Abstract
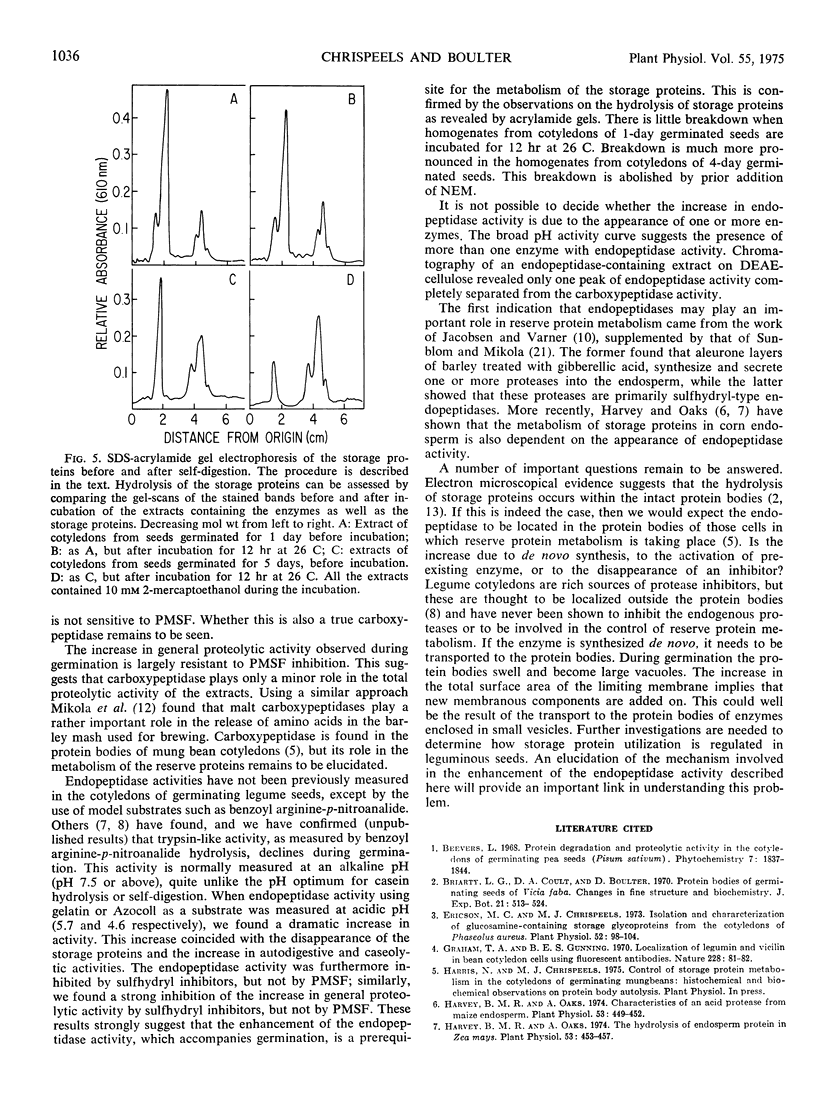
The autodigestive proteolytic activity of extracts of cotyledons of mung beans (Phaseolus aureus Roxb.) increased 4- to 5-fold during germination. A similar increase was found in the ability of these extracts to digest added casein or mung bean globulins. The increase occurred after a 2-day lag during the next 2 to 3 days of germination and coincided with the period of rapid storage protein breakdown. To understand which enzyme(s) may be responsible for this increase in proteolytic activity, the hydrolytic activity of cotyledon extracts toward a number of synthetic substrates and proteins was measured. Germination was accompanied by a marked decline in leucine aminopeptidase, while carboxypeptidase increased about 50%. There were no dramatic changes in either α-mannosidase or N-acetyl-β-glucosaminidase, enzymes which may be involved in the metabolism of the carbohydrate moieties of the reserve glycoproteins. The increase in general proteolytic activity was closely paralleled by a 10-fold increase in endopeptidase activity. This activity was inhibited by sulfhydryl reagents such as N-ethylmaleimide. Studies with inhibitors of proteolytic enzymes showed that reagents which blocked sulfhydryl groups also inhibited the rise in general proteolytic activity. Our results suggest that the appearance of a sulfhydryl-type endopeptidase activity is a necessary prerequisite for the rapid metabolism of the reserve proteins which accompanies germination.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ericson M. C., Chrispeels M. J. Isolation and Characterization of Glucosamine-containing Storage Glycoproteins from the Cotyledons of Phaseolus aureus. Plant Physiol. 1973 Aug;52(2):98–104. doi: 10.1104/pp.52.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham T. A., Gunning B. E. Localization of legumin and vicilin in bean cotyledon cells using fluorescent antibodies. Nature. 1970 Oct 3;228(5266):81–82. doi: 10.1038/228081a0. [DOI] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. Characteristics of an Acid protease from maize endosperm. Plant Physiol. 1974 Mar;53(3):449–452. doi: 10.1104/pp.53.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey B. M., Oaks A. The Hydrolysis of Endosperm Protein in Zea mays. Plant Physiol. 1974 Mar;53(3):453–457. doi: 10.1104/pp.53.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Dure L. S., 3rd The developmental biochemistry of cottonseed embryogenesis and germination. I. Purification and properties of a carboxypeptidase from germinating cotyledons. J Biol Chem. 1972 Aug 25;247(16):5034–5040. [PubMed] [Google Scholar]
- Jacobsen J. V., Varner J. E. Gibberellic Acid-induced synthesis of protease by isolated aleurone layers of barley. Plant Physiol. 1967 Nov;42(11):1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ory R. L., Henningsen K. W. Enzymes associated with protein bodies isolated from ungerminated barley seeds. Plant Physiol. 1969 Nov;44(11):1488–1498. doi: 10.1104/pp.44.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shaw D. C., Wells J. R. Radiochemical determination of a unique sequence around the reactive serine residue of a di-isopropyl phosphorofluoridate-sensitive plant carboxypeptidase and a yeast peptidase. Biochem J. 1972 Jun;128(2):229–235. doi: 10.1042/bj1280229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Schidlovsky G. Intracellular Distribution of Proteins in Pea Cotyledons. Plant Physiol. 1963 Mar;38(2):139–144. doi: 10.1104/pp.38.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visuri K., Mikola J., Enari T. M. Isolation and partial characterization of a carboxypeptidase from barley. Eur J Biochem. 1969 Jan;7(2):193–199. doi: 10.1111/j.1432-1033.1969.tb19591.x. [DOI] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Association of lysosomal activity with aleurone grains in plant seeds. Arch Biochem Biophys. 1968 Mar 20;124(1):466–471. doi: 10.1016/0003-9861(68)90354-8. [DOI] [PubMed] [Google Scholar]
- Yomo H., Srinivasan K. Protein Breakdown and Formation of Protease in Attached and Detached Cotyledons of Phaseolus vulgaris L. Plant Physiol. 1973 Dec;52(6):671–673. doi: 10.1104/pp.52.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yomo H., Varner J. E. Control of the formation of amylases and proteases in the cotyledons of germinating peas. Plant Physiol. 1973 Apr;51(4):708–713. doi: 10.1104/pp.51.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]


