Abstract
Gestational trophoblastic neoplasia (GTN) is a spectrum of diseases including partial and complete hydatidiform moles, placental site trophoblastic tumor, and choriocarcinoma. One of the most important considerations is recognition of the possibility of GTN after molar pregnancy or even normal pregnancy. It is common practice to use chest x-ray for the detection of pulmonary metastasis. Computed tomography imaging of the lungs is ordered if lung lesions are noted on chest x-rays. However, understanding the limitations of chest x-rays is important for detecting smaller pulmonary lesions. We present a patient with GTN and pulmonary metastasis after having received 2 negative chest x-rays.
Keywords: Gestational trophoblastic neoplasia, Molar pregnancy, Pulmonary metastasis
Introduction
Gestational trophoblastic neoplasia (GTN) is a spectrum of diseases including partial and complete hydatidiform moles, placental site trophoblastic tumor, and choriocarcinoma. One of the most important considerations is recognition of the possibility of GTN after molar pregnancy or even normal pregnancy [1], [2], [3]. It is a common practice to use chest x-ray for the detection of pulmonary metastasis. Computed tomography (CT) imaging of the lungs is ordered if lung lesions are noted on chest x-rays. However, understanding the limitations of chest x-rays is important for detecting smaller pulmonary lesions. We present a patient with GTN and pulmonary metastasis after having received 2 negative chest x-rays.
Case
A 32-year-old indigent Hispanic woman, gravida 2 para 1-0-1-1, status post Suction, Dilation and Curettage (SD&C) 3 months before for molar pregnancy presented with heavy vaginal bleeding to the emergency department. Surgical pathology from the SD&C had revealed a complete hydatidiform mole with atypical trophoblastic epithelium. The chest x-ray at the time of the surgical evacuation 3 months before was unremarkable. Before the surgery, the beta-human chorionic gonadotropin (hCG) level was 248,270 milli-international units/milliliter (mIU/mL). One week after the surgery, the beta-hCG level had decreased to 971 mIU/mL, but there was inconsistent follow-up of the beta-hCG titers due to multiple social issues. Immediately after the SD&C, she received postmolar contraception of 150 milligrams (mg) depo medroxyprogesterone intramuscularly. Of note, her obstetrical history included an uncomplicated vaginal delivery 7 years before.
On examination in the emergency room, she was somnolent, but alert and oriented. Her heart rate was 117 beats per minute, and remaining vital signs were normal. She appeared pale, and capillary refill was 3 seconds. Vaginal examination revealed red, thick, mucinous discharge with clear, jelly-like substance, and no active bleeding. The uterus was 10 weeks in size and tender to palpation. There were no palpable adnexal masses.
The patient was admitted to the gynecology service. Her beta-hCG level was 4512 mIU/mL and hemoglobin level was 6.2 grams/deciliter (g/dL). Pelvic ultrasound was interpreted by radiology as no evidence of intrauterine pregnancy with an 11 × 5 × 7 centimeters (cm) sized uterus. The endometrium was distended with hyperechoic material with low resistance waveform measuring 3 × 4 cm. The right ovary was normal and the left ovary was not visualized. Her admission chest x-ray was unremarkable.
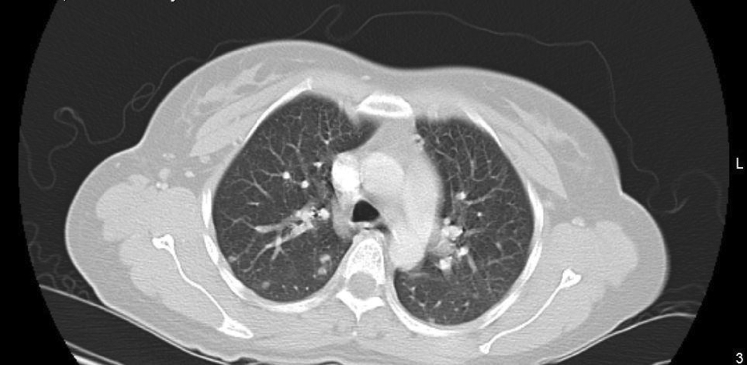
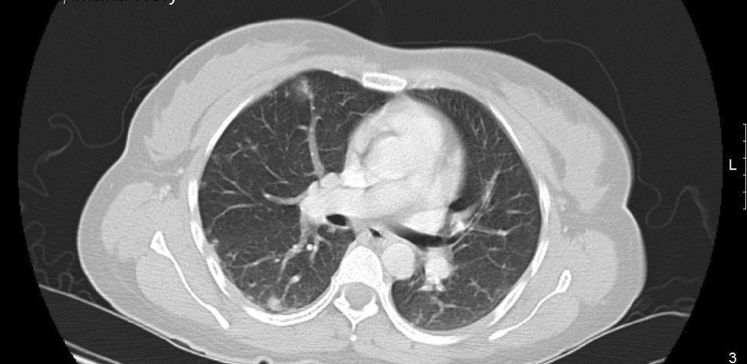
The patient continued to experience vaginal bleeding and her repeat hemoglobin level was 4.8 g/dL. She received 2 units of packed red blood cells. Because of her elevated beta-hCG without evidence of intrauterine pregnancy on ultrasound, additional testing was ordered for possible metastasis of GTN. CT imaging of her chest revealed small nodules, some with ground glass halos, scattered throughout both lungs (Figs. 1 and 2). These findings were interpreted by radiology as consistent with metastatic lung disease from choriocarcinoma.
Fig. 1.
Upper lung.
Fig. 2.
Middle lung.
The patient continued to have vaginal spotting and received a total of 8 units of packed red blood cells for acute blood loss anemia. After the patient hemodynamically stable, the Hematology & Oncology team was consulted for further management of her disease. She was assigned a stage 3 favorable low risk prognosis according to the International Federation of Gynecology and Obstetrics criteria [4]. Based on this staging, decision was made to treat her with 50 mg methotrexate intramuscularly on days 1, 3, 5, and 7 and 7.5 mg folic acid orally on days 2, 4, 6, and 8 for rescue therapy. We monitored her hematologic, hepatic, and renal indices during her chemotherapy. The patient followed-up with Hematology & Oncology for subsequent chemotherapy and further beta-hCG monitoring. The patient has since been in surveillance with Hematology & Oncology. Vaginal bleeding had completely subsided and her most recent beta-hCG level was less than 5 mIU/mL.
Discussion
The patient's elevated beta-hCG level was not due to a new pregnancy because she received postmolar depo medroxyprogesterone after her SD&C. The patient received 2 chest x-rays 3 months apart that were both unremarkable for pathology. We could have collected biopsy samples of the pulmonary lesions for histological review; however, this was not performed because of the patient's history, clinical picture, and CT confirmation of scattered small nodules in both lungs. The CT imaging was sufficient for the diagnosis of metastatic disease. A histological diagnosis would not have altered our management and a biopsy could have resulted in excessive bleeding. No further metastases were found on additional testing. The patient's noncompliance with postmolar surveillance may have contributed to the delay in diagnosis. However, she was compliant with subsequent outpatient chemotherapy treatments. Post-treatment imaging was not obtained because her vaginal bleeding had completely subsided and her beta-hCG levels were decreasing appropriately.
Conclusion
The risk for developing GTN is 0.1% in the general female population. The common presentation is persistent vaginal bleeding after either a normal or molar pregnancy along with an elevated beta-hCG level [5]. It is a common practice to cease further evaluation of pulmonary metastasis when initial chest x-rays are negative and patients do not exhibit significant respiratory distress. Although chest x-rays may be a good initial step for evaluation of pulmonary metastases, they may miss smaller lesions in the setting of someone whose beta hCG levels have plateaued or continued to rise. Three cases described by Moodley and Moodley [6] involved women with normal chest X-rays and subsequent CT imaging-confirmed pulmonary metastases. This case highlights the need of obstetricians and gynecologists to use CT imaging to evaluate patients with postmolar persistently elevated beta-hCG levels, especially if their clinical picture indicates a possibility of malignant GTN. In these cases, despite a normal chest x-ray, it is imperative to complete a full metastatic survey and order CT imaging of the head, chest, abdomen, and pelvis if available. By increasing this practice, pulmonary metastasis of GTN may be diagnosed earlier and more accurately.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Lurain J.R., Brewer J.I., Torok E.E., Halpern B. Natural history of hydatidiform mole after primary evacuation. Am J Obstet Gynecol. 1983;145:591–595. doi: 10.1016/0002-9378(83)91202-4. [DOI] [PubMed] [Google Scholar]
- 2.Berkowitz R.S., Goldstein D.P. Chorionic tumors. N Engl J Med. 1996;335:1740–1748. doi: 10.1056/NEJM199612053352306. [DOI] [PubMed] [Google Scholar]
- 3.Soper J.T., Clarke-Pearson D., Hammond C.B. Metastatic gestational trophoblastic disease: prognostic factors in previously untreated patients. Obstet Gynecol. 1988;71:338–343. [PubMed] [Google Scholar]
- 4.FIGO Oncology Committee FIGO staging for gestational trophoblastic neoplasia 2000. FIGO Oncology Committee. Int J Gynecol Obstet. 2002;77:285–287. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- 5.Soper J. Gestational trophoblastic disease. Obstet Gynecol. 2006;108:176–187. doi: 10.1097/01.AOG.0000224697.31138.a1. [DOI] [PubMed] [Google Scholar]
- 6.Moodley M., Moodley J. Evaluation of chest X-ray findings to determine metastatic gestational trophoblastic disease according to the proposed new staging system: a case series. J Obstet Gynaecol. 2004;24:287–288. doi: 10.1080/01443610410001660878. [DOI] [PubMed] [Google Scholar]