Abstract
We show that androgens, testosterone and 5α-dihydrotestosterone (DHT), acutely (∼40 min) provoke the mechanical potentiation of spontaneous and agonist-induced contractile activity in mouse colonic longitudinal smooth muscle. The results using flutamide, finasteride, cycloheximide, and actinomycin D indicate that androgen-induced potentiation is dependent on androgen receptors, requires reduction of testosterone to DHT, and occurs independently of transcriptional and translational events. Using permeabilized colonic smooth muscle preparations, we could demonstrate that mechanical potentiation is entirely due to calcium sensitization of contractile machinery. In addition, DHT (10 nm) increased phosphorylation of both 20-kDa myosin light chain (LC20) [regulatory myosin light chain, (MLC)] and CPI-17 (an endogenous inhibitor of MLC phosphatase). Paralleling these findings, inhibition of Rho-associated Rho kinase (ROK) and/or protein kinase C (PKC) with, respectively, Y27632 and chelerythrine, prevented LC20 phosphorylation and abolished calcium sensitization. In addition, inhibition of ROK prevents CPI-17 phosphorylation, indicating that ROK is located upstream PKC-mediated CPI-17 modulation in the signalling cascade. Additionally, androgens induce a rapid activation of RhoA and its translocation to the plasma membrane to activate ROK. The results demonstrate that androgens induce sensitization of colonic smooth muscle to calcium through activation of ROK, which in turn, activates PKC to induce CPI-17 phosphorylation. Activation of this pathway induces a potent steady stimulation of LC20 by inhibiting MLC phosphatase and displacing the equilibrium of the regulatory subunit towards its phosphorylated state. This is the first demonstration that colonic smooth muscle is a physiological target for androgen hormones, and that androgens modulate force generation of smooth muscle contractile machinery through nongenomic calcium sensitization pathways.
Androgens are powerful physiological modulators of intestinal contractile activity. This effect is mediated by non-genomic induction of calcium sensitization linked to regulatory myosin-light chain phosphorylation.
Compelling evidence accumulated over the last two decades have demonstrated that, apart from being genotropic inducers of cell differentiation and sexual development, androgens may exert a number of nongenomic effects in target and nontarget tissues. Such nongenomic effects are characterized by a time frame not sufficiently long to allow gene transcription/translation and are often initiated at the plasma membrane by interaction with membrane embedded receptors or binding proteins even in cells lacking androgen receptors (ARs) (reviewed in Refs. 1 and 2). Nongenomic actions of androgens have been described in a variety of cell types and tissues, including osteoblasts (3), macrophages (4), T cells (5), kidney cells (6), granulosa lutenizing cells (7), Sertoli cells (8), and skeletal muscle (9), among others. However, the molecular mechanisms by which androgens and their receptors transduce cellular signals to provoke these nongenomic effects are considerably diverse and only partially understood. Thus, androgens can activate tyrosine kinase c-Src through interaction of AR with the SH3 domain of c-Src (10, 11), MAPK signaling cascade via the Ras pathway (10, 11, 12), phosphatidylinositol-3 kinase/Akt pathway in a ligand-binding independent manner (13), cAMP/protein kinase A by interaction with steroid hormone binding globulin receptor (14) and phospholipase C/inositol 1,4,5 triphosphate (IP3) pathway via activation of a pertussis toxin-sensitive G protein-coupled membrane AR (9). Rapid actions of androgens may also occur due to direct modulation of ion-channels, namely voltage-gated L-type Ca2+-channels (15, 16), large-conductance Ca2+- and voltage-activated K+-channels (17), and ATP-sensitive K+-channels (18), as well as by modulation of plasmalemmal active transporters like Ca2+-ATPase (19).
Perhaps the most common and best studied acute action of androgens is the modulation of calcium homeostasis. Indeed, androgens are able to modify the intracellular Ca2+ levels within seconds to minutes in a number of cell systems, including muscle cells like cardiomyocytes (20), skeletal muscle cells (9), and vascular smooth muscle cells (15, 21). Alteration of calcium homeostasis in muscle cells in response to androgens may occur secondarily to modulation of either calcium release from IP3-sensitive stores, altered calcium entry across the plasma membrane, or less frequently, may the changes in [Ca2+]i be a consequence of modulated interplay between both calcium sources (reviewed in Ref. 2).
In vascular smooth muscle from aorta, coronary, and cerebral arteries, androgens reduce vascular tone and induce rapid vasodilating effects both in vivo and in vitro partly by negative modulation of voltage-gated calcium channels (21, 22, 23, 24). However, a major concern on the physiological relevance of acute effects of androgens reported to date is that, in most cases, conclusions were drawn based on experiments conducted at unphysiological androgen levels (>1 μm) and only few studies were performed within a physiological range (10–25 nm). On the other hand, though androgen (and estrogens) acute effects on vasculature have been extensively studied, it is noteworthy that only few studies have been performed on smooth muscle from other origins like visceral smooth muscle, especially from the gastrointestinal wall. This is striking because gastrointestinal motility is governed by complex physiological mechanisms involving pathways known to be rapidly modulated by androgens (25). In this sense, only two recent reports from our laboratory have pointed out that androgens may affect acutely the contractile activity of mouse ileum (26, 27). In addition, other recent work from our group has provided evidence that natural and synthetic estrogens, as well as triphenylethylene antiestrogens, cause a fast reversible relaxation of smooth muscle tone from proximal intestine mainly by inhibiting L-type calcium channels, therefore interfering with the generation of oscillatory electric rhythm characteristic of peristaltic activity (28, 29, 30). This suggests that gastrointestinal muscles might be physiological targets for sex hormones. Nonetheless, whether androgens could be physiological modulators of distal intestine motility is a question that remains largely unexplored. Thus, the aim of the present study was to explore the potential modulation of colonic contractile activity by androgens. Our results demonstrate, for the first time, that physiological concentrations of androgens induce a potent stimulatory effect of colonic muscle mechanical activity. This potentiation effect is dependent on the presence of AR and takes place on a nongenomic basis through identified molecular pathways leading to calcium sensitization. The physiological relevance of these findings is also discussed.
Results
Androgens stimulate colonic smooth muscle contractile activity
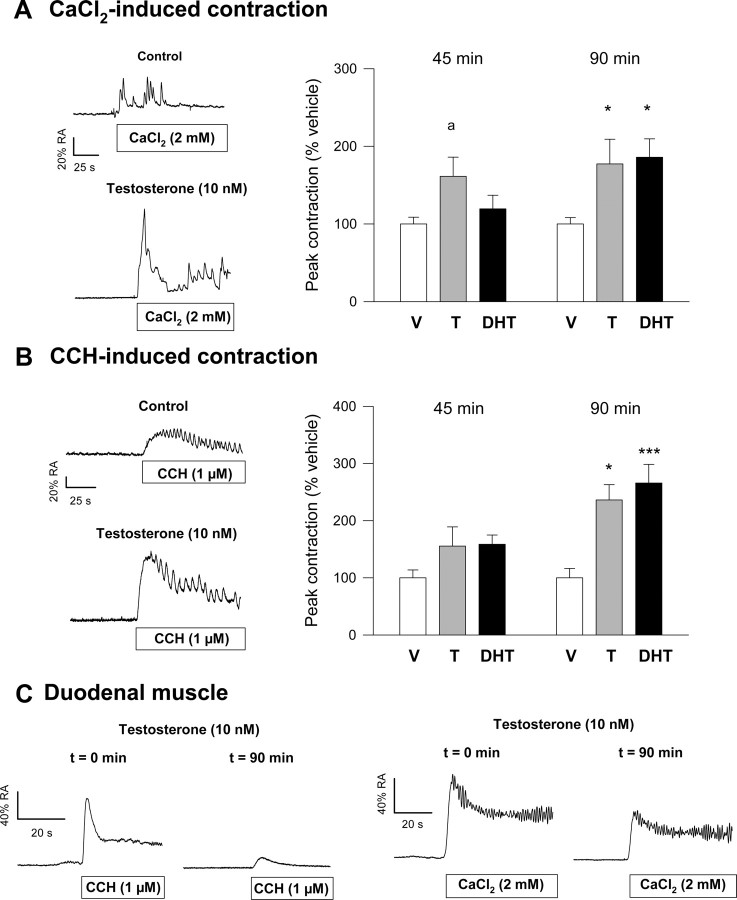
Under control resting conditions, colonic longitudinal muscle responds to both application of CaCl2 (2.0 mm) and the muscarinic agonist carbachol (CCH) (1 μm). Results illustrated in Fig. 1 show that contractile responses were consistent with a rapid increase of isometric tension followed by a sustained phase often featured by a peristaltic pattern. Preincubation of colonic muscle with physiological concentrations of testosterone (T) (10 nm) or dihydrotestosterone (DHT) (10 nm) for 45 or 90 min brought about a considerable increase of the contractile responses to both CaCl2 and CCH (Fig. 1, A and B, left panels). The increase in isometric tension was dependent on the time of preincubation and maximally achieved (average 216% for both stimuli) around 90 min after application of either androgen (Fig. 1, A and B, right panels). The stimulatory effect of androgens was specific for colonic muscle and was not observed in duodenal tissues (Fig. 1C). Androgen-induced effects on colonic tissues could also be observed on records of spontaneous activity, where exposure to the steroids caused both, an increase in isometric peak-to-peak amplitude (average 249%) and in the pattern of peristaltic activity compared with the control [dimethylsulfoxide (DMSO)-treated] period (Fig. 2A).
Fig. 1.
Effects of androgens on contractile responses of colonic and duodenal longitudinal smooth muscles. A and B, Left panels, Representative recordings of the effects of T on contractile responses elicited by CaCl2 (A) and CCH (B). Tissues were exposed to T (10 nm) for 90 min. The contractile responses to CaCl2 and CCH were recorded at the beginning of the experiment (Control) and 90 min after exposure to the hormone. External stimuli were applied after a 5-min period in which tissues were maintained under calcium-free conditions. Right panels, Summary of the effects of preincubation with T (10 nm) and DHT (10 nm) on CaCl2-induced (A) and CCH-induced (B) contractions measured at 45 and 90 min. C, Effects of preincubation of duodenal smooth muscle with T (10 nm) on CaCl2- and CCH-induced contractions. Results in right panels of A and B represent the mean ± sem of 15 different experiments. Statistical significance was assessed by one-way ANOVA, followed by Student’s t test. a, P < 0.1; *, P < 0.05; and ***, P < 0.005 compared with vehicle (V), respectively.
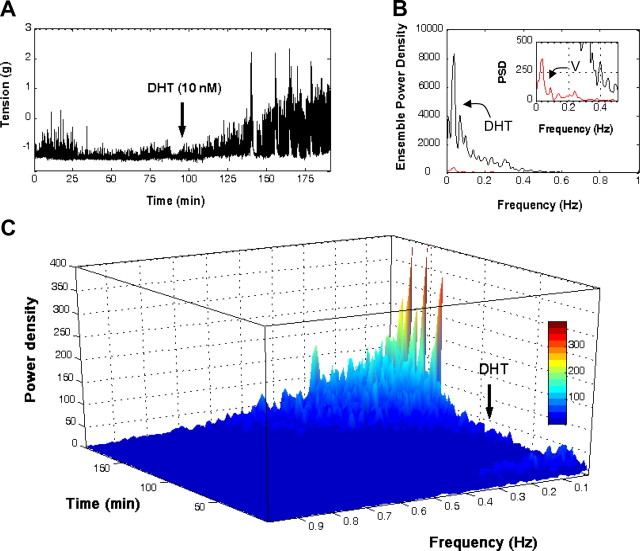
Fig. 2.
Androgens alter the frequency spectrum of colonic peristaltic activity. A, Representative recording showing the time course of spontaneous activity in the presence of vehicle DMSO (0.1%) and after application of DHT (10 nm). B, Frequency analyses using the FFT obtained 90 min after incubation with vehicle (red trace) or DHT (blue trace). PSD, Power spectral density. C, Application of the STFT to the contractile signal shown in A. Results are representative of another five different experiments. Color scale (gray) encodes for spectral densities. For details, see Materials and Methods and Results.
To quantitatively assess the effect of androgens on the pattern of colonic peristaltic activity, we applied Fourier spectral methods to long-lasting continuous records of spontaneous activity (31). Analyses were performed at different times over continuous 180-min records sampled at 20 Hz, using consecutive signal windows (2048 data/window) taken 1 h after exposure to either DMSO or DHT. The outcomes of these analyses using the Fast Fourier Transform (FFT) revealed that, paralleling the increase in contractile activity, DHT dramatically altered the frequency spectrum of spontaneous peristaltic activity. First, DHT considerably increased the energy of the dominant frequency component observed in control tissues, which was in the range 0.039–0.048 Hz, corresponding to 2.34–2.88 cpm (Fig. 2B). Second, DHT altered the power density spectrum of spontaneous activity by extending the range of significant harmonic components toward higher frequencies up to 0.1269 Hz (7.61 cpm). Often, these changes were accompanied by the generation of a new low frequency high-energy component in the range 0.0097–0.0195 Hz. Because there appear to exist nonstationary events within the spontaneous contractile signals, we have also applied the Short-Time Fourier Transform (STFT) to obtain the spectrotemporal representation of mechanical signals. An illustrative example of these analyses is shown in Fig. 2C. Here, application of the STFT algorithm to whole records of spontaneous activity revealed that the low frequency high-energy component was generated approximately 49 min after exposure to DHT, whereas androgen-induced higher frequency components appeared earlier and gradually spread toward higher frequencies after 39-min stimulation (Fig. 2C).
Androgen-induced stimulation of colonic smooth muscle contractility is dependent on ARs but nongenomically mediated
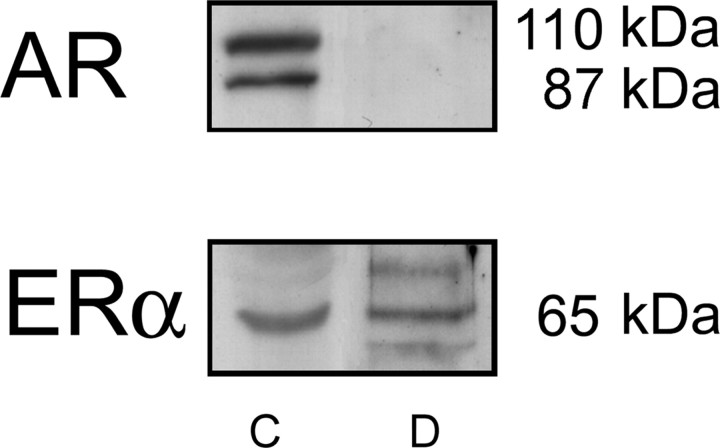
The fact that T and DHT treatments potentiated mouse colonic muscle contractile activity led us to test for the presence of ARs in colonic tissues. Western blotting using a specific polyclonal antibody directed to AR (Fig. 3) revealed a strong band at the expected molecular mass of the canonical AR (∼110 kDa), as well as an additional band at about 87 kDa, likely corresponding to a N terminally truncated AR form of this protein (32). In contrast, no AR expression was observed in duodenal protein extracts. Membranes were then reprobbed with a specific polyclonal rabbit antibody directed to estrogen receptor α (ERα), which recognized a specific ERα protein in both tissues, therefore confirming our previous data in duodenum (29).
Fig. 3.
Analysis of AR expression in colon and duodenum. Total protein extracts from colonic (C) and duodenal (D) smooth muscle were electrophoresed on SDS-PAGE and processed for Western blot analysis, using a specific polyclonal antibody to AR. As a control of protein load, membranes were reblotted with an antibody directed to ERα. Molecular messes of the different specific bands recognized by the antibodies are indicated. Results are representative of five experiments.
The participation of ARs on the potentiation of colonic contractile activity by androgens was pharmacologically demonstrated using the nonsteroidal antiandrogen flutamide (FLU). FLU (10 μm) was allowed to preincubate for 60 min before the application of DHT. The results showed that FLU completely prevented androgen-induced potentiation of both CaCl2- and CCH-induced contractions on intact colonic preparations (Table 1), therefore demonstrating that AR activation is an absolute requirement for the stimulatory mechanism. To assess the potential involvement of cell membrane in the response, we used a macromolecular impermeable T conjugate, T-BSA. Our results showed that incubation of colonic muscle with T-BSA over the same time course than T and in a concentration range between 10 and 500 nm failed to reproduce the contractile stimulation induced by androgens of both CaCl2-induced and CCH-induced contractions (Table 2 and Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), suggesting that intracellular AR(s) might be responsible for the mechanical potentiation of colonic longitudinal muscle.
Table 1.
Effects of FLU on contractile responses to CaCl2 and CCH measured at 45 and 90 min after exposure to DHT
| CaCl2-induced contraction | CCH-induced contraction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | (n) | 90 min | (n) | 45 min | (n) | 90 min | (n) | |||||||
| DMSO (0.1%) | 100.00 ± 8.69 | 12 | 100.00 ± 8.10 | 13 | 100.00 ± 13.75 | 12 | 100.00 ± 16.44 | 10 | ||||||
| DHT (10 nm) | 119.39 ± 17.53 | 13 | 185.89 ± 23.781 | 9 | 158.66 ± 16.27 | 13 | 265.82 ± 32.762 | 19 | ||||||
| FLU (10 μm) + DHT (10 nm) | 100.99 ± 8.05 | 8 | 80.93 ± 15.143 | 8 | 131.14 ± 16.65 | 8 | 102.17 ± 20.703 | 7 | ||||||
Data are expressed as mean ± sem.
P < 0.05.
P < 0.005 vs. vehicle (DMSO).
P < 0.05 vs. DHT.
Table 2.
Effects of T-BSA conjugate on contractile responses to CaCl2 and CCH
| CaCl2-induced contraction | CCH-induced contraction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | (n) | 90 min | (n) | 45 min | (n) | 90 min | (n) | |||||||
| BSA (4 nm) | 100.00 ± 9.49 | 4 | 100.00 ± 10.41 | 4 | 100.00 ± 14.95 | 4 | 100.00 ± 13.69 | 4 | ||||||
| T-BSA (100 nm) | 95.84 ± 10.21 | 4 | 122.52 ± 9.69 | 4 | 90.21 ± 10.96 | 4 | 110.84 ± 17.50 | 4 | ||||||
Data are expressed as mean ± sem.
To explore the involvement of transcriptional and/or translational processes in the effect of androgens, we have assayed the effect of a cycloheximide (10 μm) plus actinomycin D (10 μm) mixture on CaCl2- and CCH-induced contractions (Table 3). The results showed that preincubation with the inhibitory cocktail for 45 min before application of vehicle (DMSO) or DHT failed to prevent the potentiation of contractile responses to agonists induced by androgens, demonstrating the nongenomic nature of this effect.
Table 3.
Effects of cycloheximide (CHX) plus actinomycin D (ACTD) on contractile responses to CaCl2 and CCH measured at 45 and 90 min after exposure to T
| CaCl2-induced contraction | CCH-induced contraction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | (n) | 90 min | (n) | 45 min | (n) | 90 min | (n) | |||||||
| Vehicle | 100.00 ± 8.70 | 12 | 100.00 ± 8.10 | 13 | 100.00 ± 13.75 | 12 | 100.00 ± 16.48 | 10 | ||||||
| T (10 nm) | 161.29 ± 24.701 | 11 | 177.26 ± 31.832 | 6 | 155.54 ± 33.69 | 11 | 236.26 ± 26.794 | 10 | ||||||
| CHX (10 μm) + ACTD (10 μm) + T (10 nm) | 157.05 ± 25.10 | 6 | 171.05 ± 22.043 | 5 | 156.25 ± 27.30 | 6 | 180.28 ± 39.182 | 5 | ||||||
Data are expressed as mean ± sem. Vehicle was DMSO (0.1%).
P < 0.1.
P < 0.05.
P < 0.01.
P < 0.005 vs. vehicle.
Reduction of T to DHT is required to initiate the stimulatory effect of androgens
Given that DHT is, at least, as effective as T in stimulating colonic mechanical activity, we addressed the question of whether T has to be converted to DHT. In many androgen-responsive tissues, the reduction of T to the more potent androgen DHT is catalyzed by a membrane-bound nicotinamide adenine dinucleotide phosphate-dependent enzyme, the steroid 5α-reductase (EC 1.3.99.5). In human and rodent tissues, steroid 5α-reductase isozymes can be irreversibly inhibited by the 4-azasteroid finasteride (FIN) (33). Thus, we assayed the effects of this pharmacological tool by preincubation (for 30 min) of colonic muscle strips with FIN (10 μm) before the application of T (10 nm). We observed that potentiation of both CaCl2- and CCH-induced contractions by T were markedly inhibited by the steroidal antagonist at all times recorded (Table 4), indicating that reduction of T to the physiological metabolite DHT was a necessary requisite to trigger the stimulatory effect of androgens on colonic muscle.
Table 4.
Effects of FIN on contractile responses to CaCl2 and CCH measured at 45 and 90 min after exposure to T
| CaCl2-induced contraction | CCH-induced contraction | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 45 min | (n) | 90 min | (n) | 45 min | (n) | 90 min | (n) | |||||||
| Vehicle | 100.00 ± 9.31 | 8 | 100.00 ± 7.21 | 9 | 100.00 ± 15.29 | 10 | 100.00 ± 14.20 | 8 | ||||||
| FIN (10 μm) | 93.43 ± 16.08 | 6 | 90.09 ± 16.271 | 6 | 121.17 ± 19.73 | 6 | 142.21 ± 8.351 | 5 | ||||||
| T (10 nm) | 200.41 ± 32.31 | 6 | 181.54 ± 32.592 | 6 | 186.70 ± 56.17 | 6 | 227.84 ± 20.183 | 6 | ||||||
| FIN (10 μm) + T (10 nm) | 101.31 ± 9.53 | 6 | 92.75 ± 8.831 | 6 | 100.54 ± 14.62 | 6 | 144.73 ± 22.301 | 6 | ||||||
Data are expressed as mean ± sem. Vehicle was DMSO (0.1%).
P < 0.05 vs. testosterone.
P < 0.05.
P < 0.005 vs. vehicle.
Calcium sensitization is involved in androgen-induced mechanical potentiation
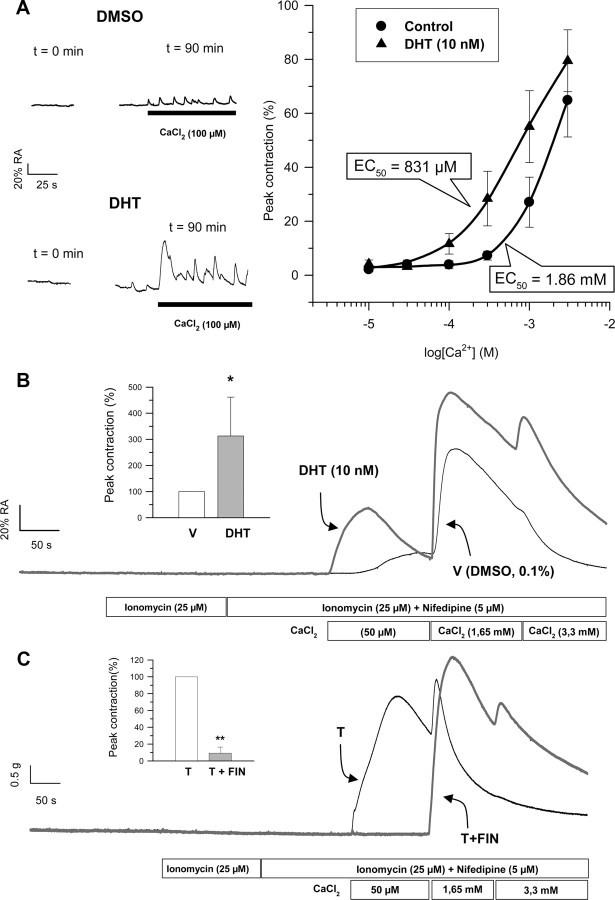
Calcium dependence responses of intact colonic muscle in the presence of DHT (10 nm) is shown in Fig. 4A. The analyses revealed a displacement of EC50 values from 1.86 mm in control tissues to 0.83 mm in the presence of DHT. In fact, at subthreshold external calcium concentrations, i.e. 100 μm, the presence of DHT induced significant phasic and peristaltic responses to CaCl2 that were undetectable in control tissues (Fig. 4A, left panel), indicating that androgens have changed calcium dependence of contractile responses. The fact that androgens stimulated contractile responses to stimuli involving different calcium sources (i.e. extracellular medium via L-type calcium channels and sarcoplasmic reticulum through activation of IP3 receptor) led us to consider the possibility that a phenomenon of calcium sensitization might underlie the stimulatory effect of androgens. To explore this hypothesis, we used a protocol of calcium permeabilization of colonic muscle by using ionomycin (25 μm) in the presence of the calcium-channel blocker nifedipine (5 μm) as described in Ref. 26 . Under these conditions, we have observed that, unlike DMSO-treated tissues, where virtually no change in isometric tension was observed in response to the clamped subthreshold calcium pulse (50 μm), colonic tissues preexposed to DHT (10 nm, 90 min) developed a significant increase in phasic contractile force (Fig. 4B). Considering that under these conditions, L-type calcium channels were blocked by the presence of the dihidropyridine nifedipine, changes in tension are entirely due to calcium influx through ionomycin pores. These results strongly indicate that androgens have induced mechanisms of Ca2+ sensitization in colonic smooth muscle. In addition, the contractile response of permeabilized colonic muscle to calcium (50 μm) was absolutely dependent on the reduction of T to DHT, as demonstrated by the complete blockade of the contractile response by 30-min preincubation with 10 μm FIN (Fig. 4C).
Fig. 4.
Androgens induce Ca2+ sensitization in colonic smooth muscle. A, Calcium dependence curves for control and DHT (10 nm) measured as peak responses to CaCl2 in longitudinal colonic muscle. Tissues were allowed to preincubate with control or DHT for 90 min before application of the corresponding calcium pulse. Traces on the left panel illustrate typical responses to calcium (100 μm) at time 0 and after 90 min preincubation with DHT. B, Typical responses of ionomycin-permeabilized colonic longitudinal muscle to extracellular calcium (50 μm, 1.6 mm, and 3.3 mm) preincubated with vehicle (V) (DMSO, 0.1%, 90 min; trace in black) or DHT (10 nm, 90 min; trace in dark gray). Inset, A summary of the results from another seven experiments. *, P < 0.05 compared with V, respectively. C, Effects of FIN on DHT-induced calcium sensitization. Tissues were allow to preincubated with FIN (10 μm) for 30 min or DMSO (0.1%) before incubation with DHT (10 nm) and then submitted to the permeabilization maneuver and calcium challenges at the concentrations indicated. Inset, A summary of the results from another four experiments. **, P < 0.01 compared with DHT. Recordings (control and experimental) in B and C were obtained in smooth muscle preparations from the same animals.
Several laboratories have shown that Ca2+ sensitization in smooth muscle in response to neurohumoral agonists or GTPγS is molecularly linked to changes in the phosphorylation state of the regulatory subunit of 20-kDa myosin light chain (LC20) (34, 35, 36, 37, 38). Therefore, we have monitored the phosphorylation levels of LC20 by using antiphospho-LC20 specific antibody that recognizes the phosphorylated Ser19 of the regulatory light chain. Longitudinal strips of colonic tissues from the same animals were preexposed to either DMSO or DHT (10 nm) and submitted to Western blot analyses. The results showed that DHT induced a time-dependent increase in colonic LC20 phospho-Ser19 vs. total LC20, as compared with control tissues (Fig. 5A), which paralleled the gradual development of mechanical potentiation. The effect of DHT was specific for colonic muscle and was not observed in duodenal tissues (Fig. 5B), paralleling our previous findings (26).
Fig. 5.
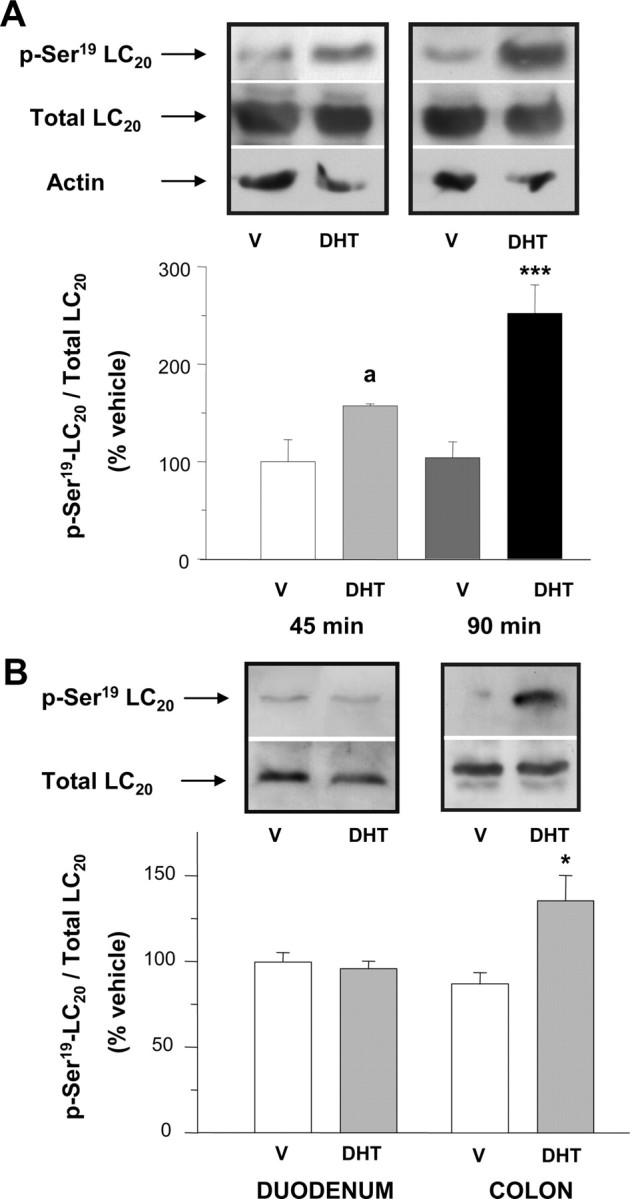
Androgens induce the phosphorylation of LC20 in colonic muscle. A, Western blot analyses for total and phosphorylated-Ser19 LC20 (p-Ser19-LC20) in colonic muscle extracts. In each experiment, equivalent colonic segments obtained from the same animal were exposed for 45 or 90 min to either vehicle (V) (DMSO, 0.1%) or DHT (10 nm). Top, Representative assay after immunoblotting with either antiphospho-LC20 or total LC20 antibodies. α-Actin was used as a control of protein load. Bottom, Densitometric values of phospho-LC20 relative to total LC20. Values were normalized to the average of immunosignals obtained with V-treated tissues (V). a and ***, P < 0.1 and P < 0.005 compared with V, respectively. B, Comparison of the effects of preincubation with V (DMSO, 0.1%; 90 min) or DHT (10 nm, 90 min) on the levels of total LC20 and p-Ser19-LC20 between colonic and duodenal segments. Colonic and duodenal segments were obtained from the same animals. Top, Representative assays after immunoblotting with either antiphospho-LC20 or total LC20 antibodies. Bottom, Densitometric values of phospho-LC20 relative to total LC20 and normalized to average immunosignals obtained with V. *, P < 0.05 vs. V-treated tissues. Four assays were performed for each type of experiment and intestinal segment in A and B.
Deciphering the mechanism of calcium sensitization
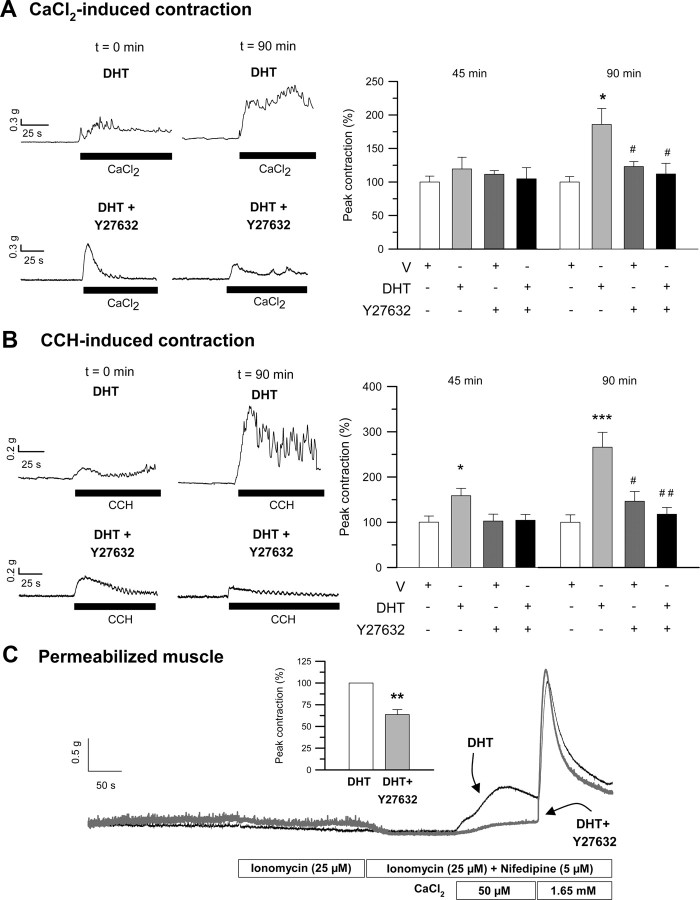
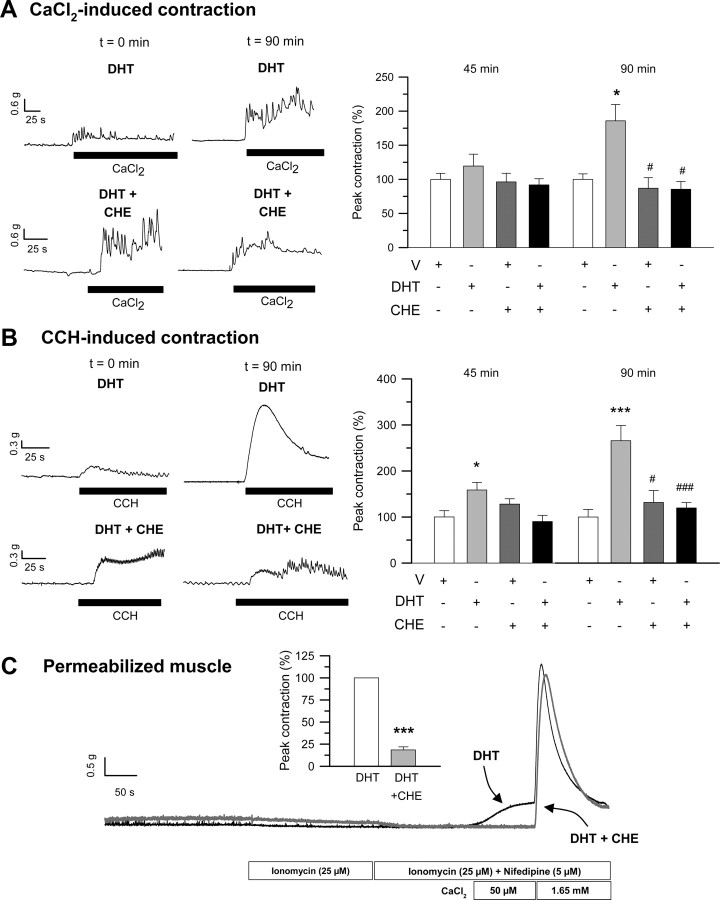
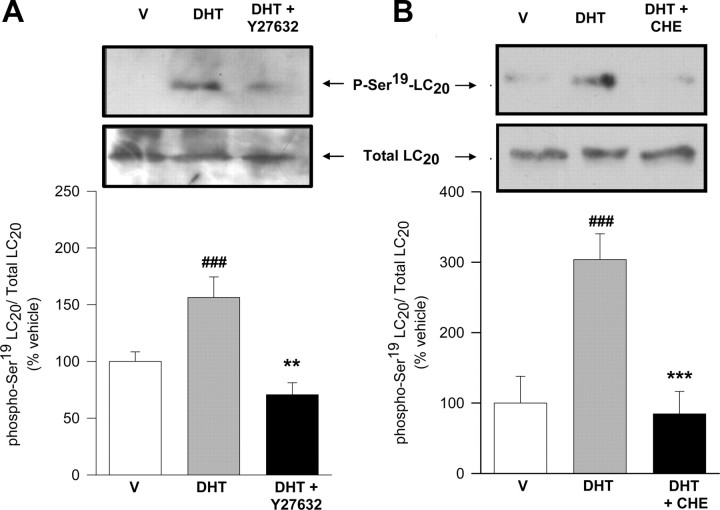
Several intracellular mechanisms have been postulated to underlie calcium sensitization and LC20 phosphorylation in different types of smooth muscles, these involving activation of protein kinase C (PKC) and/or Rho-kinase pathways (38, 39, 40, 41). We have assessed the participation of either Ca2+-sensitizing pathways in the stimulation of colonic contractile activity by androgens. First, we used a pharmacological approach by assessing the effects of preincubation with the general PKC inhibitor chelerythrine (CHE) (1–10 μm) and the specific Rho-associated Rho kinase (ROK) antagonist Y27632 (10 μm) on intact and permeabilized longitudinal smooth muscle preparations. The results shown in Figs. 6, A and B, and 7, A and B, demonstrate that inhibition of both PKC and ROK abolished DHT-induced potentiation of CaCl2- and CCH-triggered contractions. Using permeabilized preparations, we could also demonstrate that both antagonists effectively prevented DHT-induced calcium sensitization as reflected by the reduction of the amplitude of contractile responses to CaCl2 compared with control DHT-treated tissues (Figs. 6C and 7C). These results strongly suggest that both sensitization pathways are involved in the potentiation of mechanical force induced by DHT in colonic longitudinal muscle. We further explored the phosphorylation state of the regulatory subunit of LC20 in Ser19 in the presence of both inhibitors by Western blot analysis (Fig. 8). The outcomes showed that both Y27632 (Fig. 8, left panel) and CHE (Fig. 8, right panel), used at the same concentrations and incubation times that abolished contractile effects of DHT, dramatically diminished the levels of phospho-Ser19-LC20 vs. total LC20 in the presence of DHT to levels comparable with those of DMSO-treated tissues, providing the molecular demonstration that both pathways are involved in the sensitization mechanism triggered by androgens in colonic muscle.
Fig. 6.
Involvement of RhoA-associated ROK in the induction of mechanical potentiation and calcium sensitization elicited by androgens. A and B, Left panels, Illustrative recordings, taken at time 0 and 90 min, showing the effect of 45-min preincubation with the specific inhibitor of ROK, Y27632 (10 μm), on DHT-induced (10 nm) potentiation of contractile responses to CaCl2 and CCH. Right panels, Summary of the effects of preincubation with Y27632 on DHT-induced stimulation of CaCl2-triggered (A) and CCH-triggered (B) contractions as measured at 45 and 90 min (n = 6). * and ***, P < 0.05 and P < 0.005 compared with vehicle (V), respectively; # and ##, P < 0.05 and P < 0.01 vs. DHT. C, Effects of Y27632 (10 μm) preincubation (45 min) on DHT-induced calcium sensitization in permeabilized colonic muscle. Inset, A summary of the results, expressed as mean ± sem, from another four experiments. In each experiment, equivalent segments of colonic muscle obtained from the same animal were preexposed to either V or Y27632 (10 μm) for 45 min before incubation with DHT (10 nm, 90 min). **, P < 0.01 vs. DHT.
Fig. 7.
Involvement of PKC in the induction of mechanical potentiation and calcium sensitization elicited by androgens. A and B, Left panels, Illustrative recordings, taken at time 0 and 90 min, showing the effect of 20-min preincubation with the generic inhibitor of PKC, CHE (1 μm), on DHT-induced (10 nm) potentiation of contractile responses to CaCl2 and CCH. Right panels, Summary of the effects of preincubation with CHE on DHT-induced stimulation of CaCl2-triggered (A) and CCH-triggered (B) contractions as measured at 45 and 90 min (n = 4). * and ***, P < 0.05 and P < 0.005 compared with vehicle (V), respectively; # and ###, P < 0.05 and P < 0.005 vs. DHT. C, Effects of CHE (1 μm) preincubation (10 min) on DHT-induced calcium sensitization in permeabilized colonic muscle. Inset, A summary of the results, expressed as mean ± sem, from another three experiments. In each experiment, equivalent segments of colonic muscle obtained from the same animal were preexposed to either V or CHE (1 μm) for 45 min before incubation with DHT (10 nm, 90 min). ***, P < 0.005 vs. DHT.
Fig. 8.
ROK and PKC are involved in DHT-induced phosphorylation of LC20 in colonic muscle. Western blot analyses for total LC20 and phosphorylated Ser19 LC20 (p-Ser19-LC20) in muscle extracts. In each experiment, equivalent segments from colon obtained from the same animal were incubated in the presence of vehicle (V) (DMSO, 0.1%), DHT (10 nm), or DHT+Y27632 (left panel) or V (DMSO, 0.1%), DHT (10 nm), or DHT+CHE (right panel). Tissues exposed to Y27632 (10 μm) or CHE (1 μm) were allowed to preincubate for 45 or 20 min, respectively, with the inhibitor before application of DHT for additional 90 min. Total lysates were analyzed for changes in LC20 phosphorylation using antiphospho(Ser19)-LC20 antibody, followed by reblotting with an antitotal-LC20 antibody. Top, Representative assays after immunoblotting with antiphospho-LC20 and total LC20 antibodies. Bottom, Densitometric values of phospho-LC20 relative to total LC20 expressed as percentage of V. ###, P < 0.005 vs. V; ** and ***, P < 0.01 and P < 0.005 vs. DHT-treated tissues. Four assays were performed under each condition.
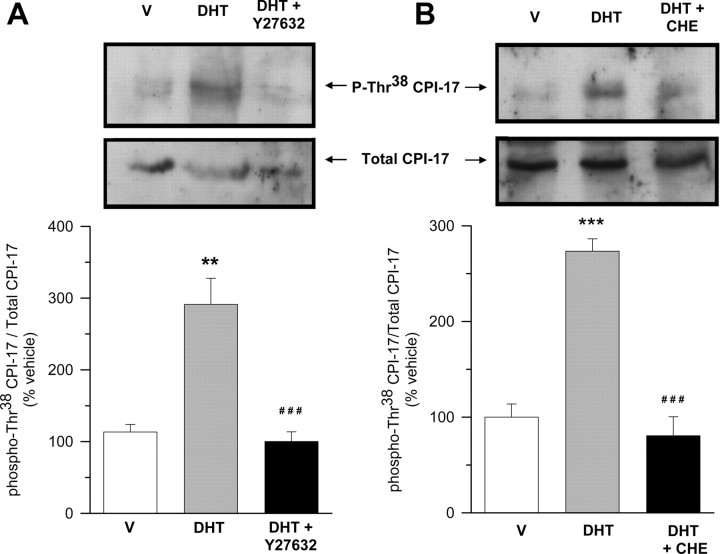
It has been established that PKC-mediated calcium sensitization in smooth muscle preparations from different origins involves phosphorylation of CPI-17, an endogenous inhibitor of myosin light chain phosphatase (MLCP) (42, 43). PKC phosphorylates CPI-17 at Thr38 and activates it to cause inhibition of MLCP through binding to its catalytic subunit (44, 45). Given that PKC inhibition completely prevented androgen-induced mechanical potentiation, calcium sensitization and LC20 phosphorylation, we thought worthwhile to assess the possible inhibition of MLCP in the signaling pathway triggered by androgens in colonic preparations. Longitudinal strips of colonic tissues were preexposed for 90 min to either DMSO or DHT (10 nm) and submitted to Western blot analyses using a specific antibody that recognized the phosphorylated Thr38 of CPI-17. The results shown in Fig. 9 revealed that DHT brought about a significant increase in phospho-Thr38CPI-17 vs. total CPI-17 within the same time course than mechanical stimulation. Moreover, inhibition of PKC with chelerytrine (1 μm) returned CPI-17 phosphorylation levels to vehicle values (Fig. 9, right panel). Surprisingly, the experiments aimed at determining the effects of Y27632 (10 μm) on the phosphorylation status of CPI-17 demonstrated that ROK inhibition abolished the increase in Thr38CPI-17 vs. total CPI-17 induced by DHT, therefore closely mimicking the effect of CHE (Fig. 9, left panel). These results demonstrate that activation of both PKC and ROK pathways are integrated within the same signaling route and that inhibition of either pathway is sufficient to prevent the stimulatory effect of androgens on calcium sensitization and mechanical activity of colonic muscle.
Fig. 9.
ROK and PKC are involved in DHT-induced phosphorylation of CPI-17 in colonic muscle. Western blot analyses for total CPI-17 and phosphorylated Thr38 CPI-17 (p-Thr38-CPI-17) in colonic muscle extracts. In each experiment, equivalent segments from colon obtained from the same animal were incubated in the presence of vehicle (V) (DMSO, 0.1%), DHT (10 nm), or DHT+Y27632 (left panel) or V (DMSO, 0.1%), DHT (10 nm), or DHT+CHE (right panel). Tissues exposed to Y27632 (10 μm) or CHE (1 μm) were allowed to preincubate for 45 or 20 min, respectively, with the inhibitor before application of DHT for additional 90 min. Total lysates were analyzed for changes in CPI-17 phosphorylation using antiphospho(Thr38)-CPI-17 antibody, followed by reblotting with an antitotal-CPI-17 antibody. Top, Representative assays after immunoblotting with antiphospho-CPI-17 and total CPI-17 antibodies. Bottom, Densitometric values of phospho-CPI-17 relative to total CPI-17 expressed as percentage of V. ** and ***, P < 0.01 and P < 0.005 vs. V; ###, P < 0.005 vs. DHT-treated tissues. Four assays were performed under each condition.
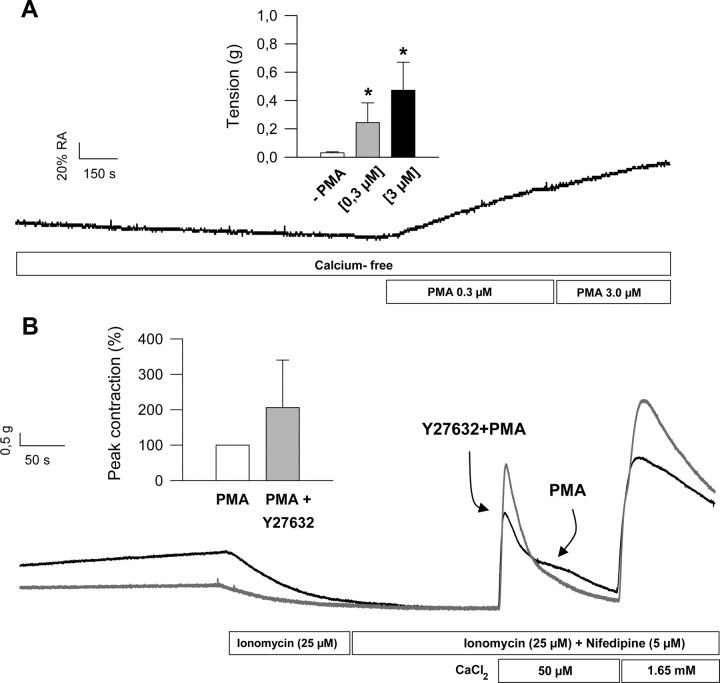
The results also suggest that PKC activation and CPI-17 phosphorylation take place downstream ROK activation in the signaling pathway. The demonstration that ROK and PKC are located in series came from another subset of experiments using the specific PKC activator phorbol 12-myristate 13-acetate (PMA). The initial experiments showed that preexposure of colonic muscle to PMA (0.3–3 μm for 10 min) elicited a significant concentration-dependent increase of muscle tension in the absence of extracellular calcium, indicating that activation of PKC induced calcium sensitization clearly observed at subthreshold intracellular calcium (Fig. 10A). In the second set of experiments, permeabilized colonic longitudinal muscles were exposed to PMA (0.3 μm for 10 min) but in the presence of Y27632 (10 μm for 30 min before addition of PMA). The results illustrated in Fig. 10B shows that PKC activation induced calcium sensitization of colonic muscle to a similar magnitude than androgens, but inhibition of ROK failed to prevent sensitization of contractile machinery.
Fig. 10.
A, PMA induces Ca2+ sensitization in colonic smooth muscle. Illustrative response of colonic longitudinal muscle to extracellular increasing concentrations of PMA (0.3–3 μm) under extracellular calcium-free conditions. Inset, A summary of the results from four different dose-response experiments. *, P < 0.05 vs. vehicle (DMSO, 0.1%). B, Inhibition of ROK does not prevent PMA-induced Ca2+ sensitization in colonic smooth muscle. Illustrative response of ionomycin-permeabilized colonic longitudinal muscle to extracellular calcium pulses. Tissues were preincubated with Y27632 (10 μm, 45 min; black trace) or vehicle (dark gray trace) before being exposed to PMA (0.3 μm, 10 min) and submitted to the permeabilization maneuver and calcium challenges at the concentrations indicated. Inset, A summary of the results from another three experiments. Recordings (PMA and PMA + Y27632) were obtained from colonic smooth muscle preparations from the same animal.
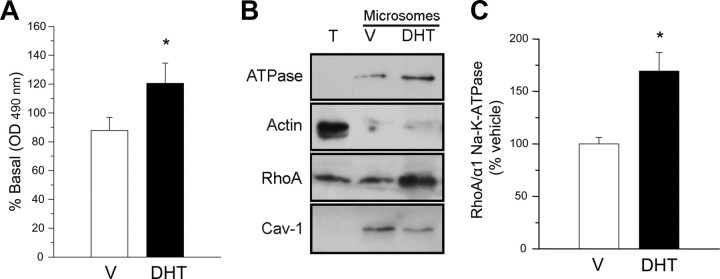
A critical step in the activation of ROK pathway is the activation of the small G protein RhoA and its translocation to the plasma membrane. Our results shown in Fig. 11A demonstrate that androgens activate RhoA by stimulating the exchange of GDP for GTP after 20-min exposure to DHT (10 nm). The effect was transient, and the levels of activated RhoA (GTP·RhoA) in the plasma membrane returned to basal levels after 1-h exposure (data not shown). Furthermore, we could also observe that androgens significantly induce the enrichment of RhoA protein (relative to the levels of plasma membrane markers α1 Na+/K+ ATPase or caveolin-1) in the microsomal fraction of colonic tissues exposed to DHT (10 nm) as compared with control vehicle-treated tissues (Fig. 11, B and C) and within the same time course than RhoA activation, indicating that active RhoA translocates to the plasma membrane upon acute treatment with the androgen.
Fig. 11.
Androgens elicit RhoA activation and translocation to the plasma membrane. A, Determination of activated RhoA (GTP·RhoA) in colonic muscle preparations preexposed to vehicle (V) (DMSO, 0.1%) or DHT (10 nm, 20 min). *, P < 0.05 compared with V. B, Representative assay after immunoblotting with anti-RhoA and plasma membrane markers (α1 Na+/K+-ATPase and caveolin-1) in total lysates (T) and microsomal fractions from colonic muscle. The cytosolic marker α-actin was used as a control of microsomes purity. Colonic tissues were exposed to V (DMSO 0.1%) or DHT (10 nm). C, Densitometric values of RhoA relative to α1 Na+/K+-ATPase expressed as percentage of V. *, P < 0.05 vs. V-treated tissues. Four assays were performed under each condition in all experiments.
Discussion
The data reported in the present study demonstrate for the first time that both T and its active metabolite DHT are potent modulators of colonic contractile activity. Used at physiological concentrations (100 pm to 10 nm), androgens induce the potentiation of mechanical responses triggered by different external stimuli, i.e. CaCl2 and CCH (average 240%), and also stimulate the spontaneous peristaltic activity by increasing the peak-to-peak amplitude of contractile signals and by altering the pattern of peristaltism. The analyses of peristaltic signals in the frequency domain using Fourier analyses techniques revealed that androgens increased the energy of the dominant frequency component characteristic of control tissues, which was in the range 2.34–2.88 cpm. The spectrotemporal representation of colonic peristaltic signals showed that androgen-induced alteration of frequency components takes place approximately 40 min after exposure to androgens and was often accompanied by the generation of a new high energy low-frequency component (0.58–1.17 cpm). These changes indicate that androgens have altered the excitation-contraction coupling in longitudinal colonic muscle (31). Interestingly, none of these effects could be observed in duodenal tissues, indicating that the acute mechanical effects of androgens on smooth muscle are restricted to the more distal parts of the intestine.
The finding that androgens exert a potent stimulatory influence on intestinal contractile activity is clearly in contrast with the effects reported for other sex hormones on visceral smooth muscles, where estrogens induce a fast reversible relaxation of mouse intestinal peristaltic activity (29), as well as inhibition of rat and human uterine muscles (46, 47), rabbit airway muscle (48), and rat genitourinary muscle (49). These observations strongly point to the existence of gender differences in the behavior of visceral smooth muscles to sex hormones and, in particular, in the distal intestine, where estrogens and androgens cause opposite effects (26, 27). It is also remarkable that most effects reported for androgens on different types of smooth muscle, including vascular muscle (21, 24, 50, 51, 52, 53, 54), involve the relaxation of smooth muscle mostly by inhibiting calcium influx through direct interaction of androgen molecules with voltage-dependent L-type calcium channels or by activation of different potassium conductances (16, 17, 21, 23, 53, 54). However, in most cases, these acute effects of androgens can only be observed at supraphysiological doses, which have raised serious concerns about the physiological significance of these pharmacological effects. In contrast, we have found that low physiological concentrations of T or DHT are effective in stimulating intestinal contractile activity, suggesting that colonic muscle might be a target organ for androgens and the presence of high affinity receptors for androgens in colonic smooth muscle cells.
We demonstrate here the presence of two different inmunoreactive bands in mouse colonic muscle; one corresponding to the canonical AR (110 kDa) and another protein of about 87 kDa, likely corresponding to AR-A, a N-terminal truncated form of AR (32). Interestingly, these two proteins are also expressed in the mouse ileum (26), but not in the duodenum, paralleling our present observation that duodenal tissues are irresponsive to androgens. The involvement of such ARs in the mechanical response to androgens was demonstrated pharmacologically by using the nonsteroidal antiandrogen FLU (55), which completely suppressed the stimulatory effects of androgens on the contractile responses to external calcium and CCH, and demonstrating the participation of AR in the initial molecular steps of androgen actions leading to mechanical potentiation. Membrane ARs have been associated with the activation of acute responses to androgens in a number of target and nontarget tissues including liver, prostate cancer cells, macrophages, lymphocytes, breast cancer cells, epididymus, and vascular endothelium (56, 57, 58, 59, 60, 61). However, the AR involved in the mechanical stimulation of colonic longitudinal muscle is likely to be the intracellular canonic receptor because T conjugated to BSA (10–500 nm) failed to mimic the effects of the unconjugated hormone. Nonetheless, the participation of putative membrane ARs can not be totally excluded given that T needs to be converted to DHT in the colonic muscle by the 5α reductase (see below), which might not physically accessible to the impermeable conjugate interacting from the extracellular side of the membrane. Our present results also demonstrated that androgens induce mechanical potentiation through nongenomic pathways. Thus, inhibition of transcriptional and translational processes with actinomycin D plus cycloheximide failed to prevent the effect of androgens. This was an important issue given the delayed time course of the response (functionally detectable after ∼40 min) and the ability of androgens (and T-BSA) to induce the transcriptional activation of early gene expression (62).
We also demonstrate here that T has to be converted into the physiological metabolite DHT in colonic tissues. Indeed, blockade of steroid 5α reductases by preincubation with micromolar concentrations of the 4-azasteroid FIN (33, 63) prevented the mechanical potentiation induced by T, also providing the first evidence that colonic muscle might express some of the 5α reductase isozymes responsible for catalyzing the reduction of T to DHT (64). Accordingly, preliminary data from our laboratory have shown the significant expression of mRNAs coding for 5α reductases types I and III in colonic muscle (data not shown). In line with this, the facts that DHT is not aromatizable and that estrogens cause a relaxing response in colonic muscle rule out the putative aromatase-mediated conversion of T to estradiol in the contractile potentiation induced by androgens in the colon, though aromatase expression have been demonstrated in mouse colonic tissues, in particular at the epithelial lining (65), which could affect smooth muscle via a paracrine route.
The mechanisms by which androgens provoke the mechanical potentiation of colonic muscle appear to involve a phenomenon of calcium sensitization. Over the last two decades, a number of laboratories have demonstrated that androgens are able to modulate the intracellular Ca2+ homeostasis within seconds to minutes in different cell systems (3, 4, 5, 6, 7, 8, 9, 15, 20). The mechanisms of androgen-induced alteration of calcium dynamics vary considerably and depend on the cell type. Thus, in skeletal muscle, T induces fast (<1 min) intracellular Ca2+ oscillations, which begin as Ca2+ transients initiated in the cytosol and propagate as waves of Ca2+ in the cytoplasm and nucleus (9, 66). This complex Ca2+ signal, which depends on an interplay between IP3-sensitive stores and extracellular Ca2+ influx, is unaffected by the AR-antagonist cyproterone acetate and apparently mediated by a pertussis toxin-sensitive G protein-coupled membrane receptor activated by T-BSA (9). Conversely, in A7r5 vascular smooth muscle cells and HEK cells stably transfected with the α1C subunit of the human cardiovascular L-type Ca2+ channel, physiological doses of T reduce calcium influx through selective inhibition of L-type calcium channels without altering Ca2+ mobilization from intracellular stores nor capacitative Ca2+ entry (15, 23). This inhibitory action of T is independent on the presence of ARs but rather is due to a direct molecular interaction of the hormone with the channel pore. Interestingly, a single point mutation (T1007Y) in α1C cardiovascular L-type Ca2+ channel almost abolishes both nifedipine and T sensitivities in A7r5 cells (16).
Our present data show that androgen-induced potentiation of colonic muscle is neither due to changes in conductive calcium influx nor increased calcium release from intracellular stores nor altered frequency of calcium oscillations. In fact, the displacement on the force/[Ca2+] and calcium dependence relationships strongly pinpoint to an increase in the calcium sensitivity of contractile machinery (35, 36, 37). Using ionomycin-permeabilized smooth muscle preparations, we could demonstrate that androgens effectively trigger a phenomenon of calcium sensitization in colonic longitudinal muscle. This increase in mechanical force in response to T was completely prevented by preincubation with FIN, providing the irrefutable evidence that DHT was the androgen molecule responsible for calcium sensitization (Fig. 4C). Calcium sensitization has been observed in response to natural and synthetic neurohumoral agonists like acetylcholine, histamine, ATP, CCH, neurokinin A, and GTPγS in different intestinal muscle preparations, including rat proximal and distal colon (67), canine colon (68), guinea pig ileum (41), and mouse distal ileum (26). In most cases, calcium sensitization has been molecularly associated to changes in the degree of LC20 phosphorylation, in particular at Ser19. Here, we demonstrate that androgens acutely stimulate colonic muscle LC20-Ser19 phosphorylation in a time-dependent manner that is consistent with the development of contractile potentiation and calcium sensitization. To our knowledge, our present data provides the first demonstration that androgens are physiological inducers of calcium sensitization in colonic muscle.
In smooth muscle cells, phosphorylation of LC20 at Ser19 is primarily governed by the activity of the Ca2+-calmodulin dependent MLC kinase but also in a calcium-independent manner by the regulation of MLCP activity (69, 70). Evidence accumulated over the last two decades has led to the notion that agonists inducing calcium sensitization modify MLCP at different levels, from direct phosphorylation of the myosin binding subunit of MLCP to activation of CPI-17 the endogenous inhibitor of the catalytic subunit of MLCP (PP1c) (45, 71, 72, 73, 74). Intracellular mechanisms linking receptor activation to Ca2+ sensitization and MLCP inhibition in smooth muscle cells have been investigated in several laboratories. The results obtained suggest the existence of at least two main pathways. One, the Rho pathway, involves RhoA, a small GTP-binding protein whose activation allows its interaction with ROK, leading to its activation (75, 76). The other is the PKC pathway (42, 43). The activation of PKC activates CPI-17, a smooth muscle-specific protein kinase inhibitor of MLC phosphatase (44). Both protein kinases, ROK and CPI-17, induce Ca2+ sensitization in smooth muscle by inhibiting the activity of MLCP, resulting in an increase in the level of LC20 phosphorylation (77). However, the extent of the contribution of the two pathways in Ca2+ sensitization differs with the type of smooth muscle and animal species.
We have explored the participation of either pathway in the effects of androgens on longitudinal colonic muscle. Using a pharmacological approach, we have observed that inhibition of ROK and PKC with the specific inhibitors Y27632 and CHE, respectively, abolished the stimulatory action of androgens on CaCl2- and CCH-induced contractions. Using ionomycin-permeabilized preparations, we could also demonstrate that androgen-induced calcium sensitization was also hampered by preincubation with these inhibitors. Paralleling these results, inhibition of either PKC or ROK dramatically reduced the levels of phospho-Ser19-LC20 vs. total LC20 in the presence of DHT to levels comparable with those of DMSO-treated tissues, providing the molecular demonstration that both pathways are involved in the sensitization mechanism triggered by androgens in colonic muscle. Notably, the coexistence of both, RhoA and PKC sensitization mechanisms, is in agreement with recent data from rat distal colon, where agonist-induced Ca2+ sensitization involves activation of RhoA and PKC pathways (38), although in the same study, only the RhoA pathway was involved in the sensitization of proximal colon. Interestingly, in β-escin-permeabilized preparations of the guinea-pig ileum, CCH-induced Ca2+ sensitization was abolished by Y27632 and HA 1077 (another inhibitor of ROK), whereas the activator of PKC phorbol 12,13-dibutyrate, failed to induce Ca2+ sensitization (41, 78). Similar results were obtained in the rat (40) and mouse (26) ileum, suggesting that only the RhoA-mediated pathway contributes to Ca2+ sensitization in the more proximal intestinal smooth muscles, i.e. ileal and proximal colon segments. Yet, this is logical in that intestinal smooth muscle is highly specialized in a multitude of ways, and the pathways for calcium sensitization reflects the intricacies involved in smooth muscle function.
Our present data also showed that androgens induce a significant phosphorylation of CPI-17 at Thr38 (∼200% above vehicle values) and that inhibition of PKC with CHE completely revert the increase in the phosphorylation state of CPI-17. Phosphorylation of CPI-17 at Thr38 by PKC converts this protein to a potent inhibitor of the catalytic activity of PP1c but also the MLCP holoenzyme (79, 80) behaving as a pseudosubstrate directly binding to the catalytic center of PP1c (80). Inhibition of MLCP induced by androgens would displace the phosphorylation/dephosphorylation of LC20 toward its Ser19 phosphorylated form eventually triggering the calcium sensitization mechanism. Unexpectedly, inhibition of ROK with Y27632 also prevented PKC-mediated phosphorylation of CPI-17. These observations indicate that CPI-17 is a convergence point at the induction of calcium sensitization in colonic muscle triggered by androgens and probably other neurohumoral agents. The results also point to a topological disposition of both kinases in the signaling pathway. Thus, the fact that inhibition of both ROK and PKC completely hampered the contractile potentiation, calcium sensitization, and LC20 phosphorylation induced by androgens indicate that both pathways have to be located in series and not parallel as it has been demonstrated in rat distal colon (38), but necessarily PKC and its target protein CPI-17 should be located downstream of ROK. One crucial observation demonstrating this topology was that in ionomycin-permeabilized colon muscle, blockade of ROK with Y27632 could be overcome by the phorbol ester PMA, with concomitant induction of calcium sensitization (Fig. 10). The most straightforward explanation for these findings is that ROK is located upstream of PKC in the signaling pathway.
It has been firmly established that the small GTPase RhoA is implicated in the enhancement of calcium sensitivity in different types of smooth muscles by GTP and agonists (reviewed in Refs. 76 and 77). Upon stimulation with different extracellular signals, cytosolic GDP·RhoA, the inactive form of RhoA, is converted to GTP·RhoA through GDP/GTP exchange reaction and targeted to the membrane through its C-terminal geranyl-geranylated tail, rendering RhoA capable to interact with downstream targets, including ROK (reviewed in Refs. 76 and 81). Because androgens activated ROK to cause calcium sensitization and mechanical potentiation of colonic muscle, we tested for the degree of RhoA activation in response to androgens. We observed that DHT caused a time-dependent generation of GTP·RhoA peaking at 20 min after androgen exposure, which agrees with the time course of mechanical potentiation as inferred from Fourier analyses (Fig. 2). Our data also showed that androgens stimulated the translocation of RhoA to the microsomal fraction (Fig. 11), suggesting that androgens modify the intracellular dynamics of RhoA to trigger calcium sensitization of longitudinal smooth muscle. Consistently, the translocation of active RhoA during agonist stimulation has been demonstrated in smooth muscle (82, 83), including colonic smooth muscle cells (84).
Finally, the observations highlighted here might have physiological implications in terms of gender differences in colonic transit times, which seem to be lower in females than in males, especially during the women luteal phase of menstrual cycle (85, 86, 87) and during pregnancy (88). It can be envisaged that chronic low levels of T circulating in male plasma might account for gender differences in relation to colonic transit times due to the calcium sensitization of colonic contractile machinery in men. Also, colorectal motility disorders like chronic constipation due to slow transit, one of the most frequent and difficult to treat subtypes of constipation, are more common in female than in male patients (89), a finding that correlates with up-regulated expression of progesterone receptors in colonic cells (90). Interestingly, recent studies on animal models of chronic inflammatory bowel diseases have shown that secondary to increased proinflamatory cytokines IL-1β and TNF-α, there exist a down-regulation of CPI-17 resulting in decreased smooth muscle contractility (91, 92). Such down-regulation of CPI-17 and intestinal dismotility has also been observed in human patients suffering ulcerative colitis (91).
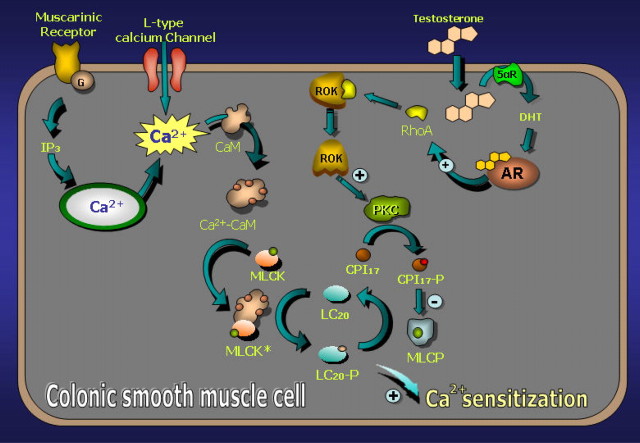
In summary, our data demonstrate that androgens are powerful nongenomic inducers of calcium sensitization and mechanical potentiation of colonic longitudinal muscle. At physiological concentrations, T is converted in the colonic smooth muscle cell to the powerful physiological androgen DHT and activates ARs. Once activated, ARs trigger a cascade of signaling events leading sequential activation of ROK and PKC, which eventually induce the phosphorylation of CPI-17 to inhibit MLCP. Consequently, the phosphorylation state of LC20 is displaced toward its Ser19 phosphorylated form by the prevalence of the MLC kinase over MLCP, and the contractile apparatus develops calcium sensitization. A hypothetical model illustrating the events in the induction of calcium sensitization in colonic smooth muscle cells by androgens is depicted in Fig. 12. From the heuristic point of view, these results provide one of the few physiologically relevant scenarios for the emerging field of nongenomic actions of steroid hormones.
Fig. 12.
Proposed cellular model illustrating the mechanisms of Ca2+ sensitization in mouse colonic muscle induced by androgens. For details see Discussion. CaM, Calmodulin.
Materials and Methods
Animals and tissues
Male Swiss CD1 mice weighting 25–30 g were killed under diethyl ether anesthesia. Distal colon and duodenal segments (1.5 cm long) were removed, cleaned up of mesenteries, and immediately placed in aerated cold physiological salt solution (PSS), containing (in mm): 126 NaCl; 4.5 KCl; 1.0 MgSO4; 2.0 CaCl2; 0.56 NaH2PO4; 1.44 Na2HPO4, adjusted to pH 7.4; and 15.0 glucose. Longitudinal strips of intestinal tissues were mounted in water-jacketed organ baths and incubated in aerated PSS at 37 C. Bath solutions were replaced every 15 min. Tissues were equilibrated at a resting tension value of 0.5 g. The dynamic contractile range (RA%) was recorded for each muscle strip at the beginning of the experiment. RA% was calculated as the difference between maximal contraction produced by 50 mm KCl, and the resting tension measured at zero nominal calcium and expressed as percentage (RA%) or grams. Ca2+-free solutions were made by replacing all CaCl2 from the standard saline solution and supplemented with 25 μm EGTA.
All procedures were performed in accordance with the European Community and Universidad de La Laguna Ethics Committee guidelines for the care of laboratory animals.
Measurement of muscle tension
The isometric tension of isolated colonic strips was measured using an isometric force transducer (TRI110; Letica, Barcelona, Spain) connected to a DC amplifier (Letica). Voltage signals were digitized at a sampling rate 20 Hz using an A/D card (LabPC+; National Instruments, Austin, TX) and stored onto the computer using a data acquisition and analysis program written by one of the authors (M.D.). Data were low-pass filtered at 5 Hz and analyzed using computer routines included in the acquisition software.
Spontaneous peristaltic activity of colonic muscle was recorded in PSS for 90 min (control activity) before exposure to androgens for additional 90 min. T or DHT were dissolved in DMSO and added directly to the organ bath at the final concentration of 10 nm. Muscle contractile activity was recorded throughout the experiments (up to 210 min). Comparison of spontaneous activity between periods was assessed by frequency analysis using Fourier methods (see below).
For the study of CaCl2-induced and CCH-induced contractions, intact colonic preparations were first incubated in Ca+2-free solutions for 5 min and then exposed to either 2.0 mm CaCl2 or 1.0 μm CCH, respectively. The resulting peak contraction was used as a control value for subsequent measurements. Afterwards, the solution was replaced with fresh PSS and left for 5 min to stabilize. Tissues were then exposed to DMSO or androgens (T or DHT) at desired concentration for different times (45 and 90 min). At the end of these periods, bath solutions were replaced with Ca2+-free solutions for 5 min, and the peak contraction elicited by a second application of 2.0 mm CaCl2 or 1.0 μm CCH was measured. Bath solutions were then replaced and washed three times with fresh PSS and left in the presence of vehicle or androgen until next measurement. Antagonists (FLU, FIN, CHE, Y27632, and cycloheximide plus actinomycin D) were allowed to preincubate for 20–60 min (depending on the chemical) before the initial application of androgens and maintained thereafter at the same concentrations in the bath.
Muscle permeabilization
Experiments in calcium-permeabilized smooth muscles were performed after the methodology described previously by our group (26). Colonic strips were incubated in the permeabilization solution (PS) containing: 25 μm ionomycin; 1.6 mm EGTA; 126 mm NaCl; 4.5 mm KCl; 1.2 mm MgCl2; 20 mm Tris-HCl, pH 7.4; and 15 mm glucose. To obtain simultaneous recordings of control and experimental conditions within the same animal, colonic tissues were cut into two halves (∼0.4 cm long), equilibrated at a resting tension of 0.5 g, and incubated for 30 min in aerated PSS. Afterwards, tissues were exposed to appropriate concentrations of either vehicles or androgens for 90 min, washed three times with Ca2+-free solution, and finally incubated in PS. Colonic preparations were incubated for 5 min in PS and exposed to nifedipine (5 μm) for additional 2 min before the application of the first calcium pulse (free extracellular [Ca2+], 50 μm). Afterwards, tissues were then exposed to a calcium pulse of 1.6 mm (occasionally 3.3. mm) to achieve maximal contractions. The RA% was determined for each muscle strip at the beginning of the permeabilization procedure.
Isolation of smooth muscle microsomes
Colonic strips were immersed in liquid nitrogen and then homogenized in lysis buffer (25 mm HEPES; 150 mm NaCl; 10 mm MgCl2; 1 mm EDTA; 87% glycerol; 1 mm Na3VO4; 25 mm NaF; 1× protease inhibitor cocktail; and 0.01% phosphatase inhibitor cocktail, pH 7.5) using a tissue homogenizer and ultracentrifuged at 100,000 × g for 60 min at 4 C. The pellet was resuspended in 100 μl Mg2+ lysis buffer (Upstate, Lake Placid, NY) and centrifuged at 14,000 × g for 10 min at 4 C, and the supernatant, containing the microsomal fraction, was collected and stored frozen until analyses.
Activation of RhoA
For the analyses of RhoA activation, colonic smooth muscle strips from the same animal were incubated in PSS at different times with either DHT (10 nm) or vehicle (DMSO, 0.1%). Microsomal fractions were then isolated, and the amount of GTP-bound RhoA (activated RhoA) was inmunodetected using a G-lisa RhoA activation assay (Cytoskeleton, Inc., Denver, CO).
Western blot analyses
For total protein extraction, male murine intestinal tissues were first homogenized in sodium dodecyl sulfate (SDS) lysis buffer (62.5 mm Tris-HCl; 2.3% SDS; and 10% glycerol, pH 6.8) supplemented with 1× protease inhibitor cocktail, 0.01% phosphatase inhibitor cocktail, using a tissue homogenizer. Homogenates were kept on ice for 15 min, and then centrifuged at 13,000 × g for 15 min at 4 C. Proteins recovered in the supernatants were analyzed by SDS-PAGE. An aliquot of each protein extract was quantified using a commercial DC protein assay. β-Mercaptoethanol (5%) and bromophenol blue (0.001%) were then added, and samples were boiled at 95 C for 5 min. Equal amounts of extracted protein (60 μg or more) were electrophoresed on 12.5% SDS-PAGE, transferred to Hybond-P membranes, and proceeded for Western blotting. Membranes were first preincubated at room temperature for 1 h in 5% blotting grade blocker nonfat dry milk (Bio-Rad, Hercules, CA) in Tris-buffered saline (TBS) with 0.1% Tween 20 (TBS-T) or, alternatively, in 5% BSA diluted in TBS-T for the incubation with antibodies against phospho-proteins. Membranes were then incubated with the corresponding specific primary antibody overnight at 4 C with gentle agitation. The dilutions of the different antibodies were: 1:150 for anti-AR antibody; 1:200 for anti-ERα, antiregulatory LC20, anti-RhoA, and antitotal CPI17 antibodies; 1:700 for antiphospho-myosin light chain 2 (Ser19) antibody; 1:1000 for anti-α1 subunit of Na+/K+ ATPase antibody; 1:200 for anticaveolin-1 antibody; and 1:5000 for antiactin antibody. After incubation, membranes were washed several times (10 min each) in TBS with 0.05% Tween 20, and incubated for 2 h at room temperature with the specific horseradish peroxidase-linked secondary antibody diluted in nonfat dry milk solution or in BSA. Goat antirabbit or rabbit antimouse antibodies were diluted at 1:5000, and rabbit antigoat secondary antibody was diluted 1:1000. After washing in TBS-T for several times (10 min each), specific bands corresponding to proteins recognized by the different antibodies were visualized with the Amersham enhanced chemiluminescence kit (Amersham Biosciences, Arlington Heights, IL).
Statistical and mathematical analyses
Results are expressed as mean ± sem. Differences between sample means were assessed by ANOVA or Kruskal-Wallis test followed by either Student-Newman-Keuls t test, post hoc Tukey significant difference or Games-Howell test where appropriate. Comparison between segments from the same animals (permeabilized muscles) was assessed by paired t test or Wilcoxon signed-rank test. Calcium dependence curves were fitted to logistic equations using nonlinear regression analysis tools provided in SigmaPlot version 8.0 software (Jandel Scientific, Corte Madera, CA). Free calcium concentrations were calculated using EQCAL, specific software for multiple equilibrium calculation (Biosoft, Cambridge, UK). Analyses in the frequency domain were assessed using the FFT and STFT algorithms using MatLab 6.5 package (The Mathworks, Inc., Natick, MA) following the procedures described in Refs. 28 and 31 .
Materials
T, 5α-DHT, FLU, CCH, CHE, FIN, PMA, actinomycin D, cycloheximide, and the monoclonal antibody against actin were obtained from Sigma-Aldrich (Biosigma, Madrid, Spain). Y27632 was purchased from Tocris Cookson Ltd. (Bristol, UK). Protease (Complete) and phosphatase inhibitor cocktails were obtained from Roche Diagnostics (Mannheim, Germany). The rabbit polyclonal anti-AR antibody (N-20), raised against a peptide mapping at the N terminus of AR of human origin, the rabbit polyclonal anti-ERα antibody (MC-20) raised against a region at the vicinity of the ligand binding domain, the goat polyclonal antibody recognizing LC20, the mouse monoclonal antibody directed to RhoA, the goat polyclonal antibody against phosphorylated (Thr38) CPI-17, and the polyclonal antibody against caveolin-1 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The rabbit polyclonal antibody against Ser19 phosphorylated LC20 was from Cell Signaling Technology (Barcelona, Spain). The rabbit polyclonal antibody against native CPI-17 and the mouse monoclonal antibody directed to α1 subunit of Na+/K+ ATPase were from Upstate. The antirabbit and antigoat horseradish peroxidase-linked whole antibodies, the Hybond-P poly(vinylidene difluoride) membranes, and the enhanced chemiluminescence Western blotting kit were from Amersham Biosciences.
Acknowledgments
We thank Lupe Acosta for excellent technical assistance and generous collaboration throughout the development of this study.
NURSA Molecule Pages:
Ligands: Dihydrotestosterone | Testosterone.
Footnotes
This work was supported by Grant SAF2007-66148-C02-02 from the Ministerio de Educación y Ciencia (MEC), Spain. J.M.-A. holds a fellowship from MEC.
Disclosure Summary: The authors have nothing to disclose.
First Published Online March 5, 2010
Abbreviations: AR, Androgen receptor; CCH, carbachol; CHE, chelerythrine; CPI-17, endogenous inhibitor of MLCP; DHT, dihydrotestosterone; DMSO, dimethylsulfoxide; ERα, estrogen receptor α; FFT, Fast Fourier Transform; FIN, finasteride; FLU, flutamide; IP3, inositol 1,4,5 triphosphate; LC20, 20-kDa myosin light chain; MLCP, myosin light chain phosphatase; PKC, protein kinase C; PMA, phorbol 12-myristate 13-acetate; PP1c, catalytic subunit of MLCP; PS, permeabilization solution; PSS, physiological salt solution; RA%, dynamic contractile range; ROK, Rho-associated Rho kinase; SDS, sodium dodecyl sulfate; STFT, Short-Time Fourier Transform; T, testosterone; TBS, Tris-buffered saline; TBS-T, TBS with 0.1% Tween 20.
References
- 1.Foradori CD, Weiser MJ, Handa RJ2008. Non-genomic actions of androgens. Front Neuroendocrinol 29:169–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michels G, Hoppe UC2008. Rapid actions of androgens. Front Neuroendocrinol 29:182–198 [DOI] [PubMed] [Google Scholar]
- 3.Lieberherr M, Grosse B1994. Androgens increase intracellular calcium concentration and inositol 1,4,5-trisphosphate and diacylglycerol formation via a pertussis toxin- sensitive G-protein. J Biol Chem 269:7217–7223 [PubMed] [Google Scholar]
- 4.Guo Z, Benten WP, Krücken J, Wunderlich F2002. Nongenomic testosterone calcium signaling. Genotropic actions in androgen receptor-free macrophages. J Biol Chem 277:29600–29607 [DOI] [PubMed] [Google Scholar]
- 5.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F1999. Functional testosterone receptors in plasma membranes of T cells. FASEB J 13:123–133 [DOI] [PubMed] [Google Scholar]
- 6.Couchourel D, Leclerc M, Filep J, Brunette MG2004. Testosterone enhances calcium reabsorption by the kidney. Mol Cell Endocrinol 222:71–81 [DOI] [PubMed] [Google Scholar]
- 7.Machelon V, Nomé F, Tesarik J1998. Nongenomic effects of androstenedione on human granulosa luteinizing cells. J Clin Endocrinol Metab 83:263–269 [DOI] [PubMed] [Google Scholar]
- 8.Lyng FM, Jones GR, Rommerts FF2000. Rapid androgen actions on calcium signaling in rat sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol Reprod 63:736–747 [DOI] [PubMed] [Google Scholar]
- 9.Estrada M, Espinosa A, Müller M, Jaimovich E2003. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 144:3586–3597 [DOI] [PubMed] [Google Scholar]
- 10.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC2001. Nongenotropic, sex-nonspecific signalling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- 11.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F2000. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterziel H, Mink S, Schonert A, Becker M, Klocker H, Cato AC1999. Rapid signalling by androgen receptor in prostate cancer cells. Oncogene 18:6322–6329 [DOI] [PubMed] [Google Scholar]
- 13.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ2003. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85α, androgen receptor, and Src. J Biol Chem 278:42992–43000 [DOI] [PubMed] [Google Scholar]
- 14.Rosner W, Hryb D, Khan MS, Nakhla AM, Romas NA1999. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J Steroid Biochem Mol Biol 69:481–485 [DOI] [PubMed] [Google Scholar]
- 15.Hall J, Jones RD, Jones TH, Channer KS, Peers C2006. Selective inhibition of L-type Ca2+ channels in A7r5 cells by physiological levels of testosterone. Endocrinology 147:2675–2680 [DOI] [PubMed] [Google Scholar]
- 16.Scragg JL, Dallas ML, Peers C2007. Molecular requirements for L-type Ca2+ channel blockade by testosterone. Cell Calcium 42:11–15 [DOI] [PubMed] [Google Scholar]
- 17.Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ2001. Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281:H1720–H1727 [DOI] [PubMed]
- 18.Seyrek M, Yildiz O, Ulusoy HB, Yildirim V2007. Testosterone relaxes isolated human radial artery by potassium channel opening action. J Pharmacol Sci 103:309–316 [DOI] [PubMed] [Google Scholar]
- 19.Zylińska L, Gromadzińska E, Lachowicz L1999. Short-time effects of neuroactive steroids on rat cortical Ca2+-ATPase activity. Biochim Biophys Acta 1437:257–264 [DOI] [PubMed] [Google Scholar]
- 20.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, Diaz-Araya G, Jaimovich E, Lavandero S2006. Testosterone induces an intracellular calcium increase by a nongenomic mechanism in cultured rat cardiac myocytes. Endocrinology 147:1386–1395 [DOI] [PubMed] [Google Scholar]
- 21.Jones RD, English KM, Jones TH, Channer KS2004. Testosterone-induced coronary vasodilatation occurs via a non-genomic mechanism: evidence of a direct calcium antagonism action. Clin Sci 107:149–158 [DOI] [PubMed] [Google Scholar]
- 22.Montaño LM, Calixto E, Figueroa A, Flores-Soto E, Carbajal V, Perusquía M2008. Relaxation of androgens on rat thoracic aorta: testosterone concentration dependent agonist/antagonist L-type Ca2+ channel activity, and 5β-dihydrotestosterone restricted to L-type Ca2+ channel blockade. Endocrinology 149:2517–2526 [DOI] [PubMed] [Google Scholar]
- 23.Scragg JL, Jones RD, Channer KS, Jones TH, Peers C2004. Testosterone is a potent inhibitor of L-type Ca2+ channels. Biochem Biophys Res Comm 318:503–506 [DOI] [PubMed] [Google Scholar]
- 24.Yue P, Chatterjee K, Beale C, Poole-Wilson PA, Collins P1995. Testosterone relaxes rabbit coronary arteries and aorta. Circulation 91:1154–1160 [DOI] [PubMed] [Google Scholar]
- 25.Horowitz A, Menice CB, Laporte R, Morgan KG1996. Mechanisms of smooth muscle contraction. Physiol Rev 76:967–1003 [DOI] [PubMed] [Google Scholar]
- 26.González-Montelongo MC, Marín R, Gómez T, Díaz M2006. Androgens differentially potentiate mouse intestinal smooth muscle by non-genomic activation of polyamine synthesis and Rho-kinase activation. Endocrinology 147:5715–5729 [DOI] [PubMed] [Google Scholar]
- 27.González-Montelongo MC, Marín R, Gómez T, Díaz M 1 October 2009. Androgens are powerful non-genomic inducers of calcium sensitization in visceral smooth muscle. Steroids 10.1016/j.steroids. 2009.09.012 [DOI] [PubMed]
- 28.Díaz M2002. Triphenylethylene antiestrogen-induced acute relaxation of mouse duodenal muscle. Possible involvement of Ca2+ channels. Eur J Pharmacol 445:257–266 [DOI] [PubMed] [Google Scholar]
- 29.Díaz M, Ramírez CM, Marin R, Marrero-Alonso J, Gómez T, Alonso R2004. Acute relaxation of mouse duodenun by estrogens: evidence for an estrogen receptor-independent modulation of muscle excitability. Eur J Pharmacol 501:161–178 [DOI] [PubMed] [Google Scholar]
- 30.Díaz M, Marrero-Alonso J, García Marrero B, Marín R, Gómez T, Alonso R2006. Cellular and molecular basis for acute nongenomically mediated actions of SERMs. In: Cano A, Calaf i Alsina J, Dueñas-Diez JL, eds. Selective estrogen receptor modulators. Berlin, Heidelberg: Springer-Verlag; 79–102
- 31.Díaz M2007. Application of Fourier linear spectral analyses to the characterization of smooth muscle contractile signals. J Biochem Biophys Methods 70:803–808 [DOI] [PubMed] [Google Scholar]
- 32.Wilson CM, McPhaul MJ1996. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol 120:51–57 [DOI] [PubMed] [Google Scholar]
- 33.Stoner E1990. The clinical development of a 5α-reductase inhibitor, finasteride. J Steroid Biochem Mol Biol 37:375–378 [DOI] [PubMed] [Google Scholar]
- 34.Kitazawa T, Gaylinn BD, Denney GH, Somlyo AP1991. G-protein-mediated Ca2+ sensitization of smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 266:1708–1715 [PubMed] [Google Scholar]
- 35.Somlyo AP, Himpens B1989. Cell calcium and its regulation in smooth muscle. FASEB J 3:2266–2276 [DOI] [PubMed] [Google Scholar]
- 36.Somlyo AP, Somlyo AV1994. Signal transduction and regulation in smooth muscle. Nature 372:231–236 [DOI] [PubMed] [Google Scholar]
- 37.Somlyo AP, Somlyo AV2003. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83:1325–1358 [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi T, Kushida M, Hirayama N, Kitayama M, Fujita A, Hata F2004. Mechanisms involved in carbachol-induced Ca2+ sensitization of contractile elements in rat proximal and distal colon. Br J Pharmacol 142:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kitazawa T, Eto M, Woodsome TP, Khalequzzaman M2003. Phosphorylation of the myosin phosphatase targeting subunit and CPI-17 during Ca2+ sensitization in rabbit smooth muscle. J Physiol 546:879–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loirand G, Cario-Toumaniantz C, Chardin P, Pacaud P1999. The Rho-related protein Rnd1 inhibits Ca2+ sensitization of rat smooth muscle. J Physiol 516:825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swärd K, Dreja K, Susnjar M, Hellstrand P, Hartshorne DJ, Walsh MP2000. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J Physiol 522:33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitazawa T, Takizawa N, Ikebe M, Eto M1999. Reconstitution of protein kinase C-induced contractile Ca2+ sensitization in Triton X-100-demembranated rabbit arterial smooth muscle. J Physiol 520:139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Eto M, Lee MR, Morita F, Yazawa M, Kitazawa T1998. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol 508:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitazawa T, Eto M, Woodsome TP, Brautigan DL2000. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem 275:9897–9900 [DOI] [PubMed] [Google Scholar]
- 45.Niiro N, Koga Y, Ikebe M2003. Agonist-induced changes in the phosphorylation of the myosin- binding subunit of myosin light chain phosphatase and CPI17, two regulatory factors of myosin light chain phosphatase, in smooth muscle. Biochem J 369:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez A, Cantabrana B, Hidalgo A1993. Estrogen and antiestrogen non-genomic effect in rat uterus contraction in calcium-free solution. Gen Pharmacol 24:391–395 [DOI] [PubMed] [Google Scholar]
- 47.Kostrzewska A, Laudánski T, Batra S1993. Effect of ovarian steroids and diethylstilbestrol on the contractile responses of the human myometrium and intramyometrial arteries. Eur J Pharmacol 233:127–134 [DOI] [PubMed] [Google Scholar]
- 48.Pang JJ, Xu XB, Li HF, Zhang XY, Zheng TZ, Qu SY2002. Inhibition of β-estradiol on trachea smooth muscle contraction in vitro and in vivo. Acta Pharmacol Sin 23:273–277 [PubMed] [Google Scholar]
- 49.Ratz PH, McCammon KA, Altstatt D, Blackmore PF, Shenfeld OZ, Schlossberg SM1999. Differential effects of sex hormones and phytoestrogens on peak and steady state contractions in isolated rabbit detrusor. J Urol 162:1821–1828 [PubMed] [Google Scholar]
- 50.Kouloumenta V, Hatziefthimiou A, Paraskeva E, Gourgoulianis K, Molyvdas P2006. Non-genomic effect of testosterone on airway smooth muscle. Br J Pharm 149:1083–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafayette SS, Vladimirova I, Garcez-do-Carmo L, Monteforte PT, Caricati Neto A, Jurkiewicz A2008. Evidence for the participation of calcium in non-genomic relaxations induced by androgenic steroids in rat vas deferens. Br J Pharmacol 153:1242–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perusquía M, Villalón CM1999. Posible role of Ca2+ channels in the vasodilating effect of 5β-dihydrotestosterone in rat aorta. Eur J Pharmacol 371:169–178 [DOI] [PubMed] [Google Scholar]
- 53.Perusquía M, Navarrete E, Jasso-Kamel J, Montaño LM2005. Androgens induce relaxation of contractile activity in pregnant human myometrium at term: a nongenomic action on L-type calcium channels. Biol Reprod 73:214–221 [DOI] [PubMed] [Google Scholar]
- 54.Tep-areenan P, Kendall DA, Randall MD2002. Testosterone-induced vasorelaxation in the rat mesenteric arterial bed is mediated predominantly via potassium channels. Eur J Pharmacol 135:735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh SM, Gauthier S, Labrie F2000. Androgen receptor antagonists (antiandrogens) structure-activity relationships. Curr Med Chem 7:211–247 [DOI] [PubMed] [Google Scholar]
- 56.Benten WP, Lieberherr M, Sekeris CE, Wunderlich F1997. Testosterone induces Ca2+ influx via non-genomic surface receptors in activated T cells. FEBS Lett 407:211–214 [DOI] [PubMed] [Google Scholar]
- 57.Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F1999. Testosterone signalling through internalizable surface receptors in androgen receptor-free macrophages. Mol Biol Cell 10:3113–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Felden F, Leheup B, Fremont S, Bouguerne R, Egloff M, Nicolas JP, Grignon G, Gueant JL1992. The plasma membrane of epididymal epithelial cells has a specific receptor which binds to androgen-binding protein and sex steroid-binding protein. J Steroid Biochem Mol Biol 42:279–285 [DOI] [PubMed] [Google Scholar]
- 59.Figueroa-Valverde L, Luna H, Castillo-Henkel C, Muñoz-Garcia O, Morato-Cartagena T, Ceballos-Reyes G2002. Synthesis and evaluation of the cardiovascular effects of two, membrane impermeant, macromolecular complexes of dextran-testosterone. Steroids 67:611–619 [DOI] [PubMed] [Google Scholar]
- 60.Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E2002. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J 16:1429–1431 [DOI] [PubMed] [Google Scholar]
- 61.Konoplya EF, Popoff EH1992. Identification of the classical androgen receptor in male rat liver and prostate cell plasma membranes. Int J Biochem 24:1979–1983 [DOI] [PubMed] [Google Scholar]
- 62.Sommer A, Haendler B2003. Androgen receptor and prostate cancer: Molecular aspects and gene expression profiling. Curr Opin Drug Discov Devel 6:702–711 [PubMed] [Google Scholar]
- 63.Bull HG, Garcia-Calvo M, Andersson S, Baginsky WF, Chan HK, Ellsworth DE, Miller RR, Stearns RA, Bakshi RK, Rasmusson GH, Tolman RL, Myers RW, Kozarich JW, Harris GS1996. Mechanism-based inhibition of human steroid 5α-reductase by finasteride: enzyme-catalyzed formation of NADP-dihydrofinasteride, a potent bisubstrate analog inhibitor. J Am Chem Soc 118:2359–2365 [Google Scholar]
- 64.Russell DW, Wilson JD1994. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem 63:25–61 [DOI] [PubMed] [Google Scholar]
- 65.Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML1999. Estrogen synthesis in human colon cancer epithelial cells. J Steroid Biochem Mol Biol 71:223–230 [DOI] [PubMed] [Google Scholar]
- 66.Estrada M, Espinosa A, Gibson CJ, Uhlen P, Jaimovich E2005. Capacitative calcium entry in testosterone-induced intracellular calcium oscillations in myotubes. J Endocrinol 184:371–379 [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi T, Sumiyoshi M, Kitayama M, Hirayama N, Fujita A, Hata F2001. Origin of Ca2+ necessary for carbachol-induced contraction in longitudinal muscle of the proximal colon of rats. Jpn J Pharmacol 87:309–317 [DOI] [PubMed] [Google Scholar]
- 68.Sato K, Leposavic R, Publicover NG, Sanders KM, Gerthoffer WT1994. Sensitization of the contractile system of canine colonic smooth muscle by agonists and phorbol ester. J Physiol 481:677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Colburn JC, Michnoff CH, Hsu LC, Slaughter CA, Kamm KE, Stull JT1988. Sites phosphorylated in myosin light chain in contracting smooth muscle. J Biol Chem 263:19166–19173 [PubMed] [Google Scholar]
- 70.Hartshorne D1987. Biochemistry of the contractile process in smooth muscle. In: Johnson LR, ed. Physiology of the gastrointestinal tract. New York: Raven Press, Ltd.; 423–482
- 71.Hartshorne DJ, Ito M, Erdödi F1998. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil 19:325–341 [DOI] [PubMed] [Google Scholar]
- 72.Hirano K, Derkach DN, Hirano M, Nishimura J, Kanaide H2003. Protein kinase network in the regulation of phosphorylation and dephosphorylation of smooth muscle myosin light chain. Mol Cell Biochem 248:105–114 [DOI] [PubMed] [Google Scholar]
- 73.Ichikawa K, Ito M, Hartshorne DJ1996. Phosphorylation of the large subunit of myosin phosphatase and inhibition of phosphatase activity. J Biol Chem 271:4733–4740 [DOI] [PubMed] [Google Scholar]
- 74.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K1996. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248 [DOI] [PubMed] [Google Scholar]
- 75.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K1996. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271:20246–20249 [DOI] [PubMed] [Google Scholar]
- 76.Fukata Y, Amano M, Kaibuchi K2001. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22:32–39 [DOI] [PubMed] [Google Scholar]
- 77.Somlyo AP, Somlyo AV2000. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522:177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Otto B, Steusloff A, Just I, Aktories K, Pfitzer G1996. Role of Rho proteins in carbachol-induced contractions in intact and permeabilized guinea-pig intestinal smooth muscle. J Physiol 496:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eto M, Senba S, Morita F, Yazawa M1997. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett 410:356–360 [DOI] [PubMed] [Google Scholar]
- 80.Senba S, Eto M, Yazawa M1999. Identification of trimeric myosin phosphatase (PP1M) as a target for a novel PKC-potentiated protein phosphatase-1 inhibitory protein (CPI17) in porcine aorta smooth muscle. J Biochem 125:354–362 [DOI] [PubMed] [Google Scholar]
- 81.Kaibuchi K, Kuroda S, Amano M1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem 68:459–486 [DOI] [PubMed] [Google Scholar]
- 82.Gong MC, Fujihara H, Somlyo AV, Somlyo AP1997. Translocation of RhoA associated with Ca2+ sensitization of smooth muscle. J Biol Chem 272:10704–10709 [DOI] [PubMed] [Google Scholar]
- 83.Miyazaki K, Yano T, Schmidt DJ, Tokui T, Shibata M, Lifshitz LM, Kimura S, Tuft RA, Ikebe M2002. Rho-dependent agonist-induced spatio-temporal change in myosin phosphorylation in smooth muscle cells. J Biol Chem 277:725–734 [DOI] [PubMed] [Google Scholar]
- 84.Patil SB, Bitar KN2006. RhoA- and PKC-α-mediated phosphorylation of MYPT and its association with HSP27 in colonic smooth muscle cells. Am J Physiol Gastrointest Liver Physiol 290:G83–G95 [DOI] [PubMed]
- 85.Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, Sperber AD2006. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology 130:1435–1446 [DOI] [PubMed] [Google Scholar]
- 86.Harari D, Gurwitz JH, Avorn J, Bohn R, Minaker KL1996. Bowel habit in relation to age and gender. Findings from the National Health Interview Survey and clinical implications. Arch Intern Med 156:315–320 [PubMed] [Google Scholar]
- 87.Jung HK, Kim DY, Moon IH2003. Effects of gender and menstrual cycle on colonic transit time in healthy subjects. Korean J Intern Med 18:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cullen G, O'Donoghue D2007. Constipation and pregnancy. Best Pract Res Cl Ga 21:807–818 [DOI] [PubMed] [Google Scholar]
- 89.Choung RS, Locke 3rd GR, Schleck CD, Zinsmeister AR, Talley NJ2007. Cumulative incidence of chronic constipation: a population-based study 1988–2003. Aliment Pharmacol Ther 26:1521–1528 [DOI] [PubMed] [Google Scholar]
- 90.Cong P, Pricolo V, Biancani P, Behar J2007. Abnormalities of prostaglandins and cyclooxygenase enzymes in female patients with slow-transit constipation. Gastroenterology 133:445–453 [DOI] [PubMed] [Google Scholar]
- 91.Ohama T, Hori M, Fujisawa M, Kiyosue M, Hashimoto M, Ikenoue Y, Jinno Y, Miwa H, Hatsumoto T, Murata T, Ozaki H2008. Downregulation of CPI-17 contributes to dysfunctional motility in chronic intestinal inflammation model mice and ulcerative colitis patients. J Gastroenterol 43:858–865 [DOI] [PubMed] [Google Scholar]
- 92.Ohama T, Hori M, Momotani E, Iwakura Y, Guo F, Kishi H, Kobayashi S, Ozaki H2007. Intestinal inflammation downregulates smooth muscle CPI-17 through induction of TNF-α and causes motility disorders. Am J Physiol Gastrointest Liver Physiol 292:G1429–G1438 [DOI] [PubMed]