Abstract
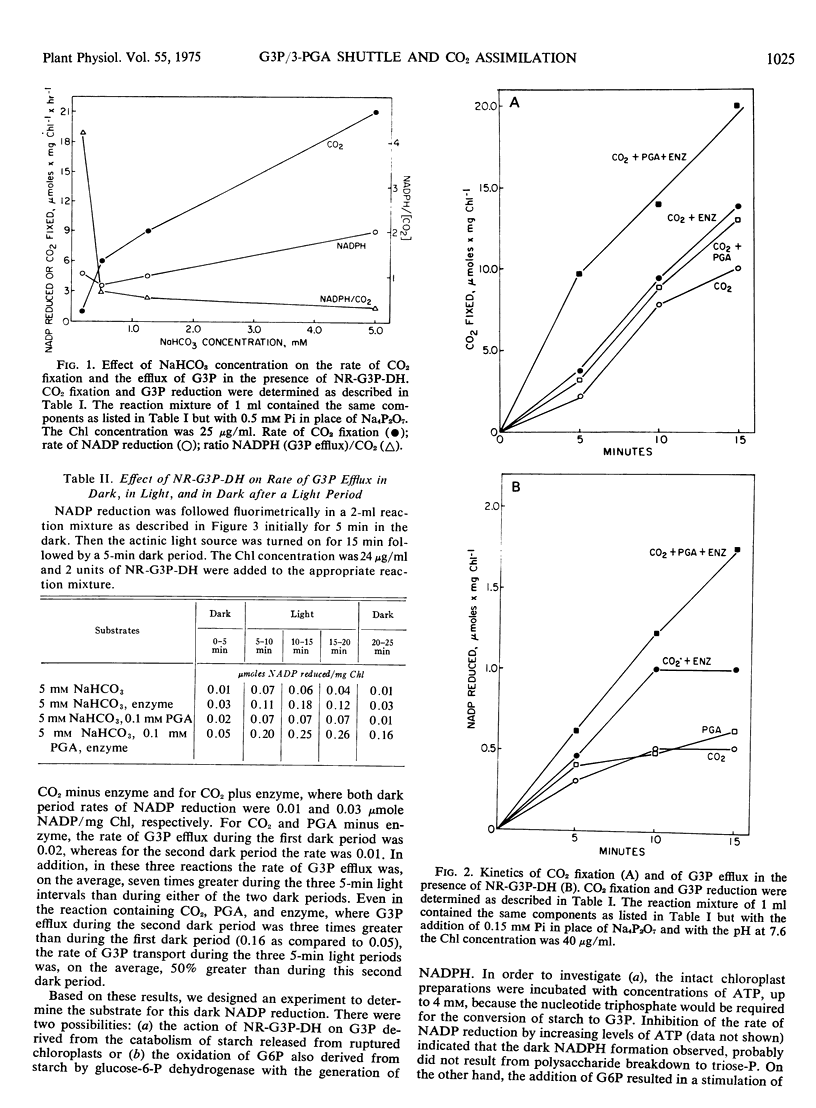
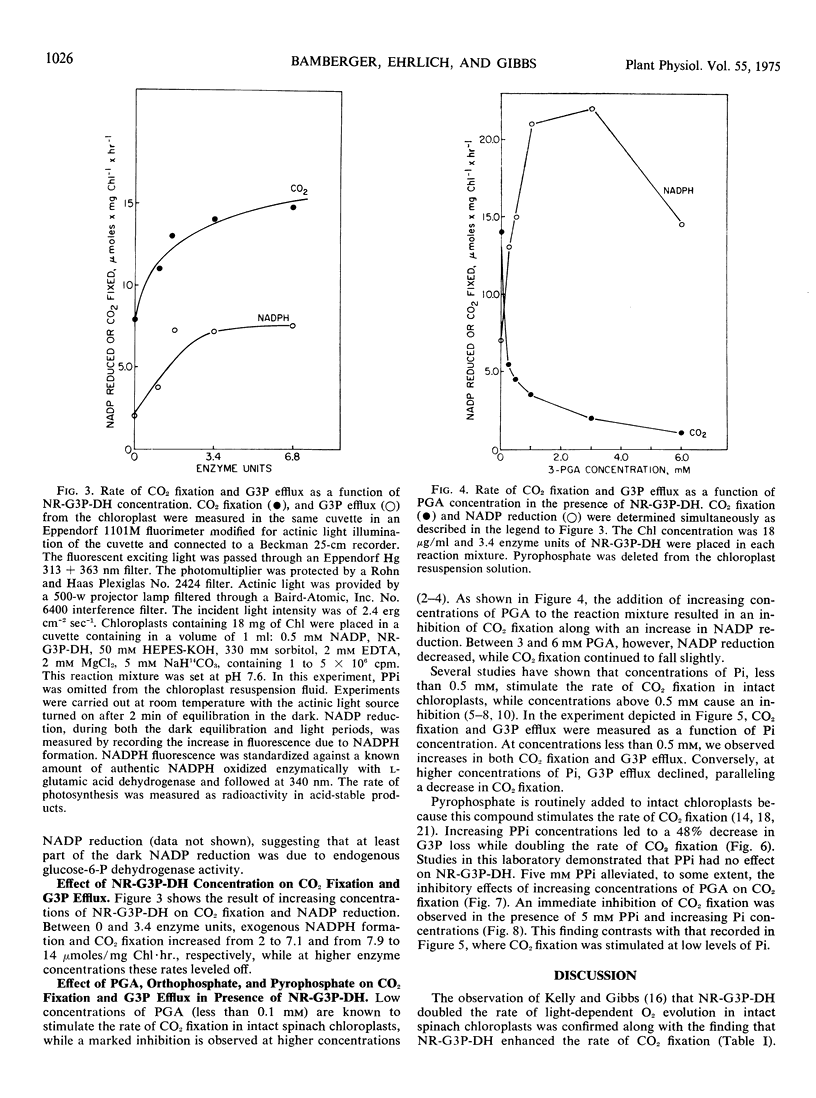
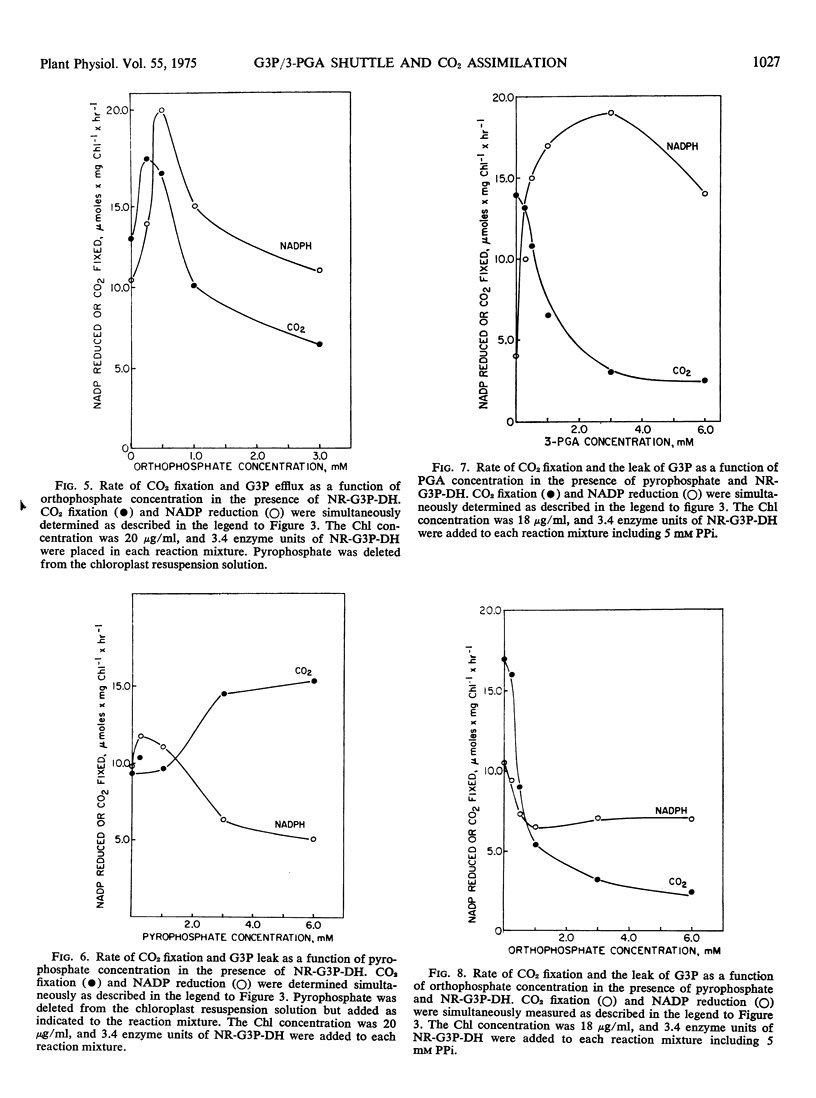
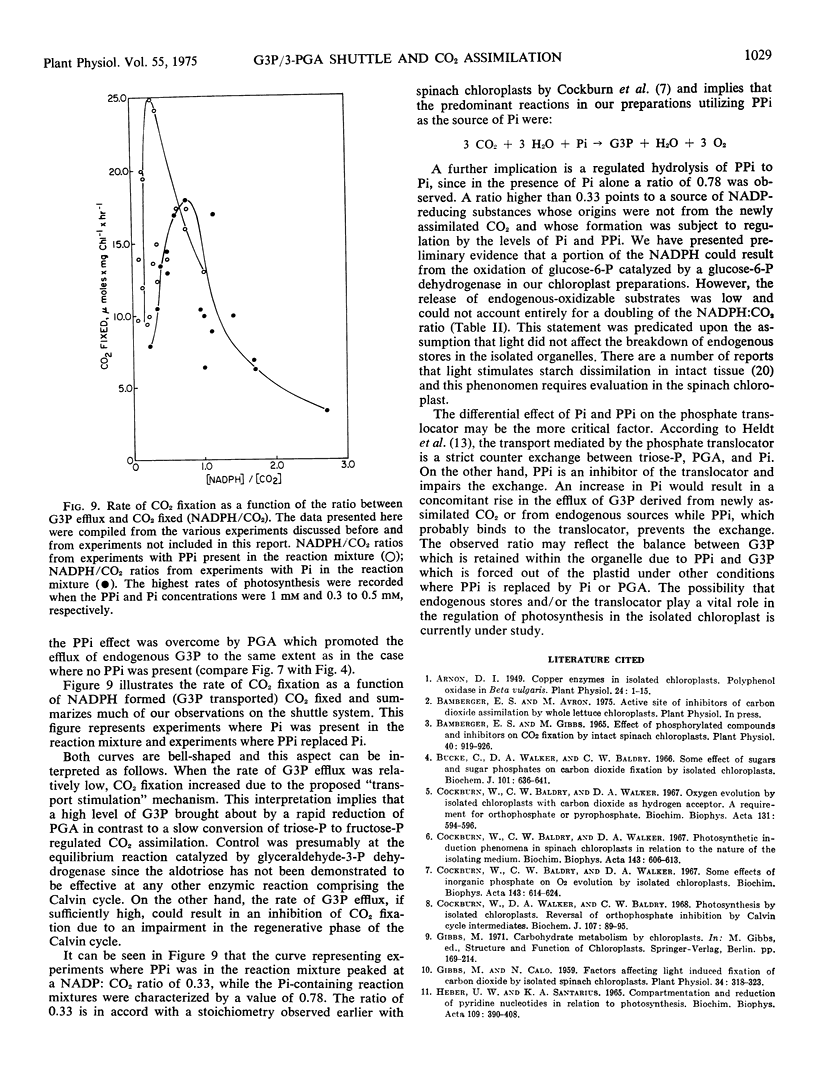
The regulation of CO2 assimilation by intact spinach (Spinacia oleracea) chloroplasts by exogenous NADP-linked nonreversible d-glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.9) was investigated. This dehydrogenase mediated a glyceraldehyde 3-phosphate/glycerate 3-phosphate shuttle for the indirect transfer of NADPH from chloroplast to the external medium. The rate of NADPH formation in the medium reflected glyceraldehyde 3-phosphate efflux from the chloroplast. Increasing enzyme concentrations stimulated NADP reduction and, in turn, CO2 fixation. Pyrophosphate increased CO2 fixation by apparently inhibiting glyceraldehyde 3-phosphate efflux. Increasing the glycerate 3-phosphate concentration above 0.1 mm stimulated glyceraldehyde 3-phosphate efflux but inhibited CO2 fixation. Addition of up to 0.5 mm orthophosphate enhanced both glyceraldehyde 3-phosphate efflux and CO2 fixation while each was inhibited by higher orthophosphate concentrations. The mechanism by which the extent of glyceraldehyde 3-phosphate efflux regulated the rate of CO2 fixation in chloroplasts was discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberger E. S., Gibbs M. Effect of Phosphorylated Compounds and Inhibitors on CO(2) Fixation by Intact Spinach Chloroplasts. Plant Physiol. 1965 Sep;40(5):919–926. doi: 10.1104/pp.40.5.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucke C., Walker D. A., Baldry C. W. Some effects of sugars and sugar phosphates on carbon dioxide fixation by isolated chloroplasts. Biochem J. 1966 Dec;101(3):636–641. doi: 10.1042/bj1010636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Oxygen evolution by isolated chloroplasts with carbon dioxide as the hydrogen acceptor. A requirement for orthophosphate or pyrophosphate. Biochim Biophys Acta. 1967 May 9;131(3):594–596. doi: 10.1016/0005-2728(67)90022-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Walker D. A., Baldry C. W. Photosynthesis by isolated chloroplasts. Reversal of orthophosphate inhibition by Calvin-cycle intermediates. Biochem J. 1968 Mar;107(1):89–95. doi: 10.1042/bj1070089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M., Calo N. Factors Affecting Light Induced Fixation of Carbon Dioxide by Isolated Spinach Chloroplasts. Plant Physiol. 1959 May;34(3):318–323. doi: 10.1104/pp.34.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. A mechanism for the indirect transfer of photosynthetically reduced nicotinamide adenine dinucleotide phosphate from chloroplasts to the cytoplasm. Plant Physiol. 1973 Dec;52(6):674–676. doi: 10.1104/pp.52.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly G. J., Gibbs M. Nonreversible d-Glyceraldehyde 3-Phosphate Dehydrogenase of Plant Tissues. Plant Physiol. 1973 Aug;52(2):111–118. doi: 10.1104/pp.52.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan R. E., Gibbs M. Comparative Enzymology of the Glyceraldehyde 3-Phosphate Dehydrogenases from Pisum sativum. Plant Physiol. 1974 Sep;54(3):312–319. doi: 10.1104/pp.54.3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenn J. D., Lilley R. M., Walker D. A. Inorganic pyrophospatase and photosynthesis by isolated chloroplasts. I. Characterisation of chloroplast pyrophosphatase and its relation to the response to exogenous pyrophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):586–595. doi: 10.1016/0005-2728(73)90218-1. [DOI] [PubMed] [Google Scholar]
- Stocking C. R., Larson S. A chloroplast cytoplasmic shuttle and the reduction of extraplastid NAD. Biochem Biophys Res Commun. 1969 Oct 8;37(2):278–282. doi: 10.1016/0006-291x(69)90731-1. [DOI] [PubMed] [Google Scholar]


