Abstract
Spasticity is common following stroke; however, high subject variability and unreliable measurement techniques limit research and treatment advances. Our objective was to investigate the use of shear wave elastography (SWE) to characterize the spastic reflex in the biceps brachii during passive elbow extension in an individual with spasticity. The patient was a 42-year-old right-hand-dominant male with history of right middle cerebral artery-distribution ischemic infarction causing spastic left hemiparesis. We compared Fugl-Meyer scores (numerical evaluation of motor function, sensation, motion, and pain), Modified Ashworth scores (most commonly used clinical assessment of spasticity), and SWE measures of bilateral biceps brachii during passive elbow extension. We detected a catch that featured markedly increased stiffness of the brachialis muscle during several trials of the contralateral limb, especially at higher extension velocities. SWE was able to detect velocity-related increases in stiffness with extension of the contralateral limb, likely indicative of the spastic reflex. This study offers optimism that SWE can provide a rapid, real-time, quantitative technique that is readily accessible to clinicians for evaluating spasticity.
Keywords: Muscle spasticity, Stroke, Ultrasound, Skeletal muscle, Upper extremity
Introduction
An estimated 795,000 Americans experience stroke every year [1], and stroke incidence is expected to increase as the population ages [2]. It is estimated that the prevalence of spasticity after stroke ranges from 18% to 39% [3], [4], [5], and spasticity-associated functional limitations create significant burdens on survivors and caregivers [6]. Health care costs for individuals with stroke who develop spasticity are estimated to be fourfold higher than those without spasticity [7]. However, high subject variability and indeterminate measurement techniques limit research investigation and treatment advances [8], [9].
Though classically considered to have increased stiffness resulting solely from the over-active velocity-dependent stretch reflex, chronically spastic muscles associated with stroke appear to also have increased nonreflex stiffness when compared to the side of the body ipsilateral to the lesioned hemisphere, as well as healthy controls [9], [10]. Clinically, spasticity is diagnosed and monitored using the 5-point Modified Ashworth Scale (MAS): a simple technique that requires no equipment, though is subjective, qualitative, and varies widely with muscle groups [11], [12]. Though the precise mechanism behind spasticity is not known, we now recognize a variety of biomechanical changes within skeletal muscle connective tissue that likely limit the effectiveness of a simplistic tool, such as the MAS, for evaluating spasticity in chronic stroke [13], [14]. Electromyography or biomechanical measures may offer more reliable, quantitative information, though are impractical for routine clinical use [14], [15], [16]. Furthermore, elevated muscle tone in persons with spasticity may not be related to activation of the muscle groups in question [17], [18].
A variety of imaging-based elastography techniques have emerged with great promise for skeletal muscle evaluation, including ultrasound elastography and magnetic resonance elastography [18], [19], [20], [21], [22]. Strain elastography, a qualitative measure of relative stiffness, is also available but offers little advantage over the MAS, as neither offers a quantitative, objective measure [21], [23], [24]. The two quantitative imaging modalities, magnetic resonance elastography and ultrasound shear wave elastography (SWE), show good agreement in both phantoms and tissues, though SWE is especially promising for its flexibility, accessibility, and real-time results [25], [26], [27]. For this reason, SWE may be uniquely suited for evaluating pathologic alterations in stiffness of individual muscles, especially for quantifying spasticity [18], [28], [29], [30], [31].
This study evaluated the feasibility of using SWE to characterize the spastic reflex during passive elbow extension in an individual with spasticity caused by stroke. We hypothesized that SWE would capture heightened skeletal muscle stiffness, representing the spastic reflex, during passive elbow range of motion.
Methods
The subject was a 42-year-old right-hand-dominant male who experienced thromboembolic right middle cerebral artery occlusion, acutely treated with tissue plasminogen activator and endovascular recanalization. We evaluated him 10 months later, when he was receiving outpatient physiotherapy but no medical therapy for spasticity. His body mass index was 29.2 kg/m2. He provided informed consent, and all study procedures were approved by the institutional review board. Prior to biomechanical and ultrasound testing, an experienced, licensed, neuromuscular occupational therapist evaluated upper limb function and spasticity using the Fugl-Meyer assessment and MAS.
We fixed an L7-4 linear-array ultrasound transducer (Philips Healthcare, Andover, MA) over the midbelly of the biceps brachii using a custom-molded apparatus. The apparatus attached to the subject's arm and maintained even, minimal, and continuous contact pressure between the ultrasound transducer and subject's arm via liberal coupling gel. We tested the side ipsilateral to the lesioned hemisphere first and aligned the ultrasound transducer with the long axis of the biceps. We encouraged the subject to remain as relaxed as possible for the duration of testing. The study included three sets of passive elbow extension trials from 90° to 165° extension (180°=full extension) using a Humac (Computer Sports Medicine Inc, Stoughton, MA) dynamometer to carefully control extension velocities at 5°/s, 20°/s, 40°/s, and 60°/s then repeating for subsequent trials. Synchronizing through the dynamometer, we obtained SWE measurements at 105°, 120°, 135°, 150°, and 165°, using the Verasonics (Verasonics Inc, Kirkland, WA) ultrasound system. To evaluate any lingering changes in stiffness, we obtained a series of measurements at 1-second intervals with the arm held at 165°. A focused ultrasound push beam with duration of 400 μs produced shear waves that were detected using plane wave imaging with a frame rate of 5.85 kHz for 14.8 ms.
Two-dimensional shear wave speed maps of the muscle were reconstructed using the time-of-flight approach based on local cross-correlation of the shear wave signal [32]. Shear wave speed is a quantitative measure of tissue stiffness and can be converted to shear modulus using the equation
where μ is shear modulus, cs is shear wave propagation velocity, and ρ is density, which can be assumed to be 1000 kg/m3 for all soft tissues [33]. We selected two regions of interest, for evaluating shear wave speed in the biceps and brachialis, as indicated in Figures 1 and 2.
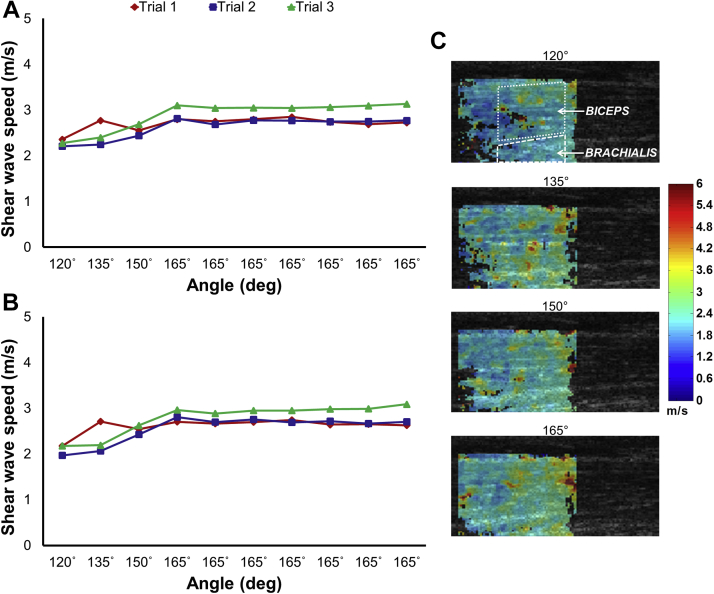
Fig. 1.
Shear wave speeds, ultrasound images, and elastograms for 60°/s ipsilateral elbow extension trials. (A) Ipsilateral biceps; (B) ipsilateral brachialis; (C) ultrasound images and elastograms from trial 1 with sample regions of interest demonstrated in the first panel.
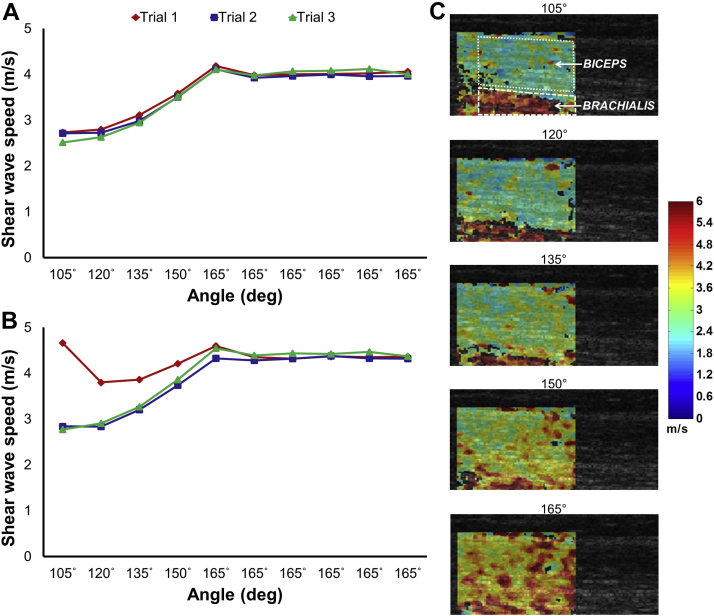
Fig. 2.
Shear wave speeds, ultrasound images, and elastograms for 60°/s contralateral elbow extension trials. (A) Contralateral biceps; (B) contralateral brachialis; note peak in stiffness, likely representative of spastic reflex, at 105° for trial 1 in the contralateral brachialis at 60°/s. (C) Ultrasound images and elastograms from trial 1 with sample regions of interest demonstrated in the first panel; note deep area of increased stiffness early in elbow extension that corresponds with peak shown in (B).
Results
The subject had a Fugl-Meyer motor function score of 41 (normal: 66), with primary deficits in the contralateral upper forearm (25/35), wrist (3/10), and hand (9/14). His MAS for the right and left sides was 0 and 1.
A sample set of bilateral elastograms and associated shear wave speeds during 60°/s extensions for the ipsilateral side are presented in Figure 1. The results for the contralateral side are included in Figure 2, which demonstrates consistently higher stiffness when compared to the ipsilateral limb—an effect present throughout all trials, regardless of elbow extension speed, that is best demonstrated by the 165° plateau. Most notably, at higher velocities, the contralateral brachialis experienced a catch with increased stiffness, as in Figure 2B (trial 1; 105°)—an effect that dissipated with successive extension trials.
Discussion
This study represents one example from several pilot studies demonstrating the feasibility of using SWE to characterize the spastic reflex during passive elbow extension following chronic stroke. Highly variable from 1 day to the next, and even throughout a given day, spasticity can be very challenging for clinicians to monitor and diagnose. Furthermore, patients experience spasticity significantly more often than clinicians and investigators are able to detect with currently available measures, thus limiting our ability to adequately treat their symptoms [34]. Though our subject did not experience profound spasticity or impairment, SWE was able to detect velocity-related increases in stiffness with extension of the contralateral limb, likely indicative of the spastic reflex. Additionally, an increase in passive stiffness appears unrelated to spasticity, as seen by the 165°-plateau region in Figure 2, though may have clinical and functional implications. This pattern of heightened stiffness for the contralateral side when the arm is held in extension was consistent throughout all trials and may facilitate using SWE as a clinical tool, noting that specialized dynamometers would not be necessary for obtaining measurements of the static arm. These findings show promise for future investigations and clinical applications using SWE to quantify and characterize the spastic reflex associated with stroke, as well as changes in passive mechanical properties.
Spasticity is classically defined as a velocity-dependent resistance to stretch; however, a variety of factors contribute to its clinical manifestation [13], [14]. As a more thorough understanding of the neuromuscular sequelae of stroke and other pathologies affecting sensorimotor systems continues to evolve, a lack of precise, quantitative measurement techniques will continue to limit treatment and progress toward improving function and independence for individuals with spasticity. This study found brief periods of increased muscle stiffness during trials of increased extension velocity, possibly related to altered viscoelasticity of skeletal muscle following stroke. As is classically found with spasticity, this increased stiffness displayed conditioning effects with repeated elbow extension. Interestingly, SWE identified focal regions of marked elevations in stiffness and presumed contraction in the deeper brachialis muscle, while the overlying biceps brachii did not show concomitant elevations in stiffness. Previous techniques for evaluating spasticity are often limited in their ability to localize specific causative muscles, instead identifying muscle groups associated with a given joint's function. Future work should investigate how this differential activation may guide directed clinical intervention to improve function and quality of life.
This feasibility study has several limitations. We studied a single individual with stroke-related spasticity on a single day. Collecting electromyography or torque may provide additional information about the nature of muscle stiffness during a spastic event. Limitations with equipment synchronization prevented evaluation of extension velocities greater than 60°/s—relatively slow to consistently elicit a spastic reflex. Furthermore, an initial synchronization error prevented the collection of SWE values at 105° for the ipsilateral side, though we are confident these missed data points are of little significance. Difficulties with equipment transport limited our evaluation to an ambulatory, community-dwelling subject, and we likely measured chronic biomechanical changes in addition to the elevated stretch reflex that is a hallmark of spasticity. Fortunately, assessing individuals in the acute setting following stroke when the spastic reflex is more pronounced will become much more feasible as SWE technologies are increasingly utilized on commercially available clinical ultrasound machines.
SWE should continue to be used to evaluate spasticity in stroke, as well as a variety of other neuromuscular conditions, such as multiple sclerosis, spinal cord injury, and cerebral palsy, to further characterize the alterations to passive and active skeletal muscle stiffness with both acute and chronic spasticity. Additionally, future work should investigate the reliability and repeatability of SWE measures in spasticity, to ensure such a technique is truly superior to classic clinical evaluation tools, such as the MAS and others. This study offers optimism that SWE can provide a rapid, real-time, quantitative technique that is readily accessible to clinicians for evaluating spastic muscle.
Acknowledgments
This work was supported by the National Center for Research Resources and Mayo Clinic CTSA (grant number UL1RR024150); and the National Institute on Aging (grant number F30AG044075).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Adams R.J., Berry J.D., Brown T.M. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolominsky-Rabas P.L., Heuschmann P.U., Marschall D., Emmert M., Baltzer N., Neundörfer B. Lifetime cost of ischemic stroke in Germany: results and national projections from a population-based stroke registry: the Erlangen Stroke Project. Stroke. 2006;37:1179–1183. doi: 10.1161/01.STR.0000217450.21310.90. [DOI] [PubMed] [Google Scholar]
- 3.Sommerfeld D.K., Gripenstedt U., Welmer A.-K. Spasticity after stroke: an overview of prevalence, test instruments, and treatments. Am J Phys Med Rehabil. 2012;91:814–820. doi: 10.1097/PHM.0b013e31825f13a3. [DOI] [PubMed] [Google Scholar]
- 4.Watkins C.L., Leathley M.J., Gregson J.M., Moore A.P., Smith T.L., Sharma A.K. Prevalence of spasticity post stroke. Clin Rehabil. 2002;16:515–522. doi: 10.1191/0269215502cr512oa. [DOI] [PubMed] [Google Scholar]
- 5.Lundström E., Terént A., Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. 2008;15:533–539. doi: 10.1111/j.1468-1331.2008.02114.x. [DOI] [PubMed] [Google Scholar]
- 6.Zorowitz R.D., Gillard P.J., Brainin M. Poststroke spasticity: sequelae and burden on stroke survivors and caregivers. Neurology. 2013;80:S45–S52. doi: 10.1212/WNL.0b013e3182764c86. [DOI] [PubMed] [Google Scholar]
- 7.Lundström E., Smits A., Borg J., Terént A. Four-fold increase in direct costs of stroke survivors with spasticity compared with stroke survivors without spasticity: the first year after the event. Stroke. 2010;41:319–324. doi: 10.1161/STROKEAHA.109.558619. [DOI] [PubMed] [Google Scholar]
- 8.Platz T., Eickhof C., Nuyens G., Vuadens P. Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil Rehabil. 2005;27:7–18. doi: 10.1080/09638280400014634. [DOI] [PubMed] [Google Scholar]
- 9.Mirbagheri M.M., Settle K., Harvey R., Rymer W.Z. Neuromuscular abnormalities associated with spasticity of upper extremity muscles in hemiparetic stroke. J Neurophysiol. 2007;98:629–637. doi: 10.1152/jn.00049.2007. [DOI] [PubMed] [Google Scholar]
- 10.Sinkjaer T., Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain. 1994;117(Pt 2):355–363. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- 11.Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67:206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 12.Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964;192:540–542. [PubMed] [Google Scholar]
- 13.Fellows S.J., Ross H.F., Thilmann A.F. The limitations of the tendon jerk as a marker of pathological stretch reflex activity in human spasticity. J Neurol Neurosurg Psychiatr. 1993;56:531–537. doi: 10.1136/jnnp.56.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R.T.S., Pandyan A.D., Sharma A.K. Biomechanical measurement of post-stroke spasticity. Age Ageing. 2006;35:371–375. doi: 10.1093/ageing/afj084. [DOI] [PubMed] [Google Scholar]
- 15.Sunnerhagen K.S., Olver J., Francisco G.E. Assessing and treating functional impairment in poststroke spasticity. Neurology. 2013;80:S35–S44. doi: 10.1212/WNL.0b013e3182764aa2. [DOI] [PubMed] [Google Scholar]
- 16.Pandyan A.D., Price C.I., Rodgers H., Barnes M.P., Johnson G.R. Biomechanical examination of a commonly used measure of spasticity. Clin Biomech (Bristol, Avon) 2001;16:859–865. doi: 10.1016/s0268-0033(01)00084-5. [DOI] [PubMed] [Google Scholar]
- 17.Dietz V., Quintern J., Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981;104:431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- 18.Zhao H., Ren Y., Wu Y.-N., Liu S.Q., Zhang L.-Q. Ultrasonic evaluations of achilles tendon mechanical properties poststroke. J Appl Physiol. 2009;106:843–849. doi: 10.1152/japplphysiol.91212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debernard L., Robert L., Charleux F., Bensamoun S.F. Analysis of thigh muscle stiffness from childhood to adulthood using magnetic resonance elastography (MRE) technique. Clin Biomech (Bristol, Avon) 2011;26:836–840. doi: 10.1016/j.clinbiomech.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Debernard L., Robert L., Charleux F., Bensamoun S.F., Bensamoun S.F. Characterization of muscle architecture in children and adults using magnetic resonance elastography and ultrasound techniques. J Biomech. 2011;44:397–401. doi: 10.1016/j.jbiomech.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 21.Brandenburg J.E., Eby S.F., Song P., Zhao H., Brault J.S., Chen S. Ultrasound elastography: the new frontier in direct measurement of muscle stiffness. Arch Phys Med Rehabil. 2014;95:2207–2219. doi: 10.1016/j.apmr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gennisson J.-L., Deffieux T., Macé E., Montaldo G., Fink M., Tanter M. Viscoelastic and anisotropic mechanical properties of in vivo muscle tissue assessed by supersonic shear imaging. Ultrasound Med Biol. 2010;36:789–801. doi: 10.1016/j.ultrasmedbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Park G.-Y., Kwon D.R. Sonoelastographic evaluation of medial gastrocnemius muscles intrinsic stiffness after rehabilitation therapy with botulinum toxin a injection in spastic cerebral palsy. Arch Phys Med Rehabil. 2012;93:2085–2089. doi: 10.1016/j.apmr.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Inami T., Kawakami Y. Assessment of individual muscle hardness and stiffness using ultrasound elastography. J Phys Fit Sports. 2016;5:313–317. [Google Scholar]
- 25.Bensamoun S.F., Wang L., Robert L., Charleux F., Latrive J., Tho M.H. Measurement of liver stiffness with two imaging techniques: magnetic resonance elastography and ultrasound elastometry. J Magn Reson Imaging. 2008;28:1287–1292. doi: 10.1002/jmri.21523. [DOI] [PubMed] [Google Scholar]
- 26.Dutt V., Kinnick R.R., Muthupillai R., Oliphant T.E., Ehman R.L., Greenleaf J.F. Acoustic shear-wave imaging using echo ultrasound compared to magnetic resonance elastography. Ultrasound Med Biol. 2000;26:397–403. doi: 10.1016/s0301-5629(99)00166-0. [DOI] [PubMed] [Google Scholar]
- 27.Oudry J., Chen J., Glaser K.J., Miette V., Sandrin L., Ehman R.L. Cross-validation of magnetic resonance elastography and ultrasound-based transient elastography: a preliminary phantom study. J Magn Reson Imaging. 2009;30:1145–1150. doi: 10.1002/jmri.21929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eby S., Zhao H., Song P., Vareberg B.J., Kinnick R., Greenleaf J.F. Quantitative evaluation of passive muscle stiffness in chronic stroke. Am J Phys Med Rehabil. 2016;95(12):899–910. doi: 10.1097/PHM.0000000000000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eby S.F., Cloud B.A., Brandenburg J.E., Giambini H., Song P., Chen S. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol, Avon) 2015;30:22–27. doi: 10.1016/j.clinbiomech.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S.S.M., Spear S., Rymer W.Z. Quantifying changes in material properties of stroke-impaired muscle. Clin Biomech (Bristol, Avon) 2015;30:269–275. doi: 10.1016/j.clinbiomech.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S.S.M., Gaebler-Spira D., Zhang L.-Q., Rymer W.Z., Steele K.M. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech (Bristol, Avon) 2016;31:20–28. doi: 10.1016/j.clinbiomech.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanter M., Bercoff J., Athanasiou A., Deffieux T., Gennisson J.-L., Montaldo G. Quantitative assessment of breast lesion viscoelasticity: initial clinical results using supersonic shear imaging. Ultrasound Med Biol. 2008;34:1373–1386. doi: 10.1016/j.ultrasmedbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Sarvazyan A.P., Rudenko O.V., Swanson S.D., Fowlkes J.B., Emelianov S.Y. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol. 1998;24:1419–1435. doi: 10.1016/s0301-5629(98)00110-0. [DOI] [PubMed] [Google Scholar]
- 34.Sköld C., Levi R., Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]