Abstract
A 7-year-old boy with a history of spasticity, global developmental delay, and seizures was given the general diagnosis of cerebral palsy at an early age. Chromosomal array analysis performed at an outside center was normal. The patient's family sought neurodevelopmental pediatric care at a new institution following a move out of state. Electroencephalography confirmed abnormal epileptogenic activity. Brain magnetic resonance imaging showed findings consistent with a tubulin gene defect (tubulinopathy) and of focal cortical dysplasia, as well as evidence of a remote occipital lobe injury. This case report describes the various brain magnetic resonance findings suggestive of a tubulin gene defect and raises the possibility of focal cortical dysplasia manifesting as a result of tubulin dysfunction.
Keywords: Tubulinopathy, Seizure, Focal cortical dysplasia, Magnetization transfer, Developmental delay
Introduction
Tubulin genes have an important role in appropriate cortical cerebral development during embryonic and fetal life. Development is reliant upon a complex series of well-timed events requiring normal cell proliferation, cellular migration from sight of origin to final end point, and postmigration development [1]. Tubulin mutations have been linked to multiple neuronal malformations. These malformations known as tubulinopathies demonstrate a multitude of neuronal manifestations such as polymicrogyria, corpus callosum agenesis, cerebellar dysplasia, and basal ganglia dysmorphia [2], [3], [4], [5], [6], [7], [8]. Clinically, these patients can develop ocular abnormalities, cognitive deficits, speech and gross motor delay, and gait ataxia. The severity of the brain malformations is directly related to the degree of clinical manifestations [2], [4], [6]. Epilepsy is also a common clinical presentation; however, in contrast to cognitive and motor deficiency, the severity of brain abnormalities is unrelated to the severity of seizures [2], [4], [5]. The majority of tubulinopathies follow an autosomal dominant inheritance pattern, and 95% of mutations are de novo [2].
Focal cortical dysplasia (FCD) is a malformation of the cortex in a single area with potential for inducing seizure activity [1]. Under the microscope, FCD manifests as dysmorphic neurons within dislaminated cortex [1]. Here, we describe the case of a child with spasticity and seizures who has brain magnetic resonance imaging findings consistent with both a tubulinopathy and with FCD. To the best of our knowledge, there has been no previously described association between tubulin defects and FCD. This case raises the possibility of a molecular connection between the two disorders, perhaps related to the dependence of cortical cellular organization on tubulin function.
Case report
A 7-year-old boy with a history of cerebral palsy, global developmental delays, and seizures presented to our institution for continued care after management at an outside institution. He was born at term by cesarean delivery for posterior breech presentation and was referred for neurodevelopmental assessment at age 6 months for concerns that he was not reaching normal developmental milestones. At the outside institution, a brain magnetic resonance imaging (MRI) was done at 8 months of age (images were unavailable) reportedly showing “multifocal cortical dysplasia, atrophic left occipital pole, dysmorphic globus pallidus with lack of normal development of the internal capsule, and also nonobstructive dilation of the ventricles.” Early work-up at that time included a 105K chromosomal microarray analysis and protein O-mannosyltransferase 2 gene sequencing, which were negative.
At age 4 years, his gross and fine motor skills were markedly delayed: he was able to crawl, able to jump if holding onto something, and he could stand by pulling onto things but was unable to walk unassisted. He could throw with both hands and occasionally catch. He was not able to dress himself, he could scribble, and he could feed himself but not using utensils. His speech was limited to a few single words, and he would gesture with pointing to indicate what he wanted.
Clinical examination at age 4 years was most notable for hypertonia in the upper and lower extremities, giving him a diagnosis of spastic quadriplegic cerebral palsy (gross motor function classification system II/III). He was also noted to have mild esotropia when focusing. Swallowing was difficult, and he had chronic sialorrhea.
Electroencephalography at age 5 years showed frequent epileptiform discharges in wake and in all stages of sleep, most notably in the occipital leads bilaterally, without any observed clinical correlate. Later at age 7 years, he began taking Keppra for episodes concerning for seizures. These episodes included teeth grinding, body stiffness, 1-minute periods of nonresponsiveness, followed by sleepiness.
Imaging findings
Brain MR imaging at our institution was performed at age 7 years. Magnetic resonance imaging of the brain without contrast demonstrated multiple cortical and noncortical abnormalities. The ventricles were moderately dilated (Figs. 1 and 2A). The anterior left lateral anterior ventricular horn demonstrated a hooked appearance, and the left lateral ventricular atrium was asymmetrically dilated relative to the right side (Fig. 1). There was dysgenesis of the anterior limbs of the internal capsules bilaterally with associated dysmorphic basal ganglia; in particular, the striatum is distorted with enlarged caudate and bulbous lateral margins (Fig. 1). Bilateral perisylvian polymicrogyria (Fig. 2B) was noted along with nodular heterotopia in the right frontoparietal region. The corpus callosum was uniformly thinned (Fig. 2C), and there was absence of the septum pellucidum. The midbrain and pons had diminished volume, and a sagittal cleft was observed in anterior pons (Figs. 1C and 2C). The superior cerebellar vermis showed a disorganized folia pattern (Figs. 1C and 3A), and there was dysgenesis of the right cerebellar hemisphere (not shown).
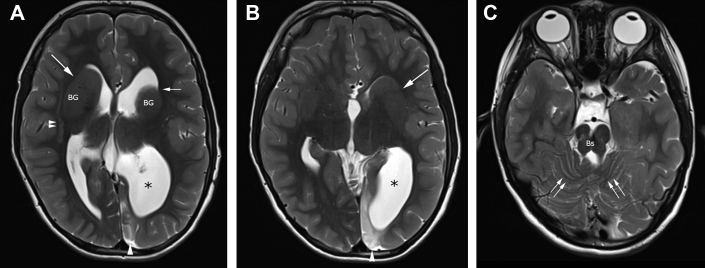
Fig. 1.
Axial T2-weighted brain MR imaging showing abnormal configuration of ventricles and abnormal internal capsule typical of tubulinopathy. Images at (A) level of the lateral ventricles and (B) third ventricle show a disproportionately large left lateral ventricle atrium (*), and frontal horns have an abnormal hooked morphology (small single arrow), associated with dysmorphic, bulbous basal ganglia (BG), and dysplasia of anterior limbs of internal capsules (long arrows). Linear band subcortical heterotopia [double arrowheads in (A)] is also evident. Left occipital parenchymal high signal (single arrowhead) is related to remote cortical insult, presumably vascular. (C) Image at the level of posterior fossa shows a hypoplastic brainstem (Bs) and cerebellar dysplasia evidenced by disorganized cerebellar folia (double arrows). MR, magnetic resonance.
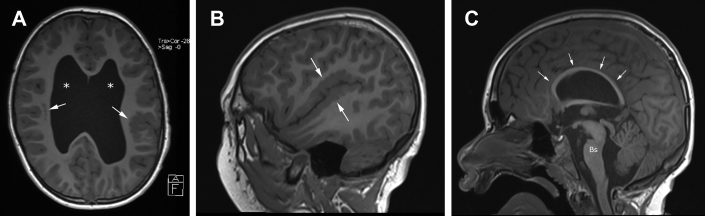
Fig. 2.
T1-weighted brain MR images showing polymicrogyria and thinning of corpus callosum, typical of tubulinopathy. (A) Axial image at level of lateral ventricles shows abnormally enlarged lateral ventricles (*), bilateral perisylvian polymicrogyria (arrows), and right-sided subcortical heterotopia. (B) Sagittal image along Sylvian fissure shows perisylvian polymicrogyria (arrows). (C) Sagittal midline image demonstrates uniform thinning of the corpus callosum (small arrows). Bs, brainstem; MR, magnetic resonance.
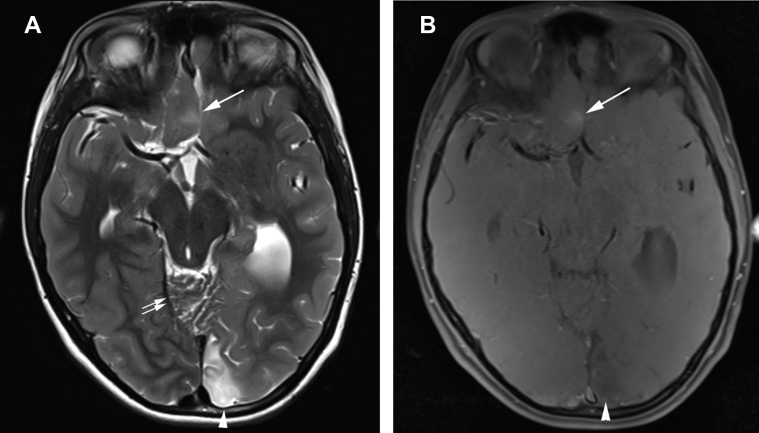
Fig. 3.
T2-weighted and T1-weighted spin echo magnetization transfer images show focal cortical dysplasia in inferior right frontal cortex. (A) Axial T2-weighted image and (B) axial T1 spin echo mag transfer demonstrate abnormally bright signal and blurring of the gray-white matter interface (arrow), typical of focal cortical dysplasia. Left occipital encephalomalacia (arrowhead) and cerebellar dysplasia [double arrows in (A)] are noted.
Increased T2 signal intensity was noted in the medial left occipital lobe, without restricted diffusion, consistent with gliosis (Figs. 1 and 3A). In the right medial inferior frontal lobe was a focus of increased signal on a T1-magnetization transfer sequence with associated gray-white matter blurring on T1 spin echo and increased T2 signal intensity, consistent with FCD (Fig. 3). The hippocampal formations had normal architecture. The orbits and optic nerves were normal. Noncontrast magnetic resonance angiogram of the head was normal, with note only of an absent right posterior communicating artery (not shown).
Discussion
Tubulin is a complex protein that acts as the building block of microtubules, which are polymers that comprise the cytoskeleton and play a critical role in the migration of developing neurons early in brain development [3]. Defects in tubulin genes have been associated with various cortical malformations, grouped into the five following patterns: (1) microlissencephaly; (2) agyria-pachygyria (lissencephaly); (3) central (often perisylvian) pachygyria and polymicrogyria-like cortical dysplasia; (4) generalized polymicrogyria-like cortical dysplasia; and (5) simplified gyral pattern with area of focal polymicrogyria [3]. Various pattern types have been associated with specific tubulin gene malformations. Microlissencephaly and lissencephaly are more commonly seen in defects of TUBA1A, whereas central pachygyria, polymicrogyria, and a simplified gyral pattern are more commonly seen in patients with defects in TUBB2B, TUBB3, and TUBB5 [2], [4], [5], [7], [9], [10]. Optic nerve hypoplasia and septo-optic dysplasia have been noted in other cases of tubulinopathy related to TUBA8 mutations [1], [5], [11].
In addition to these particular neuronal migrational anomalies, tubulin defects result in shared manifestations including corpus callosum agenesis, basal ganglia dysplasia, and cerebellar hypoplasia [1], [3], [5], [6], [8], [12]. Recognition of dysplastic caudate and putamen with apparent lack of the anterior limb of the internal capsule and multiple cortical abnormalities should raise suspicion of an underlying tubulinopathy [3], [13].
FCD refers to an often epileptogenic cortical malformation demonstrating a focus of abnormal lamination classified by the International League Against Epilepsy into type 1 or 2 depending on presence of dysmorphic neurons [1]. Disorganized neurochemistry may also play a role in the epileptogenic effects [1], [14]. Findings on MRI include blurring of the gray-white matter junction, abnormal gyral or sulcal pattern, cortical thickening, and T2 signal hyperintensity of gray or subcortical white matter [15]. Some FCD lesions are associated with increased signal on T1-magnetization sequences [15], [16]. In the patient described here, there was one such focus in the right frontal lobe. To the best of our knowledge, there has been no previously described association between tubulin defects and FCD. Given the widespread brain malformations seen with tubulin gene defects, it is reasonable to surmise that if the development of FCD was secondary to tubulin malfunction, then this abnormality would be a routinely seen feature. Therefore, it is likely the presence of FCD in this case is simply coincidental. It is conversely conceivable that previously published tubulinopathy cases had unrecognized FCD amidst the widespread gyral anomalies. At our institution, patients presenting with seizure are evaluated with brain MR imaging on a 3 tesla platform, to augment the sensitivity of the study for detection of FCD and subtle polymicrogyria. A T1-magnetization transfer sequence is also routinely included within the MR brain protocol for evaluation of patients with seizures.
Nevertheless, the dependence of cellular organization at the cortical level on tubulin function does create a potential connection between FCD and tubulin defects. Though the MR appearance in the current case is characteristic of a tubulin defect, the underlying defect in this case is not defined and may represent a unique defect resulting in deformities typical of tubulin dysfunction and well as producing FCD. A number of genetic defects associated with FCD have been identified [17], [18], [19], [20], [21], [22], [23]. These include both germline mutations (Dishevelled Egl-10 and Pleckstrin domain-containing protein 5, DEPDC5; nitrogen permease regulator-like proteins, NPRL2 and NPRL3; GTPase-activating protein activity toward Rags complex 1, GATOR1) and spontaneous mutations (rapamycin/mTOR signal transduction pathway; phosphatidylinositol 3-kinase PIK3). There is currently no known overlap in these genetic defects with the TUBA or TUBB mutations. The intricate intracellular signaling pathways, if disrupted by a mutation such as these associated with focal epilepsy, could theoretically influence the function or assembly of tubulin; however, there is no discrete link in the currently recognized genetics of these two entities. It is intriguing to consider that the cortical tubers in tuberous sclerosis are indistinguishable from type 2 FCD, and the pathophysiology of these tuberous sclerosis cortical tubers is thought to involve abnormal radial glial function, which could be imagined to be disrupted by tubulin dysfunction.
The patient described here had the additional finding of left occipital parenchymal signal abnormality and thinning, suggestive of a remote injury, presumably vascular. Furthermore, the EEG showed activity predominately in the occipital regions, implying epileptogenic spikes originating from the site of remote injury rather than from the focus of cortical dysplasia anteriorly.
At the prior institution, a 105K chromosomal array was performed and showed no abnormalities. However, chromosomal microarrays do not test for all chromosomal abnormalities. For example, they do not detect balanced chromosomal rearrangements, point mutations, and duplications or deletions of DNA segments within a single gene. In order to detect tubulin gene mutations, a serial single gene test or a multigene panel including the specific genes of interest can be used [2], [24]. Our patient has several clinical and radiologic findings that are highly suggestive of a TUBB mutation, with polymicrogyria being the dominant pattern [4], [9]; however, specific genetic testing sensitive for many of the tubulinopathies has not been performed. Genetic counseling has been recommended to the patient's family based on the brain MR findings of this case.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Barkovich A.J., Guerrini R., Kuzniecky R.I., Jackson G.D., Dobyns W.B. A developmental and genetic classification for malformations of cortical development: update 2012. Brain. 2012;135:1348–1369. doi: 10.1093/brain/aws019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahi-Buisson N, Cavallin M. Tubulinopathies overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace S.E., et al. GeneReviews 1993-2016. Seattle, Seattle (WA): University of Washington.
- 3.Bahi-Buisson N., Poirier K., Fourniol F., Saillour Y., Valence S., Lebrun N. The wide spectrum of tubulinopathies: what are the key features for the diagnosis? Brain. 2014;137:1676–1700. doi: 10.1093/brain/awu082. [DOI] [PubMed] [Google Scholar]
- 4.Cushion T.D., Paciorkowski A.R., Pilz D.T., Mullins J.G., Seltzer L.E., Marion R.W. De novo mutations in the beta-tubulin gene TUBB2A cause simplified gyral patterning and infantile-onset epilepsy. Am J Hum Genet. 2014;94(4):634–641. doi: 10.1016/j.ajhg.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cushion T.D., Dobyns W.B., Mullins J.G., Stoodley N., Chung S.K., Fry A.E. Overlapping cortical malformations and mutations in TUBB2B and TUBA1A. Brain. 2013;136:536–548. doi: 10.1093/brain/aws338. [DOI] [PubMed] [Google Scholar]
- 6.Kato M. Genotype-phenotype correlation in neuronal migration disorders and cortical dysplasias. Front Neurosci. 2015;9:181. doi: 10.3389/fnins.2015.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laquerriere A., Gonzales M., Saillour Y., Cavallin M., Joyē N., Quēlin C. De novo TUBB2B mutation causes fetal akinesia deformation sequence with microlissencephaly: an unusual presentation of tubulinopathy. Eur J Med Genet. 2016;59:249–256. doi: 10.1016/j.ejmg.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Oegema R., Cushion T.D., Phelps I.G., Chung S.K., Dempsey J.C., Collins S. Recognizable cerebellar dysplasia associated with mutations in multiple tubulin genes. Hum Mol Genet. 2015;24:5313–5325. doi: 10.1093/hmg/ddv250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrini R., Mei D., Cordelli D.M., Pucatti D., Franzoni E., Parrini E. Symmetric polymicrogyria and pachygyria associated with TUBB2B gene mutations. Eur J Hum Genet. 2012;20:995–998. doi: 10.1038/ejhg.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poirier K., Lebrun N., Broix L., Tian G., Saillour Y., Boscheron C. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdollahi M.R., Morrison E., Sirey T., Molnar Z., Hayward B.E., Carr I.M. Mutation of the variant alphatubulin TUBA8 results in polymicrogyria with optic nerve hypoplasia. Am J Hum Genet. 2009;85:737–744. doi: 10.1016/j.ajhg.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amir T., Poretti A., Boltshauser E., Huisman T.A.G.M. Differential diagnosis of ventriculomegaly and brainstem kinking on fetal MRI. Brain Dev. 2016;38:103–108. doi: 10.1016/j.braindev.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Chang B.S. Tubulinopathies and their brain malformation syndromes: every tub on its own bottom. Epilepsy Curr. 2015;15:65–67. doi: 10.5698/1535-7597-15.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tassi L., Garbelli R., Colombo N., Bramerio M., Lo Russo G., Mai R. Electroclinical, MRI and surgical outcomes in 100 epileptic patients with type II FCD. Epileptic Disord. 2012;14:257–266. doi: 10.1684/epd.2012.0525. [DOI] [PubMed] [Google Scholar]
- 15.Kadom N., Trofimova A., Vezina G.L. Utility of magnetization transfer T1 imaging in children with seizures. AJNR Am J Neuroradiol. 2013;34:895–898. doi: 10.3174/ajnr.A3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rugg-Gunn F.J., Eriksson S.H., Boulby P.A., Symms M.R., Barker G.J., Duncan J.S. Magnetization transfer imaging in focal epilepsy. Neurology. 2003;60:1638–1645. doi: 10.1212/01.wnl.0000065891.93179.cc. [DOI] [PubMed] [Google Scholar]
- 17.Ishida S., Picard F., Rudolf G., Noé E., Achaz G., Thomas P. Mutations of DEPDC5 cause autosomal dominant focal epilepsies. Nat Genet. 2013;45:552–555. doi: 10.1038/ng.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dibbens L.M., de Vries B., Donatello S., Heron S.E., Hodgson B.L., Chintawar S. Mutations in DEPDC5 cause familial focal epilepsy with variable foci. Nat Genet. 2013;45:546–551. doi: 10.1038/ng.2599. [DOI] [PubMed] [Google Scholar]
- 19.Ricos M.G., Hodgson B.L., Pippucci T., Saidin A., Ong Y.S., Heron S.E. Mutations in the mammalian target of rapamycin pathway regulators NPRL2 and NPRL3 cause focal epilepsy. Ann Neurol. 2016;79:120–131. doi: 10.1002/ana.24547. [DOI] [PubMed] [Google Scholar]
- 20.Weckhuysen S., Marsan E., Lambrecq V., Marchal C., Morin-Brureau M., An-Gourfinkel I. Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia. 2016;57:994–1003. doi: 10.1111/epi.13391. [DOI] [PubMed] [Google Scholar]
- 21.Mirzaa G.M., Campbell C.D., Solovieff N., Goold C.P., Jansen L.A., Menon S. Association of MTOR mutations with developmental brain disorders, including megalencephaly, focal cortical dysplasia, and pigmentary mosaicism. JAMA Neurol. 2016;73:836–845. doi: 10.1001/jamaneurol.2016.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terrone G., Voisin N., Abdullah Alfaiz A., Cappuccio G., Vitiello G., Guex N. De novo PIK3R2 variant causes polymicrogyria, corpus callosum hyperplasia and focal cortical dysplasia. Eur J Hum Genet. 2016;24:1359–1362. doi: 10.1038/ejhg.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Møller R.S., Weckhuysen S., Chipaux M., Marsan E., Taly V., Bebin E.M. Germline and somatic mutations in the MTOR gene in focal cortical dysplasia and epilepsy. Neurol Genet. 2016;2:e118. doi: 10.1212/NXG.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miclea D., Peca L., Cuzmici Z., Pop I.V. Genetic testing in patients with global developmental delay/intellectual disabilities. A review. Clujul Med. 2015;88:288–292. doi: 10.15386/cjmed-461. [DOI] [PMC free article] [PubMed] [Google Scholar]