Abstract
Xanthogranulomatous pyelonephritis (XGPN) is an atypical long-term pyelonephritis with destruction of renal parenchyma and a long-term inflammatory infiltrate of macrophages. Reported presentations of transitional cell carcinoma (TCC) are different. A 73-year-old woman presented with loin pain, prostration, and fever. Computed tomography scan revealed poor cortical enhancement of the kidney, but some of the images bore resemblance to the characteristic “bear's paw” sign, consistent with XGPN with a 7-cm perinephric collection. She was provisionally diagnosed as severe acute pyelonephritis, possibly XGPN, with abscess. In view of the poor clinical condition, decision was made to perform nephrectomy. Histology revealed a G3pT4 high grade TCC with perineural and vascular invasion and reactive xanthogranulomatous inflammatory response. There are few reports of concomitant XGPN and TCC affecting the kidney. However, there has not been any mention of XGPN and TCC presenting as acute pyelonephritis and perinephric abscess so far.
Keywords: Transitional cell carcinoma, Xanthogranulomatous, Pyelonephritis
Introduction
Xanthogranulomatous pyelonephritis (XGPN) is an atypical form of long-term pyelonephritis, characterized by the destruction of renal parenchyma and replacement with a long-term inflammatory infiltrate of macrophages, known as xanthoma cells. Commonly reported presentations of transitional cell carcinoma (TCC) arising from renal pelvis are macroscopic or microscopic hematuria, flank pain, renal or clot colic, mass, constitutional symptoms, or occasionally incidental diagnosis from radiological investigations for other pathologies. We report an unusual case of high-grade TCC presenting with a clinical and pathological presentation of XGPN and perinephric abscess.
Case report
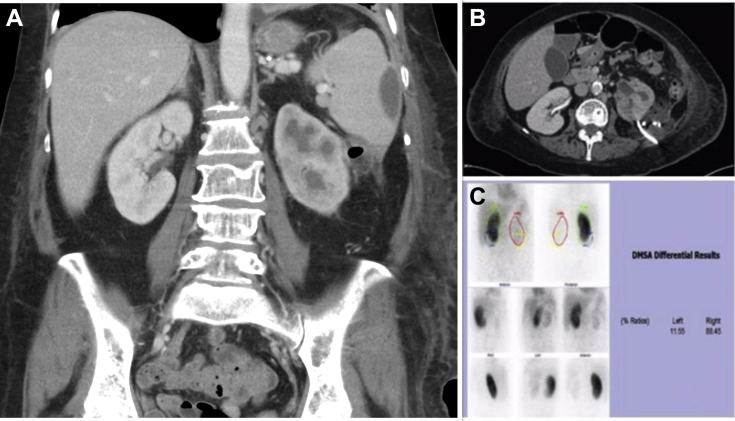
A 73-year-old woman presented as an emergency with left loin pain, prostration, and fever. The history revealed of recurrent Escherichia coli urinary tract infection (UTI) over the preceding few months, that was treated with multiple courses of antibiotics by her primary care physician. Physical examination revealed tachycardia (110/min), flushed facies, and a distended abdomen with left flank tenderness, but no guarding or peritonitis. Serum parameters were suggestive of acute infection with a neutrophilia (23.3 × 109/L), mild anemia (hemoglobin 98 g/dL), and elevated C-reactive protein levels (400 mg/dL). Computed tomography scan with intravenous contrast revealed poor cortical enhancement of the left kidney, but some of the images bore resemblance to the characteristic “bear's paw” sign, consistent with XGPN. A 7 cm perinephric and perisplenic collection with gas locules posterolateral to the left kidney, and multiple small para aortic nodes were also noted (Fig. 1A.). Chest x-ray was suggestive of left lower lobe consolidation and pleural effusion.
Fig. 1.
(A) Coronal computed tomography (CT) image at presentation showing perinephric and perisplenic collection with “bear's paw” sign of the left kidney. (B) Axial CT image after percutaneous drainage with nonresolution of perinephric collection. (C) DMSA renal scan shows poor tracer uptake in left kidney consistent with poor function. DMSA, dimercaptosuccinic acid.
Case hypothesis
She was given a provisional diagnosis of severe acute pyelonephritis, possibly XGPN, with perinephric abscess. Parenteral antibiotics (ceftriaxone and metronidazole) were initiated with supportive care. A percutaneous drain was inserted under computed tomography guidance into the perinephric collection (Fig. 1B). Purulent fluid was drained, from which E. coli infection was cultured. Despite optimal supportive measures, only marginal clinical improvement was noted over the next few days. She continued to experience high grade fever, flank pain, leukocytosis, and serum acute phase reactants continued to rise. Despite repeated saline flushes, output from the drain was low and repeat imaging revealed unresolved pyelonephritis and perinephric and peri splenic collection. In view of the continuing poor clinical condition, a decision was made to perform nephrectomy and thorough lavage of perinephric and perisplenic area. This was further supported by the poor function (11%) of the left kidney on dimercaptosuccinic acid renogram (Fig. 1C.). Nephrectomy and lavage was undertaken through a retroperitoneal approach. The procedure and postoperative recovery were uneventful.
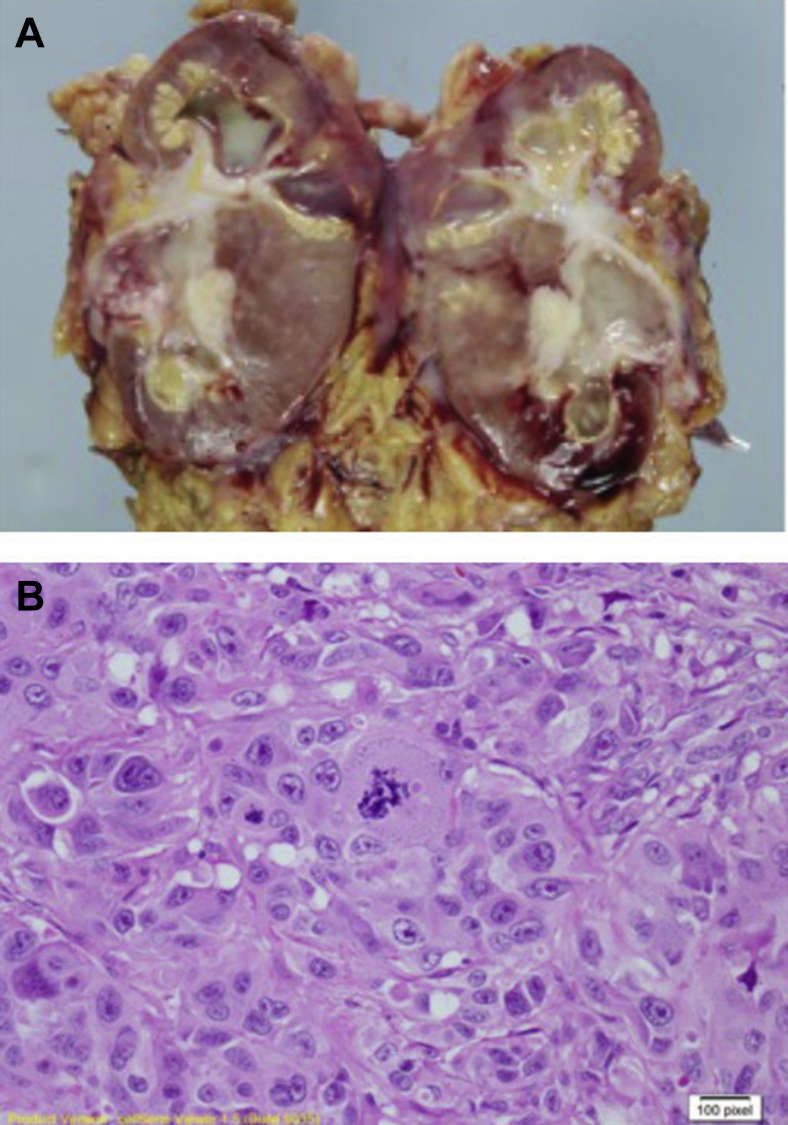
Histology revealed a G3pT4 high-grade TCC traversing the cortex and medulla with capsular penetration, perineural and vascular invasion, and a xanthogranulomatous inflammatory stromal response (Figs. 2A and B). Resection margins were free of tumor.
Fig. 2.
(A) Macroscopic appearance of nephrectomy specimen. (B) 1 × 40 Microscopic appearance—histology shows high grade transitional cell carcinoma, xanthogranulomatous change, and lipid-laden foamy macrophages.
Discussion
Acute presentation with fever, loin pain, hemodynamic instability, and elevated acute phase reactants on the background of recurrent UTI is not typical of upper tract TCC. The presentation led us to consider severe acute pyelonephritis (with perinephric abscess) as a provisional diagnosis. Imaging also pointed to aggressive infection with pus. The usual radiological signs of upper tract urothelial neoplasm (a soft tissue lesion with low grade enhancement, collecting system filling defect, stipple sign, phantom calyx [1], [2]) were absent in this case. Renal and perinephric abscesses are life threatening conditions that demand prompt resuscitation and definitive management. We managed this as a severe infective process, guided by history and findings, and attempted conservative management, with percutaneous drainage. With the exception of multiloculated abscesses, percutaneous drainage of perinephric abscesses may be successful and is the first line in management of perirenal pus; open drainage and possibly extirpative surgery and associated morbidity may be avoided with this approach in number of patients [3].
We only progressed to surgical intervention because percutaneous drainage was unsuccessful, and a diagnosis of XGPN was most likely. This was fortuitous, as surgery led to a histological diagnosis of TCC that would have otherwise been delayed or missed.
XGPN results from long-term infection of the kidney with widespread destruction of the parenchyma. Recurrent Proteus sp., E. coli, or Pseudomonas sp. infection underlie most cases, and calculus disease is often associated. In the present case, recurrent E. coli UTI had been present over the past few months. XGPN is also more often encountered in elderly females and may present with constitutional features and flank pain [4], as was the presentation in our case. The radiological characteristics include the classic “bear's paw” sign and perinephric extension may be noted.
Histology in our case reported UC and a reactive xanthogranulomatous inflammatory stromal response. There are only few reports of concomitant XGPN and TCC affecting the kidney [5], [6], [7]. However, as far as we are aware, there has not been any mention of XGPN and TCC presenting as acute pyelonephritis and perinephric abscess. Obstruction, long-term inflammation, and mechanical irritation by stones have been postulated as culprits in the pathogenesis of TCC in the presence of XGPN [5]. Eighty percent of reported XGPN have an associated staghorn calculus, but not in this case. Renal cell carcinoma has been described in association with XGPN [8], but not in the presence of overt clinical infection. Transitional cell carcinoma arising in the renal pelvis accounts for 5% of urothelial cancers. Tumor grade and (more importantly) stage are important prognostic determinants. TCC invading the renal parenchyma has lower local failure and better cancer-specific and recurrence-free survival rates than TCC invading the peripelvic or perinephric fat. This has been attributed to the thick renal parenchyma that is considered protective against local tumor spread from TCC [9]. In this case, the urothelial cancer exhibited early invasion through the renal parenchyma into perinephric space. This may be attributable to loss of parenchymal integrity secondary to xanthogranulomatous inflammation. This theory is difficult to validate; the other case reports in the literature report one localized presentation [7] and one case with widespread metastasis [6].
Conclusion
XGPN despite being a long-term granulomatous disease may present acutely with features of aggressive infection. Rarely, TCC may be associated with XGPN, and the only radiographic findings may be those of XGPN. Both radiologist and surgeon should be aware of the possibility of underlying TCC.
Footnotes
Authors' contributions: FVO, Urology Fellow, Royal Adelaide Hospital, manuscript and references; KD, Urology Fellow, Royal Adelaide Hospital, manuscript and references; SP, Radiology Department, Royal Adelaide Hospital, radiology images; PC, Pathology Department, Royal Adelaide Hospital, pathology report, micro- and macropathology images; NRB, Consultant Urology, Royal Adelaide Hospital, senior author and manuscript editor.
Acknowledgments: Thanks to Dr Penny Cohen for providing the macro- and micropathological images.
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Dyer R.B., Chen M.Y., Zagoria R.J. Classic signs in uroradiology. Radiographics. 2004;24(Suppl. 1):S247–S280. doi: 10.1148/rg.24si045509. [DOI] [PubMed] [Google Scholar]
- 2.Vikram R., Sandler C.M., Ng C.S. Imaging and staging of transitional cell carcinoma: part 2, upper urinary tract. AJR Am J Roentgenol. 2009;192(6):1488–1493. doi: 10.2214/AJR.09.2577. [DOI] [PubMed] [Google Scholar]
- 3.El-Nahas A.R., Faisal R., Mohsen T., Al-Marhoon M.S., Abol-Enein H. What is the best drainage method for a perinephric abscess? Int Braz J Urol. 2010;36(1):29–37. doi: 10.1590/s1677-55382010000100005. [DOI] [PubMed] [Google Scholar]
- 4.Dwivedi U.S., Goyal N.K., Saxena V., Acharya R.L., Trivedi S., Singh P.B. Xanthogranulomatous pyelonephritis: our experience with review of published reports. ANZ J Surg. 2006;76(11):1007–1009. doi: 10.1111/j.1445-2197.2006.03919.x. [DOI] [PubMed] [Google Scholar]
- 5.Val-Bernal J.F., Castro F. Xanthogranulomatous pyelonephritis associated with transitional cell carcinoma of the renal pelvis. Urol Int. 1996;57(4):240–245. doi: 10.1159/000282924. [DOI] [PubMed] [Google Scholar]
- 6.Godec C.J., Murrah V.A. Simultaneous occurrence of transitional cell carcinoma and urothelial adenocarcinoma associated with xanthogranulomatous pyelonephritis. Urology. 1985;26(4):412–415. doi: 10.1016/0090-4295(85)90197-9. [DOI] [PubMed] [Google Scholar]
- 7.Tseng C.W., Chen W.N.J., Juang G.D., Huang T.I.S. Staghorn calculi and xanthogranulomatous pyelonephritis associated with transitional cell carcinoma. Urol Sci. 2015;26:69–71. [Google Scholar]
- 8.Huisman T.K., Sands J.P. Focal xanthogranulomatous pyelonephritis associated with renal cell carcinoma. Urology. 1992;39(3):281–284. doi: 10.1016/0090-4295(92)90307-i. [DOI] [PubMed] [Google Scholar]
- 9.Park J., Ha S.H., Min G.E., Song C., Hong B., Hong J.H. The protective role of renal parenchyma as a barrier to local tumor spread of upper tract transitional cell carcinoma and its impact on patient survival. J Urol. 2009;182(3):894–899. doi: 10.1016/j.juro.2009.05.040. [DOI] [PubMed] [Google Scholar]