Abstract
We recently reported on the overexpression of Runx2 (Cbfa1/AML3), an osteoblast-specific transcription factor, in human papillary thyroid cancer tissues. We report here that normal thyrocytes also express Runx2 and that Runx2+/− mice are in a hypothyroid state. To clarify the mechanism, we studied the effects of small interfering RNA-mediated silencing of Runx2 on thyroid-specific gene expression in FRTL-5 cells. Lowering the levels of Runx2 had no effect on the amount of Na+/I− symporter mRNA but markedly decreased the amount of thyroglobulin (Tg) mRNA. A Runx2 binding consensus sequence is present on the Tg gene promoter, and gel-shift assay revealed that Runx2 binds to this region. Reporter assay showed that deletion of the region or introduction of a mutation into the binding site significantly impairs promoter function. These results indicate that Runx2 deficiency in mice causes decreased Tg expression and a novel type of hypothyroidism.
Normal thyrocytes express Runx2, an osteoblast-specific transcription factor. Runx2 deficiency in mice causes decreased thyroglobulin expression in thyroid glands and a novel type of hypothyroidism.
Runx transcription factors determine cell fate and regulate lineage-specific proliferation and differentiation. Runx1 is essential for definitive hematopoiesis, and Runx3 is involved in gut development (1, 2). Runx2 is a key regulator of bone development, being necessary for the maturation of chondrocytes and osteoblasts (3, 4). Runx2-null mice die at birth with the absence of a mineralized skeleton (5, 6).
With regard to nonosteoblastic cells, human smooth vascular cells undergo a spontaneous osteo/chondrocytic conversion and begin expressing Runx2 in vitro (7). It is hypothesized that progressive changes in the expression of genes encoding bone-associated proteins are involved in the regulation of vascular mineralization.
Development of calcifying foci is a fairly common finding in the human thyroid in pathological states. In particular, psammoma bodies, very fine calcifications, are known to be characteristic to papillary carcinoma and are of a diagnostic value (8, 9). Thus, the high frequency of calcification in human papillary thyroid carcinoma prompted us to study whether osteocalcin and/or Runx2 genes are expressed in malignant thyroid epithelial cells, and we found that Runx2 (Cbfa1/AML3) is overexpressed in human papillary thyroid cancer tissues (10).
To study the role of Runx2 in thyrocytes, we analyzed thyroid function in Runx2+/− mice (heterozygous mice) and found that these mice were in a hypothyroid state.
Results and Discussion
Mean height and weight of 12-wk-old male Runx2+/− mice (n = 9) were essentially the same as those of wild-type mice (n = 7) (Table 1). However, total T4 and total T3 levels of the former were significantly lower than those of the latter. Serum free T4 levels of the former were also significantly lower than those of the latter, and serum TSH levels of the Runx2+/− mice were higher than those of wild-type mice. Mean maximum diameter of the right and left lobes of the thyroid glands from Runx2+/− mice were also larger than that from wild-type mice (Table 1 and Fig. 1, A and B), whereas 125I uptake activity in Runx2+/− mice was significantly lower than that in wild-type mice (Table 1).
Table 1.
Male Runx2+/− mice and their thyroid function
| Wild-type mice (n = 7) | Heterozygous mice (n = 9) | |
|---|---|---|
| Height (cm) | 7.4 ± 0.11 | 7.7 ± 0.09 |
| Weight (g) | 17.3 ± 0.46 | 17.5 ± 0.67 |
| Total T4 (μg/dl) | 4.16 ± 0.15 | 3.21 ± 0.133 |
| Total T3 (ng/ml) | 0.72 ± 0.018 | 0.63 ± 0.0162 |
| Free T4 (ng/dl) | 1.65 ± 0.06 | 1.24 ± 0.063 |
| TSH (μU/ml) | 2.23 ± 0.6 | 5.01 ± 0.552 |
| Size of thyroid glands (mm) | 2.30 ± 0.09 | 2.90 ± 0.103 |
| 125I uptake (×104 cpm) | 15.9 ± 1.0 | 10.7 ± 1.22 |
| Rectal temperature (room, C) | 39.5 ± 0.19 | 38.1 ± 0.141 |
| Rectal temperature (cold exposure, C) | 38.3 ± 0.20 | 36.9 ± 0.153 |
Height was measured from nose to base of the tail. Size of thyroid glands is the mean of maximum diameters of the right and left lobes. For 125I uptake, the radioactivity in the thyrotracheal unit was determined using a γ-counter. For cold exposure, rectal temperature was determined 120 min after exposed to 4 C.
P < 0.05.
P < 0.01.
P < 0.001.
Fig. 1.
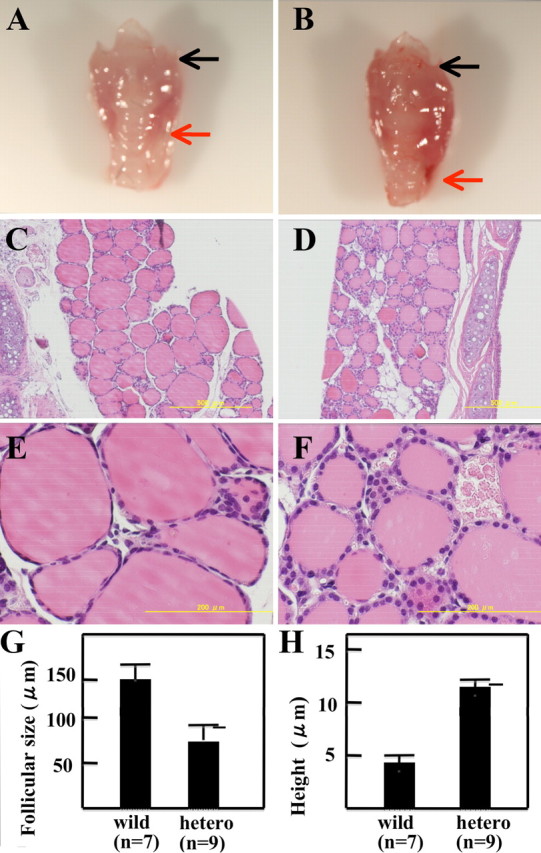
Thyroid glands from wild-type and Runx2+/− mice. Macroscopic views of thyroid from 12-wk-old wild-type (A) and Runx2+/− (B) mice observed under a stereomicroscope (Olympus SZX7, Tokyo, Japan). Black arrows indicate the upper poles, and red arrows indicate the lower poles of the left lobe of the thyroid glands. Histology on low magnification in wild-type (C) and Runx2+/− (D) mice and on high magnification in wild-type (E) and Runx2+/− (F) mice. Tissues were fixed with 4% formaldehyde and embedded in paraffin. Sections (5 μm) were stained with hematoxylin-eosin. G, Mean follicular size of thyroid glands from wild-type and Runx2+/− mice. Maximum diameters of 200 follicles of the thyroid glands from both groups were measured. H, Mean height of thyroid epithelial cells from both groups. Maximum heights of 500 epithelial cells from wild-type and Runx2+/− mice were measured. Data are means ± sd. *, P < 0.001.
We exposed Runx2+/− and wild-type mice (age, 12 wk) to a cold environment (4 C) and measured time-dependent changes in rectal temperature. Rectal temperatures of wild-type (n = 7) and Runx2+/− (n = 9) mice before exposure to cold were 39.5 ± 0.19 C and 38.1 ± 0.14 C, respectively, the latter being significantly lower than the former (P < 0.05). After 120 min of exposure to cold, rectal temperature in wild-type mice decreased slightly to 38.3 ± 0.20 C, but that in Runx2+/− mice decreased rapidly to 36.9 ± 0.15 C (P < 0.001) (Table 1). In rodents, serum thyroid hormone levels are well correlated with rectal temperature (11). These results, in addition to the low T4 and T3 levels in Runx2+/− mice (Table 1), indicate that Runx2+/− mice are in overt, rather than subclinical, hypothyroidism.
Figure 1 shows the morphological changes in the thyroid glands of 12-wk-old Runx2+/− mice. As compared with wild-type mice (Fig. 1A), the thyroid glands from Runx2+/− mice were enlarged (Fig. 1B and Table 1). Microscopically, thyroid glands of Runx2+/− mice consisted of smaller follicles, and their mean maximum follicle diameter (75 ± 4.9 μm) was significantly shorter than that in wild-type mice (154 ± 8.3 μm, Fig. 1G). Furthermore, the height of thyroid epithelial cells in Runx2+/− mice was higher than that in wild-type mice (Fig. 1, E and F). When the heights of 500 cells from Runx2+/− and wild-type mice were measured, mean height in the Runx2+/− mice (12.1 ± 0.1 μm) was significantly greater than that in wild-type mice (4.9 ± 0.2 μm, Fig. 1H). Collins and Capen (12) reported that thyroid follicular cells become more columnar and the follicular lumens become smaller by TSH treatment; thus, these results suggest that thyroid epithelial cells in Runx2+/− mice were stimulated by TSH but were in dyshormonogenesis.
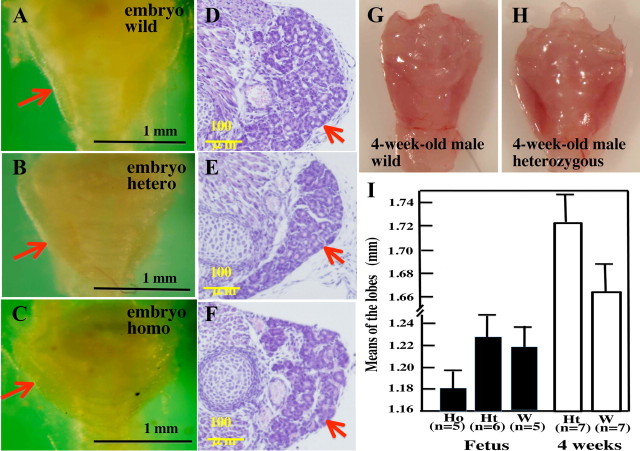
Figure 2, A–F, shows macroscopic and microscopic views of thyroid glands from late embryos (d 19) of wild-type, Runx2+/−, and Runx2−/− (homozygous) mice. Even in the absence of Runx2, thyroid glands were formed in the normal position (Fig. 2C), and follicle formation was observed (Fig. 2F). The mean maximum diameter of the right and the left lobes of the thyroid glands in Runx2−/− mice tended to be smaller than that in wild-type mice, but the difference was not significant (Fig. 2, C and I). The mean maximum diameter of 4-wk-old Runx2+/− mice tended to be larger than that of wild-type mice (Fig. 2, G and H), but the difference was not significant (Fig. 2I).
Fig. 2.
Thyroid gland development in Runx2-deficient mice. Thyroid glands from wild-type (A and D) (n = 5), Runx2+/− (B and E) (n = 6), and Runx2−/− (C and F) (n =5) mouse embryos at d 19. Thyrotracheal units were observed with a stereomicroscope (A–C). Tissues were then fixed with 4% formaldehyde and embedded in paraffin. Sections (5 μm) were stained with hematoxylin-eosin (D–F). Red arrows indicate thyroid glands. G and H, Thyroid glands of 4-wk-old male wild-type (G) and Runx2+/− (H) mice. I, Mean maximum diameter of the right and left lobes of thyroid glands from Runx2-deficient mice. Black bars are means ± sd of the lobes from Runx2−/− homozygous (Ho; n = 5), Runx2+/− heterozygous (Ht; n = 6), and wild-type (W; n = 5) mouse embryos, and white bars are those from 4-wk-old male heterozygous (n = 7) and male wild-type (n = 7) mice.
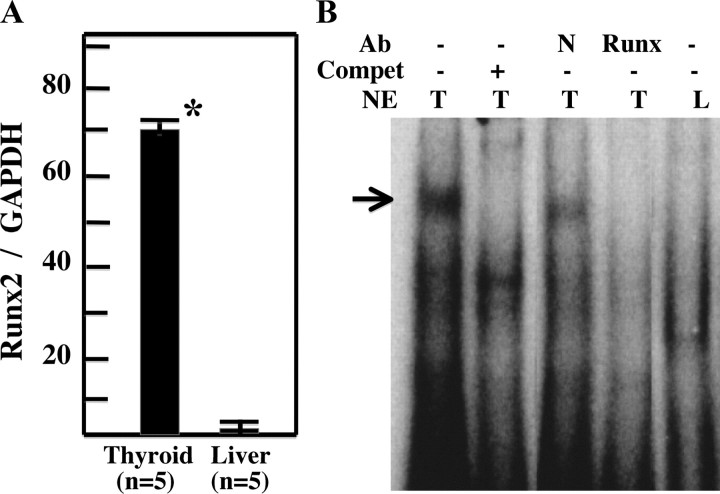
Figure 3A shows the results of quantitative PCR analysis of Runx2 from thyroid glands of 12-wk-old wild-type mice. When the relative Runx2/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratio was studied, the thyroid expressed about 100-fold more Runx2 mRNA than the liver (70.0 ± 13.0 vs. 0.67 ± 0.033, P < 0.001). We thus carried out EMSA using a double-stranded oligonucleotide (Oligo) OC corresponding to the Runx2 binding site on the osteocalcin promoter (3) and nuclear extracts from thyroid glands from wild-type mice. Oligo OC formed a protein/DNA complex with nuclear extracts from the thyroid but not from the liver. This formation was abolished by addition of wild-type Oligo and was diminished by the addition of anti-Runx2 antibody (Fig. 3B). These results indicate that normal thyrocytes also express Runx2.
Fig. 3.
Expression of Runx2 in normal thyroid glands. A, Quantitative PCR for Runx2 in thyroid gland (n = 5) and liver (n = 5) from 12-wk-old wild-type mice. Quantitative PCR was carried out as described in the Materials and Methods. Means of Runx2/GAPDH ± sd are indicated. *, P < 0.001. B, EMSA with Oligo OC. Nuclear extracts (NE) from thyroid gland (T) and liver (L) from wild-type mice were incubated with radiolabeled Oligo OC, which corresponds to the Runx2 binding site on the osteocalcin gene promoter. Protein/DNA complexes are indicated by black arrows. Complex formation was inhibited by addition of self-competitor (compet) and diminished by incubation with anti-Runx2 antibody (Runx) (1:250) but not with control serum (N). Arrow indicates Runx2/Oligo complex. Ab, Antibody.
To study the cause of hypothyroidism in Runx2+/− mice, we studied the effects of small interfering RNA (siRNA)-mediated silencing of Runx2 on thyroid-specific gene expression in FRTL-5 cells, functional thyroid epithelial cells. After transfecting pSilencer-siRunx2 into FRTL-5 cells and then selecting with antibiotics, we successfully established cell lines stably expressing siRNA of Runx2 (FRTL-5/siRunx2 cells). From these cells, mRNA was prepared and transferred onto a cellulose acetate membrane. Figure 4 shows the results of Northern blot analysis for Runx2 mRNA. The amount of Runx2 mRNA in the cells was markedly decreased and almost undetectable when compared with that in FRTL-5/negative control (NC) cells, indicating that Runx2 gene expression was successfully silenced.
Fig. 4.
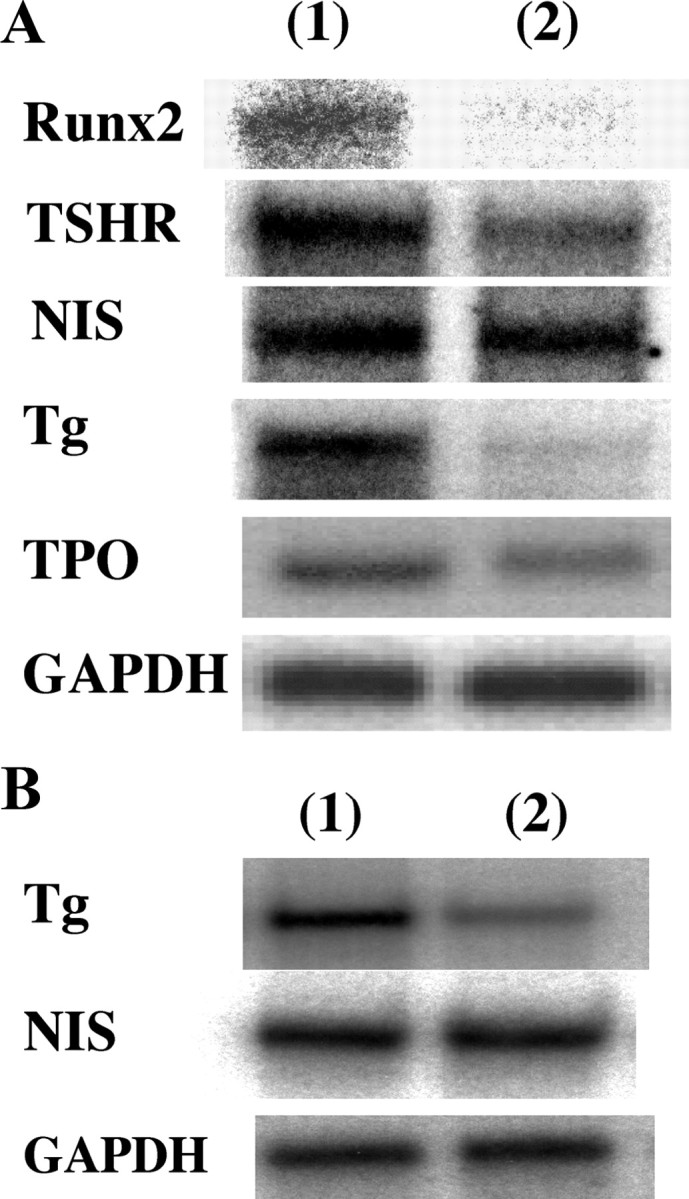
Effects of siRNA-mediated silencing of Runx2 on thyroid-specific gene expression in thyrocytes. A, mRNA (1 μg) from FRTL-5 cells stably transfected with pSilencer NC (lane 1) or with pSilencer-siRunx2 (lane 2) was transferred to cellulose acetate membranes and hybridized with [32P]cDNAs for Runx-2, TSHR, NIS, Tg, TPO, and GAPDH. B, Tg and NIS mRNA levels in wild-type (lane 1) and heterozygous (lane 2) mice. mRNA (1 μg) from thyroid glands of wild-type and Runx2+/− mice was transferred to the membrane, and levels of Tg and NIS mRNA were also determined.
In FRTL-5/siRunx2 cells, mRNA expression of Na+/I− symporter (NIS) and thyroid peroxidase (TPO) is almost equal to that in FRTL-5/NC cells. However, the mRNA level of mouse TSH receptor (TSHR) in FRTL-5/siRunx2 cells was lower than that in FRTL-5/NC cells, whereas mRNA expression of thyroglobulin (Tg) was markedly lower in FRTL-5/siRunx2 cells than in FRTL-5/NC cells (Fig. 4A). Indeed, the mRNA level of Tg in thyroid glands from Runx2+/− mice is about 40% lower than that from wild-type mice, but NIS mRNA level in Runx2+/− mice is equal to that in wild-type mice (Fig. 4B). These results suggest that Runx2 is involved in the regulation mechanism of Tg gene expression in thyroid epithelial cells.
Figure 5A schematically depicts the Tg promoter structure and the 5′-upstream sequence from region A. Musti et al. (13) analyzed the structure and function of the rat Tg promoter. They found that for thyroid-specific expression of the Tg gene, binding of thyroid transcription factor (TTF)-1, TTF-2, and Pax-8 to the promoter regions A, B, K, and C is required.
Fig. 5.
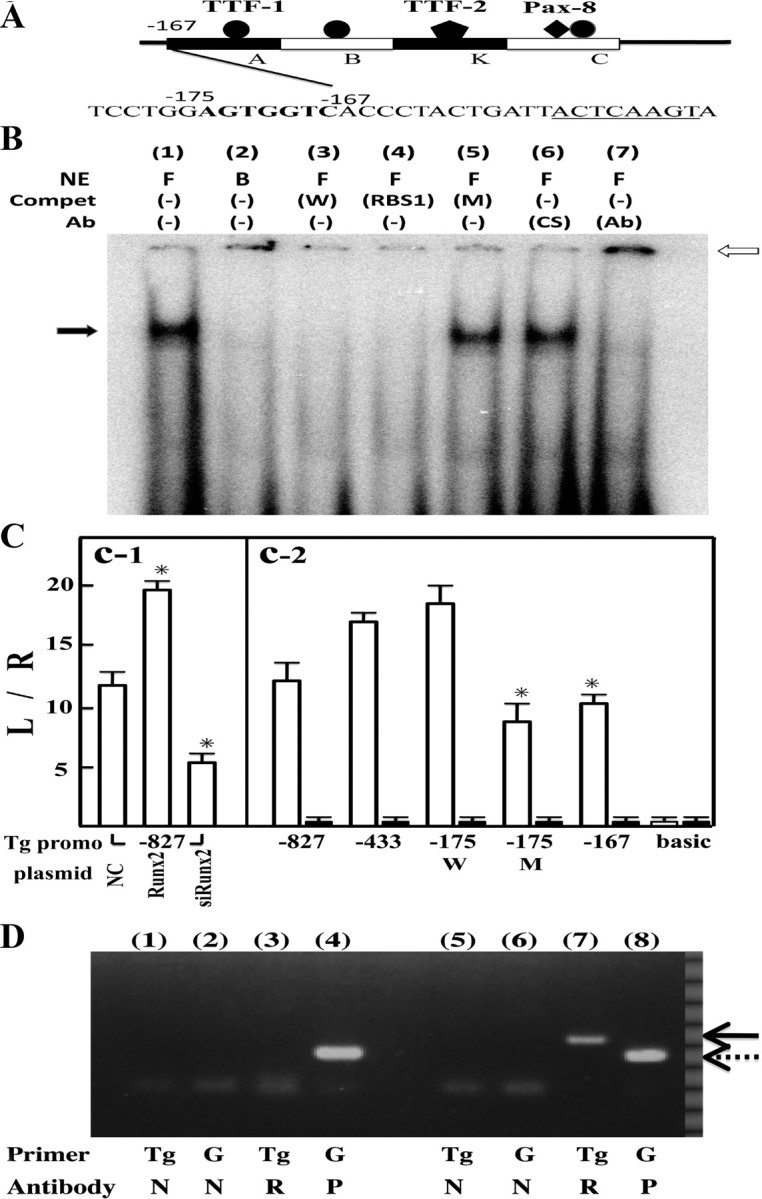
Runx2 binding to Tg promoter region. Panel A, Scheme of Tg promoter structure and 5′-upstream sequence from region A. Rat Tg gene promoter (from −167 to −1 bp; transcription start site is designated as +1), which is essential for thyroid-specific expression, consists of regions A, B, K, and C. Runx2 binding consensus sequence, AGTGGTC, shown in bold, is located just upstream from region A. The underlined sequence is the TTF-1 binding site in region A. Panel B, EMSA with Oligo W. Nuclear extracts (NE) from FRTL-5 cells (F) and BRL-3A liver cells (B) were incubated with radiolabeled Oligo W, which corresponds to −178 to −167 bp of rat Tg promoter. Protein/DNA complex is indicated by black arrows. Complex formation was inhibited by the addition of self-competitor (W) and Oligo RBS1 containing Runx2 binding consensus sequences but not by Oligo M (M) to which mutations were introduced (lanes 3–5). In lanes 6 and 7, radiolabeled Oligo W was incubated with control serum (CS) (lane 6) or anti-Runx2 antibody (Ab) (1:250, lane 7). The supershifted band is indicated by a white arrow. Panel C, Promoter activity of rat Tg-luciferase chimera plasmids. C-1, pTg-Luc (−827) (2 μg) was cotransfected with 2 μg of pSilencer NC, pcDNA3-Runx2 (Runx2), or pSilencer-siRunx2 (siRunx2) into FRTL-5 cells by electroporation. Data are means of triplicate assays. *, P < 0.01 vs. NC. C-2, Luciferase activities in lysates from FRTL-5 cells (white bars) and BRL-3A cells (black bars) after transfection with pTg-Luc (−827) and its deletion mutants, as indicated. Data are means of triplicate assays. pTg-Luc (−175M) has a mutation in the Runx-2 binding site. *, P < 0.01 vs. pTg-Luc(−175W). All cells were cotransfected with 100 ng pRL-SV40 to normalize transfection efficiency. Data are means ± sd. Panel D, ChiP assays of thyroid gland material. Liver (lanes 1–4) and thyroid glands (lanes 5-8) were cross-linked with 1% formaldehyde and then precipitated with anti-Runx2 antibody (2.5 μg, R), anti-RNA polymerase II antibody [2.5 μg, positive control (P)], and normal mouse IgG [1 μg, NC (N)]. Precipitated DNA fragments were amplified using primers for Tg promoter or for GAPDH promoter (G). Solid arrow indicates Tg promoter cDNA, and dotted arrow indicates GAPDH promoter cDNA.
Immediately upstream from region A (from −175 to −169 bp), we identified the Runx2 binding consensus sequence, AGTGGTC, which coincides with the Runx2 binding site on the bone sialoprotein gene promoter (site 4) (14). We synthesized a double-stranded Oligo containing this sequence (Oligo W) and carried out EMSA. A protein/DNA complex was formed with nuclear extracts from FRTL-5 cells but not from BRL-3A liver cells. This formation was abolished by the addition of Oligo W or Oligo RBS1, which contains another Runx2 binding sequence on the bone sialoprotein gene promoter (site 1), but not by Oligo M, to which we introduced mutations. When we added anti-Runx-2 antibody to the reaction mixture, the protein/DNA complex was successfully supershifted (Fig. 5B), indicating that Runx2 is able to bind to this region.
We then carried out a reporter assay using chimera constructs of the Tg gene promoter (−822 bp) and luciferase gene [pTg-Luc(−822)], and its 5′-deletion mutants. When pTg-Luc(−822) was cotransfected with the Runx2 expression vector, pcDNA3-Runx2, or silencing vector, pSilencer-Runx2, into FRTL-5 cells, Runx2 stimulated luciferase activity, but its silencing inhibited enzyme activity (Fig. 5C-1), indicating that Runx2 is involved in the regulation of Tg gene expression. To identify the Runx2 binding site, pTg-Luc(−822) and its deletion mutants were transiently transfected into FRTL-5 cells and BRL-3A cells. From −822 to −175 bp, promoter activities in FRTL-5 cells increased gradually, but the enzyme activity of pTg-Luc(−167) was lower than that of pTg-Luc(−175W). When mutations were introduced into the putative Runx2 binding site [pTg-Luc(−175M)], activity was also significantly lower than that of pTg-Luc(−175W) (Fig. 5C-2). These results indicate that Runx-2 binds to the upstream site of region A and stimulates Tg gene expression in thyrocytes.
To extrapolate to the in vivo status, we further carried out chromatin immunoprecipitation (ChIP) assays on thyroid gland material from wild-type mice. After cross-linking the tissues with 1% formaldehyde followed by brief sonication, protein/DNA complexes were precipitated with anti-RNA polymerase II antibody (positive control) or anti-Runx2 antibody. As shown in Fig. 5D, anti-RNA polymerase II antibody precipitated the GAPDH promoter fragment from the thyroid and liver, whereas anti-Runx2 antibody precipitated the Tg promoter segment only from the thyroid.
Congenital hypothyroidism (CH) is the most common neonatal metabolic disorder and, if untreated, can result in severe neurodevelopmental impairment. In about 85% of cases, CH is associated with developmental defects of the thyroid gland, referred to as thyroid dysgenesis (agenesis, ectopic thyroid, and hypoplasia). Thyroid dysgenesis is typically sporadic and occurs in a familial manner in only 2% of cases.
This type of disorder can be caused by mutations in TTFs (e.g. TTF-1 and -2 and Pax-8) that are essential for the development and function of thyrocytes (15). Mutations in the TSHR gene also induce thyroid dysgenesis (16).
Defects in the steps required for thyroid hormone synthesis within thyroid epithelial cells are referred to as dyshormonogenesis and account for about 10–15% of CH cases. In contrast to thyroid dysgenesis, affected patients typically present with goitrous enlargement of the thyroid. Dyshormonogenesis can be caused by mutations in thyroid-specific genes such as TPO, Tg, NIS, pendrin, and thyroid oxidase 2 (THOX2). However, the prevalence of identifiable disease-causing mutations is low, and still unidentified genes have been postulated to induce CH (15). Runx-2+/− mice showed high serum TSH, low T4, and low iodide uptake activity with enlarged thyroid glands. Therefore, CH in Runx2+/− mice belongs to the dyshormonogenesis type.
In this report, we showed that Runx2 binds to the Tg gene promoter region, from −175 to −168 bp, and stimulates its expression in thyrocytes. Musti et al. (13) reported that the region from −167 to −160 bp in the Tg promoter is a very important cis-element, because the Tg promoter (−160 to +39)/CAT chimeric completely lacks enzyme activity in FRTL-5 cells (12). They revealed that a ubiquitous factor binds to this region (17). Because Runx2 binds just upstream of this ubiquitous factor binding site, these two factors may be correlated with one another. However, the ubiquitous factor has not been identified to date, and thus further elucidation is necessary to clarify the issue.
In addition to the effects of Runx2 on Tg gene expression, which we believe is a major cause of hypothyroidism in Runx2+/− mice, silencing of Runx2 gene expression in FRTL-5 cells decreases TSHR mRNA levels (Fig. 2). In thyrocytes, iodide is taken up by NIS and then organized into Tg molecules in the follicular lumen (18). Because TSH is the major regulator for both steps (19), low levels of TSHR mRNA may confer the low iodide uptake activity observed in Runx2+/− mice (Table 1). Thus, we cannot exclude the possibility that decreased levels of TSHR would result in dyshormonogenesis in Runx2+/− mice.
In humans, Runx2 gene mutations induce cleidocranial dysplasia (CCD) (20). Clinical features of CCD include delayed closure of skull suture, hypoplastic or aplastic clavicles, and dental anomalies (21). There have been few reports discussing the relationship between CCD and CH, but Chen et al. (22) reported a 4-yr-old boy with CCD and CH, in whom radioiodide uptake activity was diffusely decreased. Future studies will be needed to clarify thyroid status in human CCD.
Materials and Methods
Animals
All studies were approved by the Animal Research Committee of the University of Yamanashi. C57BL6 Runx2+/− mice (5) were kindly donated by Prof. T. Komori of Nagasaki University and were bred to generate experimental animals. To determine the genotypes of the mice, we carried out PCR using tail DNA as a template with the following primers: sense, 5′-CAAGCGAAACATCGC ATCGAGC-3′, and antisense, 5′-AAAGCACAGGGAAGCGGTCAGC-3′, for the Neo gene; and sense, 5′-CCGCACGACAACCGCACCAT-3′, and antisense, 5′-AGCCACCAAGGCTGGAGTC TT-3′, for the Runx2 gene (6). Thyroid size and function were studied using 12-wk-old male mice. For the study of thyroid gland development, we also studied the size and the morphology of 4-wk-old male mice and late embryos at d 19. Rectal temperature was measured using a digital thermometer (TD-300; Shibaura Electronics Co., Ltd., Tokyo, Japan).
Cell culture and silencing of Runx2 gene expression
FRTL-5 cells (CRL8395; ATCC, Manassas, VA) were cultured in Ham F12 containing 6H (10 ng/ml insulin, 0.4 ng/ml cortisol, 5 mg/ml transferrin, 10 ng/ml gylcyl-l-histidyl-l-lysine, 10 ng/ml somatostatin, and 10 mU/ml TSH; Sigma-Aldrich, Inc., St. Louis, MO) and 5% calf serum. BRL-3A liver cells were also obtained from American Type Culture Collection. Runx2 siRNA was generated in the cells using the pSilencer 4.1-CMV hygro vector (Applied Biosystems, Inc., Carlsbad, CA). Oligonucleotides, 5′-GATCCTTCAACG ATCTGAGATTTTTTCAAGAGAAAAATCTCAGATCGTTGAACC-3′ and 5′-AGCTTGGTTCAACGATCTGAGATTTTTCTCTTGAAAAAATCTCAGATCGTTGA AG-3′ were annealed and ligated into the BamH1/HindIII site of pSilencer 4.1-CMV hygro (pSilencer-siRunx2). The construct and pSilencer 4.1-CMV hygro-negative control (Applied Biosystems) were transfected into cultured cells with the Gene Pulser (Gene Pulser Xcell; Bio-Rad, Tokyo, Japan) at 250 V-750 μF. Stable transformants were established by adding 300 μg/ml hygromycin B (Wako Pure Chemicals, Inc., Ltd., Osaka, Japan) to the culture medium.
Northern blot analysis and quantitative PCR
mRNAs from cultured cells and thyroid glands (n = 5) and liver (n = 5) from 12-wk-old wild-type mice were prepared by RNeasy Mini kit (QIAGEN, Inc., Valencia, CA) and further purified with Oligotex-dT30 (Takara Bio, Inc., Shiga, Japan). Human Runx2 cDNA in pBluescript was also donated by Prof. T. Komori. The XhoI/SacII fragment containing the full coding region was ligated into pcDNA 3.1. TSHR, NIS, Tg, TPO, and GAPDH cDNAs, amplified by PCR, were subcloned into pcDNA 3.1 and used as probes. Blotted filters were hybridized with 32P-labeled cDNA probes. mRNA (1 μg) was electrophoresed on a 1% agarose gel, transferred onto a cellulose acetate membrane, and hybridized with 32P-labeled cDNAs, as described previously (23). For quantitative PCR, mRNA (1 μg) was reverse transcribed into cDNA with AML reverse transcriptase (Takara Bio). Quantitative PCR was carried out with Rotor-Gene Q (QIAGEN) using TaqMan probes (Applied Biosystems) for mouse Runx2 (Rn01512296_A1) and GAPDH (MA999999_g1).
EMSA and reporter assay
Nuclear extracts were prepared from mouse tissues (100 mg of thyroid gland or liver) or cultured cells (24). The protocol for EMSA was as described elsewhere (24). Synthesized double-stranded Oligo OC (sense, 5′-CTAGCGAGTATTGTGGTTAATT CG-3′, and antisense, 5′-CTAGCGTATTAACCACAATACTCG-3′) (3), corresponding to the Runx2 binding site on the osteocalcin gene promoter, and Oligo W (sense, 5′-CATGTGGAGTGGTC AC-3′, and antisense, 5′-CATGGTGACCACTCCA-3′), corresponding to the Runx2 binding site on the Tg promoter (−178 to −167 bp), were labeled with [α-32P]dCTP and klenow fragment and purified using a Quick Spin column (Roche Molecular Biochemicals, Indianapolis, IN). In experiments using an antibody against Runx2 (M-70; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), nuclear extracts from the cells were incubated with antiserum or control serum for 30 min at room temperature before adding the labeled probes. Oligo M (sense, 5′-CATGTGG AGTACTCAC-3′, and antisense, 5′-CATGGTGAGTACTCCA-3′) and Oligo RBS1 (sense, 5′-CTAGCGAGTAT TGTGGTTAATACG-3′, and antisense, 5′-CTAGCGTATTAACCA CAATACTCG-3′), corresponding to the Runx2 binding site (site 1) on the bone sialoprotein gene promoter, were also synthesized and used as competitors. For reporter assay, genomic sequences of the Tg gene from −827 to +72, −433 to +72, −175 to +72, and −169 to +72 (10) were ligated into the BglII/HindIII site of the pGL3 basic vector (Promega Corp., Madison, WI). These Tg-luciferase (Luc) chimera constructs were designated pTg-Luc (−827), pTg-Luc (−433), pTg-Luc (−175W), and pTg-Luc (−167), respectively. To generate pTg-Luc (−175M), which has a mutation in the Runx2 binding sequence, we carried out PCR with a forward primer having a mutated sequence. All plasmids were purified using a Plasmid Maxi kit (QIAGEN, Chatsworth, CA). Two micrograms of reporter and 100 ng pRL-SV40 were cotransfected into FRTL-5 cells or BRL-3A cells with electroporation as described above, and after 72 h, firefly and renilla luciferase activities were assayed with the dual-luciferase reported assay system (Promega).
In vivo ChIP assay
In vivo tissue ChIP assay was carried out using thyroid gland (75 mg) or liver (75 mg) from wild-type mice with the EpiQuik tissue ChIP kit (Epigentek Group, Inc., Brooklyn, NY). After cross-linking tissues with 1% formaldehyde, protein/DNA complexes were precipitated with normal mouse IgG (1 μg, NC), anti-RNA polymerase II antibody (2.5 μg, positive control), or anti-Runx2 antibody (2.5 μg, M-70; Santa Cruz Biotechnology), and precipitated DNA fragments were amplified by PCR using the following primers: for Tg promoter, sense, 5′-TCCACAGTTTTATGATCGAACTTCT-3′, and antisense, 5′-GCTCAACAAAGTAGA GACCCACAAG-3′; and for GAPDH promoter, sense, 5′-TGG AACAGGGAGGAGCAGAGAG CA-3′, and antisense, 5′-TCATCGCGGCTTTACGGG-3′.
Measurement of thyroid hormones and 125I uptake
Serum-free T4, free T3, total T3, and total T4 levels were assayed using the ECLusis system (Roche Diagnostic Co., Tokyo, Japan). Mouse TSH was assayed using the rat TSH ELISA kit (AKRTS-010; Shibayagi, Gunma, Japan). 125I uptake by thyroid glands was measured by administering 104 Bq of 125INa (GE Healthcare, Tokyo, Japan) into the peritoneal space. After 24 h, mice were anesthetized with pentobarbital, the thyrotracheal unit was resected, and radioactivity was measured with a γ-counter (Autowell Gamma System, ARC-380; Aloka, Tokyo, Japan).
Statistical analysis
Statistical analysis was carried out by one-way ANOVA and Student’s t test.
Footnotes
Disclosure Summary: The authors have nothing to disclose.
First Published Online April 7, 2010
Abbreviations: CCD, Cleidocranial dysplasia; CH, congenital hypothyroidism; ChIP, chromatin immunoprecipitation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Luc, luciferase; NC, negative control; NIS, Na+/I− symporter; Oligo, oligonucleotide; siRNA, small interfering RNA; Tg, thyroglobulin; TPO, thyroid peroxidase; TSHR, TSH receptor; TTF, thyroid transcription factor.
References
- 1.de Bruijn MF, Speck NA2004. Core-binding factors in hematopoiesis and immune function. Oncogene 24:4238–4248 [DOI] [PubMed] [Google Scholar]
- 2.Fukamachi H, Ito K, Ito Y2004. Runx3−/− gastric epithelial cells differentiate into intestinal type cells. Biochem Biophys Res Commun 321:58–64 [DOI] [PubMed] [Google Scholar]
- 3.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G1997. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89:747–754 [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi A, Komori T, Suda T2000. Regulation of osteoblast differentiation mediated by bone morphologic proteins, Hedgehogs, and Cbfa-1. Endocr Rev 21:393–411 [DOI] [PubMed] [Google Scholar]
- 5.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T1997. Targeted disruption of Cbfa-1 resulted in a complete lack of bone formation owing to maturation arrest of osteoblasts. Cell 89:755–764 [DOI] [PubMed] [Google Scholar]
- 6.Yoshida CA, Yamamoto H, Fujita T, Furuichi T, Ito K, Inoue K, Yamana K, Zanma A, Takada K, Ito Y, Komori T2004. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev 18:952–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM2003. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol 23:489–494 [DOI] [PubMed] [Google Scholar]
- 8.Carcangiu ML, Zampi G, Pupi A, Castagnoli A, Rosai J1985. Papillary carcinoma of the thyroid: a clinicopathologic study of 241 cases treated at the University of Florence, Italy. Cancer 55:805–828 [DOI] [PubMed] [Google Scholar]
- 9.Johannessen JV, Sobrinho-Simões M1980. The origin and significance of thyroid psammoma bodies. Lab Invest 43:287–296 [PubMed] [Google Scholar]
- 10.Endo T, Ohta K, Kobayashi T2008. Expression and function of Cbfa-1/Runx2 in thyroid papillary carcinoma cells. J Clin Endocrinol Metab 93:2409–2412 [DOI] [PubMed] [Google Scholar]
- 11.Endo T, Kobayashi T2008. Thyroid-stimulating hormone receptor in brown adipose tissues is involved in the regulation of thermogenesis. Am J Physiol Endocrinol Metab 295:E514–E518 [DOI] [PubMed]
- 12.Collins WT, Capen CC1980. Ultrastructural and functional alterations of the rat thyroid gland produced by polychlorinated biphenyls compared with iodide excess and deficiency, and thyrotropin and thyroxine administration. Virchows Arch B Cell Pathol Incl Mol Pathol 33:213–231 [DOI] [PubMed] [Google Scholar]
- 13.Musti AM, Ursini VM, Avvedimento EV, Zimarino V, Di Lauro R1987. A cell type specific factor recognizes the rat thyroglobulin promoter. Nucleic Acids Res 15:8149–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javed A, Barnes GL, Jasanya BO, Stein JL, Gerstenfeld L, Lian JB, Stein GS2001. Runt homology domain transcription factors (Runx, Cbfa, and AML) mediated repression of the bone sialoprotein promoter: evidence for promoter-context-dependent activity of Cbfa proteins. Mol Cell Biol 21:2891–2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SM, Chatterjee VK2005. Genetics of congenital hypothyroidism. J Med Genet 42:379–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunthornthepvarakui T, Gottschalk ME, Hayashi Y, Refetoff S1995. Resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. N Engl J Med 332:155–160 [DOI] [PubMed] [Google Scholar]
- 17.Sinclair AJ, Lonigro R, Civitareale D, Ghibelli L, Di Lauro R1990. The tissue-specific expression of the thyroglobulin gene requires interaction between thyroid-specific and ubiquitous factors. Eur J Biochem 193:311–318 [DOI] [PubMed] [Google Scholar]
- 18.Dohán O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, Ginter CS, Carrasco N2003. The sodium/iodide Symporter (NIS): characterization, regulation, and medical significance. Endocr Rev 24:48–77 [DOI] [PubMed] [Google Scholar]
- 19.Fassler CA, Dunn JT, Anderson PC, Fox JW, Dunn AD, Hite LA, Moore RC, Kim PS1988. Thyrotropin alters the utilization of thyroglobulin’s hormonogenic sites. J Biol Chem 263:17366–17371 [PubMed] [Google Scholar]
- 20.Rodan GA, Harada S1997. The missing bone. Cell 89:677–680 [DOI] [PubMed] [Google Scholar]
- 21.Mundlos S, Mulliken JB, Abramson DL, Warman ML, Knoll JH, Olsen BR1995. Genetic mapping of cleidocranial dysplasia and evidence of a microdeletion in one family. Hum Mol Genet 4:71–75 [DOI] [PubMed] [Google Scholar]
- 22.Chen BH, Chen LY, Jaw TH, Chao MC1998. Cleidocranial dysplasia: a rare case associated with congenital hypothyroidism and severe neonatal hyperbilirubinemia. Kaohsiung J Med Sci 14:53–57 [PubMed] [Google Scholar]
- 23.Endo T, Ohta K, Haraguchi K, Onaya T1995. Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J Biol Chem 270:10833–10837 [DOI] [PubMed] [Google Scholar]
- 24.Endo T, Kaneshige M, Nakazato M, Ohmori M, Harii N, Onaya T1997. Thyroid transcription factor-1 activates the promoter activity of rat thyroid Na+/I− symporter gene. Mol Endocrinol 11:1747–1755 [DOI] [PubMed] [Google Scholar]