Abstract
Estrogen receptor-related receptor α (ERRα) is an orphan member of the nuclear receptor superfamily of transcription factors. ERRα is highly expressed in the prostate, especially in prostate stromal cells. However, little is known about the regulation and function of ERRα, which may contribute to the progression of prostatic diseases. We previously found that prostaglandin E2 (PGE2) up-regulated the expression of aromatase in prostate stromal cells. Here we show that PGE2 also up-regulates the expression of ERRα, which, as a transcription factor, further mediates the regulatory effects of PGE2 on the expression of aromatase. ERRα expression was up-regulated by PGE2 in prostate stromal cell line WPMY-1, which was mediated mainly through the protein kinase A signaling pathway by PGE2 receptor EP2. Suppression of ERRα activity by chlordane (an antagonist of ERRα) or small interfering RNA knockdown of ERRα blocked the increase of expression and promoter activity of aromatase induced by PGE2. Overexpression of ERRα significantly increased aromatase expression and promoter activity, which were further augmented by PGE2. Chromatin immunoprecipitation assay demonstrated that ERRα directly bound to the aromatase promoter in vivo, and PGE2 enhanced the recruitment of ERRα and promoted transcriptional regulatory effects on aromatase expression in WPMY-1. 17β-Estradiol concentration in WPMY-1 medium was up-regulated by ERRα expression, and that was further increased by PGE2. Our results provided evidence that ERRα contributed to local estrogen production by up-regulating aromatase expression in response to PGE2 and provided further insights into the potential role of ERRα in estrogen-related prostatic diseases.
This study demonstrates that estrogen receptor-related receptor α (ERRα) regulates estradiol production in response to prostaglandin E2 via up-regulation of aromatase expression in prostate stromal cells.
Estrogen receptor-related receptor α (ERRα) is a member of the ERR family, which belongs to the third superfamily of nuclear receptors (1). ERRα was the first orphan nuclear receptor identified with a DNA binding domain similar to that of estrogen receptor α (ERα). Interconnections between ERRα and the estrogen signaling pathways have been well documented (2). Because ERRα is an orphan receptor, one remaining question is how its transcriptional activity is regulated. Naturally occurring estrogens, such as 17β-estradiol (E2), estrone, and estriol, do not serve as its ligands. ERRα interacts with steroid receptor coactivator (SRC) proteins in the absence of a ligand (3). Although the natural specific ligands for ERRα have not been identified, toxaphene and chlordane, two organochlorine pesticides with estrogen-like activity, behave as antagonists for ERRα (4). As a nuclear receptor, ERRα can bind to the consensus palindromic estrogen response elements (EREs), as well as ERR elements (ERREs) (5, 6). ERRα interacts with human ERα through protein-protein contacts and modulates the estrogenic response (7). ERRα target genes include lactoferrin (7), osteopontin (8, 9), and pS2 (10). Recently, the common ERRα/ERα target genes have been identified by genome-wide analyses (11). ERRα is widely distributed in the developing embryo and adult tissues, especially abundant in the uterus, prostate, brain, heart, skeletal muscle, and brown adipose tissue. In the prostate, ERRα is detected in almost all prostate cell lines, including prostate epithelial and stromal cells (12). Although the exact function of ERRα has not been defined precisely, increasing evidences suggest that ERRα plays an important role in estrogen-related diseases including breast cancer and prostate cancer. ERRα is considered as a biomarker of unfavorable clinical outcome and a potential therapeutic target for breast cancer (13, 14, 15). Increased expression of ERRα is also a negative prognostic predictor in prostate cancer (16).
Aromatase is a key enzyme catalyzing the conversion of androgens to estrogens, leading to the elevated level of estrogen. Emerging evidence suggests that estrogens are implicated in the etiology of not only breast cancer, but also prostatic diseases (17, 18). Aromatase is mainly expressed in prostate stromal cells, and its overexpression may lead to an increased estrogen exposure in the prostate (19). We recently found that prostaglandin E2 (PGE2) significantly stimulated aromatase expression in prostate stromal cells through epithelial-stromal interactions (19). However, the molecular mechanism by which PGE2 stimulates aromatase expression in prostate stromal cells has not been well defined. Our hypothesis that ERRα mediates PGE2 induced-aromatase expression is mainly based on the following rationales: first, in our preliminary experiments, we found that the expression of ERRα was induced by conditioned medium of benign prostatic hyperplasic cell line (BPH-1) but not by that of prostate cancer cell lines (data not shown), which is similar to the regulation of aromatase in prostate stromal cells. Second, previous studies suggested the regulatory action of ERRα on promoters I.3/II of the human aromatase gene in breast cancer cells (20, 21, 22). In this study, we investigated the regulation of ERRα expression and its transcriptional activity by PGE2 and proposed a novel mechanism that ERRα mediated PGE2-induced aromatase expression in prostate stromal cells.
Results
PGE2 stimulates the expression of ERRα through PGE2 receptor EP2 and protein kinase A (PKA) signaling pathway in WPMY-1 prostate stromal cells
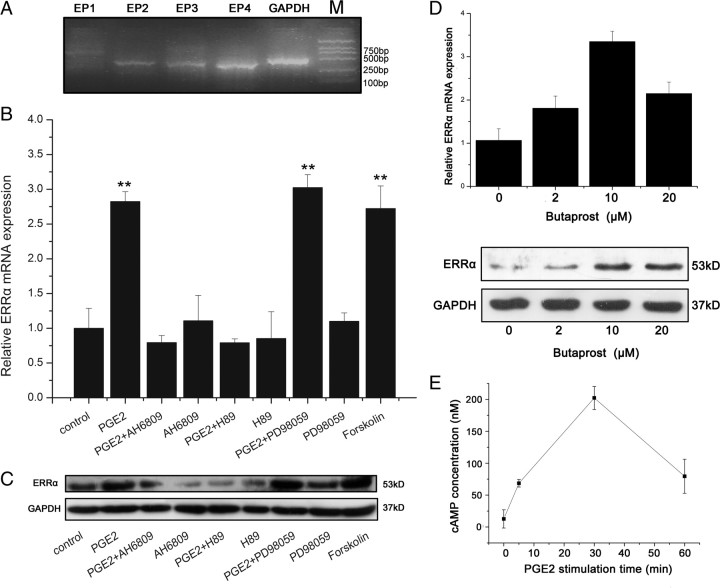
PGE2 is synthesized by cyclooxygenase-2 (COX-2) and secreted through epithelial-stromal interactions during the progression of the prostatic diseases such as BPH and prostate cancer (23, 24, 25, 26, 27). Cellular effects of PGE2 are mediated through the PGE2 receptors. As shown in Fig. 1A, WPMY-1, a human normal prostate stromal cell line, expressed three PGE2 receptors EP2, EP3, and EP4. This corresponds to the pattern of prostate primary stromal cells (19). The regulation of ERRα expression by PGE2 was investigated in prostate stromal cell line WPMY-1. As shown in Fig. 1, B and C, PGE2 significantly up-regulated ERRα expression at both mRNA and protein levels. When the cells were pretreated with AH6809, an antagonist of EP2, the up-regulation of ERRα expression by PGE2 was almost completely blocked. By contrast, butaprost, a specific agonist of EP2, increased ERRα expression in a dose-dependent manner (Fig. 1D), indicating that EP2 mediates the effect of PGE2-induced ERRα expression. Because EP2 downstream signaling is coupled to an increase in cAMP production and the PKA pathway, we further examined whether PGE2 regulated intracellular cAMP production and whether PKA signaling pathway was involved in PGE2-induced ERRα expression. As shown in Fig. 1E, PGE2 significantly increased cAMP concentration in a time-dependent manner in WPMY-1 cells. Forskolin, a cAMP activator, also induced ERRα expression (Fig. 1, B and C). Selective PKA inhibitor H89 blocked the up-regulation of ERRα expression by PGE2, whereas blockade of the MAPK pathway by MEK inhibitor PD98059 had no effect. These results suggested that PGE2 acted through EP2 and PKA/cAMP signaling pathway to increase ERRα expression.
Fig. 1.
PGE2 induces the expression of ERRα through the PGE2 receptor EP2 and the PKA signaling pathway in WPMY-1 cells. A, RT-PCR analysis of mRNA expression of PGE2 receptor subtypes (EP1–EP4) in WPMY-1 cells. B, Real-time RT-PCR analysis of the effects of PGE2 and/or the EP2 antagonist, the inhibitor of PKA, the inhibitor of MAPK pathway or forskolin on mRNA expression of ERRα in WPMY-1 cells. Cells were pretreated with or without AH6809 (10 μm) or H89 (0.5 μg/ml) or PD98059 (25 nm) for 30 min, and then treated with PGE2 (1 μm), or Forskolin (1 μm) for 12 h. Total RNA was extracted and real-time RT-PCR was performed using ERRα and housekeeping gene primers. Values represent mean ± sd of three independent experiments. **, P < 0.01 compared with untreated controls. C, Western blot analysis of the effects of PGE2 and/or the EP2 antagonist, the inhibitor of the PKA, the inhibitor of MAPK pathway or forskolin on protein expression of ERRα in WPMY-1 cells. Cells were preincubated for 30 min in the absence or presence of DMSO (vehicle), AH6809 (10 μm), H89 (0.5 μg/ml), or PD98059 (25 nm) followed by addition of PGE2 (1 μm), or forskolin (1 μm) for 24 h. Whole cell lysates were subjected to Western blot analysis using antibodies against ERRα and GAPDH. The experiments were performed in duplicate. Similar results were obtained from separate experiments. D, Effect of butaprost on ERRα expression. WPMY-1 cells were treated with 2, 10 or 20 μm butaprost. After 12 h or 24 h, total RNA or protein was extracted and analyzed by real-time RT-PCR or Western blot, respectively. D, Upper panel, Real-time PCR data. Values represent means ± sd (n = 4) of three independent experiments. *, P < 0.05 compared with untreated controls. D, Lower panel, Western blot analysis was performed using antibodies to ERRα or GAPDH. The experiments were performed in duplicate. Similar results were obtained from separate experiments. E, Effect of PGE2 on cAMP production. WPMY-1 cells were treated with 1 μm PGE2 in DMEM supplemented with 1% FBS and cell lysates were collected at the indicated time points. Concentrations of cAMP were quantified by using a kit (cAMP-Glo Assay, V1501; Promega). Values represent mean ± sd (n = 4).
ERRα antagonist and ERRα small interfering RNA (siRNA) block the expression and promoter activity of aromatase induced by PGE2 in WPMY-1 cells
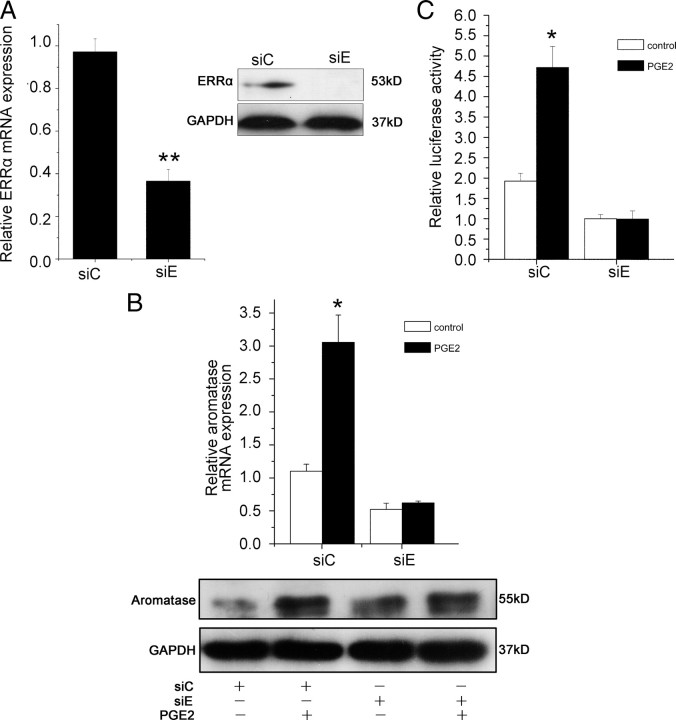
When WPMY-1 cells were exposed to chlordane, a selective ERRα antagonist, at a concentration of 10 μm for 30 min, PGE2-induced aromatase mRNA expression was inhibited by at least 60%, whereas chlordane alone had no significant effect (Fig. 2A, upper panel). We also found a similar effect of aromatase expression in protein level (Fig. 2A, lower panel). A similar finding was observed in primary prostate stromal cells (data not shown).
Fig. 2.
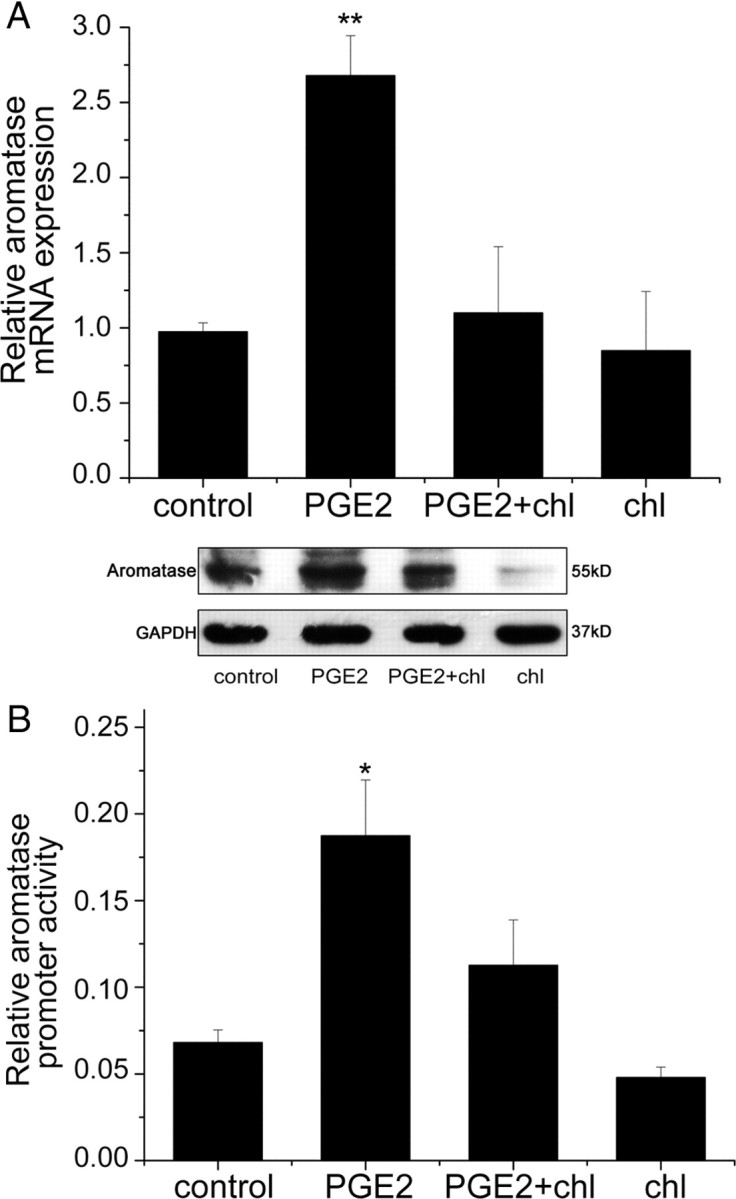
Inhibitory effect of the ERRα antagonist chlordane (chl) on aromatase expression and its promoter activity in PGE2-treated prostate stromal cells. A, WPMY-1 cells were pretreated for 30 min with or without 10 μm chlordane and treated with PGE2. After 24 h or 48 h, total RNA or protein was extracted and real-time RT-PCR or Western blot was performed, respectively. A, Upper panel, Real-time RT-PCR. Values represent mean ± sd (n = 4). **, P < 0.01 compared with untreated controls. A, Lower panel, Western blot analysis was performed using antibodies to aromatase or GAPDH. The experiments were performed in duplicate. Similar results were obtained from separate experiments. B, Cells were transfected with aromatase promoter luciferase reporter plasmid in 24-well plates for 6 h and pretreated with vehicle or chlordane (10 μm) for 30 min and then treated with PGE2 (1 μm) for 24 h. Luciferase activity was measured using dual-luciferase assay and normalized to the control renilla luciferase activity. Values represent mean ± sd (n = 4). *, P < 0.05 compared with untreated controls.
The aromatase gene promoters are complex and tissue specific. The transcriptional regulation of aromatase expression and its promoter usage have been documented and promoter I.3/II was found to be activated in breast cancer (20, 21, 22). However, the regulation of aromatase in the prostate remains unclear. Because it is widely accepted that promoter II (PII) is gonad specific and is the proximal to the promoter I.3 (PI.3) within 1 kb upstream of the transcriptional start site, we cloned the aromatase promoters PI.3 and PII into a luciferase reporter plasmid as described in Materials and Methods. The luciferase activity of this reporter plasmid was significantly increased when WPMY-1 cells were stimulated with PGE2. However, when WPMY-1 cells were treated with PGE2 plus chlordane, the activation of the aromatase promoter was inhibited (Fig. 2B). Chlordane alone had no effect on the aromatase promoter activity. These results suggested that ERRα was required for PGE2-induced aromatase expression and promoter activity.
As an organochlorine pesticide, chlordane at high concentrations may be toxic to cells. To further confirm the role of ERRα in the pathway of PGE2 induced aromatase expression in prostate stromal cells, we transfected ERRα siRNA into WPMY-1 cells. As shown in Fig. 3A, siRNA suppressed ERRα expression at both mRNA and protein levels. Next, we evaluated the effect of ERRα siRNA on PGE2-induced aromatase expression and promoter activity in WPMY-1 cells. As shown in Fig. 3B, knockdown of ERRα gene expression by siRNA led to a significant decrease in aromatase expression at both mRNA and protein levels [ERRα siRNA (siE) control vs. control siRNA (siC) control]. Furthermore, PGE2 stimulation of aromatase expression was blocked by ERRα siRNA. We also found a similar effect of ERRα siRNA on PGE2-stimulated promoter activity of aromatase in WPMY-1 cells (Fig. 3C). Therefore, we postulated that ERRα was required for PGE2-induced aromatase expression in prostate stromal cells.
Fig. 3.
Suppression of PGE2-induced the expression and promoter activity of aromatase by siRNA against ERRα. A, Cells in six-well plate were transfected with 25 pmol/well of siE or siC and grown for 24 and 48 h. ERRα mRNA expression was analyzed by real-time RT-PCR and protein levels were determined by Western blot. A, Upper panel, Real-time RT-PCR. Values represent mean ± sd (n = 3). **, P < 0.01 compared with untreated controls. A, Lower panel, Western blot analysis was performed using antibodies to ERRα or GAPDH. The experiments were performed in duplicate. Similar results were obtained from separate experiments. **, P < 0.01 compared with untreated controls. B, Cells in six-well plate were transfected with 25 pmol/well of siE or siC and then treated PGE2 (1 μm) or vehicle. After 24 h, total RNA was extracted and real-time RT-PCR was performed (B, upper panel). Values represent mean ± sd (n = 4). *, P < 0.05 compared with untreated controls. After 48 h, cells were lysated and Western blot was performed using antibodies to aromatase and GAPDH in duplicate (B, lower panel). Similar results were obtained from separate experiments. C, siE or siC were transfected into WPMY-1 cells together with the aromatase promoter luciferase reporter plasmid and then stimulated PGE2 (1 μm) or vehicle. Twenty-four hours later, luciferase activity was measured and normalized to renilla control activity. Results are the mean ± sd of three values. *, P < 0.05 compared with untreated controls.
Overexpression of ERRα enhances the effect of PGE2 on aromatase expression and its promoter activity in WPMY-1 cells
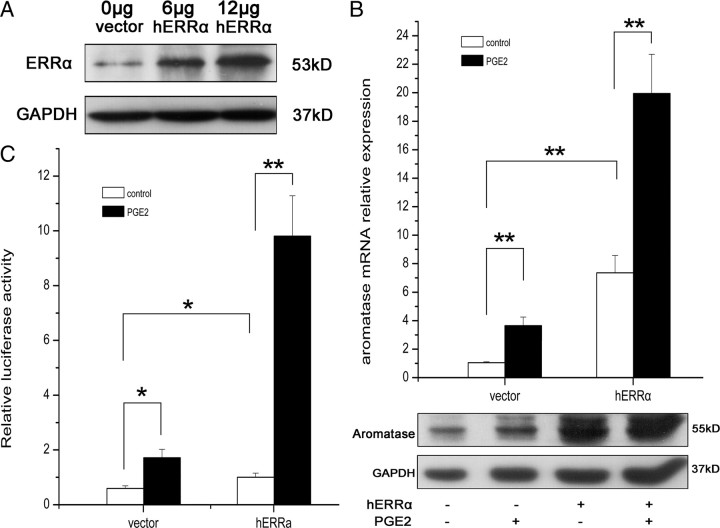
To confirm the hypothesis that ERRα was involved in the induction of aromatase by PGE2, we transfected an ERRα expression plasmid into WPMY-1 cells, which were then treated with 1 μm PGE2 or vehicle for 24 h, and examined gene expressions of ERRα and aromatase. ERRα protein was increased in a plasmid dose-dependent manner compared with the control (Fig. 4A). As expected, up-regulation of ERRα dramatically stimulated aromatase expression at both mRNA and protein levels compared with the control, and PGE2 further enhanced the effect of ERRα on aromatase expression (Fig. 4B). We also performed the luciferase assay to detect the effect of ERRα overexpression on aromatase promoter activity stimulated by PGE2. Similarly, overexpression of ERRα also dramatically increased aromatase promoter activity, which was further enhanced by PGE2 in WPMY-1 cells (Fig. 4C).
Fig. 4.
Overexpression of ERRα increases the aromatase expression and its promoter activity induced by PGE2 in WPMY-1 cells. A, Western blot analysis of ERRα protein expression after transfection with ERRα expression plasmid into WPMY-1 cells for 24 h. B, Cells in six-well plates were transfected with ERRα expression plasmid and then treated with PGE2 (1 μm) or vehicle. After 24 h, total RNA was extracted and aromatase mRNA levels were determined by real-time RT-PCR (B, upper panel). Values represent mean ± sd (n = 4). **, P < 0.01 compared with untreated controls. After 48 h, cells were lysed and Western blot was performed using antibodies to aromatase and GAPDH in duplicate (B, lower panel). Similar results were obtained from separate experiments. C, ERRα expression plasmid or vector were transfected into WPMY-1 cells together with the aromatase promoter luciferase plasmid and then stimulated with PGE2 (1 μm) or vehicle for 24 h. Luciferase activity was measured and normalized to renilla control activity. Results are the mean ± sd of three values. *, P < 0.05; **, P < 0.01 compared with untreated controls.
ERRα binds to a specific region of aromatase promoter in vivo, and its recruitment is enhanced by PGE2
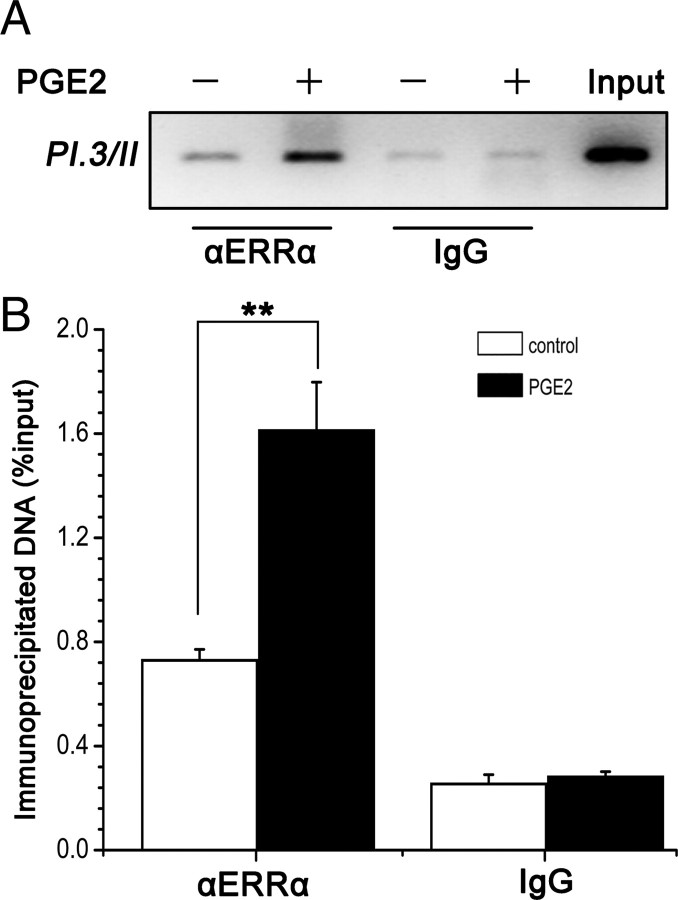
The luciferase assay results showed that ERRα transcriptionally activated aromatase promoter, indicating that PI.3 and PII may play an important role in regulation of PGE2-induced aromatase expression in prostate stromal cells. Meanwhile, it was reported that ERRα can interact with silencer 1 (S1) in exon I.3 (20, 21, 22), which was within the cloned fragment of the aromatase promoter in the reporter plasmid. To confirm the direct interaction between ERRα and the aromatase promoter in vivo, we performed chromatin immunoprecipitation (ChIP) experiment targeting a specific 118-bp region (−197/−80) of the aromatase promoter I.3/II in WPMY-1 cells. As shown in Fig. 5A, in the absence of PGE2, a low level of ERRα binding to aromatase promoter can be detected using an anti-ERRα antibody and the binding was much enhanced compared with that using nonimmune mouse IgG. Treatment with PGE2 enhanced the recruitment of ERRα to the aromatase promoter I.3/II site. Similar results were shown by quantitative real-time PCR analysis (Fig. 5B). These findings indicated that ERRα specifically bound to the aromatase promoter and transcriptionally activated its expression and that PGE2 enhanced the recruitment of ERRα to the aromatase promoter.
Fig. 5.
PGE2 enhances ERRα recruitment to aromatase gene promoter in WPMY-1 cells. WPMY-1 cells were treated with PGE2 (1 μm) for 24 h. The recruitment of ERRα to aromatase promoter region was examined by ChIP analysis with anti-ERRα specific antibodies. IgG was used as control. PI.3/PII regions were amplified by conventional PCR (A) followed by electrophoresis or real-time PCR (B). Results of real-time PCR were expressed as percentage of input. Values represent mean ± sd (n = 3). *, P < 0.05 compared with untreated controls.
ERRα contributes to local E2 production by WPMY-1 cells, and the induction is increased by PGE2
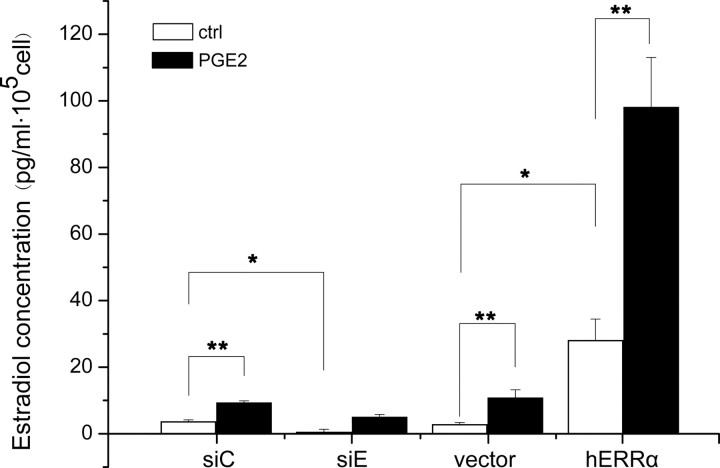
Because aromatase is an enzyme that converts androgen to estrogen, measuring the production of estrogen in culture medium after addition of the substrate androgen to cultured cells has been used as an alternative nonradioactive method for aromatase activity (28). To investigate whether the production of E2 by WPMY-1 cells was regulated by ERRα, we cultured WPMY-1 cells with testosterone and measured the E2 levels in the culture medium with an eletrochemiluminescence immunoassay (ECLIA). As shown in Fig. 6, knockdown of ERRα by siRNA led to a significant decrease in E2 production (siE control vs. siC control), whereas up-regulation of ERRα expression dramatically increased E2 production (vector control vs. ERRα control). PGE2 further enhanced the effect of ERRα on E2 levels (control vs. PGE2). These findings provided direct evidence that ERRα contributed to the local estrogen production by up-regulating the expression of aromatase in response to PGE2.
Fig. 6.
ERRα modulates local E2 production in the culture media of WPMY-1 cells. WPMY-1 cells were transfected with ERRα expression plasmid or ERRα siRNA for 6 h, and then stimulated with or without PGE2 (1 μm) for 48 h. At the end of the incubation period, testosterone (10 nm) was added, and cells were incubated for 24 h, and cultured media were collected and analyzed for E2 concentration using Estradiol II kit. Values represent the mean ± sd (n = 3). *, P < 0.05; **, P < 0.01 compared with the untreated controls.
Discussion
Up to now, natural ligands for ERRα remain unknown. In vitro experiments have demonstrated that interactions between ERRα and coactivators such as peroxisome proliferator-activated receptor γ coactivator-1 α (29, 30, 31, 32) or members of the p160 family (3) are sufficient for transcriptional activation of ERRα and additional external compounds are not required, which suggested that ERRα may function as a constitutive activator (33). ERRα activity can be inhibited upon binding to the organochlorine pesticides such as toxaphene and chlordane (4). These observations indicated that the modulators of ERRα transcriptional activity may be pivotal for understanding the regulation of its function. It was previously shown that estrogen stimulated ERRα expression through the multiple hormone response element half-sites (34). Moreover, orphan nuclear receptors can be regulated by vitamin A and its derivatives, and by prostaglandins (35). Consistently, our results are the first to identify that PGE2 up-regulates the orphan nuclear receptor ERRα mRNA and protein expression in prostate stromal cells (Fig. 1, B and C).
PGE2 elicits its functions via PGE2 receptors (EPs) (36). In WPMY-1 cells, all subtypes of G protein-coupled EP receptors except EP1 have been detected (Fig. 1A). Our data showed that the EP2 antagonist AH6809 totally abrogated the induction of ERRα expression by PGE2 (Fig. 1, B and C), whereas EP2 agonist EP2 butaprost mimicked the effect of PGE2 on ERRα expression (Fig. 1D), indicating that EP2 mediated the effect of PGE2 in prostate stromal cells. Activation of EP2 usually stimulates adenylate cyclase, resulting in an increased intracellular cAMP formation (36, 37, 38). In the present study, PGE2 induced the cAMP production in WPMY-1 cells (Fig. 1E) and the cAMP mimetic forskolin alone up-regulated the ERRα expression (Fig. 1, B and C). In addition, the PKA inhibitor H89 abrogated the stimulatory effect of PGE2 on ERRα expression, whereas the MAPK inhibitor PD98059 had no significant effect (Fig. 1, B and C). It is worth noting that not only the expression of ERRα, but also the transcription activity of the ERRα gene promoter, can be enhanced by cAMP through increasing its interaction with PKA and its coactivators at the surfactant protein A (SP-A) promoter (39).
Aromatase is the key enzyme catalyzing the conversion of androgens to estrogens. In the prostate, aromatase is mainly expressed in stromal cells and its overexpression may lead to an increased estrogen exposure in the prostate (40, 41, 42). Microarray expression profiling and quantitative RT-PCR studies showed a significant positive correlation between aromatase and ERRα mRNA expression in breast cancer, suggesting that ERRα is a key regulator of aromatase expression in these cells (43). Moreover, overexpression of ERRα stimulates aromatase promoter I.3 in breast cancer cells (21). In the prostate, we found that the conditioned medium from the benign prostatic hyperplasia cell line BPH-1 induced ERRα expression in prostate stromal cells (data not shown). This parallels aromatase expression reported previously (19) and suggests a close correlation between ERRα and aromatase in the prostate. In the present study, we showed that overexpression of ERRα induced aromatase expression and its promoter activity in prostate stromal cells (Fig. 4); and ChIP assay also confirmed that ERRα can bind to the aromatase promoter I.3/II (Fig. 5). These results identified aromatase as a target gene of ERRα in prostate stromal cells. Expression of aromatase is driven by tissue-specific promoters (22). In the stroma of nonmalignant breast and prostate tissue specimens, aromatase expression was shown to be driven exclusively by promoter PII, while in the malignant epithelial cells, other promoters (I.4 and I.3) were used (44). In the present study, we identified the region between −197 and −80 of PI.3/II of aromatase promoter as the binding site of ERRα in the prostate stromal cells, which is consistent with that in breast cancer cells (20, 21, 22).
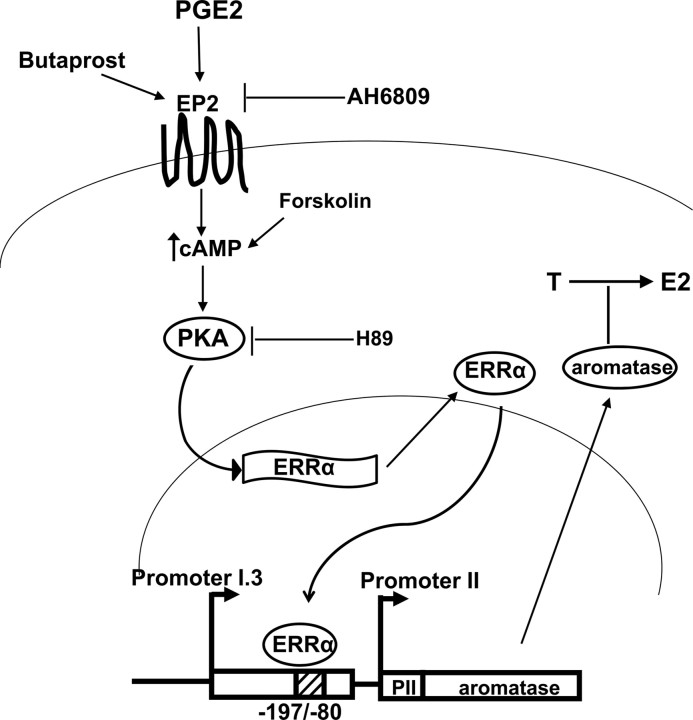
In the breast, PGE2 stimulates the aromatase expression in adipose cells via EP1 and EP2, both of which mediate important but different signaling pathways (45). Our group recently found that PGE2 significantly stimulated aromatase expression in prostate stromal cells through epithelial-stromal interactions (19), but the molecular mechanism has not been elucidated. Studies from several groups have identified transcription factors regulated by the PKA and PKC pathways, and involved in activation of aromatase promoter I.3/II, the key promoter driving aromatase expression (46, 47, 48). More importantly, ERRα has been demonstrated by our data to be a transcription factor induced by PGE2 through the PKA pathway (Fig. 1). We therefore investigated whether ERRα played a critical role in PGE2-induced expression of the aromatase gene. Knockdown of ERRα expression completely inhibited PGE2-induced aromatase expression and promoter activity in prostate stromal cells (Fig. 3). By contrast, overexpression of ERRα significantly enhanced the effect (Fig. 4). Based on the results of the present study that PGE2 stimulation of ERRα expression is responsible for the stimulation of aromatase expression via promoter I.3/II in prostate stromal cells, we propose a novel molecular mechanism by which ERRα mediates the effect of PGE2 on local estrogen production in human prostate stromal cells. The relationship between PGE2, ERRα, PKA, aromatase and estrogen is illustrated in Fig. 7. Similar to our findings, Zhou et al. (49) found that in breast adipose stromal cells PGE2 may increase aromatase expression by inducing the expression of another orphan nuclear receptor, liver receptor homologue-1. Clyne et al. (50) showed that liver receptor homologue-1, which is regulated by PKA- and PKC-dependent pathways, binds to and stimulates the steroidogenic factor-1 site (−136/−124) in the aromatase promoter I.3/II.
Fig. 7.
A schematic representation illustrating the relationship between PGE2, PKA, ERRα, aromatase and estrogen. PGE2 binds to PGE2 receptor EP2 located in the cell membrane of prostate stromal cells, leading to an increased production of cAMP. Then PKA is activated after binding to cAMP and results in up-regulation of ERRα expression. Both the EP2 agonist butaprost and cAMP activator forskolin mimics PGE2-induced ERRα expression in prostate stromal cells, which is blocked by the EP2 antagonist AH6809 and PKA inhibitor H89. Up-regulated ERRα interacts with the specific region (−197/−80) of the aromatase promoter, leading to transactivation of aromatase. The increased expression of aromatase then catalyzes the conversion from testosterone (T) into E2, leading to increased estrogen concentration.
Increased production of PGE2 by COX-2 is thought to be one of the key events involved in chronic inflammation in BPH and prostate cancer (23, 24, 25). Meanwhile, recent studies found a positive association between PGE2 levels and an increase of the stromal component of the prostate (26). Because an increase of the stromal component is the most common finding in BPH, PGE2 may be associated with this frequent disease. Moreover, cDNA microarray results have shown that COX-2 expression was up-regulated in BPH tissues when compared with normal prostate tissues (27). Overexpression of COX-2 has also been found in the benign cell line BPH-1 when compared with prostate cancer cell lines LNCaP, DU-145, and PC-3 (19). The molecular signaling pathways induced by PGE2 have not been documented yet in prostate stromal cells. Our results link this pathway to estrogens. Estrogen has been implicated in prostatic diseases, which was considered to be not only a treatment option but also one of the hormonal risk factors for prostate cancer (51, 52, 53). Epidemiological and experimental studies have also demonstrated a possible role of estrogen in prostate cancer (54). At the cellular level, increased prostatic estrogen exposure occurs through high aromatase activity particularly in prostatic stroma (40, 42). In this study, we identified a signaling pathway in which ERRα regulated the aromatase gene expression and contributed to the local concentration of estrogen in prostate stromal cells. Although aromatase gene was mainly expressed in the prostate stromal cells, estrogenic action targets not only prostate stromal cells but also prostate epithelial cells. The use of aromatase knockout mice (ArKO) and aromatase inhibitors has been proven to be useful in understanding the importance of local prostatic estrogen production and its relation to prostate carcinogenesis (17, 51). It is reported that the increased expression of ERRα is a negative prognostic predictor in human prostate cancer (16), which indicated that ERRα may play a role in the development of prostate cancer. However, the molecular mechanism by which ERRα participated in this process remains unclear. Further investigations on ERRα-mediated pathways are needed.
In summary, our results provided the first evidence that PGE2 further increases the local concentration of estrogens in prostate stroma, through up-regulation of ERRα and subsequent up-regulation of aromatase.
Materials and Methods
Cell culture and treatment
The immortalized prostate stromal cell line WPMY-1 was purchased from the American Type Culture Collection (ATCC, Manassas, VA), and cultured in DMEM (Sigma, St. Louis, MO) supplemented with 5% fetal bovine serum (FBS; Invitrogen, Carlsbad, CA) and 100 mg/ml penicillin/streptomycin (Hyclone, Logan, UT). Upon reaching 60–70% confluence, the cultures were changed to DMEM supplemented with 1% FBS and cultured continuously for at least 24 h. Then the cells were treated with PGE2 (1 μm) and/or AH6809 (10 μm), butaprost (2, 10, 20 μm), H89 (0.5 μg/ml), PD98059 (25 nm), chlordane (10 μm), or forskolin (1 μm). After 12 or 24 h, cells were harvested for RNA or protein extraction, respectively.
RNA extraction and RT-PCR
Total RNA was isolated from the cells using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. The reverse transcription was then performed at 37 C for 2 h as described previously (57). The 20-μl reaction solution included 1–2 μg of RNA, 0.5 mm of deoxy-NTPs, 5 μm of random hexamers, 10 mm of dithiothreitol, 200 U Moloney murine leukemia virus reverse transcriptase, and 20 U ribonuclease inhibitor (all from Promega, Madison, WI). RT-PCR was performed to detect the mRNA expression of PGE2 receptors in WPMY-1 cells. Real-time PCR was performed to determine the levels of mRNA for ERRα and aromatase in WPMY-1 cells treated with different stimulators. A SYBR Green I-based real-time quantitative PCR was carried out on a DNA Engine Opticon Continuous Fluorescence Detection System (Opticon Monitor II; MJ Research, Inc., Waltham, MA). The relative gene expression was determined using the comparative CT method and normalized to a housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used are listed in Table 1 and were synthesized by Sangon (Shanghai, China).
Table 1.
Primers used for PCR
| Gene or promoter region | Primer sequences (5′–3′) | Product length (bp) | Annealing temperature (C) |
|---|---|---|---|
| GAPDH | |||
| Forward | GGGGAGCCAAAAGGGTCATCATCT | 457 | 58 |
| Reverse | GACGCCTGCTTCACCACCTTCTTG | ||
| Aromatase | |||
| Forward | GAATATTGGAAGGATGCACAGACT | 343 | 58 |
| Reverse | GGGTAAAGATCATTTCCAGCATGT | ||
| ERRα | |||
| Forward | AAAGTGCTGGCCCATTTCTAT | 100 | 60 |
| Reverse | CCTTGCCTCAGTCCATCAT | ||
| EP1 | |||
| Forward | CGCTATGAGCTGCAGTACCC | 508 | 58 |
| Reverse | CCAGGATCTGGTTCCAGGAG | ||
| EP2 | |||
| Forward | CAACCTCATCCGCATGCAC | 421 | 58 |
| Reverse | CTCAAAGGTCAGCCTG | ||
| EP3 | |||
| Forward | ACCCGCCTCAACCACTCCTACACA | 410 | 58 |
| Reverse | ATGGCGCTGGCGATGAACAAC | ||
| EP4 | |||
| Forward | CCTCCTGAGAAAGACAGTGCT | 367 | 58 |
| Reverse | AAGACACTCTCTGAGTCCT | ||
| PI.3/II | |||
| Forward | CCCACTCAAGGGCAAGAT | 118 | 58 |
| Reverse | CAAGGAAGCCCAAGAAAG |
Western blot analysis
Cellular protein was extracted with RIPA buffer and was quantified using the Bradford method. An equal amount of protein (20–40 μg) was loaded to each well. After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane. The membrane was blocked with 13% nonimmune serum for 1 h at room temperature, and then incubated with anti-ERRα mouse monoclonal antibody (1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA; sc-65715) or anti-aromatase rabbit polyclonal antibody (1:400, BA1562; Boster, Wuhan, China) at 4 C overnight. After washing six times with Tris-buffered saline with 0.1% Tween 20, the membrane was incubated with the secondary antibodies (goat-antimouse horseradish peroxidase-conjugated IgG 1:5000 (Bio-Rad, Hercules, CA; 1706516), or goat-antirabbit peroxidase-conjugated IgG 1:10000 (BA1054; Boster) at room temperature for 1 h, developed by enhanced chemiluminescence and visualized with Hyperfilm-ECL (Amersham, Piscataway, NJ).
Plasmid
The ERRα expression plasmid was a gift from Professor J. M. Vanacker (Institut de Génomique Fonctionnelle de Lyon, Université de Lyon, Université Lyon 1, France). Renilla luciferase plasmid pRL-TK was purchased from Promega. The luciferase reporter plasmid aromatase pro-Luc plasmid was constructed by inserting an aromatase promoter fragment into pGL3-basic vector (Promega). The fragment of PI.3/II was obtained from the human genome by long and accurate PCR using a commercially available kit (Takara, Dalian, China) with the following primers: forward 5′-TGAGAGAAGCCTGCCACAACCAT-3′ and reverse 5′-TCCTTGACCTCAGAGGGGGCAAT-3′.
cAMP Measurement
WPMY-1 cells were seeded in six-well plates as described above. For the time course experiment, cells were treated with PGE2 (1 μm) for 0, 5, 30, and 60 min. After treatment, cells were washed once with PBS and lysed. The lysates were subsequently collected and subjected to cAMP luminescence assay according to the manufacturer’s instruction (cAMP-Glo Assay; Promega V1501).
Transfection and luciferase assays
Transfection and luciferase assays were performed using the protocols described previously (56). Before transient transfection, WPMY-1 cells were seeded in 24-well plates at a density of 80–90% with DMEM supplemented with 1% FBS. After attachment, WPMY-1 were transfected with 0.6 μg plasmid/well (0.4 μg aromatase pro-Luc plasmid or the promoter-less pGL3-basic plasmid and 0.2 μg of reference pRL-TK plasmid), 0.2 μg/well ERRα expression plasmid or 30 pmol ERRα siRNA (described below) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Cells were incubated for at least 6 h and then treated with PGE2 (1 μm) and/or chlordane (10 μm). For the luciferase assay, cell lysates were prepared after 24 h stimulation and dual-luciferase assays (Promega E1910) were performed using a luminometer (TD20/20; Turner Designs, Sunnyvale, CA). Firefly luciferase activities were corrected for variations in transfection efficiencies as determined by assaying cell extracts for renilla luciferase activities.
For the assay of ERRα expression, 48 h after the transfection with ERRα expression plasmid, cells were treated with PGE2 (1 μm) and/or chlordane (10 μm) for 24 h, and RNA was extracted as described above.
siRNA of ERRα, transfection, and treatment
The sequences of the siRNAs used to target ERRα are as follows: sense 5′-GGCAGAAACCUAUCUCAGGUU-3′, antisense 5′-CCUGAGAUAGGUUUCUGCCUC-3′. The sequences of the siRNA control are as follows: 5′-UUCUCCGAACGUGUCACGUTT-3′, antisense 5′-ACGUGACACGUUCGGAGAATT-3. Annealed siRNAs were transfected with Lipofectamine 2000 (Invitrogen) into WPMY-1 cells in six-well plates. After 24 h, cells were treated with PGE2 (1 μm) and/or chlordane (10 μm) for additional 24 h. RNA was extracted and mRNA levels for the indicated genes were determined by PCR and normalized to the GAPDH mRNA level.
ChIP
ChIP experiments were performed according to previously published protocols with minor modifications (58, 59, 60). Briefly, WPMY-1 cells were grown in DMEM containing 1% FBS in 100-mm dishes for 24 h. Then the cells were treated with or without PGE2 (1 μm) for 24 h. For a ChIP experiment, cells were cross-linked in culture medium containing 1% formaldehyde for 10 min at room temperature. Cross-linking was arrested by addition of glycine for 10 min at room temperature (final concentration 125 mm). Cells were washed with PBS, scraped off in PBS, and harvested by centrifugation at 300 × g for 3 min. Pellets were resuspended in sodium dodecyl sulfate lysis buffer, sonicated to 300–500 bp, and centrifuged at 11,000 × g for 30 min. A small portion (1%) of the cross-linked, sheared chromatin was saved as input, and the remainder was precleared by incubating with 50 μl preblocked protein-A-agarose/Salmon Sperm DNA (Upstate Biotechnology, Lake Placid, NY) for 1 h. The precleared chromatin was then immunoprecipitated with 0.6 μg anti-ERRα antibody (Santa Cruz; sc-65715) or control IgG with rotation overnight at 4 C. After an additional incubation with 50 μl protein-A-agarose for 5 h at 4 C, immunocomplexes were washed and eluted from the beads with the elution buffer (1% sodium dodecyl sulfate and 0.1 m NaHCO3). Cross-linking was reversed overnight at 65 C, and DNA was purified using QIAquick column (Qiagen, Valencia, CA). ChIP samples were analyzed by standard PCR and real-time PCR using primers located in the PI.3/PII region (see Table 1).
Measurement of E2 levels in the medium of WPMY-1
WPMY-1 cells were seeded into a 24-well plate at a density of 2.5 × 105 per well and cultured in 500 μl of DMEM containing 1% charocol/dextran-treated FBS. After attachment, cells were transfected with ERRα expression plasmid or ERRα siRNA and incubated for 6 h, and then treated with PGE2 (1 μm) or vehicle. After 48 h, the medium was replaced with fresh medium supplemented with 1% charocol/dextran-treated FBS and 10 nm testosterone was added to each well. WPMY-1 cells were incubated for another 24 h. The cultured medium were collected, centrifuged and stored at −20 C. Concentrations of E2 in medium were measured using a specific Estradiol II kit (catalog no. 03000079 122; Roche, Indianapolis, IN) according to the manufacturer’s protocol. Briefly, 35 μl of each sample was incubated with an E2-specific biotinylated antibody followed by incubation after addition of streptavidin-coated microparticles and an E2 derivative labeled with a ruthenium complex. The microparticles in the reaction mixture were captured on the surface of the electrode magnetically. Chemiluminescent emission induced by a voltage to the electrode was measured by a photomultiplier analyzer (cobas e 411; Roche). E2 concentration was calculated based on a calibration curve.
Statistical analysis
Data are expressed as mean ± sd. Significance was assessed using Student’s paired t test. *, P < 0.05 was considered as significant.
Acknowledgments
We thank Prof. Vanacker (Université de Lyon, France) for the gift of ERRα expression plasmid.
NURSA Molecule Pages:
Nuclear Receptors: ERR-α.
Footnotes
This work was supported by National Basic Research Programs (973 Programs, No. 2009CB918904, No. 2010CB945003), the National Natural Science Foundation of China (Grant No. 30872592), Joint Research Fund for Overseas Chinese, Hong Kong and Macau Scholars (No. 30928027), and the Major Project of Tianjin Sci-Tech Support Program (No. 09CKFSF00800).
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 29, 2010
Abbreviations: BPH-1, Benign prostatic hyperplasic cell line; ChIP, chromatin immunoprecipitation; COX-2, cyclooxygenase-2; E2, 17β-estradiol; EP2–EP4, PGE2 receptors; ERα, estrogen receptor α; ERRα, estrogen receptor-related receptor α; EREs, estrogen response elements; ERREs, ERR elements; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGE2, prostaglandin E2; PI.3, promoter I.3; PII, promoter II; PKA, protein kinase A; siC, control siRNA; siE, ERRα siRNA; siRNA, small interfering RNA; SRC, steroid receptor coactivator; WPMY-1, prostate stromal cell line.
References
- 1.Giguère V, Yang N, Segui P, Evans RM1988. Identification of a new class of steroid hormone receptors. Nature 331:91–94 [DOI] [PubMed] [Google Scholar]
- 2.Giguère V2002. To ERR in the estrogen pathway. Trends Endocrinol Metab 13:220–225 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Z, Teng CT2000. Estrogen receptor-related receptor α1 interacts with coactivator and constitutively activates the estrogen response elements of the human lactoferrin gene. J Biol Chem 275:20837–20846 [DOI] [PubMed] [Google Scholar]
- 4.Yang C, Chen S1999. Two organochlorine pesticides, toxaphene and chlordane, are antagonists for estrogen-related receptor α-1 orphan receptor. Cancer Res 59:4519–4524 [PubMed] [Google Scholar]
- 5.Johnston SD, Liu X, Zuo F, Eisenbraun TL, Wiley SR, Kraus RJ, Mertz JE1997. Estrogen-related receptor α 1 functionally binds as a monomer to extended half-site sequences including ones contained within estrogen-response elements. Mol Endocrinol 11:342–352 [DOI] [PubMed] [Google Scholar]
- 6.Vanacker JM, Bonnelye E, Chopin-Delannoy S, Delmarre C, Cavaillès V, Laudet V1999. Transcriptional activities of the orphan nuclear receptor ERR α (estrogen receptor-related receptor-α). Mol Endocrinol 13:764–773 [DOI] [PubMed] [Google Scholar]
- 7.Yang N, Shigeta H, Shi H, Teng CT1996. Estrogen-related receptor, hERR1, modulates estrogen receptor-mediated response of human lactoferrin gene promoter. J Biol Chem 271:5795–5804 [DOI] [PubMed] [Google Scholar]
- 8.Zirngibl RA, Chan JS, Aubin JE2008. Estrogen receptor-related receptor α (ERRα) regulates osteopontin expression through a non-canonical ERRα response element in a cell context-dependent manner. J Mol Endocrinol 40:61–73 [DOI] [PubMed] [Google Scholar]
- 9.Vanacker JM, Delmarre C, Guo X, Laudet V1998. Activation of the osteopontin promoter by the orphan nuclear receptor estrogen receptor related α. Cell Growth Differ 9:1007–1014 [PubMed] [Google Scholar]
- 10.Lu D, Kiriyama Y, Lee KY, Giguère V2001. Transcriptional regulation of the estrogen-inducible pS2 breast cancer marker gene by the ERR family of orphan nuclear receptors. Cancer Res 61:6755–6761 [PubMed] [Google Scholar]
- 11.Deblois G, Hall JA, Perry MC, Laganière J, Ghahremani M, Park M, Hallett M, Giguère V2009. Genome-wide identification of direct target genes implicates estrogen-related receptor α as a determinant of breast cancer heterogeneity. Cancer Res 69:6149–6157 [DOI] [PubMed] [Google Scholar]
- 12.Cheung CP, Yu S, Wong KB, Chan LW, Lai FM, Wang X, Suetsugi M, Chen S, Chan FL2005. Expression and functional study of estrogen receptor-related receptors in human prostatic cells and tissues. J Clin Endocrinol Metab 90:1830–1844 [DOI] [PubMed] [Google Scholar]
- 13.Ariazi EA, Clark GM, Mertz JE2002. Estrogen-related receptor α and estrogen-related receptor γ associate with unfavorable and favorable biomarkers, respectively, in human breast cancer. Cancer Res 62:6510–6518 [PubMed] [Google Scholar]
- 14.Suzuki T, Miki Y, Moriya T, Shimada N, Ishida T, Hirakawa H, Ohuchi N, Sasano H2004. Estrogen-related receptor α in human breast carcinoma as a potent prognostic factor. Cancer Res 64:4670–4676 [DOI] [PubMed] [Google Scholar]
- 15.Stein RA, Chang CY, Kazmin DA, Way J, Schroeder T, Wergin M, Dewhirst MW, McDonnell DP2008. Estrogen-related receptor α is critical for the growth of estrogen receptor-negative breast cancer. Cancer Res 68:8805–8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujimura T, Takahashi S, Urano T, Kumagai J, Ogushi T, Horie-Inoue K, Ouchi Y, Kitamura T, Muramatsu M, Inoue S2007. Increased expression of estrogen-related receptor α (ERRα) is a negative prognostic predictor in human prostate cancer. Int J Cancer 120:2325–2330 [DOI] [PubMed] [Google Scholar]
- 17.Ellem SJ, Risbridger GP2006. Aromatase and prostate cancer. Minerva Endocrinol 31:1–12 [PubMed] [Google Scholar]
- 18.Wu Q, Shi J, Chen L, Wang CY, Park I, Lee C, Zhang J2008. Regulation of proliferation and differentiation of prostatic stromal cells by oestradiol through prostatic epithelial cells in a paracrine manner. BJU Int 101:497–502 [DOI] [PubMed] [Google Scholar]
- 19.Wu Q, Zhou Y, Chen L, Shi J, Wang CY, Miao L, Klocker H, Park I, Lee C, Zhang J2007. Benign prostatic hyperplasia (BPH) epithelial cell line BPH-1 induces aromatase expression in prostatic stromal cells via prostaglandin E2. J Endocrinol 195:89–94 [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Zhou D, Chen S1998. Modulation of aromatase expression in the breast tissue by ERR α-1 orphan receptor. Cancer Res 58:5695–5700 [PubMed] [Google Scholar]
- 21.Yang C, Yu B, Zhou D, Chen S2002. Regulation of aromatase promoter activity in human breast tissue by nuclear receptors. Oncogene 21:2854–2863 [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE2009. Regulation of breast cancer-associated aromatase promoters. Cancer Lett 273:15–27 [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Bergh A, Damber JE2004. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate 61:60–72 [DOI] [PubMed] [Google Scholar]
- 24.Wang W, Bergh A, Damber JE2005. Cyclooxygenase-2 expression correlates with local chronic inflammation and tumor neovascularization in human prostate cancer. Clin Cancer Res 11:3250–3256 [DOI] [PubMed] [Google Scholar]
- 25.Cai Y, Lee YF, Li G, Liu S, Bao BY, Huang J, Hsu CL, Chang C2008. A new prostate cancer therapeutic approach: combination of androgen ablation with COX-2 inhibitor. Int J Cancer 123:195–201 [DOI] [PubMed] [Google Scholar]
- 26.Larré S, Tran N, Fan C, Hamadeh H, Champigneulles J, Azzouzi R, Cussenot O, Mangin P, Olivier JL2008. PGE2 and LTB4 tissue levels in benign and cancerous prostates. Prostaglandins Other Lipid Mediat 87:14–19 [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Dunn T, Ewing C, Sauvageot J, Chen Y, Trent J, Isaacs W2002. Gene expression signature of benign prostatic hyperplasia revealed by cDNA microarray analysis. Prostate 51:189–200 [DOI] [PubMed] [Google Scholar]
- 28.Ohno K, Araki N, Yanase T, Nawata H, Iida M2004. A novel nonradioactive method for measuring aromatase activity using a human ovarian granulosa-like tumor cell line and an estrone ELISA. Toxicol Sci 82:443–450 [DOI] [PubMed] [Google Scholar]
- 29.Laganière J, Tremblay GB, Dufour CR, Giroux S, Rousseau F, Giguère V2004. A polymorphic autoregulatory hormone response element in the human estrogen-related receptor α (ERRα) promoter dictates peroxisome proliferator-activated receptor γ coactivator-1α control of ERRα expression. J Biol Chem 279:18504–18510 [DOI] [PubMed] [Google Scholar]
- 30.Huss JM, Kopp RP, Kelly DP2002. Peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) coactivates the cardiac-enriched nuclear receptors estrogen-related receptor-α and -γ. Identification of novel leucine-rich interaction motif within PGC-1α. J Biol Chem 277:40265–40274 [DOI] [PubMed] [Google Scholar]
- 31.Ichida M, Nemoto S, Finkel T2002. Identification of a specific molecular repressor of the peroxisome proliferator-activated receptor γ Coactivator-1 α (PGC-1α). J Biol Chem 277:50991–50995 [DOI] [PubMed] [Google Scholar]
- 32.Schreiber SN, Knutti D, Brogli K, Uhlmann T, Kralli A2003. The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor α (ERRα). J Biol Chem 278:9013–9018 [DOI] [PubMed] [Google Scholar]
- 33.Xie W, Hong H, Yang NN, Lin RJ, Simon CM, Stallcup MR, Evans RM1999. Constitutive activation of transcription and binding of coactivator by estrogen-related receptors 1 and 2. Mol Endocrinol 13:2151–2162 [DOI] [PubMed] [Google Scholar]
- 34.Liu D, Zhang Z, Gladwell W, Teng CT2003. Estrogen stimulates estrogen-related receptor α gene expression through conserved hormone response elements. Endocrinology 144:4894–4904 [DOI] [PubMed] [Google Scholar]
- 35.Sladek R, Giguère V2000. Orphan nuclear receptors: an emerging family of metabolic regulators. Adv Pharmacol 47:23–87 [DOI] [PubMed] [Google Scholar]
- 36.Narumiya S, Sugimoto Y, Ushikubi F1999. Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226 [DOI] [PubMed] [Google Scholar]
- 37.Steinert D, Küper C, Bartels H, Beck FX, Neuhofer W2009. PGE2 potentiates tonicity-induced COX-2 expression in renal medullary cells in a positive feedback loop involving EP2-cAMP-PKA signaling. Am J Physiol Cell Physiol 296:C75–C87 [DOI] [PubMed]
- 38.Funahashi K, Cao X, Yamauchi M, Kozaki Y, Ishiguro N, Kambe F2009. Prostaglandin E2 negatively regulates AMP-activated protein kinase via protein kinase A signaling pathway. Prostaglandins Other Lipid Mediat 88:31–35 [DOI] [PubMed] [Google Scholar]
- 39.Liu D, Benlhabib H, Mendelson CR2009. cAMP enhances estrogen-related receptor α (ERRα) transcriptional activity at the SP-A promoter by increasing its interaction with protein kinase A and steroid receptor coactivator 2 (SRC-2). Mol Endocrinol 23:772–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krieg M, Nass R, Tunn S1993. Effect of aging on endogenous level of 5 α-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab 77:375–381 [DOI] [PubMed] [Google Scholar]
- 41.Hiramatsu M, Maehara I, Ozaki M, Harada N, Orikasa S, Sasano H1997. Aromatase in hyperplasia and carcinoma of the human prostate. Prostate 31:118–124 [DOI] [PubMed] [Google Scholar]
- 42.Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP2004. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab 89:2434–2441 [DOI] [PubMed] [Google Scholar]
- 43.Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H2007. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer Res 67:3945–3954 [DOI] [PubMed] [Google Scholar]
- 44.Chen S, Itoh T, Wu K, Zhou D, Yang C2002. Transcriptional regulation of aromatase expression in human breast tissue. J Steroid Biochem Mol Biol 83:93–99 [DOI] [PubMed] [Google Scholar]
- 45.Richards JA, Brueggemeier RW2003. Prostaglandin E2 regulates aromatase activity and expression in human adipose stromal cells via two distinct receptor subtypes. J Clin Endocrinol Metab 88:2810–2816 [DOI] [PubMed] [Google Scholar]
- 46.Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE2007. Prostaglandin E(2) induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH(2)-terminal kinase in adipose fibroblasts. Cancer Res 67:8914–8922 [DOI] [PubMed] [Google Scholar]
- 47.Evans CT, Corbin CJ, Saunders CT, Merrill JC, Simpson ER, Mendelson CR1987. Regulation of estrogen biosynthesis in human adipose stromal cells. Effects of dibutyryl cyclic AMP, epidermal growth factor, and phorbol esters on the synthesis of aromatase cytochrome P-450. J Biol Chem 262:6914–6920 [PubMed] [Google Scholar]
- 48.Mendelson CR, Corbin CJ, Smith ME, Smith J, Simpson ER1986. Growth factors suppress and phorbol esters potentiate the action of dibutyryl adenosine 3′,5′-monophosphate to stimulate aromatase activity of human adipose stromal cells. Endocrinology 118:968–973 [DOI] [PubMed] [Google Scholar]
- 49.Zhou J, Suzuki T, Kovacic A, Saito R, Miki Y, Ishida T, Moriya T, Simpson ER, Sasano H, Clyne CD2005. Interactions between prostaglandin E(2), liver receptor homologue-1, and aromatase in breast cancer. Cancer Res 65:657–663 [PubMed] [Google Scholar]
- 50.Clyne CD, Speed CJ, Zhou J, Simpson ER2002. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem 277:20591–20597 [DOI] [PubMed] [Google Scholar]
- 51.Ricke WA, Wang Y, Cunha GR2007. Steroid hormones and carcinogenesis of the prostate: the role of estrogens. Differentiation 75:871–882 [DOI] [PubMed] [Google Scholar]
- 52.Bosland MC2000. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr:39–66 [DOI] [PubMed]
- 53.Griffiths K2000. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate 45:87–100 [DOI] [PubMed] [Google Scholar]
- 54.Prins GS2008. Endocrine disruptors and prostate cancer risk. Endocr Relat Cancer 15:649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Hess MW, Thurnher M, Hobisch A, Radmayr C, Cronauer MV, Hittmair A, Culig Z, Bartsch G, Klocker H1997. Human prostatic smooth muscle cells in culture: estradiol enhances expression of smooth muscle cell-specific markers. Prostate 30:117–129 [DOI] [PubMed] [Google Scholar]
- 56.Wang CY, Shi JD, Yan CH, Wu Q, Klocker H, Park I, Lee C, Zhang J2007. Development of a cell-isolation method for human prostatic smooth muscle cells based on cell type-specific activation of the SM22 gene promoter. BJU Int 99:183–188 [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Wang CY, Yang R, Shi J, Fu R, Chen L, Klocker H, Zhang J2008. Real-time quantitative RT-PCR assay of prostate-specific antigen and prostate-specific membrane antigen in peripheral blood for detection of prostate cancer micrometastasis. Urol Oncol 26:634–640 [DOI] [PubMed] [Google Scholar]
- 58.Wang CY, Shi JD, Zhu Y, Zhang J2005. Application of chromatin immunoprecipitation assay in deciphering DNA-protein interactions. Heriditas (Yi Chuan) 27:801–807 [PubMed] [Google Scholar]
- 59.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 60.Shang Y, Brown M2002. Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]