Abstract
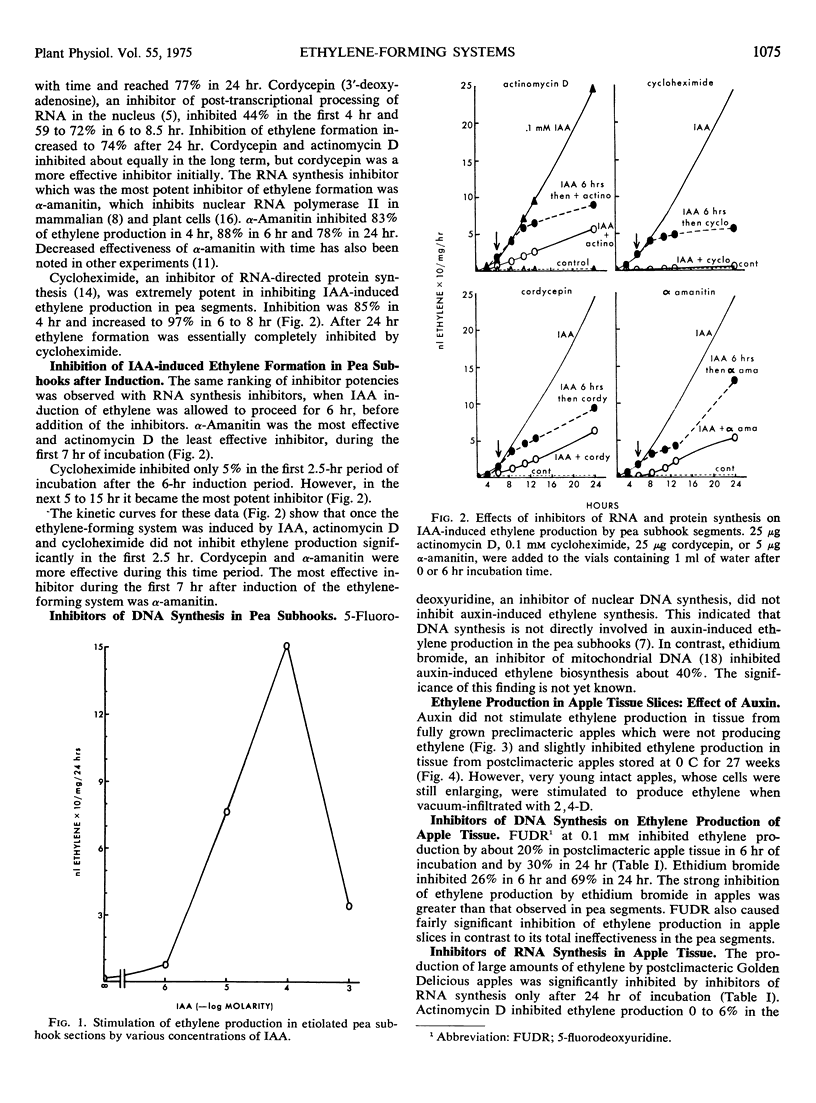
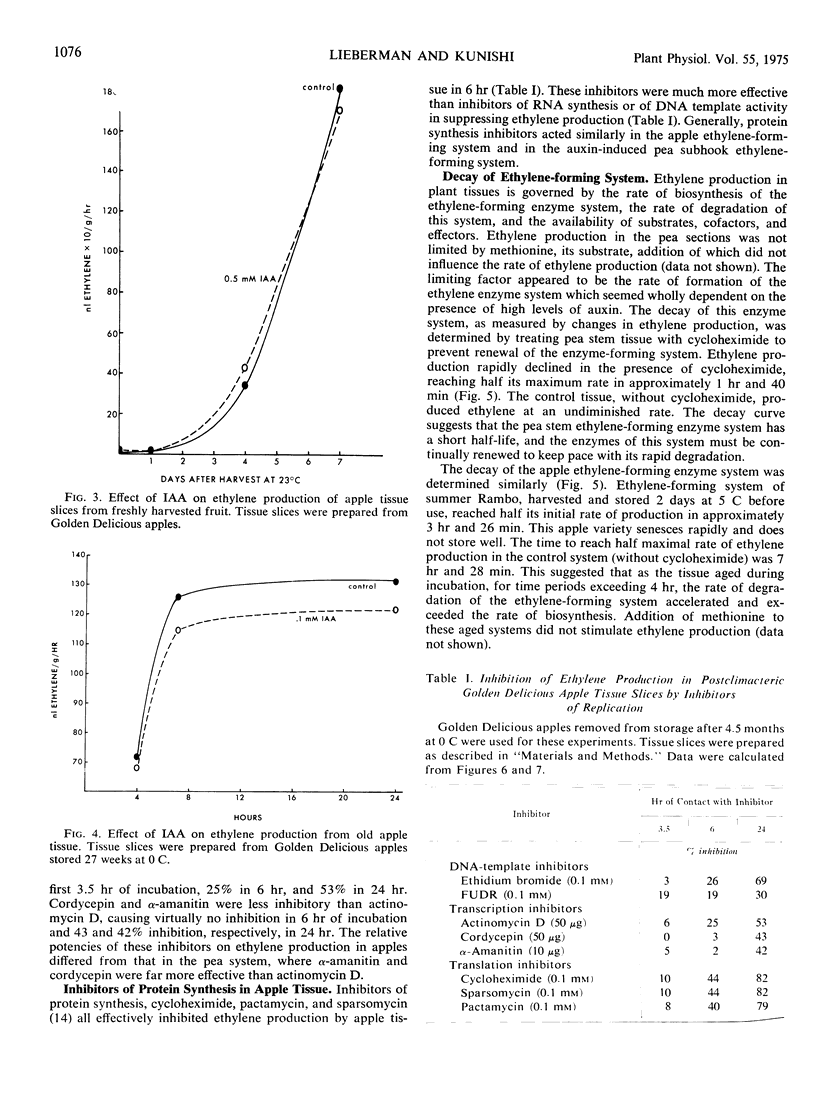
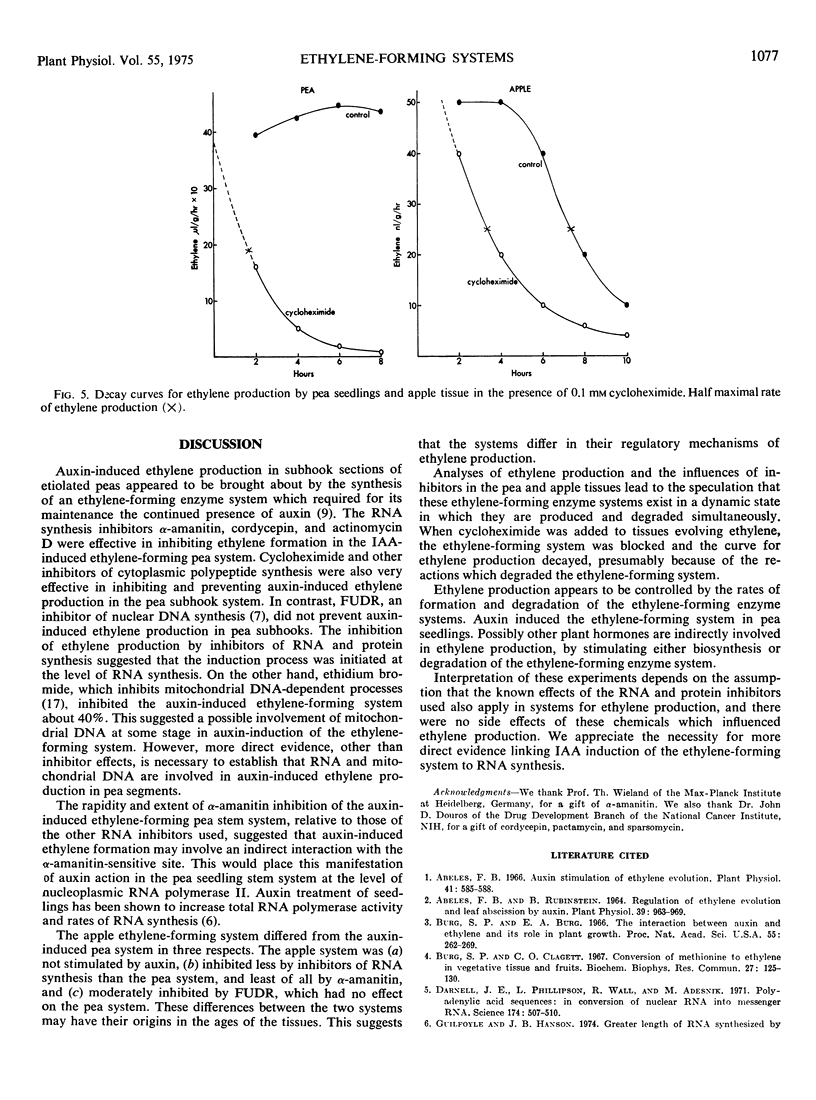
Auxin-induced ethylene formation in etiolated pea (Pisum sativum L. var. Alaska) stem segments was inhibited by inhibitors of RNA and protein synthesis. Kinetics of the inhibitions is described for actinomycin D, cordycepin, α-amanitin, and cycloheximide. α-Amanitin was the most potent and fast-acting inhibitor, when added before induction or 6 hours after induction of the ethylene-forming system. The ethylene-forming system of postclimacteric apple (Malus sylvestris L.) tissue, which is already massively induced, was not further stimulated by auxin. Ethylene production in apples was inhibited least by α-amanitin and most by actinomycin D. The relative responses of the ethylene system in apples to RNA inhibitors were different from the ethylene system of pea stems. However, the protein synthesis inhibitor, cycloheximide, appeared to act equally in both tissue systems. The effect of cycloheximide on ethylene production in postclimacteric apple tissue, already producing large quantities of ethylene, suggests a dynamic regulating system for the synthesis and degradation of the ethylene-forming system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B. Auxin stimulation of ethylene evolution. Plant Physiol. 1966 Apr;41(4):585–588. doi: 10.1104/pp.41.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964 Nov;39(6):963–969. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. The interaction between auxin and ethylene and its role in plant growth. Proc Natl Acad Sci U S A. 1966 Feb;55(2):262–269. doi: 10.1073/pnas.55.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Clagett C. O. Conversion of methionine to ethylene in vegetative tissue and fruits. Biochem Biophys Res Commun. 1967 Apr 20;27(2):125–130. doi: 10.1016/s0006-291x(67)80050-0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Haber A. H., Schwarz O. J. A method for testing the specificity of inhibitors of deoxyribonucleic Acid synthesis in growth studies. Plant Physiol. 1972 Mar;49(3):335–337. doi: 10.1104/pp.49.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Specific action of alpha-amanitin on mammalian RNA polymerase protein. Nature. 1970 Jan 3;225(5227):60–62. doi: 10.1038/225060b0. [DOI] [PubMed] [Google Scholar]
- Kang B. G., Newcomb W., Burg S. P. Mechanism of Auxin-induced Ethylene Production. Plant Physiol. 1971 Apr;47(4):504–509. doi: 10.1104/pp.47.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Kunishi A. Stimulation of ethylene production in apple tissue slices by methionine. Plant Physiol. 1966 Mar;41(3):376–382. doi: 10.1104/pp.41.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro L., Novello F., Stirpe F. Inhibition of ribonucleic acid and of protein synthesis in the organs of rats and mice poisoned with alpha-amanitin. Biochim Biophys Acta. 1973 Aug 24;319(2):188–198. doi: 10.1016/0005-2787(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Pestka S. Inhibitors of ribosome functions. Annu Rev Microbiol. 1971;25:487–562. doi: 10.1146/annurev.mi.25.100171.002415. [DOI] [PubMed] [Google Scholar]
- Strain G. C., Mullinix K. P., Bogorad L. RNA polymerases of maize: nuclear RNA polymerases. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2647–2651. doi: 10.1073/pnas.68.11.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylber E., Vesco C., Penman S. Selective inhibition of the synthesis of mitochondria-associated RNA by ethidium bromide. J Mol Biol. 1969 Aug 28;44(1):195–204. doi: 10.1016/0022-2836(69)90414-8. [DOI] [PubMed] [Google Scholar]


