptake compared with vehicle, respectively. High concentrations (1000 nm) of QRFP-43 and -26 (Fig. 2D) further increased FA uptake by 62 ± 5 and 58 ± 6% (P < 0.01), respectively, supporting a role for GPR103b in regulating FA uptake in 3T3-L1 adipocytes.
We next investigated the role of GPR103b on differentiated adipocyte functional response, using ISO-induced lipolysis. Neither QRFP-43 nor QRFP-26 had an effect on basal lipolysis (data not shown); however, both peptides inhibited ISO-elicited lipolysis in a dose-dependent manner, with IC50s of 2.3 ± 1.2 and 1.1 ± 1.0 nm, respectively (Fig. 2E). In contrast, QRFP-28n, which displays weak affinity for GPR103 (4), had no effect on ISO-induced lipolysis (Fig. 2E).
Effects of GPR103b silencing on QRFP-dependent responses in differentiated 3T3-L1 adipocytes
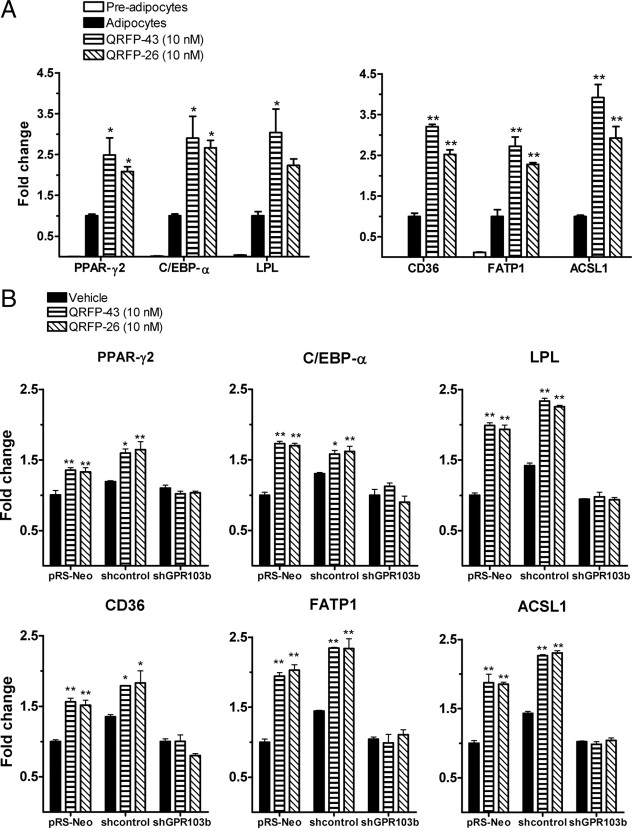
To determine the involvement of GPR103b in mediating the effects of QRFP on adipocytes, we used retroviral-delivered short hairpin RNA (shRNA) to knockdown the expression of GPR103b in 3T3-L1 adipocytes. As shown in Fig. 3, A and B, an infection with a shRNA sequence specific for GPR103b (shGPR103b) decreased the expression of GPR103b by 67% (P < 0.05), at the mRNA level, and by 46% (P < 0.05) at the protein level, compared with an infection with the empty vector (pRS-Neo). Interestingly, an infection with a nontargeting shRNA (shcontrol) increased the mRNA of GPR103b without affecting its protein expression. The knockdown of GPR103b did not affect 3T3-L1 differentiation compared with empty vector and nontargeting shRNA infections as shown by ORO staining of differentiated cells (Fig. 3C). The decrease in GPR103b immunofluorescence following specific shRNA infection is shown by a left shift in the fluorescence histogram compare to empty vector and nontargeting shRNA infections (Fig. 3D). Specific GPR103b knockdown abolished the increase in FA uptake as shown in Fig. 3E. Neither control infections, with empty vector or nontargeting shRNA sequence, modulated QRFP-43 and -26 abilities to increase FA uptake. The GPR103b knockdown did not affect insulin-mediated FA uptake, which was used as a positive control. These results suggest that triglyceride accumulation and FA uptake induced by QRFP-43 and -26 in differentiated adipocytes is mediated by the GPR103b receptor. Furthermore, specific GPR103b knockdown abolished the antilipolytic effects of QRFP-43 and -26 on ISO-induced lipolysis (Fig. 3F). In agreement, an infection with the empty vector or nontargeting shRNA did not affect the antilipolytic effect of QRFP peptides on ISO-induced lipolysis. In the latter case, both QRFP-43 and -26 inhibited ISO-induced lipolysis in a dose-dependent manner, supporting that the inhibitory effect of QRFP on β-adrenergic agonist-stimulated lipolysis is GPR103b-dependent.
Fig. 3.
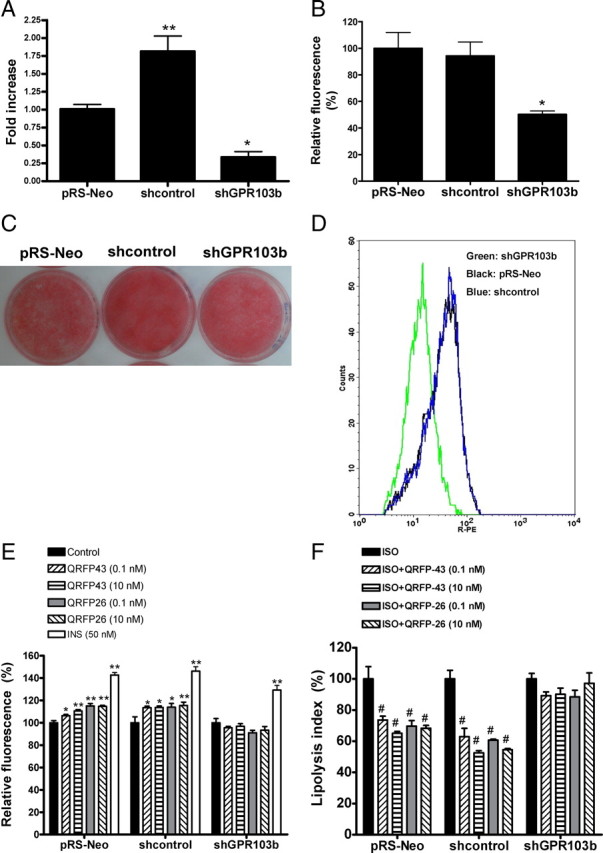
Effects of GPR103b silencing on QRFP-dependent responses in differentiated 3T3-L1. A, qPCR of GPR103b in 3T3-L1 infected with shRNAs. Expression in nontargeting shRNA cells (shcontrol) and specific GPR103b shRNA cells (shGPR103b) are presented as fold change relative to empty vector (pRS-Neo) expression set to 1 (n = 4). B, Bar graphs of protein detection in percent change (±sem, n = 4) in mean fluorescence intensity over fluorescence in empty vector infected cells set at 100%, as assessed by flow cytometry of GPR103b in empty vector, nontargeting shRNA or specific GPR103b shRNA-infected and differentiated 3T3-L1 cells. C, Triglyceride accumulation in empty vector, nontargeting shRNA, or specific GPR103b shRNA-infected 3T3-L1 cells. ORO staining has been performed on differentiated untreated cells. D, Detection of GPR103b in infected and differentiated 3T3-L1 cells by flow cytometry immunofluorescence. The left shift of shGPR103b cells in the fluorescence histogram shows a decrease in GPR103b expression compared with pRS-Neo and shcontrol cells (n = 4). E, C12-BODIPY-labeled FA uptake in pRS-Neo, shcontrol, or shGPR103b in infected and differentiated 3T3-L1 cells following 1-h starvation and 2-h treatment with insulin (INS, 50 nm) or QRFP peptides (0.1 and 10 nm) (n = 4). F, Inhibition of ISO-induced lipolysis by QRFP-43 and -26. pRS-Neo, shcontrol, or shGPR103b infected 3T3-L1 cells were differentiated, and ISO-elicited lipolysis was inhibited with QRFP peptides at 0.1 and 10 nm (n = 3). Data are expressed as lipolysis index. #, P < 0.05 vs. ISO; *, P < 0.05 and **, P < 0.01 vs. their respective control.
GPR103b activation increases adipogenic genes in 3T3-L1 adipocytes
Using qPCR analysis, we tested the expression of adipogenic genes in differentiated 3T3-L1 adipocytes incubated for 2 d with 10 nm QRFP-26 or QRFP-43. The expression of peroxisome proliferator-activated receptor-γ (PPAR-γ) and CCAAT/enhancer binding protein-α (C/EBP-α) as transcription factors involved in adipocyte differentiation was quantified, as well as that of CD36 and fatty acid transport protein-1 (FATP1) as long-chain FA transporters, of LPL, involved in lipid uptake, and of acyl-CoA synthetase long-chain family member 1 (ACSL1), involved in intracellular triglyceride formation. Treatments with QRFP-43 and QRFP-26 increased the expression of PPAR-γ by 2.2- and 1.7-fold (P < 0.05), of C/EBP-α by 2.7- and 2.4-fold (P < 0.05), of CD36 by 3.0- and 2.2-fold (P < 0.01), of LPL by 2.9- (P < 0.05) and 2.0-fold, of FATP1 by 2.6- (P < 0.01) and 2.1-fold (P < 0.01), and of ACSL1 by 3.7- (P < 0.01) and 2.6-fold (P < 0.01), respectively, over nontreated differentiated 3T3-L1 adipocytes (Fig. 4A). Treatment of empty vector and nontargeting shRNA infected 3T3-L1 cells has also shown a significant increase of the expression of PPAR-γ, C/EBP-α, CD36, LPL, FATP1, and ACSL1. However, specific knockdown of GPR103b abolished the induction of these genes by QRFP (Fig. 4B). Rosiglitazone treatment (10 μm) was used as a positive control for the induction of these genes (see Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Fig. 4.
Adipogenic gene expression in 3T3-L1-differentiated cells following QRFP treatment. Differentiated 3T3-L1 cells (A) or empty vector, nontargeting shRNA, and specific GPR103b shRNA-differentiated 3T3-L1 cells after retroviral infection (B) were treated or not with QRFP-43, -26 (10 nm) or vehicle for 2 d, and the total number of RNAs were assayed by qPCR. Data are presented as fold increase over vehicle (10% FBS)-treated cells (n = 3). Gene expression levels were quantified by SYBR Green real-time PCR. Relative gene expression was calculated using the 2−ΔΔCt method. *, P < 0.05 and **, P < 0.01 vs. control (vehicle).
Effect of diet-induced obesity on GPR103b and QRFP expression in adipose tissue depots
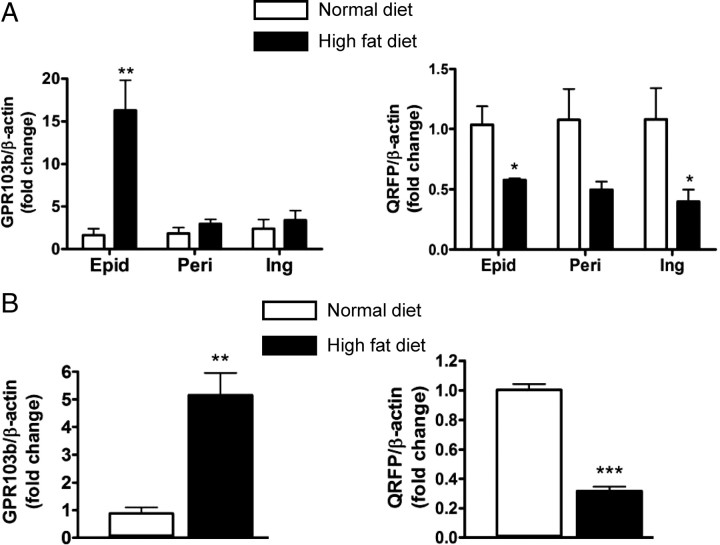
To determine the relevance of GPR103b and QRFP pathway in vivo, we investigated their expression levels in different adipose tissue depots in a mouse model of diet-induced obesity (DIO). Using qPCR, we measured the mRNA levels of both GPR103b and QRFP in epididymal, perirenal, and inguinal (sc) fat depots from mice fed a HFD or normal diet for 24 wk. DIO increased by 16-fold the expression of GPR103b in epididymal fat depots compared with mice fed a normal diet, while no significant increase was found in perirenal and inguinal fat depots (Fig. 5A). In contrast, DIO decreased the expression of QRFP by 46 ± 0.2% (P < 0.05) in epididymal, 58 ± 0.3% in perirenal, and 69 ± 0.3% (P < 0.05) in inguinal fat depots (Fig. 5A). Fat depots are heterogeneous in their cell composition. To confirm that DIO was associated with changes in QRFP/GPR103b expression in adipocytes, epididymal adipocytes from mice fed normal chow or HFD for 18 wk were isolated and mRNA levels were determined. DIO increased GPR103b expression by 5.2-fold (P < 0.01) in isolated adipocytes, whereas QRFP expression was decreased by 60%, as observed for the epididymal fat (Fig. 5B).
Fig. 5.
Expression of GPR103b and pre-QRFP in adipose depot from mice fed a high-fat diet. A, qPCR of GPR103b and pre-QRFP in epididymal (Epid), perirenal (Peri), and inguinal (Ing) fat depots from mice fed a HFD (D12492; Research Diet Inc.) or normal diet (D12450B) for 24 wk. B, qPCR analysis of GPR103b and pre-QRFP in isolated primary adipocytes from epididymal fat pads of mice fed a HFD or normal diet for 18 wk. Data (mean ± sem) are presented as fold change relative to control (normal diet) expression (n = 3–4). Relative gene expression was calculated using the 2−ΔΔCt method. *, P < 0.05; **, P < 0.01; and ***, P < 0.001 vs. normal diet.
QRFP-induced GPR103 activation inhibits ISO-induced lipolysis in human omental adipocytes
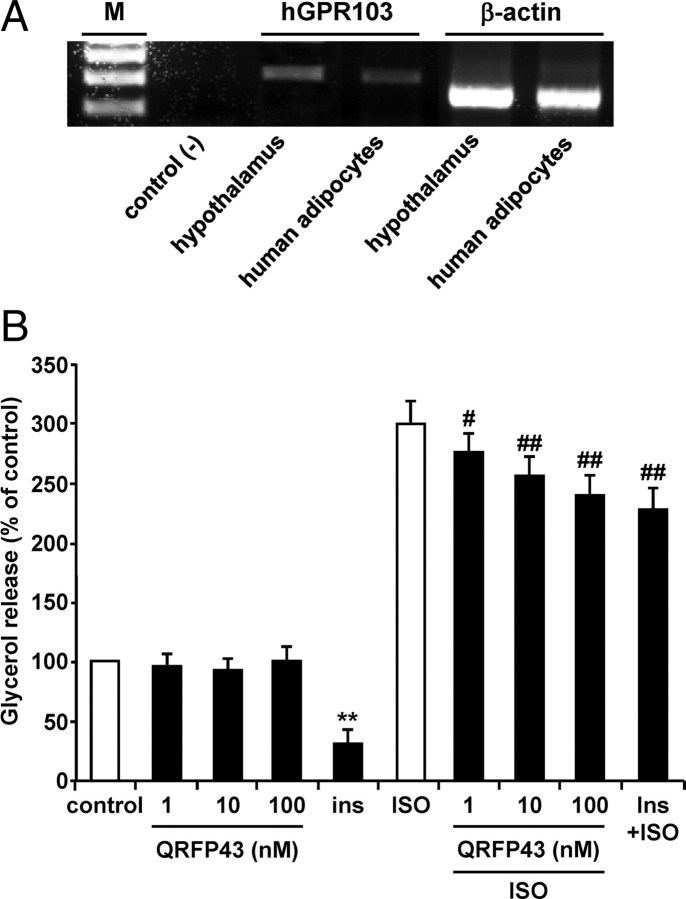
Adipocytes obtained from human omental fat were found to express hGPR103 mRNA (Fig. 6A). The effect of QRFP-43 was then investigated on ISO-induced lipolysis in human omental adipocytes. ISO increased lipolysis by 3-fold over control, as assessed by glycerol release in the culture medium, after 2-h incubation (Fig. 6B). QRFP-43 dose-dependently counteracted the lipolytic effect of ISO by significantly reducing glycerol release. Interestingly, at the highest concentration tested (100 nm), QRFP-43 antilipolytic effect, although being less potent, was similar to that exerted by 100 nm insulin, which was used as positive control. By contrast, whereas insulin showed antilipolytic action even in the absence of ISO, QRFP-43 alone had no effect. These results suggest that GPR103 counteracts lipolysis in human omental adipocytes by inhibiting ISO-induced glycerol release.
Fig. 6.
Expression of hGPR103 and lipolysis in human omental adipocytes. A, GPR103 mRNA expression in human omental adipocytes. PCR amplification products of human hypothalamus were used for positive control. As expected, the amplified products corresponded to 318 bp for hGPR103 and 230 bp for β-actin. B, Lipolysis was measured in adipocytes preincubated with or without either QRFP-43 (1–100 nm) or insulin (100 nm) for 40 min and then incubated with ISO (1 μm) for an additional 80 min. Results are expressed as percentage of control in basal (left) or stimulated (right) conditions (control glycerol release is of 33.75 μm). The means ± sem of five independent experiments are shown. **, P < 0.01 vs. control; #, P < 0.05; ##, P < 0.01 vs. ISO.
Discussion
In the present study, G protein-coupled receptor 103b and its ligands, QRFP-43 and -26, have been identified as new peripheral regulators of adipogenesis. Indeed, the RFamide peptides QRFP-43 and -26, known for their role in the central regulation of appetite (3, 6), are now shown to function as adipogenic factors in peripheral WAT. We also show that the GPR103b subtype, previously identified as a hypothalamic receptor with orexigenic activity (3), is expressed in WAT. Its physiological relevance is supported by specific changes in gene expression levels following long-term high-fat feeding in mice and the differential expression pattern of the QRFP/GPR103b receptor pathway in different fat depots. Human omental fat also expresses the GPR103 receptor, and QRFP-43 relevantly inhibits ISO-induced lipolysis in human adipocytes.
The growing problem of obesity has forced investigators to focus on adipocytes as target. The quest for a cure for this condition has allowed the discovery and characterization of new GPCR peptidic ligands expressed and released by adipocytes that play a role in adipogenesis in an autocrine/paracrine manner. Among these, some are peptides also known for their central orexigenic effect, like NPY working through NPY1R (20) and NPY2R (21), and ghrelin working through GHS-R1a (22). These peptides increase adipogenesis to yield a global anabolic effect in agreement with their orexigenic attributes. Using an in vitro model (e.g. the 3T3-L1 cell line), a well-established reliable model of adipogenesis (23), we examined the mRNA expression levels of GPR103b and QRFP before and after the induction of adipocyte differentiation. Whereas differentiated 3T3-L1 adipocytes express exclusively the GPR103b receptor but not the GPR103a receptor subtype, neither subtypes were present in preadipocytes. Yet, the expression of GPR103b and QRFP, as measured by qPCR, increased during adipocyte differentiation. These data correlated with the detection of GPR103b protein expression measured by flow cytometry immunofluorescence in differentiated 3T3-L1 adipocytes.
The activation of GPR103b by QRFP was found to increase intracellular triglyceride accumulation in 3T3-L1 cells, which have been committed toward differentiation as shown by an approximate 1.5-fold increase in ORO-stained lipid droplets in QRFP-treated cells. In contrast, no effect of QRFP has been observed in nondifferentiated 3T3-L1 cells, in accordance with the absence of GPR103b in naive preadipocytes (data not shown). Both QRFP-26 and -43 regulated triglyceride metabolism in mature 3T3-L1 adipocytes, as shown by a potent antilipolytic action on ISO-induced lipolysis. The QRFP-28n fragment, which features weak binding affinity for GPR103 (4), was unable to inhibit ISO-induced lipolysis, thereby supporting a specific role for GPR103 in mediating the effect of QRFP. Using a retroviral-delivered shRNA to knockdown GPR103b, we demonstrated that both QRFP-43 and -26 could not inhibit ISO-induced lipolysis neither stimulate FA uptake in GPR103b knockdown cells. Overall, these results suggest that GPR103b promotes anabolism by increasing fat storage and by decreasing triglyceride hydrolysis in adipocytes. These observations suggest that QRFP may play a role in triglyceride metabolism similar to that of NPY and ghrelin. Indeed, both ghrelin (22, 24) and NPY (25) have been reported to inhibit ISO-induced lipolysis in isolated fat cells. In addition, both NPY and ghrelin have been reported to increase adipogenesis (21, 26) and food intake (27, 28, 29). In contrast, other RFamide peptides like NPFF, NPAF, and NPSF were shown to inhibit, rather than increase, adipocyte differentiation, by up-regulating the expression of Id3, a transcription factor that inhibits adipogenesis (15).
The role of LPL in catalyzing the hydrolysis of lipoproteins to generate free fatty acids that can be transported in adipocytes following an anabolic process is well established. LPL is regulated by hormones and nutrients in a tissue-specific manner (30, 31, 32). Among the hormones involved in the regulation of LPL activity, insulin was shown to regulate LPL activity in adipocytes by posttranscriptional and posttranslational mechanisms (19, 33, 34). Insulin decreases the intracellular pool of LPL and increases the cell surface-associated LPL released by heparin (33). Heparin-releasable LPL activity was increased significantly following treatment of 3T3-L1 adipocytes with QRFP-26. This increase of LPL activity promoted by QRFP was less than that mediated by insulin with a 3.4-fold increase in heparin-releasable LPL activity, in agreement with previous studies (19). In parallel to this observation, LPL activity within 3T3-L1 cells decreased after insulin and QRFP-26 treatment (data not shown). However, LPL mRNA expression was up-regulated by QRFP, suggesting a regulatory effect of QRFP on LPL expression and release. The LPL activity correlated with FA uptake and storage in adipocytes. Using RNA interference, Gonzalez and Orlando (35) demonstrated that a 50% loss of LPL activity led to a reduction of 80% in intracellular lipids. The relevancy of increased LPL activity by QRFP is confirmed by an increase in the cellular uptake of fluorescent free FA (C12-BODIPY) in 3T3-L1 adipocytes, as measured by flow cytometry (Fig. 2D). This increased cellular uptake of lipids was associated with an increase in FA transport proteins, CD36 and FATP1, mRNA levels that have been involved in the process of FA uptake in adipocytes (36). QRFP also induced the increase of ACSL1 expression, an enzyme which converts intracellular free long-chain FA into fatty acyl-CoA esters (37, 38, 39). This increase of ACSL1 correlated with the effect of QRFP on the increased expression of key transcription factors, such as PPAR-γ and C/EBP-α (Fig. 4), which control the expression of proteins involved in adipogenesis (40, 41, 42). In agreement, the up-regulation of adipogenic genes by QRFP was inhibited when GPR103b was knocked down. Taken together, these data suggest that genes involved in the storage of lipids within adipocytes are up-regulated in a GPR103b-dependent manner following a treatment with QRFP.
Our results show that the expression of GPR103b and QRFP are modulated in a state of obesity. In a mouse model of DIO, a model mimicking the most prevalent type of human obesity (43), the expression of GPR103b was strikingly increased in epididymal fat tissue compared with mice fed a normal diet, whereas no difference was observed in perirenal and inguinal fat tissues (Fig. 5A). The expression of GPR103b followed the same pattern in adipocytes isolated from epididymal fat tissue of DIO mice. This observation supports a role for GPR103b in metabolically active fat tissue such as abdominal fat, whereas its function appears to be less apparent in sc (inguinal) fat tissue, with a limited role in triglyceride metabolism (44). Using the same DIO mouse model, QRFP gene expression levels were found to be decreased in epididymal, perirenal, and inguinal fat tissues as well as in isolated adipocytes from HFD-fed mice. The down-regulation of QRFP mRNA in a state of obesity suggests that the expression of this peptide may be decreased by proinflammatory cytokines like TNF-α, as shown in differentiated 3T3-L1 cells (Supplemental Fig. 2), in a similar manner to that of adiponectin in a state of obesity (45, 46).
The unique human form of GPR103 is expressed in omental fat tissue and appears to mediate an inhibitory effect on ISO-induced lipolysis as documented in human isolated adipocytes. This inhibition was similar to that seen with ISO-induced lipolysis in 3T3-L1 cells. Interestingly, in contrast to insulin, QRFP did not show any effect on basal lipolysis of human omental adipocytes.
The pathophysiological relevancy of GPR103b in obesity has not been determined so far. A mouse with a gene deletion for GPR103a has been generated (8), but their body weight was normal. This is in agreement with the fact that GPR103a is not expressed in murine adipocytes. It also suggests that the role of GPR103a in the hypothalamus could be less important than that of GPR103b in the regulation appetite. Along that line, a GPR103b-deficient or a QRFP-deficient mouse would be helpful to assess the relative role of QRFP peptides in obesity. Insights regarding the intracellular pathways activated by GPR103, including cAMP production (3), pertussis toxin-sensitive Gi-protein (10), and Gq-protein (calcium mobilization) pathways (5) have been proposed in different cell types. Additional studies will be necessary to delineate the signaling pathways mediating the acute antilipolytic effect associated with GPR103b activation in adipocytes.
In this work, we reported a novel role for GPR103b as a peripheral modulator of adipogenesis that could function with QRFPs in an autocrine/paracrine manner. Nevertheless, more exhaustive in vivo studies are needed to confirm GPR103b and QRFP as potential targets for the treatment of obesity.
Materials and Methods
Animals
All experimental protocols were approved by the Institutional Animal Ethics Committee of the Université de Montréal, in accordance with the Canadian Council on Animal Care guidelines for use of experimental animals and the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (A5213-01). Wild-type C57BL/6 mice were weaned at 4 wk old and put on either normal chow (D12450B, 10% calories from fat) or a HFD (60% kcal from fat, D12492), from Research Diets Inc. (New Brunswick, NJ) for 18 or 24 wk as a model of diet-induced obesity. At 22 or 28 wk old, mice were killed by CO2 asphyxiation and adipose tissues from perirenal, inguinal, and epididymal depots were collected and kept at −80 C until RNA extraction.
Materials
QRFP peptides were obtained from a custom synthesis at the W.M. Keck Biotechnology Resource Center (New Haven, CT). Insulin, dexamethasone, adenosine, ISO, FA-free BSA (A7030), ORO, and 3-isobutyl-1-methylxanthine (IBMX) were from Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada). Cell culture media, fetal bovine serum (FBS), TRIzol, and PCR reagents were from Invitrogen (Burlington, Ontario, Canada). Rabbit IgG isotype control was from U.S. Biological (Swampscott, MS). R-phycoerythrin goat antirabbit IgG and 4,4-difluoro-5-methyl-4-bora-3a-diaza-3-indacene-3-dodecanoic acid (C12-BODIPY 3823) were from Molecular Probes Inc. (Eugene, OR). Rosiglitazone was from Cayman Chemical Co. (Ann Arbor, MI).
Cell culture
Murine 3T3-L1 preadipocytes (CL-173) were obtained from American Type Culture Collection (Manassas, VA). At confluence (designated as d 0), differentiation was induced by culturing cells with DMEM supplemented with 10% FBS, 167 nm (1 μg/ml) insulin, 1 μm dexamethasone, and 0.5 mm IBMX for 3 d (d 3). Differentiated cells were then cultured in DMEM with 10% FBS containing 167 nm insulin for 2 d (d 5). Cells were then were maintained in DMEM with 10% FBS for an additional 2 d (d 7).
mRNA expression of GPR103b during 3T3-L1 differentiation
Total RNA was extracted with TRIzol LS reagent and treated with DNase I Amplification Grade according to manufacturer’s instructions. cDNA was synthesized from DNase-treated total RNA with random primers and Moloney murine leukemia virus reverse transcriptase. For RT-PCR, primers for GPR103a, sense: 5′-TCT TTG GCA ACT CTC TGG TCA TC-3′, antisense: 5′-CTT CGG GTA GTG TAC TGC CAC T-3′; GPR103b, sense: 5′-CGA TAT CAA GTG GTG TGA ACA GCC-3′, antisense: 5′-GGG TCT CTT GTA GCC CAG GT-3′); QRFP, sense: 5′-TCT GCC GTC CTT ACC ATC TCA-3′, antisense: 5′-TCT CAG GAC TGT CCC AAA GGA G-3′; and RNA 18S, sense: 5′-GGA CCA GAG CGA AAG CAT TTG CC-3′, antisense: TCA ATC TCG GGT GGC TGA ACG C-3′) were used to assess mRNA expression levels at different stages during 3T3-L1 adipocytes differentiation, using Taq DNA polymerase. PCR products were separated on 1.5% agarose gel. qPCR of GPR103b and β-actin (sense: 5′-ATTACTGCTCTGGCTCCTA-3′, antisense: 5′-TCTGCTGGAAGGTGGACA-3′) were performed using a Platinum SYBR Green Supermix-UDG with Rox. Gene expression levels were quantified by SYBR Green real-time PCR using Rotor-Gene 3000 (Corbett Research, Mortlake, New South Wales, Australia). GPR103b mRNA levels were normalized to β-actin mRNA, and relative gene expression was calculated using the comparative Ct (2−ΔΔCt) method. Data are presented relative to the expression found in hypothalamus. A detailed description of primer sequences is found in Supplemental Table 1.
Detection of GPR103b by flow cytometry immunofluorescence in 3T3-L1 adipocytes
GPR103b was detected by a polyclonal rabbit antimouse GPR103b antibody generated in our laboratory by using the peptide GPR103b [Glu (400) to Cys (414)] coupled to keyhole limpet hemocyanin as immunogen. The specific anti-GPR103b Igs were purified by affinity on 6% cross-linked agarose coupled to the GPR103b [Glu (400) to Cys (414)] peptide. Trypsinized 3T3-L1 adipocytes were fixed with 2% formaldehyde in PBS and permeabilized with 0.5% Tween 20 in 0.02% BSA-PBS. Cells were first stained with rabbit antimouse GPR103b IgG (or preimmune purified IgG and rabbit IgG isotype used as controls; 20 μg/ml in permeabilization buffer) and then with R-phycoerythrin goat antirabbit IgG (4 μg/ml in permeabilization buffer). Fluorescence intensity was measured by flow cytometry with a FACSCalibur Becton Dickinson flow cytometer (BD Biosciences; San Jose, CA), and the results were analyzed using CellQuest software (BD).
ORO staining
At d 2 of differentiation, 3T3-L1 cells were cultured in DMEM with 10% FBS and incubated with 167 nm insulin and 10 nm QRFP-43 or QRFP-26 for 4 d. Cells were fixed for 1 h with 10% buffered formalin and stained with a solution of 0.6% (wt/vol) ORO in 60% isopropanol for 1 h. Cells were then washed 3 times, alternating between 60% isopropanol and water, and then photographed using Axio Imager microscope (Carl Zeiss Inc., Thornwood, NY). ORO stain was then eluted with isopropanol and quantified by measuring the optical density at 510 nm. The results are expressed as fold increase over control.
Fatty acid uptake (BODIPY 3823)
4,4-Difluoro-5-methyl-4-bora-3a-diaza-3-indacene-3-dodecanoic acid (C12-BODIPY 3823)-labeled FA was used for measuring long-chain FA uptake in differentiated 3T3-L1 adipocytes. Cells were starved for 1 h and then incubated with QRFP peptides (0.1, 10, and 1000 nm). After 2 h of treatment, cells were washed with PBS and incubated with PBS containing 20 μm FA-free BSA (Sigma A8806) and 10 μm C12-BODIPY- labeled FA for 2 min at 37 C. Cells were then washed extensively with PBS containing 0.1% BSA. Cells were suspended in cold DMEM buffered with 25 mm HEPES to pH 7.5 for cytometric analysis. BODIPY fluorescence intensity was measured by flow cytometry with a FACSCalibur flow cytometer, and the results were analyzed using CellQuest software.
LPL enzyme activity assay
Two days after the induction of differentiation, 3T3-L1 cells were incubated in DMEM supplemented with 5% of heat inactivated (55 C for 30 min) FBS for 24 h. Cells were then incubated, or not, with 167 nm insulin and QRFP-26 (10 and 1000 nm) for 2 d. Heparin (10 UI/ml) was added to the incubation media, which was collected after 30 min. The heparin-releasable LPL activity was measured with the LPL activity kit (Roar Biomedical Inc., New York, NY) using a nonfluorescent substrate emulsion that becomes intensely fluorescent upon interaction with LPL, according to the manufacturer’s instructions. Results are expressed as fold increase of relative enzyme activity normalized to DNA content.
Lipolysis experiments
3T3-L1 adipocytes were incubated at 37 C for 1 h with serum-free DMEM containing 200 nm adenosine as described by Viswanadha and Londos (47). The inhibitory effect of QRFP (preincubated for 30 min) on ISO-induced lipolysis in 3T3-L1 adipocytes was assessed in DMEM supplemented with 2% FA-free BSA containing adenosine deaminase (1 U/ml). After 30 min stimulation with ISO, the incubation medium was collected for free glycerol measurement using the free glycerol reagent (Sigma F6428) and total protein by the BCA protein assay (Pierce, Rockford, IL). The amount of glycerol released was calculated as μmol/mg protein and expressed as a percentage of ISO-induced lipolysis. The lipolysis index was calculated as follows: (sample free glycerol concentration − basal concentration)/ (ISO-elicited free glycerol concentration − basal concentration).
Knockdown of GPR103b in 3T3-L1 adipocytes
Specific shRNA constructs against mouse GPR103b were custom made by Origene Technologies (Rockville, MD). HuSH 29-mer for GPR103b were provided in the pRS plasmid driven by U6-RNA promoter. Selection of a single shRNA sequence for the production of retrovirus was based on the ability of the sequence to silence the expression of GPR103b mRNA in HEK293T cells transiently cotransfected with pFLAG-CMV2-mGPR103b generated in-house. The targeted sequence selected for retrovirus production was 5′-CATTTGTCCAGTGCACTGCCATTGTGACA-3′. Retroviruses (Moloney murine leukemia virus) were produced by transfecting HEK293T Phoenix Ampho cells (Orbigen, San Diego, CA) with the pRS-shRNA plasmids using Lipofectamine 2000 (Invitrogen). Medium containing infectious retroviruses was harvested 36 h after transfection, centrifuged to remove cell debris, and filtered on a 0.45-μm filter. The infection of 3T3-L1 preadipocytes with shGPR103b retroviruses has been carried out by adding retrovirus to the culture cells in the presence of 8 μg/ml Polybrene for 2 d. Control cells were infected with an empty vector retrovirus (pRS-Neo) and a nontargeting shRNA retrovirus (shcontrol). Infected cells were selected for their stable integration of the shRNA with 5 μg/ml puromycin. Infected 3T3-L1 cells were then differentiated in adipocytes as described above.
Isolation of primary adipose cells from mouse fat pads
For primary adipocytes isolation, epididymal, perirenal, and inguinal fat pads were minced and digested in Krebs-Ringer-Bicarbonate-HEPES buffer [20 mm HEPES (pH 7.4), 120 mm NaCl, 4.7 mm KCl, 1.2 mm K2HPO4, 2.5 mm CaCl2, 1.2 mm MgSO4, 24 mm NaHCO3] saturated with CO2 and containing 6 mm glucose, 1% BSA, and type 2 collagenase (2 mg/g tissue) (Sigma C6885). The cell suspension was filtered through a nylon mesh, and isolated adipocytes were washed three times with warm Krebs-Ringer-Bicarbonate-HEPES buffer containing 1% BSA. The adipocytes were kept at −80 C until RNA extraction.
Human subjects
Adipose tissue samples were obtained from obese adult subjects undergoing laparoscopic antiobesity surgery. Specimens of adipose tissue (2–5 g) from the abdominal omental region were obtained at the beginning of surgery (n = 5). No selection was made for age, sex, or body mass index. Age was between 40 and 50 yr. These subjects were included for studies on adipocyte differentiation. The study protocol was approved by the Local Ethical Committee (Department of Internal Medicine, University of Turin), and all subjects gave their written informed consent.
Expression of hGPR103 and lipolysis experiments in human omental adipocytes
Adipose tissue was minced into small pieces and digested at 37 C for 2 h in DMEM/Ham’s F12 medium with 750 μg/ml type 1 collagenase (Sigma-Aldrich, Milan, Italy). The tissue was then diluted with PBS and filtered through a 100-μm nylon mesh. The stromal cells were separated from the floating mature adipocytes by centrifugation. Total RNA was extracted as described above, and GPR103 mRNA was detected by RT-PCR in human omental mature adipocytes using the following primers: sense 5′-TAG GAT CAC CCA TGT GGC ACG T-3′ and antisense 5′-AAG AGA GCC ACC ACT GTC ACC ATC-3′, as previously described (10). The PCR products were analyzed on a 1.5% agarose gel stained with ethidium bromide. Amplification of human β-actin mRNA served as internal control, sense 5′-GGT CAT CTT CTC GCG GTT GGC CTT GGG GT-3′ and antisense 5′-CCC CAG GCA CCA GGG CGT GAT-3′. For lipolysis experiments, 40 μl of mature adipocytes were then collected and transferred into a 96-well plate containing 100 μl of Lipolysis Assay Kit Buffer (Zen-Bio, Research Triangle Park, NC) in the presence or absence of either QRFP-43 (1, 10, 100 nm) or insulin (100 nm). After 40 min, lipolysis was induced by addition of ISO (1 μm) for 80 min. Following the manufacturer’s instructions, 50 μl of medium were then transferred into a new 96-well plate for glycerol measurement. Results are expressed as percentage of glycerol release in the basal condition used as control.
Statistical analysis
Data are expressed as mean ± sem. Data were analyzed using nonlinear regression analysis software PRISM (GraphPad, San Diego, CA), and significance was tested using a one-way ANOVA with Dunnett’s post test. P < 0.05 was considered as statistically significant. For expression in isolated adipocytes and in different fat pads, an unpaired Student’s t test was used.
Acknowledgments
We thank Petra Pohankova (Faculty of Pharmacy, Université de Montréal) for her skillful technical assistance.
Footnotes
This work was supported by an educational grant from Aeterna-Zentaris, Inc. M.M. is a recipient of a scholarship from the Fonds de Recherche en Santé du Québec (FRSQ) and from the Groupe de Recherche Universitaire sur le Médicament (GRUM). C.J. is a recipient of a scholarship from the Rx&D Foundation and the Canadian Institutes of Health Research (CIHR). M.J.S. is a scholar from the FRSQ.
Disclosure Summary: H.O. received grant support from Aeterna-Zentaris, Inc. M.M., C.J., R.G., D.G., E.E., E.G., M.J.S., and S.M. have nothing to declare.
First Published Online June 9, 2010
M.M. and C.J. contributed equally to this work and should be considered as joint first authors.
Abbreviations: ACSL1, Acyl-CoA synthetase long-chain family member 1; C12-BODIPY 3823, 4,4-difluoro-5-methyl-4-bora-3a-diaza-3-indacene-3-dodecanoic acid; C/EBP-α, CCAAT/enhancer binding protein- α; DIO, diet-induced obesity; FA, fatty acid; FATP1, fatty acid transport protein-1; FBS, fetal bovine serum; GPR103, G protein-coupled receptor 103; HFD, high-fat diet; IBMX, 3-isobutyl-1-methylxanthine; ISO, isoproterenol; LPL, lipoprotein lipase; NPY, neuropeptide Y; ORO, Oil Red O; PPAR-γ, peroxisome proliferator-activated receptor-γ; qPCR, quantitative real-time PCR; shRNA, short hairpin RNA; WAT, white adipose tissue.
References
- 1.Lee DK, Nguyen T, Lynch KR, Cheng R, Vanti WB, Arkhitko O, Lewis T, Evans JF, George SR, O'Dowd BF2001. Discovery and mapping of ten novel G protein-coupled receptor genes. Gene 275:83–91 [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Luo L, Gustafson EL, Yadav D, Laverty M, Murgolo N, Vassileva G, Zeng M, Laz TM, Behan J, Qiu P, Wang L, Wang S, Bayne M, Greene J, Monsma Jr F, Zhang FL2003. Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. J Biol Chem 278:27652–27657 [DOI] [PubMed] [Google Scholar]
- 3.Chartrel N, Dujardin C, Anouar Y, Leprince J, Decker A, Clerens S, Do-Régo JC, Vandesande F, Llorens-Cortes C, Costentin J, Beauvillain JC, Vaudry H2003. Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci USA 100:15247–15252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukusumi S, Yoshida H, Fujii R, Maruyama M, Komatsu H, Habata Y, Shintani Y, Hinuma S, Fujino M2003. A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem 278:46387–46395 [DOI] [PubMed] [Google Scholar]
- 5.Takayasu S, Sakurai T, Iwasaki S, Teranishi H, Yamanaka A, Williams SC, Iguchi H, Kawasawa YI, Ikeda Y, Sakakibara I, Ohno K, Ioka RX, Murakami S, Dohmae N, Xie J, Suda T, Motoike T, Ohuchi T, Yanagisawa M, Sakai J2006. A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci USA 103:7438–7443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moriya R, Sano H, Umeda T, Ito M, Takahashi Y, Matsuda M, Ishihara A, Kanatani A, Iwaasa H2006. RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinology 147:2916–2922 [DOI] [PubMed] [Google Scholar]
- 7.Primeaux SD, Blackmon C, Barnes MJ, Braymer HD, Bray GA2008. Central administration of the RFamide peptides, QRFP-26 and QRFP-43, increases high fat food intake in rats. Peptides 29:1994–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baribault H, Danao J, Gupte J, Yang L, Sun B, Richards W, Tian H2006. The G-protein-coupled receptor GPR103 regulates bone formation. Mol Cell Biol 26:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navarro VM, Fernández-Fernández R, Nogueiras R, Vigo E, Tovar S, Chartrel N, Le Marec O, Leprince J, Aguilar E, Pinilla L, Dieguez C, Vaudry H, Tena-Sempere M2006. Novel role of 26RFa, a hypothalamic RFamide orexigenic peptide, as putative regulator of the gonadotropic axis. J Physiol 573:237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egido EM, Hernández R, Leprince J, Chartrel N, Vaudry H, Marco J, Silvestre RA2007. 26RFa, a novel orexigenic neuropeptide, inhibits insulin secretion in the rat pancreas. Peptides 28:725–730 [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Wada T, Miyazaki R2008. Analgesic effects of intrathecally administered 26RFa, an intrinsic agonist for GPR103, on formalin test and carrageenan test in rats. Neuroscience 157:214–222 [DOI] [PubMed] [Google Scholar]
- 12.Lectez B, Jeandel L, El-Yamani FZ, Arthaud S, Alexandre D, Mardargent A, Jégou S, Mounien L, Bizet P, Magoul R, Anouar Y, Chartrel N2009. The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus. Endocrinology 150:2342–2350 [DOI] [PubMed] [Google Scholar]
- 13.Zhang Q, Qiu P, Arreaza MG, Simon JS, Golovko A, Laverty M, Vassileva G, Gustafson EL, Rojas-Triana A, Bober LA, Hedrick JA, Monsma Jr FJ, Greene JR, Bayne ML, Murgolo NJ2007. P518/Qrfp sequence polymorphisms in SAMP6 osteopenic mouse. Genomics 90:629–635 [DOI] [PubMed] [Google Scholar]
- 14.Fukusumi S, Fujii R, Hinuma S2006. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides 27:1073–1086 [DOI] [PubMed] [Google Scholar]
- 15.Herrera-Herrera ML, Salazar-Olivo LA2008. RFamide neuropeptides inhibit murine and human adipose differentiation. Biochem Biophys Res Commun 377:29–34 [DOI] [PubMed] [Google Scholar]
- 16.Lefrere I, De CP, Camelin JC, Le Lay S, Mercier N, Elshourbagy N, Bril A, Berrebi-Bertrand I, Feve B, Krief S2002. Neuropeptide AF and FF modulation of adipocyte metabolism. Primary insights from functional genomics and effects on β-adrenergic responsiveness. J Biol Chem 277:39169–39178 [DOI] [PubMed] [Google Scholar]
- 17.Dockray GJ2004. The expanding family of RFamide peptides and their effects on feeding behaviour. Exp Physiol 89:229–235 [DOI] [PubMed] [Google Scholar]
- 18.Bechtold DA, Luckman SM2007. The role of RFamide peptides in feeding. J Endocrinol 192:3–15 [DOI] [PubMed] [Google Scholar]
- 19.Spooner PM, Chernick SS, Garrison MM, Scow RO1979. Insulin regulation of lipoprotein lipase activity and release in 3T3-L1 adipocytes. Separation and dependence of hormonal effects on hexose metabolism and synthesis of RNA and protein. J Biol Chem 254:10021–10029 [PubMed] [Google Scholar]
- 20.Yang K, Guan H, Arany E, Hill DJ, Cao X2008. Neuropeptide Y is produced in visceral adipose tissue and promotes proliferation of adipocyte precursor cells via the Y1 receptor. FASEB J 22:2452–2464 [DOI] [PubMed] [Google Scholar]
- 21.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z2007. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13:803–811 [DOI] [PubMed] [Google Scholar]
- 22.Choi K, Roh SG, Hong YH, Shrestha YB, Hishikawa D, Chen C, Kojima M, Kangawa K, Sasaki S2003. The role of ghrelin and growth hormone secretagogues receptor on rat adipogenesis. Endocrinology 144:754–759 [DOI] [PubMed] [Google Scholar]
- 23.Ntambi JM, Young-Cheul K2000. Adipocyte differentiation and gene expression. J Nutr 130:3122S–3126S [DOI] [PubMed] [Google Scholar]
- 24.Muccioli G, Pons N, Ghè C, Catapano F, Granata R, Ghigo E2004. Ghrelin and des-acyl ghrelin both inhibit isoproterenol-induced lipolysis in rat adipocytes via a non-type 1a growth hormone secretagogue receptor. Eur J Pharmacol 498:27–35 [DOI] [PubMed] [Google Scholar]
- 25.Valet P, Berlan M, Beauville M, Crampes F, Montastruc JL, Lafontan M1990. Neuropeptide Y and peptide YY inhibit lipolysis in human and dog fat cells through a pertussis toxin-sensitive G protein. J Clin Invest 85:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson NM, Gill DA, Davies R, Loveridge N, Houston PA, Robinson IC, Wells T2004. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 145:234–242 [DOI] [PubMed] [Google Scholar]
- 27.Clark JT, Kalra PS, Crowley WR, Kalra SP1984. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115:427–429 [DOI] [PubMed] [Google Scholar]
- 28.Clark JT, Kalra PS, Kalra SP1985. Neuropeptide Y stimulates feeding but inhibits sexual behavior in rats. Endocrinology 117:2435–2442 [DOI] [PubMed] [Google Scholar]
- 29.Tschöp M, Smiley DL, Heiman ML2000. Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- 30.Preiss-Landl K, Zimmermann R, Hämmerle G, Zechner R2002. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr Opin Lipidol 13:471–481 [DOI] [PubMed] [Google Scholar]
- 31.Merkel M, Eckel RH, Goldberg IJ2002. Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res 43:1997–2006 [DOI] [PubMed] [Google Scholar]
- 32.Amri EZ, Teboul L, Vannier C, Grimaldi PA, Ailhaud G1996. Fatty acids regulate the expression of lipoprotein lipase gene and activity in preadipose and adipose cells. Biochem J 314(Pt 2):541–546 [DOI] [PMC free article] [PubMed]
- 33.Semenkovich CF, Wims M, Noe L, Etienne J, Chan L1989. Insulin regulation of lipoprotein lipase activity in 3T3-L1 adipocytes is mediated at posttranscriptional and posttranslational levels. J Biol Chem 264:9030–9038 [PubMed] [Google Scholar]
- 34.Knutson VP2000. The release of lipoprotein lipase from 3T3-L1 adipocytes is regulated by microvessel endothelial cells in an insulin-dependent manner. Endocrinology 141:693–701 [DOI] [PubMed] [Google Scholar]
- 35.Gonzales AM, Orlando RA2007. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab (Lond) 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abumrad N, Coburn C, Ibrahimi A1999. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta 1441:4–13 [DOI] [PubMed] [Google Scholar]
- 37.Shimomura I, Tokunaga K, Jiao S, Funahashi T, Keno Y, Kobatake T, Kotani K, Suzuki H, Yamamoto T, Tarui S1992. Marked enhancement of acyl-CoA synthetase activity and mRNA, paralleled to lipoprotein lipase mRNA, in adipose tissues of Zucker obese rats (fa/fa). Biochim Biophys Acta 1124:112–118 [DOI] [PubMed] [Google Scholar]
- 38.Richards MR, Harp JD, Ory DS, Schaffer JE2006. Fatty acid transport protein 1 and long-chain acyl coenzyme A synthetase 1 interact in adipocytes. J Lipid Res 47:665–672 [DOI] [PubMed] [Google Scholar]
- 39.Gargiulo CE, Stuhlsatz-Krouper SM, Schaffer JE1999. Localization of adipocyte long-chain fatty acyl-CoA synthetase at the plasma membrane. J Lipid Res 40:881–892 [PubMed] [Google Scholar]
- 40.Cowherd RM, Lyle RE, McGehee Jr RE1999. Molecular regulation of adipocyte differentiation. Semin Cell Dev Biol 10:3–10 [DOI] [PubMed] [Google Scholar]
- 41.Kudo M, Sugawara A, Uruno A, Takeuchi K, Ito S2004. Transcription suppression of peroxisome proliferator-activated receptor γ2 gene expression by tumor necrosis factor α via an inhibition of CCAAT/ enhancer-binding protein delta during the early stage of adipocyte differentiation. Endocrinology 145:4948–4956 [DOI] [PubMed] [Google Scholar]
- 42.Patel SR, Murphy KG, Thompson EL, Patterson M, Curtis AE, Ghatei MA, Bloom SR2008. Pyroglutamylated RFamide peptide 43 stimulates the hypothalamic-pituitary-gonadal axis via gonadotropin-releasing hormone in rats. Endocrinology 149:4747–4754 [DOI] [PubMed] [Google Scholar]
- 43.Tschöp M, Heiman ML2001. Rodent obesity models: an overview. Exp Clin Endocrinol Diabetes 109:307–319 [DOI] [PubMed] [Google Scholar]
- 44.Lafontan M, Berlan M2003. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci 24:276–283 [DOI] [PubMed] [Google Scholar]
- 45.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B2003. Regulation of adiponectin by adipose tissue- derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab 285:E527–E533 [DOI] [PubMed]
- 46.Fasshauer M, Klein J, Neumann S, Eszlinger M, Paschke R2002. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun 290:1084–1089 [DOI] [PubMed] [Google Scholar]
- 47.Viswanadha S, Londos C2006. Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res 47:1859–1864 [DOI] [PubMed] [Google Scholar]