Abstract
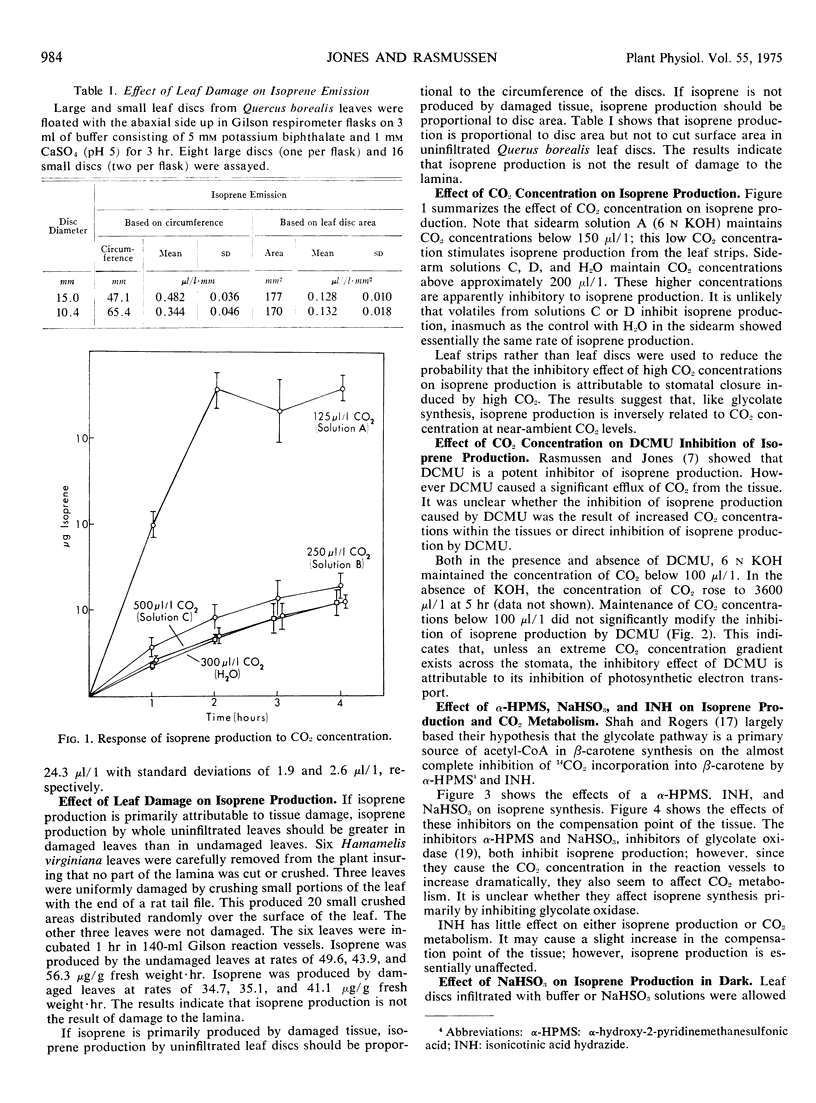
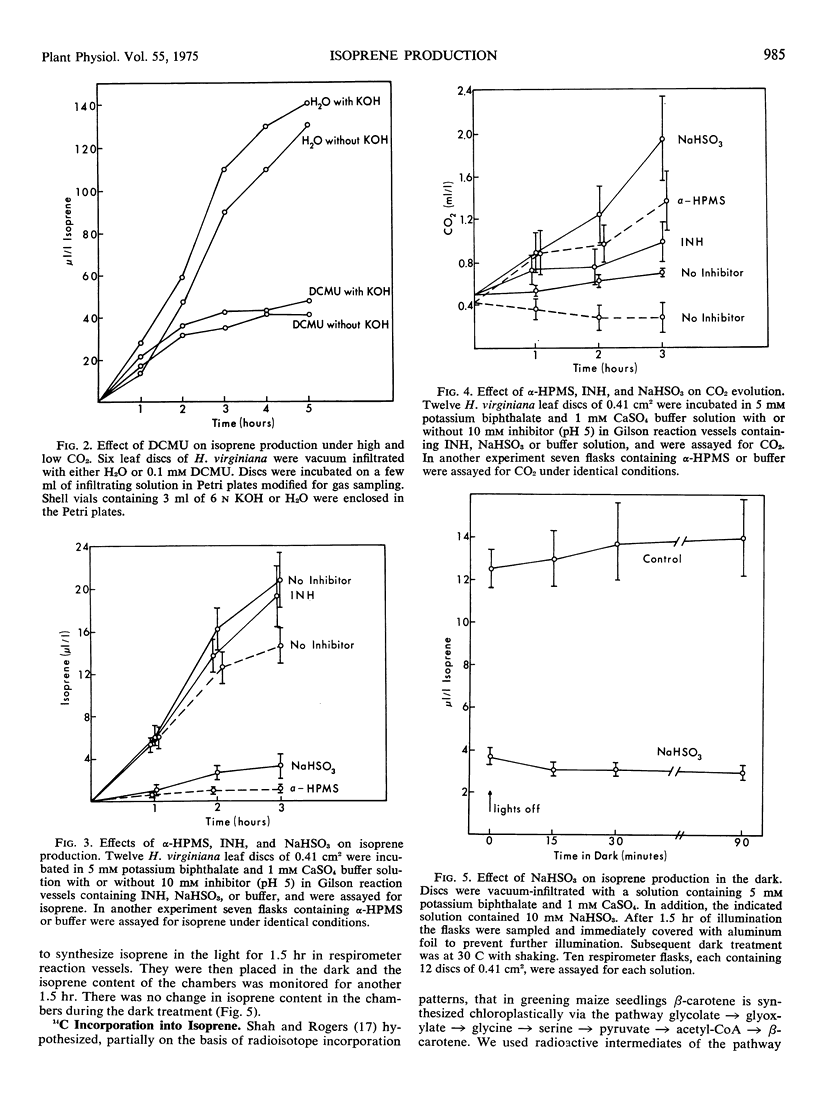
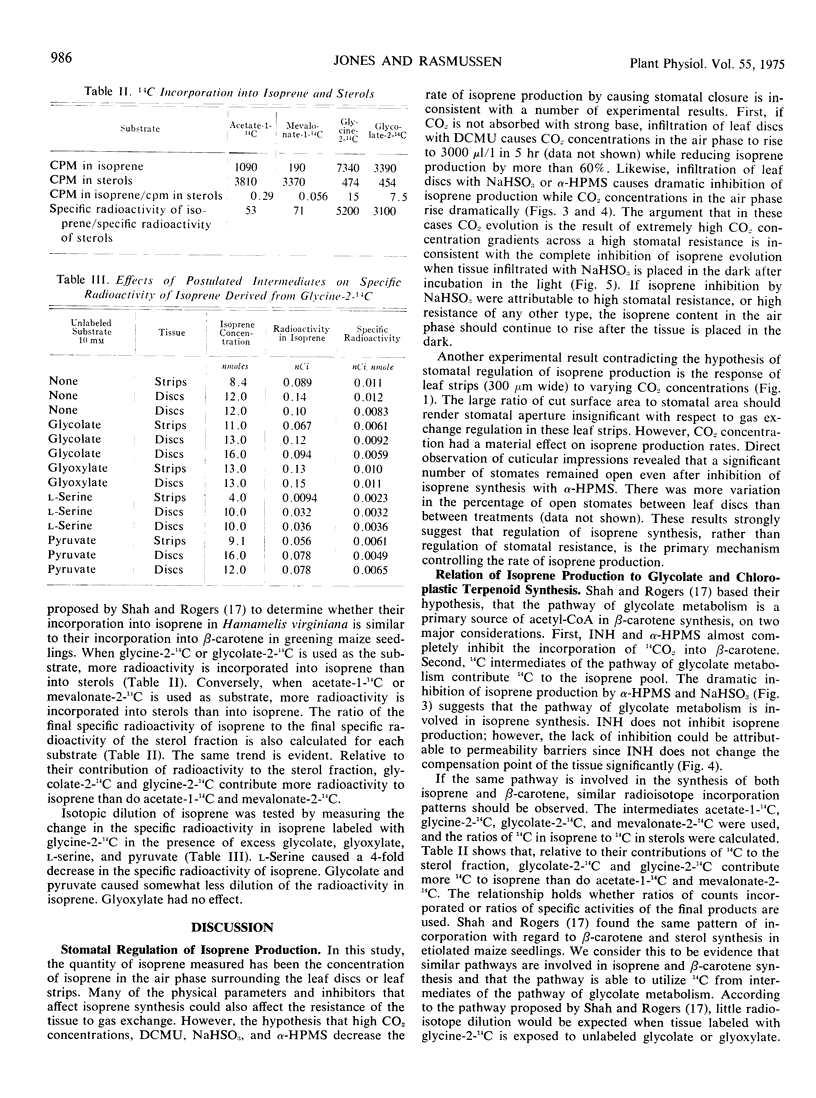
Isoprene production by Hamamelis virginiana L. and Quercus borealis Michx. leaves was studied. When ambient CO2 concentrations were maintained with bicarbonate buffers, the rate of isoprene production at 125 microliters per liter of CO2 was approximately four times that at 250 microliters per liter of CO2. Isoprene production was drastically inhibited by 97% O2. Dichlorodimethylphenylurea (0.1 mm), NaHSO3 (10 mm), and α-hydroxy-2-pyridinemethanesulfonic acid (10 mm) inhibited isoprene production but increased the compensation point of the tissue. Isonicotinic acid hydrazide neither inhibited isoprene emission nor increased the compensation point of the tissue significantly. Inhibition of isoprene production does not seem to correlate with stomatal resistance. Isoprene was labeled by intermediates of the glycolate pathway, and similarities are noted between the biosynthesis of isoprene and that of β-carotene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellyard P. W., Gibbs M. Inhibition of photosynthesis by oxygen in isolated spinach chloroplasts. Plant Physiol. 1969 Aug;44(8):1115–1121. doi: 10.1104/pp.44.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut Z., Gibbs M. Glycolate formation in intact spinach chloroplasts. Plant Physiol. 1970 Apr;45(4):470–474. doi: 10.1104/pp.45.4.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. M., Gibbs M. Photosynthetic intermediates, the warburg effect, and glycolate synthesis in isolated spinach chloroplasts. Plant Physiol. 1974 Jun;53(6):790–797. doi: 10.1104/pp.53.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. P., Rogers L. J. Compartmentation of terpenoid biosynthesis in green plants. A proposed route of acetyl-coenzyme A synthesis in maize chloroplasts. Biochem J. 1969 Sep;114(2):395–405. doi: 10.1042/bj1140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shain Y., Gibbs M. Formation of glycolate by a reconstituted spinach chloroplast preparation. Plant Physiol. 1971 Sep;48(3):325–330. doi: 10.1104/pp.48.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. The relationship of glycolic acid to respiration and photosynthesis in tobacco leaves. J Biol Chem. 1959 Dec;234:3077–3081. [PubMed] [Google Scholar]
- Zelitch I. Comparison of the effectiveness of glycolic Acid and glycine as substrates for photorespiration. Plant Physiol. 1972 Jul;50(1):109–113. doi: 10.1104/pp.50.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I., Walker D. A. The Role of Glycolic Acid Metabolism in Opening of Leaf Stomata. Plant Physiol. 1964 Sep;39(5):856–862. doi: 10.1104/pp.39.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]


