Abstract
Switching branded to generic medications has become a common cost-containment measure. Although this is an important objective for health care systems worldwide, the impact of this practice on patient outcomes needs to be carefully considered. We reviewed the literature summarizing the potential clinical and economic consequences of switching from branded to generic medications on patient outcomes. A literature search of peer-reviewed articles published 2003–2013 using key words of “generic switching” or “substitution” was conducted using PubMed, OvidSP, and ScienceDirect. Of 30 articles identified and reviewed, most were related to the diseases of the central nervous system, especially epilepsy. Based on our review, potential impacts of switching fell into 3 broad categories: patient attitudes and adherence, clinical and safety outcomes, and cost and resource utilization. Although in many cases generics may represent an appropriate alternative to branded products, this may not always be the case. Specifically, several studies suggested that switching may negatively impact medication adherence, whereas other studies found that generic switching was associated with poorer clinical outcomes and more adverse events. In some instances, switching accomplished cost savings but did so at increased total cost of care because of increased physician visits or hospitalizations. Although in many cases generics may represent an appropriate alternative, mandatory generic switching may lead to unintended consequences, especially in certain therapeutic areas. Although further study is warranted, based on our review, it may be medically justifiable for physicians and patients to retain the right to request the branded product in certain cases.
Keywords: switching, generic medication, branded medication, adherence, outcome
INTRODUCTION
An increase in medical expenditures has led health care providers and authorities to implement measures to minimize costs and maximize cost savings. Brand-name medications are typically 30%–60% more expensive than their generic counterparts.1,2 As a result, an increased use of cheaper generic prescription drugs as alternatives to more expensive branded products is encouraged by health authorities worldwide. In contrast to innovator drugs, comprehensive clinical trial evidence is not mandatory for approval of generic drugs by national authorities.2 However, generic drugs approved by national regulatory authorities must be bioequivalent to the brand-name version. In essence, the bioequivalence of 2 products is determined by their relative comparability in terms of pharmacokinetic and pharmaceutical equivalence. Specifically, products are bioequivalent if the pharmacokinetic properties of the comparator compound fall within prescribed limits relative to the reference compound. For example, the US Food and Drug Administration (FDA) and European regulations consider the products to be bioequivalent if the mean maximum concentration achieved, the time at which that concentration is achieved, and the area under the concentration–time curve for the generic product falls within 80%–125% of the innovator or branded product, when administered under a fed or fasting state.3,4
To conduct such tests, the 2 products must be pharmaceutically equivalent in that they contain the same amount of active ingredients, are of the same dosage form, and are given by the same route of administration,5 meaning they are comparable in quality, intended use, and clinical efficacy. The FDA found no significant difference between branded and generic drugs in a review of bioequivalence data from 2070 single-dose clinical pharmacokinetic trials of orally administered generics that they had approved over a period of 12 years (1996–2007).6 However, certain limitations apply to the FDA-stipulated bioequivalence requirements for generic drugs. First, these studies are conducted in healthy adult volunteers rather than in patients within the therapeutic indication for which the drug is used. Therefore, the data generated are not reflective of any variations associated with disease, gender, and age.7–10 Second, these studies do not measure the ratios of the active compound/metabolite; this may be very important for those drugs where the metabolite is as active as the parent compound (as is the case with venlafaxine and its metabolite O-desmethylvenlafaxine).
Despite this, there can be differences in formulation between the innovator brand-name drug and a generic counterpart. It is not a regulatory requirement that the “inactive” ingredients in a generic version be identical to those in the innovator formulation.11,12 However, small changes or impurities in the excipients used in a formulation can alter the properties of a medication and lead to unexpected adverse effects on drug absorption, bioavailability, efficacy, and safety.13,14 Certain excipients are not tolerated by some patients, including lactose and gluten, and certain dyes.7 Furthermore, the appearance, taste, allergenicity, and shelf life may differ between generic and branded medications because of variations in the salt or ester of the active ingredient in the formulations.15,16
In the majority of patients and for the majority of medications, generic switching is a means to obtain similar therapeutic benefit at considerably lower costs, without any problems. Indeed, generic prescribing already accounts for 83% of prescriptions in the United Kingdom.17,18 In the United States, generic medicines account for over 70% of prescriptions dispensed.19 However, several researchers have reported patient concerns related to generic medicines.20–24 Many investigations center on the impact of the relative cheapness of generic medications on attitudes toward effectiveness,25,26 with some reports showing that patients did not perceive a generic drug to be as effective, or work at all, in comparison with original branded drugs.27,28 Generic switching has the potential to interfere with a patient's usual medication regimen and impact adherence,29,30 which can affect clinical and safety outcomes and the total costs of care.31–34 One of the main problems is that generic medicines often have different names, sizes, shapes, colors, tastes, smells, and packaging to one another, and to their branded medicine, which can cause anxiety, insecurity, and confusion among certain patient populations and lead to reduced adherence.33,35
Branded to generic switching for classes of drugs with narrow therapeutic indices,36 such as antiepileptic, antiarrhythmic, thyroid medications, and anticoagulants, is particularly problematic and may result in adverse clinical outcomes.5,37–39 Generic switching could result in subtherapeutic levels or increased toxicity after small changes in dosage level.39 When treating epilepsy, a slight change in dosing because of substitution can result in a whole spectrum of serious adverse events, including hospitalization or even death.37,39–41 Generic drugs in modified- and sustained-release formulations may not exhibit the same pharmacokinetic profiles as the branded drugs,2 biologicals, and biosimilars.7 Subgroups of people who have been well controlled on a branded drug for a long period, those being treated for a serious or chronic illness, those with symptom-free conditions (such as hypertension), patients being treated for psychological disorders, those taking multiple medications, and the elderly have less favorable attitudes toward generic substitution.38,42–45
Based on these observations, we conducted a comprehensive review to summarize the available evidence from the published literature to assess the potential negative clinical and economic consequences of switching from branded to generic medications on patient outcomes. Our objective was to highlight the importance of considering patient, global, economic, and outcome-related consequences beyond the obvious drug acquisition costs when undertaking brand to generic switching.
MATERIALS AND METHODS
A literature search of peer-reviewed articles published in English between January 1, 2003, and March 31, 2013, was conducted using PubMed, OvidSP, and ScienceDirect databases with the purpose of identifying all relevant original research articles on generic substitution. The search terms used were a combination of words related to “brand to generic switching,” “generic substitution,” “poor adherence,” and “poor clinical outcome.” Based on title and abstract, articles were identified for further analysis. In addition, a manual screen of the reference lists of any identified articles was conducted.
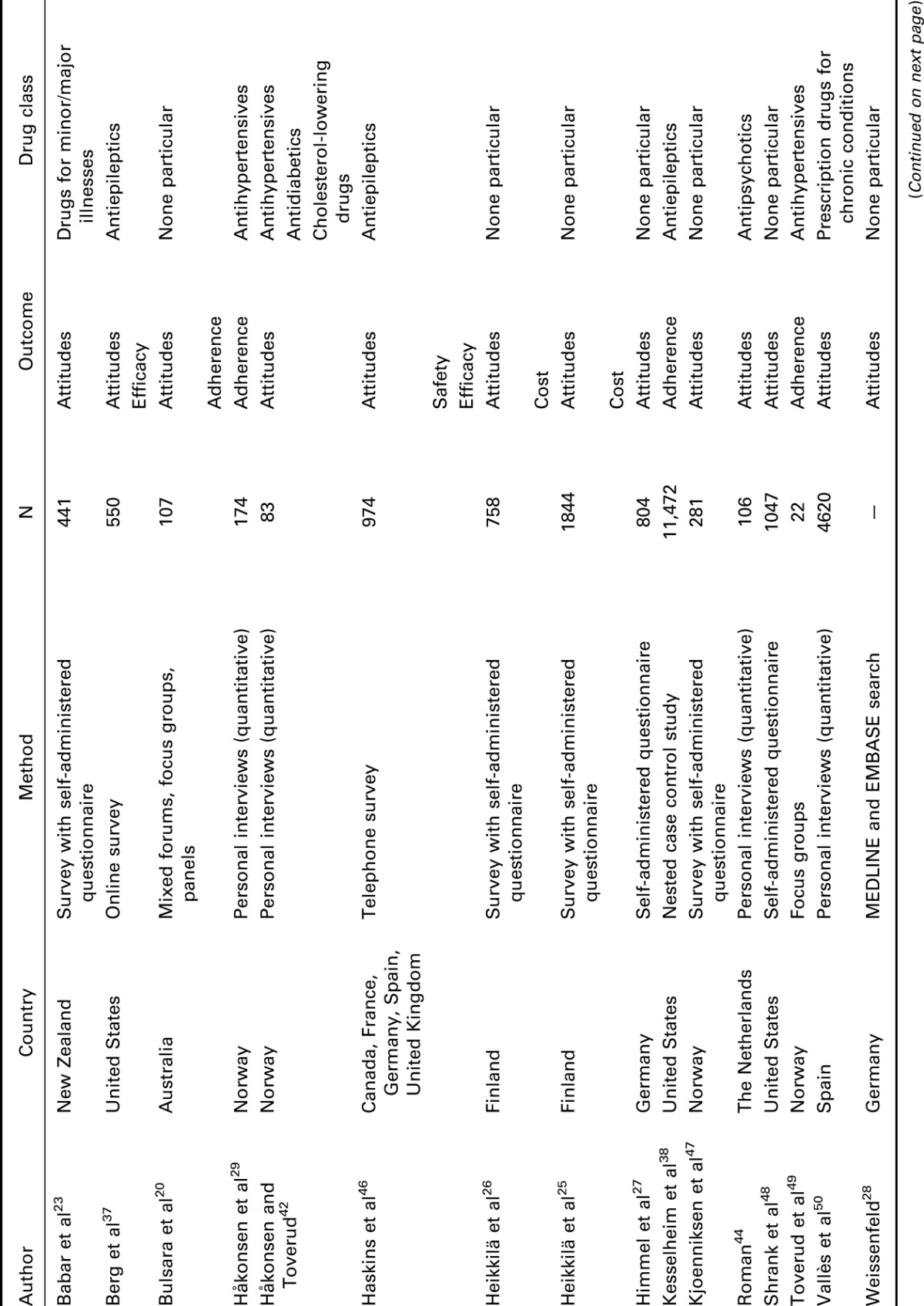
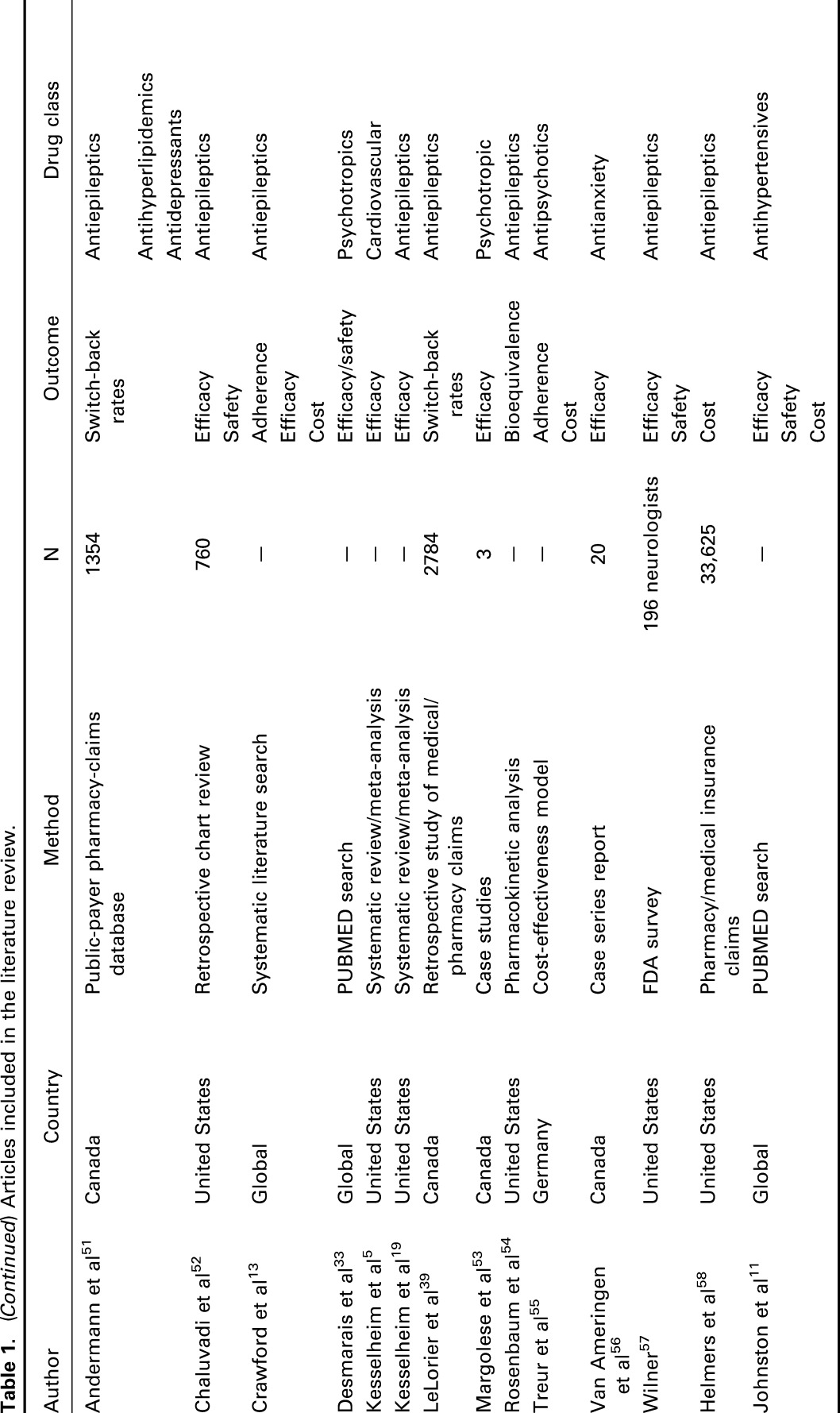
A total of 127 relevant articles were screened based on our search terms, among which 30 articles discussed the therapeutic equivalence of generic medications and their brand-name versions. An overview of the articles included in this review is presented in Table 1, listed alphabetically according to first author, year of publication, country where the study was conducted, the research methodology, number of participating subjects, and drug classification. The results were grouped according to theme and are described in detail in the main text. Of the articles identified and reviewed, most were related to diseases of the central nervous system, in particular, epilepsy. Overall, the potential impact of switching fell into 3 broad categories: patient attitudes and medication adherence, clinical and safety outcomes, and cost and resource utilization; these are discussed in the Results section.
Table 1.
Articles included in the literature review.
RESULTS
Patients' attitudes and medication adherence
Several questionnaire-based surveys have suggested that switching from branded to generic medications could negatively impact adherence because of concerns and confusion among patients otherwise stable on branded medications. The Aston Medication Adherence Study (AMAS) surveyed participants in the United Kingdom to establish the extent of nonadherence to prescribed generic medication within 4 treatment areas (dyslipidemia, type 2 diabetes, hypothyroidism, and prophylaxis of thrombosis).35 Almost all patients reported changes to their medication including changes in the color of tablets received, changes in the shape of tablets, changes in the taste of medicines, and changes in packaging. Patients reported that this led to confusion and distress. Their low price was equated to poor quality. A number of patients believed that it was important for pharmacists to inform patients of changes to their generic medicines.
Another study surveyed primary care patients with hypertension (n = 804) about their thoughts on generic drug use.27 Nearly 30% were not satisfied with the information given by their doctor and 37% of patients expressed general skepticism toward generic drugs because they were cheaper. Patients younger than 60 years, those who were chronically ill, and/or without higher education more frequently expressed this opinion. About 30% of patients had become accustomed to the color or shape of their substituted drug. Twelve percent claimed a diminished effect of their medication after the switch and 13% reported the occurrence of new adverse events. More than half of the individuals who were aware that their medications had been substituted (30% of the overall sample) were reported to be skeptical about the switching.
Two surveys were conducted in Finland after the substitution reform that became effective in 2003. In the first survey, 28% of customers accepted and 72% of customers refused generic substitution.26 The main reason for this was lower costs and the fact that the generic medication was recommended by the pharmacist. The most common reasons for refusing substitution were positive experiences with drugs used previously and a wish to discuss the substitution with their physician first. In the second survey, 81% of the participants considered cheaper generics to be as effective as branded medications and 85% did not consider generic substitution as a risk to drug safety.25 In both surveys, males and patients younger than 60 years were identified as those individuals most likely to feel positive toward generic switching.
In a Norwegian survey conducted by Kjønniksen et al,47 36% of the participants (n = 281) reported 1 or more negative experiences with generic substitution, for example, more adverse events or a reduced therapeutic effect, and 21% of the participants claimed an overall negative experience with switching. The negative experiences did not seem to be associated with the age or gender of survey participants or the number of drugs. Participants who had received information from their general physician or the pharmacy about generic substitution were more likely to have switched (P < 0.001). In total, 41% preferred not to change their medication without financial savings.
Weissenfeld et al28 pooled data from 8 studies (n = 12,463). Generic substitution was generally accepted by over two-thirds of the study population. However, among the patients being treated for psychological diseases who had had their medication substituted to a generic version, 34% experienced additional adverse events because of their poor perceptions of the generic drug. This is termed the nocebo effect.
In a further survey, the attitude of participants (n = 441) toward the use of generic medicines was determined by their knowledge of generics, whether it was recommended by a pharmacist, and their type of illness. Patients were more inclined to change to generics for minor problems, such as hay fever, cold, or flu (78%) rather than for major issues, such as heart problems, diabetes, or asthma (59%). The switch in medication was more likely to be accepted by patients who were younger, educated, and had sufficient knowledge about generic drugs for both minor and major problems.23 In a study of elderly patients (n = 107), participants showed considerable misgivings regarding the use of generic medicines,20 highlighting their doubts about the extent of the pharmaceutical industry's influence on health care professionals, their suspicions of foreign generic drug manufacturers, skepticism that the generics would achieve the same therapeutic effect, and concerns over changes in packaging. The participants also thought that general physicians need to discuss more actively a switch to a generic medication with their patients as this plays an important role in their decision to accept the substitution.
Personal interviews, using a semistructured questionnaire, were conducted with 174 Norwegian patients (50–80 years) who had had their branded antihypertensive drug substituted with a nonbranded counterpart. Overall, 29% of the patients reported feeling anxious when they first started using the generic medication.29,49 Two of the 3 survey participants noticed that the generically substituted product had a different size, color, or shape compared with their original brand-name medication. One in 3 participants claimed that generic switching made keeping track of their medications increasingly demanding. Some thought it was easier to recognize and identify the brand-name drug because the different nonbranded products frequently feature similarities in the appearance of packaging and tablets. Others had experienced the nonbranded product as harder to swallow because of a lack of coating or a greater size. With respect to medical outcome and effectiveness, 8% of the patients believed that the effect of the substituted drug differed, and all of these, except 1, thought the effect was poorer after generic substitution. Fifteen percent of patients claimed they experienced new or more side effects.
The effect of change in the external appearance of generic antiepileptic medications on adherence was studied in a nested case–control study of 11,472 patients with epilepsy compared with >50,000 controls.38 Seven different antiepileptic drugs studied were produced in a total of 37 colors; 4 drugs were produced in a single shape, and the remaining 3 were produced in 2 different shapes. A change in pill color will significantly increase the odds of nonpersistence; odds of color discordance were 53% higher for those who were nonadherent than for those who were adherent {adjusted odds ratio, 1.53 [95% confidence interval (CI): 1.07–2.18]}. The odds of shape discordance were also greater in patients than in controls, but the difference was not statistically significant.
A US national survey evaluated patients' perceptions about generic medications. Patients surveyed (n = 1047) agreed that generic drugs were less costly and better value than innovator branded drugs, with similar safety. However, although 56% believed that in general US patients ought to use more generically substituted medications, only 37.6% would opt for generics themselves.48
In a survey of psychoses/schizophrenia patients (n = 106), 73% of the respondents stated that they would be unlikely to take a generic antipsychotic medication. Among these, 50% attributed this decision to a difference in packaging, whereas 28% claimed that they had no faith in the efficacy of the generic drug as it was not prescribed by their psychiatrist.44 They also thought that generic substitution should take place only with the knowledge and agreement of the psychiatrist and the patient.
One study has investigated the effects of providing information to patients. In a multicenter study, the effect of patient education on the use of generically substituted medications in general practice, including verbal information and providing hand outs, helped 99% of participants to accept their generic substitution. However, patient acceptance of substitution was once again less for drugs acting on the central nervous system compared with drugs used for other conditions.50
Impact on clinical and safety outcomes
Kesselheim et al5 conducted a meta-analysis of 47 studies (38 were randomized controlled trials) involving 9 different classes of cardiovascular drugs (α-blockers, angiotensin-converting enzyme inhibitors, antiplatelet agents, β-blockers, calcium channel blockers, diuretics, and statins). No evidence of superiority was found for original branded medications versus generically substituted medications.
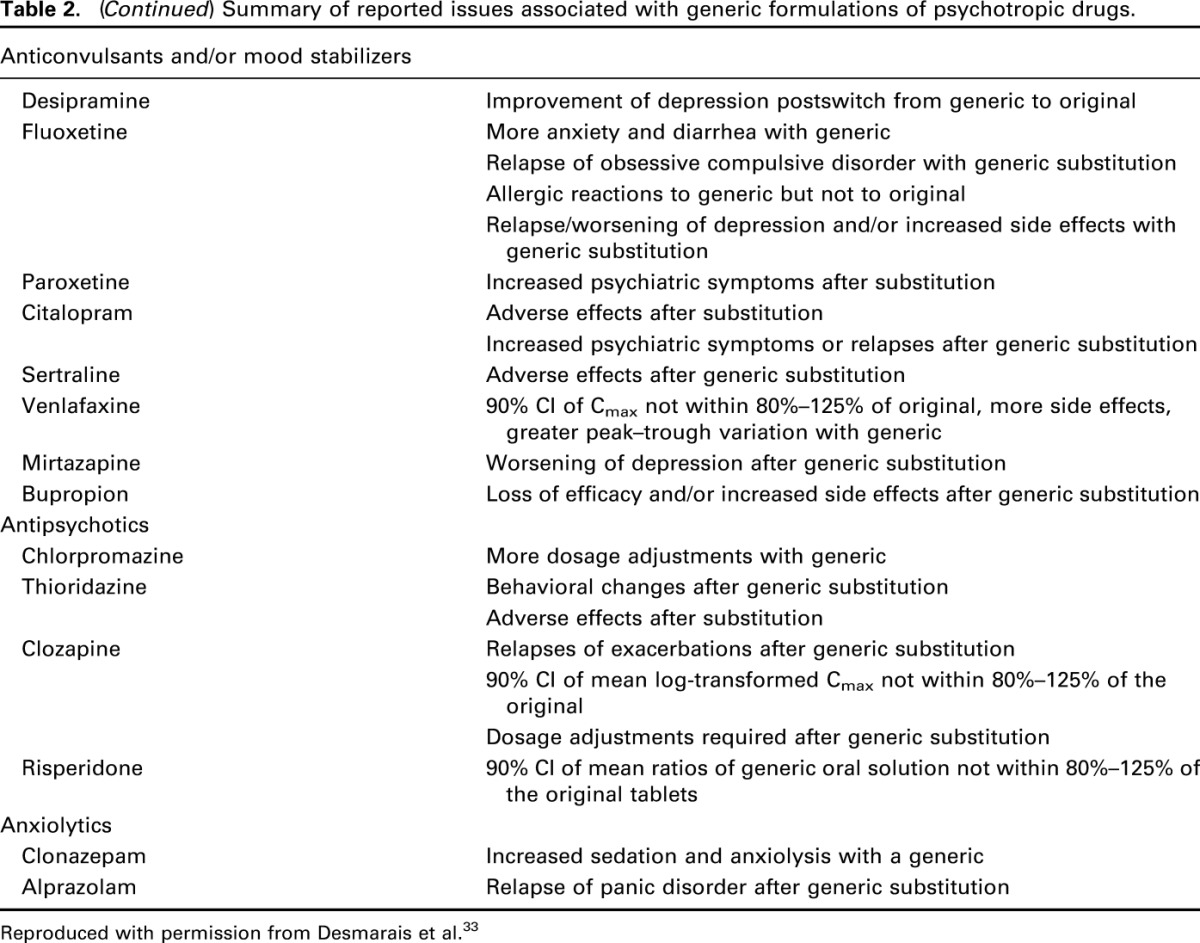
In a further meta-analysis of 9 randomized controlled trials, Kesselheim et al19 found no difference in the odds ratio (1.0; 95% CI: 0.7–1.4), of uncontrolled seizures for patients receiving generic drugs versus those receiving branded drugs. However, some retrospective case–control studies with antiepileptic drugs have yielded data suggestive of therapeutic inequivalence with higher adverse events and higher use of medical resources from loss of efficacy. Table 2 lists different adverse consequences reported in patients administered generic psychotropic drugs including antiepileptics.
Table 2.
Summary of reported issues associated with generic formulations of psychotropic drugs.

Crawford et al13 conducted a systematic literature search of 70 articles of antiepileptic drugs after generic substitution. They found therapeutic failure associated with increased seizure frequency in 25% of study subjects after a brand-to-generic switch. Another large survey was conducted in 974 patients and 435 physicians from Canada, the United Kingdom, Germany, France, and Spain in patients with epilepsy.46 In total, 58% of participants declared that they had concerns about the safety and efficacy of generic antiepileptic drugs; of these, 23% believed that substitution was linked to breakthrough seizures, whereas 58% felt uncomfortable taking a generically substituted drug.
In a retrospective review of 760 epileptic patients, a significant proportion of patients taking generic levetiracetam needed a switch back to the original brand-name drug because of an increase in seizure frequency and adverse events, including blurred vision, headache, depression, memory loss, aggression, and mood swings.52 Similarly, substitution to generic phenytoin was associated with decreased seizure control compared with the branded version. Switching from branded to generic phenytoin also resulted in 22%–31% lower plasma drug levels with generic drug compared with the brand-name drug.54
A questionnaire conducted by the FDA among 196 neurologists reported decreased clinical efficacy and increased seizure frequency and adverse events among their patients after generic substitution of antiepileptic drugs. Approximately two-thirds reported increased seizure frequency and 56% reported increased adverse events.57
In another survey, cases of loss of seizure control followed by generic substitution were reviewed among physicians.37 In total, 50 patients who were well controlled on a branded antiepileptic drug experienced a breakthrough seizure or had an increase in seizure frequency after generic switching. Almost half of the patients on the generic drug had lower drug serum levels at the time of the breakthrough seizure. Clearly, the studies referred to above underscore a clear theme of citing safety, toxicity, or efficacy concerns where brand-to-generic switching is concerned in the area of antiepileptic medications. However, as noted below, outcomes for this particular condition can also translate into cost-based concerns that are not favorable to brand-to-generic switching.
In a further survey of 1354 epileptic patients, high switch-back rates from generics to brand-name antiepileptic drugs (12.9%–20%) were reported compared with antihypertensive agents and antidepressants (1.5%–2.9%).51 Again, this was because of increased toxicity and/or loss of seizure control associated with the use of generic antiepileptic drugs.
In a large retrospective study of medical and pharmacy claims, LeLorier et al39 measured the percentage of patients who switched from a generic drug to a brand-name drug among users of antiepileptic drugs compared with other therapeutic drug classes. Antiepileptic drug users were more inclined to switch back to branded medications (20.8%–44.1%) compared with patients taking generic antihypertensive agents or statins, where fewer patients switched back to their branded drugs (7.7%–9.1%).
In contrast to antiepileptic drugs, antipsychotics, antidepressants, and benzodiazepines are generally believed to have a somewhat broader therapeutic window, and dosing is adjusted based on clinical efficacy or rate-limiting adverse events. Desmarais et al33 conducted a thorough literature search to evaluate the effectiveness, tolerability, adherence, and economics of generic psychotropic medications. Table 2 lists different adverse consequences and adherence issues reported in patients administered generic psychotropic drugs. Relative to the widespread use of generics in these psychotropic medication classes, only a few case reports exist on clinical inequalities, such as switch-related increases in adverse events or re-emergence of symptoms. For example, re-emerging or worsening symptoms were observed in 20 patients after a brand-to-generic switching of citalopram. Emerging symptoms included suicidal ideation, increase in anxiety, obsessive thoughts, compulsions, irritability, and depressive thoughts. Although the previous treatment response was restored after switching back to the innovator brand-name drug, the time required for restoration was greater than the time taken for the re-emergence (0.7–12 weeks vs. 0.58 weeks, respectively).56
Impact on cost and resource use
Helmers et al58 reported the outcome of a large study (n = 33,625) where generic antiepileptic drug treatment was associated with higher total medical service and direct health care costs than with branded drug use, despite the generic drugs being less expensive. After adjustment for confounding factors, the overall annual health care costs for patients receiving therapy with generics were 25.8% higher [adjusted cost differences (95% CI): $3254 ($2403–$4105), P < 0.05] than for patients treated with brand-name products, because of higher health care costs. In another study, compared with the use of the brand-name product, use of topiramate multiple-generic products was correlated with a higher utilization of other prescription drugs, higher hospitalization rates, and longer hospital stays. Also, the risk of head injury and the overall annual health care cost per patient were higher with multiple-generics use than with brand-name use.51
Treur et al55 also estimated the health–economic consequences after the generic substitution of risperidone in schizophrenic patients. In Germany, substituting risperidone with generic products in patients with schizophrenia was found to be not cost-effective if it involved a reduction in adherence rates. Patients who become nonadherent after generic substitution had poorer symptom control, increasing the probability of requiring treatment in a more intensive and expensive facility (eg, hospital). Keeping a patient with schizophrenia on the brand-name risperidone product rather than switching to a generic version was found to be cost-effective (assuming a 40% reduction in medication costs), providing the incremental probability for nonadherence after generic substitution was greater than 5.2%.
Johnston et al11 reviewed a large number of studies in the treatment of hypertension. They found that the treatment of hypertension was often associated with cost-containment measures, and switching to generics was commonly observed to reduce the drug acquisition costs. However, in certain cases, switching antihypertensive caused extra clinic visits, additional laboratory tests, and potential hospitalization because of cardiovascular events from uncontrolled hypertension.
Each of the above highlights the complexities involved when evaluating the economic benefits of switching to generics. Total cost of care and, in some cases, potential indirect costs are among the more comprehensive measures of the true cost of switching to generics. Indeed, each case, condition, or therapeutic entity may represent a unique circumstance requiring careful study before considering its true impact.
DISCUSSION
Brand-to-generic switching is a common practice across all therapeutic areas with the main aim of cost savings. According to the FDA, the cost of a generic drug can be 80%–85% lower than the innovator's branded product; hence generic drugs can be rapidly substituted after patent expiration with estimated cost savings to consumers of $8 to $10 billion a year.40
Although in many cases generics may represent an appropriate alternative, mandatory generic switching may lead to unintended consequences. The present review highlights that generic substitution may lead to concerns among patients, which can lead to challenges in drug adherence. The studies reviewed highlight the existence of negative attitudes toward generic products in a sizeable minority (∼33%) of the patients. Some patients believed that lower prices translated into poorer quality, whereas others believe that economic savings were the reason that they had accepted the generic product. Changes in physical attributes of their medicine added to their uncertainty. Some patients reported a different effect or new or more adverse events. In the case of chronic illnesses, patients who were familiar with their drugs by appearance were less prepared to accept the switch.
Many patients who were surveyed believed that general physicians and pharmacists should discuss generic substitution more actively with them and wished to receive further information, as this would impact their decision to accept the substitution. Furthermore, the research implied that patients would generally prefer the medications to be prescribed by their physician.
Clearly, there are differences in the success rates, either measured by switch-back rates or overall costs of care, prevalence of side effects, toxicity, and/or efficacy based on the therapeutic class of agents undergoing the switch. This is particularly relevant to agents that have narrower therapeutic indices compared with other classes of drugs. For example, in the treatment of epilepsy, significant issues have been documented after generic switching, including breakthrough seizures, more adverse events, and increased cost of care because of extra physician visits or hospitalizations.
Given these observations, the significance of considering the overall impact of generic substitution on medication adherence should be carefully considered for each indication and circumstance relative to their presumed economic benefit. Adherence to treatment should be focused on several levels, including the patient, the health care provider, and the health care scheme. Patients require the knowledge, attitudes, and skills to adhere to an appropriately prescribed treatment regimen. Similarly, health care providers are required to follow established treatment guidelines, make certain that patients are aware and understand the reason for their prescribed medications (branded or generic), any potential adverse effects, interactions with other drugs, and how their medicine should be taken, as well as make certain that the suggested treatment regimen is the simplest possible. If the health care system insurer or the patient cannot afford the branded medication, then the justification and support for generic switching should be explained to the patient, so as not to adversely affect adherence. With a thoughtful approach, the true cost of the medications and consequences of generic switching may be more appropriately considered.
To the best of our ability, we have attempted to include examples of what has been published on the impact of generic substitution. Although we did note a publication bias toward the reporting of negative findings, we do believe that our review is of value as it heightens awareness of the issues surrounding generic substitution.
In summary, caution and careful consideration should be given when implementing a generic switching policy. Based on our review, in certain therapeutic areas, physicians and patients should retain the right to request the branded product when medically justifiable. Generic switching should be assessed on an individual basis, and with disclosure of possible consequences to the patient and a plan to implement the change and assess its ultimate impact through education and monitoring.53
Footnotes
Supported by Pfizer Inc. Editorial support was provided by Sarah Knott of Engage Scientific Solutions and funded by Pfizer Inc. Additional support, composed of copyediting a late draft of the manuscript, was provided by Anne Jakobsen, MSc, and Abegale Templar, PhD, of Engage Scientific and funded by Pfizer Inc.
R. J. Straka was a paid consultant to Pfizer Inc in connection with this study and development of this manuscript. D. J. Keohane and L. Z. Liu are employees of Pfizer Inc.
REFERENCES
- 1.Zarowitz BJ. The generic imperative. Geriatr Nurs. 2008;29:223–226. [DOI] [PubMed] [Google Scholar]
- 2.Lewek P, Kardas P. Generic drugs: the benefits and risks of making the switch. J Fam Pract. 2010;59:634–640. [PubMed] [Google Scholar]
- 3.FDA. Therapeutic Equivalence of Generic Drugs: Letter to Health Practitioners. Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm073182.htm. Accessed July 22, 2013. [Google Scholar]
- 4.Verbeeck RK. Bioequivalence, therapeutic equivalence and generic drugs. Acta Clin Belg. 2009;64:379–383. [DOI] [PubMed] [Google Scholar]
- 5.Kesselheim AS, Misono AS, Lee JL, et al. Clinical equivalence of generic and brand-name drugs used in cardiovascular disease: a systematic review and meta-analysis. JAMA. 2008;300:2514–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davit BM, Nwakama PE, Buehler GJ, et al. Comparing generic and innovator drugs: a review of 12 years of bioequivalence data from the United States Food and Drug Administration. Ann Pharmacother. 2009;43:1583–1597. [DOI] [PubMed] [Google Scholar]
- 7.Association of the British Pharmaceutical Industry (ABPI). Pharmaceutical Price Regulation Scheme (PPRS), London, 2014. Available at: https://www.gov.uk/government/publications/pharmaceutical-price-regulation-scheme-2014. Accessed March 18, 2014. [Google Scholar]
- 8.Blier P. Brand versus generic medications: the money, the patient and the research. J Psychiatry Neurosci. 2003;28:167–168. [PMC free article] [PubMed] [Google Scholar]
- 9.Blier P. Generic medications: another variable in the treatment of illnesses. J Psychopharmacol. 2007;21:459–460. [DOI] [PubMed] [Google Scholar]
- 10.Blier P. Generic substitution for psychotropic drugs. CNS Spectr. 2009;14:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Johnston A, Stafylas P, Stergiou GS. Effectiveness, safety and cost of drug substitution in hypertension. Br J Clin Pharmacol. 2010;70:320–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai K, Fujita M, Ogata H. International harmonization of bioequivalence studies and issues shared in common. Yakugaku Zasshi. 2000;120:1193–1200. [DOI] [PubMed] [Google Scholar]
- 13.Crawford P, Feely M, Guberman A, et al. Are there potential problems with generic substitution of antiepileptic drugs? A review of issues. Seizure. 2006;15:165–176. [DOI] [PubMed] [Google Scholar]
- 14.Borgheini G. The bioequivalence and therapeutic efficacy of generic versus brand-name psychoactive drugs. Clin Ther. 2003;25:1578–1592. [DOI] [PubMed] [Google Scholar]
- 15.Davies G. Changing the salt, changing the drug. Pharm J. 2001;266:322–323. [Google Scholar]
- 16.Verbeeck RK, Kanfer I, Walker RB. Generic substitution: the use of medicinal products containing different salts and implications for safety and efficacy. Eur J Pharm Sci. 2006;28:1–6. [DOI] [PubMed] [Google Scholar]
- 17.Duerden MG, Hughes DA. Generic and therapeutic substitutions in the UK: are they a good thing? Br J Clin Pharmacol. 2010;70:335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker M, Candy D, Kownacki S, et al. Automatic Generic Substitution—Clinical Implications for Patients. Available at: http://www.kidney.org.uk/documentlibrary/AutomaticGenericSubstitutionClinicalImplicationsForPatientsJuly09.pdf. Accessed March 10, 2014. [Google Scholar]
- 19.Kesselheim AS, Stedman MR, Bubrick EJ, et al. Seizure outcomes following the use of generic versus brand-name antiepileptic drugs: a systematic review and meta-analysis. Drugs. 2010;70:605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulsara C, McKenzie A, Sanfilippo F, et al. Not the full Monty: a qualitative study of seniors' perceptions of generic medicines in Western Australia. Aust J Prim Health. 2010;16:240–245. [DOI] [PubMed] [Google Scholar]
- 21.Figueiras MJ, Cortes MA, Marcelino D, et al. Lay views about medicines: the influence of the illness label for the use of generic versus brand. Psychol Health. 2010;25:1121–1128. [DOI] [PubMed] [Google Scholar]
- 22.Hassali MA, Shafie AA, Jamshed S, et al. Consumers' views on generic medicines: a review of the literature. Int J Pharm Pract. 2009;17:79–88. [PubMed] [Google Scholar]
- 23.Babar ZU, Stewart J, Reddy S, et al. An evaluation of consumers' knowledge, perceptions and attitudes regarding generic medicines in Auckland. Pharm World Sci. 2010;32:440–448. [DOI] [PubMed] [Google Scholar]
- 24.Quintal C, Mendes P. Underuse of generic medicines in Portugal: an empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy. 2012;104:61–68. [DOI] [PubMed] [Google Scholar]
- 25.Heikkilä R, Mäntyselkä P, Ahonen R. Do people regard cheaper medicines effective? Population survey on public opinion of generic substitution in Finland. Pharmacoepidemiol Drug Saf. 2011;20:185–191. [DOI] [PubMed] [Google Scholar]
- 26.Heikkilä R, Mäntyselkä P, Hartikainen-Herranen K, et al. Customers' and physicians' opinions of and experiences with generic substitution during the first year in Finland. Health Policy. 2007;82:366–374. [DOI] [PubMed] [Google Scholar]
- 27.Himmel W, Simmenroth-Nayda A, Niebling W, et al. What do primary care patients think about generic drugs? Int J Clin Pharmacol Ther. 2005;43:472–479. [DOI] [PubMed] [Google Scholar]
- 28.Weissenfeld J, Stock S, Lüngen M, et al. The nocebo effect: a reason for patients' non-adherence to generic substitution? Pharmazie. 2010;65:451–456. [PubMed] [Google Scholar]
- 29.Håkonsen H, Eilertsen M, Borge H, et al. Generic substitution: additional challenge for adherence in hypertensive patients? Curr Med Res Opin. 2009;25:2515–2521. [DOI] [PubMed] [Google Scholar]
- 30.Thiebaud P, Patel BV, Nichol MB, et al. The effect of switching on compliance and persistence: the case of statin treatment. Am J Manag Care. 2005;11:670–674. [PubMed] [Google Scholar]
- 31.Ansell BJ. Not getting to goal: the clinical costs of noncompliance. J Manag Care Pharm. 2008;14:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bainbridge JL, Ruscin JM. Challenges of treatment adherence in older patients with Parkinson's disease. Drugs Aging. 2009;26:145–155. [DOI] [PubMed] [Google Scholar]
- 33.Desmarais JE, Beauclair L, Margolese HC. Switching from brand-name to generic psychotropic medications: a literature review. CNS Neurosci Ther. 2011;17:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. Adherence to Long-term Therapies. Available at: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Accessed July 23, 2013. [Google Scholar]
- 35.AMAS. Generic Medicine Switches Confuse Patients and Reduce Adherence. Available at: http://www.gabionline.net/Reports/Generic-medicine-switches-confuse-patients-and-reduce-adherence. Accessed June 19, 2013. [Google Scholar]
- 36.Banahan BF, Bonnarens JK, Bentley JP. Generic substitution of NTI drugs: issues for formulary Committee consideration. Formulary. 1998;33:1082–1096. [Google Scholar]
- 37.Berg MJ, Gross RA, Tomaszewski KJ, et al. Generic substitution in the treatment of epilepsy: case evidence of breakthrough seizures. Neurology. 2008;71:525–530. [DOI] [PubMed] [Google Scholar]
- 38.Kesselheim AS, Misono AS, Shrank WH, et al. Variations in pill appearance of antiepileptic drugs and the risk of nonadherence. JAMA Intern Med. 2013;173:202–208. [DOI] [PubMed] [Google Scholar]
- 39.LeLorier J, Duh MS, Paradis PE, et al. Clinical consequences of generic substitution of lamotrigine for patients with epilepsy. Neurology. 2008;70:2179–2186. [DOI] [PubMed] [Google Scholar]
- 40.FDA. Facts About Generic Drugs. Available at: http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/UnderstandingGenericDrugs/ucm167991.htm. Accessed July 30, 2013. [Google Scholar]
- 41.Hottinger M, Liang BA. Deficiencies of the FDA in evaluating generic formulations: addressing narrow therapeutic index drugs. Am J Law Med. 2012;38:667–689. [DOI] [PubMed] [Google Scholar]
- 42.Håkonsen H, Toverud EL. Special challenges for drug adherence following generic substitution in Pakistani immigrants living in Norway. Eur J Clin Pharmacol. 2011;67:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kjønniksen I, Lindbaek M, Granås AG. Patients' experiences with and attitudes to generic substitution [in Norwegian]. Tidsskr Nor Laegeforen. 2005;125:1682–1684. [PubMed] [Google Scholar]
- 44.Roman B. Patients' attitudes towards generic substitution of oral atypical antipsychotics: a questionnaire-based survey in a hypothetical pharmacy setting. CNS Drugs. 2009;23:693–701. [DOI] [PubMed] [Google Scholar]
- 45.Silkey B, Preskorn SH, Golbeck A, et al. Complexity of medication use in the Veterans Affairs healthcare system: Part II. Antidepressant use among younger and older outpatients. J Psychiatr Pract. 2005;11:16–26. [DOI] [PubMed] [Google Scholar]
- 46.Haskins LS, Tomaszewski KJ, Crawford P. Patient and physician reactions to generic antiepileptic substitution in the treatment of epilepsy. Epilepsy Behav. 2005;7:98–105. [DOI] [PubMed] [Google Scholar]
- 47.Kjoenniksen I, Lindbaek M, Granas AG. Patients' attitudes towards and experiences of generic drug substitution in Norway. Pharm World Sci. 2006;28:284–289. [DOI] [PubMed] [Google Scholar]
- 48.Shrank WH, Cox ER, Fischer MA, et al. Patients' perceptions of generic medications. Health Aff (Millwood). 2009;28:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toverud EL, Røise AK, Hogstad G, et al. Norwegian patients on generic antihypertensive drugs: a qualitative study of their own experiences. Eur J Clin Pharmacol. 2011;67:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallès JA, Barreiro M, Cereza G, et al. A prospective multicenter study of the effect of patient education on acceptability of generic prescribing in general practice. Health Policy. 2003;65:269–275. [DOI] [PubMed] [Google Scholar]
- 51.Andermann F, Duh MS, Gosselin A, et al. Compulsory generic switching of antiepileptic drugs: high switchback rates to branded compounds compared with other drug classes. Epilepsia. 2007;48:464–469. [DOI] [PubMed] [Google Scholar]
- 52.Chaluvadi S, Chiang S, Tran L, et al. Clinical experience with generic levetiracetam in people with epilepsy. Epilepsia. 2011;52:810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolese HC, Wolf Y, Desmarais JE, et al. Loss of response after switching from brand name to generic formulations: three cases and a discussion of key clinical considerations when switching. Int Clin Psychopharmacol. 2010;25:180–182. [DOI] [PubMed] [Google Scholar]
- 54.Rosenbaum DH, Rowan AJ, Tuchman L, et al. Comparative bioavailability of a generic phenytoin and Dilantin. Epilepsia. 1994;35:656–660. [DOI] [PubMed] [Google Scholar]
- 55.Treur M, Heeg B, Möller HJ, et al. A pharmaco-economic analysis of patients with schizophrenia switching to generic risperidone involving a possible compliance loss. BMC Health Serv Res. 2009;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Ameringen M, Mancini C, Patterson B, et al. Symptom relapse following switch from Celexa to generic citalopram: an anxiety disorders case series. J Psychopharmacol. 2007;21:472–476. [DOI] [PubMed] [Google Scholar]
- 57.Wilner AN. Therapeutic equivalency of generic antiepileptic drugs: results of a survey. Epilepsy Behav. 2004;5:995–998. [DOI] [PubMed] [Google Scholar]
- 58.Helmers SL, Paradis PE, Manjunath R, et al. Economic burden associated with the use of generic antiepileptic drugs in the United States. Epilepsy Behav. 2010;18:437–444. [DOI] [PubMed] [Google Scholar]


