Abstract
Hunting for meat was a critical step in all animal and human evolution. A key brain-trophic element in meat is vitamin B3 / nicotinamide. The supply of meat and nicotinamide steadily increased from the Cambrian origin of animal predators ratcheting ever larger brains. This culminated in the 3-million-year evolution of Homo sapiens and our overall demographic success. We view human evolution, recent history, and agricultural and demographic transitions in the light of meat and nicotinamide intake. A biochemical and immunological switch is highlighted that affects fertility in the ‘de novo’ tryptophan-to-kynurenine-nicotinamide ‘immune tolerance’ pathway. Longevity relates to nicotinamide adenine dinucleotide consumer pathways. High meat intake correlates with moderate fertility, high intelligence, good health, and longevity with consequent population stability, whereas low meat/high cereal intake (short of starvation) correlates with high fertility, disease, and population booms and busts. Too high a meat intake and fertility falls below replacement levels. Reducing variances in meat consumption might help stabilise population growth and improve human capital.
Keywords: Meat, nicotinamide, evolution, NAD(H), vitamin B3, Malthus, fertility, immunological tolerance, longevity
Rich fellas …their kids die out but we keep a-comin …we’ll go on forever, Pa, cos we’re the people
The Grapes of Wrath, Steinbeck, 1939.
Introduction
Malthusian demographic projections predict that the rate of population increase will inevitably (postponed in man by improvements in agricultural and medical technology) lead to intense competition between individuals for food resources and reproductive success.1–4 This led Darwin to his theory of natural selection and the concept of the survival of the fittest. However, was this competition just about calories? We will argue that it was really about an optimal supply of nicotinamide/nicotinamide adenine dinucleotide (NAD) important not only for the energy supply but also for the metabolic and genetic regulation and growth of big brains. The demographic success of humans relative to other omnivorous primates, or, as individuals or groups competing with each other, being down to success in obtaining this supply, largely from meat. Nicotinamide adenine dinucleotide powered brains with cognitive workspaces and the physical wherewithal to obtain NAD precursors in an ‘NAD World’.5,6
Demographic events that we discuss in the light of meat intake closely relate to Cohen’s ‘Four Evolutions in human population growth’ covering hunter-gatherers whose populations doubled over hundreds of thousands of years to early agriculturists that doubled over thousands of years to later agriculturists that doubled over hundreds of years to the modern day when populations in developing countries can treble within lifetimes.7
Taking an ecological view of human history was first proposed by Aldo Leopold8 in A Sand County Almanac and taken up by Alfred Crosby9 in The Columbian Exchange and his followers10–13 including Anthony McMichael’s14 recent Climate Change and the Health of Nations. These authors emphasise historical exchanges of food and disease, particularly infectious disease, and the effect of climate on crop yield: here, we expand on the effect of changing nutrition on intelligence, longevity, and reproductive behaviour in more specific detail.15–17 A very long perspective helps our argument beginning with the original rise of the animal kingdom and costly brains.
Meat, NAD, and the Cambrian
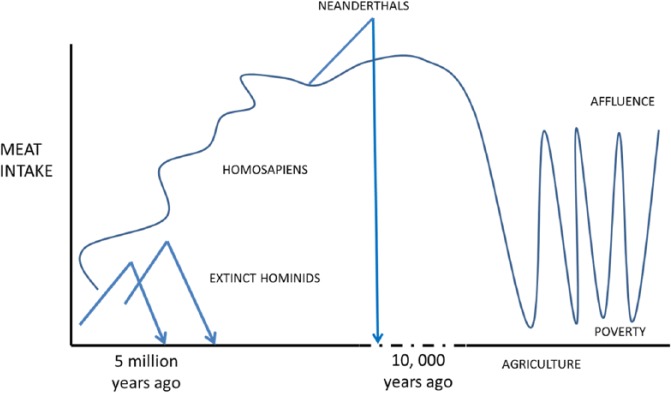
Our argument begins with the Cambrian explosion18,19 (Figure 1). This era is known as Darwin’s dilemma as evolution progressed so fast – a veritable gallop of morphological complexity and genomic variation. The Cambrian was, in essence, an explosion of (vertebrate) brains and mineralised skeletons allowing calculated movement and burrowing for food. Consciousness, sentience, primal emotions, qualia, high arousal, and mental maps whether visual, tactile, sound, pain, taste, and lingering smells leading to memory maps, and so on, necessarily evolved for predation, as did raptorial appendages, in an escalatory arms race. (This evolutionary route was not used by all species as many survived on genetically programmed reflex actions that avoid the high costs of complex nervous systems.) Bacteria that eat worms use NAD as a ‘food signal’ to open their mouths, but if NAD is unavailable they stop reproducing and enter a developmental and reproductive arrest phase, mediated by serotonin, to survive.20 The next evolutionary step was macropredator worms hunting worms and arthropods hunting fish and included widespread cannibalism, often at defined developmental phases.21,22 Carnivory became pervasive and our own evolution can be traced from these times.23,24 Even the clever invertebrates, such as octopi, hunted and they had been preceded in the long Precambrian by the earliest animals with recognisable heads, and ancestral neurones and ganglia, some of whom developed prey capture as their foraging style.25
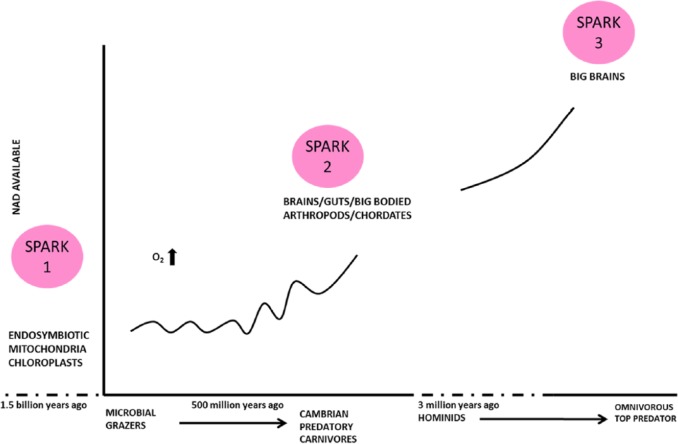
Figure 1.
Evolution seen as the acquisition of NAD. Mitochondrial usage and rising O2 levels followed by animals hunting for NAD followed by even bigger human brains. NAD indicates nicotinamide adenine dinucleotide.
Rising oxygen levels in the atmosphere would have allowed better adenosine triphosphate (ATP) production from NAD(H) + O2 reactions but preceded the Cambrian.26–30 One can take this ‘metabolic acceleration’ argument over the importance of hydrogen metabolism and NAD(H) availability back further to the origins of life, given that it is a redox cofactor used by all living organisms, and this fits with ideas over the importance of the endosymbiotic acquisition of mitochondria and the arrival of visible multicellular eukaryocytes.31–33
Well after the Cambrian, dinosaurs flourished, but a series of volcanic eruptions followed by a large asteroid struck the Yucatan 65 million years ago and caused widespread climate change and extinctions; this is thought to have first affected herbivorous prey and consequently the demise of clever ‘red in tooth and claw’ carnivorous dinosaurs such as Tyrannosaurus rex.34,35 Amniotes, then mammals and primates then took the lead again with improving nutrition (more nicotinamide-riboside in milk and early weaning to animal products) for their young building big brains in an increasingly complex and variable environment requiring problem-solving skills aided by social learning and cooperation.36,37 Strategic and social hunters have to take account of the habits of their prey and competitive groups whether other carnivores or ‘Machiavellian’ members of their own species. Whether one is a proponent of the ecological, social, technological, or general intelligence hypothesis for the evolution of high energy requiring high neuronal count brains, ideas overlap if the original task was clever foraging for difficult-to-obtain micronutrients and high-quality energy in a variable environment with new opportunities and new dangers.38–43
Meat, NAD, and Human Evolution
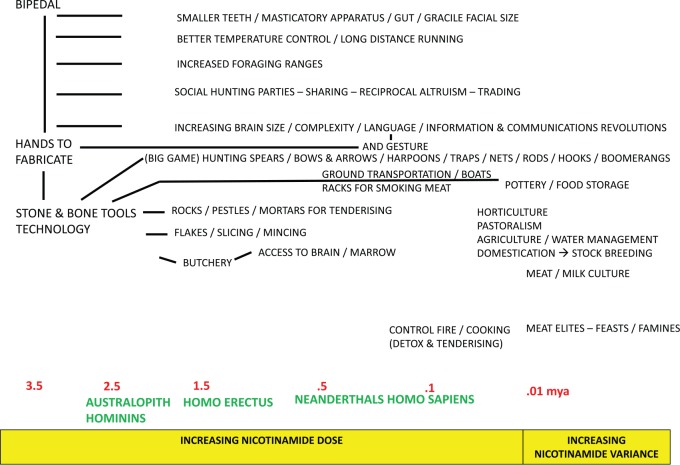
Archaeological and palaeo-ontological evidence indicate that hominins increased meat consumption and developed the necessary fabricated stone tools while their brains and their bodies evolved for a novel foraging niche and hunting range, at least 3 million years ago. This ‘cradle of mankind’ was centred around the Rift Valley in East Africa where the variable climate and savannah conditions, with reductions in forests and arboreal living for apes, may have required clever and novel foraging in an area where overall prey availability but also predator dangers were high44–50 (Figure 2). Tools helped hunting and butchery and reduced time and effort spent chewing as did cooking later.51 Another crucial step may have been the evolution of a cooperative social unit with divisions of labour, big enough to ensure against the risks involved in hunting large game and the right size to succeed as an ambush hunter – with the requisite prosocial and altruistic skills to also share the spoil across sexes and ages.52 The ambitious transition from prey to predator hunting the then extensive radiation of megaherbivores so big that they are normally considered immune to carnivores, needed advanced individual and social cognition as humans do not have the usual physical attributes of a top predator.53–59 Adult human requirements to run such big brains are impressive enough, but during development, they are extraordinarily high with 80% to 90% of basal metabolic rate necessary in neonates – this is probably not possible after weaning without the use of animal-derived foods.51,60,61
Figure 2.
Our early evolution in a nutshell. Steadily increasing nicotinamide powered biological and cultural evolution.
Such a climb up the food chain is unusual as most species go for the easy calories as they increase in body size.52,62,63 Sometimes, this climb down the food chain can be extreme with some, for instance, in the order Carnivora becoming specialist herbivores.64–66 Close examples among primates that have taken this approach are gorillas and orangutans and extinct robust hominids such as Paranthropus – none known for their particularly big brains or sociality relative to chimpanzees. Chimpanzees went for a high-quality diet as did early Homo. Homo sapiens whose brains enlarged markedly simultaneously managed high reproductive rates with large dependent children with long developmental periods and high longevity.67–69 Energy constraints were lifted to achieve this suite of costly traits without always having large trade-offs, at least when the NAD supply allowed.60,70
As a consequence, man may have been part responsible for the wave of megafaunal herbivore extinctions starting in Australasia some 50 000 years ago in a diaspora out of Africa probably driven by hunting parties in search of meat –even if climate and loss of high-protein clover-like foods caused some of the die-off of animals such as the wooly mammoths.71,72 Later came the technically more difficult over-fishing and near extinctions or extinctions of oceanic megafauna and fauna such as the dodo and giant tortoises less than 20 years after they were discovered by meat-hungry sailors. Present-day near extinctions of other primates and meso-carnivores for bush-meat leave ‘empty forests’. All this meat eating changed the geological and physical nature of the world long before the onset of agriculture and marks the true beginning of the Anthropocene where now 90% of mammals are either human or our domesticates73 (Figure 3).
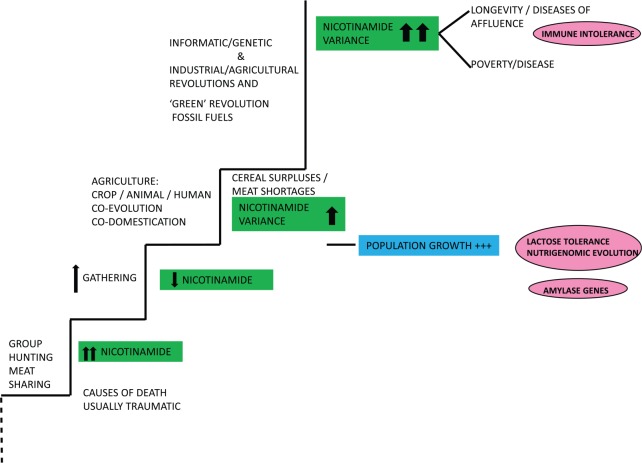
Figure 3.
Our later evolution in the light of oscillations in the nicotinamide supply. Variances provoked population booms and some busts with friction over meat resources between populations and between social classes.
Omnivorous man
Omnivory, such as by farming, first evolved in insects and later by convergent evolution in Eocene primates and is their defining trait74–76 (Figure 4). Our more remote ancestors were herbivores eating grasses, digested by gut symbionts, then frugivores and insectivores eating enough animal protein to supply vitamin B12 and some nicotinamide – either by chance on fruit or more actively, such as fishing for termites. Hominids and early Homo increased meat intake but remained omnivores not carnivores, despite being top predators.77,78 Finding new plant foods and the tension between neophobia vs neophilia (as laid out in the ‘omnivore’s dilemma’) involved dangers that need avoiding to avoid plant toxicity from secondary compounds evolved to deter herbivores.79–82 We were helped by evolving a complex detoxification enzymic system (such as cytochrome P450 now also used to metabolise many drugs) that includes detoxification from excess nicotinamide by nicotinamide N-methyltransferase and culturally acquired cooking methods to avoid toxicity, for example, from cyanogens and improve extraction of calories and some micronutrients (including nicotinamide).83–85 Cooking helped tenderise meat and made plants more edible and nutritionally available – such as the culturally learnt alkalinisation of maize that releases nicotinamide from its bound form niacytin – a process known as nixtamalization.86 Processes for storage and preserving, such as smoking, drying, and salting of meat, or fermentation, whether with co-evolved yeasts and lactobacilli (of cereals to produce bread and beer or of milk to produce cheeses), also improved the nicotinamide supply.87,88 Tolerance of milk drinking throughout adult life – a genetic change that followed the culturally lead domestication of animals for their milk and the potent nicotinamide-riboside in particular – may speak for strong selection pressures for nicotinamide.31
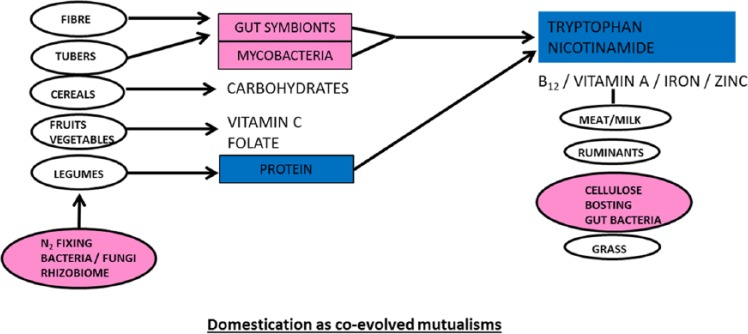
Figure 4.
Omnivores and domesticated diets. Domestication of animals and plants but also of ourselves, our microbiome, and the microbiomes of our domesticates whether of ruminant cattle or the mycorrhiza of legumes.
Being omnivorous has several advantages, an obvious one being switching diet depending on availability, rather than starving. Diet (and altered nutrimicrobiomes) can affect individual behaviour and increase variability within a population – striking in isogenomic social insect castes but also generally true.89–91 Omnivores can use diet to self-regulate behaviour – Argentine ants change from protein-rich to carbohydrate-rich diets when they settle down and increase population size – just as we did).92
Plant and animal cultivation
A key series of plant and animal pre-domestication gardening or pet-like relationships followed by co-evolved domestication happened, including of ourselves.93,94 This transition was slower than previously thought – 3000 years to fix the non-shattering spikelet of wheat – but still speaks for significant selection pressures – not convincingly explained95–98 (Figure 5). Only a tiny proportion of available plants were selected – some 3% of 5000 species with less than 20 plants now providing most of the world’s food supply. Some selection must have been unconscious from metabolic needs and some conscious based on taste, toxicity, harvesting characteristics, or tameness.99–101
Figure 5.
Steady rise of meat/nicotinamide in surviving hominids until the Neanderthals. Homo sapiens survived, but only just, and then we subjected ourselves to large meat variances with demographic consequences.
Why there is sudden interest in plants is unclear even if the when, where, and how is becoming clearer. Farming, for instance, was facilitated by a benign climate at the end of the recent ice age and the warmer, wetter, higher carbon dioxide early Holocene 9000 to 12 000 years ago. This heralded the dawn of food production rather than food gathering and urban civilisation – almost simultaneously, and independently, in 8 to 10 major centres. In the context of our 200 000-year history (‘an eerie synchronicity’), this suggests a single driving evolutionary advantage adapted to local circumstances.102–105
We make the case that the change to an agricultural lifestyle co-evolved as the change in diet changed our (tryptophan-nicotinamide) biochemistry and immunology favouring tolerance of the foetus, increased fertility and higher, denser, populations. Population size and density are important to a social species. More people particularly young people enable more division of labour while avoiding labour shortages – even if the price is hard work, more disease, inter-group stress, and social stratification with rich elites.106 Perhaps, an earlier evolutionary pressure for bigger brains relaxed (brains did atrophy) under domestication in favour of interactive social brains and specialisation.107,108
Change in diet changes serotonin and other neurotransmitters directly and indirectly through altering the gut microbiome (an overall trend from Bacteroides to Prevotella enterotype on more plant-based diets) influencing cognition and mood and potentially increasing social cohesion, collectivism, and sedentism.109–117 Cross infection with symbionts is an advantage when they add to the supply of nutrients of living closer together with less than perfect hygiene, but risks dysbioses. Increased transmission of pathogens and species jumping from domesticates’ vectors led to then emerging ‘crowd’ diseases, such as measles and smallpox, and slash and burn agriculture encourages mosquitoes and malaria explaining why many of these diseases became common at this time.118–120 Pathogens and dysbioses have a ‘Malthusian’ role in reducing population size particularly and may target NAD-deficient individuals on a poor diet.121 This could, seen from one perspective, be beneficial and better than starving and damaging the ecological and farming niche or eating seed corn in desperation – putting eventual recovery at risk. Ecological damage made deliberately and politically by empires being a major factor in their population collapses, although once a popular alternative hypothesis, has proven difficult to confirm.70,122,123
Demographic Transitions
The key features of demographic transitions comprise usually (but not always) improving economics followed by decreased mortality, first in adults and then in infants.124–126 Then, nearly always, this is followed by a delayed decline in fertility from up to 8 births per woman to replacement levels of around 2 (this lag causes the disequilibrium and the population explosion). Population then stabilises but can implode as fertility drops below replacement levels (if not bolstered by immigration or infertility treatment).127–130 This pattern has been and still is being replicated in all parts of the world – even if circumstances and timescales differ in detail. The pattern is almost a demographic law even though the mechanism remains hotly disputed.131–133 During the transition period, population increases often over a century – although some recently have been faster. A historical minimum is a 2-fold increase with an average of an 8-fold increase. Fourfold increases are predicted for China and India, but record-breaking 10-fold to 18-fold increases or more (up to 30-fold) are projected in several countries mainly in sub-Saharan Africa where meat intake remains very low.134,135
Thomas Doubleday136 in his Great General Law made while contemporaneously observing the United Kingdom’s 19th century transition stated that the first decline in fertility happened in the wealthy, whose food habits at that time included far more meat eating, and led him to favour a biological cause for the relative sterility of the rich. We will take his argument a step further and argue that more meat in diet provides the biochemical basis for increased longevity; then the usual (but not inevitable) further improvement in dosage leads to a decrease in fertility as well as improved cognition and education – that then play their part in further decreases in fertility. The length of the lag between increased longevity and decreased fertility is critical to the size of the population explosion and depends, we think, on the speed at which the meat intake (and nicotinamide dose) increases.137–139
Meat – The Key Ingredient is Nicotinamide
Is meat special? In some cultures, they recognise a ‘meat hunger’ and it is a prized food in all cultures with cattle capital often used as a sign of wealth and used for dowries. Great effort and costs are made to hunt and obtain hunting grounds or pastureland, through raids or warfare when necessary. Domesticated animals include omnivores at the cost of them eating human animal product fodder let alone crops. We have argued, as have others, that there have always been easier and safer ways of obtaining calories, such as foraging for tubers.48,140 Vitamin B12 can only be obtained from meat, but an insectivorous diet, even one obtained inadvertently from eating fruit, would have sufficed. Similarly, iron, zinc, and vitamin A are best sourced from meat, but not much is enough to satisfy requirements. Certainly, one does not need to hunt big game. An argument for protein has been found wanting as most essential amino acid needs can be met from plants. However, a case can be made for needing animal protein for tryptophan that is otherwise hard to obtain – tryptophan is a source of nicotinamide, and dietary deficiency also has effects on serotonin and therefore social behaviour.141–144 We have made the case for vitamin B3 – nicotinamide – as this is the key vitamin that is missing in cases of over-reliance on a single plant, usually maize, and little or no meat. Nicotinamide as an essential component of NAD/NADH (reduced NAD) drives the electron transport chain converting the free energy of the electromotive force in to a proton gradient across the mitochondrial inner membrane driving ATP production and controlling pH and other voltage-coupled processes.145–148 Simultaneously, many NAD-coupled redox reactions as well as more recently discovered NAD-consuming reactions are known to be important for cell development, repair, and ageing: NAD is a master controller of much of metabolism necessary for running large bodies and brains and restoring stem cells.70,149–155
High energy has long been recognised as crucial for expanding ape brain size but has often been considered to be paid for by reducing energy spent on large guts, inefficient locomotion, and less chewing.156–158 Storing more fat – another human feature – would be a reserve store of calories and nicotinamide without having to use auto-carnivory of muscle, liver, or other organs where the longer term price is high. Our metabolism runs faster with higher energy expenditure measured as calories burnt (27% more than chimps and more than that in gorillas): this can only happen by boosting mitochondrial function. Increasing the supply of NAD would be a way of accomplishing such a feat.159,160
Lessons From Pellagra – The Forgotten Meat Deficiency State
The past is never dead. It’s not even past. (William Faulkner)
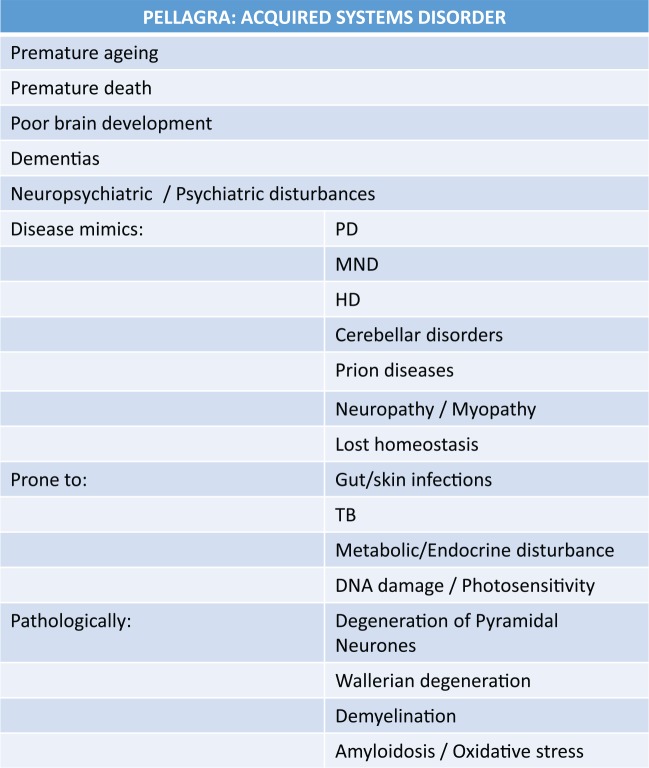
Pellagra causes brain atrophy and subsequent low IQ/dementia, poor social behaviour, and gut dysbiosis.161–163 It has often been considered an atavistic model of human evolution164; in other words, evolution running in reverse as a truly degenerative phenomenon – homo without the sapiens. Pellagra was known as the ‘lazy’ disease with emotional, cognitive, and physical stunting alongside many degenerative disease mimics (as we define them now) (Figure 6). Comments about pellagrins as degenerates were made in the early European literature and the more recent American literature describing the pellagra-prone southern states around a century ago. Clinically undiagnosed cases had impaired IQ with average army Confederate recruits being in the ‘moron’ group (unlike Union recruits) and been felt to be in part responsible for the ‘white trash’ phenomenon with racial differences being both an additional factor in causing the Civil War and in determining the outcome. Such views fuelled the eugenic movement as genetics were thought to be causal and it was believed that such people had too many children.165 The high fecundity observed among sub-clinical pellagrins that caused much of this friction may be relevant to our argument.
Figure 6.
Pellagra has an extra-ordinary wide phenotype. Many mimic modern diseases of ageing. Premature ageing may have been traded off against greater fecundity when the diet is poor. NAD disruption may be a final common pathway of ageing diseases whether from dietary under- or over-dosage or high NAD consumption from stressors. NAD indicates nicotinamide adenine dinucleotide.
Golberger proved that pellagra was dietary in origin and due to a lack of meat and milk, not genetic or infectious, and that eventually influenced thinking that southerners were not genetically degenerate and deserved help.166,167 Finally, the biochemical basis and treatment with replacement nicotinic acid was discovered in the 1940s.168
1850 – UK Data
We now take the data available from the demographic transition in the United Kingdom, 1850–1950, to look at correlations between meat and both fertility and longevity alongside height and IQ as a general marker of health to advance our hypothesis. The period from around 1800 had led to the population doubling to 18 million, despite conditions deteriorating culminating in the ‘hungry 1840s’ (perhaps, a boom with a bust narrowly avoided as conditions improved as the buoyant economy allowed high meat imports). This period had very poor harvests probably triggered by the eruption of Mt Tambora in Indonesia in 1815 with falls in average temperatures and lost summers and El Niño consequences.169 This period is well documented in literature with Charles Dickens Hard Times and William Cobbett’s Rural Rides describing the plight of both city and rural poor as did Freidrich Engels’ The Condition of the Working Class in England. Relevant legislation such as the repeal of the corn laws and new poor and education laws was enacted: relevantly for us the Births and Registration Act culminated in William Farr’s classification system and means that we have clean data from a time when modern medical or contraceptive interventions were not possible.
Methods
Sources for data
All meat data were collated from The Meat Trade in Britain 1840–1914 by Perren,170 Eating Meat: Evolution, Patterns, and Consequences by Smil,171 and The Atlas of Food Who Eats What, Where, and Why by Millstone and Lang.172 Raw data sets for fertility and death were sourced from OPCS (1995),173 The Health of Adult Britain 1841–1994 Charlton and Murphy,174 and Millstone and Lang.172 Life expectancy circa 1850 data were from The Population History of England 1541–1871 by Wrigley and Schofield175 and Ecological Public Health: The 21st Century’s Big Idea?’ by Rayner and Lang.176 Birth rate data was from Hardy.177 Data for diarrhoea were derived from Mortality in England and Wales from 1848 to 1947 by Logan178 and Millstone and Lang.172 IQ and literacy data were from Some British Pioneers of Social Medicine by Greenwood179 and ‘National IQS predict differences in scholastic achievement’ in 67 countries by Lynn et al.180 Fertility data were collated from The Central Intelligence World Factbook.181 World Population Growth Rates data were collated from World Population Prospects in McMichael14 and correlated to recent meat data taken from Weis in Pritchard et al182 and nicotinamide trends from Hiza and Bente.
Statistics
Exploratory analysis was conducted on these data to identify relationships between meat consumption and other variables by conducting scatter plots. The correlation between meat consumption and other variables was analysed using the Pearson correlation coefficient. All statistics were conducted using SPSS (version 21).
Results
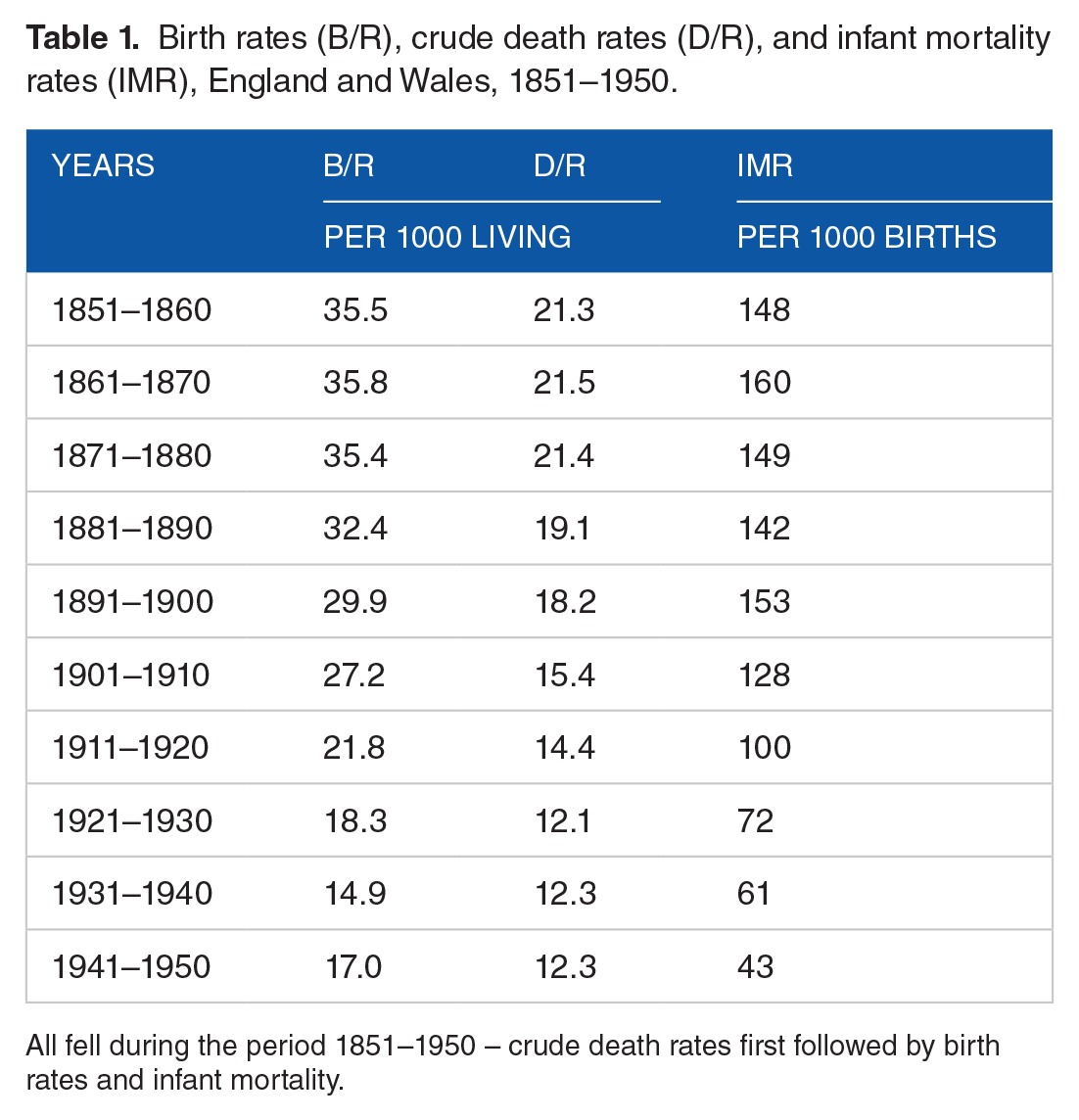
During the period 1850–1950, in the United Kingdom, birth rates fell very significantly as did overall death rates and with a short lag infant mortality rates (Table 1).
Table 1.
Birth rates (B/R), crude death rates (D/R), and infant mortality rates (IMR), England and Wales, 1851–1950.
Life expectancy increased very significantly and trended with increased meat consumption P > .065 and correlates more strongly across the world today P > .0001 (Table 2; Figures 7 and 8).
Table 2.
Life expectancy in England and Wales, 1838–1952.
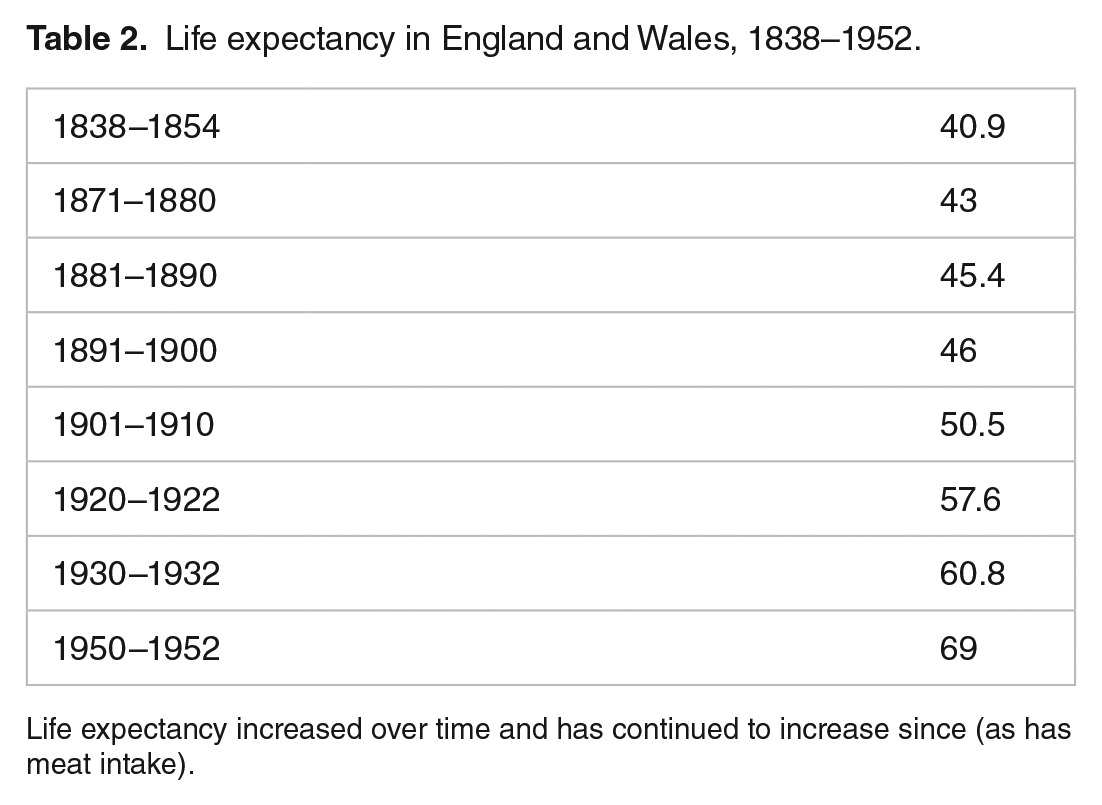
Figure 7.
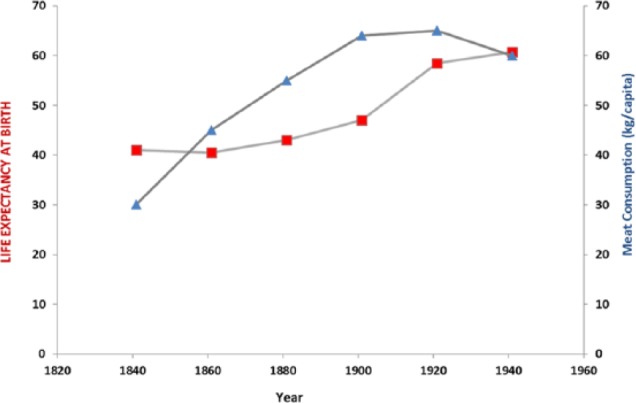
Life expectancy plotted against meat consumption 1820–1960. Overall life expectancy increased and positively trended with increased meat consumption (r = 0.685; P = .067).
Figure 8.
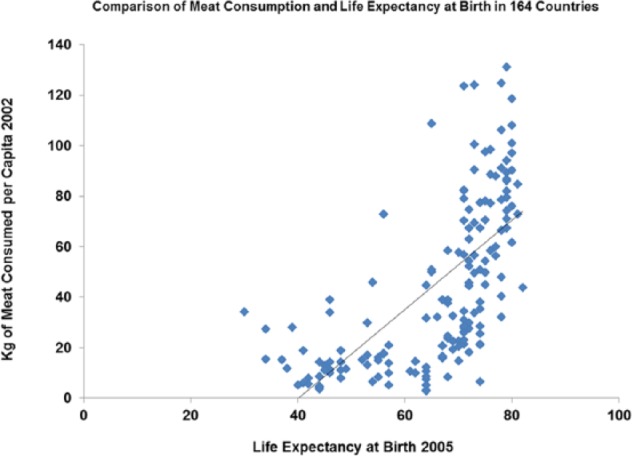
Life expectancy plotted against meat consumption now is significant (r = 0.641; P < .001).
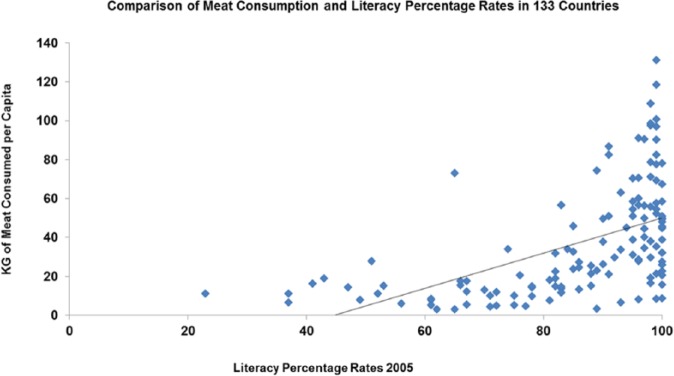
Death rates falling between 1850 and 1960 in the United Kingdom correlate with meat consumption P > .001, as does the drop in fertility rate P > .001 (Figure 9). The death rate falls first and the fertility rate continues to fall until it reaches just above or below the replacement rate. Fertility and meat or nicotinamide intake across the contemporary world correlates negatively (P < .01) (Figure 10). Measures of good physical and mental health whether height or literacy correlate with meat intake in the United Kingdom between 1850 and 1950 and in the contemporary world – all P<0.001 (Figures 11 to 14).
Figure 9.
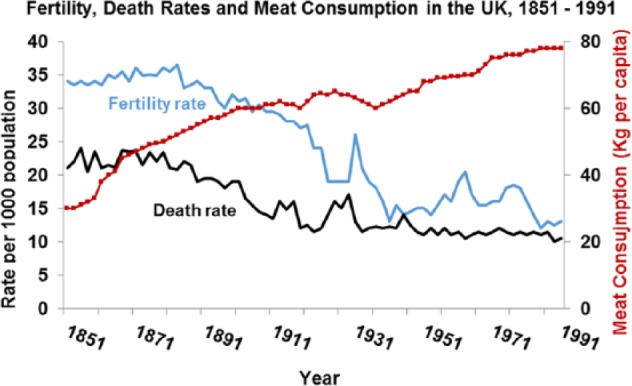
Death and fertility rates plotted against meat consumption. Overall death rates and infant mortality fell and correlated strongly with increased meat consumption (for fertility rate vs meat r = −0.815; P < .001; for death rate vs meat r = −0.864; P < .001).
Figure 10.
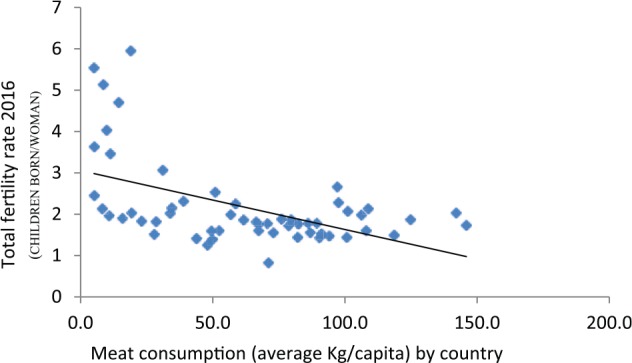
Fertility and meat across the contemporary world (P < .01).
Figure 11.
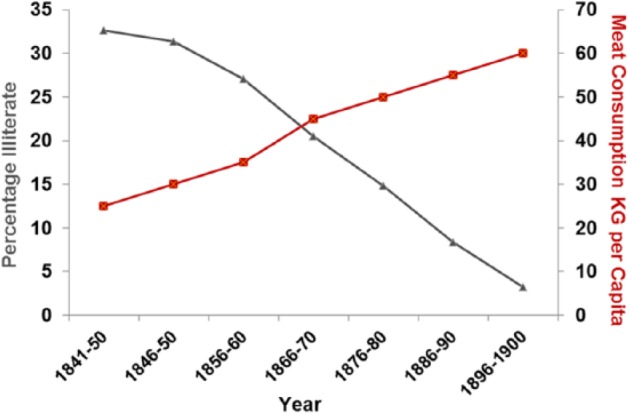
Literacy rates plotted against meat consumption in the United Kingdom 1850–1900 (r = −0.988; P < 0.001).
Figure 14.
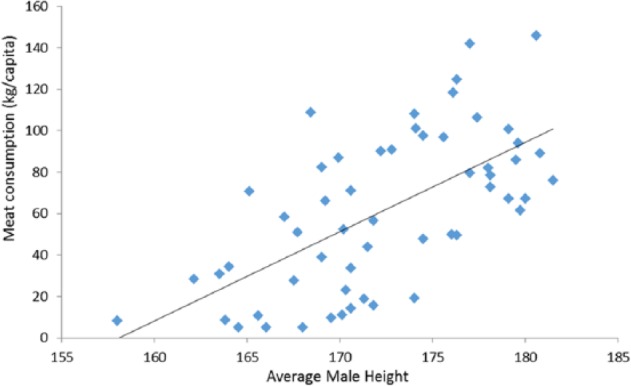
Increased height correlates strongly with higher meat intake currently (r = 0.635; P < .001).
Recent percentage declines in population growth rates significantly correlate with average rise in meat and nicotinamide intake (P < .01) (Figure 15).
Figure 15.
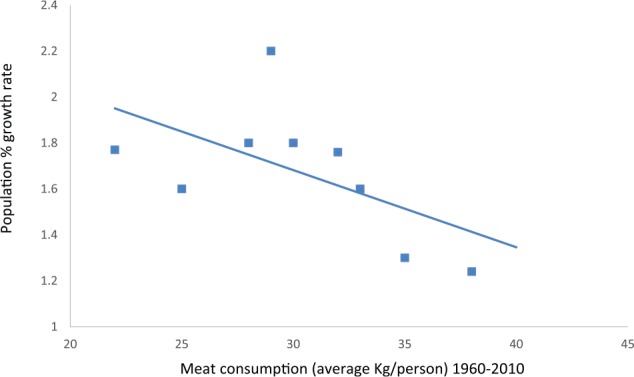
Recent percentage population growth rates correlate with average rise in meat and nicotinamide intake (P < .01).
Having seen these supportive correlations between meat and therefore nicotinamide intake and lower fertility and markers of health and intelligence and therefore longevity during this modern period for which there are data, we now return to our 100 000–200 000 years of evolution and history with meat intake and population growth in mind and then will propose a biologically plausible biochemical mechanism.
Hunter-gatherer times
Meat intake was high in all hunter-gatherer groups beginning some 700 000 years ago and only ending relatively recently on evolutionary timescales, assuming that contemporary studies reflect the conditions under which early humans obtained meat with a relatively light workload.183 Dietary animal protein intake among the San of the Kalahari Desert is approximately 30 to 50 g per person per day (developing countries now average 7–10 g per person per day) derived from 17 of 55 edible animals along with an eclectic yet selective collection of wild fresh vegetables consuming 23 of 85 plant species that they know to be edible.184 The Hadza of Tanzania may be a better model for pre-history as they live in the same kinds of environments, ie, game-rich savannas, steppes, and open forests – not deserts and jungles.185 On this analogy, early human beings are estimated to get 50 to 250 g of meat protein per person per day – high even by modern affluent standards.
Population size by the time that humans had gone global around 50 000 years ago suggests a total of maybe 3 to 4 million people and densities of around 1 person/km2. Given the long time periods involved, this suggests a very gentle rate of population growth, at no time having exponential phases. It has, up till now, been considered a mystery as to why this was so low, given that it is well below the carrying capacity of the land.186 High meat intake during this very long time period was linked with low fertility supporting our hypothesis. Kung San hunter-gatherers who turn to sedentism and agriculture increase their fertility rates.187
Neolithic agricultural revolution
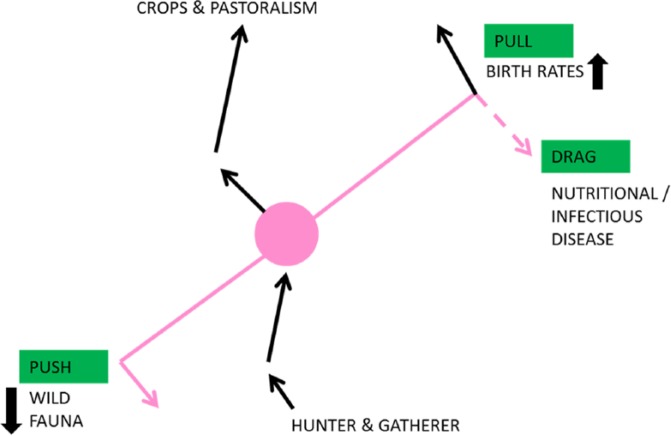
Plant domestication began in the Jordan Valley around 9500 bc with rye, barley, and wheat in the ‘fertile crescent’ using the waters and flood plains from the Euphrates, Tigris, and the Nile. Animal husbandry started a little later in the Zagros Mountains of Iran/Iraq and in North Africa.188 Goats were first domesticated, then pigs and sheep around 7000 years ago, and the largest bovids domesticated from aurochs into cattle herds 6000 years ago with signs of dairy farming between the Sahara and Mesopotamia between 4 and 3000 bc.189–192 Despite domestication of animals, there is general agreement that the increased use of plant foods decreased the dependence on animal protein by around 50%193–197 (Figure 16).
Figure 16.
Decreased meat availability from climate change or over-hunting may have pushed the agriculture revolution, but a bigger pull may have come from increased fecundity.
Populations exploded once the area under crops was large enough to have an effect around 5000 bc doubling every 1000 years, and populations became denser as a consequence.198 Estimates, including from sets of mitochondrial genomes, suggest some 4 to 5 million in 10 000 bc rising to 190 million by ad 500 largely at the first birthplaces of agriculture in Europe and Asia and later within the Roman and Chinese (Han) empires.199,200 The latter is possible due to a benign climate known as the ‘Roman Warm’ from around 300 bc lasting some 800 years.
Evidence suggests that this demic explosion caused the diffusion of agriculture and farmers rather than that the idea, through cultural diffusion, spread. Indeed, the idea may not have been popular as agriculture by all accounts is harder work than being a hunter-gatherer and there are highly significant detrimental consequences for height and health.201–205 Many tooth and bone infections (including tuberculosis [TB]) along with stunting show up in the palaeopathological record and are all associated with deficient animal proteins or iron deficiency – although pellagra itself has no diagnostic features in bone.206–208 Despite all of this, farmers outreproduced hunter-gatherers supporting our argument and in a biological sense were ‘fitter’.209–211
Roman empire to the middle ages: decline and fall – of fertility
The origins of the Middle Ages were one with the decline and fall of the Roman Empire. Rome’s population peaked at 500 000 persons – numbers not to be attained again for several centuries in European cities.212–219 Augustus was so concerned about the falling birth rate among the wealthy, who ate large amounts of meat that in 19 bc, he penalised the childless; the overall phenomenon (later picked up by Doubleday) is well documented by Polybius who predicted that this loss of fertility would affect the ability of Rome to remain masters of the world. Declining numbers across the Western Empire were evident from the spread of agri deserti (‘abandoned fields’) and abandoned villas.220 Finlay, a 19th century American historian of Rome, repeated this analysis coming to the same conclusion and pointed out that it was also true of his contemporary oligarchy in France and was implicit in the use of the Latin term proletariat (proles = offspring) that the poorest had the higher fertility attesting to the antiquity of the observation (described even earlier by writers in Sparta and later again in explanations of the decline of the Habsburg and Egyptian empires).221,222
Rome was highly dependent on imported grain much from ‘bread-baskets’ in Egypt. The diet of the average person, unlike the wealthy, was not heavily meat dependent. Others have wondered if poor diet later pre-disposed Romans to the bubonic plague whose long pandemic lasted from 541 to 750 during the reign of Justinian. Exhausted soil and poor summers may have played a part leaving the empire prone to exogenous attacks whether from plague or Vandals from the west and the rise of Islam in the East. Byzantine eyewitness accounts by Procopius during the Gothic War suggest pellagra: ‘skin livid to black faces with a dreadful sort of insane stare and flesh consumed by starvation – such was the manner in which famine visited the land’. ‘Barbarians’ were mixed farmers (such as the Goths) or nomadic tribes (such as the Huns) with livestock playing a more prominent role in their culture and diet.
We propose that the rise of the Roman empire and its population boom was related to a successful crop agriculture feeding the proletariat. High meat intake among wealthy roman citizens eventually led to their low fertility, and simultaneously among the poor, eventually pellagra-like conditions led to a population bust from disease. Finally, they were overcome by formidable meat–eating barbarians.
Domesday Book to Black Death and its aftermath
The 300-year period up to 50 years before the Black Death in 1348 benefited from good climatic conditions with high solar irradiance and positive El Niño conditions fuelling good harvests and high cereal crop yields.223 Population boomed from approximately 1.5 million in England to a medieval peak of 5 million. Southeast Asian and Indian empires along with Song China population booms were similar and were famously very cereal dependent.224–228
Meat intake was very low just before the plague as rough pasture was turned to arable and climate change to cold wet dark summers led to harvest failures with famines – such as the famine of 1314.223,229–231 Poor pastureland triggered diseases in sheep (scab) and cattle plagues (probably rinderpest) that decimated the meat supply before the plagues. Yersinia pestis had evolved remarkably quickly from Yersinia pseudotuberculosis in around 20 000 years and has a very active NAD metabolism. It is spread by fleas normally on rodents, the natural host, advancing from Asia to Europe causing massive mortality rates with the major outbreak in 1348 and at least 4 sequels before the end of the century.232–234
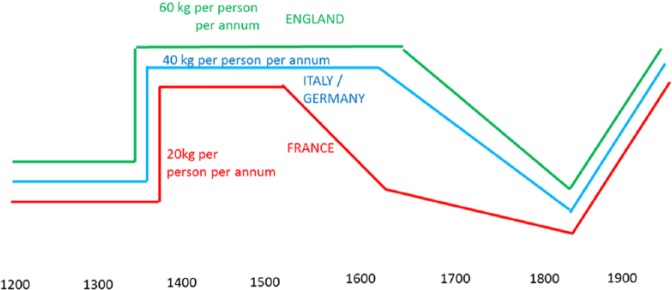
Because of the resultant population decline to 2 million people, arable land was turned back to pasture (as so much grain was no longer required).235,236 Meat became more available to the survivors as production stayed stable or increased with the invention of the plough. Meat and milk intake doubled between 1300 and 1450 but varied within Europe (Figure 17). Despite a survivor benefit that included higher wages and more housing for couples contemplating marriage, fertility remained paradoxically low and the population did not rebound taking two centuries to climb back to the medieval peak.237 This is a good example of a high meat diet leading to low fertility rates and an increase in human capital.
Figure 17.
Meat intake was not the same in every European country. This may explain the relative success of England during this long period up to the industrial revolution.
Geographic variation: the New World
The ‘fertile crescent’ was lucky having significant choice of both domesticable plants and animals, but other parts of the world were less fortunate.238 Diets often became dependent on single cereal staples all with problems that are usually solved by adequate meat or dairy intake. Wheat is deficient in lysine/isoleucine, rice in protein and vitamin A, millet or sorghum deficient in protein. Maize is deficient in nicotinamide and tryptophan.
As the last habitable continent colonised, the Americas were slower (4000 rather than 10 000 years ago) to take up crops with several reverses (elsewhere unusual except in marginal zones sensitive to climate change such as for the Norse in Greenland) back to hunter-gathering lifestyles – suggesting that the risk-benefit ratio of the agricultural revolution was tighter than in the Old World.
The pre-Columbian New World became reliant on maize once the minute cobs attracted human interest and became the principal focus of artificial selection and domestication. Maize first appeared around 9000 years ago in Mexico having descended from teosinte and may have been first attractive for its sugary stalks that could be chewed or fermented. Maize has advantages as it grows in difficult hydrological conditions and returns 25 to 100 (1000 in modern times) grains per grain planted (compared with 5 grains for wheat),239 relevant as the continent was prone to droughts and poor soils that did not make agriculture easy.240 Micronutrient deficiencies were partly corrected by adding beans and squash to the diet and co-evolved cooking measures involving alkali releasing some nicotinamide from maize. Despite this cultural adaptation, presumably under strong selection pressure, the risk of sub-clinical or clinical pellagra must have always higher than in other parts of the world – as it would have been if maize was exported without the learnt cooking adaptation.
The original Americans had potato, squash, beans, chillies, tomatoes, peanuts, avocado, and manioc (cassava) and plants with medicinal, eg, quinine, and hallucinogenic potential for ritual or recreational purposes, e.g., cocaine, mescaline, chocolate and tobacco.241,242 Tobacco is of interest as nicotine is so closely related to nicotinic acid and other hallucinogenic compounds intersect with tryptophan – serotonin metabolism perhaps acting as pharmacological meat/nicotinamide substitutes.243–245
Their agricultural revolution was also harder as there were few herbivorous animal species, namely, the turkey, lhama, guinea pig, and dog suitable for domestication. Unlike the New World, this domestication of animals happened with a lag of 1000 to 2000 years after plants putting the whole society at risk of an unbalanced diet. Impressive though, the Aztec, Inca, and Mayan empires were they were considerable, later than equivalent cultures, in the Middle East and some never developed full literacy – a gap of approximately 2000 years – and when the two worlds eventually clashed the older culture with a long history of higher availability of meat and earlier successful domestication ‘won’. Columbus first noted these discrepancies ‘I saw neither sheep nor goats nor any other beast’.
Such plant-based pre-Columbian societies such as the Maya are well known for population booms and busts adding to our argument.246–248 Pre-Columbian archaeology suggests large but often unstable populations with evidence of disease in bony material. Tuberculosis was certainly present, and bone pathology shows chronic infection, iron deficiency, and stunting suggesting a deficiency of animal products in diet – pellagra itself is not known to have any specific bony changes.249,250
Capturing human slaves for cannibalism are well-documented.251,252 This speaks for a meat shortage and a meat hunger. The enigma is explained of Aztec human sacrifice and wars taking prisoners -–rather than the more usual land for agricultural usage – as there were no domesticated herbivores, so there was no point. This was a politically and religiously sanctioned form of human cannibalism fronted by meat-requiring gods. Meat went preferentially to the nobles, and the commoners only obtained meat at feasts or if they were responsible for human captures gaining the right to eat human flesh as a reward.
The heavy dependence of the Americas on plants and a boom-bust population history with poor human capital supports our hypothesis.
The great divergence and enrichment: the smart European ‘Miracle’
Although some think of the roots of economic growth (perhaps 100 times that of the agricultural era) and the dominance or Europe and Great Britain in particular as being a product of the Industrial Revolution, its roots date from the aftermath of the Black Death.253,254 This could not have happened without high intellectual function with perhaps 10 000 very well-educated Europeans with enough time and longer lives to participate in the search for useful knowledge about nature and the environment.255–257 Others have wondered if the rise of the British and later American empires was related to diet with some implicating a sugar or caffeine ‘rush’ but others pointing to ‘beef-eaters’ – certainly, both empires consumed high quantities of meat.258–260 Earlier and relative to other parts of the world, Europeans commanded more working capital in the form of livestock that they ate often in prodigious quantities especially if rich. Literacy rates were considerably higher than elsewhere and technological and decision-making superiority played significant roles in the conquest of America. Superior health may have been just as decisive.261,262
The Columbian Exchange
This major global event is well known to have caused population busts in the New World but population explosions in Europe.263 The New World populations were decimated by imported disease, but their own poor constitution from their relatively poor low meat/high maize diet may have contributed. Livestock were later imported and populations eventually stabilised, although not fast, suggesting that the extra meat did not lead to another population boom.264,265
Maize was imported to Europe, and although often considered a food fit for animals, not humans (by decree in France), it did not stop its popularity and later spread to Asia and particularly Africa. Population booms followed. Maize was directly held responsible for the outbreaks and first descriptions of Pellagra in Spain and Italy in the 18th century in poor people on a very low meat diet.266
Easter Island
For those who prefer case studies from microcosms, the story of Easter Island may be relevant: islands cannot support big game, and as plant agriculture developed strongly, Easter islanders’ population boomed. Few animal domesticates, however, resulted in them becoming reliant on eating rats that are, however, competing omnivores, eg, over seagull eggs. This desperate meat poverty may have caused booms and busts followed by a final collapse.267
Ireland
The Irish famine around mid-19th century may also have been a low meat population boom with too high a reliance on the potato and little meat. Although potato blight was blamed (alongside the attitude of the British Government), this was ultimately another Malthusian population bust.268,269 Interestingly, even within Ireland, the Irish ate the most potatoes and had the highest population growth and later declines – such exceptional fertility rates continued after emigration to New England and reliance on Indian maize.270,271
China
China’s demographics have been considered, even by Malthus, to be different. But we would argue a country well known (until very recently) to have had a low meat diet and exceptional population growth – 200 to 1400 million people between 1700 and 2000 – supports our hypothesis.272 Differences claimed for China include a collective rather than an individualistic attitude but that in itself may be influenced by a low meat diet. Collective and coercive attitudes have been prominent from high use of late marriages and at times female infanticide or state intervention with one child policies. These preventive checks may have kept fertility rates below the maximum of 8 but were still high at around 6 children per eligible woman. Malthusian checks with multiple famines up till the Great Leap Forward 1958-1961 causing severe population busts are well recorded demonstrating that this is another cereal-dependent boom-bust population.
Meat intake has improved markedly in recent times and mortality has fallen from 1950 with fertility following from 1970.273 We argue that more meat, with its biological effect on fertility and higher IQ not primarily moral restraint or state intervention, is now responsible for this more controlled demographic transition.
1950
Meat and demographic transitions continue across the world, such as in China, but stall or fail to complete in other places, notably Africa.274–276 Africa, despite being the ‘cradle of mankind’ subsequently, was unlucky in both the availability of good animal and plant domesticates and its climate. Africa has many parasites and the aridity that helped initially became too extreme with much of the continent becoming desert. Tsetse fly and trypanosomiasis were the bane of cattle and man and with rinderpest could decimate the meat supply with outbreaks of pellagra described in the south.277–281 Malaria of course was, and is, a major issue and has known interactions with NAD metabolism as do the evolved protective haemoglobinopathies: high meat areas appear to escape human-biting mosquitoes (who bite the animals instead) and individuals have a better constitution so see a decline in disease rates as a result.282–289 Low meat areas prone to pellagra or famines are prone to malaria in the past even in temperate zones in Europe and America a century ago (even though other dietary deficiencies such as that of iron protect).290 Meat intake is still very low in many parts of Africa, and over-reliance on maize remains a prominent feature.291 One has to consider Africa as a high-risk area for nicotinamide deficiency with resultant classic boom-bust demographics as history repeats itself, currently, in spades.292–296
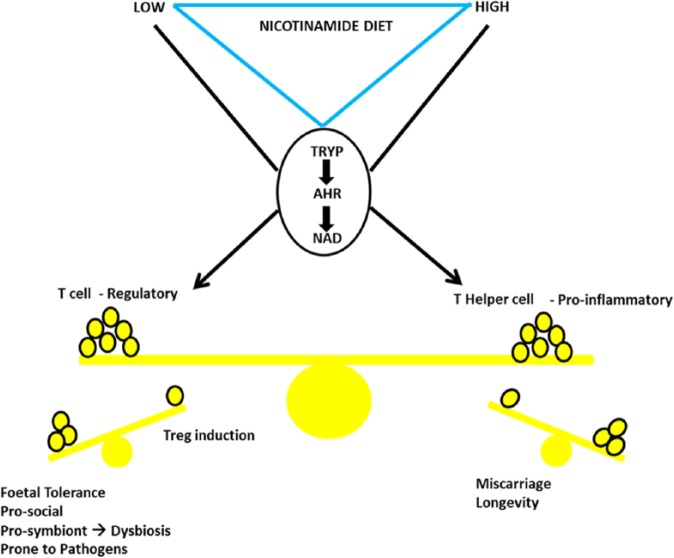
Correlation to mechanism: the nicotinamide/tryptophan/kynurenine immune tolerance pathway and fertility – maternal acceptance of the foetus
So, in broad consistent but somewhat anecdotal historical terms, with more specific data from one transition period in the United Kingdom, 1850–1950, our hypothesis is supported, but is there a mechanism? (Figure 18).
Figure 18.
Low nicotinamide in diet biases the ‘de novo’ pathway towards catabolising tryptophan to nicotinamide. This increases tolerance of the foetus but risks illness and deaths from dysbioses and pathogens.
Nicotinamide, though, classified as a vitamin is more complicated than just looking to diet as some is provided by the microbiome (in gut or perhaps from TB) and also as there is an intrinsic ‘de novo’ pathway as it is synthesised, rather inefficiently, as a degradation product of the essential amino acid tryptophan.243 Both these backup sources are used when the diet is poor. Activation of the ‘de novo’ pathway affects the immune system and thus is also known as the ‘immune tolerance’ or ‘disease tolerance’ pathway. This pathway allows symbionts to flourish – that produces nicotinamide/nicotinic acid – but affects adversely the ability to resist pathogens.297,298 This pathway can be neurotoxic, although this ‘autocarnivory’ may release precursors to nicotinamide as another short-term fix.144,299–302
This pathway is the biochemical site of immune privilege.303–310 Importantly, for our argument, this immune privilege includes transgenerational tolerance to the allogenic foetus that one would normally expect to be rejected by the mother as ‘foreign’. When this pathway is suppressed, inflammation is followed by spontaneous abortion (as can also happen for some cancers and organ transplants).311 Indoleamine 2,3-dioxygenase 1 (IDO-1), the rate-limiting enzyme in this pathway, is produced in trophoblast cells that result in selective apoptosis of T cells.312 Systemic inhibition of IDO in pregnant mice results in immune abortion of allogenic foetuses through mechanisms that involve Tregs.313 Adoptive transfer of pregnancy-induced Tregs prevent fetal rejection in murine abortion models, and human pregnancy is associated with an increased number of immunosuppressive Tregs.314 Indoleamine 2,3-dioxygenase is highly expressed in the placenta and serum during pregnancy and encourages trophoblast proliferation, migration, and invasion essential to placental and fetal development, and its inhibition whether by pharmacological blockade or high nicotinamide in diet will result in an inflammatory reaction causing early abortion or later pre-eclampsia and fetal loss.315–319 Dietary interactions with fertility have indeed been recognised, although not specifically in the context of nicotinamide that may have a regulatory role affecting fertility in a positive or negative direction depending on dietary and other contexts.320–331
This mechanism using the ‘de novo’ pathway evolved long before humans being present in all animals (affecting how much nicotinamide is needed from diet) and may be a method of population control. It might explain why top-predator/carnivore populations do not relentlessly expand to the estimated carrying capacity of the land even when prey is plentiful – the ‘prudent predator’ (as we were in hunter-gatherer days). This avoids species abusing keystone status and exponential growth of populations with eventual extinction from decimation of prey. This pathway also allows a switch from quantity (what ecologists call ‘r’ selection hedging bets with large numbers of offspring) to quality (‘K’ selection) of fewer offspring depending on ecological context. Namely, the latter higher quality route is used when there is a better diet with more nicotinamide – a defining feature of our history.332,333
NAD and fertility
NAD itself when extracellular and working through adenosine diphosphate ribosylation of surface proteins such as CD38 and the ART2 and prinoreceptors can shape regulatory T cells and immune tolerance at times killing T cells and working on host-symbiont, host-tumour, and host-fetal interactions.152,313,314,334–340 NAD influences the endocrine system and oxytocin affecting social and sexual interactions.341 Central oxytocin release through a NAD-CD38 pathway affects mating and social/sexual pair bonding, maternal nurturing, and therefore, chances of infant survival and their own later (parental) behaviour – let alone having peripheral effects on labour and lactation.342–345 NAD consumers such as SIRTs are associated with the hypothalamic-pituitary-gonadotrophin and stress axis and developmental oocyte and spermatogenesis and with both male and female infertility.346,347 SIRTs are involved as sensors and guardians of the redox state in the key issue of oocyte ageing and the natural decline in fertility with age ending with the menopause – given that ovarian lifespan is the main determinant of reproductive lifespan.348,349 Poly (ADP-ribose) polymerases (PARPs) upregulated by oestrogen in the uterus have an important role in implantation of an embryo.350,351
Dietary Aversions
Pregnancy dietary aversions are also of interest as the commonest single aversion is of animal products, particularly meat.351 Morning sickness and meat aversion could during the first trimester affect the outcome of pregnancy and fertility rates. Cravings usually involve non-meat items and when they do involve animal products may be influenced by evolution having to balance the suppressed immunological needs of pregnancy with the nutritional needs of both the mother and the foetus if seriously deficient.318,352–355
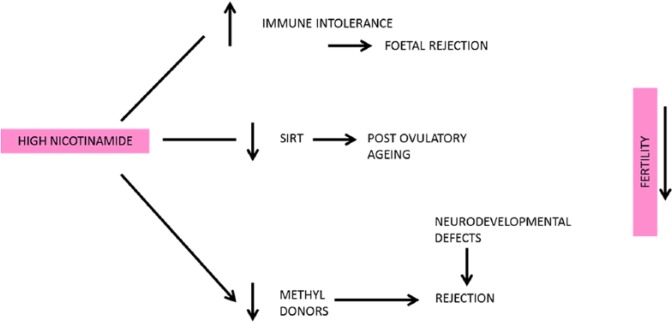
In summary, we therefore have biochemical mechanisms (there may be others such as the rise of polycystic ovary syndrome) whereby those on a low-nicotinamide diet could have a higher fertility due to lower chances of rejection at many stages of pregnancy (Figure 19). Clearly if taken too far to frank pellagra or starvation fertility falls – in the famine in 1940–1942 in occupied Athens for instance births declined by nearly 50% as it does in frank pellagrins.269
Figure 19.
High nicotinamide could influence fertility rates by various compounding mechanisms.
NAD, survival, and ageing
Fertility is a key issue in demographics but so is survival and ageing.356 Ageing, in general, as in oocytes in particular, have long been associated with declining mitochondrial function and free radical production.357 Pellagra caused premature death and premature ageing. Much recent research supports a major role for good mitochondrial function and the NAD-sirtuin axis in ageing. Dietary intake of nicotinamide, nicotinamide-riboside, and nicotinamide mononucleotide improves metabolic health and restores mitochondrial function whether measured by respiration, membrane potential, ATP production apoptosis, autophagy, or the unfolded response with less degeneration and more active stem cells.358 Supplementation works for genetic versions of premature ageing and defects in DNA repair with resulting SIRT inactivation. NAD levels decline with age in part due to diet and in part due to increased requirements to repair damage particularly to DNA, using PARPs.358 High nicotinamide in diet and therefore NAD levels reduces the need for symbionts and the risk of the relationship becoming dysbiotic and improves resistance to pathogens. All improve the chances of survival to older ages.359
Conclusions
Meat eating and high nicotinamide dose have been important throughout our evolution and may explain why when we are on an optimal diet, we have enough energy and the mechanisms to run big costly brains as well as high reproductive rates and long lives without always having to trade one off against the other.360–362 Meat eating correlates with low fertility. This was true in the long pre-Neolithic and has remained a consistent trend in all the examples that we can find. With the exception of the pre-Neolithic when trauma (rather than disease) caused many deaths at all ages, high meat eating also correlates with physical and mental health and longevity, the other main driver of population growth.
We have drawn attention to a biochemical mechanism whereby high nicotinamide in diet switches off the ‘de novo’ pathway stopping in-house production of nicotinamide from tryptophan and causing immune intolerance, including of the foetus and dietary-induced infertility (Figure 19). The population explosion related to the Neolithic agricultural revolution and lower meat and higher cereal diets and high fertility was just the first example of this commonplace and causative correlation that continues to this day363. High fertility and enlarging populations when nicotinamide dose is low had many advantages (at least in the past) but is traded off against poorer health with more chronic infections and lack of resistance to pathogens and reduced height and cognitive skills.364 Picking quantity or quality offspring to survive has depended on diet throughout our demographic history and remains the main driver of inequality between nations and individuals.365,366
There is a general consensus that there are too many exceptions to link any one aspect of progress and social modernity such as educational or economic improvements or even birth control to be truly causative of the decline in fertility.367 The incontravertible law is that the mortality decrease happens first with the logical implication that the then apparently inevitable fall in fertility occurs by the same basic mechanism. We argue that modest improvements in home economics allows poor families to improve diet and they will prioritise spending on animal products (Engel’s law). An improved nicotinamide dose first decreases mortality from gut infections, TB, and classic pathogens, and then at a higher dose reduces fertility creating a natural lag responsible for the population increase. The length of the lag depends on whether the meat/nicotinamide supply continues to improve and at what tempo. Many propose increasing literacy and access to better birth control devices to speed demographic transitions. We argue that diet needs dealing with first and the rest will follow367–369 (Figure 20 & 21).370
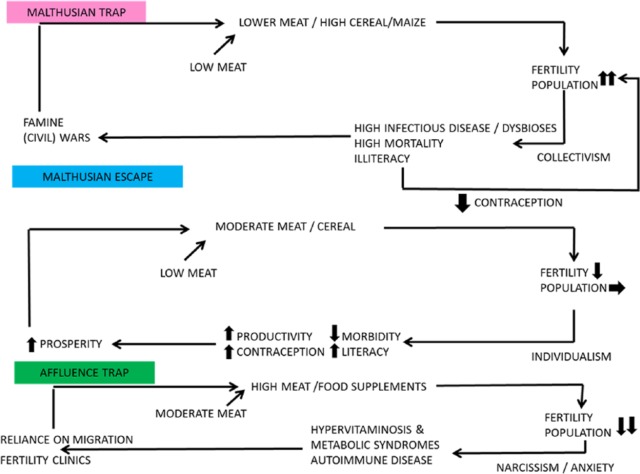
Figure 20.
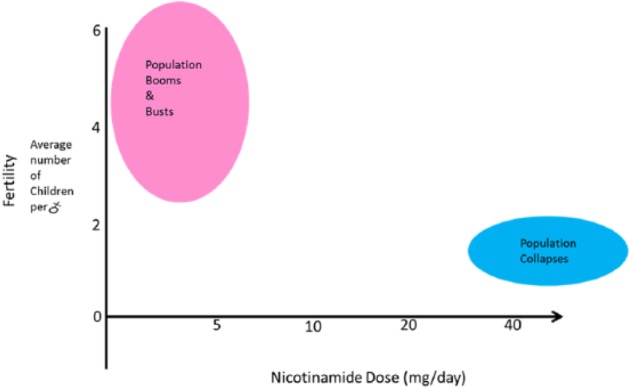
A summary of the demographic effects of nicotinamide dose on fecundity Moderation works.
Figure 21.
Classic Malthusian trap and deadlock on low-nicotinamide diet. Malthusian escape on a moderate nicotinamide diet. Affluence trap on a high-nicotinamide diet – rise of fertility clinics and necessary immigration.
Such ‘Enlightenments’ that also come with scientific and technological change, democracy, and emancipation may always have related first to better diet not better institutions or markets.367–370 Many think that our economic and intellectual breakthrough started in the period after the Black Death. Meat intake was high then fell but got a second wind after 1850. In contrast, other countries such as China had a much lower meat intake and although made promising scientific starts, and indeed may have been ahead for a while, never quite managed to pull off an early industrial or cultural revolutions or demographic transition.371
Better diet with more meat/nicotinamide closer to our evolutionary norm helps cognition. The French anthropologist Lévi-Strauss commented that foods must be ‘good to think’ before they can be ‘good to eat’.372,373 Usually, the emphasis has been on the social, symbolic, and cultural nature of our food-consuming behaviour – rarely positive in the case of meat eating. Meat eating as one gets richer is often seen as a penchant, or worse, for example gutting linked to, ‘demonic males’ or violence for violence’s sake rather than that there is a real ‘meat hunger’.374 Being in denial about our need for animal products and not accepting that ‘meat hunger’ is biological and not a matter of taste, or cultural history, or perversity, or to signal status/wealth/masculinity or our war-like nature does not help375.
Meat is expensive requiring many resources whether land, water, or grain affecting the ‘green-house’ effect, so increasing meat in diet looks highly counter-intuitive on a sustainability agenda given its ecological ‘hoofprint’.376–378 Traditional farming for animal husbandry used grassland not suitable for crops.379 Those days are being re-visited as part of value ‘farm to fork’ local chains, but other technologies need to be developed. Agroecology is an established field and aims to nourish not just feed populations on evidence-based rather than ideological grounds.380 This has to be better than driving a desperate market for bushmeat that is as damaging to forests as clearing them for agriculture – or one that leads to poor game management where human hunters eliminate all fauna larger than rats. Poverty is the biggest polluter borne out by Kuznets curves showing that as countries develop pollution increases but then decreases.381
Re-distributing micronutrient supplements or preferably meat as a meat ‘entitlement’ would be a parallel approach. Clean water in countries in the past was introduced as an entitlement for rich and poor, not really from good social motives but because the poor were thought of as a source of typhoid and cholera: better food for the poor is not yet considered in the light of reducing risks for the rich but if it was, being a clear source of (emerging) infection or violence, this could provide the motivation to share more, as we did in our evolutionary past.382 Mitigation of environmental effects could come from re-distribution of meat, eliminating the extremes, or advances in artificial meat production or other sources of animal protein not commonly used, such as insects.383,384 Meat moderation and ‘flexitarian’ diets are, after all, a policy already followed by the ‘healthy-wealthy’ in a life cycle approach that should begin with concentrating on the first 1000 days of life - and may be both the healthiest and the one most likely to be environmentally and economically sustainable.385–389 We make the case that history has taught us that a dietary approach modestly increasing animal products for the poor rather than cereal subsidies work and may be the necessary and sufficient approach to control population size and improve human capital even if there is a conceptual and political mountain to climb.390
Figure 12.
Literacy rates plotted against meat consumption in the contemporary world across nations (r = 0.531; P < .001).
Figure 13.
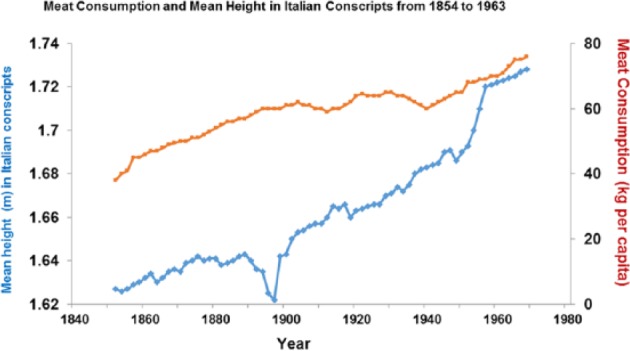
Increased height correlates strongly with higher meat intake in the past (r = 1; P < .001).
Acknowledgments
A.C.W. would like to thank Mike Hammond and the Richardson family for their support and Robin Dunbar for earlier discussions.
Footnotes
PEER REVIEW: Five peer reviewers contributed to the peer review report. Reviewers’ reports totalled 1091 words, excluding any confidential comments to the academic editor.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is funded by QEHB Charities.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
ACW and LJH analysed the data, agree with manuscript results and conclusions, and made critical revisions and approved final version. ACW wrote the first draft of the manuscript. LJH contributed to the writing of the manuscript.
REFERENCES
- 1.Kaack LH, Katul GG. Fifty years to prove Malthus right. Proc Natl Acad Sci U S A. 2013;110:4161–4162. doi: 10.1073/pnas.1301246110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura M, Bhatnagar A, Sadoshima J. Overview of pyridine nucleotides review series. Circ Res. 2012;111:604–610. doi: 10.1161/CIRCRESAHA.111.247924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clutton-Brock T. Mammal Societies. Hoboken, NJ: Wiley; 2016. [Google Scholar]
- 4.Mayhew RJ. Malthus. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]
- 5.Morowitz HJ. Energy Flow in Biology: Biological Organization as a Problem in Thermal Physics. Woodbridge, CT: Ox Bow Press; 1979. [Google Scholar]
- 6.Shipman L. Metabolism: totally addicted to NAD+ Nat Rev Cancer. 2016;16:70. doi: 10.1038/nrc.2015.19. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MN. The Food Crisis in Prehistory: Overpopulation and the Origins of Agriculture. New Haven, CT: Yale University Press; 1977. [Google Scholar]
- 8.Leopold A. A Sand County Almanac, and Sketches Here and There. Oxford, UK: Oxford University Press; 1949. [Google Scholar]
- 9.Crosby AW. The Columbian Exchange: Biological and Cultural Consequences of 1492. Westport, CT: Greenwood Publishing Group; 2003. [Google Scholar]
- 10.McNeill JR, McNeill WH. The Human Web: A Bird’s-Eye View of World History. New York, NY: W.W. Norton; 2003. [Google Scholar]
- 11.Acemoglu D, Robinson JA. Why Nations Fail: The Origins of Power, Prosperity and Poverty. London, England: Profile Books; 2012. [Google Scholar]
- 12.Barnes E. Diseases and Human Evolution. Albuquerque, NM: University of New Mexico Press; 2007. [Google Scholar]
- 13.Fernández-Armesto F, Kulshrestha JP. Food: A History. London, England: Macmillan; 2001. [Google Scholar]
- 14.McMichael A. Climate Change and the Health of Nations. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 15.Nutrition and Metabolism. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 16.Cockburn A. The Evolution and Eradication of Infectious Diseases. Baltimore, MD: Johns Hopkins Press; 1963. [Google Scholar]
- 17.Ewald PW. Evolution of Infectious Disease. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 18.Abouheif E, Zardoya R, Meyer A. Limitations of metazoan 18S rRNA sequence data: implications for reconstructing a phylogeny of the animal kingdom and inferring the reality of the Cambrian explosion. J Mol Evol. 1998;47:394–405. doi: 10.1007/pl00006397. [DOI] [PubMed] [Google Scholar]
- 19.Fox D. What sparked the Cambrian explosion? Nature. 2016;530:268–270. doi: 10.1038/530268a. [DOI] [PubMed] [Google Scholar]
- 20.Mylenko M, Boland S, Penkov S, et al. NAD+ is a food component that promotes exit from dauer diapause in Caenorhabditis elegans. PLoS ONE. 2016;11:e0167208. doi: 10.1371/journal.pone.0167208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crook M, Mcreynolds MR, Wang W, Hanna-Rose W. An NAD(+) biosynthetic pathway enzyme functions cell non-autonomously in C elegans development. Dev Dyn. 2014;243:965–976. doi: 10.1002/dvdy.24139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schutt B. Eat Me: A Natural and Unnatural History of Cannibalism. London, England: Profile Books; 2017. [Google Scholar]
- 23.Wray GA. Molecular clocks and the early evolution of metazoan nervous systems. Phil Trans R Soc B. 2015;370:20150046. doi: 10.1098/rstb.2015.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown FD, Prendergast A, Swalla BJ. Man is but a worm: chordate origins. Genesis. 2008;46:605–613. doi: 10.1002/dvg.20471. [DOI] [PubMed] [Google Scholar]
- 25.Han J, Morris SC, Ou Q, Shu D, Huang H. Meiofaunal deuterostomes from the basal Cambrian of Shaanxi (China) Nature. 2017;542:228–231. doi: 10.1038/nature21072. [DOI] [PubMed] [Google Scholar]
- 26.Battistuzzi FU, Billing-Ross P, Murillo O, Filipski A, Kumar S. A protocol for diagnosing the effect of calibration priors on posterior time estimates: a case study for the Cambrian explosion of animal Phyla. Mol Biol Evol. 2015;32:1907–1912. doi: 10.1093/molbev/msv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell MA. Origin of metazoan phyla: Cambrian explosion or proterozoic slow burn? Trends Ecol Evol. 1997;12:1–2. doi: 10.1016/s0169-5347(96)30048-7. [DOI] [PubMed] [Google Scholar]
- 28.Lane N. Power, Sex, Suicide: Mitochondria and the Meaning of Life. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 29.Lane N. Life Ascending: The Ten Great Inventions of Evolution. London, England: Profile Books; 2010. [Google Scholar]
- 30.Thomas AL. The breath of life – did increased oxygen levels trigger the Cambrian explosion? Trends Ecol Evol. 1997;12:44–45. doi: 10.1016/s0169-5347(96)30065-7. [DOI] [PubMed] [Google Scholar]
- 31.Sulovari A, Chen YH, Hudziak JJ, Li D. Atlas of human diseases influenced by genetic variants with extreme allele frequency differences. Hum Genet. 2017;136:39–54. doi: 10.1007/s00439-016-1734-y. [DOI] [PubMed] [Google Scholar]
- 32.Lane N. Bioenergetic constraints on the evolution of complex life. Cold Spring Harb Perspect Biol. 2014;6:a015982. doi: 10.1101/cshperspect.a015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lane N, Martin WF. Eukaryotes really are special, and mitochondria are why. Proc Natl Acad Sci U S A. 2015;112:E4823. doi: 10.1073/pnas.1509237112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brusatte S, Luo ZX. Ascent of the Mammals. Sci Am. 2016;314:28–35. doi: 10.1038/scientificamerican0616-28. [DOI] [PubMed] [Google Scholar]
- 35.Brusatte SL, Butler RJ, Barrett PM, et al. The extinction of the dinosaurs. Biol Rev. 2015;90:628–642. doi: 10.1111/brv.12128. [DOI] [PubMed] [Google Scholar]
- 36.McNally L, Brown SP, Jackson AL. Cooperation and the evolution of intelligence. Proc R Soc B: Biol Sci. 2012;279:3027–3034. doi: 10.1098/rspb.2012.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs DE. The Cambrian explosion. Curr Biol. 2015;25:R864–R868. doi: 10.1016/j.cub.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 38.Dunbar RI, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 39.van der Bijl W, Kolm N. Why direct effects of predation complicate the social brain hypothesis. Bioessays. 2016;38:568–577. doi: 10.1002/bies.201500166. [DOI] [PubMed] [Google Scholar]
- 40.Leonard WR, Robertson ML. Comparative primate energetics and hominid evolution. Am J Phys Anthropol. 1997;102:265–281. doi: 10.1002/(SICI)1096-8644(199702)102:2<265::AID-AJPA8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 41.Isler K, Van Schaik CP. How our ancestors broke through the gray ceiling. Curr Anthropol. 2012;53:S453–S465. [Google Scholar]
- 42.Sayol F, Maspons J, Lapiedra O, Iwaniuk AN, Székely T, Sol D. Environmental variation and the evolution of large brains in birds. Nat Commun. 2016;7 doi: 10.1038/ncomms13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stankowich T, Romero AN. The correlated evolution of antipredator defences and brain size in mammals [published online ahead of print January 11, 2017] Proc R Soc B: Biol Sci. doi: 10.1098/rspb.2016.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein RG. The Human Career: Human Biological and Cultural Origins. 3rd ed. Chicago, IL: University of Chicago Press; 2009. [Google Scholar]
- 45.Grine FE, Fleagle JG, Leakey RE. The First Humans: Origin and Early Evolution of the Genus Homo. Dordrecht, The Netherlands: Springer; 2009. [Google Scholar]
- 46.Laird MF, Vogel ER, Pontzer H. Chewing efficiency and occlusal functional morphology in modern humans. J Hum Evol. 2016;93:1–11. doi: 10.1016/j.jhevol.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Nowell A, Davidson I. Stone Tools and the Evolution of Human Cognition. Boulder, CO: University Press of Colorado; 2010. [Google Scholar]
- 48.Milton K. The critical role played by animal source foods in human (Homo) evolution. J Nutr. 2003;133:3886S–3892S. doi: 10.1093/jn/133.11.3886S. [DOI] [PubMed] [Google Scholar]
- 49.Pickering T. Rough and Tumble: Aggression, Hunting, and Human Evolution. Berkeley, CA: University of California Press; 2013. [Google Scholar]
- 50.Maslin M. The Cradle of Humanity: How the Changing Landscape of Africa Made Us So Smart. Oxford, UK: Oxford University Press; 2017. [Google Scholar]
- 51.Crittenden AN, Schnorr SL. Current views on hunter-gatherer nutrition and the evolution of the human diet. Am J Phys Anthropol. 2017;162:84–109. doi: 10.1002/ajpa.23148. [DOI] [PubMed] [Google Scholar]
- 52.Bowles S, Gintis H. A Cooperative Species: Human Reciprocity and Its Evolution. Princeton, NJ: Princeton University Press; 2011. [Google Scholar]
- 53.Ardrey R. The Hunting Hypothesis: A Personal Conclusion Concerning the Evolutionary Nature of Man. New York, NY: Fontana Press; 1977. [Google Scholar]
- 54.Cornélio AM, de Bittencourt-Navarrete RE, de Bittencourt Brum R, Queiroz CM, Costa MR. Human brain expansion during evolution is independent of fire control and cooking. Front Neurosci. 2016;10:167. doi: 10.3389/fnins.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hart D, Sussman RW. Man the Hunted: Primates, Predators, and Human Evolution Expanded ed. Boulder, CO: Westview Press; 2008. [Google Scholar]
- 56.Jones M. Feast: Why Humans Share Food. Oxford, UK: Oxford University Press; 2008. [Google Scholar]
- 57.McCorriston J, Harrower M, Martin L, Oches E. Cattle cults of the Arabian Neolithic and early territorial societies. Am Anthropol. 2012;114:45–63. doi: 10.1111/j.1548-1433.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 58.Tattersall I. Masters of the Planet: The Search for Our Human Origins. New York, NY: St. Martin’s Press; 2012. [Google Scholar]
- 59.Terborgh J, Estes JA. Trophic Cascades: Predators, Prey, and the Changing Dynamics of Nature. Washington, DC: Island Press; 2013. [Google Scholar]
- 60.Marean CW. The transition to foraging for dense and predictable resources and its impact on the evolution of modern humans [published online ahead of print June 13, 2016] Proc R Soc B: Biol Sci. doi: 10.1098/rstb.2015.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hublin JJ, Richards MP. The Evolution of Hominin Diets: Integrating Approaches to the Study of Palaeolithic Subsistence. Dordrecht, The Netherlands: Springer; 2009. [Google Scholar]
- 62.Herculano-Houzel S. The Human Advantage: A New Understanding of How Our Brain Became Remarkable. Cambridge, MA: MIT Press; 2016. [Google Scholar]
- 63.Wynn T, Coolidge FL. Archeological insights into hominin cognitive evolution. Evol Anthropol. 2016;25:200–213. doi: 10.1002/evan.21496. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Wu Q, Ma S, Ma T, et al. Comparative genomics reveals convergent evolution between the bamboo-eating giant and red pandas. Proc Natl Acad Sci U S A. 2017;114:1081–1086. doi: 10.1073/pnas.1613870114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hohmann G, Robbins MM, Boesch C. Feeding Ecology in Apes and Other Primates. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 66.Sussman RW, Tab Rasmussen D, Raven PH. Rethinking primate origins again. Am J Primatol. 2013;75:95–106. doi: 10.1002/ajp.22096. [DOI] [PubMed] [Google Scholar]
- 67.Smaers JB, Gómez-Robles A, Parks AN, Sherwood CC. Exceptional evolutionary expansion of prefrontal cortex in great apes and humans. Curr Biol. 2017;27:714–720. doi: 10.1016/j.cub.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Lyras GA, Giannakopoulou A, Kouvari M, Papadopoulos GC. Evolution of gyrification in carnivores. Brain Behav Evol. 2017;88:187–203. doi: 10.1159/000453104. [DOI] [PubMed] [Google Scholar]
- 69.Gómez-Robles A, Smaers JB, Holloway RL, Polly PD, Wood BA. Brain enlargement and dental reduction were not linked in hominin evolution. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1608798114. 201608798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pontzer H, Brown MH, Raichlen DA, et al. Metabolic acceleration and the evolution of human brain size and life history. Nature. 2016;533:390–392. doi: 10.1038/nature17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wroe S, Field JH, Archer M, et al. Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea) Proc Natl Acad Sci U S A. 2013;110:8777–8781. doi: 10.1073/pnas.1302698110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Willerslev E, Davison J, Moora M, et al. Fifty thousand years of Arctic vegetation and megafaunal diet. Nature. 2014;506:47–51. doi: 10.1038/nature12921. [DOI] [PubMed] [Google Scholar]
- 73.Johnson AW, Earle TK. The Evolution of Human Societies: From Foraging Group to Agrarian State. Stanford, CA: Stanford University Press; 2000. [Google Scholar]
- 74.Harding RSO, Teleki G. Omnivorous Primates: Gathering and Hunting in Human Evolution. New York, NY: Columbia University Press; 1981. [Google Scholar]
- 75.Krebs JR. The gourmet ape: evolution and human food preferences. Am J Clin Nutr. 2009;90:707S–711S. doi: 10.3945/ajcn.2009.27462B. [DOI] [PubMed] [Google Scholar]
- 76.Allen JS. The Omnivorous Mind: Our Evolving Relationship with Food. Cambridge, MA: Harvard University Press; 2012. [Google Scholar]
- 77.Rindos D. The Origins of Agriculture: An Evolutionary Perspective. New York, NY: Academic Press; 1984. [Google Scholar]
- 78.Marlowe F. The Hadza: Hunter-Gatherers of Tanzania. Berkeley, CA: University of California Press; 2010. [Google Scholar]
- 79.Armelagos GJ. Brain evolution, the determinates of food choice, and the omnivore’s dilemma. Crit Rev Food Sci Nutr. 2014;54:1330–1341. doi: 10.1080/10408398.2011.635817. [DOI] [PubMed] [Google Scholar]
- 80.Freedman PH. Food: The History of Taste. Berkeley, CA: University of California Press; 2007. [Google Scholar]
- 81.Levenstein H. Fear of Food: A History of Why We Worry about What We Eat. Chicago, IL: University of Chicago Press; 2012. [Google Scholar]
- 82.Shepherd GM. Neurogastronomy: How the Brain Creates Flavor and Why It Matters. New York, NY: Columbia University Press; 2013. [Google Scholar]
- 83.Jackson LC. Two evolutionary models for the interactions of dietary organic cyanogens, hemoglobins, and falciparum malaria. Am J Hum Biol. 2002;2:521–532. doi: 10.1002/ajhb.1310020508. [DOI] [PubMed] [Google Scholar]
- 84.Danielson PB. The cytochrome P450 superfamily: biochemistry evolution and drug metabolism in humans. Curr Drug Metab. 2002;3:561–597. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 85.Nebert DW, Wikvall K, Miller WL. Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120431. doi: 10.1098/rstb.2012.0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carmody RN, Weintraub GS, Wrangham RW. Energetic consequences of thermal and nonthermal food processing. Proc Natl Acad Sci U S A. 2011;108:19199–19203. doi: 10.1073/pnas.1112128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamang JP, Kailasapathy K. Fermented Foods and Beverages of the World. New York, NY: CRC Press; 2010. [Google Scholar]
- 88.Carmody RN, Dannemann M, Briggs AW, et al. Genetic evidence of human adaptation to a cooked diet. Genome Biol Evol. 2016;8:1091–1103. doi: 10.1093/gbe/evw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ernst UR, Van Hiel MB, Depuydt G, Boerjan B, De Loof A, Schoofs L. Epigenetics and locust life phase transitions. J Exp Biol. 2015;218:88–99. doi: 10.1242/jeb.107078. [DOI] [PubMed] [Google Scholar]
- 90.Kapheim KM, Rao VD, Yeoman CJ, et al. Caste-specific differences in hindgut microbial communities of honey bees (Apis mellifera) PLoS ONE. 2015;10:e0123911. doi: 10.1371/journal.pone.0123911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strachecka A, Olszewski K, Bajda M, Demetraki-Paleolog J. Natural larval diet differently influences the pattern of developmental changes in DNA 5-methyl-cytosine levels in Apis mellifera queens as compared with workers and drones. Biochemistry (Mosc) 2015;80:1019–1025. doi: 10.1134/S0006297915080076. [DOI] [PubMed] [Google Scholar]
- 92.Kay AD, Zumbusch T, Heinen JL, Marsh TC, Holway DA. Nutrition and interference competition have interactive effects on the behavior and performance of Argentine ants. Ecology. 2010;91:57–64. doi: 10.1890/09-0908.1. [DOI] [PubMed] [Google Scholar]
- 93.Wilson PJ. The Domestication of the Human Species. New Haven, CT: Yale University Press; 1991. [Google Scholar]
- 94.Perez SI, Postillone MB, Rindel D. Domestication and human demographic history in South America. Am J Phys Anthropol. 2017 doi: 10.1002/ajpa.23176. [DOI] [PubMed] [Google Scholar]
- 95.Balick MJ, Cox PA. Plants, People, and Culture: The Science of Ethnobotany. New York, NY: Scientific American Library; 1997. [Google Scholar]
- 96.Harlan JR. Crops and Man. Madison, WI, USA: American Society of Agronomy & Crop Science Society of America; 1975. [Google Scholar]
- 97.Harris S. What Have Plants Ever Done for Us? Western Civilization in Fifty Plants. Oxford, UK: Bodleian Library; 2015. [Google Scholar]
- 98.Hodder I. The Domestication of Europe. London, England: Blackwell; 1990. [Google Scholar]
- 99.Budiansky S. The Covenant of the Wild: Why Animals Chose Domestication. New York, NY: William Morrow; 1992. [Google Scholar]
- 100.Larson G, Piperno DR, Allaby RG, et al. Current perspectives and the future of domestication studies. Proc Natl Acad Sci U S A. 2014;111:6139–6146. doi: 10.1073/pnas.1323964111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurihara K. Umami the fifth basic taste: history of studies on receptor mechanisms and role as a food flavor. Biomed Res Int. 2015;2015:189402. doi: 10.1155/2015/189402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heiser CB. Seed to Civilization: The Story of Food. Cambridge, MA: Harvard University Press; 1990. [Google Scholar]
- 103.Cauvin J, Watkins T. The Birth of the Gods and the Origins of Agriculture. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 104.Hsu E, Harris S. Plants, Health And Healing: On the Interface of Ethnobotany and Medical Anthropology. Oxford, UK: Berghahn Books; 2012. [Google Scholar]
- 105.Mazoyer M, Roudart L, Membrez JH. A History of World Agriculture: From the Neolithic Age to the Current Crisis. London, England: Earthscan; 2006. [Google Scholar]
- 106.Boyd R, Richerson PJ. Culture and the Evolutionary Process. Chicago, IL: University of Chicago Press; 1988. [Google Scholar]
- 107.Bednarik RG. Doing with less: hominin brain atrophy. Homo. 2014;65:433–449. doi: 10.1016/j.jchb.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Henriksen R, Johnsson M, Andersson L, Jensen P, Wright D. The domesticated brain: genetics of brain mass and brain structure in an avian species. Sci Rep. 2016;6:34031. doi: 10.1038/srep34031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proc Natl Acad Sci U S A. 2010;107:17433–17438. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cryan JF. Microbial Endocrinology. Dordrecht, The Netherlands: Springer; 2014. [Google Scholar]
- 112.Heijtz RD, Wang S, Anuar F, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McAuliffe K. This Is Your Brain on Parasites: How Tiny Creatures Manipulate Our Behavior and Shape Society. Boston, MA: Houghton Mifflin Harcourt; 2016. [Google Scholar]
- 114.Trammell SA, Brenner C. NNMT: a bad actor in fat makes good in liver. Cell Metab. 2015;22:200–201. doi: 10.1016/j.cmet.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vickers M, Breier B, McCarthy D, Gluckman P. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 116.Moeller AH, Caro-Quintero A, Mjungu D, et al. Cospeciation of gut microbiota with hominids. Science. 2016;353:380–382. doi: 10.1126/science.aaf3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valle Gottlieb MG, Closs VE, Junges VM, Schwanke CHA. Impact of human aging and modern lifestyle on microbiota [published online ahead of print January 13, 2016] Crit Rev Food Sci Nutr. doi: 10.1080/10408398.2016.1269054. [DOI] [PubMed] [Google Scholar]
- 118.Spencer HG, Zuk M. For host’s sake: the pluses of parasite preservation. Trends Ecol Evol. 2016;31:341–343. doi: 10.1016/j.tree.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 119.Start D, Gilbert B. Host-parasitoid evolution in a metacommunity. Proc Biol Sci. 2016;283:20160477. doi: 10.1098/rspb.2016.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shah S. The Fever: How Malaria Has Ruled Humankind for 500,000 years. New York, NY: Farrar, Straus and Giroux; 2010. [Google Scholar]
- 121.Brown PJ, Inhorn MC. The Anthropology of Infectious Disease: International Health Perspectives. Abingdon, UK: Taylor & Francis; 2013. [Google Scholar]
- 122.Diamond J. COLLAPSE: How Societies Choose. New York, NY: Penguin Books; 2005. [Google Scholar]
- 123.McAnany PA, Yoffee N. Questioning Collapse: Human Resilience, Ecological Vulnerability, and the Aftermath of Empire. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 124.Landry A. La révolution démographique:études et essais sur les problèmes de la population Librairie du Recueil Sirey, société anonyme. Oxford, UK: Oxford, Blackwell publishing; 1934. [Google Scholar]
- 125.Teitelbaum MS. The British Fertility Decline: Demographic Transition in the Crucible of the Industrial Revolution. Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 126.Titmuss RM, Titmuss K. Parents Revolt: A Study of the Declining Birth-rate in Acquisitive Societies. London, England: Secker and Warburg; 1947. [Google Scholar]
- 127.Caldwell C, Caldwell B. Demographic Transition Theory. Dordrecht, The Netherlands: Springer; 2006. [Google Scholar]
- 128.Demeny PG, McNicoll G. Encyclopedia of Population. New York, NY: Macmillan; 2003. [Google Scholar]
- 129.Dyson T. Population and Development: The Demographic Transition. London, England: Zed Books; 2010. [Google Scholar]
- 130.Hollingsworth TH. Historical Demography. London, England: Hodder and Stoughton; 1969. [Google Scholar]
- 131.Kirk D. Demographic transition theory. Pop Stud. 1986;50:361–387. doi: 10.1080/0032472031000149536. [DOI] [PubMed] [Google Scholar]
- 132.Banks JA. Prosperity and Parenthood: A Study of Family Planning Among the Victorian Middle Classes. London, England: Routledge & Kegan Paul; 1954. [Google Scholar]
- 133.Soloway RA. Demography and Degeneration: Eugenics and the Declining Birthrate in Twentieth-Century Britain. Chapel Hill, NC: University of North Carolina Press; 2014. [Google Scholar]
- 134.Omran AR. The epidemiologic transition theory. A preliminary update. J Trop Pediatr. 1983;29:305–316. doi: 10.1093/tropej/29.6.305. [DOI] [PubMed] [Google Scholar]
- 135.Szreter S. The idea of demographic transition and the study of fertility change: a critical intellectual history. Popul Dev Rev. 1993;19:659–701. [Google Scholar]
- 136.Doubleday T. The True Law of Population: Shewn to Be Connected With the Food of the People. London, England: Smith, Elder & Co.; 1853. [Google Scholar]
- 137.Fraser EDG, Rimas A. Empires of Food: Feast, Famine and the Rise and Fall of Civilizations. London, England: Arrow Books; 2011. [Google Scholar]
- 138.Livi-Bacci M. Population and Nutrition: An Essay on European Demographic History. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 139.Ponting C. A New Green History of the World: The Environment and the Collapse of Great Civilizations. New York, NY: Random House; 2011. [Google Scholar]
- 140.Rothman JM, Raubenheimer D, Bryer MA, Takahashi M, Gilbert CC. Nutritional contributions of insects to primate diets: implications for primate evolution. J Hum Evol. 2014;71:59–69. doi: 10.1016/j.jhevol.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 141.Hulsken S, Martin A, Mohajeri MH, Homberg JR. Food-derived serotonergic modulators: effects on mood and cognition. Nutr Res Rev. 2013;26:223–234. doi: 10.1017/S0954422413000164. [DOI] [PubMed] [Google Scholar]
- 142.Lemieux GA, Cunningham KA, Lin L, Mayer F, Werb Z, Ashrafi K. Kynurenic acid is a nutritional cue that enables behavioral plasticity. Cell. 2015;160:119–131. doi: 10.1016/j.cell.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Strasser B, Gostner JM, Fuchs D. Mood, food, and cognition: role of tryptophan and serotonin. Curr Opin Clin Nutr Metab Care. 2016;19:55–61. doi: 10.1097/MCO.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 144.Zhang WQ, Smolik CM, Barba-Escobedo PA, et al. Acute dietary tryptophan manipulation differentially alters social behavior, brain serotonin and plasma corticosterone in three inbred mouse strains. Neuropharmacology. 2015;90:1–8. doi: 10.1016/j.neuropharm.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chini CC, Tarragó MG, Chini EN. NAD and the aging process: role in life, death and everything in between [published online ahead of print November 5, 2016] Mol Cell Endocrinol. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guarente L. The resurgence of NAD+ Science. 2016;352:1396–1397. doi: 10.1126/science.aag1718. [DOI] [PubMed] [Google Scholar]
- 147.Pétriacq P, Ton J, Patrit O, Gakiere B. NAD acts as an integral regulator of multiple defense layers. Plant Physiol. 2016;172:1465–1479. doi: 10.1104/pp.16.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Preyat N, Leo O. Reassessing the role of NAD as a prosurvival factor. Mol Cell Oncol. 2016;3:e1062591. doi: 10.1080/23723556.2015.1062591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Son MJ, Kwon Y, Son T, Cho YS. Restoration of mitochondrial NAD+ levels delays stem cell senescence and facilitates reprogramming of aged somatic cells. Stem Cells. 2016;34:2840–2851. doi: 10.1002/stem.2460. [DOI] [PubMed] [Google Scholar]
- 150.Wu LE, Sinclair DA. Restoring stem cells – all you need is NAD+ Cell Res. 2016;26:971–972. doi: 10.1038/cr.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yin TC, Voorhees JR, Genova RM, et al. Acute axonal degeneration drives development of cognitive, motor, and visual deficits after blast-mediated traumatic brain injury in mice. Eneuro. 2016;3 doi: 10.1523/ENEURO.0220-16.2016. ENEURO. 0220-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Montserrat-de la Paz S, Naranjo MC, Lopez S, Abia R, Muriana FJG, Bermudez B. Niacin and its metabolites as master regulators of macrophage activation. J Nutr Biochem. 2017;39:40–47. doi: 10.1016/j.jnutbio.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 153.Bonkowski MS, Sinclair DA. Slowing ageing by design: the rise of NAD+ and sirtuin-activating compounds. Nat Rev Mol Cell Biol. 2016;17:679–690. doi: 10.1038/nrm.2016.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Srivastava S. Emerging therapeutic roles for NAD+ metabolism in mitochondrial and age-related disorders. Clin Transl Med. 2016;5:25. doi: 10.1186/s40169-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–110. doi: 10.1016/j.nbd.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Isler K, van Schaik CP. The expensive brain: a framework for explaining evolutionary changes in brain size. J Hum Evol. 2009;57:392–400. doi: 10.1016/j.jhevol.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 157.Aiello LC, Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36:199–221. [Google Scholar]
- 158.Navarrete A, van Schaik CP, Isler K. Energetics and the evolution of human brain size. Nature. 2011;480:91–93. doi: 10.1038/nature10629. [DOI] [PubMed] [Google Scholar]
- 159.Isler K. Metabolic acceleration in human evolution. Cell Metab. 2016;24:5–6. doi: 10.1016/j.cmet.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 160.Gibbons A. Why humans are the high-energy apes. Science. 2016;352:639. doi: 10.1126/science.352.6286.639. [DOI] [PubMed] [Google Scholar]
- 161.Harris HF. Pellagra. BiblioBazaar. Los Angeles, USA: Hardpress; 2016. [Google Scholar]
- 162.Niles GMC. Pellagra: An American Problem. Philadelphia, PA: W.B. Saunders Company; 1912. [Google Scholar]
- 163.Williams A, Ramsden D. Hydrogen symbioses in evolution and disease. QJM. 2007;100:451–459. doi: 10.1093/qjmed/hcm045. [DOI] [PubMed] [Google Scholar]
- 164.Turnbull C. Mountain People. New York, NY: Simon & Schuster; 1987. [Google Scholar]
- 165.Woodward JJ. The Medical and Surgical History of the War of the Rebellion, 1861–1865. Washington, DC: U.S. Government Printing Office; 1879. [Google Scholar]
- 166.Daniels J. A Southerner Discovers the South. London, England: Macmillan; 1938. [Google Scholar]
- 167.Rajakumar K. Pellagra in the United States: a historical perspective. South Med J. 2000;93:272–277. [PubMed] [Google Scholar]
- 168.Elvehjem CA, Madden RJ, Strong FM, Wolley DW. The isolation and identification of the anti-black tongue factor. J Biol Chem. 1937;2002;277:e22. [PubMed] [Google Scholar]
- 169.Oppenheimer C. Eruptions That Shook the World. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 170.Perren R. The Meat Trade in Britain, 1840–1914. London, England: Routledge & Kegan Paul; 1978. [Google Scholar]
- 171.Smil V. Eating meat: evolution, patterns. and consequences. Popul Dev Rev. 2002;28:599–639. [Google Scholar]
- 172.Millstone E, Lang T. The Atlas of Food Who Eats What, Where, and Why. London, England: Earthscan; 2008. [Google Scholar]
- 173.Office of Population Censuses and Surveys . Updates. London: OPCS; 1995. [Google Scholar]
- 174.Charlton J, Murphy M. The Health of Adult Britain 1841–1994. London, UK: Stationery Office Books; 1997. [Google Scholar]
- 175.Wrigley EA, Schofield RS, Schofield R. The Population History of England 1541–1871. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 176.Lang T, Rayner G. Ecological public health: the 21st century’s big idea? BMJ. 2012;345:17–20. doi: 10.1136/bmj.e5466. [DOI] [PubMed] [Google Scholar]
- 177.Hardy A. Health and Medicine in Britain Since 1860. New York, NY: Palgrave Macmillan; 2001. [Google Scholar]
- 178.Logan WPD. Mortality in England and Wales from 1848 to 1947: a survey of the changing causes of death during the past hundred years. Popul Stud. 1950;4:132–178. [Google Scholar]
- 179.Greenwood M. Some British pioneers of social medicine. Br J Soc Med. 1948;2:74. [Google Scholar]
- 180.Lynn R, Meisenberg G, Mikk J, Williams A. National IQS predict differences in scholastic achievement in 67 countries. J Biosoc Sci. 2007;39:861–874. doi: 10.1017/S0021932007001964. [DOI] [PubMed] [Google Scholar]
- 181.Agency CI. The World Factbook 2014–15. Washington, DC: U.S. Government Printing Office; 2015. [Google Scholar]
- 182.Pritchard B, Ortiz R, Shekar M. Routledge Handbook of Food and Nutrition Security. Abingdon, UK: Taylor & Francis; 2016. [Google Scholar]
- 183.Kelly RL. The Lifeways of Hunter-Gatherers: The Foraging Spectrum. Cambridge, UK: Cambridge University Press; 2013. [Google Scholar]
- 184.Scott S, Duncan CJ. Demography and Nutrition: Evidence from Historical and Contemporary Populations. Hoboken, NJ: John Wiley & Sons; 2008. [Google Scholar]
- 185.Kiple KF. The Cambridge World History of Food: 2. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- 186.Bettinger RL. Hunter-Gatherer Foraging: Five Simple Models. Clinton Corners, NY: Eliot Werner Publications; 2009. [Google Scholar]
- 187.Betzig L. Eusociality: from the first foragers to the first states. Introduction to the special issue. Hum Nat. 2014;25:1–5. doi: 10.1007/s12110-013-9187-7. [DOI] [PubMed] [Google Scholar]
- 188.Childe VG. Man Makes Himself. London, England: Watts; 1936. [Google Scholar]
- 189.Clutton-Brock J. Animals as Domesticates: A World View Through History. East Lansing, MI: Michigan State University Press; 2012. [Google Scholar]
- 190.Clutton-Brock J. The Walking Larder: Patterns of Domestication, Pastoralism, and Predation. Abingdon, UK: Taylor & Francis; 2014. [Google Scholar]
- 191.O’Connor T. Animals as Neighbors: The Past and Present of Commensal Animals. East Lansing, MI: Michigan State University Press; 2014. [Google Scholar]
- 192.Zeuner FE. A History of Domesticated Animals. London, England: Hutchinson; 1963. [Google Scholar]
- 193.Armstrong JE. How the Earth Turned Green: A Brief 38-Billion-Year History of Plants. Chicago, IL: University of Chicago Press; 2014. [Google Scholar]
- 194.Atkins PJ, Bowler IR. Food in Society: Economy, Culture, Geography. London, England: Arnold; 2001. [Google Scholar]
- 195.Brothwell DR, Brothwell P. Food in Antiquity: A Survey of the Diet of Early Peoples. Baltimore, MD: Johns Hopkins University Press; 1969. [Google Scholar]
- 196.Grigg DB. The Agricultural Systems of the World: An Evolutionary Approach. Cambridge, UK: Cambridge University Press; 1974. [Google Scholar]
- 197.Murphy DJ. People, Plants & Genes: The Story of Crops and Humanity. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 198.Bocquet-Appel JP, Bar-Yosef O. The Neolithic Demographic Transition and Its Consequences. Dordrecht, The Netherlands: Springer; 2008. [Google Scholar]
- 199.Thorpe IJ. The Origins of Agriculture in Europe. Abingdon, UK: Taylor & Francis; 2003. [Google Scholar]
- 200.Llamas B, Willerslev E, Orlando L. Human evolution: a tale from ancient genomes. Phil Trans R Soc B. 2017;372:20150484. doi: 10.1098/rstb.2015.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sanders T, Reddy S. Nutritional implications of a meatless diet. Proc Nutr Soc. 1994;53:297–307. doi: 10.1079/pns19940035. [DOI] [PubMed] [Google Scholar]
- 202.Sauer CO. Agricultural Origins and Dispersals – Primary Source Edition. Charleston, USA: BiblioLife; 2014. [Google Scholar]
- 203.Wells C. Prehistoric and historical changes in nutritional diseases and associated conditions. Prog Food Nutr Sci. 1975;1:729–779. [PubMed] [Google Scholar]
- 204.Wells S. Pandora’s Seed: The Unforeseen Cost of Civilization. New York, NY: Penguin Books; 2011. [Google Scholar]
- 205.Gignoux CR, Henn BM, Mountain JL. Rapid, global demographic expansions after the origins of agriculture. Proc Natl Acad Sci U S A. 2011;108:6044–6049. doi: 10.1073/pnas.0914274108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Warinner C, Rodrigues JFM, Vyas R, et al. Pathogens and host immunity in the ancient human oral cavity. Nat Genet. 2014;46:336–344. doi: 10.1038/ng.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Webb S. Palaeopathology of Aboriginal Australians: Health and Disease Across a Hunter-Gatherer Continent. Cambridge, UK: Cambridge University Press; 2009. [Google Scholar]
- 208.Aufderheide AC, Rodríguez-Martín C, Langsjoen O. The Cambridge Encyclopedia of Human Paleopathology. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 209.Bourke AFG. Principles of Social Evolution. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 210.Zohary D, Hopf M, Weiss E. Domestication of Plants in the Old World: The Origin and Spread of Domesticated Plants in Southwest Asia, Europe, and the Mediterranean Basin. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 211.Cavalli-Sforza LL, Cavalli-Sforza F. The Great Human Diasporas: The History of Diversity and Evolution. New York, NY: Addison-Wesley; 1995. [Google Scholar]
- 212.Brown P. The Rise of Western Christendom: Triumph and Diversity, AD 200–1000. Hoboken, NJ: Wiley; 2012. [Google Scholar]
- 213.Heather P. The Fall of the Roman Empire: A New History of Rome and the Barbarians. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 214.Hornblower S, Spawforth A. The Oxford Companion to Classical Civilization. Oxford, UK: Oxford University Press; 1998. [Google Scholar]
- 215.Little LK. Plague and the End of Antiquity: The Pandemic of 541–750. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- 216.Mitchell S. A History of the Later Roman Empire, AD 284–641. Hoboken, NJ: Wiley; 2014. [Google Scholar]
- 217.Sarris P. Empires of Faith: The Fall of Rome to the Rise of Islam, 500–700. Oxford, UK: Oxford University Press; 2011. [Google Scholar]
- 218.van Bath BS. The Agrarian History of Western Europe, A.D. 500–1850. London, England: Arnold; 1963. [Google Scholar]
- 219.Wickham C. Framing the Early Middle Ages: Europe and the Mediterranean, 400–800. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 220.DeMolen RL Herlihy D. One thousand years; Western Europe in the Middle Ages. Boston, MA: Houghton Mifflin Harcourt; 1974. [Google Scholar]
- 221.Wilkinson T. The Rise and Fall of Ancient Egypt. London, England: Bloomsbury Publishing; 2013. [Google Scholar]
- 222.Finlay G. Greece Under the Rome. London, England: J.M. Dent & Sons; 1907. [Google Scholar]
- 223.Darby HC. Domesday England. Cambridge, UK: Cambridge University Press; 1986. [Google Scholar]
- 224.Abel W. Agricultural Fluctuations in Europe: From the Thirteenth to the Twentieth Centuries. London, England: Methuen; 1935. [Google Scholar]
- 225.Moote AL, Moote DC. The Great Plague: The Story of London’s Most Deadly Year. Baltimore, MD: Johns Hopkins University Press; 2008. [Google Scholar]
- 226.Cohn SK. The Black Death Transformed: Disease and Culture in Early Renaissance Europe. New York, NY: Bloomsbury Academic; 2003. [Google Scholar]
- 227.Cantor NF. In the Wake of the Plague: The Black Death and the World It Made. New York, NY: Simon & Schuster; 2015. [Google Scholar]
- 228.Slack P. The Impact of Plague in Tudor and Stuart England. London, England: Routledge & Kegan Paul; 1985. [Google Scholar]
- 229.Dyer C. Standards of Living in the Later Middle Ages: Social Change in England C 1200–1520. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 230.Jordan WC. The Great Famine: Northern Europe in the Early Fourteenth Century. Princeton, NJ: Princeton University Press; 1997. [Google Scholar]
- 231.Lamb HH. Climate, History and the Modern World. London, England: Routledge; 1995. [Google Scholar]
- 232.Herlihy D, Cohn SK. The Black Death and the Transformation of the West. Cambridge, MA: Harvard University Press; 1997. [Google Scholar]
- 233.Ziegler P. The Black Death. Boston, MA: Faber & Faber; 2013. [Google Scholar]
- 234.Zinsser H. Rats, Lice and History. Boston, MA: Little Brown; 1934. [Google Scholar]
- 235.Freeman H. The Black Death: History’s Most Effective Killer. New York, USA: Hourly History; 2016. (CreateSpace Independent Publishing Platform). [Google Scholar]
- 236.Horrox R. The Black Death. Manchester, UK: Manchester University Press; 1994. [Google Scholar]
- 237.Ashworth W. An Economic History of England 1870–1939. Abingdon, UK: Taylor & Francis; 2013. [Google Scholar]
- 238.Dillehay TD. From Foraging to Farming in the Andes: New Perspectives on Food Production and Social Organization. Cambridge, UK: Cambridge University Press; 2011. [Google Scholar]
- 239.Fussell BH. The Story of Corn. Albuquerque, NM: University of New Mexico Press; 1992. [Google Scholar]
- 240.Gill RB. The Great Maya Droughts: Water, Life, and Death. Albuquerque, NM: University of New Mexico Press; 2000. [Google Scholar]
- 241.Black W. Psychopathic Cultures and Toxic Empires. Books Noir, Limited; Scotland: Frontline Noir; 2015. [Google Scholar]
- 242.Schultes RE, Raffauf RF. The Healing Forest: Medicinal and Toxic Plants of the Northwest Amazonia. Portland, OR: Dioscorides Press; 1990. [Google Scholar]
- 243.Bollwerk EA, Tushingham S. Perspectives on the Archaeology of Pipes, Tobacco and other Smoke Plants in the Ancient Americas. Dordrecht, The Netherlands: Springer; 2015. [Google Scholar]
- 244.Furst PT. Hallucinogens and Culture. San Francisco, CA: Chandler & Sharp; 1976. [Google Scholar]
- 245.Wilbert J. Tobacco and Shamanism in South America. New Haven, CT: Yale University Press; 1993. [Google Scholar]
- 246.Culbert TP. The Classic Maya Collapse. Albuquerque, NM: University of New Mexico Press; 1983. [Google Scholar]
- 247.Demarest A. Ancient Maya: The Rise and Fall of a Rainforest Civilization. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 248.Fernández-Armesto F. The Americas: A Hemispheric History. New York, NY: Modern Library; 2006. [Google Scholar]
- 249.Buikstra JE. Prehistoric Tuberculosis in the Americas. IL, USA: Center for Amer Archeology Pr; 1981. (Northwestern University Archeological Program). [Google Scholar]
- 250.Grob GN. The Deadly Truth: A History of Disease in America. Cambridge, MA: Harvard University Press; 2009. [PubMed] [Google Scholar]
- 251.Bremmer JN. The Strange World of Human Sacrifice. Belgium: Peeters; 2007. [Google Scholar]
- 252.Carrasco D. City of Sacrifice: The Aztec Empire and the Role of Violence in Civilization. Boston, MA: Beacon Press; 1999. [Google Scholar]
- 253.Allen RC. The British Industrial Revolution in Global Perspective: How Commerce Created the Industrial Revolution and Modern Economic Growth. Oxford, UK: Oxford University Press; 2006. [Google Scholar]
- 254.Braudel F. Capitalism and Material Life, 1400–1800. London, England: Fontana; 1974. [Google Scholar]
- 255.Pomeranz K. The Great Divergence: China, Europe, and the Making of the Modern World Economy. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- 256.Berg AD, Muscat RJ. The Nutrition Factor: Its Role in National Development [By] Alan Berg (Portions With Robert J Muscat) Washington, DC: Brookings Institution; 1973. [Google Scholar]
- 257.Drummond JC, Wilbraham A. The Englishman’s Food; Five Centuries of English Diet. London, England: Pimlico; 1957. [Google Scholar]
- 258.Ferguson N. Empire: How Britain Made the Modern World. London, England: Allen Lane; 2003. [Google Scholar]
- 259.Bennett MK, Peirce RH. Change in the American national diet, 1879–1959. Food Res I Stud. 1961;2:95–119. [Google Scholar]
- 260.Preston SH, Haines MR. Fatal Years: Child Mortality in Late Nineteenth-Century America. Princeton, NJ: Princeton University Press; 2014. [Google Scholar]
- 261.Carrasco D. The History of the Conquest of New Spain by Bernal Diaz del Castillo. Albuquerque, NM: University of New Mexico Press; 2009. [Google Scholar]
- 262.Castillo BD, Cohen J. The Conquest of New Spain. New York, NY: Penguin Books; 2003. [Google Scholar]
- 263.Cook ND. Demographic Collapse: Indian Peru, 1520–1620. Cambridge, UK: Cambridge University Press; 1981. [Google Scholar]
- 264.Earle R. The Body of the Conquistador: Food, Race and the Colonial Experience in Spanish America, 1492–1700. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 265.Melville E. A Plague of Sheep: Environmental Consequences of the Conquest of Mexico. Cambridge, UK: Cambridge University Press; 1997. [Google Scholar]
- 266.Bryan CS, Mull SR. Pellagra Pre-Goldberger: Rupert Blue, Fleming Sandwith, and The ‘Vitamine Hypothesis’. Trans Am Clin Climatol Assoc. 2015;126:20–45. [PMC free article] [PubMed] [Google Scholar]
- 267.Flenley J, Bahn P. The Enigmas of Easter Island. Oxford, UK: Oxford University Press; 2003. [Google Scholar]
- 268.Goss EM, Tabima JF, Cooke DE, et al. The Irish potato famine pathogen Phytophthora infestans originated in central Mexico rather than the Andes. Proc Natl Acad Sci U S A. 2014;111:8791–8796. doi: 10.1073/pnas.1401884111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Gráda CÓ. Famine: A Short History. Princeton, NJ: Princeton University Press; 2009. [Google Scholar]
- 270.Foster RF. Modern Ireland, 1600–1972. New York, NY: Penguin Books; 1989. [Google Scholar]
- 271.Bollet AJ. Plagues & Poxes: The Impact of Human History on Epidemic Disease. Dordrecht, The Netherlands: Springer; 2004. [Google Scholar]
- 272.He B. Studies on the Population of China, 1368–1953. Cambridge, MA: Harvard University Press; 1959. [Google Scholar]
- 273.Dikötter F. Mao’s Great Famine: The History of China’s Most Devastating Catastrophe, 1958–62. London, England: Bloomsbury Publishing; 2010. [Google Scholar]
- 274.Canning D, Raja S, Yazbeck AS. Africa’s Demographic Transition: Dividend Or Disaster? Washington, DC: World Bank Publications; 2015. [Google Scholar]
- 275.Daunton MJ. Progress and Poverty: An Economic and Social History of Britain, 1700–1850. Oxford, UK: Oxford University Press; 1995. [Google Scholar]
- 276.Caballero B, Popkin BM. The Nutrition Transition: Diet and Disease in the Developing World. New York, NY: Academic Press; 2002. [Google Scholar]
- 277.Roeder P, Mariner J, Kock R. Rinderpest: the veterinary perspective on eradication. Phil Trans R Soc B. 2013;368:20120139. doi: 10.1098/rstb.2012.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Krafsur E. Tsetse flies: genetics, evolution, and role as vectors. Infect Genet Evol. 2009;9:124–141. doi: 10.1016/j.meegid.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Simo G, Njiokou F, Mbida JM, et al. Tsetse fly host preference from sleeping sickness foci in Cameroon: epidemiological implications. Infect Genet Evol. 2008;8:34–39. doi: 10.1016/j.meegid.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 280.Ilemobade A. Tsetse and trypanosomosis in Africa: the challenges, the opportunities. Onderstepoort J Vet Res. 2009;76:35–40. doi: 10.4102/ojvr.v76i1.59. [DOI] [PubMed] [Google Scholar]
- 281.Nash TAM. Africa’s Bane: The Tsetse Fly. London, England: Collins; 1969. [Google Scholar]
- 282.Zerez CR, Tanaka KR. Impaired erythrocyte NAD synthesis: a metabolic abnormality in thalassemia. Am J Hematol. 1989;32:1–7. doi: 10.1002/ajh.2830320102. [DOI] [PubMed] [Google Scholar]
- 283.Mesquita I, Varela P, Belinha A, et al. Exploring NAD+ metabolism in host-pathogen interactions. Cell Mol Life Sci. 2016;73:1225–1236. doi: 10.1007/s00018-015-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.O’Hara JK, Kerwin LJ, Cobbold SA, et al. Targeting NAD+ metabolism in the human malaria parasite Plasmodium falciparum. PLoS ONE. 2014;9:e94061. doi: 10.1371/journal.pone.0094061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Perry GH. Parasites and human evolution. Evol Anthropol. 2014;23:218–228. doi: 10.1002/evan.21427. [DOI] [PubMed] [Google Scholar]
- 286.Sabbatani S, Manfredi R, Fiorino S. Malaria infection and human evolution. Infez Med. 2010;18:56–74. [PubMed] [Google Scholar]
- 287.Loy DE, Liu W, Li Y, et al. Out of Africa: origins and evolution of the human malaria parasites Plasmodium falciparum and Plasmodium vivax. Int J Parasitol. 2017;47:87–97. doi: 10.1016/j.ijpara.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.van Zwieten R, Verhoeven AJ, Roos D. Inborn defects in the antioxidant systems of human red blood cells. Free Radic Biol Med. 2014;67:377–386. doi: 10.1016/j.freeradbiomed.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 289.Magnani M, Stocchi V, Canestrari F, et al. Redox and energetic state of red blood cells in G6PD deficiency, heterozygous beta-thalassemia and the combination of both. Acta Haematol. 1986;75:211–214. doi: 10.1159/000206127. [DOI] [PubMed] [Google Scholar]
- 290.Kiple KF. The Cambridge World History of Human Disease. Cambridge, UK: Cambridge University Press; 1999. [Google Scholar]
- 291.Warman A. Corn & Capitalism: How a Botanical Bastard Grew to Global Dominance. Chapel Hill, NC: University of North Carolina Press; 2003. [Google Scholar]
- 292.Fanelli J. Asymmetric Demography and the Global Economy: Growth Opportunities and Macroeconomic Challenges in an Ageing World. New York, NY: Palgrave Macmillan; 2015. [Google Scholar]
- 293.Davis M. Late Victorian Holocausts: El Niño Famines and the Making of the Third World. London, England: Verso Books; 2002. [Google Scholar]
- 294.Collier P. The Bottom Billion: Why the Poorest Countries are Failing and What Can Be Done About It. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- 295.Floud R, Humphries J, Johnson PAJ. The Cambridge Economic History of Modern Britain. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 296.Goldstone JA, Kaufmann EP, Toft MD. Political Demography: How Population Changes Are Reshaping International Security and National Politics. New York, NY: Oxford University Press; 2012. [Google Scholar]
- 297.Jaronen M, Quintana FJ. Immunological relevance of the coevolution of IDO1 and AHR. Front Immunol. 2014;5:521. doi: 10.3389/fimmu.2014.00521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 299.Breda C, Sathyasaikumar KV, Sograte Idrissi S, et al. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci U S A. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Engin A, Engin AB. Tryptophan Metabolism: Implications for Biological Processes, Health and Disease. Dordrecht, The Netherlands: Springer; 2015. [Google Scholar]
- 301.Yeung AW, Terentis AC, King NJ, Thomas SR. Role of indoleamine 2,3-dioxygenase in health and disease. Clin Sci (Lond) 2015;129:601–672. doi: 10.1042/CS20140392. [DOI] [PubMed] [Google Scholar]
- 302.Bessede A, Gargaro M, Pallotta MT, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–190. doi: 10.1038/nature13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Adar T, Grisaru-Granovsky S, Ben Ya’acov A, Goldin E, Bar-Gil Shitrit A. Pregnancy and the immune system: general overview and the gastroenterological perspective. Dig Dis Sci. 2015;60:2581–2589. doi: 10.1007/s10620-015-3683-z. [DOI] [PubMed] [Google Scholar]
- 304.Badawy AA. Tryptophan metabolism, disposition and utilization in pregnancy. Biosci Rep. 2015;35:e00261. doi: 10.1042/BSR20150197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Liu Q, Tian Fj, Xie Qz, Zhang J, Liu L, Yang J. Fyn plays a pivotal role in fetomaternal tolerance through regulation of Th17 cells. Am J Reprod Immunol. 2016;75:569–579. doi: 10.1111/aji.12498. [DOI] [PubMed] [Google Scholar]
- 306.Gobert M, Lafaille JJ. Maternal-fetal immune tolerance, block by block. Cell. 2012;150:7–9. doi: 10.1016/j.cell.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 308.Mellor AL, Munn DH. Tryptophan catabolism prevents maternal T cells from activating lethal anti-fetal immune responses. J Rreprod Immunol. 2001;52:5–13. doi: 10.1016/s0165-0378(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 309.Mellor AL, Sivakumar J, Chandler P, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 310.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 311.Nilsen RM, Bjørke-Monsen AL, Midttun O, et al. Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet Gynecol. 2012;119:1243–1250. doi: 10.1097/AOG.0b013e318255004e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Bongaarts J, Potter RE. Fertility, Biology, and Behavior: An Analysis of the Proximate Determinants. London, England: Elsevier Science; 2013. [Google Scholar]
- 313.Zong S, Li C, Luo C, et al. Dysregulated expression of IDO may cause unexplained recurrent spontaneous abortion through suppression of trophoblast cell proliferation and migration. Sci Rep. 2016;6:19916. doi: 10.1038/srep19916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Routy JP, Routy B, Graziani GM, Mehraj V. The kynurenine pathway is a double-edged sword in immune-privileged sites and in cancer: implications for immunotherapy. IJTR. 2016;9:67–77. doi: 10.4137/IJTR.S38355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Sperber H, Mathieu J, Wang Y, et al. The metabolome regulates the epigenetic landscape during naive-to-primed human embryonic stem cell transition. Nature Cell Biology. 2015;17:1523–1535. doi: 10.1038/ncb3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Mor G, Kwon JY. Trophoblast-microbiome interaction: a new paradigm on immune regulation. Am J Obstet Gynecol. 2015;213:S131–S137. doi: 10.1016/j.ajog.2015.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Barnea ER, Almogi-Hazan O, Or R, et al. Immune regulatory and neuroprotective properties of preimplantation factor: from newborn to adult. Pharmacol Ther. 2015;156:10–25. doi: 10.1016/j.pharmthera.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 318.Li F, Fushima T, Oyanagi G, et al. Nicotinamide benefits both mothers and pups in two contrasting mouse models of preeclampsia. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1614947113. 201614947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lash GE, Ernerudh J. Decidual cytokines and pregnancy complications: focus on spontaneous miscarriage. J Reprod Immunol. 2015;108:83–89. doi: 10.1016/j.jri.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 320.Maklakov AA, Hall MD, Simpson SJ, et al. Sex differences in nutrient-dependent reproductive ageing. Aging Cell. 2009;8:324–330. doi: 10.1111/j.1474-9726.2009.00479.x. [DOI] [PubMed] [Google Scholar]
- 321.Solon-Biet SM, Walters KA, Simanainen UK, et al. Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci U S A. 2015;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 323.Han CS, Dingemanse NJ. You are what you eat: diet shapes body composition, personality and behavioural stability. BMC Evol Biol. 2017;17:8. doi: 10.1186/s12862-016-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.D’Ippolito S, Tersigni C, Marana R, et al. Inflammosome in the human endometrium: further step in the evaluation of the ‘maternal side’. Fertil Steril. 2016;105:111–118. e1–4. doi: 10.1016/j.fertnstert.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 325.Di Simone N. Insights into the role of Helicobacter pylori infection in preeclampsia: from the bench to the bedside. Immun Interact Reprod Cycle. 2015. https://doi.org/10.3389/fimmu.2014.00484. [DOI] [PMC free article] [PubMed]
- 326.Tersigni C, Castellani R, De Waure C, et al. Celiac disease and reproductive disorders: meta-analysis of epidemiologic associations and potential pathogenic mechanisms. Hum Reprod Update. 2014;20:582–593. doi: 10.1093/humupd/dmu007. [DOI] [PubMed] [Google Scholar]
- 327.Karayiannis D, Kontogianni MD, Mendorou C, Douka L, Mastrominas M, Yiannakouris N. Association between adherence to the Mediterranean diet and semen quality parameters in male partners of couples attempting fertility. Hum Reprod. 2016;32:215–222. doi: 10.1093/humrep/dew288. [DOI] [PubMed] [Google Scholar]
- 328.Riley JK, Jungheim ES. Is there a role for diet in ameliorating the reproductive sequelae associated with chronic low-grade inflammation in polycystic ovary syndrome and obesity? Fertil Steril. 2016;106:520–527. doi: 10.1016/j.fertnstert.2016.07.1069. [DOI] [PubMed] [Google Scholar]
- 329.Chiu Y, Afeiche M, Gaskins A, et al. Sugar-sweetened beverage intake in relation to semen quality and reproductive hormone levels in young men. Hum Reprod. 2014;29:1575–1584. doi: 10.1093/humrep/deu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Pearce K, Tremellen K. Influence of nutrition on the decline of ovarian reserve and subsequent onset of natural menopause. Hum Fertil. 2016;19:173–179. doi: 10.1080/14647273.2016.1205759. [DOI] [PubMed] [Google Scholar]
- 332.Stearns SC. The Evolution of Life Histories. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- 333.Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evol Anthropol. 1993;1:191–194. [Google Scholar]
- 334.Grahnert A, Klein C, Schilling E, Wehrhahn J, Hauschildt S. Review: NAD+: a modulator of immune functions. Innate Immun. 2011;17:212–233. doi: 10.1177/1753425910361989. [DOI] [PubMed] [Google Scholar]
- 335.Hubert S, Rissiek B, Klages K, et al. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207:2561–2568. doi: 10.1084/jem.20091154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zhou XL, Xu JJ, Ni YH, et al. SIRT1 activator (SRT1720) improves the follicle reserve and prolongs the ovarian lifespan of diet-induced obesity in female mice via activating SIRT1 and suppressing mTOR signaling. J Ovarian Res. 2014;7:97. doi: 10.1186/s13048-014-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Marino J, Paster J, Benichou G. Allorecognition by T lymphocytes and allograft rejection. Front Immunol. 2016;7:582. doi: 10.3389/fimmu.2016.00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Vasiljevic A, Poreau B, Bouvier R, et al. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome and recurrent intrauterine fetal death. Lancet. 2015;385:2120. doi: 10.1016/S0140-6736(15)60773-5. [DOI] [PubMed] [Google Scholar]
- 339.Fushima T, Sekimoto A, Oe Y, et al. Nicotinamide ameliorates a preeclampsia-like condition in mice with reduced uterine perfusion pressure. Am J Physiol: Renal. 2016;312:F366–F372. doi: 10.1152/ajprenal.00501.2016. [DOI] [PubMed] [Google Scholar]
- 340.Tissier ML, Handrich Y, Dallongeville O, Robin J-P, Habold C. Diets derived from maize monoculture cause maternal infanticides in the endangered European hamster due to a vitamin B3 deficiency [published online ahead of print January 18, 2017] Proc R Soc B: Biol Sci. doi: 10.1098/rspb.2016.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bartz JA, McInnes LA. CD38 regulates oxytocin secretion and complex social behavior. Bioessays. 2007;29:837–841. doi: 10.1002/bies.20623. [DOI] [PubMed] [Google Scholar]
- 342.Feldman R, Monakhov M, Pratt M, Ebstein RP. Oxytocin pathway genes: evolutionary ancient system impacting on human affiliation, sociality, and psychopathology. Biol Psychiatry. 2016;79:174–184. doi: 10.1016/j.biopsych.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 343.Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- 344.Krol KM, Monakhov M, Lai PS, Ebstein RP, Grossmann T. Genetic variation in CD38 and breastfeeding experience interact to impact infants’ attention to social eye cues. Proc Natl Acad Sci U S A. 2015;112:E5434–E5442. doi: 10.1073/pnas.1506352112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Young LJ. Regulating the social brain: a new role for CD38. Neuron. 2007;54:353–356. doi: 10.1016/j.neuron.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 346.Bell EL, Nagamori I, Williams EO, et al. SirT1 is required in the male germ cell for differentiation and fecundity in mice. Development. 2014;141:3495–3504. doi: 10.1242/dev.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Nair RR, Verma P, Singh K. Immune-endocrine crosstalk during pregnancy. Gen Comp Endocrinol. 2016;242:18–23. doi: 10.1016/j.ygcen.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 348.Porter B, Babbar S, Ye SQ, Maulik D. The role of nicotinamide phosphoribosyltransferase in pregnancy: a review. Am J Perinatol. 2016;33:1327–1336. doi: 10.1055/s-0036-1582448. [DOI] [PubMed] [Google Scholar]
- 349.Tatone C, Di Emidio G, Vitti M, et al. Sirtuin functions in female fertility: possible role in oxidative stress and aging. Oxid Med Cell Longev. 2015;2015:659687. doi: 10.1155/2015/659687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Joshi A, Mahfooz S, Maurya VK, et al. ARP1 during embryo implantation and its upregulation by oestradiol in mice. Reproduction. 2014;147:765–780. doi: 10.1530/REP-13-0588. [DOI] [PubMed] [Google Scholar]
- 351.Fessler DM. Reproductive immunosupression and diet. An evolutionary perspective on pregnancy sickness and meat consumption. Curr Anthropol. 2002;43:19–61. doi: 10.1086/324128. [DOI] [PubMed] [Google Scholar]
- 352.Belzer LM, Smulian JC, Lu SE, Tepper BJ. Food cravings and intake of sweet foods in healthy pregnancy and mild gestational diabetes mellitus. A prospective study. Appetite. 2010;55:609–615. doi: 10.1016/j.appet.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 353.Cardwell MS. Pregnancy sickness: a biopsychological perspective. Obstet Gynecol Surv. 2012;67:645–652. doi: 10.1097/OGX.0b013e31826ff6c5. [DOI] [PubMed] [Google Scholar]
- 354.Hill A, Cairnduff V, McCance D. Nutritional and clinical associations of food cravings in pregnancy. J Hum Nutr Diet. 2015;29:281–289. doi: 10.1111/jhn.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.McKerracher L, Collard M, Henrich J. Food aversions and cravings during pregnancy on Yasawa Island, Fiji. Hum Nat. 2016;27:296–315. doi: 10.1007/s12110-016-9262-y. [DOI] [PubMed] [Google Scholar]
- 356.Riley JC. Rising Life Expectancy: A Global History. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 357.James EL, Lane JA, Michalek RD, Karoly ED, Parkinson EK. Replicatively senescent human fibroblasts reveal a distinct intracellular metabolic profile with alterations in NAD+ and nicotinamide metabolism. Sci Rep. 2016;6:38489. doi: 10.1038/srep38489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Bennett CF, Kaeberlein M. The mitochondrial unfolded protein response and increased longevity: cause, consequence, or correlation? Exp Gerontol. 2014;56:142–146. doi: 10.1016/j.exger.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–465. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 360.Fogel RW. The Escape From Hunger and Premature Death, 1700–2100: Europe, America, and the Third World. Cambridge, UK: Cambridge University Press; 2004. [Google Scholar]
- 361.Karlen A. Man and Microbes: Disease and Plagues in History and Modern Times. New York, NY: Simon & Schuster; 1996. [Google Scholar]
- 362.Mercer A. Infections, Chronic Disease, and the Epidemiological Transition: A New Perspective. Rochester, NY: University of Rochester Press; 2014. [Google Scholar]
- 363.Lutz W, Butz WP, Samir KC. World Population and Human Capital in the Twenty-First Century. Oxford, UK: Oxford University Press; 2014. [Google Scholar]
- 364.Lynn R. Race Differences in Intelligence: An Evolutionary Analysis. Athens, Greece: Washington Summit Books; 2006. [Google Scholar]
- 365.Bailey RL, West KP, Jr, Black RE. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33. doi: 10.1159/000371618. [DOI] [PubMed] [Google Scholar]
- 366.Marmot M. The Health Gap: The Challenge of an Unequal World. London, England: Bloomsbury Publishing; 2015. [DOI] [PubMed] [Google Scholar]
- 367.Boulding KE. The Meaning of the Twentieth Century: The Great Transition. New York, NY: Harper & Row; 1964. [Google Scholar]
- 368.Bengtsson T, Campbell C, Lee JZ. Life Under Pressure: Mortality and Living Standards in Europe and Asia, 1700–1900. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- 369.Mokyr J. A Culture of Growth: The Origins of the Modern Economy. Princeton, NJ: Princeton University Press; 2016. [Google Scholar]
- 370.Athanasiou T. Divided Planet: The Ecology of Rich and Poor. Athens, GA: University of Georgia Press; 1998. [Google Scholar]
- 371.Needham J. The Grand Titration: Science and Society in East and West. London, England: Routledge; 2013. [Google Scholar]
- 372.Baggini J. The Virtues of the Table: How to Eat and Think. London, England: Granta Publications; 2014. [Google Scholar]
- 373.Lévi-Strauss C. Des Symboles et leurs doubles. Paris: Plon; 1989. [Google Scholar]
- 374.Wrangham RW, Peterson D. Demonic Males: Apes and the Origins of Human Violence. Boston, MA: Houghton Mifflin Harcourt; 1996. [Google Scholar]
- 375.Sahn DE. The Fight Against Hunger and Malnutrition: The Role of Food, Agriculture, and Targeted Policies. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 376.D’Silva J, Webster J. The Meat Crisis: Developing More Sustainable Production and Consumption. London, England: Earthscan; 2010. [Google Scholar]
- 377.Friel S, Dangour AD, Garnett T, et al. Public health benefits of strategies to reduce greenhouse-gas emissions: food and agriculture. Lancet. 374:2016–2025. doi: 10.1016/S0140-6736(09)61753-0. [DOI] [PubMed] [Google Scholar]
- 378.McMichael AJ, Powles JW, Butler CD, Uauy R. Food, livestock production, energy, climate change, and health. Lancet. 2007;370:1253–1263. doi: 10.1016/S0140-6736(07)61256-2. [DOI] [PubMed] [Google Scholar]
- 379.Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: the 2012 revision. FAO; (ESA Working paper Rome). http://www.fao.org/docrep/016/ap106e/ap106e.pdf. Published 2012. [Google Scholar]
- 380.Anderson K. Globalization’s effects on world agricultural trade, 1960–2050. Proc R Soc B: Biol Sci. 2010;365:3007–3021. doi: 10.1098/rstb.2010.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Lorente DB, Alvarez-Herranz A. Economic growth and energy regulation in the environmental Kuznets curve. Environ Sci Pollut Res Int. 2016;23:16478–16494. doi: 10.1007/s11356-016-6773-3. [DOI] [PubMed] [Google Scholar]
- 382.Troesken W. Water, Race, and Disease. Cambridge, MA: MIT Press; 2004. [Google Scholar]
- 383.Brunori G, Galli F, Barjolle D, et al. Are local food chains more sustainable than global food chains? considerations for assessment. Sustainability. 2016;8:449. [Google Scholar]
- 384.Global Panel on Agriculture and Food Systems for Nutrition . Food Systems and Diets: Facing the Challenges of the 21st Century. London, UK: Global Panel on Agriculture and Food Systems for Nutrition; 2016. [Google Scholar]
- 385.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 386.Bhutta ZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382:452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 387.Agus DB. The Lucky Years: How to Thrive in the Brave New World of Health. New York, NY: Simon & Schuster; 2016. [Google Scholar]
- 388.Acharya T, Fanzo J, Gustafson D, et al. Assessing sustainable nutrition security: the role of food systems. Washington, DC: http://ilsirf.org/wp-content/uploads/sites/5/2016/07/2014_Acharya_et_al-SNS.pdf. Published 2014. [Google Scholar]
- 389.Bucher T, Collins C, Rollo ME, et al. Nudging consumers towards healthier choices: a systematic review of positional influences on food choice. Br J Nutr. 2016;115:2252–2263. doi: 10.1017/S0007114516001653. [DOI] [PubMed] [Google Scholar]
- 390.May JF. World Population Policies: Their Origin, Evolution, and Impact. Dordrecht, The Netherlands: Springer; 2012. [Google Scholar]