Abstract
The shape-dependent activity of gold nanoparticles (AuNP) was studied by testing them as electrocatalysts for the notoriously slow non-enzymatic oxidation of glucose in neutral solutions. The AuNP of spherical and irregular (including polyhedral) morphologies were synthesized and attached to glassy carbon electrodes with chitosan. Voltammetric and mass spectrometric studies showed that the irregular AuNP were more catalytically active toward the oxidation of glucose to gluconic acid. No obvious differences between both morphologies were found based on their X-ray diffraction patterns and HRTEM images suggesting that the crystallographic orientation alone did not account for their catalytic properties. While both morphologies contain the (111) crystallographic planes that are catalytic toward glucose oxidation, the better activity of irregular AuNP was ascribed to a higher surface density of incipient gold oxide acting as a fast redox mediator for glucose oxidation. Supporting this, the AuNP of both morphologies oxidized glucose after their anodic activation, although not to the same extent. The amperometric (0.30 V) determination of glucose at electrodes made of irregular AuNP yielded a wide linear calibration plot (0.20–110 mM; R2, 0.998), sensitivity of 66 μA M−1 cm−2, limit of detection of 100 μM (S/N, 3), and a response time below 5 s. The advantage of low-cost irregular AuNP over macro gold is that they are catalytic toward glucose oxidation without any need for prior activation.
Keywords: HEPES gold nanoparticles, morphology-dependent electrocatalysis, irregular vs. spherical morphology, oxidation of glucose in neutral solutions
Graphical abstract

1. Introduction
Recently, there has been an explosion of interest in the dependence of the optical, magnetic, and electronic properties of metal nanoparticles on their morphology (size and shape). Such dependence has been explored in photonic, therapeutic, diagnostic, photovoltaic, and catalytic applications [1,2]. In the present work, we investigate gold nanoparticles (AuNP) with different morphologies as electrocatalysts for the non-enzymatic oxidation of glucose at physiological pH 7.40.
The oxidation of glucose is important in the development of glucose sensors for monitoring diabetes, glucose fuel cells for powering medical implants, and reactors for the synthesis of specialty chemicals (e.g. gluconic acid) [3,4]. However, in contrast to the enzymatic oxidation, the direct oxidation of glucose in neutral solutions is difficult because of the slow kinetics at carbon electrodes. Several transition metal-based electrodes have been designed, including gold, to facilitate the non-enzymatic oxidation of glucose in neutral solutions [5–16]. In this context, the research with single crystals of gold has shown that their low-index crystallographic planes (e.g. (111), (110), (100)) catalyze the oxidation of glucose [17,18].
Here, we test two hypotheses of the shape-dependent catalytic activity of AuNP.
the non-spherical morphology of AuNP should promote the presence of diverse crystallographic surface planes including those with low Miller indices that facilitate the adsorption and oxidation of glucose
the non-spherical AuNP with corners and edges should support a higher surface density of gold-oxygen sites acting as redox mediators for glucose oxidation
These hypotheses were tested with irregular AuNPHEPES and mostly spherical AuNPCIT that were prepared by using the HEPES and sodium citrate (CIT), respectively, as reducing agents. The nanoparticles and their properties were studied by using the electrochemistry, X-ray powder diffraction, transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and electrospray ionization collision-induced dissociation (ESI-CID) mass spectrometry.
2. Experimental
2.1. Reagents and solutions
Tetrachloroauric acid (HAuCl4•3H2O), 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), sodium citrate, polysaccharide chitosan (CHIT, MW ~ 1× 106 Da; ~80 % deacetylation), methanol, and ethanol were purchased from Sigma-Aldrich. The chitosan solution (0.10 wt. %) was prepared by dissolving chitosan flakes in a hydrochloric acid solution and following previously established protocol [19]. All solutions were prepared using 18-MΩ-cm deionized water that was purified with a Synergy Millipore cartridge system.
2.2. Synthesis of gold nanoparticles (AuNP)
The AuNPHEPES were synthesized by mixing 10.0 mL of 100 mM HEPES solution with 1.0 mL of 10.3 mM HAuCl4•3HO solution at room temperature. In contrast to literature protocols [20, 21], no pH adjustment was done during the present synthesis. Within 3 min, a color of unstirred mixture changed from light yellow to dark brown. The brown precipitate settled down overnight and the supernatant was removed leaving ~1.0 mL of the concentrated colloidal suspension of AuNPHEPES. The centrifugation was avoided because it caused excessive agglomeration of nanoparticles.
The AuNPCIT were synthesized by following a classical citrate method [22]. The 1.0 mL of 10.3 mM HAuCl4•3H2O solution was reacted with 10.0 mL of boiling 120 mM sodium citrate. The mixture was stirred and boiled for 10 min while color changed from light yellow to red. After cooling to room temperature, the mixture was centrifuged at 3,000 rpm for 10 min and supernatant was removed leaving ~1.0 mL of the concentrated colloidal suspension of AuNPCIT.
2.3. Preparation of AuNPHEPES and AuNPCIT electrodes
The electrodes were prepared by mixing a 40-μL aliquot of AuNPHEPES or 120-μL aliquot of AuNPCIT suspension with a 15-μL aliquot of chitosan solution on the surface of a glassy carbon electrode, and evaporating water for 2 h at room temperature. This resulted in a uniform film of chitosan holding gold nanoparticles on the electrode surface.
2.4. Electrochemical measurements
The cyclic voltammetry and amperometry experiments were performed by using a CHI 832 workstation (CH Instruments, Inc.). The measurements were performed in the three-electrode system. The working electrode was a 3.0-mm-diameter glassy carbon electrode that was modified with either AuNPHEPES or AuNPCIT. In control experiments, the bare glassy carbon and gold (2.0-mm diameter) disc electrodes served as working electrodes. The auxiliary electrode was made of platinum wire and the reference electrode was the Ag/AgCl/3MNaCl (Bioanalytical Systems, Inc., BAS). Before their use, the glassy carbon and gold electrodes were wet polished on an Alpha A polishing cloth (Mark V Lab) with successively smaller particles (0.30 and 0.05 μm diameter) of alumina. The alumina slurry was removed from the electrode surface by sonication in deionized water and methanol.
All experiments were performed at room temperature (21 ± 1 °C) using a pH 7.40 phosphate buffer solution (0.10 M) as a background electrolyte. The experiments were repeated minimum three times and the means of measurements are presented with the relative standard deviation.
2.5. Microscopic and X-ray measurements
The microscopic data were collected with a JEOL 2010F and JEOL 1230 (120 kV) using GATAN digital micrograph. The aliquot of water-suspended gold nanoparticles was placed onto a formvar-coated copper TEM grid (300 mesh, TedPella Inc.) and dried at room temperature prior to analysis. X-ray spectra of gold nanoparticles were recorded by using a PANalytical Empyrean diffractometer (Cu Kα radiation; λ = 0.15418 nm; step size, 0.0001°; 2θ linearity ± 0.01° over the whole angular range) with a standard proportional detector and a PIXcel 3D detector.
2.6. Electrospray ionization mass spectrometric analysis
Before analysis, the electrolyzed glucose solution (500 μM) was diluted 10-fold with an aqueous solution of methanol (50 % v/v). Gluconic acid standard was prepared using the same mixed solvent. The data were collected on an LTQ-Orbitrap Elite mass spectrometer equipped with a nanospray source (Thermo Scientific) and operated in the negative ionization mode (flow rate, 0.50 μL min−1; source voltage, 2.2 kV; vaporizer temperature, 180 °C; capillary temperature, 200 °C, 60K resolution). The calibration was performed externally using Pierce® ESI Negative Ion Calibration Solution (Thermo Scientific). The mass spectra were an average of 2 minutes of scans with an automatic gain control target of 1 × 106 ions or maximum injection time of 100 ms and were processed using Qual Browser Xcalibur™ 2.2 (Thermo Fisher Scientific Inc.). The m/z 195 peak of gluconic acid was isolated and fragmented by collision-induced dissociation (CID) using 20 eV with a 100 ms activation time.
3. Results and discussion
3.1. X-ray and microscopic characterization of AuNPHEPES and AuNPCIT
The AuNPHEPES were synthesized by using Good’s zwitterionic buffer HEPES. The piperazine ring of HEPES generated nitrogen-centered free radicals that reduced gold ions to metallic gold [20]. The ring was also responsible for the adsorption of HEPES on different gold facets, with the weakest binding on the (111) plane [21]. Such a reduction and shape-directing abilities of HEPES allowed synthesizing non-spherical nanoparticles quickly without any need for additional reductants or surfactants.
Figure 1A shows that the reduction of HAuCl4 with HEPES yielded agglomerated AuNPHEPES that displayed a mixture of different shapes and sizes (15–100 nm diameter). In contrast, the reduction of tetrachloroaurate with citrate resulted in mostly spherical AuNPCIT with a narrower size distribution of 10–15 nm diameter (Figure 1B).
Figure 1.

TEM images of (A) AuNPHEPES, and (B) AuNPCIT. Bars represent 20 nm.
The X-ray diffraction patterns were used to characterize the crystalline structures of AuNPHEPES and AuNPCIT. Figure 2 shows that both types of nanoparticles displayed well defined Bragg peaks corresponding to the reflections from (111), (200), (220), (311) and (222) facets that are characteristic for a face-centered cubic structure of gold. The peak corresponding to the (111) plane was the most intense in both AuNPHEPES and AuNPCIT.
Figure 2.

X-ray diffraction patterns for (A) AuNPHEPES, and (B) AuNPCIT.
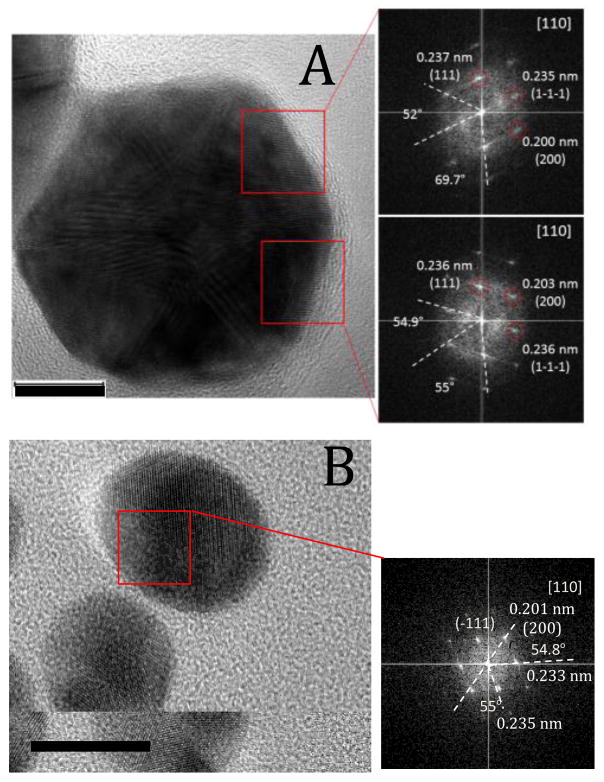
The relative peak intensities did not depend on the method of particle preparation. For example, the intensity ratio of (200) and (111) peaks for the AuNPHEPES and AuNPCIT was the same and equal to 0.29. This value is lower than the standard value for macro gold (0.52) indicating that the (111) plane was the dominant orientation with the same highest fraction in both AuNPHEPES and AuNPCIT. This was further confirmed by the similarity of their HRTEM images (Figure 3) that show that a prevailing lattice spacing was on average equal to 0.235 nm, which is characteristic for the (111) plane of bulk gold. In summary, no obvious differences were found in X-ray diffraction patterns and HRTEM images of AuNPHEPES and AuNPCIT suggesting that they adopt similar crystallographic orientations.
Figure 3.
HRTEM and Fast Fourier Transform images of (A) AuNPHEPES, and (B) AuNPCIT. Bars represent 10 nm.
3.2. Characterization of AuNPHEPES and AuNPCIT electrodes
The electrodes were prepared by attaching gold nanoparticles to glassy carbon electrodes with a film of polysaccharide chitosan. Chitosan was used because it (1) strongly adhered to the surface of glassy carbon preventing the loss of nanoparticles from the electrode surface in stirred solutions, (2) protected nanoparticles from deactivating by the adsorption of impurities from a solution, and (3) provided an inert and electrochemically silent matrix that prevented further agglomeration of nanoparticles on the electrode surface.
The nanoparticle load in a chitosan film on the electrode surface was calculated assuming that HAuCl4 was completely reduced to Au0. It was equal to 81 and 120 μg (±20%) in the AuNPHEPES and AuNPCIT electrodes, respectively. The active surface area of each electrode was determined based on the charge necessary to reduce its gold oxide that was formed by cycling the electrode potential to 0.90 V and using a conversion factor of 400 μC cm−2 [23]. It was equal to 0.12 and 0.051 cm2 (±15%) for AuNPHEPES and AuNPCIT electrodes, respectively. The lower active surface area of AuNPCIT electrode, regardless of its larger nanoparticle load, suggested a lower surface coverage of active gold oxide.
The capacitance of each electrode/solution interface was calculated by dividing the background current that was recorded in the double layer region at 0.30 V by the scan rate. The capacitance of AuNPCIT electrode (569 μF cm−2, ±15%) was larger than that of AuNPHEPES electrode (225 μF cm−2, ±15%). Apparently, smaller AuNPCIT could store more electrical charge at the interface than the larger agglomerated AuNPHEPES. The capacitance of both AuNPCIT than AuNPHEPES electrodes was much larger than that of the chitosan-coated glassy carbon (56 μF cm−2, ±10%)) or conventional gold (64 μF cm−2, ±10%)) electrodes. The larger capacitance indicated successful modification of glassy carbon electrodes with gold nanoparticles.
3.3. Voltammetric studies of glucose oxidation at AuNPHEPES and AuNPCIT electrodes
The cyclic voltammograms were recorded in two potential windows starting at −0.20 V and going to either 0.50 or 0.90 V in order to minimize or promote, respectively, the formation of surface gold oxide at higher potentials. Figure 4 shows the voltammograms that were recorded at as prepared AuNPHEPES and AuNPCIT electrodes in the narrower potential window. In the presence of glucose in a solution, the AuNPHEPES electrode displayed a distinct anodic current peak at 0.30 V (Figure 4A, red line a) while the AuNPCIT electrode showed only a minimal response to glucose (Figure 4B, red line b). In a control experiment, the conventional macro gold electrode showed no voltammetric response to glucose (Figure 4C, red line c). When adjusted for the difference in the active surface area of the electrodes, the peak current density at 0.30 V was more than five times larger at the AuNPHEPES (51 μA cm−2, ±15%) than at AuNPCIT (9 μA cm−2, ±15%) electrode.
Figure 4.

Cyclic voltammograms recorded at (A) AuNPHEPES, (B) AuNPCIT, and (C) conventional macro gold electrodes. Traces a, b, and c were recorded in the presence of 50 mM glucose in a solution. Traces a1, b1, and c1 represent the corresponding background voltammograms. Background electrolyte, pH 7.40 phosphate buffer solution (0.050 M). Scan rate, 50 mV s−1.
The explanation of higher activity of AuNPHEPES in terms of our first hypothesis is rather problematic. The argument of more catalytic crystallographic orientation of AuNPHEPES is not likely because no obvious differences between AuNPHEPES and AuNPCIT were found in X-ray and HRTEM experiments (vide supra). The argument of different surface-capping species (HEPES vs. citrate) is perhaps also not adequate because of the stronger competitive adsorption of polymeric chitosan chains. Indeed, a strong adsorption of chitosan on the surface of nanoparticles has been shown to facilitate their solubilization in water [24]. Also, from the purely geometric argument, the smaller AuNPCIT should be more active considering their higher surface-to-volume ratio and, thus, higher content of coordinatively unsaturated surface atoms of gold.
To explain differences in the catalytic activity of AuNPHEPES and AuNPCIT, we formulated a second hypothesis that considered the presence of gold-oxygen surface sites acting as redox mediators of glucose oxidation. The hypothesis was based on the differences in the background voltammograms of AuNPHEPES and AuNPCIT electrodes (Figure 5). Figure 5 shows that, in contrast to AuNPCIT, the AuNPHEPES electrode displays surface activity in the form of two voltammetric peaks in the double layer region at 0.20 and 0.40 V. The position of the first peak (0.20 V) coincides with the onset of glucose oxidation (Figure 4A, red line a). Such a correlation is typically a mark of the redox mediation by active surface species.
Figure 5.

Background cyclic voltammograms recorded at (a) AuNPHEPES, and (b) AuNPCIT electrodes in a pH 7.40 phosphate buffer solution (0.050 M). Scan rate, 50 mV s−1.
For macro gold electrodes, the redox mediation has been explained in terms of the “incipient hydrous oxide adatom mediator” model [25]. The model postulates that the high-energy surface atoms of gold are oxidized to a hydrous oxide, which mediates the anodic electrode processes. In the extension of this model to gold nanoparticles, we hypothesize that the activity of AuNPHEPES is also due to the redox active sites made of incipient hydrous oxide. We postulate further that the surface coverage of such sites is dependent on the nanoparticle morphology. Apparently, the anisotropic morphology of AuNPHEPES with corners and edges favors the formation of higher density of catalytic gold oxide than the more isotropic morphology of AuNPCIT. This is supported by recent studies, which showed that the kinetics at metal nanoparticles increase with the rising number of surface atoms at the corners and edges [26].
The small response of spherical AuNPCIT to glucose (Figure 4B) indicates that the contribution of a catalytic (111) plane to glucose oxidation is limited in the present case. More important seems to be the presence of surface redox active sites, which are scarce on the surface of AuNPCIT as indicated by a featureless background voltammogram of AuNPCIT electrode (Figure 5, blue line b). Indeed, it has been shown that the surface coverage of gold by the incipient oxide can be as low as a sub-monolayer [27], which is below a detection limit of cyclic voltammetry.
To confirm that the observed catalytic activity was due to the gold and not some impurities, the effects of exposing AuNPHEPES and AuNPCIT electrodes to higher potentials were investigated. Figure 6 shows the cyclic voltammograms that were recorded in a glucose solution at electrodes that were previously cycled ten times between −0.20 and 0.90 V in a glucose-free background solution (traces b). Both AuNPHEPES and AuNPCIT electrodes were activated by such a treatment and displayed a glucose current that was six times larger than that recorded before their exposure to higher potentials (traces a). One can hypothesize that such anodic activation was caused by the formation of a multilayer gold oxide at >0.50 V and its incomplete reduction at <0.50 V leaving the remnants of incipient hydrous oxide with a higher surface coverage than that on as prepared (not activated) nanoparticles. We have shown a similar activation pattern in the case of macro gold [11]. It should be noted that the anodic activation did not bring the catalytic activity of AuNPCIT to that of AuNPHEPES (Figure 6, traces b). This further underlined the importance of morphology-sensitive surface chemistry of gold nanoparticles in determining their catalytic properties.
Figure 6.
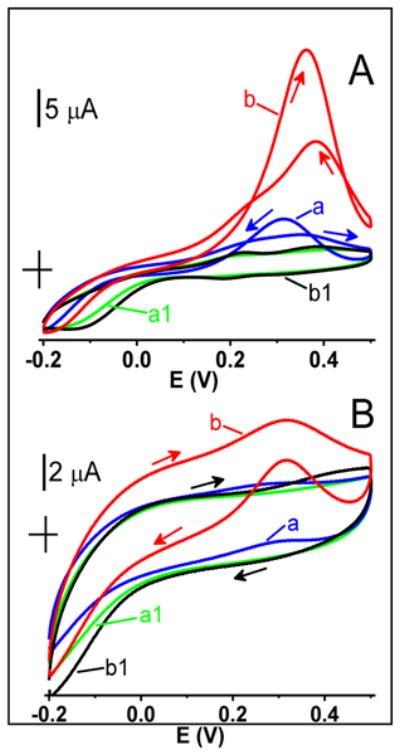
Cyclic voltammograms recorded in a 50 mM glucose solution at (A) AuNPHEPES, and (B) AuNPCIT electrodes before (blue traces a) and after (red traces b) activation by cycling their potential 10 times between −0.20 and 0.90 V in a background electrolyte solution. Green traces a1 and black traces b1 represent the corresponding background voltammograms. Background electrolyte, pH 7.40 phosphate buffer solution (0.050 M). Scan rate, 50 mV s−1.
The mechanism of glucose oxidation at the incipient gold oxide probably involves a removal of labile C1 hemiacetal hydrogen from a glucose molecule as a rate-determining step on a way to gluconolactone, which finally undergoes hydrolysis to gluconic acid. The involvement of slow removal of hemiacetal hydrogen has been confirmed by isotopic studies [18].
3.4. Amperometric determination of glucose at AuNPHEPES and AuNPCIT electrodes
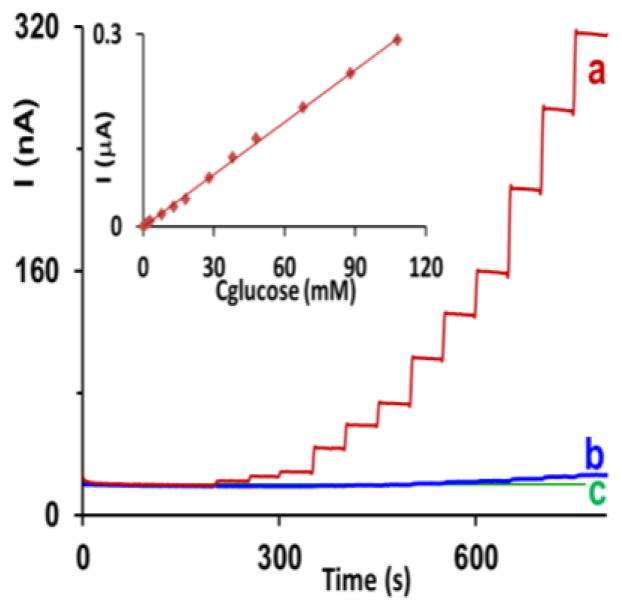
Figure 7 (line a) shows a typical amperometric trace for the determination of glucose at non-activated AuNPHEPES electrodes. The most interesting is the unusually wide range of linear response to glucose (0.20–110 mM; R2, 0.998; inset). The sensitivity of electrode to glucose was equal to 66 μA M−1 cm−2. The limit of detection was equal to 100 μM (S/N, 3) and a response time was below 5 s. Consistent with the voltammetric data, the amperometric responses of AuNPCIT (line b) and conventional macro gold (line c) electrodes to glucose were inadequate for analytical purposes.
Figure 7.
Amperometric response of the non-activated (a) AuNPHEPES, (b) AuNPCIT, and (c) conventional macro gold electrodes to the additions of glucose aliquots (1.0, 2.0, 3.0, 8.0, 13.0, 18, 28, 38, 48, 68, 88, 108 mM) to a stirred pH 7.40 phosphate buffer solution. Inset: Calibration plot for a red trace a. Potential, 0.30 V.
The gold electrodes are known to deactivate in the presence of chloride ions in a solution. While the AuNPHEPES electrodes showed such sensitivity to chlorides, they could be used in the presence of physiological concentration of NaCl (0.10 M). Under such conditions, the sensitivity of glucose detection decreased by an order of magnitude; however, the glucose signal remained stable at this lower level. The coating of gold electrodes with Nafion and diluting analyzed samples should further decrease the effects of chlorides on their characteristics.
The response of five different AuNPHEPES electrodes to 20 mM glucose was within ±10% documenting a good reproducibility of electrode preparation. The electrodes retained 80% of the initial glucose current even after 2 h of continuous electrolysis in a vigorously stirred solution of 20 mM glucose. If necessary, their activity could be restored by a brief anodic activation. The electrode shelf life was at least a couple of months. In summary, the AuNPHEPES electrodes are a cost-effective addition to the toolbox of electrochemical detectors for chromatographic analyses.
3.5. Mass spectrometry of electrolyzed glucose solutions
Figure 8A shows the ESI mass spectrum of a glucose solution after a 2-h electrolysis at an AuNPHEPES electrode. The substrate glucose and the final product of electrolysis, gluconic acid, were detected as singly deprotonated ions at m/z 179 and 195, respectively. Other peaks in the mass spectrum were due to common contaminants in the methanol/water solvent and were also detected in the blank. The identity of the gluconic acid as the product of electrolysis was further confirmed by using collision-induced dissociation of the peak at m/z 195. The almost identical tandem mass spectra of the electrolysis product (Figure 8B) and gluconic acid standard (Figure 8C) positively identified the transformation of glucose to gluconic acid at an AuNPHEPES electrode. Fragment peaks at m/z 177, 159, 129, and 99 were due to the successive loss of 1 and 2 –H2O and then 1 and 2 –HCHO moieties, respectively [28].
Figure 8.
(A) Negative ESI mass spectrum of glucose solution after two-hour electrolysis at AuNPHEPES electrode at 0.30 V. Peaks at m/z 179 and 195 correspond to singly deprotonated species [M− H+]− of glucose and gluconic acid, respectively. Other peaks are due to contaminants in the background solvent and were also present in the blank. (B) Tandem mass spectrum of gluconic acid electrolytic product. (C) Tandem mass spectrum of gluconic acid standard.
4. Conclusions
The zwitterionic buffer HEPES acted as a reducing and shape-directing agent yielding the irregular morphologies of gold nanoparticles (AuNPHEPES) that facilitated the oxidation of glucose in neutral solutions. The regular spherical morphology of nanoparticles was minimally active toward the glucose oxidation under such conditions. A model of catalytic incipient hydrous oxide that was originally developed for conventional macro gold could explain such behavior. In the nanoparticle version of this model, the irregular morphologies of AuNPHEPES with corners and edges supported a higher surface density of incipient gold oxide that acted as a fast redox mediator for the oxidation of glucose. In contrast to photonic applications that typically require uniform nanoparticles, the electrocatalysis was successfully completed with a batch of AuNPHEPES aggregates of different shapes and sizes. The AuNPHEPES are a cost-effective alternative to the expensive single crystals of gold and conventional macro gold for the electrooxidation and determination of glucose. The AuNPHEPES do not require any prior activation for such electrocatalysis, which is important because the anodic activation of gold is not always feasible [11].
Highlights.
Morphology-sensitive electrocatalysis at gold nanoparticles (AuNP)
AuNP polyhedral morphologies active toward the oxidation of glucose to gluconate
Redox-mediation by gold-oxygen sites on the surface of AuNP
Anodic activation of macro and nano gold toward glucose oxidation
Acknowledgments
This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (G12MD007591).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li N, Zhao P, Astruc D. Anistropic gold nanoparticles: synthesis, properties, applications, and toxicity. Angew Chem Int Ed. 2014;53:1756–1789. doi: 10.1002/anie.201300441. [DOI] [PubMed] [Google Scholar]
- 2.Tang Y, Cheng W. Metallic nanoparticles as advanced electrocatalysts. Sci Adv Mat. 2012;4:784–797. [Google Scholar]
- 3.Toghil KE, Compton RG. Electrochemical non-enzymatic glucose sensors: A perspective and an evaluation. Int J Electrochem Sci. 2010;5:1246–1301. [Google Scholar]
- 4.Kerzenmacher S, Ducree J, Zengerle R, von Stetten F. Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J Power Sources. 2008;182:1–17. [Google Scholar]
- 5.Bolzan AE, Iwasita T, Vielstich W. On the electrochemical oxidation of glucose. J Electrochem Soc. 1987;134:3052–3058. [Google Scholar]
- 6.Bindra DS, Wilson GS. Pulsed amperometric detection of glucose in biological fluids at a surface-modified gold electrode. Anal Chem. 1989;61:2566–2570. doi: 10.1021/ac00197a022. [DOI] [PubMed] [Google Scholar]
- 7.Castro Luna AM, de Mele MFL, Arvia AJ. The electro-oxidation of glucose on microcolumnar gold electrodes in different neurtral solutions. J Electroanal Chem. 1992;323:149–162. [Google Scholar]
- 8.Gorski W, Kennedy RT. Electrocatalyst for non-enzymatic oxidation of glucose in neutral saline solutions. J Electroanal Chem. 1997;424:43–48. [Google Scholar]
- 9.Mullens C, Pikulski M, Agachan S, Gorski W. Synergistic effects in multicomponent electrocatalysts: The Pb-Ir-O system. J Am Chem Soc. 2003;125:13602–13608. doi: 10.1021/ja0366843. [DOI] [PubMed] [Google Scholar]
- 10.Tominaga M, Shimazoe T, Nagashima M, Taniguchi I. Electocatalytic oxidation of glucose at gold nanoparticle-modified carbon electrodes in alkaline and neutral solutions. Electrochem Commun. 2005;7:189–193. [Google Scholar]
- 11.Wooten M, Shim JH, Gorski W. Amperometric determination of glucose at conventional vs. nanostructured gold electrodes in neutral solutions. Electroanalysis. 2010;22:1275–1277. [Google Scholar]
- 12.Xie F, Huang Z, Chen C, Xie Q, Huang Y, Qin C, Liu Y, Su Z, Yao S. Preparation of Au-film electrodes in glucose-containg Au-electroplating aqueous bath for high-performance nonenzymatic glucose sensor and glucose/O2 fuel cell. Electrochem Commun. 2012;18:108–111. [Google Scholar]
- 13.Das AK, Raj CR. Shape and surface structure-dependent electrocatalytic activity of Au nanoparticles. Electrochim Acta. 2013;107:592–598. [Google Scholar]
- 14.Das AK, Raj CR. Shape-controlled growth of surface-confined Au nanostructures for electroanalytical applications. J Electroanal Chem. 2014;717:140–146. [Google Scholar]
- 15.Boopathi S, Kumar SS, Phani KLN. Generation of active sites on gold nanostructured surface through ultrasuound-assisted direct electrodeposition and its effect on enzyme-less glucose electrooxidation. ChemElectoChem. 2014;1:1189–1197. [Google Scholar]
- 16.Gougis M, Ma D, Mohamedi M. Tungsten oxide-Au nanosized film composites for glucose oxidation and sensing in neutral medium. Inter J Nanomed. 2015;10:2939–2950. doi: 10.2147/IJN.S73770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adzic RR, Hsiao MW, Yeager EB. Electrochemical oxidation of glucose on single crystal gold surfaces. J Electrochem Chem. 1989;260:475–485. [Google Scholar]
- 18.Hsiao MW, Adzic RR, Yeager EB. Electrochemical oxidation of glucose on single crystal and polycrystalline gold surfaces in phosphate buffer. J Electrochem Soc. 1996;143:759–767. [Google Scholar]
- 19.Zhang M, Gorski W. Amperometric ethanol biosensors based on chitosannad+-alcohol dehydrogenase films. Electroanalysis. 2011;23:1856–1862. [Google Scholar]
- 20.Habib A, Tabata M, Wu YG. Formation of gold nanoparticles by Good’s buffers. Bull Chem Soc Jpn. 2005;78:262–269. [Google Scholar]
- 21.Xie, Lee JY, Wang DIC. Seedles, surfactantless, high-yield synthesis of branched gold nanocrystals in HEPES buffer solution. Chem Mater. 2007;19:2823–2830. [Google Scholar]
- 22.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–15707. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 23.Trasatti S, Petrii OA. Real surface area measurements in electrochemistry. Pure Appl Chem. 1991;63:711–734. [Google Scholar]
- 24.Zhang M, Smith A, Gorski W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal Chem. 2004;76:5045–5050. doi: 10.1021/ac049519u. [DOI] [PubMed] [Google Scholar]
- 25.Burke LD. Scope for new applications for gold arising from the electrocatalytic behavior of its metastable surface state. Gold Bulletin. 2004;37:125–135. [Google Scholar]
- 26.Narayanan R, El-Sayed MA. Catalysis with transition metal nanoparticles in colloidal solution: Nanoparticle shape dependence and stability. J Phys Chem B. 2005;109:12663–12676. doi: 10.1021/jp051066p. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues-Lopez J, Alpuche-Aviles MA, Bard AJ. Interrogation of surfaces for the quantification of adsorbed species on electrodes: oxygen on gold and platinum in neutral media. J Am Chem Soc. 2008;130:16985–16995. doi: 10.1021/ja8050553. [DOI] [PubMed] [Google Scholar]
- 28.Lu M, Liu Y, Helmy R, Martin GE, Dewald HD, Chen H. Online investigation of aqueous-phase electrochemical reactions by desorption electrospray ionization mass spectrometry. J Am Soc Mass Spectrom. 2015;26:1676–1685. doi: 10.1007/s13361-015-1210-2. [DOI] [PubMed] [Google Scholar]