Abstract
Latent Autoimmune Diabetes in Adults (LADA) is a slowly progressing form of immune-mediated diabetes that combines phenotypical features of type 2 diabetes (T2D) with the presence of islet cell antigens detected in type 1 diabetes (T1D). Heterogeneous clinical picture have led to the classification of patients based on the levels of antibodies against glutamic acid decarboxylase 65 (GADA) that correlate with clinical phenotypes closer to T1D or T2D when GADA titers are high or low, respectively. To date, LADA etiology remains elusive despite numerous studies investigating on genetic predisposition and environmental risk factors. To our knowledge, this is the first study aimed at evaluation of a putative role played by Mycobacterium avium subsp. paratuberculosis (MAP) as an infective agent in LADA pathogenesis. MAP is known to cause chronic enteritis in ruminants and has been associated with autoimmune disorders in humans. We analyzed seroreactivity of 223 Sardinian LADA subjects and 182 healthy volunteers against MAP-derived peptides and their human homologs of proinsulin and zinc transporter 8 protein. A significantly elevated positivity for MAP/proinsulin was detected among patients, with the highest prevalence in the 32-41-year-old T1D-like LADA subgroup, supporting our hypothesis of a possible MAP contribution in the development of autoimmunity.
Introduction
Latent Autoimmune Diabetes in Adults (LADA) is a slowly developing subtype of immune-mediated diabetes characterized by a combination of clinical features typical to type 2 diabetes (T2D) and reactivity to islet cell antigens commonly detected in type 1 diabetes (T1D). Antibodies (Abs) against glutamic acid decarboxylase 65 (GADA65Ab) and protein tyrosine phosphatase IA-2 (IA-2A) are the most conclusive biomarkers [1] that, along with insulin independence period of at least 6 months following disease onset, permit to distinguish LADA from other forms of diabetes. Although general diagnostic criteria has been widely discussed [2, 3], GADA titers remain a benchmark for discrimination between phenotypes recognized among LADA patients presenting clinical characteristics closer to classical T1D or T2D [4].
It is estimated that LADA accounts for almost 10% of newly diagnosed adult-onset diabetes cases [1], therefore its importance in terms of prevalence exceeds that of T1D. Nonetheless, LADA is far less investigated and its etiology remains so far poorly understood. Even though some studies reported increased frequency of predisposing genotypes such as the T2D-associated variant in TCF7L2 transcription factor [5] or HLA T1D-susceptibility haplotypes [6, 7], major occurrence of less protective genotypes [8] and familial clustering linked to LADA pathogenesis [9], hereditary factors alone cannot explain progression towards β-cell failure in subjects at low genetic risk. Despite its phenotypically heterogeneous nature [10], LADA is a distinct entity frequently associated with other endocrine disorders [11]. The scientific literature describes environmental risk factors related to dietary habits and lifestyle [12, 13, 14, 15, 16], however, in contrast to T1D, scarce information is provided regarding infectious agents putatively contributing to LADA. In this study we focused our attention on a possible role of Mycobacterium avuim subsp. paratuberculosis (MAP) as an environmental agent at play in LADA pathogenesis. MAP is a globally widespread trigger of a chronic granulomatous enteritis (Johne’s disease) in ruminant animals, able to survive inside host macrophages by overcoming the mechanism of phagocytosis [17] and can be easily transmitted to humans with contaminated water, meat and dairy products [18]. We have previously demonstrated a high prevalence of Abs targeting MAP-derived epitopes and their human homologs of proinsulin (PI) and zinc transporter 8 protein (ZnT8) in T1D at-risk subjects reaching 43% for MAP/PI homologous pair in Sardinian infants [19] and exceeding 62% for MAP/ZnT8 pair in youth from mainland Italy [20]. Interestingly, no significant anti-MAP seroreactivity was associated to T2D [21].
The present study describes humoral responses to the same peptide selection in Sardinian LADA patients with further evaluation based on age-related classification and disease phenotype. We attempted to investigate whether our earlier results may reflect the hypothesis of common factors underlying T2D and LADA etiology [22] and help in identifying the risk for autoimmune comorbidities.
Methods
Subjects
Two hundred and twenty three LADA patients (n = 104 men and n = 119 women, mean age 55.22±10.71 years) and 182 age-matched healthy blood donors (HCs; n = 102 males and n = 80 females, mean age 48.13±10.35 years) attending the Transfusion Medicine Department were enrolled in the present study. LADA patients have been referred as a part of a multicentric study, from five Diabetic Units of the island of Sardinia, Italy. The clinical features, autoimmune markers, and progression toward insulin dependence in these patients have been described in detail previously [23]. Any history of autoimmune or inflammatory disorders preceding blood collection from HCs was unknown. Diagnosis of LADA was performed upon analyses for the presence of circulating classical islet autoantibodies (GADA and IA2A), age at onset and period of insulin independence according to the Immunology of Diabetes Society criteria. Venous whole blood was drawn after obtaining written informed consent from all study participants and subjected to plasma separation through sedimentation method for further identification of Abs against MAP peptides and their human homologs deriving from ZnT8 and PI. All patients were free from insulin therapy at the moment of blood collection and none of them developed insulin dependence for at least 6 months following diagnosis. The study protocols were approved by the Bioethical Committee of the University Hospital of Sassari and all methods were performed in accordance with institutional and governmental guidelines and regulations.
Diabetes-related autoantibodies
Levels of anti-GAD65 and anti-IA-2 autoantibodies were determined by radioligand assays employing respective CentAK® anti-GAD65 and CentAK® anti-IA-2 commercial kits (Medipan GMBH, 15827 Dahlewitz, Germany) according to the manufacturer's instruction. GADA titers were used to classify LADA patients in two groups based on a threshold established as previously reported [23], giving 80 subjects with high GADA values assigned to LADA1 subtype and 118 individuals with low GADA levels grouped as LADA2 subtype. Relative data was not available for 25 subjects.
Peptide antigens
Peptides MAP2404c70-85 (RGFVVLPVTRRDVTDV), MAP1,4αgbp157-173 (GTVELLGGPLAHPFQPL), MAP3865c133–141 (LAANFVVAL) and MAP3865c125-133 (MIAVALAGL) along with their respective human-derived homologs PI46-61 (RGFFYTPKTRREAEDL), PI64-80 (GQVELGGGPGAGSLQPL), ZnT8186-194 (VAANIVLTV), and ZnT8178-186 (MIIVSSCAV) were synthesized at >85% purity (LifeTein, South Plainfield, NJ 07080, USA) confirmed by HPLC. After reconstitution to 10mM concentration in sterile DMSO, the peptides were stored at -20°C in single-use aliquots for further assays.
Enzyme-linked immunosorbent assay
Abs specific for MAP1,4αgbp/PI, MAP2404c/PI and MAP3865c/ZnT8 homologous epitope pairs were detected in plasma samples by indirect enzyme-linked immunosorbent assays, as described previously [24]. Each test included a highly responsive serum as a positive reference for data normalization with Abs reactivity set at 1,0 arbitrary units (U/ml) and a negative control to which serum was not added. Optimal positivity thresholds were established at 0.74–0.80 U/ml for MAP/PI and 0.81–0.86 U/ml for MAP/ZnT8 antigen pairs based on the receiver operating characteristic (ROC) curves with specificity set at 90% for all peptides. Mann-Whitney U test (95% CI, two-tailed) and Fisher’s exact test were applied to determine statistical significance of the data using Graphpad Prism Version 6.02 (GraphPad Software Inc., La Jolla, CA 92037, USA). Variances of separated groups of LADA1 and LADA2 patients along with HCs were compared employing one-way ANOVA on ranks.
Results
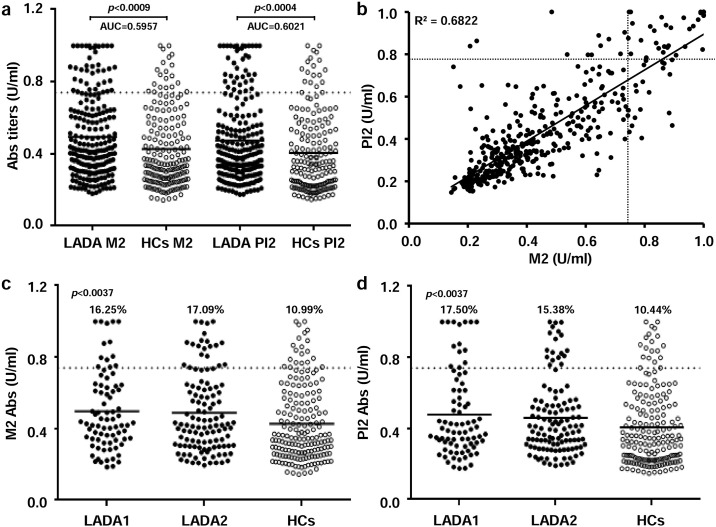
After a single-peptide analysis, we did not find significant differences in responsiveness to the analyzed antigens between LADA subjects and HCs with the exception of MAP2404c70-85/PI46-61 pair (Fig 1), for which Abs prevalence characterized by high statistical significance exceeded 17% in LADA patients and 9% among HCs (p<0.0009 for MAP2404c and p<0.0004 for the homologous PI, Fig 2a). Fisher’s exact test performed based on the numbers of positive and negative subjects further confirmed the significant results when comparing LADA patients and HCs (p<0.028 for MAP2404c70-85 and p<0.039 for PI46-61). Moreover, a high degree of correlation (R2 = 0.68; Fig 2b) was detected between Abs titers recognizing the two homologs in either patients or HCs suggestive of cross-reactivity.
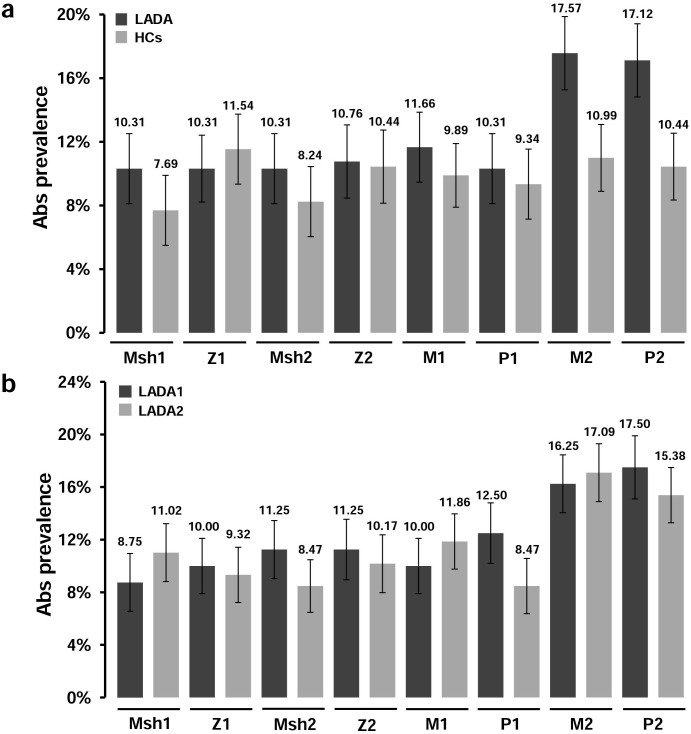
Fig 1. Prevalence of Abs against MAP, proinsulin and ZnT8 homologous epitopes in LADA patients and HCs.
The histograms represent percentages of subjects with Abs positivity to selected epitopes upon single-antigen analysis; sera were tested in duplicate for their reactivity against plate-coated peptides: MAP3865c133–141 (Msh1); ZnT8186-194 (Z1); MAP3865c125-133 (Msh2); ZnT8178-186 (Z2), MAP1,4αgbp157-173 (M1); PI64-80 (PI1); MAP2404c70-85 (M2); PI46-61 (PI2). (a) Dark bars represent LADA patients; light grey bars correspond to HCs. (b) Abs prevalence among LADA 1 (dark bars) and LADA 2 (light grey bars) patient groups. Relative percentages and standard deviations are reported for each bar.
Fig 2. Prevalence and correlation of Abs specific for MAP2404c70-85/PI46-61 peptide pair in LADA patients and HCs.
(A) Distribution of Abs values for MAP2404c70-85 (M2) and PI46-61 (PI2) antigens. Horizontal bars specific for LADA and HCs groups correspond to means. AUC and p values (CI 95%) are indicated above the distributions. (B) Correlation between Abs recognizing MAP2404c70-85 and PI46-61 homologous epitopes in LADA subjects and HCs. Each dot represents Abs detected in one sample; in both graphics the dotted lines indicate positivity thresholds established for each assay based on the ROC analysis. (C) Distributions relative to Abs titers of M2 and (D) PI2 in LADA 1 and LADA 2 patients with HCs as reference; p values calculated by Kruskal-Wallis test as well as percentages of Abs prevalence in each group are reported above the distributions. Only statistically significant results are presented.
Responses to the six remaining peptides were distributed evenly among LADA subjects at the level of 10.31–11.66%. HCs displayed somewhat lower Abs positivity towards both MAP-derived peptides homologous to ZnT8 accounting for 7.69%, whereas percentages of anti-ZnT8 Abs were similar to those observed in the patient group. Similarly, prevalence of Abs directed against MAP1,4αgbp157-173/PI64-80 epitope pair among HCs remained slightly below 10%. Analysis of separated groups defined by high and low GADA titers (LADA1 and LADA2, respectively) confirmed the above mentioned results with respect to HCs, however no differences in mean values or Abs prevalence were found between the two disease phenotypes (Fig 2c and 2d). In fact, MAP2404c70-85/PI46-61 homologs were recognized by 15.00–16.25% of patients in each LADA group (p<0.0037), whereas immune reactivity towards the other selected peptides appeared leveled off at 8.47–12.50% (Fig 1b).
Data relative to other islet autoantibodies were available for 188 patients; among them 43 individuals resulted positive to IA-2A, of which 12 (30.23%, Table 1) had anti-MAP Abs and those directed against MAP2404c70-85/PI46-61 epitopes were detected in 9 subjects (25.58%). IA-2A-negative patients showed 18.71% of general anti-MAP Abs prevalence that decreased to 16.77% when only the statistically significant pair of homologs was considered. After division of patients in disease subtypes, we obtained 8 IA-2A-positive LADA1 and 35 LADA2 patients among which 3 and 10 subjects, respectively, with anti-MAP/PI Abs. Patients without elevated IA-2A titers had Abs against MAP/PI antigens in 13 cases for LADA1 and 15 for LADA2 (19.70% and 18.99%, respectively).
Table 1. Reactivity MAP, ZnT8 and PI antigens in relation to IA-2A titers.
| IA-2A | Patients | Anti-MAP Abs | M2/PI2 Abs* | |
|---|---|---|---|---|
| LADA | + | 43 | 12 (30.23%) | 9 (25.58%) |
| - | 145 | 30 (18.71%) | 28 (16.77%) | |
| LADA1 | + | 8 | 3 (37.50%) | 3 (37.50%) |
| - | 66 | 13 (19.70%) | 12 (18.18%) | |
| LADA2 | + | 35 | 10 (28.57%) | 8 (22.86%) |
| - | 79 | 15 (18.99%) | 12 (15.19%) |
*Antigens for which statistically significant values were obtained.
74 LADA1 and 114 LADA2 subjects with complete clinical data were selected from the study population and further classified in four age groups: 32–41, 42–51, 52–61 and ≥62 years, for which we calculated Abs prevalence either to all peptides together or to the homologous pair MAP2404c70-85/PI46-61 separately (Table 2). Seroreactivity was evaluated between patients and HCs as well as in distinct LADA phenotypes with regard to age at onset and at the time of blood collection. The youngest and the oldest patients were both diagnosed with LADA1 at 32 and 77 years old, respectively. In all disease groups, the highest reactivity to at least one of the analyzed epitopes was registered in the youngest individuals, even though only slightly lower prevalence was observed after 61 years for LADA2; the weakest responses corresponded to the 42–51 age range except for LADA1 for which the lowest seroreactivity occurred in the oldest subjects. When Abs against only MAP2404c70-85/PI46-61 homologs was taken into account, we assigned the highest prevalence to the 32–41 group for LADA1 and ≥61 year old patients for LADA2; for both phenotypes quite high positivity with significant values was maintained in the 52–61 group (p<0.0001). Independently from the analyzed peptides, HCs presented a progressive loss of Abs with age.
Table 2. Seroreactivity to MAP, ZnT8 and PI epitopes in different age groups and during insulin independence period.
Percentages of patients positive to the analyzed epitopes are reported along with the relative numbers of seroreactive subjects given in brackets. Mann-Whitney U test was employed to calculate statistical significance.
| Age group (years) | LADA patients | LADA 1 | LADA 2 | HCs | |||
|---|---|---|---|---|---|---|---|
| Onseta | Sample collectionb | Onset | Sample collection | Onset | Sample collection | Sample collection | |
| Abs prevalence for 8 peptides (%) | |||||||
| 32–41 | 34.37 (11) | 32.26 (10) | 40.00(6) | 45.45 (5) | 29.41 (5) | 27.78 (5) | 38.46 (20) |
| 42–51 | 8.51 (4) | 17,78 (8) | 11.11 (2) | 20.00 (4) | 6.89 (2) | 16.00 (4) | 18.46 (12) |
| 52–61 | 25.93 (21) | 21.43 (12) | 25.93(7) | 23.53 (4) | 25.93 (14) | 20.51 (8) | 10.64 (5) |
| >61 | 21.43 (6) | 20.69 (12) | 7.14 (1) | 11.54 (3) | 33.33 (5) | 27.28 (9) | 11.12 (2) |
| Abs prevalence for MAP2404c70-85/PI46-61 (%) | |||||||
| 32–41 | 25.00 (8) | 22.58 (7) | 33.33 (5) | 36.36 (4) | 17.65 (3) | 16.67 (3) | 21.15 (11) |
| 42–51 | 8.51 (4) | 17,78 (8) | 11.11 (2) | 20.00 (4) | 6.89 (2) | 16.00 (4) | 10.77 (7) |
| 52–61* | 23.46 (19) | 17.86 (10) | 25.93 (7) | 23.53 (4) | 22.22 (12) | 15.38 (6) | 6.38 (3) |
| >61 | 21.43 (6) | 20.69 (12) | 7.14 (1) | 11.54 (3) | 33.33 (5) | 27.28 (9) | 0.00 |
| Insulin-free periodc | Abs prevalence for MAP2404c70-85/PI46-61 (%) | ||||||
| ≤12 | 17.39 (4) | - | 14.28 (1) | - | 18.75 (3) | - | - |
| 13–24 | 23.53 (4) | - | 0.00 | - | 33.33 (4) | - | - |
| 25–36 | 18.19 (2) | - | 16.67 (1) | - | 20.00 (1) | - | - |
| 37–48 | 17.10 (13) | - | 18.75 (6) | - | 15.90 (7) | - | - |
| >48 | 14.28 (2) | - | 25.00 (1) | - | 10.00 (1) | - | - |
* Statistically significant.
a Samples collected at the time of LADA diagnosis.
b Samples collected during a control medical visit following LADA onset.
c Expressed in months.
Clinical history regarding insulin-free period after LADA diagnosis was complete for 142 patients. Most of them (n = 127; 63.68% with LADA2 phenotype) required insulin therapy within 4 consecutive years and only 23 subjects undergone insulin treatment within 12 months following LADA onset (69.57% with LADA2). Differences in anti-MAP positivity between periods of insulin independence reached 1% until 48 months when both LADA phenotypes were analyzed together (Table 2) with the exception of insulin treatment started after 13–24 months characterized by the highest seroreactivity; after splitting of patients according to LADA subtypes, LADA1 subjects showed Abs prevalence increasing with length of insulin-free periods from 14.28% to 25%, while opposite trends were observed for LADA2 decreasing from 18.75% to 10% of seropositive individuals. However, a few positive cases in each period do not permit to draw significant conclusions.
Although sex-related analysis did not reveal associations with high titers of Abs against the selected peptides, the number of males and females varied among LADA phenotypes based on patients’ age at onset. Men accounted for 60% of LADA1 subjects between 32 and 51 years old, whereas proportions in older groups were close to 1:1. In contrast, individuals assigned as LADA2 and aged 42–51 or older than 61 years at diagnosis were characterized by 1:2 male to female ratio.
Discussion
We here demonstrated the increased prevalence of Abs targeting a portion belonging to a MAP-derived putative regulator for proline utilization (MAP2404c) and its human PI homolog with high sequence identity corresponding to contiguous fragments of B chain and C-peptide in Sardinian LADA patients. The study cohort was recruited from the local population known for an elevated incidence of autoimmune diseases [25] that has previously shown a significant reactivity against glucan branching protein of MAP (MAP1,4-αgbp) and the homologous peptide derived from B chain of human PI in subjects suffering from Hashimoto’s thyroiditis [26]. Likewise, responses to the same epitope pairs were even higher in Sardinian infants at risk for T1D [19] and new-onset children from mainland Italy [20]. In a similar fashion, increased seroreactivity to ZnT8 transmembrane regions and their homologous MAP peptides (MAP3865c) has been observed in either Sardinian or Italian cohorts at different disease stages [27, 28, 29, 30]. These results are in line with the ubiquitous presence of MAP in the environment and the frequency of exposure resulting in a straightforward transmission pathway to humans. Detection of Abs may indicate past contact with MAP following to consumption of contaminated food; most likely the intracellular form of MAP acquires enhanced virulence for humans after passage through bovine macrophages present in milk and cheese [31]. On the other hand, raised anti-MAP Abs titers have been found in individuals at continual interaction with infected animals such as veterinarians or livestock breeders; seropositivity among HCs and subjects at-risk for diabetes who do not develop clinical symptoms can be explained by exposure in early childhood to the extracellular phenotype of MAP that may confer natural immunity protective against mycobacterial infection [32].
Unlike T2D patients who do not display significant anti-MAP responses [21], LADA2 was characterized by similar Abs titers as LADA1 subtype. This outcome confirms the hypothesis involving MAP in the pathogenesis of autoimmune diabetes and sustains distinct mechanisms underlying the development of LADA2 with prevailing autoimmune component despite etiological factors shared with T2D. Among genetic risk factors, single nucleotide polymorphisms (SNPs) in TCF7L2, resulting in increased expression of the transcriptional factor, have been linked to T2D and LADA phenotype with low GADA titers [33, 34], whereas other authors have not reported any association [35, 36]. Interestingly, TCF7L2 down-regulation has been observed in MAP-infected bovine monocyte-derived macrophages [37]. Data relative to TCF7L2-conferred risk were not available for our study cohort, however a future assessment based on the combination of these two factors in Sardinian population, considered its genetic homogeneity [38], may provide additional elements to complete our thesis.
Age-related analysis showed major prevalence of anti-MAP positivity for all the eight peptides in the youngest group (32–41 years) of LADA patients assessed either regardless or depending on GADA titers. When only the significant MAP/PI epitope pair was envisaged, this trend switched to the group over 61 years old for LADA2, while remained unvaried for LADA1 and for the general study population, even though the latter presented high Abs titers also in older groups. It is plausible that anti-mycobacterial Abs remain detectable at high levels even after 20 years following LADA diagnosis in a similar way to GADA titers [39], indicating a possible appearance of their peak values at a younger age. In addition, we have previously observed a comparable distribution of Abs targeting the homologous peptides in case of Hashimoto’s thyroiditis with distinct sex-related prevalence [26]. In the present study, responses to MAP antigens were not gender-associated, however Abs positivity in predisposed subjects, especially those assigned as LADA1, may suggest an increased risk for autoimmune thyroiditis. Comorbidity of both diseases has been already described and high GADA titers in males seem to be predisposing for thyroid autoimmunity [40, 41]. Moreover, males aged 32–51 years at disease onset accounted for 60% in LADA1 subgroup reflecting findings observed in case of T1D that confirm male excess in populations with high incidence such as Sardinia [42]. Male to female 1:2 ratio among LADA2 subjects was similar to distributions described for T2D.
We further assessed patients’ reactivity to the selected peptides in correlation with time interval from LADA diagnosis to insulin dependence. Abs prevalence relative to the significant MAP/PI epitope pair was distributed homogeneously among subjects who showed insulin-free period from 18 months to 4 years (within the range 17–18%); the percentage was slightly higher among patients requiring insulin treatment between 13–24 months (23.53%) and much lower in those without therapy after 4 years from LADA onset (14.28%). Surprisingly, after separated analysis of the two disease phenotypes, the decreasing anti-MAP Abs positivity trend was maintained for LADA2 subgroup and showed an inverted picture among LADA1 individuals. These results may suggest a possible association of MAP with a more severe disease course in LADA2 phenotype leading to a sooner need for insulin dosage, however a small number of patients assigned to each insulin-free period did not permit to obtain statistical significance. Moreover, no relation with GADA titers has been found in our previous studies; in contrast, MAP/PI and MAP/ZnT8 positivity has been correlated with respective classical T1D autoantibodies [19] not available for the present cohort. A deeper insight should be dedicated to this question given the increased anti-MAP responses detected among IA-2A-positive subjects.
Our work provides evidence of increased prevalence of antibodies directed against MAP epitope and its homolog derived from human proinsulin in LADA patients. Furthermore, it is in line with our previous findings highlighting elevated positivity to MAP in pediatric and adult populations with other autoimmune disorders such as T1D at different stages of β-cell immunity and Hashimoto’s thyroiditis. Further study is needed to address genetic predisposition in correlation with anti-MAP reactivity and the presence of non-islet organ-specific autoantibodies as a possible indication of comorbidities. Yet, information relative to risk factors in dietary habits may be useful in determining any correlation of LADA with sources of putative exposure to MAP.
Data Availability
Data are available from DOI:10.17605/OSF.IO/63CN8.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Hawa MI, Kolb H, Schloot N, Beyan H, Paschou SA, Buzzetti R, et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care 2003; 36(4):908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao Y, Xiang Y, Zhou Z. Diagnostic criteria of latent autoimmune diabetes in adults (LADA): a review and reflection. Front Med 2012; 6(3):243–7. 10.1007/s11684-012-0201-y [DOI] [PubMed] [Google Scholar]
- 3.Redondo M. LADA: Time for a new definition. Diabetes 2013; 62(2): 339–340. 10.2337/db12-1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falorni A, Calcinaro F. Autoantibody profile and epitope mapping in latent autoimmune diabetes in adults. Ann Y Acad Sci 2002; 958:99–106. [DOI] [PubMed] [Google Scholar]
- 5.Lukacs K, Hosszufalusi N, Dinya E, Bakacs M, Madacsy L, Panczel P. The type 2 diabetes-associated variant in TCF7L2 is associated with latent autoimmune diabetes in adult Europeans and the gene effect is modified by a meta-analysis and an individual study. Diabetologia 2012; 55(3):689–93. 10.1007/s00125-011-2378-z [DOI] [PubMed] [Google Scholar]
- 6.Cervin C, Lyssenko V, Bakhtadze E, Lindholm E, Nilsson P, Tuomi T, et al. Genetic similarities between latent autoimmune diabetes in adults, type 1 diabetes, and type 2 diabetes. Diabetes 2008; 57(5):1433–7. 10.2337/db07-0299 [DOI] [PubMed] [Google Scholar]
- 7.Desai M, Zeggini E, Horton VA, Owen KR, Hattersley AT, Lew JC, et al. An association analysis of the HLA gene region in latent autoimmune diabetes in adults. Diabetologia 2007; 50(1): 68–73. 10.1007/s00125-006-0513-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenstrom G, Berger B, Borg H, Fernlund P, Dorman JS, Sundkvist Gl. HLA-DQ genotypes in classic type 1 diabetes and in latent autoimmune diabetes of the adult. Am J Epidemiol 2002; 156(9):787–96. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson S, Midthjell K, Grill V. Influence of Family History of Diabetes on Incidence and Prevalence of Latent Autoimmune Diabetes of the Adult. Diabetes Care 2007; 30(12):3040–5. 10.2337/dc07-0718 [DOI] [PubMed] [Google Scholar]
- 10.Pes GM, Delitala AP, Errigo A, Delitala G, Dore MP. Clustering of immunological, metabolic and genetic features in latent autoimmune diabetes in adults: evidence from principal component analysis. Intern Emerg Med 2016. 11:561–567. 10.1007/s11739-015-1352-z [DOI] [PubMed] [Google Scholar]
- 11.Delitala AP, Fanciulli G, Zoledziewska M, Pitzalis M, Pusceddu P, Frongia P, et al. Allelic variant in CTLA4 is associated with thyroid failure and faster β-cell exhaustion in latent autoimmune diabetes in adults. J Diabetes 2015; 7:68–73. [DOI] [PubMed] [Google Scholar]
- 12.Østergaard JA, Laugesen E, Leslie RD. Should There be Concern About Autoimmune Diabetes in Adults? Current Evidence and Controversies. Curr Diab Rep 2016; 16(9):82 10.1007/s11892-016-0780-0 [DOI] [PubMed] [Google Scholar]
- 13.Guglielmi C, Palermo A, Pozzilli P. Latent Autoimmune Diabetes in the Aults (LADA) in Asia: from pathogenesis and epidemiology to therapy. Diabetes Metab Res Rev 2012; 2:40–6. [DOI] [PubMed] [Google Scholar]
- 14.Rasuoli B, Andersson T, Carlsson PO, Grill V, Groop L, Martinell M, et al. Use of Swedish smokeless tobacco (snus) and the risk of Type 2 and latent autoimmune diabetes of adulthood (LADA). Diabet Med 2016; [DOI] [PubMed] [Google Scholar]
- 15.Löfvenborg JE, Andersson T, Carlsson PO, Dorkhan M, Groop L, Martinell M, et al. Fatty fish consumption and risk of latent autoimmune diabetes in aduls. Nutr Diabetes 2014; 20;4:e139 10.1038/nutd.2014.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasuoli B, Andersson T, Carlsson PO, Dorkhan M, Grill V, Groop L, et al. Alcohol and the risk for latent autoimmune diabetes in adults: results based on Swedish ESTRID study. Eur J Endocrinol 2014; 171(5):535–43. 10.1530/EJE-14-0403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka S, Sato M, Onitsuka T, Kamata H, Yokomizo Y. Inflammatory cytokine gene expression in different types of granulomatous lesions during asymptomatic stages of bovine paratuberculosis. Vet Pathol 2005; 42(5):579–88. 10.1354/vp.42-5-579 [DOI] [PubMed] [Google Scholar]
- 18.Atreya R, Bülte M, Gerlach GF, Goethe R, Hornef MW, Köhler H, et al. Facts, myths and hypotheses on the zoonotic nature of Mycobacterium avium subspecies paratuberculosis. Int J Med Microbiol 2013; 304(7):858–67. [DOI] [PubMed] [Google Scholar]
- 19.Niegowska M, Paccagnini D, Mannu C, Targhetta C, Songini M, Sechi LA. Recognition of ZnT8, Proinsulin, and Homologous MAP Peptides in Sardinian Children at Risk of T1D Precedes Detection of Classical Islet Antibodies. J Diabetes Res 2016; 2016:5842701 10.1155/2016/5842701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niegowska M, Rapini N, Piccinini S, Mameli G, Caggiu E, Manca Bitti ML, et al. Type 1 Diabetes at-risk children highly recognize Mycobacterium avium subspecies paratuberculosis epitopes homologous to human Znt8 and Proinsulin. Sci Rep 2016; 29;6:22266 10.1038/srep22266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosu V, Ahmed N, Paccagnini D, Gerlach G, Fadda G, Hasnain SE, et al. Specific immunoassays confirm association of Mycobacterium avium subsp. paratuberculosis with type-1 but not type-2 diabetes mellitus. PLoS One 2009; 4(2):e4386 10.1371/journal.pone.0004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjort R, Alfredsson L, Carlsson PO, Groop L, Martinell M, Storm P, et al. Low birthweight is associated with an increased risk of LADA and type 2 diabetes: results from a Swedish case-control study. Diabetologia 2015; 58(11):2525–32. 10.1007/s00125-015-3711-8 [DOI] [PubMed] [Google Scholar]
- 23.Maioli M, Pes GM, Delitala G, Puddu L, Falorni A, Tolu F, et al. Number of autoantibodies and HLA genotype, more than high titers of glutamic acid decarboxylase autoantibodies, predict insulin dependence in latent autoimmune diabetes of adults. Eur J Endocrinol 2010; 163:541–9. 10.1530/EJE-10-0427 [DOI] [PubMed] [Google Scholar]
- 24.Masala S, Cossu D, Piccinini S, Rapini N, Mameli G, Manca Bitti ML, et al. Proinsulin and MAP3865c homologous epitopes are a target of antibody response in new-onset type 1 diabetes children from continental Italy. Pediatr Diabetes 2015; 16(3):189–95. 10.1111/pedi.12269 [DOI] [PubMed] [Google Scholar]
- 25.Sardu C, Cocco E, Mereu A, Massa R, Cuccu A, Marrosu MG, et al. Population based study of 12 autoimmune diseases in Sardinia, Italy: prevalence and comorbidity. PLoS One 2012; 7(3):e32487 10.1371/journal.pone.0032487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niegowska M, Paccagnini D, Burrai C, Palermo M, Sechi LA. Antibodies against Proinsulin and Homologous MAP Epitopes Are Detectable in Hashimoto's Thyroiditis Sardinian Patients, an Additional Link of Association. PLoS One 2015; 10(7):e0133497 10.1371/journal.pone.0133497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masala S, Cossu D, Palermo M, Sechi LA. Recognition of Zinc Transporter 8 and MAP3865c Homologous Epitopes by Hashimoto's Thyroiditis Subjects from Sardinia: A Common Target with Type 1 Diabetes? PLoS One 2014; 15; 9(5):e97621 10.1371/journal.pone.0097621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masala S, Cossu D, Palermo M, Sechi LA. Antibodies recognizing Mycobacterium avium paratuberculosis epitopes cross-react with the beta-cell antigen ZnT8 in Sardinian type 1 diabetic patients. PLoS One 2011; 6(10):e26931 10.1371/journal.pone.0026931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masala S, Cossu D, Piccinini S, Rapini N, Massimi A, Porzio O, et al. Recognition of zinc transporter 8 and MAP3865c homologous epitopes by new-onset type 1 diabetes children from continental Italy. Acta Diabetol 2014; 51(4):577–85. 10.1007/s00592-014-0558-2 [DOI] [PubMed] [Google Scholar]
- 30.Masala S, Zedda MA, Cossu D, Ripoli C, Palermo M, Sechi LA. Zinc transporter 8 and MAP3865c homologous epitopes are recognized at T1D onset in Sardinian children. PLoS One 2013; 8(5):e63371 10.1371/journal.pone.0063371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel D, Danelishvili L, Yamazaki Y, Alonso M, Paustian ML, Bannantine JP, et al. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect Immun 2006; 74(5):2849–55. 10.1128/IAI.74.5.2849-2855.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hermon-Tylor J. Mycobacterium avium subspecies paratuberculosis, Crohn's disease and the Doomsday scenario. Gut Pathol 2009; 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zampetti S, Spoletini M, Petrone A, Capizzi M, Arpi ML, Tiberti C, et al. Association of TCF7L2 gene variants with low GAD autoantibody titer in LADA subjects (NIRAD Study 5). Diabet Med 2010; 27(6):701–4. 10.1111/j.1464-5491.2010.02997.x [DOI] [PubMed] [Google Scholar]
- 34.Savic D, Ye H, Aneas I, Park SY, Bell GI, Nobrega MA. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res 2011; 21(9): 1417–1425. 10.1101/gr.123745.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettersen E, Skorpen F, Kvaløy K, Midthjell K, Grill V. Genetic Heterogeneity in Latent Autoimmune Diabetes Is Linked to Various Degrees of Autoimmune Activity. Diabetes 2010; 59(1): 302–310. 10.2337/db09-0923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barros CM, Araujo-Neto AP, Lopes TR, Barros MA, Motta FJ, Canalle R, et al. Association of the rs7903146 and rs12255372 polymorphisms in the TCF7L2 gene with type 2 diabetes in a population from northeastern Brazil. Genet Mol Res 2014; 13(3):7889–98. 10.4238/2014.September.29.1 [DOI] [PubMed] [Google Scholar]
- 37.Shin MK, Shin SW, Jung M, Park H, Park HE, Yoo HS. Host gene expression for Mycobacterium avium subsp. paratuberculosis infection in human THP-1 macrophages. Pathog Dis 2015; 15; 73(5). [DOI] [PubMed] [Google Scholar]
- 38.Di Gaetano C, Fiorito G, Ortu MF, Rosa F, Guarrera S, Pardini B, et al. Sardinians Genetic Background Explained by Runs of Homozygosity and Genomic Regions under Positive Selection. PLoS One 2014; 9(3):e91237 10.1371/journal.pone.0091237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber P, Ambrosova P, Canov P, Weberova D, Kuklinek P, Meluzinova H, et al. GAD antibodies in T1D and LADA—relations to age, BMI, c-peptide, IA-2 and HLA-DRB1*03 and DRB1*04 alleles. Adv Gerontol 2011; 24(2):312–8. [PubMed] [Google Scholar]
- 40.Delitala AP, Pes GM, Fanciulli G, Maioli M, Secchi G, Sanciu F, et al. Organ-specific antibodies in LADA patients for the prediction of insulin dependence. Endocr Res 2016; 11:1–6. [DOI] [PubMed] [Google Scholar]
- 41.Zampetti S, Capizzi M, Spoletini M, Campagna G, Leto G, Cipolloni L, et al. GADA titer-related risk for organ-specific autoimmunity in LADA subjects subdivided according to gender (NIRAD study 6). J Clin Endocrinol Metab 2012; 97(10):3759–65. 10.1210/jc.2012-2037 [DOI] [PubMed] [Google Scholar]
- 42.Gale EA, Gillespie KM. Diabetes and gender. Diabetologia 2001; 44(1):3.–. 10.1007/s001250051573 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from DOI:10.17605/OSF.IO/63CN8.