Abstract
Background
Lung-function decline is one of the possible mechanisms leading to Chronic Obstructive Pulmonary Disease (COPD).
Methods
We analyzed data obtained from two population-based surveys of adults (n = 2026) conducted in the same individuals 5–9 years (y) after their baseline examination in three Latin-American cities. Post BronchoDilator (postBD) FEV1 decline in mL/y, as %predicted/y (%P/y) and % of baseline/y (%B/y) was calculated and the influence of age, gender, BMI, baseline lung function, BD response, exacerbations rate evaluated using multivariate models.
Results
Expressed in ml/y, the mean annual postBD FEV1 decline was 27 mL (0.22%P, 1.32%B) in patients with baseline COPD and 36 (0.14%P, 1.36%B) in those without. Faster decline (in mL/y) was associated with higher baseline lung function, with significant response to bronchodilators, older age and smoking at baseline, also in women with chronic cough and phlegm, or ≥2 respiratory exacerbations in the previous year, and in men with asthma.
Conclusions
Lung function decline in a population-based cohort did not differ in obstructed and non-obstructed individuals, it was proportional to baseline FEV1, and was higher in smokers, elderly, and women with respiratory symptoms.
Introduction
Chronic Obstructive Pulmonary Diseases (COPD) is a leading cause of death worldwide. Classically, COPD has been considered a consequence of rapid lung-function decline during adult life, but more recently, it has also been considered as the consequence of poor lung development either before or after birth[1,2].
There is a great interest in identifying COPD subgroups, the so-called COPD phenotypes which are defined as ‘‘a single or a combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression or death)” [3] In the (PLATINO) study population, we have described the main characteristics of several phenotypes associated with COPD[4–6]. Recently we reported longitudinal mortality rates according to COPD status and lung function measured at baseline in population-based samples of adults living in three Latin American cities, with follow-up periods ranging from 5–9 years (y) (the PLATINO follow-up study)[7]. To our knowledge, no information is available regarding lung-function decline in Latin America or its determinants in a population-based study; we aimed to assess the decline in the Forced Expiratory Volume in the first second (FEV1) in a large sample from Latin America with a high overall response rates and robust, well-established methods.
Methods
The study protocol was approved by the Ethics Committee on Research, Pontificial Catholic University of Chile School of Medicine, by the Ethics Committee of the Maciel Hospital in Montevideo Uruguay, and by the Ethics Committee on Research of the Federal University of Sao Paulo/Sao Paulo Hospital. Study participants provided signed informed consent. The detailed methods of the PLATINO baseline[8] and follow-up studies[9] are available elsewhere. Between the years 2003 and 2005, population-based surveys were conducted, employing standardized methodology in five large Latin-American metropolitan areas: Sao Paulo (Brazil); Mexico City (Mexico); Montevideo (Uruguay); Santiago (Chile); and Caracas (Venezuela). We successfully interviewed 1,000 subjects aged 40 years or older in Sao Paulo, 1,063 in Mexico City, 943 in Montevideo, 1,208 in Santiago, and 1,357 in Caracas. Spirometry testing was performed for 963 (97.9%) subjects in Sao Paulo, 1,000 (98.3%) in Mexico City, 885 (97.1%) in Montevideo, 1,173 (99.8%) in Santiago, and 1,294 (98.4%) in Caracas[10]. The questionnaires (Spanish and Portuguese) are available at the PLATINO website: http://www.platino-alat.org.
Spirometry in the baseline and follow-up surveys was undertaken using an ultrasonic spirometer (EasyOne; ndd Medical Technologies, Zurich, Switzerland) prior to (pre- BronchoDilator [BD]) and 15 minutes after the administration of 200 μg of Salbutamol (post-BD) according to American Thoracic Society (ATS) criteria of acceptability and reproducibility [11], aiming for >90% test compliance with ATS quality criteria[8].
Follow-up studies were conducted in Montevideo, Santiago, and Sao Paulo 5, 6, and 9 years after the baseline surveys, respectively [9]. Individuals were visited at their homes based on the contact information provided by these persons during the baseline examination.
For the purposes of this work, we defined post-Bronchodilator (postBD) airflow obstruction Chronic Obstructive Pulmonary Disease [COPD] as individuals with a postBD FEV1/FVC below the Lower Limit of Normal (LLN)[12–14]–defined as the lower 5th percentile for predicted postBD FEV1/FVC based on equations derived from the 5 cities baseline study in a sub-set of healthy and never-smoker subjects[15]. We assessed, in survivors at the second evaluation, the annual decrease in spirometric lung function.
Statistical analysis
The annual decline in FEV1, was estimated by subtracting the second measurement from the first, and subsequently dividing by the number of years (and fractions) between both measurements. The FEV1 was analyzed in milliliters, but was also expressed as percentage of predicted (%P) for gender, age, and height[15], as percentage or baseline (%B), and as its natural logarithm[16] both for the preBD and postBD values.
The following potential confounders were explored in a multivariate analysis: age; gender; current smoking at baseline and at final evaluation (dichotomous and as cigarettes/day); lifetime cumulative smoking (pack-years); Body Mass Index BMI (kg/m2); years of academic education; self-reported comorbidities (heart disease, hypertension, diabetes, cerebrovascular accident and gastritis or ulcer, separately and as a count of positive comorbidities from 0–5); hour-years of exposure to biomass smoke (average number of years exposed by means of cooking multiplied by the average number of hours per day exposed); years of exposure in an occupation involving dust, smoke, or gases; the presence of chronic bronchitis (cough or phlegm on the majority of days, at least 3 months per year for >2 years, or cough and phlegm for the same time period) and previous Physician diagnosis of asthma or TB; significant response to bronchodilators (a postBD increase in FEV1 or FVC of ≥12% and of ≥200mL)[17]; baseline spirometric measurement [15], and the presence of postBD airflow obstruction (COPD). Final multivariate models included all variables showing an association with decline, at a P <0.15.
All statistical analyses were performed using Stata ver 13 statistical software (StataCorp, College Station, TX, USA) and we considered statistical significance as a P-value of <0.05.
Results
Only individuals with valid baseline spriometric data were eligible for follow-up. There were 885 eligible individuals for follow-up in Montevideo, 1173 in Santiago and 963 in Sao Paulo. Information was obtained for 758 (85.6%), 993 (84.7%) and 748 (77.7%) subjects, respectively. A total of 71 deaths in Montevideo, 95 in Santiago and 135 in Sao Paulo were identified. Among the individuals evaluated during follow-up, 2,120 had preBD and 2,026 postBD spirometry tests in both examinations. Follow-up rates for each independent-variable category were around 80% (see flowchart in S1 Appendix) [8,9].
Characteristics of the studied population in the first and second evaluations are depicted in Table 1. Compared with the first examination, individuals in the follow-up exam were older, with less current smoking, and slightly lower lung function.
Table 1. Characteristics of the studied population in the first and second evaluation (means± standard deviation [SD]).
| Variables | First evaluation | Second evaluation | P-value |
|---|---|---|---|
| Males, (% and 95%CI) | 40.9 (39.6–42.3) | 40.5 (38.9–42.1) | 0.76 |
| Age (years) | 57.4±12.1 | 62.6±11.1 | |
| Height (cm) | 160.0±9.7 | 159.8±9.8 | 0.99 |
| BMI (kg/m2) | 28.1±5.7 | 28.8±5.2 | <0.001 |
| FEV1 preBD (L) | 2.56±0.80 | 2.41±0.77 | <0.001 |
| FVC preBD (L) | 3.47±1.02 | 3.22±0.98 | <0.001 |
| FEV1 postBD (L) | 2.67±0.80 | 2.50±0.77 | <0.001 |
| FVC postBD (L) | 3.47±0.98 | 3.26±0.96 | <0.001 |
| FEV1/FVC preBD | 73.8±9.1 | 74.9±8.7 | <0.001 |
| FEV1/FVC postBD | 77.1±9.0 | 76.8±8.5 | 0.30 |
| Response to bronchodilators, % (95%CI) | 9.0 (7.9–10.1) | 9.7 (8.4–11.0) | 0.013 |
| History of asthma* (95%CI) | 15.4 (14.2–16.8) | 15.6 (14.1–17.2) | 0.86 |
| History of COPD* (95%CI) | 4.6 (3.9–5.4) | 6.3 (5.3–7.3) | 0.01 |
| Current smoker (95%CI) | 30.8 (29.1–32.4) | 24.3 (22.5–26.1) | <0.001 |
| % exposed to biomass smoke for >6months % (95%CI) | 46.2 (44.5–48.0) | 27.5 (26.0–29.1) | <0.001 |
| Hour-years in those exposed to biomass smoke (median, IQR) | 20 (5, 48) | 26 (10, 60) | <0.001 Ç |
| ≥2 exacerbations, last-year % (95%CI) | 2.6 (2.0–3.2) | 3.3 (2.6–4.1) | 0.03 |
| Previous tuberculosis, % (95%CI) | 3.4 (2.8–4.1) | 2.9 (2.2–3.7) | 0.29 |
| Job exposure to dust or smoke, % | 47.2 (45.5–49.0) | 34.0 (32.3–35.6) | <0.001 |
| Chronic cough or phlegm % | 14.5 (13.3–15.8) | 17.5 (15.4–18.9) | 0.01 |
| Chronic cough and phlegm %(95%CI) | 4.2(3.5–4.9) | 5.3(4.3–6.3) | 0.11 |
| COPD (GOLD stages 1–4), % (95%CI) | 17.1 (15.7–18.5) | 17.4 (15.8–19.1) | 0.76 |
| COPD (GOLD stages 2–4), % (95%CI) | 6.5 (5.6–7.4) | 6.6 (5.6–7.7) | 0.89 |
| COPD (FEV1/FVC<LLN), % (95%CI) | 9.3 (8.3–10.4) | 8.6 (7.4–9.9) | 0.37 |
BMI (Body Mass Index) = weight/ height2; SD = Standard Deviation; preBD = before bronchodilator; postBD = after bronchodilator. 95%CI = 95% confidence interval; IQR = interquartile range; GOLD = Global Initiative for Chronic Obstructive Lung Disease. LLN = lower limit of normal; 95%CI = 95% confidence interval. Asthma-COPD overlap = medical diagnosis of asthma (first definition) plus FEV1 /FVC<0.7 post-BD or wheezing in the last year plus response to bronchodilator plus FEV1/FVC<0.7 in the second definition. Bronchodilator response is the increase in FVC or FEV1 of ≥12% and of ≥200mL Chronic bronchitis was cough or phlegm on the majority of days for >3 months in 1 year for >2 consecutive years.
*Self-reported previous physician diagnosis of the condition.
**Student t tests on the equality of means
ÇMann-Whitney U tests, two independent samples. Individuals described are those with spirometric testing at baseline (column one) and follow up (column two, see Methods).
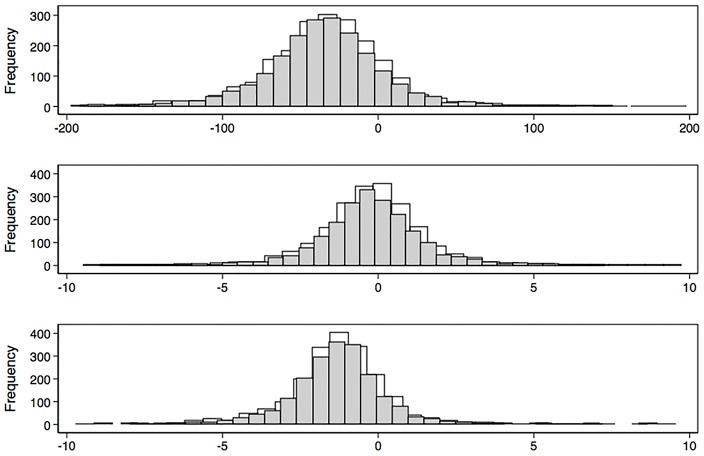
Decline in spirometric function was heterogeneous (Fig 1), and some individuals did not demonstrate any decline. We observed a second postBD measurement lower than the first, (negative change or decline) in 86% of participants for FEV1 expressed in mL, in 58% if function is expressed as %P, and in 86% if expressed as %B. In 5.9% of participants, postBD FEV1 increased >20 mL/y during the follow-up (5.6% in women, 6.3% in men, and 9.9% in those with postBD airflow obstruction).
Fig 1. Distribution of pre-bronchodilator (preBD) (gray bars) and post-bronchodilator (postBD) (light bars) Forced Expiratory Volume in the first second (FEV1) in mL/y (upper graph); as percentage of predicted (%P, middle graph); and as percentage of baseline (lower graph).
Decline varied considerably regardless of in which units is expressed, including individuals with no decline or increase in measurements from baseline.
In Table 2, we describe the mean annual decline of FEV1, expressed in milliliters, as %P and as %B in women and men. Women declined, on average, for postBD FEV1, 31 mL/y (0.17%P/y, 1.33%B/y), whereas men declined on average for postBD FEV1 42 mL/y (0.29%P/y, 1.31%B/y). Decline in men was higher than in women in mL/y but was not statistically different if expressed as %P/y, as %B/y (Table 2), or as Δz scores, or as Δlog spirometric values (See Supplementary material, S1–S5 Tables). Decline of postBD measurements >40 mL/y, for FEV1, was observed in 34% of women, and in 48%, of men respectively. Similarly, the proportion of individuals with decline of postBD measurements >100 mL/y were higher in men: it was observed in 2.5%, of women, and in 8.5%, of men respectively. Variance of the lung function decline (SD) in the population was significantly higher (P<0.001) for preBD measurements than for posBD measurements, and for men than for women (Table 2).
Table 2. Mean annual decline FEV1 (SD), with 95% confidence intervals (95%CI) and 5th percentiles (P5).
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Mean annual drop (SD) | 95%CI, Mean annual decline | 5th percentile all | 5th percentile healthy | Mean annual drop (SD) | 95%CI, Mean annual decline | 5th percentile all | 5th percentile healthy |
| preBD FEV1 (mL/y) | -29.3 (39.6) | -31.4, -27.1 | -88 | -100 | -40.5 (49.1) | -43.8,-37.2 | -125 | -149 |
| posBD FEV1 (mL/y) | -31.4 (34.8) | -33.5, -29.3 | -81 | -91 | -41.7 (47.7) | -44.9,-38.4 | -121 | -126 |
| preBD FEV1 (%P/y) | -0.14 (1.9) | -0.24, -0.04 | -2.9 | -3.6 | -0.30 (1.7) | -0.41,-0.18 | -3.2 | -4.2 |
| posBD FEV1 (%P/y) | -0.17 (1.7) | -0.27,-0.06 | -2.7 | -3.1 | -0.29 (1.7) | -0.40,-0.18 | -3.1 | -3.7 |
| preBD FEV1 (%B/y) | -1.25 (2.39) | -1.38,-1.12 | -4.12 | -4.92 | -1.28 (2.0) | -1.41,-1.15 | -4.31 | -4.70 |
| PosBD FEV1 (% B/y) | -1.33 (1.61) | -1.43, -1.24 | -3.79 | -3.91 | -1.31 (1.6) | -1.42, -1.20 | -4.07 | -4.60 |
%P = expressed as percentage of predicted according to PLATINO reference values. %B = expressed as percentage of baseline. 95%CI = 95% confidence interval of the mean. PreBD = pre bronchodilator test; posBD = post bronchodilator test; SD = standard deviation of the sample. 5th percentile value of the whole cohort (all, 1261 women and 858 men with preBD testing, 1203 and 822 with postBD testing) or from the respiratory healthy (207 women and 104 men with preBD testing and 195, 97 with postBD testing). See the supplemental material for declines of zFEV1, FEV1/Height3 and LogFEV1
Main variables associated with lung function decline are presented in Table 3 and S1–S5 Tables. For postBD values of FEV1 (Table 3), decline in men and women was significantly higher in individuals older at baseline, in those with higher baseline lung function, in those with significant response to bronchodilators, and was milder in those with greater height (once baseline lung function was taken into account) (Table 3). When expressed as %P or %B the impact of age was eliminated or reduced but not that of baseline lung function.
Table 3. Multivariate regression coefficients (with 95% confidence intervals) for associations with the post bronchodilator Forced Expiratory Volume at one second (FEV1, mL/y) decline in the cohort.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | FEV1 (mL) | 95%CI | FEV1 (%P) | 95%CI | FEV1 (mL) | 95%CI | FEV1 (%P) | 95%CI |
| Baseline PostBD FEV1 (mL) | -0.025 | -0.031, -0.021 | -0.001 | -0.001, -0.001 | -0.014 | -0.020, -0.007 | ||
| Age (years) | -0.58 | -0.80, -0.36 | -0.613 | -0.988, -0.238 | ||||
| Height (cm) | 0.65 | 0.32, 0.98 | 0.0352 | 0.0185, 0.0519 | 0.594 | 0.068, 1.119 | 0.013* | -0.0.003, 0.03 |
| BMI (kg/m2) | 0.25* | -0.05, 0.54 | 1.140 | 0.367, 1.914 | 0.042 | 0.01, 0.06 | ||
| Cigarettes/day | -0.71 | -1.0, -0.4 | -0.0295 | -0.044, -0.015 | ||||
| Smoking at baseline (yes/no) | -11.85 | -18.89, -4.80 | -0.33 | -0.57, -0.09 | ||||
| TB | -0.661 | -1.18, -0.138 | ||||||
| >2 exacerbations last year | -14.8 | -24.2, -5.2 | -0.559 | -1.045, -0.072 | ||||
| Chronic cough & phlegm | -11.7 | -20.8, -2.6 | -0.642 | -1.11, -0.178 | ||||
| Response to BD | -11.9 | -18.6, -5.2 | —0.617 | -0.961, -0.274 | -25.160 | -39.40, -10.92 | -0.68 | -1.17, -0.19 |
95%CI = 95% confidence interval of the mean. PreBD = pre bronchodilator test; posBD = post bronchodilator test; %P = expressed as percentage of predicted according to PLATINO reference values. Variability explained by the model (adjusted R2) was 9% in women, and 4.9% in men. Bronchodilator response is the increase in FVC or FEV1 of ≥12% and of ≥200mL. Chronic cough and phlegm was cough or phlegm on the majority of days for >3 months in a year for >2 consecutive years. The presence of postBD airflow obstruction (COPD) was not associated with decline in multivariate models. TB = previous tuberculosis. Total number of individuals in the models is 2,120 for preBD and 2,026 por postBD tests.
*All variables included in the models had a P<0.15, but some of the variables in the table did not reach the statistical significance at p<0.05.
The presence of smoking at baseline or the number of cigarettes smoked, were associated with greater decline of FEV1 in men and women. Having ≥2 respiratory disease exacerbations in the previous year, and chronic cough and phlegm was associated with higher decline in FEV1 in women. Higher BMI predicted a reduced decline of FEV1 (Table 3). The presence of a FEV1/FVC<LLN (COPD) was not associated with a significant increased decline (Tables 4 and 5), after taking baseline lung function into account.
Table 4. Characteristics of subjects with and without Chronic Obstructive Pulmonary Disease (COPD, post-BD FEV1/FVC<LLN, mean±standard deviation [SD]) or 95% confidence interval (95%CI) in the whole cohort at baseline.
| Variables | NO COPD (N = 2875) |
SD (or 95%CI) |
COPD (N = 146) |
SD (or 95%CI) |
P-value* |
|---|---|---|---|---|---|
| Males, % (95%CI) | 40.6 | 39.1–41.9 | 48.6 | 39.8–57.5 | 0.06 |
| Age (years) | 56.8 | 11.8 | 63.1 | 13.1 | <0.001 |
| Height (cm) | 160.1 | 9.7 | 159.4 | 10.3 | 0.43 |
| BMI (kg/m2) | 28.1 | 5.7 | 27.9 | 5.6 | 0.75 |
| FEV1 preBD (L) | 2.60 | 0.78 | 1.79 | 0.76 | <0.001 |
| FVC preBD (L) | 3.49 | 1.01 | 3.09 | 1.11 | <0.001 |
| FEV1 postBD (L) | 2.71 | 0.78 | 1.93 | 0.76 | <0.001 |
| FVC postBD (L) | 3.48 | 0.97 | 3.32 | 1.15 | <0.001 |
| FEV1 /FVC preBD | 74.7 | 8.1 | 57.3 | 11.5 | <0.001 |
| FEV1 /FVC postBD | 78.1 | 7.7 | 57.5 | 9.4 | <0.001 |
| Deaths (95%CI) | 9.1 | 7.9–11.5 | 18.5 | 11.5–25.5 | 0.001 |
| Bronchodilator response, % (95%CI) | 7.4 | 6.4–8.4 | 39.7 | 32.0–47.5 | <0.001 |
| History of asthma, % (95%CI) | 14.3 | 13.0–15.7 | 38.4 | 30.4–46.3 | <0.001 |
| History of COPD, % (95%CI) | 4.0 | 3.2–4.7 | 17.8 | 11.1–24.5 | <0.001 |
| Current smoker, % (95%CI) | 32.9 | 30.8–35.0 | 0.000 | 0.000 | <0.001 |
| % exposed to biomass smoke of >6months | 54.2 | 51.8–56.5 | 64.4 | 56.7–72.1 | 0.02 |
| Biomass exposure, (hour-years in exposed) median, IQR | 18.6 | 43.8 | 36.3 | 95.5 | <0.001 |
| ≥1 exacerbations last year, % (95%CI) | 2.4 | 1.8–3.1 | 8.2 | 3.8–12.6 | <0.001 |
| ≥2 exacerbations last year, % (95%CI) | 3.1 | 2.4–3.8 | 7.4 | 1.4–13.4 | 0.04 |
| Chronic cough or phlegm,% (95%CI) | 14.2 | 12.9–15.4 | 22.6 | 15.6–29.6 | 0.01 |
| Chronic cough and phlegm, (95%CI) | 4.1 | 3.4–4.8 | 8.9 | 4.2–13.6 | 0.01 |
| Previous tuberculosis, % (95%CI) | 3.1 | 2.5–3.8 | 10.3 | 5.7–14.9 | <0.001 |
| Job exposure to dust or smoke, % | 47.5 | 45.4–49.7 | 58.2 | 50.4–66.1 | 0.01 |
preBD = before bronchodilator use; postBD = after bronchodilator use; COPD is a postBD FEV1/FVC<lower limit of normal according to the PLATINO reference values.
*TTest for independent groups or Fisher exact test for categorical variables; Bronchodilator response is the increase in FVC or FEV1 ≥12% &≥200mL. Chronic cough or phlegm on the majority of days for >3 months during a year for >2 years
Table 5. Lung function (FEV1) decline in participants with and without airflow obstruction (postBD FEV1/FVC<LLN), means and standard deviation (SD) or 95%CI.
| Variables | NO COPD (N = 2,025) |
SD (or 95%CI) |
COPD (N = 95) |
SD (or 95%CI) |
P-value* |
|---|---|---|---|---|---|
| Mean decline PreBD (mL/year) | -34.2 | 43.8 | -26.1 | 48.9 | 0.08 |
| Mean decline PostBD (mL/y) | -35.9 | 40.7 | -26.8 | 41.3 | 0.04 |
| Mean decline PreBD (%P/year) | -0.21 | 1.8 | -0.17 | 2.1 | 0.90 |
| Mean decline PostBD (%P/year | -0.22 | 1.6 | -0.14 | 2.0 | 0.60 |
| Mean decline PreBD (%B/year) | -1.26 | 2.2 | -1.30 | 2.8 | 0.90 |
| Mean decline PostBD (%B/year), | -1.32 | 1.5 | -1.36 | 2.1 | 0.80 |
| % with >20 mL/y increase in preBD FEV1 | 5.9 | 4.9–7.0 | 7.4 | 2.0–12.7 | 0.56 |
| % with >20 mL/y increase in postBD FEV1 | 5.7 | 4.7–6.7 | 9.9 | 3.6–16.1 | 0.10 |
| % with any preBD FEV1 decline | 84.5 | 82.8–86.2 | 77.9 | 69.6–86.2 | 0.08 |
| % with any postBD FEV1 decline | 86.0 | 84.5–87.5 | 80.2 | 72.4–88.0 | 0.12 |
| % with any preBD decline as %P | 55.8 | 53.4–58.2 | 54.7 | 44.7–64.8 | 0.84 |
| % with any postBD decline as %P | 57.7 | 55.5–59.9 | 57.1 | 46.8–67.5 | 0.91 |
| % with>40mL/y decline | 41.0 | 38.7–43.3 | 32.6 | 23.1–42.2 | 0.11 |
| % with>40mL/y postBD | 42.7 | 40.4–45.1 | 38.5 | 28.6–48.3 | 0.4 |
| % with preBD decline >3%P/y | 4.9 | 4.0–5.9 | 8.4 | 2.7–14.1 | 0.13 |
| % with postBD decline >3%/y | 4.3 | 3.4–5.2 | 3.2 | 0.0–6.7 | 0.59 |
| % with preBD decline >4%B/y | 5.5 | 4.5–6.5 | 16.8 | 9.2–24.5 | <0.001 |
| % with postBD decline >4%B/y | 4.1 | 3.2–5.0 | 7.4 | 2.0–12.7 | 0.13 |
preBD = before bronchodilator use; postBD = after bronchodilator use; COPD is a postBD FEV1/FVC<lower limit of normal according to the PLATINO reference values.
*T test for independent groups or Fisher exact test for categorical variables; %P = FEV1 expressed as percentage of predicted by the PLATINO reference values; %B = FEV1 expressed as percentage of baseline or first measurement; Results based on 2,120 individuals with two preBD spirometric tests, or 2,026 individuals with two postBD spirometry tests.
The most consistent predictors of >100mL/y decline were the size of baseline function, age, response to bronchodilators, and in men smoking at baseline. Fifth percentiles of decline are also depicted in Table 2, and these were considerably higher (more negative) in men than in women, and higher than -40 mL/y. Table 2 includes the 5th percentile of the whole cohort, and the 5th percentile of a "respiratory healthy" group[15], comprised of never smoker individuals, lacking respiratory symptoms and previous diagnosis of respiratory diseases, demonstrating slightly more negative values than in the whole cohort.
Only about 6–11% of the variability of decline in women and 2–6% in men was explained by the statistical models.
Discussion
Annual lung-function decline, one of the mechanisms leading to COPD, was estimated for a population-based cohort in three cities of Latin America. Decline was heterogeneous as previously reported in patients with COPD[18] but did not differ significantly in obstructed and non-obstructed individuals. In women, the presence of chronic bronchitis and frequent respiratory exacerbations also was associated with faster decline. As found in other longitudinal studies PostBD lung function declined faster (in mL/y) in men than in women, in individuals recruited at older age, in those with higher lung function at baseline, in current smokers, and in those with significant response to bronchodilators. A higher decline in mL/y in men and in those with higher lung function suggests that the decline is proportional to lung size[16], and models fitted to estimate loss as %P, or as %B, reduce the association of decline with baseline function. Expressing declines in mL/y may be deceiving as these tend to decrease with airflow obstruction, old age, women, and in individuals with small size, but if expressed as proportional changes, the declines may, in fact, increase[16].
We also estimated 5th percentiles of decline in the general population as it may be important for COPD risk and prevention, in that it could comprise a marker of a statistically significant and uncommon decline in the population. Fortunately, 5th percentiles of declines expressed as %P are more consistent around 3%/y, and those expressed as %B around 4%, which can be used to advantage. In our study, all declines expressed as %P, or as z-scores were statistically significant (lower than zero) meaning that the cross-sectional age-coefficient obtained from the baseline spirometry in healthy individuals[15] differs from the longitudinal age-decline.
A reduced postBD FEV1/FVC (COPD) was not associated with excessive decline if models were adjusted by baseline function, emphasizing the relevance of FEV1, rather than that of the FEV1/FVC ratio as predictors of decline, but also of mortality[7].
Individuals with previous physician diagnosis of TB, were associated with airflow obstruction and reduced baseline function in the PLATINO study[19], but they did not have a steeper lung-function decline consistently (see Online Supplementary Material). Data of South African miners exposed to silica and tuberculosis (TB), indicated that lung-function decline was proportional to the number of TB episodes[20]. However this may be different in the absence of significant exposure to silica in general population.
Similarly, individuals reporting exposure to biomass smoke or to dust at work were not more likely to have an increased rate of lung-function decline. Recently, a heterogeneous lung-function decline in a cohort of individuals with biomass smoke-associated COPD was reported[21], similar to that observed in other COPD cohorts associated with smoking[22]. In fact, only a small proportion of patients demonstrated a measurable decline.
In our study using multivariate models, individuals with a prior Physician diagnosis of asthma had significantly lower baseline FEV1 preBD, and postBD (-172 mL and -156 mL, respectively), but FEV1 declined similarly than the remaining of the cohort, in contrast with a steeper lung-function decline observed previously in asthmatics if the disease initiates in infancy, or is poorly controlled, or if the individual smokes[23].
Adult airflow obstruction may begin early in life, through poor lung development (small lungs), but also, as proposed years ago, individuals with normal -sized lungs may exhibit accelerated lung function-decline and develop obstruction[2]. Contrary to the traditional view, accelerated FEV1 decline in individuals with COPD is uncommon[22] and, in advanced COPD stages, decline in mL/y is reduced[24], although if expressed in relative terms, decline may increase[16] reinforcing the presence of a decline proportional to lung size or function and the convenience of expressing it as %B or %P.
In our study, mean FEV1 decline in COPD fell within the observed ranges in cohorts of patients with COPD[24], but, in addition the rates did not differ significantly from those of individuals without airflow obstruction, a comparison unavailable in data proceeding from cohorts of patients.
There are study limitations that should be mentioned: follow-up includes only three of the original five cities, with only two spirometric evaluations, limiting the accurate assessment of the annual individual FEV1 decline and possibly the identification of “fast decliners”[25]. In addition, participants from Mexico, likely with a higher Amerindian contribution, had a lower prevalence of COPD, although rates of decline in a cohort of Mexican patients coincide with those of the current report [21].
It is noteworthy that lung-function decline is highly variable even after several spirometric measurements[18] and also, that relevant information on lung-function decline has been produced based only on two measurements[26,27]. Although we performed only two spirometric tests, both were carried out with the same equipment, both included postBD measurements, and the test quality was good[15], with a population-based sample size sufficient for finding statistically significant and clinically relevant associations and high rates of follow-up after 5–9 years. Differently from studies based on cohorts of patients with COPD, our statistical models compared not only individuals with airflow obstruction due to their smoking experience, but also with non-obstructed individuals from general population. We reported mostly postBD spirometric measurements, which were also less variable in our data than those obtained prior to BD, and which represented maximal lung function at the testing time.
Smoking is the principal recognized cause of COPD, was a relevant risk for lung-function loss at baseline, and for lung-function decline (Table 4). Another priority comprises the diagnosis and control of asthma, a known cause of airflow obstruction and a relevant risk for exacerbations and lung function loss, and with effective interventions such as the use of inhaled corticosteroids.
In conclusion, obstructed and non-obstructed individuals had a similar lung-function decline in a population-based study from three Latin-American cities. Women with chronic cough and phlegm or with frequent respiratory exacerbations declined faster in FEV1. Anti-tobacco campaigns are the most important public health measure to prevent COPD. In addition, lung function decline should take into account baseline lung function and age, and expressing it as %P or %baseline entertains advantages over its customary expression as mL/y.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Rogelio Perez-Padilla is the guarantor of the content of the manuscript, including the data and analysis.
Marina1, Dolores Moreno3, Carmen Lisboa5, Julio Pertuze5 Oliver A. Nascimento6, Fernando Wehrmeister, Mariana R. Gazzotti6, Graciane Laender6 and Beatriz Manzano6
Data Availability
Data are attached as a Supporting Information file.
Funding Statement
This work was supported by the Asociación Latinoamericana de Tórax (ALAT), Boehringer Ingelheim GmbH, GlaxoSmithKline, and Novartis for the collection of the data during the field work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Postma DS, Bush A, van den Berge M (2015) Risk factors and early origins of chronic obstructive pulmonary disease. Lancet 385: 899–909. 10.1016/S0140-6736(14)60446-3 [DOI] [PubMed] [Google Scholar]
- 2.Lange P, Celli B, Agusti A, Boje Jensen G, Divo M, Faner R, et al. (2015) Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 373: 111–122. 10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. (2010) Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 182: 598–604. 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montes de Oca M, Halbert RJ, Lopez MV, Perez-Padilla R, Talamo C, Moreno D, et al. (2012) Chronic bronchitis phenotype in subjects with and without COPD: the PLATINO study. Eur Respir J 40: 28–36. 10.1183/09031936.00141611 [DOI] [PubMed] [Google Scholar]
- 5.Menezes AM, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. (2014) Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 145: 297–304. 10.1378/chest.13-0622 [DOI] [PubMed] [Google Scholar]
- 6.Perez-Padilla R, Fernandez R, Lopez Varela MV, Montes de Oca M, Muiño A, Tálamo C, et al. (2012) Airflow Obstruction in Never Smokers in Five Latin American Cities: The PLATINO Study. Archives of Medical Research 43: 159–165. 10.1016/j.arcmed.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 7.Menezes AM, Perez-Padilla R, Wehrmeister FC, Lopez-Varela MV, Muino A, Valdivia G, et al. (2014) FEV1 is a better predictor of mortality than FVC: the PLATINO cohort study. PLoS One 9: e109732 10.1371/journal.pone.0109732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menezes AM, Victora CG, Perez-Padilla R (2004) The Platino project: methodology of a multicenter prevalence survey of chronic obstructive pulmonary disease in major Latin American cities. BMC Med Res Methodol 4: 15 10.1186/1471-2288-4-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menezes AM, Muino A, Lopez-Varela MV, Valdivia G, Lisboa C, Jardim JR, et al. (2014) A population-based cohort study on chronic obstructive pulmonary disease in Latin America: methods and preliminary results. The PLATINO Study Phase II. Arch Bronconeumol 50: 10–17. 10.1016/j.arbres.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 10.Menezes AM, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, Valdivia G, et al. (2005) Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): a prevalence study. Lancet 366: 1875–1881. 10.1016/S0140-6736(05)67632-5 [DOI] [PubMed] [Google Scholar]
- 11.American Thoracic Society (1995) Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 152: 1107–1136. 10.1164/ajrccm.152.3.7663792 [DOI] [PubMed] [Google Scholar]
- 12.Hardie JA, Buist AS, Vollmer WM, Ellingsen I, Bakke PS, Morkve O (2002) Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J 20: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Padilla R, Hallal PC, Vazquez-Garcia JC, Muino A, Maquez M, Lopez MV, et al. (2007) Impact of bronchodilator use on the prevalence of COPD in population-based samples. COPD 4: 113–120. 10.1080/15412550701341012 [DOI] [PubMed] [Google Scholar]
- 14.Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, Kocabas A, et al. (2009) Comparison of spirometry criteria for the diagnosis of COPD: results from the BOLD study. Eur Respir J 34: 588–597. 10.1183/09031936.00164608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pérez-Padilla R, Valdivia G, Muiño A, López MV, Márquez MN, Montes de Oca M, et al. (2006) Spirometric reference values in 5 large Latin American cities for subjects aged 40 years or over. Archivos de Bronconeumología ((English Edition)) 42: 317–325. [DOI] [PubMed] [Google Scholar]
- 16.Thomsen LH, Dirksen A, Shaker SB, Skovgaard LT, Dahlback M, Pedersen JH (2014) Analysis of FEV1 decline in relatively healthy heavy smokers: implications of expressing changes in FEV1 in relative terms. COPD 11: 96–104. 10.3109/15412555.2013.830096 [DOI] [PubMed] [Google Scholar]
- 17.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. (2005) Interpretative strategies for lung function tests. Eur Respir J 26: 948–968. 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. (2011) Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med 365: 1184–1192. 10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 19.Allwood BW, Myer L, Bateman ED (2013) A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 86: 76–85. 10.1159/000350917 [DOI] [PubMed] [Google Scholar]
- 20.Hnizdo E, Singh T, Churchyard G (2000) Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 55: 32–38. 10.1136/thorax.55.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramirez-Venegas A, Sansores RH, Quintana-Carrillo RH, Velazquez-Uncal M, Hernandez-Zenteno RJ, Sanchez-Romero C, et al. (2014) FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am J Respir Crit Care Med 190: 996–1002. 10.1164/rccm.201404-0720OC [DOI] [PubMed] [Google Scholar]
- 22.Casanova C, de Torres JP, Aguirre-Jaime A, Pinto-Plata V, Marin JM, Cordoba E, et al. (2011) The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med 184: 1015–1021. 10.1164/rccm.201105-0831OC [DOI] [PubMed] [Google Scholar]
- 23.Grol MH, Gerritsen J, Vonk JM, Schouten JP, Koeter GH, Rijcken B, et al. (1999) Risk factors for growth and decline of lung function in asthmatic individuals up to age 42 years. A 30-year follow-up study. Am J Respir Crit Care Med 160: 1830–1837. 10.1164/ajrccm.160.6.9812100 [DOI] [PubMed] [Google Scholar]
- 24.Tantucci C, Modina D (2012) Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis 7: 95–99. 10.2147/COPD.S27480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson GT, Enright PL, Buist AS, Higgins MW (2000) Office spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest 117: 1146–1161. [DOI] [PubMed] [Google Scholar]
- 26.Vestbo J, Prescott E, Lange P (1996) Association of chronic mucus hypersecretion with FEV1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med 153: 1530–1535. 10.1164/ajrccm.153.5.8630597 [DOI] [PubMed] [Google Scholar]
- 27.Skloot GS, Schechter CB, Herbert R, Moline JM, Levin SM, Crowley LE, et al. (2009) Longitudinal assessment of spirometry in the World Trade Center medical monitoring program. Chest 135: 492–498. 10.1378/chest.08-1391 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
Data are attached as a Supporting Information file.