Abstract
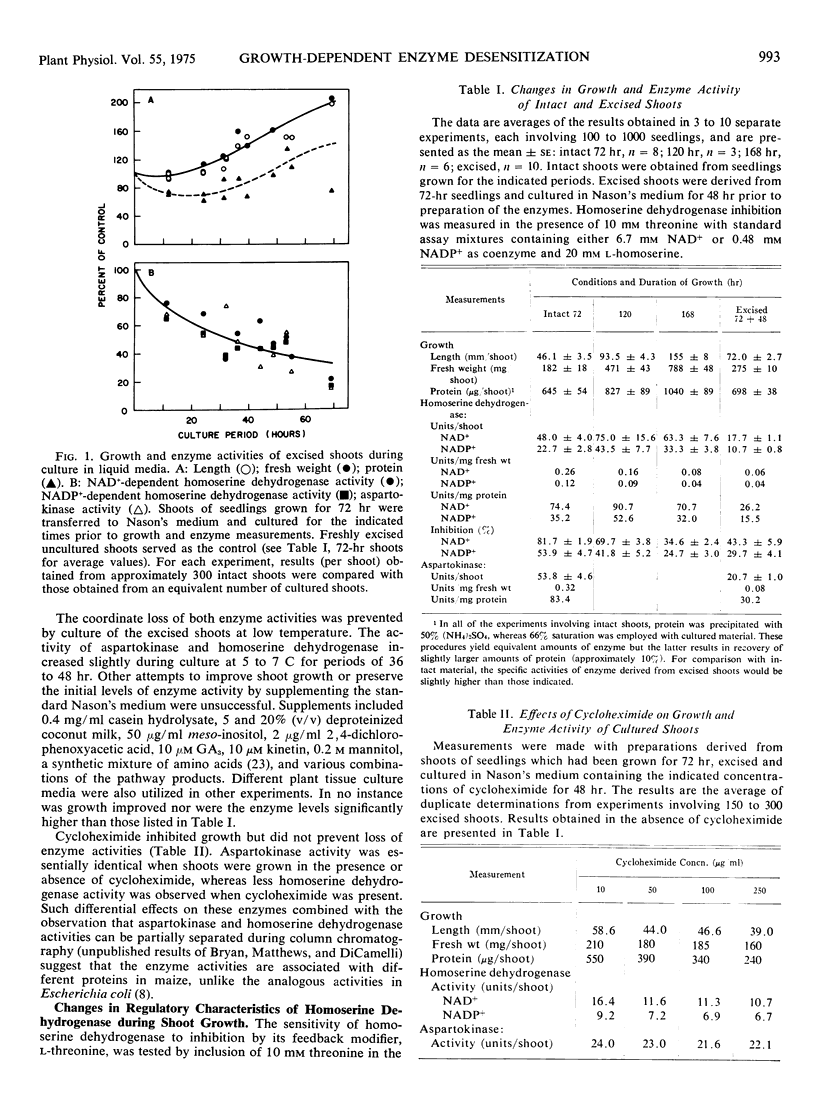
The sensitivity of homoserine dehydrogenase (EC 1.1.1.3) to inhibition by the feed-back modifier, l-threonine, was examined in preparations derived from etiolated shoots, roots, and lightgrown tissues of Zea mays L. var. earliking. A progressive decrease in enzyme sensitivity was observed during seedling growth. Enzyme derived from internode tissue retained a greater sensitivity to the effector than enzyme derived from apical portions of etiolated shoots, whereas enzyme from root tips was characteristically more sensitive than that prepared from mature cells of the root. Enzyme desensitization occurred rapidly during culture of excised shoots and the activities of both homoserine dehydrogenase and aspartokinase (EC 2.7.2.4) declined during shoot culture under a variety of conditions. The initial enzyme levels and the characteristic sensitivity of homoserine dehydrogenase were preserved during culture at 5 to 7 C, but desensitization was not prevented by inclusion of cycloheximide in the culture medium.
Results of control experiments provide evidence that desensitization occurs in vivo. No alteration of the enzyme properties was detected during extraction or concentration of sensitive or insensitive enzyme or during coextraction of enzyme from mixed populations of different age shoots; nor was a differential distribution of inhibitors or activators indicated during assay of mixed preparations. The change in enzyme sensitivity was apparent under a variety of assay conditions and was not accompanied by changes in the apparent affinity of the enzyme for the substrate, homoserine. It is suggested that systematic changes in the regulatory characteristics of certain enzymes could be an important level of metabolic regulation during cellular differentiation.
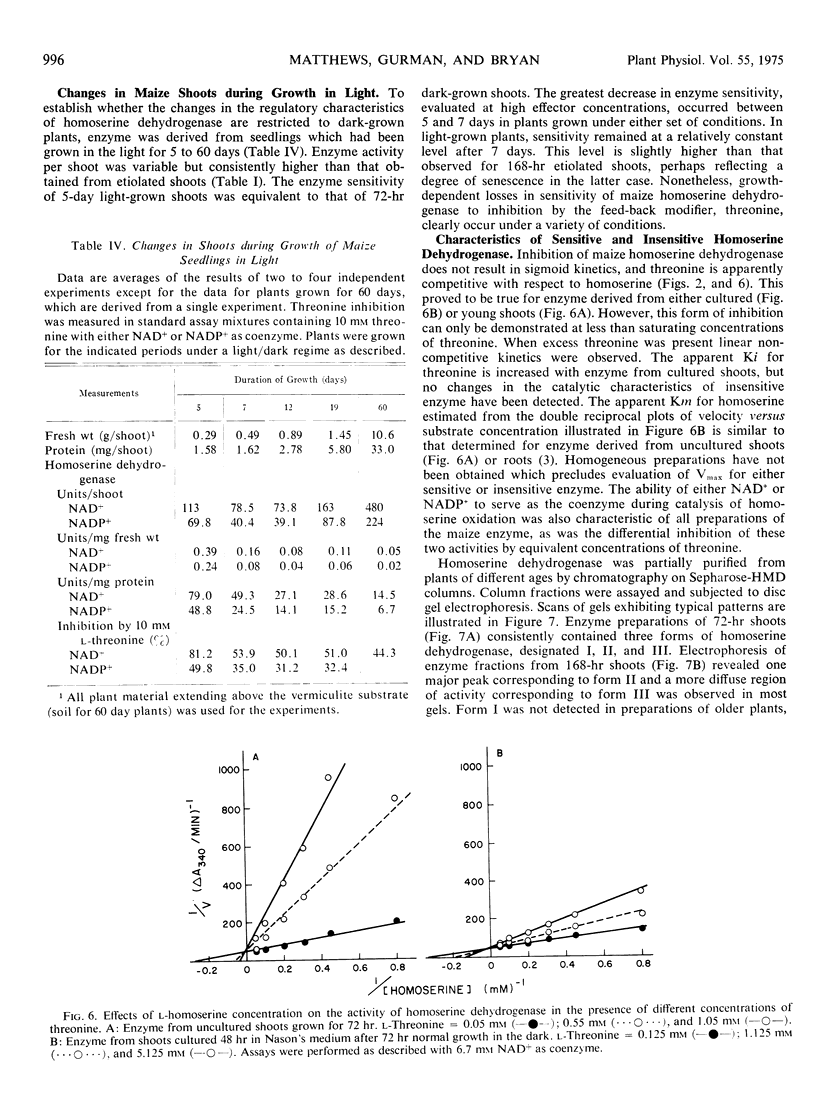
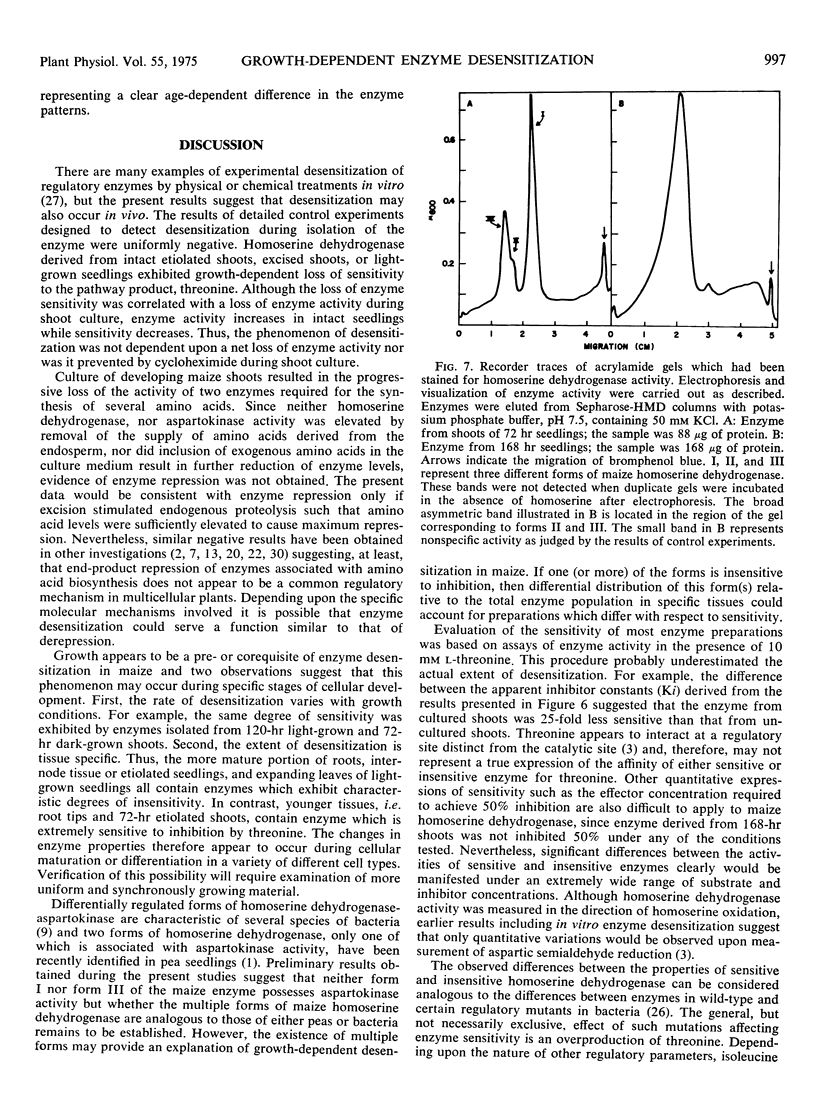
Three forms of maize homoserine dehydrogenaase were detected after acrylamide gel electrophoresis of samples derived from 72-hr shoots. Similar analysis of samples from older shoots revealed a broad asymmetric band of enzyme activity, suggesting that changes in the relative distribution of specific forms of the enzyme could be related to the growth-dependent changes in the sensitivity of maize homoserine dehydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser W. L., Murphy J. B., Delmer D. P., Mills S. E. End product control of tryptophan biosynthesis in extracts and intact cells of the higher plant Nicotiana tabacum var. Wisconsin 38. Biochim Biophys Acta. 1971 Apr 20;237(1):1–10. doi: 10.1016/0304-4165(71)90023-7. [DOI] [PubMed] [Google Scholar]
- Bryan J. K. Studies on the catalytic and regulatory properties of homoserine dehydrogenase of Zea mays roots. Biochim Biophys Acta. 1969 Feb 11;171(2):205–216. doi: 10.1016/0005-2744(69)90154-5. [DOI] [PubMed] [Google Scholar]
- Bryan P. A., Cawley R. D., Brunner C. E., Bryan J. K. Isolation and characterization of a lysine-sensitive aspartokinase from a multicellular plant. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1211–1217. doi: 10.1016/0006-291x(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. The Hydrolysis of Endosperm Protein in Zea mays. Plant Physiol. 1974 Mar;53(3):453–457. doi: 10.1104/pp.53.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oaks A., Beevers H. The Requirement for Organic Nitrogen in Zea mays Embryos. Plant Physiol. 1964 Jan;39(1):37–43. doi: 10.1104/pp.39.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks A. The synthesis of leucine in maize embryos. Biochim Biophys Acta. 1965 Nov 15;111(1):79–89. doi: 10.1016/0304-4165(65)90474-5. [DOI] [PubMed] [Google Scholar]
- Shiio I., Miyajima R., Nakamori S. Homoserine dehydrogenase genetically desensitized to the feedback inhibition in Brevibacterium flavum. J Biochem. 1970 Dec;68(6):859–866. doi: 10.1093/oxfordjournals.jbchem.a129424. [DOI] [PubMed] [Google Scholar]


