Abstract
Although genetic evidence demonstrated a requirement for Wnt5a during cartilage development, little is known about the mechanisms underlying Wnt5a-regulated chondrocyte growth and differentiation. We therefore investigated the signaling pathways by which Wnt5a influences chondrogenesis and differentiation to hypertrophy. Wnt5a treatment of chondroprogenitor cells increased chondrocyte hypertrophy and was associated with an increase in nuclear factor of activated T cells (NFAT) and a decrease in nuclear factor-κB (NF-κB) activation. In contrast, Wnt5a inhibited chondrocyte hypertrophy. This inhibition of hypertrophy occurred with the reciprocal signaling activation, in that a decrease in NFAT and an increase in NF-κB activation was observed. Furthermore, the increase in chondroprogenitor cell differentiation with Wnt5a treatment was blocked by calmodulin kinase or NFAT loss of function. In addition, the repression of chondrocyte hypertrophy observed was abrogated by NF-κB loss of function. Activation of the NFAT pathway downstream of Wnt5a also negatively regulated NF-κB activity, providing evidence of antagonism between these two pathways. Mechanistically, Wnt5a acts to increase chondrocyte differentiation at an early stage through calmodulin kinase /NFAT-dependent induction of Sox9. Conversely, Wnt5a represses chondrocyte hypertrophy via NF-κB-dependent inhibition of Runx2 expression. These data indicate that Wnt5a regulates chondrogenesis and chondrocyte hypertrophy in a stage-dependent manner through differential utilization of NFAT- and NF-κB-dependent signal transduction.
Non-canonical Wnt5a signaling stage-dependently regulates chondrogenesis and chondrocyte hypertrophy through differential utilization of NFAT- and NF-kB-dependent signal transduction.
Wnt family members are secreted glycoproteins that induce signal transduction through interaction with frizzled-containing receptor complexes (1). Noncanonical Wnt receptor/coreceptor interaction triggers distinct intracellular signaling independent of the β-catenin pathway used by canonical Wnts (2, 3, 4). The effects of noncanonical Wnts are exerted through two primary signal transduction cascades, the calcium-dependent pathway and the planar cell polarity pathway (5). In noncanonical Wnt calcium-dependent signaling, activation of phospholipase C through a G protein-dependent mechanism facilitates a rise in intracellular calcium concentrations. This increase in calcium then leads to the activation of several known calcium-sensitive effectors, including calmodulin kinase (CaMK), calcineurin (CaN), cAMP response element-binding protein (CREB), and protein kinase C (6, 7, 8). Although most evidence has been generated in Drosophila, noncanonical Wnts can also exert their effects through the planar cell polarity pathway to control cellular adhesion and motility. Through activation of the planar cell polarity pathway, noncanonical Wnts regulate cellular polarity (9). Noncanonical Wnt5a also activates phosphatidylinositol 3-kinase (PI3K)/Akt-dependent signaling, thereby potentially activating the IκB kinase (IKK)/nuclear factor-κB (NF-κB) pathway (10). In addition to the direct effects of these signal transduction pathways, Wnt5a-dependent signaling in chondrocytes also represses β-catenin-mediated signaling by promoting β-catenin degradation (11).
The noncanonical Wnt5a is expressed in chondrogenic regions during limb development (12). Genetic evidence has shown that Wnt5a is required for skeletal development (13). Postnatally, Wnt5a regulates longitudinal bone growth by controlling the recruitment of mesenchymal stem cells, chondrocyte proliferation, and the transitions into prehypertrophic and hypertrophic stages (14). In addition to an essential role during development, haploinsufficiency of Wnt5a also results in decreased bone mass and increased marrow adipogenesis, demonstrating the ability of Wnt5a to govern mesenchymal stem cell lineage commitment (15). Wnt5a has been shown to specifically promote entry into the prehypertrophic phase, whereas it conversely blocks chondrocyte hypertrophy (14, 16), thus acting in a stage-specific context. However, the mechanisms and downstream effectors governing this regulation of cartilage development are mostly uncharacterized. Available information focuses on the ability of the noncanonical pathway to regulate the function of β-catenin-mediated signaling. In the present study the noncanonical downstream effectors of Wnt5a-mediated signaling and their roles in chondrogenesis and chondrocyte maturation to hypertrophy were explored.
Sox9 is a high-mobility group domain transcription factor required for the onset of chondrogenesis and expression of Collagen type 2a (17). Inhibitors of chondrogenesis such as TNF-α and IL-1 have been shown to block Sox9 expression through an NF-κB-dependent mechanism (18). Although Sox9 is a master transcriptional regulator of chondrogenesis, additional transcriptional regulators, such as nuclear factor of activated T cells (NFAT)4, also induce chondrogenesis (19). Runx2 is a master regulator of skeletal development (20, 21). Whereas the cartilage anlagen is fully formed in Runx2-null mice, chondrocyte hypertrophy is reduced, and mineralized bone tissue development is abrogated in these animals (20, 21). Canonical Wnt signaling regulates skeletal development through differential regulation of Runx2 and Sox9 (22, 23). We have previously reported that canonical Wnt signaling enhances chondrocyte hypertrophy by induction of Runx2 transcription (24). Interestingly, although both Wnt5a and Runx2 transcripts are coexpressed in mouse limb chondrocytes, Runx2 levels are inhibited in the limbs of Wnt5a-null mice (20).
Here we describe mechanisms governing the stage-specific roles of Wnt5a in chondrocyte differentiation and further elucidate the effects that Wnt5a has on chondrogenesis and chondrocyte hypertrophy. Specifically, Wnt5a utilizes the CaN/NFAT pathway to promote chondrogenesis through induction of Sox9. Conversely, Wnt5a also represses chondrocyte hypertrophy through novel induction of NF-κB, in turn, to repress expression of Runx2. This stage-dependent activation of two distinct signal transduction pathways by this single growth factor is further controlled through NFAT-mediated antagonism of the NF-κB pathway.
Results
Wnt5a regulates chondrocyte maturation and expression of Sox9 and Runx2 in a stage-dependent manner
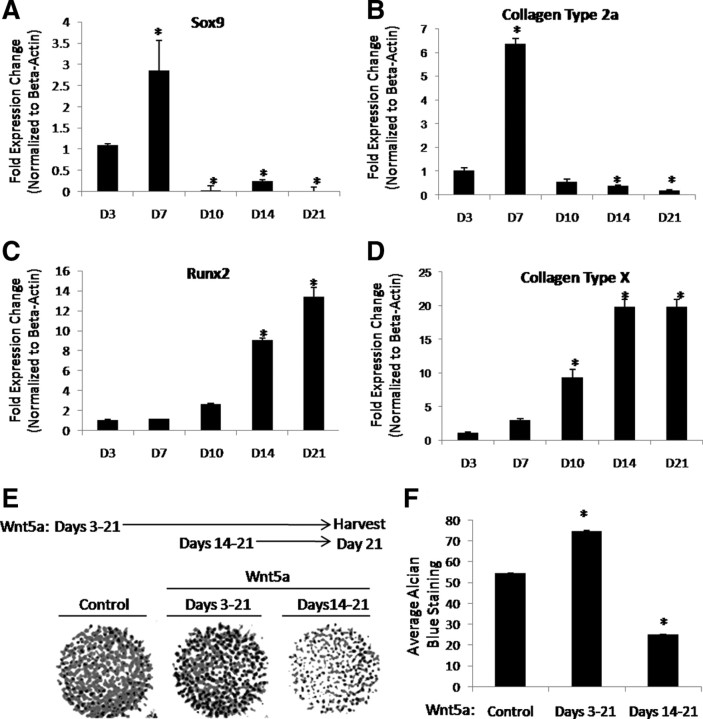
Limb bud-derived cells were harvested from embryonic d 11.5 (E11.5) mouse embryos and cultured in high-density micromass to promote chondrocyte differentiation (Fig. 1). Cultures were collected after 3–21 d and assayed for expression of Sox9, type 2a collagen, Runx2, and type X collagen by real-time RT-PCR (Fig. 1, A–D). Although expression of Sox9 and type 2a collagen, markers of the onset of chondrogenesis, were up-regulated early during differentiation, expression was markedly down-regulated with further culture time. Conversely, expression of Runx2 and type X collagen, markers of chondrocyte hypertrophy, was minimally expressed early during differentiation and then robustly induced subsequent to down-regulation of Sox9 and type 2a collagen. We further defined d 3–d 7 as the period encompassing the onset of chondrogenesis in this model system. Conversely, we defined d 14–d 21 as hypertrophic chondrocytes.
Fig. 1.
Wnt5a differentially regulates maturation of E11.5 limb bud cultures. A–D, Limb bud time course. E11.5 limb bud cells were cultured in micromass. RNA was harvested from cultures as indicated and used to assay for expression of Sox9 (A), Collagen Type 2a (B), Runx2 (C), and Collagen Type X (D) via real-time RT-PCR. *, P < 0.05. E, Wnt5a stage-dependently regulates chondrocyte maturation. Limb bud micromass cultures were treated with Wnt5a as indicated or vehicle control and cultured for 21 d and then stained with alcian blue. F, Quantitation of the average amount of alcian blue staining (±sem). *, P < 0.05.
We then selected d 3 and d 14 to determine the effects of Wnt5a on end-stage chondrocyte differentiation of chondroprogenitor and hypertrophic cells, respectively. Thus, limb bud micromass cultures were treated with Wnt5a or vehicle control at either an immature stage before the induction of Sox9 and collagen type 2a (d 3) or a hypertrophic stage (d 14). Micromass cultures were then maintained in the presence of Wnt5a through the duration of culture to determine how exposure to Wnt5a during the phases of chondrogenesis and chondrocyte hypertrophy affected overall cartilage development. An increase in proteoglycan matrix deposition, as measured by alcian blue staining, occurred when micromass cultures were treated with Wnt5a for d 3–21 of differentiation (Fig. 1, E and F). We did not observe an overt change in cell number under these conditions, indicating that the increase in proteogycan staining was due to increased differentiation and matrix deposition (data not shown). In contrast, treatment with Wnt5a from d 14–21 during hypertrophy decreased alcian blue staining (Fig. 1, E and F). These results suggest that Wnt5a regulates chondrocyte differentiation in a stage-dependent manner by promoting early chondrocyte differentiation while repressing chondrocyte hypertrophy.
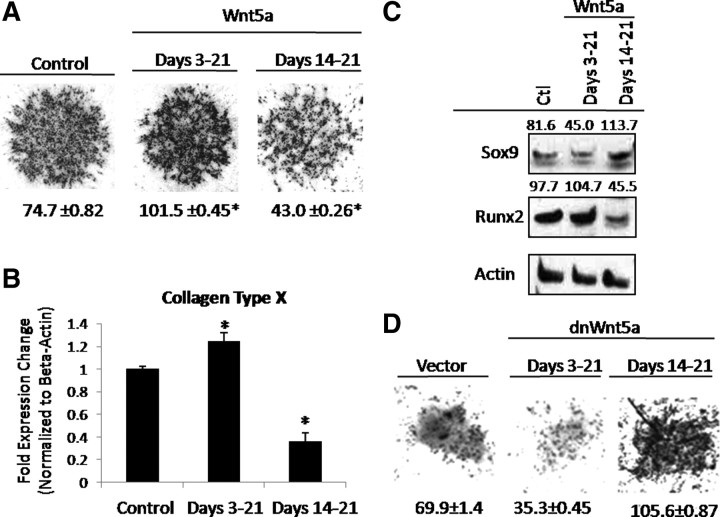
To address the molecular mechanism governing this differential effect of Wnt5a, the chondroprogenitor cell line, MLB13MYC Clone17 cells (C17), was used as a substitute to primary limb bud cultures. This cell line was established using murine limb bud-derived mesenchymal cells and therefore represents a comparable culture model to primary micromass cultures (25). Thus, when cultured in micromass, these cells also exhibit temporal changes in the chondrocyte phenotypic markers Sox9, type 2a collagen, Runx2, and type X collagen compared with the limb bud-derived cultures (data not shown). The effects of Wnt5a on chondrogenesis and chondrocyte hypertrophy of C17 micromass cultures were then assessed (Fig. 2, A and B). Consistent with the limb bud model, when C17 cultures were treated with Wnt5a during d 3–d 21, we observed an increase in alcian blue staining along with a modest but significant increase in expression of type X collagen. Conversely, treatment with Wnt5a from d 14– d 21 decreased alcian blue staining. This decrease in alcian blue staining was associated with a decrease in expression of type X collagen. We did not observe a significant change in expression of MMP13 or VEGF, implying that the decrease in proteoglycan staining was attributable to a true decrease in chondrocyte differentiation and was not due to matrix degradation (data not shown). These results mirror those obtained in primary limb bud-derived cultures and those previously described by in vivo gain and loss of Wnt5a function (14).
Fig. 2.
Wnt5a differentially regulates maturation of C17 limb bud cultures. A, Wnt5a regulates chondrocyte maturation in a stage-dependent manner. C17 micromass cultures were treated with Wnt5a or vehicle control (Ctl) as indicated and cultured for 21 d and then stained with alcian blue. Quantitation of the amount of alcian blue staining (±sem) is shown below each micromass culture. *, P < 0.05. B, C17 cells were cultured in micromass and treated with Wnt5a or vehicle control as indicated and cultured for 21 d. RNA was harvested from cultures as indicated and used to assay for expression of Collagen Type X via real-time RT-PCR (±sem). *, P < 0.05. C, Cultures were harvested, and Western blotting for levels of Sox9 and Runx2 was performed. Quantitation of the Sox9 and Runx2 signal normalized to actin is shown above each corresponding band. D, Inhibition of Wnt5a stage-dependently affects chondrocyte maturation in a stage-dependent manner. C17 micromass cultures were infected with a dnWnt5a lentivirus or vector control at an MOI of 2 as indicated. Micromass cultures were maintained for 21 d and then stained with alcian blue. Quantitation of the amount of alcian blue staining (±sem) is shown below each micromass. *, P < 0.05.
Because treatment with Wnt5a during d 3–21 during a period concomitant with induction of chondrogenic marker expression promoted chondrocyte differentiation, the ability of Wnt5a to regulate Sox9 expression in a stage-dependent manner was assessed (Fig. 2C). Limb bud micromass cultures were treated with Wnt5a or vehicle control either before the induction of Sox9 and collagen type 2a (d 3–21) or at a hypertrophic stage before maximal induction of Runx2 and type X collagen (d 14–21) as described above. Micromass cultures were then maintained in the presence of Wnt5a through the duration of culture. Treatment with Wnt5a during d 3–21 of differentiation decreased expression of Sox9, supporting the role of Wnt5a in accelerating chondrocyte differentiation. In contrast, treatment with Wnt5a at a hypertrophic stage increased Sox9 expression, suggesting again that Wnt5a inhibits chondrocyte hypertrophy.
Given that treatment with Wnt5a at a hypertrophic stage of chondrocyte differentiation repressed chondrocyte matrix deposition, the ability of Wnt5a to regulate Runx2 expression in a stage-dependent manner was examined (Fig. 2C). Micromass cultures were treated with Wnt5a either from d 3–21 or d 14–21 of differentiation as described above, and expression of Runx2 was evaluated. Treatment with Wnt5a early during differentiation modestly increased expression of Runx2, an observation in line with the role of Wnt5a in promoting early chondrocyte differentiation. In contrast, treatment with Wnt5a at a hypertrophic stage decreased Runx2 expression, suggesting that Wnt5a represses chondrocyte hypertrophy.
Because Wnt5a is expressed by chondrocytes and may act in paracrine, we further validated the differential effect of Wnt5a on chondrocyte differentiation by using a previously established dominant negative (dn) form of Wnt5a to determine the effects of loss of Wnt5a function on chondrocyte differentiation (Fig. 2D) (26). We validated the functionality of this approach and demonstrated that expression of dnWnt5a blocks Wnt5a-induced signaling (data not shown). Expression of dnWnt5a early during differentiation decreased alcian blue staining. Conversely, expression of dnWnt5a during chondrocyte hypertrophy robustly enhanced alcian blue staining. Overall, these data suggested that Wnt5a may promote early chondrocyte differentiation and acts to inhibit chondrocyte hypertrophy.
The stage-dependent effects of Wnt5a are mediated by differential use of the CaN/NFAT and IKK/NF-κB pathways
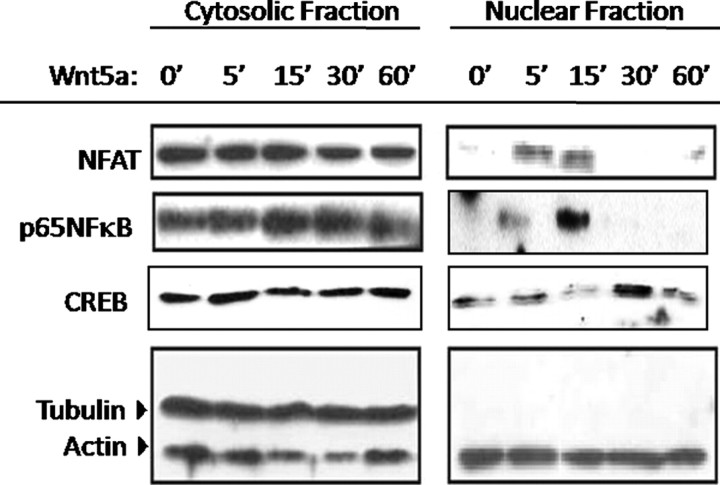
To explore the mechanism for the differential effects of Wnt5a on end-stage differentiation of chondroprogenitor cells and hypertrophic chondrocytes, we first identified potential pathways activated by Wnt5a. We assayed for activation of CREB and NFAT, two documented downstream targets of Wnt5a (6, 7). Because Wnt5a was shown to activate PI3K/Akt signaling, we also assayed for activation of the downstream transcription factor p65 NF-κB. Chondroprogenitor cells were serum starved and treated with Wnt5a for 0–60 min as shown and fractionated into cytosolic and nuclear extracts. Each fraction was then assayed for NFAT, NF-κB, and CREB levels via Western blotting (Fig. 3). Wnt5a induced transient nuclear translocation of each of the indicated transcription factors following serum starvation, indicating their potential roles as downstream effectors of Wnt5a.
Fig. 3.
Wnt5a induces nuclear translocation of NFAT and NF-κB in C17 chondrogenic cells. C17 cells were plated in six-well plates at a density of 5 × 105 cells per well and cultured in low serum medium overnight. After serum starvation, cells were treated with 50 ng/ml Wnt5a as shown. Cells were harvested into cytosolic and nuclear fractions that were probed for NFAT and NF-κB.
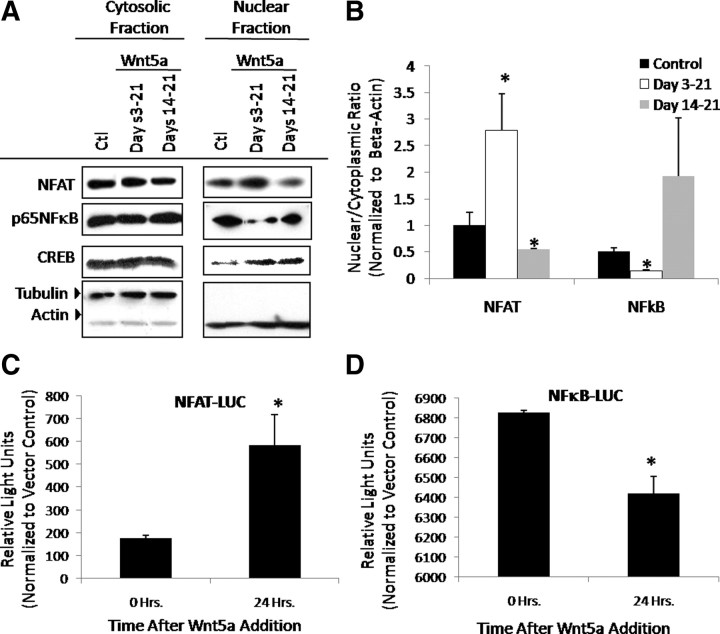
Because stage-dependent effects of Wnt5a were evident, downstream signaling cascades were then assayed to determine whether activation of Wnt5a-mediated signaling was also regulated in a stage-dependent manner (Fig. 4). To determine the overall activation state of each pathway as opposed to a transient response elicited by Wnt5a after serum starvation as described above in Fig. 3, micromass cultures were treated with Wnt5a on d 3–21 or d 14–21 as described above. Nuclear localization of the Wnt5a-responsive transcription factors NFAT and NF-κB under these conditions was then assayed and compared with control to determine whether exposure to Wnt5a early vs. late altered overall cartilage development via changes in pathway utilization (Fig. 4, A and B). Treatment with Wnt5a during d 3–d 21 enhanced nuclear NFAT. Conversely, Wnt5a treatment during d 14–21 decreased nuclear NFAT. In contrast to NFAT, treatment with Wnt5a during d 3–21 decreased nuclear NF-κB, whereas d 14–21 Wnt5a treatment led to a trend of increased NF-κB to levels compared with control conditions. Stage-dependent nuclear localization of CREB in response to early vs. late Wnt5a treatment was not observed, emphasizing the specificity of this observed response. These data suggested that the stage-dependent effects of Wnt5a may be accomplished through differential pathway activation.
Fig. 4.
Wnt5a stage-dependently utilizes the NFAT and NF-κB pathways in a stage-dependent manner. A and B, Wnt5a stage-dependently regulates NFAT and NF-κB nuclear localization in a stage-dependent manner. C17 micromass cultures were treated with Wnt5a or vehicle control (Ctl) as indicated and cultured for 21 d. Cultures were harvested and fractionated into nuclear and cytoplasmic extracts. Western blotting for levels of NFAT, NF-κB, and CREB in each fraction was performed (A), and the nuclear to cytoplasmic ratio was determined (B) Values are the mean of three independent experiments ± sem. *, P < 0.05. C and D, Wnt5a differentially regulates NFAT and NF-κB Activity. C17 cells were transfected with NFAT (C), NF-κB (D), or vector control luciferase (LUC) reporter constructs and treated with Wnt5a as indicated. After each treatment, cells were harvested in passive lysis buffer, and luciferase activity was determined. *, P < 0.05.
To further support the stage-dependent utilization of NFAT and NF-κB-dependent signaling, the responsiveness of an NFAT- and NF-κB-driven luciferase reporter constructs to Wnt5a was also ascertained (Fig. 4, C and D). Whereas Wnt5a induced luciferase activity driven by a multimerized NFAT-responsive element in C17 chondroprogenitor cells, luciferase activity downstream of the NF-κB-responsive element was repressed by Wnt5a. Thus, Wnt5a differentially used the NFAT and NF-κB pathways.
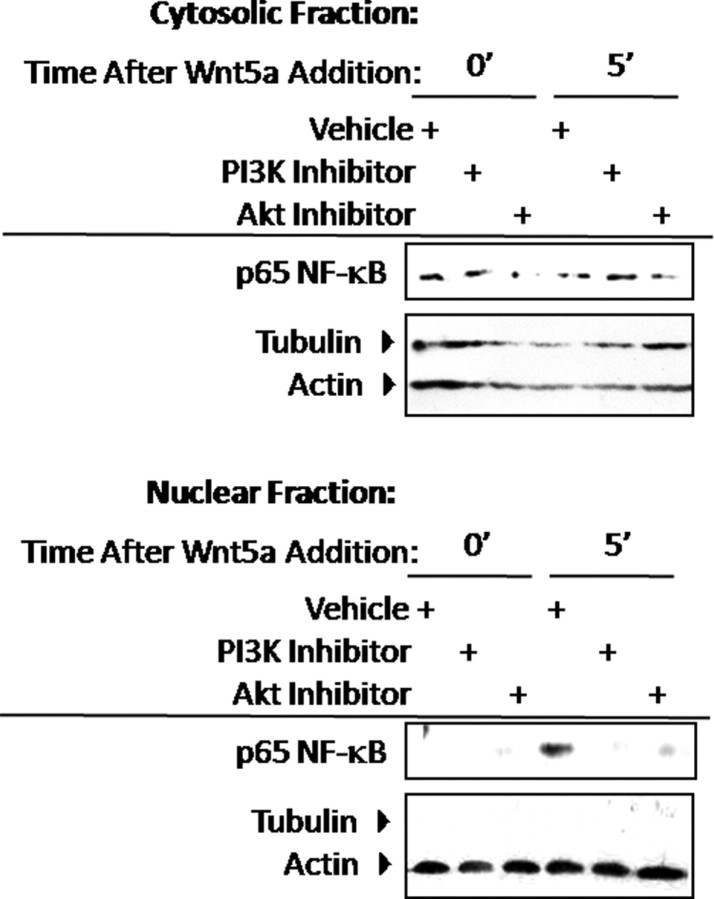
Having observed NF-κB activation in response to Wnt5a, we ascertained the upstream signaling events elicited by Wnt5a that were required to mediate NF-κB activation. Because growth factor mediated activation of NF-κB is accomplished by activation of PI3K/Akt and a previous report had demonstrated that Wnt5a can activate PI3K/Akt, we determined the role of this pathway in mediating NF-κB activation downstream of Wnt5a (Fig. 5). To explore this possibility, chondrogenic precursor cells were preincubated with each indicated inhibitor or vehicle control for 1 h under serum-starved conditions (Fig. 5). Precursor cells were then treated with Wnt5a for 0 or 5 min in the presence of each inhibitor and fractionated into nuclear and cytoplasmic extracts. Whereas treatment with Wnt5a induced nuclear translocation of NF-κB after 5 min, this response was abrogated by inhibition of PI3K or Akt (Fig. 5). NF-κB nuclear localization induced by Wnt5a was also decreased in d 14 hypertrophic chondrocytes treated with a PI3K inhibitor, indicating that the same mode of activation occurs in both immature and mature chondrocytes (data not shown).
Fig. 5.
PI3K/Akt mediates NF-κB activation in response to Wnt5a. C17 cells were serum starved and treated with Wnt5a or vehicle control in the presence of each indicated inhibitor. After Wnt5a treatment, cells were harvested and fractionated into nuclear and cytoplasmic extracts. Western blotting for levels of NF-κB in each fraction was performed.
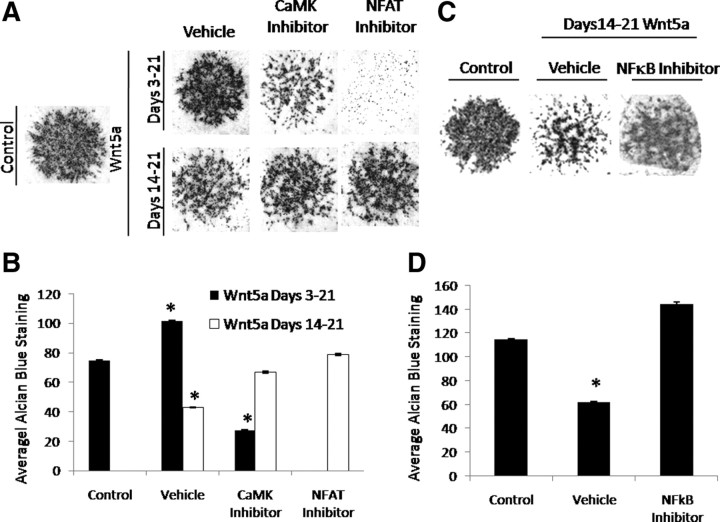
Given that Wnt5a treatment induced differential nuclear localization of both NFAT and NF-κB, the requirement of each of these signaling pathways in mediating the stage-dependent effects of Wnt5a on chondrocyte differentiation was assayed (Fig. 6). Micromass cultures were treated with Wnt5a from d 3–21 or d 14–21 in the presence of chemical inhibitors directed toward CaMK, NFAT, and NF-κB or vehicle control (Fig. 6). Although Wnt5a treatment at an immature stage enhanced alcian blue staining, this effect was significantly abrogated by inhibition of either CaMK or NFAT (Fig. 6, A and B). In contrast, the inhibitory effect of Wnt5a on late chondrocyte differentiation was prevented by CaMK or NFAT inhibition (Fig. 6, A and B). Inhibition of the NF-κB pathway on d 3–21 early during chondrocyte differentiation resulted in cellular death because NF-κB activity is required to promote survival of differentiating chondrocytes (27). However, treatment with Wnt5a during d 14–21 of differentiation decreased alcian blue staining. This effect of Wnt5a on late chondrocyte differentiation was abolished through inhibition of NF-κB (Fig. 6, C and D). These results demonstrate the requirement of the CaMK/NFAT and IKK/NF-κB pathways in mediating the differential effects of Wnt5a on chondrocyte differentiation.
Fig. 6.
NFAT and NF-κB are required for the stage-dependent effects of Wnt5a. A, The CaMK/NFAT pathway is required for the enhancement mediated by Wnt5a early during chondrocyte differentiation. C17 micromass cultures were treated with Wnt5a or vehicle control in the presence of each indicated inhibitor or vehicle control as indicated. Cultures were maintained for 21 d and then stained with alcian blue. B, Quantitation of the amount of alcian blue staining. *, P < 0.05. C, The NF-κB pathway is required for the repression mediated by Wnt5a late during chondrocyte differentiation. C17 micromass cultures were treated with Wnt5a or vehicle control in the presence of an NF-κB inhibitor or vehicle control as indicated. Cultures were maintained for 21 d and then stained with alcian blue. D, Quantitation of the amount of alcian blue staining. *, P < 0.05.
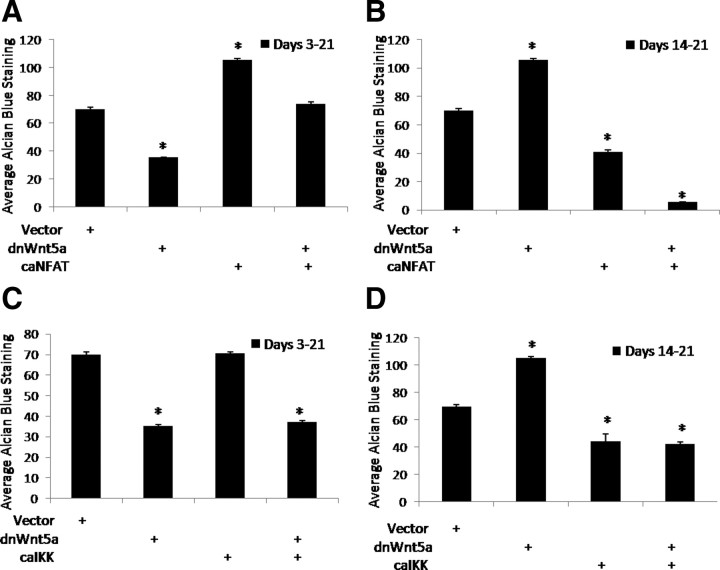
To further establish the role of the CaMK/NFAT and IKK/NF-κB pathways in promoting the stage-dependent effects of Wnt5a on chondrocyte differentiation, the ability of constitutively active (ca) forms of NFAT and IKK to circumvent the effects of dnWnt5a expression was examined (Fig. 7). Expression of dnWnt5a during d 3–21 of of chondrocyte differentiation decreased alcian blue staining. Expression of caNFAT alone early during differentiation increased alcian blue staining above vector control-infected micromass cultures (Fig. 7A). In addition, a difference between vector-infected cultures and those coinfected with dnWnt5a and caNFAT from d 3–21 was not observed. Thus, expression of caNFAT was able to abrogate the effects of Wnt5a loss of function in cultures in which expression of dnWnt5a was induced at an early stage. Conversely, expression of caNFAT in the hypertrophic chondrocyte decreased alcian blue staining. In addition, the effect of dnWnt5a expression on hypertrophic chondrocytes was abrogated by coexpression of caNFAT.
Fig. 7.
The effects of Wnt5a loss of function on differentiation are restored by constitutive activation of NFAT and IKK/NF-κB. A and B, Loss of Wnt5a function is restored by expression of caNFAT. C17 micromass cultures were infected with each indicated lentivirus or vector control at an MOI of 2. Micromass cultures were maintained for 21 d and then stained with alcian blue. Quantitation of the amount of alcian blue staining is shown below each micromass. *, P < 0.05. C and D, Loss of Wnt5a function is restored by expression of caIKK. C17 micromass cultures were infected with each indicated lentivirus or vector control at an MOI of 2. Micromass cultures were maintained for 21 d and then stained with alcian blue. Quantitation of the amount of alcian blue staining is shown below each micromass. *, P < 0.05.
Expression of caIKK during d 3–21 of chondrocyte differentiation did not change the amount of alcian blue staining compared with vector control (Fig. 7B). In addition, coexpression of caIKK with dnWnt5a during d 3–21 did not rescue the effects of dnWnt5a, demonstrating the stage-specific effects of IKK/NF-κB signaling downstream of Wnt5a. Conversely, Wnt5a loss of function in the hypertrophic chondrocyte increased proteoglycan deposition as measured by alcian blue staining. Expression of caIKK alone decreased chondrocyte differentiation above vector control infection, demonstrating the role of this pathway in repressing differentiation of hypertrophic chondrocytes. However, when d 14 micromass cultures were coinfected with dnWnt5a and caIKK, an overall decrease in alcian blue staining was observed. Therefore, the effect of dnWnt5a on hypertrophic chondrocytes was abolished by inducing NF-κB activation. Together these data support the stage-dependent role of the CaMK/NFAT and IKK/NF-κB pathways in mediating the effects of Wnt5a.
The NFAT and NF-κB pathways regulate expression of Sox9 and Runx2
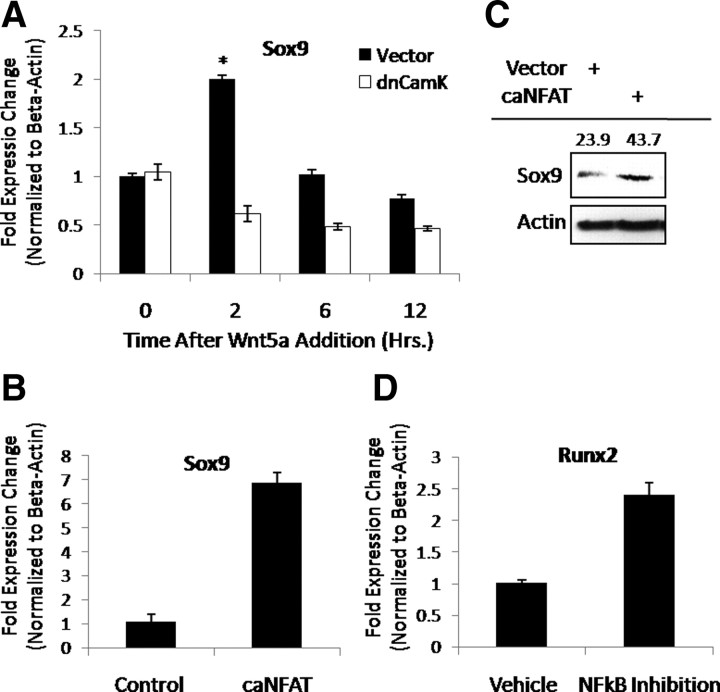
Because we had shown stage-dependent regulation of Sox9 and Runx2 expression by Wnt5a, we determined whether these transcriptional regulators were controlled by NFAT and NF-κB signaling, respectively. The ability of Wnt5a to induce expression of Sox9 downstream of the CaMK/NFAT pathway in C17 chondroprogenitor cells was first determined (Fig. 8A). C17 cells were transfected with dnCaMK or vector control and serum starved overnight. Cells were then treated with Wnt5a for 0–12 h and then harvested for total RNA and assayed for Sox9 expression via real-time RT-PCR. Treatment with Wnt5a induced a transient increase in Sox9 expression. This increase in Sox9 expression was abrogated by expression of dnCaMK. The ability of a ca form of NFAT to induce expression in the absence of Wnt5a was then examined (Fig. 8, B and C). C17 cells were transiently transfected with either caNFAT or vector control expression constructs. After transfection, cells were harvested for total RNA or protein lysates. Expression of caNFAT induced an increase in both Sox9 mRNA (data not shown) and protein levels. These data indicate that Wnt5a acts through the CaMK/NFAT pathway to affect chondrocyte differentiation by promoting chondrogenesis.
Fig. 8.
Wnt5a utilizes the CaMK/NFAT and NF-κB to regulate expression of Sox9 and Runx2. A–C, The CaMK/NFAT pathway is required for the early expression of Sox9 downstream of Wnt5a. A, C17 cells were transfected with dnCaMK or vector control and treated with Wnt5a as shown. RNA was harvested from cultures and used to assay for expression of Sox9 via real-time RT-PCR. *, P < 0.05. B, C17 cells were transfected with caNFAT or vector control. RNA was harvested from cultures and used to assay for expression of Sox9 via real-time RT-PCR. *, P < 0.05. C, C17 cells were transfected with caNFAT or vector control. After transfection, expression of Sox9 was assayed by Western blotting. Quantitation of the Sox9 signal normalized to actin is shown above each corresponding band. D, C17 cells were pretreated with an NF-κB inhibitor or vehicle control. After pretreatment, expression of Runx2 was assayed by real-time RT-PCR. *, P < 0.05.
Given the potential role of NF-κB-mediated signaling in regulating chondrocyte hypertrophy, we determined whether this signaling pathway regulated Runx2 expression (Fig. 8D). Chondroprogenitor cells were cultured in micromass to induce chondrocyte differentiation. Hypertrophic (d 14) cultures were treated with an NF-κB inhibitor or vehicle control (d 14–21). After treatment, cultures were harvested for total RNA, and expression of Runx2 was evaluated by real-time RT-PCR. Inhibition of NF-κB increased expression of Runx2 compared with vehicle control. These data indicate that the CaMK/NFAT pathway affects chondrocyte differentiation by promoting chondrogenesis, whereas the IKK/NF-κB pathway represses chondrocyte hypertrophy by inhibiting Runx2 expression.
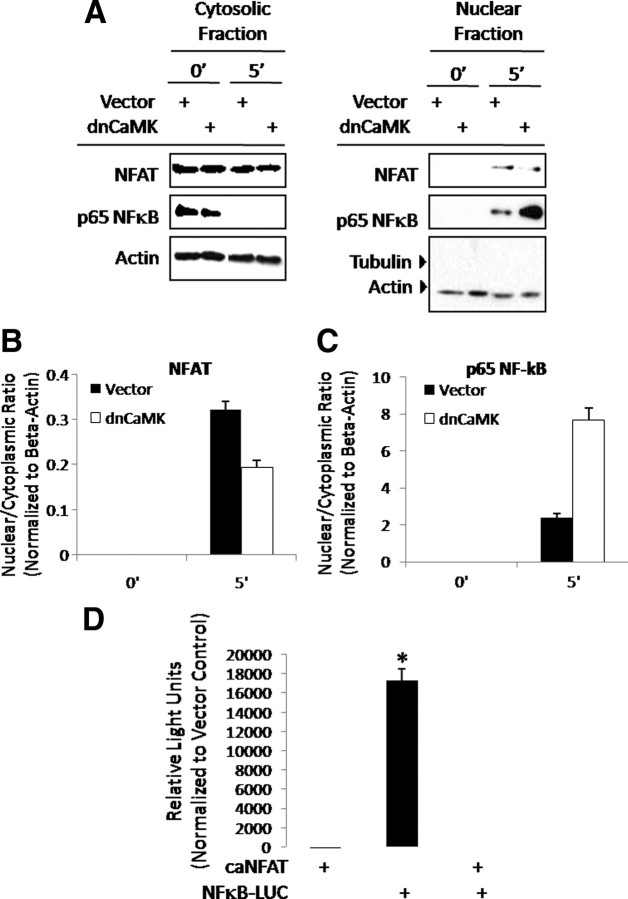
Potential cross talk between the NFAT and NF-κB pathways
In the initial analysis of downstream pathways mediating the stage-specific effects of Wnt5a, we noted that when NFAT activity is high, NF-κB is repressed. Similarly, when NF-κB activity is predominant, NFAT activation is decreased (Fig. 4). This observation suggested potential antagonism between the NFAT and NF-κB pathways. To explore this possibility, C17 cells were transiently transfected with a dnCaMK construct. Cells were then treated with Wnt5a for 0 or 5 min and fractionated into nuclear and cytoplasmic extracts. As a documented upstream regulator of NFAT activation, expression of dnCaMK blocked nuclear translocation of NFAT in response to Wnt5a as expected. In stark contrast, nuclear localization of NF-κB was markedly enhanced (Fig. 9, A–C). Because this observation suggested potential novel cross talk between these pathways, the ability of the NFAT pathway to repress NF-κB activity was therefore addressed (Fig. 9D). C17 cells were transfected with a multimerized NF-κB luciferase reporter construct or vector control. Cells were also cotransfected with either caNFAT or vector control. Whereas NF-κB-dependent luciferase activity is high compared with vector control or caNFAT expression alone, the NF-κB-driven luciferase activity was completely attenuated by coexpression of caNFAT. These data strongly suggest a role for the CaMK/NFAT pathway in negatively regulating NF-κB activity induced by Wnt5a.
Fig. 9.
Antagonism between the NFAT and NF-κB pathways. A–C, The CaMK/NFAT pathway negatively regulates NF-κB nuclear localization. C17 cells were transfected with dnCaMK or vector control and then treated with Wnt5a or vehicle control as indicated. After Wnt5a treatment, cells were harvested and fractionated into nuclear and cytoplasmic extracts. A, Western blotting for levels of NFAT and NF-κB in each fraction was performed, and the nuclear to cytoplasmic ratio was determined for NFAT (B) and NF-κB (C). Values are the mean of three independent experiments ± sem. *, P < 0.05. D, NFAT negatively regulates NF-κB activity. C17 cells were cotransfected with NF-κB or vector control luciferase (LUC) reporter constructs with caNFAT or vector control. After cotransfection, cells were treated with Wnt5a as indicated and harvested in passive lysis buffer. Luciferase activity was determined. *, P < 0.05.
Discussion
Wnt signaling plays an essential role during embryonic development and has a predominant role in governing the function of adult tissues (28). Aberrant Wnt signaling has been implicated in widespread numbers of diseases and developmental defects (28). One of the important functions mediated by Wnt signaling during embryonic development is formation of the skeletal elements. Wnt signaling during development not only regulates differentiation of mesenchymal cells but controls both chondrocyte and osteoblast differentiation during the endochondral ossification process. Although substantial information regarding the role of the canonical or β-catenin-dependent pathway, in skeletal development and chondrocyte differentiation, has been gathered, the role of the noncanonical Wnts such as Wnt5a is not as well established. The noncanonical Wnt5a is expressed in chondrogenic regions of the limb during development, and its expression is required for proper limb outgrowth (13). In addition, Wnt5a has been demonstrated to regulate cell fate decisions (15), influence chondroprogenitor proliferation, and inhibit chondrocyte hypertrophy (14, 16).
We chose the mouse micromass model system to study the effects of Wnt5a. We employed this model system because it reflects chondrocyte differentiation occurring during limb development in vivo (Fig. 1) and has been extensively characterized in our previous work (29, 30). In this system, induction of Sox9 and type 2a collagen-expressing cells represents the onset of chondrogensis, whereas expression of Runx2 and Type X Collagen defined the hypertrophic phase. In our hands, these events occurred at d 3 and d 14, respectively. These time points were therefore used to assay for the effects of Wnt5a on chondrogenesis and chondrocyte hypertrophy, respectively.
Through gain and loss of function studies in vivo, Wnt5a was shown to regulate chondrocyte entry into the proliferating phase of the developing limb, increase the number of prehypertrophic chondrocytes, and inhibit chondrocyte hypertrophy (13, 14). This observation suggested that Wnt5a may exert differential effects at various stages of chondrocyte differentiation. In support of this notion, we demonstrated that Wnt5a increased chondrocyte differentiation when supplied during the chondrogenic phase but inhibited differentiation of hypertrophic chondrocytes. Conversely, we have also shown that expression of a dn form of Wnt5a decreased early chondrocyte differentiation whereas expression of the dn form during hypertrophy enhanced chondrocyte differentiation. Thus, we have shown here that Wnt5a promotes early chondrocyte differentiation in a stage-dependent manner while repressing chondrocyte hypertrophy.
Given the differential effects of Wnt5a that we observed on early and late differentiation, we examined the specific signal transduction used during these stages of differentiation, because this was a potential mechanism for dichotomous effect of this singular growth factor. Although consistent activation of the known Wnt5a downstream effector CREB was apparent, clear differences in NFAT and NF-κB utilization were observed between chondrocytes cells treated with Wnt5a early during the chondrogenic phase or during hypertrophy. Specifically, we observed increased activation of NFAT and decreased NF-κB activity when immature chondrocytes were treated with Wnt5a. Conversely, when treated with Wnt5a at a late stage of chondrocyte differentiation, a decrease in NFAT and trend toward increased NF-κB activation were observed. These results suggested that the early effects of Wnt5a to promote chondrocyte differentiation were mediated by NFAT, whereas the ability of Wnt5a to repress chondrocyte hypertrophy was mediated via NF-κB. In support of this conclusion, we found that inhibition of NFAT or its upstream activator, CaMK (6, 7, 31), blocked the ability of Wnt5a to promote early chondrocyte differentiation. It is possible that CaMK and NFAT may control chondrocyte differentiation in separate, yet parallel, mechanisms; however, the fact that inhibition of CaMK also inhibited NFAT activity strongly argues that these components function as a linear pathway. In addition to a role during chondrogenesis, NFAT-mediated signaling likely plays a role later during differentiation. In contrast to the effects of CaMK/NFAT, inhibition of NF-κB activation abolished the ability of Wnt5a to repress late-stage chondrocyte differentiation. To further support this conclusion, when the NFAT and NF-κB-mediated pathways were constitutively activated, the effects of dnWnt5a on chondrocyte differentiation were inhibited.
The stage-dependent utilization of NFAT downstream of Wnt5a may help to explain some of the isoform-specific results previously reported regarding the role of NFAT-mediated signaling in governing chondrocyte differentiation. In two separate reports, Taschner et al. (32) and Tomita et al. (19) provided evidence that CaMK and NFAT4 promoted chondrogenesis and chondrocyte differentiation. However, a report by Ranger et al. (33) focused on the role of NFAT1 in repressing chondrogenesis. In this later study, the authors reported extraarticular cartilage formation with a global knockout of NFAT4 (33). As noted by the authors, this phenotype could be attributed to an increase in cartilage proliferation controlled by an unidentified factor or to an alteration in the cartilage progenitor cell. In addition to the potentially different effects mediated by the NFAT family members, these observed disparities in NFAT-dependent control of chondrocyte differentiation may be attributed to a stage-dependent response to NFAT-mediated signaling.
Whereas the role of Wnt5a in control of development and adult homeostasis is clear, the full range of specific effectors that facilitate noncanonical Wnt signaling has not been fully characterized. Although activation of PI3K/Akt signaling by Wnt5a, which are upstream events known to induce NF-κB activation in response to a variety of growth factors in numerous cell types, has previously been reported, this is the first report demonstrating the utilization of NF-κB by Wnt5a to control a biological response. Because NF-κB did not require the function of CaMK, activation of this pathway may be accomplished through an alternate calcium-dependent mechanism or through a calcium-independent pathway involving phospholipase C-dependent activation of PI3K/Akt.
While stage-dependent utilization of NFAT and NF-κB was apparent, we also made an additional observation. In the initial analysis of the downstream pathways mediating the differential effects of Wnt5a, a noteworthy observation was that when NFAT activity was high, NF-κB was repressed. Similarly, when NF-κB activity was predominant, NFAT activation was decreased. This contrast suggested potential antagonism between the NFAT and NF-κB pathways. We therefore explored the effect of activation of the NFAT pathway on NF-κB activity. Here we show that loss of CaMK/NFAT function promoted NF-κB activation whereas gain of NFAT function decreased NF-κB induction. There are two possible explanations for this observation. One is direct binding of NFAT to NF-κB, thereby blocking nuclear import of NF-κB. The other is stage-dependent induction of a downstream NFAT target which acts as a secondary mediator to block NF-κB function early during differentiation. Because we have observed reciprocal activation of these two pathways depending on the stage of chondrocyte differentiation, the later explanation seems more likely. Thus, we have identified potential negative cross talk between these two differentially used pathways that may be a factor governing the switch in cellular program that occurs as cells transition from the early stages of chondrocyte differentiation to hypertrophic chondrocytes. Defining the mechanisms of this cross talk warrants further investigation.
In addition to noting the stage-dependent pathway utilization of NFAT- and NF-κB-mediated effects, the mechanism by which Wnt5a potentially regulates chondrocyte differentiation downstream of these pathways was also explored. Because Wnt5a enhanced differentiation when supplied early during the chondrogenis phase, Wnt5a may regulate factors known to induce the onset of chondrogenesis. Wnt5a-null mice show a decrease in Sox9 expression in the limb mesenchyme and the palate during development, although a direct effect of Wnt5a on chondrogenesis was not reported (13, 34). We therefore determined whether Wnt5a regulated Sox9 expression by this pathway to promote chondrocyte differentiation. A role for Wnt5a inducing expression of the master chondrogenic factor Sox9 in a manner dependent on CaMK/NFAT signaling was demonstrated in this study. Putative NFAT consensus sites within both the human and mouse Sox9 promoters have been previously described, suggesting that the regulation of Sox9 by the CaMK/NFAT pathway is transcriptional (35, 36). Moreover, Colter et al. (35) also demonstrate that regions containing the NFAT consensus sites are required for Sox9 promoter activity. This observation may help to explain the shortening of the limb observed in the Wnt5a-null mice (13). In addition, data reported herein also support a role for Wnt5a in promoting mesenchymal stem cell lineage decisions toward the chondrogenic lineage and away from the adipogenic lineage as reported by Takada et al. (15).
In addition to the early effect of Wnt5a that we observed in promoting chondrocyte differentiation, treatment with Wnt5a at a late stage of differentiation decreased chondrocyte hypertrophy. Because Runx2 is a known transcriptional regulator of the onset of chondrocyte hypertrophy, we determined the role of Runx2 as a negative regulator of Wnt5a on chondrocyte maturation. In addition, transcriptional regulation via several identified NF-κB consensus sites within the Runx2 gene promoter has been previously demonstrated (37). In this report, Wnt5a stage-dependently controlled expression of Runx2 in a stage-dependent manner. Given that the negative effects of Wnt5a on hypertrophic chondrocyte differentiation were also dependent on the IKK/NF-κB pathways, Wnt5a may repress chondrocyte by negatively regulating expression of Runx2. In addition, down-regulation of NF-κB activity in the chondrogenic phase may also be essential because factors known to induce NF-κB also repress expression of Sox9 (18).
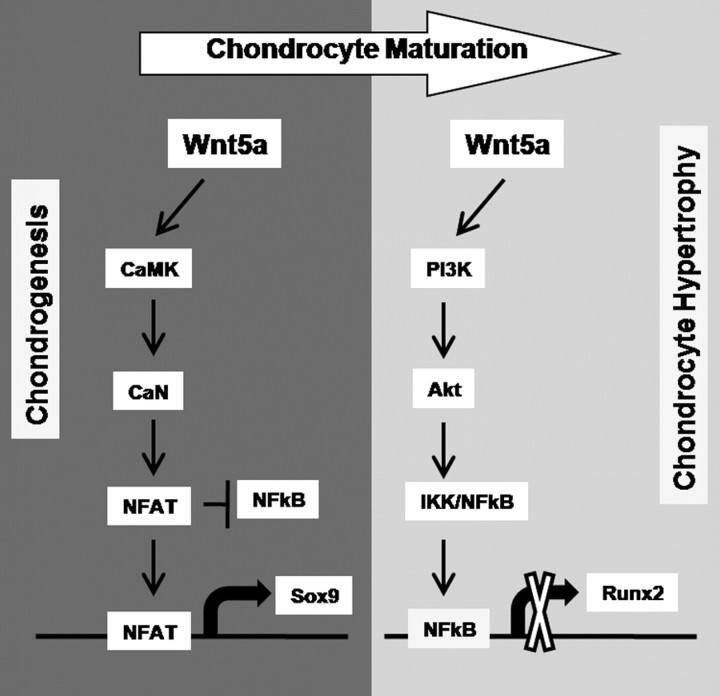
Overall we have demonstrated in this report that Wnt5a controls chondrocyte differentiation to promote chondrogenesis and repress chondrocyte hypertrophy in a stage-dependent manner (see proposed model in Fig. 10). The early effects of Wnt5a are mediated through activation of the CaMK/NFAT pathway and down-regulation of NF-κB-dependent signaling. This early effect is further regulated by negative pathway cross talk by NFAT-dependent signaling to antagonize NF-κB function. Conversely, the inhibition of chondrocyte maturation mediated by Wnt5a is accomplished through a reversal of the signal transduction pathways used by Wnt5a. These results demonstrate the importance of stage-dependent signal transduction in controlling chondrocyte differentiation regulated by Wnt5a.
Fig. 10.
Model of Wn5a stage-dependent effects on chondrocyte differentiation. Wnt5a promotes chondrogenesis through the CaMK/NFAT pathway while repressing chondrocyte maturation through the activity of NF-κB.
Materials and Methods
Limb bud micromass and cell culture system
MLB13MYC Clone17 chondrogenic precursors (C17 cells) were subcultured in DMEM, 10% fetal bovine serum (FBS), 1% pen/strep. For transfection assays, cells were serum starved in DMEM, 2% FBS, 1% penicillin/streptomycin at a density of 1 × 105 cells/cm2 overnight and treated with 50 ng/ml recombinant Wnt5a as indicated (R&D Systems, Minneapolis, MN) or harvested for luciferase assays. For micromass culture experiments, E11.5 limb bud cells or C17 cells were cultured as high-density 10-μl drops containing 2 × 105 cells in DMEM, 2% FBS supplemented with 25 μg/ml ascorbic acid, 54 μg/ml β-glycerol phosphate, and 1× insulin/transferrin/selenium (Invitrogen, Carlsbad, CA) as previously published (29). Cultures were fed every 3 d for 21 d and either fixed or harvested as described below. For d 3–d 21 treatments, micromass cultures were supplied with Wnt5a on d 3, d 7, d 10, d 14, and d 17. Wnt5a treatments for d 14–d 21were accomplished by the addition of Wnt5a on d 14 and d 17.
Alcian blue staining
Cultures were fixed with 4% paraformaldehyde for 10 min and stained with alcian blue stain (1% alcian blue, 3% acetic acid) for 2 h. Cultures were then washed twice with both ethanol and water.
Lentiviral construction
The Gateway system (Invitrogen) was used to construct lentiviruses encoding a dnWnt5a, caIKK, caNFAT, and vector control according to the manufacture’s specifications. Briefly, each cDNA was subcloned into the Gateway pENTR4 vector (Invitrogen) and used in a homologous recombination reaction to obtain a destination vector used for viral construction. Each respective lentivirus was then packaged in 293FT cells. Each virus was used by infecting each indicated culture at an multiplicity of infection (MOI) of 2 for 24 h in the presence of 1 μg/ml polybrene.
Transfection and luciferase assays
C17 cells were cultured as described above and transfected with each indicated expression or reporter construct with Lipofectamine 2000 according to the manufacturer’s specifications. The caNFAT expression construct and NFAT-reporter construct were purchased from Addgene, Inc. (Cambridge, MA; plasmids 11792 and 10959, respectively). For luciferase assays, cells were lysed in 1× passive lysis buffer for 15 min on ice. Luciferase assays were then performed using the Dual Luciferase Reporter Assay System (Promega Corp., Madison, WI) as per design.
RNA extraction and real-time RT-PCR
C17 cells or micromass cultures were differentiated as above and harvested immediately for total RNA or cultured with 50 ng/ml Wnt5a (R&D Systems) for the indicated period, rinsed with PBS, and harvested for total RNA. Total RNA from three biological replicates was harvested in TRIzol reagent (Invitrogen) and phenol/chloroform extracted from which 1 μg of RNA was reverse transcribed using the Superscript cDNA kit (Bio-Rad Laboratories, Inc., Hercules, CA). The resulting cDNA samples were used in real-time RT-PCR analysis of Sox9 (forward primer: 5′-AGGAAGCTGGCAGACCAGTA-3′; reverse primer: 5′-CGTTCTTCACCGACTTCCTC-3′); Runx2 (forward primer: 5′-AAGTGCGGTGCAAACTTTCT-3′; reverse primer: 5′-TCTCGGTGGCTGGTAGTGA-3′); collagen type 2a (forward primer: 5′-ACTGGTAAGTGGGGCAAGAC-3′; reverse primer: 5′-CCACACCAAATTCCTGTTCA-3′), collagen type X (forward primer: 5′-CTTTGTGTGCCTTTCAATCG-3′; reverse primer: 5′-GTGAGGTACAGCCTACCAGTTTT-3′); and actin (forward primer: 5′-AGATGTGGATCAGCAAGCAG-3′; reverse primer: 5′-GCGCAAGTTAGGTTTTGTCA-3′) expression as outlined in Soung et al. (29). Fold changes for each sample compared with time zero were then calculated. Each experiment was performed independently in triplicate.
Nuclear fractionation
C17 cells or micromass cultures were treated as per experimental design, washed, and harvested in ice-cold PBS. Cells were homogenized and pelleted in ice-cold PBS. Each pellet was then resuspended in 10 mm Tris-HCl (pH 8.0), 10 mm KCl, 0.1 mm EGTA, 0.1 mm EDTA, and 1× Inhibitor cocktail (Roche, Mannheim, Germany) and incubated for 15 min on ice. Cells were then lysed with the addition of 0.7% Nonidet P-40 (vol/vol). The resulting lysate was cleared by centrifugation at 14,000 rpm for 15 min at 4 C. The supernatant containing the cytoplasmic extract was retained for Western blotting. The nuclear pellet was then washed twice with ice-cold PBS and resuspended in 20 mm Tris-HCl (pH 8.0), 0.4 m NaCl, 0.1 mm EGTA, 0.1 mm EDTA, and 1× Inhibitor cocktail (Roche) and incubated on ice for 30 min. The nuclear lysate was then cleared by centrifugation at 14,000 rpm for 15 min at 4 C. The resulting supernatant was then collected as a nuclear extract and used for Western blotting.
Inhibition of signaling pathway components
For use of signaling chemical inhibitors, C17 cells or micromass cultures were treated with respective inhibitor or vehicle control as indicated. The CaMK inhibitor, K93 (Calbiochem, San Diego, CA), was used at a concentration of 2 μm. The NFAT activation inhibitor, INCA-6 (Calbiochem) was used at concentration of 40 nm, and the NF-κB inhibitor (Calbiochem) was used at a concentration of 10 nm. The PI3K inhibitor LY294002 (Cell Signaling Technology, Beverly, MA) and the Akt inhibitor (Calbiochem) were used at concentrations of 10 nm and 1 nm, respectively.
Western blotting
C17 cells of micromass cultures were treated as described and harvested as cytoplasmic or nuclear extracts. After fractionation, total protein concentrations were determined using the Bio-Rad DC assay (Bio-Rad Laboratories, Inc., Hercules, CA). After protein quantification, 1 μl β-mercaptoethanol and bromphenol blue was added to each sample, and 20 μg of protein were subjected to SDS-PAGE. Western blotting was accomplished utilizing antibodies for p65 NF-κB (Cell Signaling Technology) NFATc1 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), Sox9 (AbCam, Cambridge, MA), Runx2 (Oncogene Research, Gibbstown, NJ), actin, and tubulin (Sigma, St. Louis, MO) at a 1:1,000 dilution and corresponding secondary antibodies (Cell Signaling Technology) at a 1:10,000 dilution. Chemiluminescent detection was performed using Pierce femto reagent (Pierce Chemical Co., Rockford, IL). Blots were stripped and reprobed using Restore Western Blot Stripping Buffer (Pierce). Each experiment was repeated three times, with representative data shown. Quantitative data represent the average gray value of each corresponding band obtained from three independent experiments.
Image quantitation
Images were digitally scanned or collected using phase-contrast microscopy and converted to threshold values using Image J software. Densitometry of each image was then measured using Image J software.
Statistical analysis
Data obtained are the mean ± sem (n = 3) and are representative of three replicate experiments. The effect of treatment was compared with control values using Student’s t test to determine significant differences using Microsoft Excel software. (Microsoft Corp., Pullman, WA).
Acknowledgments
We thank Dr. M. Sen, Indian Institute of Chemical Biology (Bengal, India), for the generous gift of the dnWnt5a expression vector. Dr. K. Matsumoto, Nagoya University (Nagoya, Japan), kindly provided an expression vector for dnCaMK, whereas Dr. Y. Abu-Amer, Washington University School of Medicine provided the NF-κB reporter construct.
Footnotes
This work was supported by the University of Connecticut Health Center, Farmington, Connecticut.
Disclosure Summary: The authors have no disclosures.
First Published Online June 23, 2010
Abbreviations: ca, Constitutively active; CaMK, calmodulin kinase; CaN, calcineurin; CREB, cAMP response element-binding protein; dn, dominant negative; E11.5, embryonic d 11.5; FBS, fetal bovine serum; IKK, IκB kinase; MOI, multiplicity of infection; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; PI3K, phosphatidylinositol 3-kinase.
References
- 1.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT2003. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol 13:680–685 [DOI] [PubMed] [Google Scholar]
- 2.Huelsken J, Birchmeier W2001. New aspects of Wnt signaling pathways in higher vertebrates. Curr Opin Genet Dev 11:547–553 [DOI] [PubMed] [Google Scholar]
- 3.Kestler HA, Kühl M2008. From individual Wnt pathways towards a Wnt signalling network. Philos Trans R Soc Lond B Biol Sci 363:1333–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nusse R, Varmus HE1992. Wnt genes. Cell 69:1073–1087 [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Malbon CC2003. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science 300:1529–1530 [DOI] [PubMed] [Google Scholar]
- 6.Kühl M, Sheldahl LC, Malbon CC, Moon RT2000. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus J Biol Chem 275:12701–12711 [DOI] [PubMed] [Google Scholar]
- 7.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K2002. The Wnt/calcium pathway activates NF-AT and promotes ventral cell fate in Xenopus embryos. Nature 417:295–299 [DOI] [PubMed] [Google Scholar]
- 8.Sheldahl LC, Park M, Malbon CC, Moon RT1999. Protein kinase C is differentially stimulated by Wnt and Frizzled homologs in a G-protein-dependent manner. Curr Biol 9:695–698 [DOI] [PubMed] [Google Scholar]
- 9.Klein TJ, Mlodzik M2005. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol 21:155–176 [DOI] [PubMed] [Google Scholar]
- 10.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82–85 [DOI] [PubMed] [Google Scholar]
- 11.Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan PJ, Yang Y2003. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J Cell Biol 162:899–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loganathan PG, Nimmagadda S, Huang R, Scaal M, Christ B2005. Comparative analysis of the expression patterns of Wnts during chick limb development. Histochem Cell Biol 123:195–201 [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi TP, Bradley A, McMahon AP, Jones S1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126:1211–1223 [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Topol L, Lee H, Wu J2003. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 130:1003–1015 [DOI] [PubMed] [Google Scholar]
- 15.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, Youn MY, Takeyama K, Nakamura T, Mezaki Y, Takezawa S, Yogiashi Y, Kitagawa H, Yamada G, Takada S, Minami Y, Shibuya H, Matsumoto K, Kato S2007. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-γ transactivation. Nat Cell Biol 9:1273–1285 [DOI] [PubMed] [Google Scholar]
- 16.Hartmann C, Tabin CJ2000. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development 127:3141–3159 [DOI] [PubMed] [Google Scholar]
- 17.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B1999. Sox9 is required for cartilage formation. Nat Genet 22:85–89 [DOI] [PubMed] [Google Scholar]
- 18.Murakami S, Lefebvre V, de Crombrugghe B2000. Potent inhibition of the master chondrogenic factor Sox9 gene by interleukin-1 and tumor necrosis factor-α. J Biol Chem 275:3687–3692 [DOI] [PubMed] [Google Scholar]
- 19.Tomita M, Reinhold MI, Molkentin JD, Naski MC2002. Calcineurin and NFAT4 induce chondrogenesis. J Biol Chem 277:42214–42218 [DOI] [PubMed] [Google Scholar]
- 20.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T1997. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- 21.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ1997. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89:765–771 [DOI] [PubMed] [Google Scholar]
- 22.Day TF, Guo X, Garrett-Beal L, Yang Y2005. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8:739–750 [DOI] [PubMed] [Google Scholar]
- 23.Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C2005. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8:727–738 [DOI] [PubMed] [Google Scholar]
- 24.Dong YF, Soung do Y, Schwarz EM, O'Keefe RJ, Drissi H2006. Wnt induction of chondrocyte hypertrophy through the Runx2 transcription factor. J Cell Physiol 208:77–86 [DOI] [PubMed] [Google Scholar]
- 25.Rosen V, Nove J, Song JJ, Thies RS, Cox K, Wozney JM1994. Responsiveness of clonal limb bud cell lines to bone morphogenetic protein 2 reveals a sequential relationship between cartilage and bone cell phenotypes. J Bone Miner Res 9:1759–1768 [DOI] [PubMed] [Google Scholar]
- 26.Sen M, Chamorro M, Reifert J, Corr M, Carson DA2001. Blockade of Wnt-5A/frizzled 5 signaling inhibits rheumatoid synoviocyte activation. Arthritis Rheum 44:772–781 [DOI] [PubMed] [Google Scholar]
- 27.Park M, Yong Y, Choi SW, Kim JH, Lee JE, Kim DW2007. Constitutive RelA activation mediated by Nkx3.2 controls chondrocyte viability. Nat Cell Biol 9:287–298 [DOI] [PubMed] [Google Scholar]
- 28.Cadigan KM, Nusse R1997. Wnt signaling: a common theme in animal development. Genes Dev 11:3286–3305 [DOI] [PubMed] [Google Scholar]
- 29.Soung do Y, Dong Y, Wang Y, Zuscik MJ, Schwarz EM, O'Keefe RJ, Drissi H2007. Runx3/AML2/Cbfa3 regulates early and late chondrocyte differentiation. J Bone Miner Res 22:1260–1270 [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O'Keefe RJ2004. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-β delays hypertrophy and PGE2 inhibits terminal differentiation. Bone 34:809–817 [DOI] [PubMed] [Google Scholar]
- 31.Kühl M2004. The WNT/calcium pathway: biochemical mediators, tools and future requirements. Front Biosci 9:967–974 [DOI] [PubMed] [Google Scholar]
- 32.Taschner MJ, Rafigh M, Lampert F, Schnaiter S, Hartmann C2008. Ca2+/Calmodulin-dependent kinase II signaling causes skeletal overgrowth and premature chondrocyte maturation. Dev Biol 317:132–146 [DOI] [PubMed] [Google Scholar]
- 33.Ranger AM, Gerstenfeld LC, Wang J, Kon T, Bae H, Gravallese EM, Glimcher MJ, Glimcher LH2000. The nuclear factor of activated T cells (NFAT) transcription factor NFATp (NFATc2) is a repressor of chondrogenesis. J Exp Med 191:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen Y2008. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development 135:3871–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colter DC, Piera-Velazquez S, Hawkins DF, Whitecavage MK, Jimenez SA, Stokes DG2005. Regulation of the human Sox9 promoter by the CCAAT-binding factor. Matrix Biol 24:185–197 [DOI] [PubMed] [Google Scholar]
- 36.Ushita M, Saito T, Ikeda T, Yano F, Higashikawa A, Ogata N, Chung U, Nakamura K, Kawaguchi H2009. Transcriptional induction of SOX9 by NF-κB family member RelA in chondrogenic cells. Osteoarthritis Cartilage 17:1065–1075 [DOI] [PubMed] [Google Scholar]
- 37.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, Lian JB, Stein GS, Nanes MS2002. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2α A) is inhibited by tumor necrosis factor-α. J Biol Chem 277:2695–2701 [DOI] [PubMed] [Google Scholar]