Abstract
It is still unclear how glucocorticoids (GCs) induce apoptosis of thymocytes and T lymphoma cells. Emergence of GC-resistant lymphoma cells is a major obstacle in GC therapy, emphasizing the need for novel strategies that maintain the sensitivity of lymphoma cells to the proapoptotic effects of GC. We have undertaken a kinome study to elucidate the signal transduction pathways involved in mediating GC-induced apoptosis. Our study shows that glycogen synthase kinase (GSK3) plays a central role in promoting GC-induced apoptosis. In the absence of a ligand, GSK3α, but not GSK3β, is sequestered to the glucocorticoid receptor (GR). Exposure to GCs leads to dissociation of GSK3α from GR and subsequent interaction of GSK3α and GSK3β with the proapoptotic Bim protein, an essential mediator of GC-induced apoptosis. Chemical inhibition of GSK3 by SB216763, BIO-Acetoxime, or LiCl and GSK3 inhibition using a dominant-negative mutant of GSK3 impede this cell death process, indicating that GSK3 is involved in transmitting the apoptotic signal. GC resistance in lymphoma cells can be relieved by inhibiting the phosphatidylinositol-3 kinase-Akt survival pathway, which inactivates GSK3. Notch1, a transcription factor frequently activated in T acute lymphoblastic leukemia cells, confers GC resistance through activation of Akt. Altogether, this study illuminates the link connecting upstream GR signals to the downstream mediators of GC-induced apoptosis. Our data suggest that targeting protein kinases involved in GSK3 inactivation should improve the outcome of GC therapy.
This paper describes an important role for glycogen synthase kinase 3 (GSK3) in promoting glucocorticoid-induced apoptosis of T lymphoid cells.
Glucocorticoids (GCs) are effectively used in the treatment of various hematopoietic malignancies due to their ability to induce apoptosis of these cancerous cells. However, the mechanisms involved in GC-induced apoptosis are largely unknown. It is also poorly understood why some primary lymphomas respond to GCs with apoptotic death whereas others do not. Another enigma is the gradual acquisition of GC resistance in patients exposed to continuous GC therapy. To develop more efficient GC-based therapeutic approaches, it is of uttermost importance to understand the mechanisms regulating the cell’s susceptibility to GC-induced apoptosis.
The effects of GCs are mediated through the glucocorticoid receptor (GR), which, in its native state, is sequestered in the cytosol through interaction with the heat shock protein (Hsp) complex (1, 2). Upon ligand binding, the GR is released from the Hsp complex and translocates to the nucleus where it affects expression of numerous genes through transactivation and transrepression (2). The genomic effects of GR occur in both GC-sensitive and GC-resistant cells. So far, only few of the GC target genes, e.g. Bim, have been implicated in apoptosis (2, 3). However, the mere up-regulation of the proapoptotic Bim protein is insufficient for initiating apoptosis. Rather, it must be activated through posttranscriptional modifications and interactions with other proteins (4). Also, most GC-sensitive as well as GC-resistant lymphoma and leukemia cells innately express high basal levels of Bim (1), suggesting that the activation of Bim by GCs occurs at a posttranscriptional level. Thus, the genomic effects of GCs may modulate the cell’s propensity to undergo apoptosis but are insufficient per se to trigger the death response.
Recent data indicate that nongenomic factors are crucial for propagating the signals leading to GC-induced apoptosis (1, 2). Rapid nongenomic effects occur within minutes after exposure to GCs, which precede the nuclear effects (5, 6). A transient increase in cytosolic Ca2+ level is an early event in thymocytes after GC treatment, the prevention of which abrogates GC-induced apoptosis (7). Other nongenomic effects observed immediately after GC exposure include alterations in redox status (8), elevation of intracellular levels of hydrogen peroxide (9) and other reactive oxygen species (10), lysosomal release of cathepsin B (11), and activation of acidic and neutral sphingomyelinase with subsequent ceramide production (12). Most of these GC-induced effects originate in the mitochondria. This agrees with the observation that the synthetic GC dexamethasone (Dex) induces mitochondrial GR translocation in GC-sensitive, but not in GC-resistant lymphoid cells (13), suggesting that the instant and sustained increase in mitochondrial GR is responsible for some of the mitochondrial effects caused by GCs. Exclusive expression of GR in the mitochondria is sufficient to elicit apoptosis, even in nonhematopoietic cells (13), pointing to an essential role for mitochondrial GR in promoting GC-induced cell death.
There are also several lines of evidence that GCs affect the cell’s kinome (2). GCs induce rapid phosphorylation of GR (14, 15) and modulate the activities of some protein kinases, such as MAPKs, serum and glucocorticoid-inducible kinase 1, and Akt, depending on the cell type (16, 17, 18). Moreover, the MAPKs, ERK, p38, and Jun N-terminal kinase (JNK) are frequently inactivated through GC-mediated up-regulation of the dual specificity phosphatase-1 (MAPK phosphatase-1) (19). Also, interaction of GR with JNK leads to the inhibition of JNK (20). In contrast, overexpression studies and in vitro kinase assays showed that MAPKs, cyclin-dependent kinases (Cdks), and glycogen synthase kinase 3 (GSK3) can phosphorylate GR and modulate its transactivation activity (2, 21, 22, 23, 24, 25). The relevance of these protein kinases in modulating GR function under physiological conditions warrants further studies. GR phosphorylation requires elevation of the intracellular calcium concentration (15), but it is unclear which protein kinase(s) catalyzes the GC-induced GR phosphorylation.
Recent studies indicate that certain protein kinases antagonize GC-induced apoptosis of normal and malignant hematopoietic cells (2). These include MAPK kinase (MEK), ERK, phosphatidylinositol-3 kinase (PI3K), mammalian target of rapamycin (mTOR), and Akt (PKB) (2, 26, 27, 28, 29, 30, 31). Inhibition of one or more of these protein kinases may sensitize the cells to GC-induced apoptosis, suggesting that the apoptotic process is mediated by kinase-regulated signal transduction pathways. Also, the broad-acting protein kinase inhibitors staurosporine (32) and CHIR-258 (33) can sensitize GC-resistant lymphoma and multiple myeloma cells to GC-induced apoptosis, indicating that it is possible to overcome GC resistance by altering the cell’s kinome.
Several attempts have been made to identify protein kinases involved in mediating GC-induced apoptosis, but with conflicting results (27, 34, 35, 36, 37, 38, 39, 40, 41). Inhibition of p38 MAPK prevented GC-induced apoptosis of S49 T lymphoma and CCRF-CEM T acute lymphoblastic leukemia (T-ALL) cells (27, 34, 35, 36) but not of thymocytes, eosinophils, or multiple myeloma cells (37, 38, 39). Cdk2 was proposed to be involved in transmitting the apoptotic signals based on the findings that its activation is an early step during thymocyte apoptosis (40) and that the Cdk2 inhibitor roscovitine abrogated Dex-induced apoptosis of thymocytes (40, 41). However, Cdk2-deficient thymocytes are as sensitive to Dex-induced apoptosis as wild-type thymocytes (42), precluding an essential role for Cdk2 in this process. Roscovitine still prevented GC-induced apoptosis of Cdk2-deficient thymocytes (42), suggesting that this inhibitor acts at a different level. Indeed, roscovitine can modify the expression of hundreds of genes and alter the gene repertoire affected by Dex (23).
In the present study we have searched for protein kinase(s) transmitting the apoptotic signals induced by GC. We observed that GSK3 plays a crucial role in transmitting the apoptotic signal. In the absence of a ligand, GSK3α associates with GR. Upon Dex treatment, GSK3α dissociates from the GR, and both GSK3α and GSK3β interact with the proapoptotic Bim protein. Thus, GSK3 connects the upstream GR signals with the downstream proapoptotic effector Bim. Inhibition of GSK3 abrogates GC-induced apoptosis. GC resistance in lymphoma and leukemia cells is frequently caused by protein kinases antagonizing GSK3 and may be overcome by drugs keeping GSK3 active.
Results
Screening for protein kinases involved in mediating GC-induced apoptosis
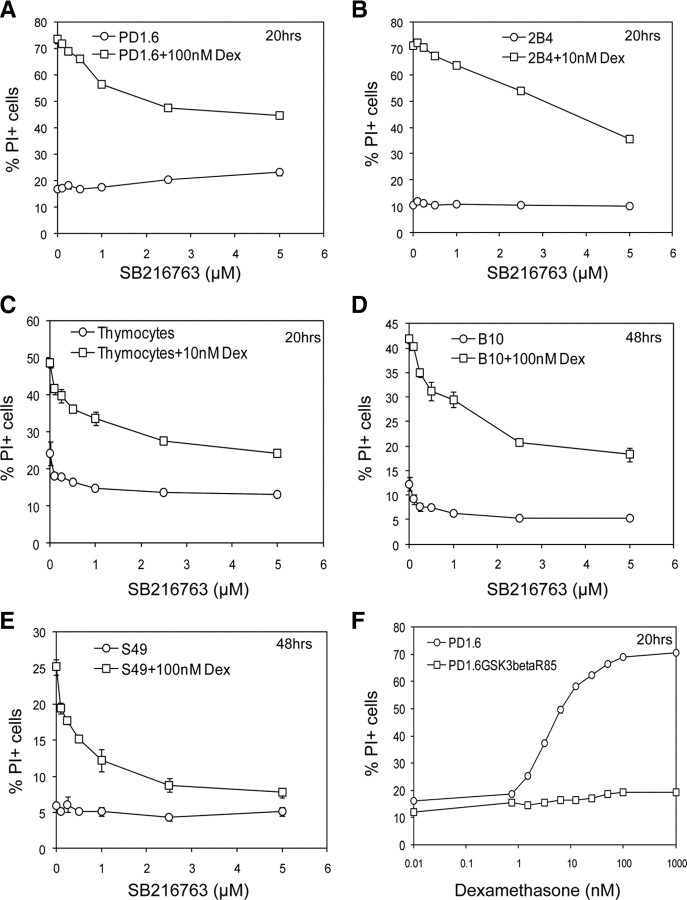
To pinpoint protein kinases involved in mediating GC-induced apoptosis, we studied the effect of various protein kinase inhibitors on this cell death process (Fig. 1). We used both highly GC-sensitive cells (e.g. thymocytes, CD4+8+ PD1.6 thymic lymphoma and 2B4 T cells), which undergo extensive GC-induced apoptosis within 20 h and partially resistant cells (e.g. CD4−8− S49 thymic lymphoma, CD4−8− B10 thymic lymphoma, and CCRF-CEM T-ALL cells), which show partial apoptotic response after 48–72 h exposure to the GC drug dexamethasone (Dex) (1, 13, 32). The use of several cell types makes it possible to distinguish the common signaling pathways from idiotypic regulatory mechanisms.
Fig. 1.
Effect of protein kinase inhibitors on GC-induced apoptosis. Thymocytes (A), PD1.6 T lymphoma (B), S49 T lymphoma (C), and CCRF-CEM T ALL (D) cells were incubated with the indicated concentrations of Dex in the absence or presence of various protein kinase inhibitors for 20 h (A–C), 48 h (C and D), or 72 h (D). Apoptosis was measured by the PI-exclusion assay, which is the most adequate assay for assessing cell death in lymphoma and leukemia cells (32 ). The results of the PI-exclusion assay correlate with the caspase-3 activation assay. PP1, a Src inhibitor; SB203580, a p38 inhibitor; PD98059, a MEK inhibitor; SP600125, a JNK inhibitor; roscovitine, a Cdk2 inhibitor; SB216763, a GSK3 inhibitor. The casein kinase I (CKI) inhibitor was obtained from Calbiochem and the casein kinase II (CKII) inhibitor is 4,5,6,7-tetrabromobenzotriazole. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. Cont, Control.
Initially, we focused on MAPKs due to the controversial data regarding the role of these kinases in GC-induced apoptosis (34, 35, 37, 38, 43, 44) and their central function in regulating thymic T cell selection (45). We observed that none of the MAPK inhibitors PD98059 (MEK inhibitor), SB203580 (p38 inhibitor), or SP600125 (JNK inhibitor) prevented GC-induced apoptosis of thymocytes or PD1.6 cells (Fig. 1, A and B), suggesting that these protein kinases are not essential for mediating the apoptotic process. In contrast to the highly GC-sensitive cells, the p38 inhibitor SB203580 inhibited, whereas the JNK inhibitor SP600125 (and to a lesser extent the MEK inhibitor PD98059), enhanced GC-induced apoptosis of the partially resistant S49 and CCRF-CEM cells (Fig. 1, C and D). The cell type-specific effect of SB203580 may be explained by the different biochemical composition of the partially resistant S49 and CCRF-CEM cells in comparison with the highly GC-sensitive thymocytes and PD1.6 cells (Ref. 1 and Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). S49 and CCRF-CEM are distinguished by the expression of the antiapoptotic Bcl-2 (1) and Notch1 (Supplemental Fig. 1k) proteins. It is likely, therefore, that adequate p38 activation is required for overcoming the resistant phenotype of S49 and CCRF-CEM cells. On the other hand, excessive activities of the JNK and MEK/ERK pathways in these cells oppose GC-induced apoptosis.
The Src inhibitor PP1 barely affected Dex-induced apoptosis of thymocytes (Fig. 1A) and CCRF-CEM cells (data not shown) but enhanced the effect of Dex on the sensitive PD1.6 cells (Fig. 1B), and sensitized S49 cells to Dex-induced apoptosis (Fig. 1C). This selective effect of PP1 indicates that Src proteins antagonize GC-induced apoptosis in some, but not all, cells. The Casein kinase I and II inhibitors did not affect Dex-induced apoptosis in the cell lines tested (Fig. 1, B and C).
Another protein kinase implicated in GC-induced apoptosis of thymocytes is Cdk2, based on the inhibitory effect of its inhibitor roscovitine at high concentration (25 μm) (40, 41). We observed that roscovitine at the concentration required to inhibit Cdk2 (10 μm) (42) only slightly reduced GC-induced apoptosis of thymocytes (Fig. 1A) and even enhanced the proapoptotic effect of Dex on PD1.6 and S49 cells (Fig. 1, B and C). The latter may be due to the cytotoxic effect of Cdk2 inhibition on proliferating malignant hematopoietic cells (46). Normal hematopoietic cells, however, are insensitive to the proapoptotic effect of roscovitine (46). In agreement with studies from the Gil-Gomez G. research group (40, 41), roscovitine at a concentration above that needed for Cdk2 inhibition (25 μm) completely blocked Dex-induced thymocyte death (Supplemental Fig. 2A). Because Cdk2-deficient thymocytes are sensitive to Dex-induced apoptosis (42), and roscovitine at high concentrations is still able to abrogate Dex-mediated cell death in these cells (42), it is likely that this inhibitor acts at a different level. Indeed, a recent study (23) shows that roscovitine modulates gene transcription.
The lack of consistency and the cell-specific effects of the above-mentioned kinase inhibitors suggest that although their target protein kinases may modulate the propensity of the cell to undergo apoptosis in response to GCs, they do not directly partake in mediating the signal transduction pathways leading to apoptotic death.
GSK3 plays a central role in mediating GC-induced apoptosis
In a further search for a protein kinase involved in GC-induced apoptosis, we observed that the potent GSK3 inhibitor LiCl prevented Dex-induced apoptosis of PD1.6 cells (Supplemental Fig. 2B). Because LiCl has additional activities other than inhibiting GSK3, we studied the effect of the two GSK3-specific inhibitors SB216763 and BIO-Acetoxime, which are widely used to determine the role of GSK3 in various biological systems. Indeed, we observed that these specific GSK3 inhibitors strongly prevented Dex-induced apoptosis of both highly GC-sensitive cells such as thymocytes (Fig. 2C), PD1.6 T lymphoma cells (Fig. 2A and Supplemental Fig. 2C) and 2B4 T cells (Fig. 2B and Supplemental Fig. 2D), and partially resistant B10 T lymphoma (Fig. 2D), S49 T lymphoma (Fig. 2E + Supplemental Fig. 2E), and CCRF-CEM T-ALL cells (Fig. 1D) in a dose-dependent manner. The inhibitory effect of SB216763 was not restricted to T lymphoid cells, because it also interfered with Dex-induced apoptosis of the MM1.S multiple myeloma cell line (Supplemental Fig. 2F). To further prove the role of GSK3 in this cell death process, we stably transfected PD1.6 cells with the kinase-inactive GSK3βR85, which acts in a dominant-negative fashion (47). PD1.6GSK3βR85 cells become resistant to Dex-induced apoptosis (Fig. 2F), thus indicating that GSK3 is essential for promoting the apoptotic response to Dex. Specific knockdown of either GSK3α or GSK3β did not lead to GC resistance (Supplemental Fig. 3), implying that one isoform may compensate for the other, and both isoforms contribute to promote apoptosis.
Fig. 2.
Dex-induced apoptosis is prevented by inhibition of GSK3. A–E, The specific GSK3 inhibitor SB216763 prevents Dex-induced apoptosis of T lymphoma cells and thymocytes. The highly GC-sensitive PD1.6 T lymphoma (A), 2B4 T hybridoma (B), and thymocytes (C), as well as the partially resistant B10 (D) and S49 (E) T lymphoma cells, were treated with various concentrations of the GSK3 inhibitor SB216763 in the presence or absence of Dex for 20 h (A–C) or 48 h (D and E). Apoptosis was measured by the PI-exclusion assay. F, PD1.6 expressing a kinase-inactive variant of GSK3 (PD1.6GSK3βR85) becomes resistant to Dex-induced apoptosis. The cells were treated for 20 h with different Dex concentrations, and apoptosis was determined by PI exclusion. P < 0.001.
GSK3α associates with GR in the absence of ligand and dissociates upon GC treatment
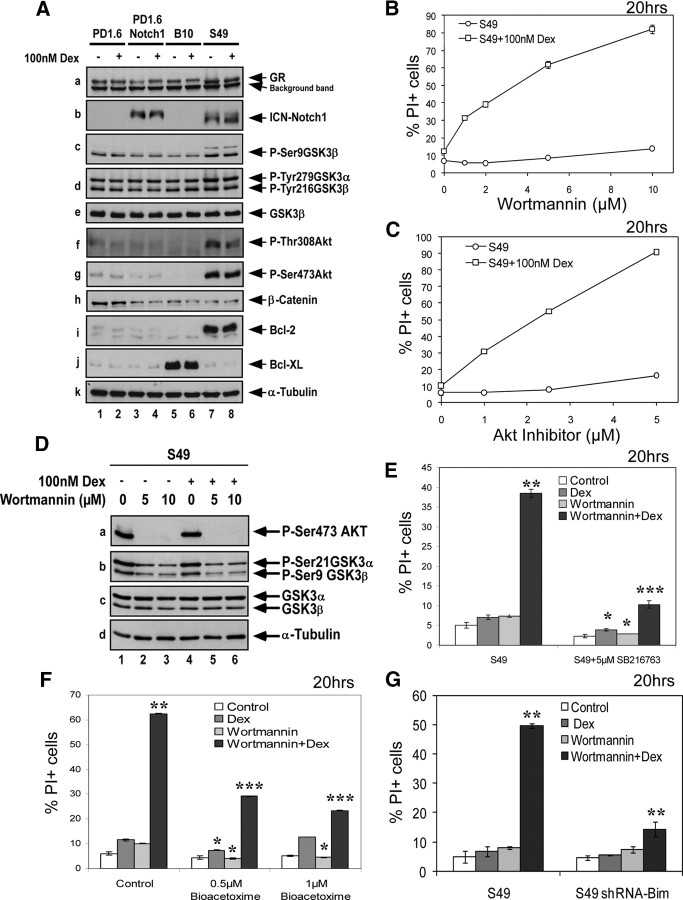
Because GSK3β activity is regulated by Ser9 and Tyr216 phosphorylations (48), we studied whether Dex affects the phosphorylation status of GSK3β. A time course follow-up using PD1.6 cells showed that Dex slightly modified phosphorylation of these two amino acid residues (Fig. 3A). In thymocytes, a transient increase in the inhibitory GSK3β Ser9 phosphorylation was observed (Fig. 3B). Thus, it is likely that another mechanism for GSK3 activation is involved. We therefore investigated the possibility that GSK3 interacts with GR. To this end, GR was immunoprecipitated from untreated and Dex-treated thymocytes or PD1.6 cells, and coimmunoprecipitated GSK3 was analyzed by Western blotting using antibodies reacting with both the α- and β-isoforms. Surprisingly, we observed that in the absence of Dex, GR interacts with GSK3α, but not GSK3β (Fig. 3B, panel a, lane 3; and Fig. 3C, panels a and b, lane 1). However, upon exposure to Dex, this interaction is immediately disrupted (Fig. 3B, panel a, lanes 4–7; and Fig. 3C, panels a and b, lane 3). A small band of GSK3α is coimmunoprecipitated with GR in thymocytes after 2 h incubation with Dex (Fig. 3B, panel a, lane 8), suggesting that GSK3α reassociates with GR at this time point. The GR-GSK3α dissociation coincides with the Dex-induced phosphorylation of GR at Ser211 (Fig. 3B, panel c). Interestingly, the GSK3 inhibitor SB216763 disrupted the GR-GSK3α interaction (Fig. 3C, panels a and b, lane 2). The GSK3 inhibitor did not affect Dex-induced phosphorylation of GR at Ser211 (Fig. 3C, panel d, lanes 3 and 4) or intracellular GR trafficking (data not shown). However, it reduced the phosphorylation of both GSK3 Ser21/9 and Tyr279/216, suggesting that it brings about a conformational change in GSK3, which prevents its interaction with phosphorylating kinases as well as with GR. Altogether, these findings demonstrate that GSK3α interacts with GR and is released from the GR complex upon GC binding.
Fig. 3.
GSK3α interacts with GR. A, The effect of Dex treatment on GSK3 phosphorylation. PD1.6 cells were untreated or treated with 100 nm Dex for the indicated time intervals and processed for Western blotting (WB) using antibodies to phospho-Ser9 GSK3β, phospho-Tyr216 GSK3β, GSK3β, or α-Tubulin. B, GSK3α interacts with native GR and is released upon Dex treatment. Thymocytes were untreated or treated with 100 nm Dex for the indicated time intervals. Total lysates were immunoprecipitated (IP) using M20 antibodies specific to GR (Santa Cruz Biotechnology) and Protein A beads. The one-step Genscript IP-WB kit was used for Western blot analysis to prevent the detection of heavy chain. Nevertheless, a band of the heavy chain is seen, as indicated. Antibodies to GSK3α/β (0011-Α, Santa Cruz Biotechnology) were applied. For the IP analysis, two pre-IP samples (lanes 1 and 9; input 1:40 of IP samples) were run to display the location of GSK3α and -β. Lane 2 contains only the antibody (Ab), which provides the location of the heavy chain. Samples of total lysates were taken before immunoprecipitation (Pre-IP), to show the initial expression levels of the indicated proteins. C, GR interacts with GSK3α in PD1.6 T lymphoma cells. PD1.6 cells were untreated (lane 1), or treated with SB216763 and/or Dex for 1 h. Immunoprecipitation was performed as described in panel B. Panels a and b are two different exposures. The GSK3α band appears just above the heavy chain band. D, Selective binding of GSK3α to GR. MCF-7 cells were transfected with 5 μg of plasmids encoding hGRα (lanes 1–3), hGSK3α (lanes 2 and 4), and/or hGSK3β.HA (hemagglutinin-labeled) (lanes 3 and 5). Empty pcDNA3 vector (5 μg) was included in samples 1, 4, and 5 to achieve equal amounts of transfected DNA. Cell lysates were immunoprecipitated with M20 antibodies against GR, and the presence of coimmunoprecipitated GSK3 was analyzed by WB as described in panel B. E, Long-term inhibition of GSK3 leads to reduced GR expression. PD1.6 cells were treated with SB216763 for 2 and 24 h, and the GR expression level was analyzed on Western blot. β-Catenin expression is shown as indication for GSK3 inhibition.
To further prove the selective interaction of GSK3α with GR, we transfected MCF-7 breast carcinoma cells with human GRα in the absence or presence of either human GSK3α or HA-tagged human GSK3β. Cell lysates were immunoprecipitated 24 h after transfection with M20 antibodies directed against GR, and the presence of coimmunoprecipitated GSK3 was analyzed by Western blotting. Figure 3D shows that GSK3α (panel a, lane 2), but not GSK3β (panel a, lane 3), coimmunoprecipitates with GR. The pull-down of GSK3α with GR is specific, because no GSK3α was detected in the immunoprecipitate in the absence of GR (Fig. 3D, lane 4). Of note, the steady-state GR level was higher in MCF-7 cells cotransfected with GSK3α and GR than in MCF-7 cells cotransfected with GSK3β and GR, or GR alone with empty vector. This finding suggests that the interaction of GSK3α with GR may affect GR expression. Indeed, an overnight incubation of PD1.6 cells with the GSK3 inhibitor SB216763, which prevents the GR-GSK3α interaction (Fig. 3C), led to reduced GR expression (Fig. 3E), without altering the GR mRNA expression level (data not shown). Shorter incubation time with SB216763 did not alter GR expression (Fig. 3E), precluding that a reduced GR expression is the reason for SB216763 inhibition of GC-induced apoptosis. Thus, GSK3 seems to play a dual role, one in promoting apoptosis and the other in maintaining GR expression.
GSK3α and GSK3β interact with Bim after GC treatment
In light of our findings that GSK3 inhibition prevents GC-induced apoptosis, and GSK3α is released from GR upon GC exposure, we raised the question whether GSK3α may transmit the apoptotic signals delivered by GCs. Because the proapoptotic Bim protein is essential for mediating GC-induced apoptosis (49, 50) and acts upstream to Bax and Bak, we asked whether GSK3 could interact with Bim. To answer this query, we immunoprecipitated Bim from untreated and Dex-treated thymocytes and 2B4 T cells and searched for coimmunoprecipitated GSK3 using the antibody reacting with both the α- and β-isoforms. A basal interaction between GSK3β and Bim is observed in thymocytes (Fig. 4A). After Dex treatment, the interaction between Bim and GSK3β is transiently enhanced peaking at 30–60 min and returns to basal after 150 min (Fig. 4A). Interestingly, an interaction between Bim and GSK3α is observed 30–60 min after adding Dex (Fig. 4A), which coincides with the release of GSK3α from native GR upon ligand binding. Also in the GC-sensitive 2B4 cells, a transient interaction between Bim and the two GSK3 isoforms is seen (Fig. 4B), albeit with different kinetics than in thymocytes (Fig. 4A). Only a fraction of the total cellular pool of GSK3α interacts with Bim, which is consistent with recent observations that GSK3 does not act as a pool but, rather, disparate fractions of GSK3 participate in different signal transduction pathways (51). The GSK3-Bim interaction is fluctuating, suggesting a dynamic association and dissociation between these proteins. Altogether, our data show that Dex treatment of lymphoid cells promotes GSK3 interaction with Bim. Thus, GSK3 connects GR-GC signaling with the intrinsic apoptotic pathway.
Fig. 4.
GSK3α and GSK3β interact with Bim after exposure of thymocytes (A) and 2B4 T cells (B) to Dex. Thymocytes (A) and 2B4 T cells (B) were either untreated or treated with 100 nm Dex for the indicated time intervals. Cell lysates were immunoprecipitated with antibodies to Bim and processed for Western blot analysis using nonreduced protein sample buffer. Coimmunoprecipitated GSK3α and GSK3β were detected by Western blotting as described in Fig. 3B.
Relieving Akt-mediated inhibition of GSK3 confers apoptotic sensitivity to Dex
Activation of the PI3K-Akt pathway is important for maintaining cell survival through inhibition of apoptosis (52). Akt affects cell survival by phosphorylating various substrates involved in regulating apoptosis, such as the proapoptotic Bad, caspase-9, and the Forkhead transcription factor FoxO3a, which stimulates bim transcription (52). In light of our finding that GSK3 mediates GC-induced apoptosis and the knowledge that Akt negatively regulates GSK3, we propose that Akt prevents Dex-induced apoptosis by inhibiting GSK3 activity. To address this possibility, we used the S49 T lymphoma that endogenously expresses constitutively active Notch1 (ICN-Notch1) and exhibits elevated Akt activity (Supplemental Fig. 1, e, f, and k; and Fig. 5A). Both the PI3K inhibitor wortmannin and the Akt inhibitor VIII strongly sensitized the resistant S49 cells to Dex-induced apoptosis (Fig. 5, B and C), indicating that Akt is the major cause for GC resistance in these cells. Wortmannin treatment leads to rapid dephosphorylation of both Akt and GSK3 (Fig. 5D). The wortmannin-mediated sensitization of S49 cells to Dex-induced apoptosis was hindered by GSK3 inhibition (Fig. 5, E and F), indicating that the sensitization is indeed effected by activation of GSK3. We further show that the proapoptotic function of GSK3 in this setting depends on Bim, because S49 cells transfected with a short hairpin RNA (shRNA) construct to Bim were resistant to the combined treatment of wortmannin and Dex (Fig. 5G). Altogether, these data demonstrate that susceptibility to GC-induced apoptosis can be regained in resistant cells by combining GC therapy with drugs that keep GSK3 active.
Fig. 5.
GC resistance of S49 cells is overcome by relieving Akt-mediated inhibition of GSK3. A, The partially resistant S49 T lymphoma cells display increased Akt activation and express elevated levels of antiapoptotic Bcl-2 and intracellular cleaved Notch1 (ICN). Western blot analysis of the highly GC-sensitive PD1.6 T lymphoma, PD1.6 cells overexpressing ICN-Notch1 (PD1.6Notch1), and the partially resistant B10 and S49 T lymphoma cells are presented. The antibody used in panel c is specific for phospho-Ser9 GSK3β. It also detects a slower migrating band in S49 cells, which may be phospho-Ser21 GSK3α. B and C, Inhibition of the PI3K-Akt axis by either wortmannin (B) or the Akt inhibitor VIII (C) confers GC sensitivity on S49 cells. S49 cells were treated with 100 nm Dex alone or in the presence of increasing concentrations of either wortmannin (C) or Akt inhibitor VIII (D) for 20 h before the extent of apoptosis was measured. The use of either drug alone had no apoptotic effect on S49 cells, whereas combined treatment induced strong apoptosis. D, The PI3K inhibitor wortmannin prevents Akt and GSK3 phosphorylation. S49 cells were either untreated or treated with wortmannin and/or Dex for 1 h. Western blot analysis was performed with antibodies to phospho-Ser473 Akt, phospho-Ser21/9 GSK3α/β, GSK3α/β, or α-tubulin. E and F, The GSK3 inhibitors SB216763 (E) and BIO-Acetoxime (F) prevent wortmannin sensitization to Dex. S49 cells were exposed to 5 μm wortmannin, 5 μm SB216763, or 0.5–1 μm BIO-Acetoxime, with or without 100 nm Dex for 20 h before apoptosis was measured. G, shRNA to Bim prevents wortmannin sensitization of S49 cells to Dex-induced apoptosis. Control S49 cells or S49 cells transfected with pSilencer-Bim were exposed to 5 μm wortmannin and/or 100 nm Dex, and the extent of apoptosis was measured 20 h later. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Notch1 confers GC resistance by activating Akt with concomitant inhibition of GSK3
Another mechanism leading to GC resistance is constitutive Notch1 signaling (53). Notch1 activation is frequently observed in T-ALL (54). The question ensuing is how does Notch1 inhibit GC-induced apoptosis? To answer this question, we stably transfected the highly GC-sensitive PD1.6 and 2B4 cells with the constitutively active ICN-Notch1. As expected (53, 55), ICN-Notch1 conferred complete GC resistance on 2B4 cells (Fig. 6A). However, it had barely any effect on PD1.6 cells (Fig. 6B). RT-PCR analyses revealed that the Notch1-regulated gene Deltex1 is up-regulated in 2B4Notch1 and S49 cells, but not in PD1.6Notch1 cells (Fig. 6C), indicating that Notch1 is not transcriptionally active in the latter. Biochemical analysis showed that Akt is activated in 2B4Notch1, but not in PD1.6Notch1 cells (Figs. 5A and 6D), which may explain the differential effect of Notch1 on the GC sensitivity of these cells. The lack of Akt activation in PD1.6 cells may be due to elevated Csk activation (our unpublished data), which abrogates the Src-PI3K-Akt signaling pathway. This conforms the observation that the Src inhibitor PP1 overcomes GC-resistance in the Notch1-expressing S49 cells (Fig. 1C and Ref. 55).
Fig. 6.
Notch1 confers GC resistance by activating Akt with subsequent inhibition of GSK3. A and B, Overexpression of ICN-Notch1 confers GC resistance on 2B4, but not on PD1.6 cells. 2B4 (A), 2B4Notch1 cells overexpressing ICN-Notch1 (A), PD1.6 (B) or PD1.6Notch1 cells overexpressing ICN-Notch1 (B) were exposed to Dex for 20 h. Apoptosis was measured by PI exclusion. C, ICN-Notch1 is transcriptionally active in 2B4 cells, but not in PD1.6 cells. RT-PCR analysis was performed on the indicated cell lines for detection of the mRNA of GR, SRG3, a gene product related to GC sensitivity (86 ), and the Notch-regulated gene Deltex1. The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was included as a control. D, 2B4Notch1 cells show elevated Akt phosphorylation. Western blot analysis of untreated or Dex-treated (100 nm, 2 h) 2B4 and 2B4Notch1 cells. E, Mcl-1 is induced in 2B4 cells overexpressing Notch1. Total extracts of the indicated cell lines were analyzed by Western blotting.
Despite enhanced Akt activation in 2B4Notch1 cells, the Ser9 phosphorylation of GSK3 is not significantly increased in comparison with that of parental 2B4 cells (Fig. 6D). Dex treatment reduces GSK3Ser9 phosphorylation both in 2B4 and 2B4Notch1 cells (Fig. 6D). However, 2B4Notch1 displays increased phosphorylation at GSK3Tyr279/216, as well as elevated β-catenin expression (Fig. 6D), which is an indication of GSK3 inhibition. Up-regulated β-catenin expression is also observed in the Notch1-positive CCRF-CEM cells (Supplemental Fig. 1j), suggesting that their GC resistance is caused by intrinsic inhibition of GSK3. Another feature of 2B4 cells transfected with ICN-Notch1 is the induction of the antiapoptotic Mcl-1 protein expression (Fig. 6E). This observation complies with data showing that overexpression of a constitutively active Akt (Myr-Akt) induces Mcl-1 expression in 2B4 cells with concomitant acquisition of GC resistance (31). The Notch1-mediated up-regulation of Mcl-1 in 2B4 cells may be related to GSK3 inhibition, because GSK3-mediated phosphorylation of Mcl-1 promotes its degradation (56). Altogether, these data show that Notch1 confers GC resistance in cells in which Akt activation is enabled, eventually leading to the inhibition of GSK3.
Discussion
The mechanisms involved in GC-induced apoptosis are poorly understood. Although the downstream effectors, Bim, Bak, and Bax, are well characterized (1, 2, 57), the signal transduction pathways linking the GR to these effectors have not been elucidated. Significant efforts have been invested in finding GC-regulated genes that are involved in the apoptotic process (reviewed in Ref. 2). However, only a few of the genes affected by GC have been directly associated with apoptosis, the most important of which is the proapoptotic Bim (3, 35, 49, 58). Up-regulation of Bim is especially crucial for conferring apoptotic competence in partially resistant cells that display low basal levels of Bim (1, 35, 49). The highly GC-sensitive cells usually carry sufficient amounts of Bim (1). Moreover, the mere up-regulation of Bim is insufficient to elicit apoptosis (4). Rather, it has to be activated by posttranslational modifications and protein-protein interactions (4). Thus, the GC-induced alterations in gene transcription may be important for twirling the proteome toward an apoptosis-prone phenotype, but is insufficient per se for triggering apoptosis (reviewed in Ref. 2). In the present study we raise the hypothesis that stimulation of GR by its ligand leads to the activation of a protein kinase that dispatches the signals required for apoptosis.
In a search for such a protein kinase, we observed a critical role for GSK3. Inhibition of GSK3 blocks GC-induced apoptosis in all of the hematopoietic cell lines tested, indicating a universal role for GSK3 in this cell death process. This is in stark contrast to p38 inhibition that only affected GC-induced apoptosis of the partially resistant S49 and CCRF-CEM cells. The latter may be explained by a role for p38 in overcoming their resistant phenotype. A recent study showed a requirement for p38 in Dex-induced up-regulation of Bim, which is necessary for sensitizing CCRF-CEM cells to GC-induced apoptosis (35). p38 may further promote apoptosis by phosphorylating Bim (59) and Bcl-2 (60). p38-mediated phosphorylation of Bim at Ser65 enhances its proapoptotic function (59), whereas p38 phosphorylation of Bcl-2 leads to its inactivation (60). This may explain why p38 is necessary for GC-induced apoptosis in the Bcl-2-positive S49 and CCRF-CEM cells. Thus, p38 appears not to be directly involved in mediating GC-induced apoptosis, but rather primes resistant T cells to be able to respond to GC.
The specific GSK3 inhibitors SB216763 and BIO-Acetoxime inhibit both GSK3α and GSK3β, which may cooperate in promoting GC-induced apoptosis. Our study shows that in the absence of ligand, GSK3α, but not GSK3β, associates with GR in the highly GC-sensitive thymocytes and PD1.6 T lymphoma cells. The reason for the isotype-specific interaction of GSK3α with GR warrants further studies. It should be noted that only a certain fraction of the total cellular pool of GSK3α is bound to the native GR. This agrees with recent observations showing a partitioned function of GSK3 (51). GC treatment leads to the dissociation of GSK3α from GR, with subsequent association of GSK3α and GSK3β to the proapoptotic Bim protein. The GSK3-Bim interaction may be important for activating the intrinsic mitochondrial apoptotic pathway. Bim is known to be an essential mediator of GC-induced apoptosis (49, 50). Our findings suggest that GSK3 links the upstream GR signaling with the downstream proapoptotic effectors of the Bcl-2 superfamily.
It is likely that other nongenomic effects induced by GCs, such as transient calcium mobilization and ceramide production (7, 12, 61), are required to fully activate GSK3. Independently, it has been demonstrated that transient increase in calcium activates GSK3β (62), and ceramide promotes dephosphorylation of GSK3 through activation of phosphatase 2A (63). Both GSK3α and -β can be activated by these nongenomic GC-induced stimuli. Specific knockdown of either GSK3α or GSK3β did not lead to GC resistance, implying that one isoform may compensate for the other and both isoforms contribute to promote apoptosis.
Previous studies from our laboratory showed an essential role for mitochondrial GR translocation in promoting GC-induced apoptosis (13). Dex induces GR translocation to the mitochondria in GC-sensitive, but not in GC-resistant lymphoma cells (13). Exclusive mitochondrial expression of GR is sufficient for eliciting apoptosis (13). The mechanisms by which mitochondria GR induce apoptosis are still obscure. It might be involved in inducing reactive oxygen species and ceramide production, which occurs in the mitochondria. On the basis of these data and the findings presented in this paper, we propose a model in which mitochondrial GR translocation cooperates with GSK3 in promoting apoptosis (Fig. 7). In the absence of a ligand, GSK3α is sequestered to GR. Upon GC treatment, GSK3α dissociates from GR. Concomitantly, GR translocates to the nucleus and the mitochondria. The nuclear GR affects the transcription of multiple genes; among them Bim is essential for apoptosis. The GR translocated to the mitochondria triggers nongenomic effects that may further activate GSK3, which we show in this paper to be important for promoting the apoptotic process. GSK3α and GSK3β associate with Bim, which acts upstream to Bax and Bak in the intrinsic apoptotic pathway.
Fig. 7.
A proposed model for GC-induced apoptosis in thymocytes and T lymphoma cells. This model is based on findings described in the present study combined with data published in the literature. In the absence of ligand, GR is sequestered in the cytosol bound to Hsps and immunophilins (no. 1). A proportion of GR also interacts with GSK3α. Upon exposure to GC, GR is released from the heat shock complex, and GSK3α dissociates from GR (no. 2). GR dimerizes and translocates to the nucleus and the mitochondria (no. 3). The nuclear translocation occurs in both sensitive and resistant T lymphoma cells, whereas mitochondrial translocation takes place only in sensitive cells (Ref. 13 ). In the nucleus, GR alters the transcription of multiple genes through transactivation and transrepression (no. 4). Of importance, the essential proapoptotic Bim is up-regulated, a process that requires proper activation of p38 and the transcription factor FoxO3 (Ref. 35 ). Bim is frequently expressed at a high basal level in many hematopoietic cells (Ref. 1 ). GR translocated to the mitochondria delivers signals (no. 5) that may activate GSK3 (no. 6). GSK3α and GSK3β interact with Bim (no. 7). Bim acts upstream to Bax and Bak (no. 7), which execute the apoptotic cascade (no. 8) (Ref. 87 ). GSK3β may also phosphorylate VDAC (no. 7) leading to dissociation of hexokinase II from the mitochondria and facilitation of Bax binding to the mitochondria (Ref. 65 ). Thus, GC-induced apoptosis is effected by a cooperation between nuclear GR (e.g. up-regulation of Bim), mitochondrial GR (dispatching nongenomic signals), and GSK3 (activating the downstream effectors of the intrinsic apoptotic pathway). AP-1, Activator protein 1; NFκB, nuclear factor-κB.
GSK3 is well known to have a wide variety of biological effects (51) and is involved both in preventing and promoting apoptosis, depending on the death signal (48). Most studies thus far focused on the β-isoform of GSK3. Overexpression of GSK3β was shown to be sufficient for inducing apoptosis in both rat-1 fibroblasts and PC12 pheochromocytoma cells (47). It is also a critical activator of neuronal apoptosis induced by various neurotoxic insults (48). GSK3β phosphorylates Bax at Ser163 and promotes its mitochondrial localization during neuronal apoptosis (64). GSK3β may also phosphorylate the voltage-dependent anion channel (VDAC), thereby preventing it from binding hexokinase II (65). Interestingly, overexpression of hexokinase II relieves Dex-induced apoptosis (66) and interferes with the ability of Bax to associate with the mitochondria (67). Thus, GC-induced GSK3 activation may shift the Bax-hexokinase II-VDAC balance, thereby fine tuning the apoptotic threshold. This mechanism explains the reduced mitochondrial hexokinase activity observed in GC-sensitive WEHI7.1 T lymphoma cells after Dex treatment (68). The ability of GSK3 to activate both Bim and Bax may clarify why Bim-deficient thymocytes are only partially resistant to GC-induced apoptosis (50).
On a further examination of various causes leading to GC resistance, we identified a common denominator converging on GSK3. For instance, Notch1, which is frequently activated in T acute lymphoblastic leukemia (69), confers GC resistance through activation of Akt with concomitant inhibition of GSK3. p56Lck was shown to link Notch1 with Akt activation (29). Notch1 function by itself requires Akt activation (70), thus creating a positive feedback loop. The fact that GSK3 phosphorylates Notch1 and down-regulates its activity (71) may explain why Notch1 does not resist long-term exposure to Dex, and Notch1-expressing cells ultimately undergo GC-induced apoptosis. This may be a reason for the more favorable treatment response to prednisone in childhood T-ALL patients carrying activated Notch1 mutations (72). Relieving GSK3 inhibition by targeting Akt is sufficient for sensitizing the Notch1-expressing S49 cells to GC-induced apoptosis. This observation is a proof that it is possible to improve GC therapy by drugs that keep GSK3 active. Thus, clinically developed Akt inhibitors should have far-reached applications in improving GC therapy of lymphomas and leukemias.
GC-induced apoptosis is not only inhibited by Akt (this paper and Ref. 28), but also by Src (this paper and Ref. 29), ERK (this paper and (Refs. 27, 30 , and 36), JNK (this paper and Refs. 27 and 36) and mTOR (27, 73, 74). GC resistance may be overcome by PP1 inhibition of Src proteins (this paper and Ref. 55). PP1 abrogates Akt phosphorylation in S49 cells with a concomitant reduction in GSK3 Ser21/9 phosphorylation (Supplemental Fig. 4). ERK may interact with and phosphorylate GSK3β at Thr43, which primes it for subsequent phosphorylation at Ser9 by p90RSK, resulting in its inactivation (75). This may explain the antagonistic effect of T cell receptor activation on GC-induced apoptosis (30). Also myelogenic leukemia cells show basal ERK activation (Supplemental Fig. 1d), which may contribute to their GC-resistant phenotype. ERK also increases the antiapoptotic effect of Bcl-2 by phosphorylating it at Ser70 (76) and promotes Bim degradation through Ser65 phosphorylation (77, 78). The JNK pathway plays a dual role in regulating cell survival and apoptosis (79). Whereas in some settings JNK-mediated phosphorylation of Bim increases its proapoptotic function (78, 80), in T-ALL cells the same phosphorylation promotes degradation of Bim (81). JNK may further prevent apoptosis by phosphorylating the proapoptotic Bad, leading to its inactivation (82). The antagonistic effect of JNK on GC-induced apoptosis is compatible with data showing that c-Jun and JNK are required for survival of T cells (83) and B lymphoblastoid cells (84). Interestingly, GC resistance in the Akt-positive S49 cells could be overcome by antagonizing either Akt or JNK, suggesting that these two kinases act in concert.
In summary, our research provides new insights into the signal transduction pathways regulating the response of T lymphoma and lymphoblastic leukemia cells to GC-induced apoptosis. It introduces the novel concept that the apoptotic response depends on proper activation of GSK3 and thus relies on the nongenomic effects of GCs. The finding that GSK3α is sequestered to the GR and dissociates from it to interact with Bim upon GC exposure fills in the missing link that connects the upstream GR activation with the downstream proapoptotic mediators of the Bcl-2 superfamily. GSK3 is also activated by elevations in intracellular calcium concentration and by ceramide (48), two signals that are seemingly triggered by GR translocated to the mitochondria (1, 2). This scenario proposes a model in which GSK3 cooperates with mitochondrial GR in activating the apoptotic machinery (Fig. 7). The p38 MAPK may assist in priming the partially resistant cells through up-regulation and activation of Bim and simultaneously antagonizing Bcl-2. Survival kinases such as Src, Akt, mTOR, and ERK antagonize GC-induced apoptosis by preventing GSK3 activation. Altogether, these findings indicate that lymphoma and leukemia therapy may be significantly improved by combining GCs with drugs that keep GSK3 active.
Materials and Methods
Cells
Mouse CD4+8+ PD1.6 thymic lymphoma (85), mouse CD4−8− B10 thymic lymphoma (85), and mouse CD4−8− S49 thymic lymphoma (kindly provided by Dr. J. Hochman, The Hebrew University of Jerusalem) were grown in DMEM supplemented with 10% heat-inactivated fetal calf serum, 2 mm glutamine, 10 mm HEPES, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol. Mouse 2B4 T cells, mouse thymocytes, human CCRF-CEM ALL [from American Type Culture Collection (Manassas, VA), no. CCL-119] and human MM1.S multiple myeloma cells were cultured in RPMI-1640 with the same supplements as for DMEM.
Stable transfection
PD1.6 cells were electroporated (250V, 950 μF) with the pEF1α-R85GSK3β plasmid (kindly provided by Dr. G. M. Cooper, Boston University, Boston, MA) and selected in 1.5 mg/ml G418 (Sigma Chemical Co., St. Louis, MO). PD1.6 and 2B4 cells were retroviral infected with MIG-ICNX vector encoding the intracellular domain of human Notch1 (kindly provided by Dr. W. Pear, Philadelphia, PA) and green fluorescent protein-positive clones were obtained by limiting dilution. PD1.6 cells were infected with pLKO lentiviral particles carrying shRNA to either GSK3α (TRCN0000010340; Open Biosystems, Huntsville, AL) or GSK3β (TRCN0000012615, Open Biosystems), or control shRNA particles. The infected PD1.6 population was enriched by selection in 4 μg/ml puromycin (Sigma). The lentivirus particles were prepared in human 293T embryonic kidney cells using the pCMV/Δ8.91 packaging vector and pMD2-VSV-G envelope construct. S49 cells were electroporated (250 V, 950 μF) with pSilencer 3.1-H1 hygro-Bim (kindly provided by Dr. Charles H. Streuli, University of Manchester, Manchester, UK) and selected in 400 μg/ml Hygromycin B (Sigma).
Transient transfection of MCF-7
Human MCF-7 breast carcinoma cells were transfected with indicated combinations of the following plasmids using the polyethylenimine method: pCMVhGRα kindly provided by Dr. A.-C. Wikström, Huddinge University Hospital, Stockholm, Sweden; hGSK3α-pMT2 and hGSK3β.HA-pcDNA3 kindly provided by Dr. J. R. Woodgett, Mount Sinai Hospital, Toronto, Canada; and empty pcDNA3 vector (Invitrogen, Carlsbad, CA).
Reagents
Dex and SB216763 were purchased from Sigma. PP1, SB203580, PD98059, PD98059, SP600123, wortmannin, and roscovitine were obtained from A.G. Scientific (San Diego, CA). The casein kinase I and II inhibitors and the Akt Inhibitor VIII were purchased from Calbiochem (La Jolla, CA).
Determination of apoptosis
Apoptosis was assessed by the propidium iodide (PI)-exclusion assay, applied to fresh cell cultures. PI at 5 μg/ml was added to the freshly harvested cell cultures immediately before flow cytometry analysis. PI+-FSClow cells are regarded as dead cells. A total of 104 events were counted by flow cytometry for each sample. We have previously shown that the data obtained from the PI-exclusion assay correlate with those obtained from the active caspase-3 assay (32).
Western blot
Total lysate was prepared by lysing 5 × 106 cells in 250 μl protein sample buffer × 1.5. Subcellular fractionation was performed as previously described (13). The following antibodies were applied: Bcl-2 (PC68) from Calbiochem; β-catenin (610153), GSK3β (G22320), phospho-Tyr216 GSK3 (612312), and phospho-Thr180/Tyr182 p38 (612280) from BD Transduction Laboratories (Franklin Lakes, NJ); phospho-Ser473 Akt (catalog no. 9271), phospho-Thr308 Akt (catalog no. 9275), phospho-Ser9 GSK3β (catalog no. 9336), phospho-Thr183/Tyr185 JNK (catalog no. 9255), phospho-Thr202/Tyr204 ERK1 and 2 (catalog no. 9101), phospho-Ser21/9 GSK3α/β (catalog no. 9331) and phospho-Ser211 GR (catalog no. 4161) from Cell Signaling Technology (Danvers, MA); Bcl-XL (H-5), GR (M20), GSK3α/β (0011-Α), and Mcl-1 (S-19) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); and Notch1 (mN1A) and α-tubulin (DM1A) from Sigma.
Coimmunoprecipitation studies
Untreated or treated cells were extracted in lysis buffer (50 mm HEPES, pH 7.4; 300 mm NaCl; 5 mm EDTA; 0.5% Nonidet P-40; 2 mm MgCl2; 10% glycerol; 10 mm sodium molybdate, 25 mm NaF; 0.2 mm Na3VO4; 2 mm phenylmethylsufonylfluoride; and 20 μg/ml aprotinin), and the clarified supernatants were incubated with antibodies to GR (M20, Santa Cruz Biotechnology) or Bim (Calbiochem) bound to protein A-Sepharose beads (Pharmacia Biotech, Piscataway, NJ). The beads were washed five times with lysis buffer, before processing for Western blot analysis. GSK3 was detected on Western blots using the one-step IP-Western kit (Genscript Corp., Piscataway, NJ) and antibodies to GSK3α/β (0011-A) (Santa Cruz Biotechnology).
RT-PCR
Total RNA was isolated using the TriReagent (MRC Molecular Research Center), and cDNA prepared using the RevertAid first strand cDNA synthesis kit (Fermentas, Inc., Glen Burnie, MD) using M-MuLV reverse transcriptase and oligo(dT)18. PCR was performed using Taq polymerase (Fermentas) and the following primers: mouse GR forward, GGAAAAGCCATTGTCAAAAGG; and reverse, TGGCCCTCTAGAGACC-ACAT; mouse SRG3 forward, CGTACTCAGGACGAATGC; and reverse, GCTGCTGACCATCAGGATCTG; mouse Deltex1 forward, GTAAGGCTTCAA-GGGGTCGCT; and reverse, CTCAGCTTGATGCGTGTATAGCC; and mouse glyceraldehyde-3-phosphate dehydrogenase forward, GGAGCCAAACGGGTCATCATCTC; and reverse, GAGGG-GCCATCCACAGTCTTCT.
Statistics
Each experiment was repeated at least three times. The P values were calculated using two-tailed paired Student’s t test.
Acknowledgments
We thank Dr. G. M. Cooper (Boston University, Boston, MA) for providing the GSK3βR85 plasmid; Dr. W. Pear (University of Pennsylvania, Philadelphia, PA) for providing the Mig-ICN-Notch1 retroviral vector; Dr. A.-C. Wikström (Huddinge University Hospital, Stockholm, Sweden) for providing the pCMVhGRα plasmid; Dr. C. H. Streuli (University of Manchester, Manchester, UK) for providing the p-Silencer 3.1-H1 hygro-Bim plasmid; and Dr. J. R. Woodgett (Mount Sinai Hospital, Toronto, Canada) for providing the hGSK3α-pMT2 and hGSK3β.HA-pcDNA3 plasmids.
NURSA Molecule Pages:
Ligands: Dexamethasone.
Footnotes
This work was supported by The Concern Foundation, Amen Foundation, The Israel Cancer Research Fund, and The Israel Cancer Association.
Disclosure Summary: There is no conflict of interest.
First Published Online April 6, 2010
R.S. and S.K.-E. contributed equally to this work.
Abbreviations: ALL, Acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CML, chronic myelogenous leukemia; Dex, dexamethasone; GC, glucocorticoid; GR, glucocorticoid receptor; GSK3, glycogen synthase kinase 3; Hsp, heat shock protein; JNK, Jun N-terminal kinase; MEK, MAPK kinase; MM, multiple myeloma; PI, propidium iodide; PI3K, phosphatidylinositol-3 kinase; PML, promyelocytic leukemia; shRNA, short hairpin RNA; T-ALL, T acute lymphoblastic leukemia; VDAC, voltage-dependent anion channel.
References
- 1.Sionov RV, Kfir S, Zafrir E, Cohen O, Zilberman Y, Yefenof E2006. Glucocorticoid-induced apoptosis revisited: a novel role for glucocorticoid receptor translocation to the mitochondria. Cell Cycle 5:1017–1026 [DOI] [PubMed] [Google Scholar]
- 2.Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E2008. Mechanisms regulating the susceptibility of hematopoietic malignancies to glucocorticoid-induced apoptosis. Adv Cancer Res 101:127–248 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Malone MH, He H, McColl KS, Distelhorst CW2003. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem 278:23861–23867 [DOI] [PubMed] [Google Scholar]
- 4.Puthalakath H, Strasser A2002. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ 9:505–512 [DOI] [PubMed] [Google Scholar]
- 5.Haller J, Mikics E, Makara GB2008. The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front Neuroendocrinol 29:273–291 [DOI] [PubMed] [Google Scholar]
- 6.Song IH, Buttgereit F2006. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol 246:142–146 [DOI] [PubMed] [Google Scholar]
- 7.Distelhorst CW2002. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ 9:6–19 [DOI] [PubMed] [Google Scholar]
- 8.Tome ME, Baker AF, Powis G, Payne CM, Briehl MM2001. Catalase-overexpressing thymocytes are resistant to glucocorticoid-induced apoptosis and exhibit increased net tumor growth. Cancer Res 61:2766–2773 [PubMed] [Google Scholar]
- 9.Tonomura N, McLaughlin K, Grimm L, Goldsby RA, Osborne BA2003. Glucocorticoid-induced apoptosis of thymocytes: requirement of proteasome-dependent mitochondrial activity. J Immunol 170:2469–2478 [DOI] [PubMed] [Google Scholar]
- 10.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G1995. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med 182:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Müller N, McPherson KG, Reichardt HM2006. Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J Immunol 176:1695–1702 [DOI] [PubMed] [Google Scholar]
- 12.Cifone MG, Migliorati G, Parroni R, Marchetti C, Millimaggi D, Santoni A, Riccardi C1999. Dexamethasone-induced thymocyte apoptosis: apoptotic signal involves the sequential activation of phosphoinositide-specific phospholipase C, acidic sphingomyelinase, and caspases. Blood 93:2282–2296 [PubMed] [Google Scholar]
- 13.Sionov RV, Cohen O, Kfir S, Zilberman Y, Yefenof E2006. Role of mitochondrial glucocorticoid receptor in glucocorticoid-induced apoptosis. J Exp Med 203:189–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismaili N, Garabedian MJ2004. Modulation of glucocorticoid receptor function via phosphorylation. Ann NY Acad Sci 1024:86–101 [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, Rogatsky I, Logan SK, Garabedian MJ2008. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol 22:1754–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herr I, Gassler N, Friess H, Büchler MW2007. Regulation of differential pro- and anti-apoptotic signaling by glucocorticoids. Apoptosis 12:271–291 [DOI] [PubMed] [Google Scholar]
- 17.Kassel O, Sancono A, Krätzschmar J, Kreft B, Stassen M, Cato AC2001. Glucocorticoids inhibit MAP kinase via increased expression and decreased degradation of MKP-1. EMBO J 20:7108–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tessier M, Woodgett JR2006. Serum and glucocorticoid-regulated protein kinases: variations on a theme. J Cell Biochem 98:1391–1407 [DOI] [PubMed] [Google Scholar]
- 19.Abraham SM, Clark AR2006. Dual-specificity phosphatase 1: a critical regulator of innate immune responses. Biochem Soc Trans 34:1018–1023 [DOI] [PubMed] [Google Scholar]
- 20.Bruna A, Nicolàs M, Muñoz A, Kyriakis JM, Caelles C2003. Glucocorticoid receptor-JNK interaction mediates inhibition of the JNK pathway by glucocorticoids. EMBO J 22:6035–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies L, Karthikeyan N, Lynch JT, Sial EA, Gkourtsa A, Demonacos C, Krstic-Demonacos M2008. Crosstalk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol 22:1331–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA2008. Glycogen synthase kinase 3β-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol 28:7309–7322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kino T, Ichijo T, Amin ND, Kesavapany S, Wang Y, Kim N, Rao S, Player A, Zheng YL, Garabedian MJ, Kawasaki E, Pant HC, Chrousos GP2007. Cyclin-dependent kinase 5 differentially regulates the transcriptional activity of the glucocorticoid receptor through phosphorylation: clinical implications for the nervous system response to glucocorticoids and stress. Mol Endocrinol 21:1552–1568 [DOI] [PubMed] [Google Scholar]
- 24.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ1997. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol 17:3947–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogatsky I, Waase CL, Garabedian MJ1998. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem 273:14315–14321 [DOI] [PubMed] [Google Scholar]
- 26.Hideshima T, Nakamura N, Chauhan D, Anderson KC2001. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 20:5991–6000 [DOI] [PubMed] [Google Scholar]
- 27.Miller AL, Garza AS, Johnson BH, Thompson EB2007. Pathway interactions between MAPKs, mTOR, PKA, and the glucocorticoid receptor in lymphoid cells. Cancer Cell Int 7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuutinen U, Postila V, Mättö M, Eeva J, Ropponen A, Eray M, Riikonen P, Pelkonen J2006. Inhibition of PI3-kinase-Akt pathway enhances dexamethasone-induced apoptosis in a human follicular lymphoma cell line. Exp Cell Res 312:322–330 [DOI] [PubMed] [Google Scholar]
- 29.Sade H, Sarin A2003. IL-7 inhibits dexamethasone-induced apoptosis via Akt/PKB in mature, peripheral T cells. Eur J Immunol 33:913–919 [DOI] [PubMed] [Google Scholar]
- 30.Tsitoura DC, Rothman PB2004. Enhancement of MEK/ERK signaling promotes glucocorticoid resistance in CD4+ T cells. J Clin Invest 113:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Pieters R, Golub TR, Armstrong SA2006. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell 10:331–342 [DOI] [PubMed] [Google Scholar]
- 32.Kfir S, Sionov RV, Zafrir E, Zilberman Y, Yefenof E2007. Staurosporine sensitizes T lymphoma cells to glucocorticoid-induced apoptosis: Role of Nur77 and Bcl-2. Cell Cycle 6:3086–3096 [DOI] [PubMed] [Google Scholar]
- 33.Trudel S, Li ZH, Wei E, Wiesmann M, Chang H, Chen C, Reece D, Heise C, Stewart AK2005. CHIR-258, a novel, multitargeted tyrosine kinase inhibitor for the potential treatment of t(4;14) multiple myeloma. Blood 105:2941–2948 [DOI] [PubMed] [Google Scholar]
- 34.Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, Thompson EB2005. p38 Mitogen-activated protein kinase (MAPK) is a key mediator in glucocorticoid-induced apoptosis of lymphoid cells: correlation between p38 MAPK activation and site-specific phosphorylation of the human glucocorticoid receptor at serine 211. Mol Endocrinol 19:1569–1583 [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Quearry B, Harada H2006. p38-MAP kinase activation followed by Bim induction is essential for glucocorticoid-induced apoptosis in lymphoblastic leukemia cells. FEBS Lett 580:3539–3544 [DOI] [PubMed] [Google Scholar]
- 36.Tanaka T, Okabe T, Gondo S, Fukuda M, Yamamoto M, Umemura T, Tani K, Nomura M, Goto K, Yanase T, Nawata H2006. Modification of glucocorticoid sensitivity by MAP kinase signaling pathways in glucocorticoid-induced T-cell apoptosis. Exp Hematol 34:1542–1552 [DOI] [PubMed] [Google Scholar]
- 37.Yoshino T, Kishi H, Nagata T, Tsukada K, Saito S, Muraguchi A2001. Differential involvement of p38 MAP kinase pathway and Bax translocation in the mitochondria-mediated cell death in TCR- and dexamethasone-stimulated thymocytes. Eur J Immunol 31:2702–2708 [DOI] [PubMed] [Google Scholar]
- 38.Chauhan D, Pandey P, Ogata A, Teoh G, Treon S, Urashima M, Kharbanda S, Anderson KC1997. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene 15:837–843 [DOI] [PubMed] [Google Scholar]
- 39.Druilhe A, Létuvé S, Pretolani M2003. Glucocorticoid-induced apoptosis in human eosinophils: mechanisms of action. Apoptosis 8:481–495 [DOI] [PubMed] [Google Scholar]
- 40.Granés F, Roig MB, Brady HJ, Gil-Gómez G2004. Cdk2 activation acts upstream of the mitochondrion during glucocorticoid induced thymocyte apoptosis. Eur J Immunol 34:2781–2790 [DOI] [PubMed] [Google Scholar]
- 41.Gil-Gómez G, Berns A, Brady HJ1998. A link between cell cycle and cell death: Bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J 17:7209–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berthet C, Rodriguez-Galan MC, Hodge DL, Gooya J, Pascal V, Young HA, Keller J, Bosselut R, Kaldis P2007. Hematopoiesis and thymic apoptosis are not affected by the loss of Cdk2. Mol Cell Biol 27:5079–5089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchetti MC, Di Marco B, Cifone G, Migliorati G, Riccardi C2003. Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood 101:585–593 [DOI] [PubMed] [Google Scholar]
- 44.Chauhan D, Hideshima T, Pandey P, Treon S, Teoh G, Raje N, Rosen S, Krett N, Husson H, Avraham S, Kharbanda S, Anderson KC1999. RAFTK/PYK2-dependent and -independent apoptosis in multiple myeloma cells. Oncogene 18:6733–6740 [DOI] [PubMed] [Google Scholar]
- 45.Werlen G, Hausmann B, Naeher D, Palmer E2003. Signaling life and death in the thymus: timing is everything. Science 299:1859–1863 [DOI] [PubMed] [Google Scholar]
- 46.Golsteyn RM2005. Cdk1 and Cdk2 complexes (cyclin dependent kinases) in apoptosis: a role beyond the cell cycle. Cancer Lett 217:129–138 [DOI] [PubMed] [Google Scholar]
- 47.Pap M, Cooper GM1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem 273:19929–19932 [DOI] [PubMed] [Google Scholar]
- 48.Beurel E, Jope RS2006. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol 79:173–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abrams MT, Robertson NM, Yoon K, Wickstrom E2004. Inhibition of glucocorticoid-induced apoptosis by targeting the major splice variants of BIM mRNA with small interfering RNA and short hairpin RNA. J Biol Chem 279:55809–55817 [DOI] [PubMed] [Google Scholar]
- 50.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, Adams JM, Strasser A1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735–1738 [DOI] [PubMed] [Google Scholar]
- 51.Kockeritz L, Doble B, Patel S, Woodgett JR2006. Glycogen synthase kinase-3-an overview of an over-achieving protein kinase. Curr Drug Targets 7:1377–1388 [DOI] [PubMed] [Google Scholar]
- 52.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, Eshraghi M, Manda KD, Wiechec E, Los M2007. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat 10:13–29 [DOI] [PubMed] [Google Scholar]
- 53.Deftos ML, He YW, Ojala EW, Bevan MJ1998. Correlating notch signaling with thymocyte maturation. Immunity 9:777–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma VM, Draheim KM, Kelliher MA2007. The Notch1/c-Myc pathway in T cell leukemia. Cell Cycle 6:927–930 [DOI] [PubMed] [Google Scholar]
- 55.Sade H, Krishna S, Sarin A2004. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem 279:2937–2944 [DOI] [PubMed] [Google Scholar]
- 56.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell 21:749–760 [DOI] [PubMed] [Google Scholar]
- 57.Almawi WY, Melemedjian OK, Jaoude MM2004. On the link between Bcl-2 family proteins and glucocorticoid-induced apoptosis. J Leukoc Biol 76:7–14 [DOI] [PubMed] [Google Scholar]
- 58.Zhang L, Insel PA2004. The pro-apoptotic protein Bim is a convergence point for cAMP/protein kinase A- and glucocorticoid-promoted apoptosis of lymphoid cells. J Biol Chem 279:20858–20865 [DOI] [PubMed] [Google Scholar]
- 59.Cai B, Chang SH, Becker EB, Bonni A, Xia Z2006. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem 281:25215–25222 [DOI] [PubMed] [Google Scholar]
- 60.De Chiara G, Marcocci ME, Torcia M, Lucibello M, Rosini P, Bonini P, Higashimoto Y, Damonte G, Armirotti A, Amodei S, Palamara AT, Russo T, Garaci E, Cozzolino F2006. Bcl-2 phosphorylation by p38 MAPK: identification of target sites and biological consequences. J Biol Chem 281:21353–21361 [DOI] [PubMed] [Google Scholar]
- 61.Greenstein S, Ghias K, Krett NL, Rosen ST2002. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin Cancer Res 8:1681–1694 [PubMed] [Google Scholar]
- 62.Hartigan JA, Johnson GV1999. Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3β-dependent pathway. J Biol Chem 274:21395–21401 [DOI] [PubMed] [Google Scholar]
- 63.Mora A, Sabio G, Risco AM, Cuenda A, Alonso JC, Soler G, Centeno F2002. Lithium blocks the PKB and GSK3 dephosphorylation induced by ceramide through protein phosphatase-2A. Cell Signal 14:557–562 [DOI] [PubMed] [Google Scholar]
- 64.Linseman DA, Butts BD, Precht TA, Phelps RA, Le SS, Laessig TA, Bouchard RJ, Florez-McClure ML, Heidenreich KA2004. Glycogen synthase kinase-3β phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J Neurosci 24:9993–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pastorino JG, Hoek JB, Shulga N2005. Activation of glycogen synthase kinase 3β disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res 65:10545–10554 [DOI] [PubMed] [Google Scholar]
- 66.Sade H, Khandre NS, Mathew MK, Sarin A2004. The mitochondrial phase of the glucocorticoid-induced apoptotic response in thymocytes comprises sequential activation of adenine nucleotide transporter (ANT)-independent and ANT-dependent events. Eur J Immunol 34:119–125 [DOI] [PubMed] [Google Scholar]
- 67.Pastorino JG, Shulga N, Hoek JB2002. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem 277:7610–7618 [DOI] [PubMed] [Google Scholar]
- 68.Tome ME, Lutz NW, Briehl MM2004. Overexpression of catalase or Bcl-2 alters glucose and energy metabolism concomitant with dexamethasone resistance. Biochim Biophys Acta 1693:57–72 [DOI] [PubMed] [Google Scholar]
- 69.Pear WS, Aster JC2004. T cell acute lymphoblastic leukemia/lymphoma: a human cancer commonly associated with aberrant NOTCH1 signaling. Curr Opin Hematol 11:426–433 [DOI] [PubMed] [Google Scholar]
- 70.McKenzie G, Ward G, Stallwood Y, Briend E, Papadia S, Lennard A, Turner M, Champion B, Hardingham GE2006. Cellular Notch responsiveness is defined by phosphoinositide 3-kinase-dependent signals. BMC Cell Biol 7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Espinosa L, Inglés-Esteve J, Aguilera C, Bigas A2003. Phosphorylation by glycogen synthase kinase-3 β down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem 278:32227–32235 [DOI] [PubMed] [Google Scholar]
- 72.Breit S, Stanulla M, Flohr T, Schrappe M, Ludwig WD, Tolle G, Happich M, Muckenthaler MU, Kulozik AE2006. Activating NOTCH1 mutations predict favorable early treatment response and long-term outcome in childhood precursor T-cell lymphoblastic leukemia. Blood 108:1151–1157 [DOI] [PubMed] [Google Scholar]
- 73.Strömberg T, Dimberg A, Hammarberg A, Carlson K, Osterborg A, Nilsson K, Jernberg-Wiklund H2004. Rapamycin sensitizes multiple myeloma cells to apoptosis induced by dexamethasone. Blood 103:3138–3147 [DOI] [PubMed] [Google Scholar]
- 74.Yan H, Frost P, Shi Y, Hoang B, Sharma S, Fisher M, Gera J, Lichtenstein A2006. Mechanism by which mammalian target of rapamycin inhibitors sensitize multiple myeloma cells to dexamethasone-induced apoptosis. Cancer Res 66:2305–2313 [DOI] [PubMed] [Google Scholar]
- 75.Ding Q, Xia W, Liu JC, Yang JY, Lee DF, Xia J, Bartholomeusz G, Li Y, Pan Y, Li Z, Bargou RC, Qin J, Lai CC, Tsai FJ, Tsai CH, Hung MC2005. Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin. Mol Cell 19:159–170 [DOI] [PubMed] [Google Scholar]
- 76.Deng X, Kornblau SM, Ruvolo PP, May Jr WS2001. Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. J Natl Cancer Inst Monogr 28:30–37 [DOI] [PubMed] [Google Scholar]
- 77.Ewings KE, Wiggins CM, Cook SJ2007. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle 6:2236–2240 [DOI] [PubMed] [Google Scholar]
- 78.Ley R, Ewings KE, Hadfield K, Cook SJ2005. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ 12:1008–1014 [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Lin A2005. Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15:36–42 [DOI] [PubMed] [Google Scholar]
- 80.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson Jr EM2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38:899–914 [DOI] [PubMed] [Google Scholar]
- 81.Leung KT, Li KK, Sun SS, Chan PK, Ooi VE, Chiu LC2008. Activation of the JNK pathway promotes phosphorylation and degradation of BimEL-a novel mechanism of chemoresistance in T-cell acute lymphoblastic leukemia. Carcinogenesis 29:544–551 [DOI] [PubMed] [Google Scholar]
- 82.Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A2004. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell 13:329–340 [DOI] [PubMed] [Google Scholar]
- 83.Shaulian E, Karin M2002. AP-1 as a regulator of cell life and death. Nat Cell Biol 4:E131–136 [DOI] [PubMed]
- 84.Hess P, Pihan G, Sawyers CL, Flavell RA, Davis RJ2002. Survival signaling mediated by c-Jun NH2-terminal kinase in transformed B lymphoblasts. Nat Genet 32:201–205 [DOI] [PubMed] [Google Scholar]
- 85.Zilberman Y, Zafrir E, Ovadia H, Yefenof E, Guy R, Sionov RV2004. The glucocorticoid receptor mediates the thymic epithelial cell-induced apoptosis of CD4+8+ thymic lymphoma cells. Cell Immunol 227:12–23 [DOI] [PubMed] [Google Scholar]
- 86.Jeon SH, Kang MG, Kim YH, Jin YH, Lee C, Chung HY, Kwon H, Park SD, Seong RH1997. A new mouse gene, SRG3, related to the SWI3 of Saccharomyces cerevisiae, is required for apoptosis induced by glucocorticoids in a thymoma cell line. J Exp Med 185:1827–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB2002. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol 3:932–939 [DOI] [PubMed] [Google Scholar]