Abstract
This work describes the depolymerization of poly(vinyl acetate-alt-sulfur dioxide) (PVAS) as initiated by chemical and mechanical stimuli. In recent years, macromolecules that are able to depolymerize in response to specific stimuli have been highly sought because of their ability to amplify signal for sensing and drug delivery. Examples include self-immolative polymers from alkoxyphenol derivatives and polyaldehydes. We show here that alternating copolymers of sulfur dioxide and vinyl acetate are able to undergo similar depolymerization into their monomer components in response to various chemical and mechanical stimuli. Certain vinyl monomers such as vinyl acetate are able to polymerize with sulfur dioxide in a perfectly alternating manner, and the resulting copolymer possesses a low ceiling temperature. We show that this polymer is able to break down into its monomer components when subjected to UV/acetone, various Reactive Oxygen Species (ROS), and ultrasonication. In the case of UV, the acetone reacted via a Norrish reaction to produce free radicals that caused clean monomer production. For ROS, the polymer showed reactivity to both oxidizing and radical-containing ROS. Through kinetic studies, these polymers were shown to proceed via a two-part, first-order kinetic model with a fast initiation phase and a slow depolymerization phase. Finally, the polymers were subjected to probe ultrasonication, and depolymerization occurred as well. Most tellingly, the polymer again showed a fast initiation step and continued to depolymerize even after ultrasonication stopped. This class of polymers shows potential for drug delivery in response to both endogenous chemical and externally-applied mechanical cues.
Interest in stimulus-responsive polymers and materials has increased in recent years, primarily owing to a wealth of potential applications in medicine and devices. For medicine in particular, efforts have focused on changing the structure of assemblies and particles in response to both endogenous molecules and externally-applied energy sources to release drugs, activate contrast agents, or initiate fluorescent signal. To attain necessary sensitivities, recent efforts have focused on amplification, in which single activation events can cause massive changes in chemical and/or physical structure. Examples for biomedical application include amphiphiles that change their self-assembly properties, charge-switching polymers for gene delivery, and phase changes in polymers with appropriate lower critical solution temperatures. In order to obtain activity at sites of interest, such polymers have been designed to react to stimuli specific to a diseased region. For example, many new sensing and imaging agents have been designed to respond to the presence of elevated Reactive Oxygen Species (ROS) as indicators of inflammation, cell signaling, and cancer.1-4 In addition, technologies have been developed that can initiate therapy at a specific location due to response to an external energy source that can be focused to a specific location.
A recent development has been the creation of polymers that spontaneously break down, or depolymerize, into their original monomer components only after activation by a specific stimulus. Such polymers have a low (e.g. < RT) ceiling temperature (Tc), which is the temperature above which depolymerization proceeds at a faster rate than polymerization. If the active chain end is capped, the polymer will be stable above its Tc, but if the chain end is activated the polymer will equilibrate back to the original monomer. Polymers with low Tc were originally developed as photoresists,5-7 but more recent examples have focused on sensing and drug delivery. For example, end-capped poly(phthalaldehyde) derivatives have been demonstrated both as substrates to generate amplified sensors capable of generating a visible response to the presence of various chemical analytes, as well as the focus of more detailed studies of the kinetics of depolymerization.8-16 In addition, polyquinonemethides, coined “self-immolative polymers” by Shabat, have been developed into both linear and branched depolymerizable units, while polymers capable of tandem reactions of cyclization and release, have shown excellent potential for drug delivery based on the degradation of linear polymers, branched polymers, and particles.17-20 Key to both of these approaches has been the use of specific end-capping groups that, when reacted with a chemical or photochemical trigger, produce a functional group that can act as an active chain end to initiate the depolymerization process. While a versatile and tunable method of obtaining sensitivity to various stimuli, this method still requires a single reaction to trigger each depolymerization event, rather than breakage along the main chain.
Here, we show that poly(vinyl acetate-alt-sulfur dioxide) (PVAS) exhibits an ability to depolymerize in response to ROS and ultrasonic waves. Alternating polymers containing sulfur dioxide and vinyl groups were originally developed as photoresists due to their ability to fragment into vaporizable fragments in response to deep-UV and x-ray irradiation.21-24 Similarly, other alternating sulfone polymers were designed for radiation sensing.25,26 The key property of PVAS for depolymerization is the perfectly alternating sulfur dioxide and vinyl acetate, which not only reduces the bond strength along the main chain (through substitution of C-C for C-S bonds),27,28 but also increases the entropy associated with depolymerization through the release of additional small molecules.7 Unless the chain propagation pathway is removed through chemical modification,29 the depolymerization is able to proceed without encountering a stable C-C-C-C bond network.
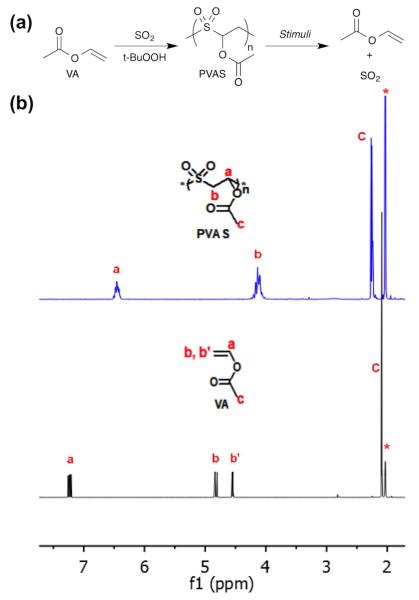
Alternating copolymerization with sulfur dioxide has been shown for terminal alkenes, vinyl carbonates, and vinyl acetate.7 In this work, vinyl acetate was cooled to −70°C, followed by condensation of SO2 into the polymerization mixture. t-Butylhydroperoxide (TBHP) initiator was added and the reaction was stirred for 2 h, followed by precipitation into methanol and warming to room temperature. The polymer was then purified by re-precipitation. True alternation was observed through analysis of 1H NMR spectra, which showed the presence of CH(OAc)-SO2 protons at 6.47 ppm but no CH(OAc)-CH2 bonds (Figure 1).
Figure 1.
(a) Scheme of synthesis of PVAS from vinyl acetate and sulfur dioxide and depolymerization back into these components. (b) 1H NMR spectra of PVAS and VA. Protons are assigned as shown; * indicates solvent peak (acetoned6).
While degradation of PVAS as film has been studied previously,7 here, the depolymerization behavior of PVAS was confirmed via photogeneration of radicals using UV light. Acetone is known to absorb UV light and generate radicals via a Norrish reaction.30 When PVAS in acetone was irradiated with UV light in the range of 320-390 nm, the PVAS showed clean degradation into free VA monomer (Figure 2, Figure S1). In comparison, PVAS irradiated at 365 nm showed little degradation. This decrease is attributed to the decreased overlap between the UV irradiation and the absorbance spectrum of acetone (λmax ~ 265 nm, λonset ~ 310 nm). Similarly, when PVAS was irradiated in other solvents with a lower UV cutoff, almost no conversion to the monomer was observed in THF, while a very small amount of degradation was observed in DMSO (Figure S2). Thus depolymerization in this case appears to proceed through activation of monomer by radical formation. The degradation of PVAS by UV/acetone was intensity-dependent as well, in which irradiation at 32-34 mW led to 60% depolymerization in 1 min, while at 18-20 mW the polymer showed little degradation after 1 min irradiation but 80% depolymerization after 5 min. More importantly, the release of free monomer was confirmed by comparing the 1H NMR spectra of the polymer and its degraded product (Figure S1).
Figure 2.
Conversion of PVAS into vinyl acetate under 320-390 nm UV light in acetone-d6. Black bars: 18-20 mW, red bars: 32-34 mW. Error bars represent standard deviation of three trials.
To analyze the behavior of PVAS as a potential scaffold for drug delivery in response to endogenous ROS, the reactivity of PVAS was tested against various biologically relevant ROS. ROS can act as a radical (e.g., superoxide, hydroxyl radical), an oxidative species (e.g., hypochlorite), or both. To test ROS reactivity, solutions of PVAS were incubated with various ROS in acetone/water, and reaction was monitored by 1H NMR spectroscopy. Figure S3 and Figure S4 show the relative reduction in CH2-S signal at 6.47 ppm by 1H NMR spectroscopy and appearance of peak corresponding to CH2=CH(OAc) at 7.25 ppm on addition of oxidative analytes within 20 min of addition. Reactions were observed for Ca(OCl)2, KO2, and NaOCl after 20 min, with greater levels observed after 24 h incubation. PVAS was not reactive to water, weak oxidizers such as hydrogen peroxide, or weak reducing agents such as glutathione (Figure S3). This reactivity profile indicates that PVAS is most sensitive to strongly oxidizing agents. The major impurities appear to be formation of acetaldehyde and acetic acid, which is expected from hydrolysis of VA. There are also peaks present that correspond to the initiation process, mostly likely due to an elimination side reaction. At basic pH, this elimination product appears to dominate, as alkenes tend to form adjacent to the sulfone as characterized by 1H NMR spectroscopy (Figure S5 and Figure S6).
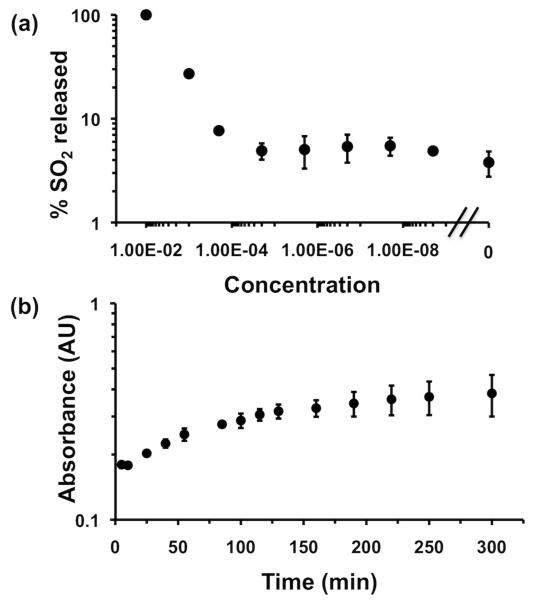
To be useful for amplified response to various stimuli, depolymerization of large polymers must be possible using small amounts of ROS. However, since in practice obtaining clear 1H NMR spectra required large concentrations (>5 mg/mL) of PVAS to obtain reliably measurable product peaks. However, although PVAS did not have a strong UV-Vis absorbance, the released sulfur dioxide had clear peaks at 260 and 330 nm (Figure S8). Using UV-Vis absorbance measurements, the release of SO2 was measured at varying concentrations of KO2 addition (Figure 4a). PVAS showed degradation over background at 200 μM KO2. In addition, PVAS showed good reactivity with sodium hypochlorite, with sensitivity down to about 2 mM (Figure S9a).
Figure 4.
(a) Sulfur dioxide release from PVAS after 24 h, incubated with varying concentrations of KO2., as measured at 260 nm. Error bars represent standard deviation of two trials. (b) Absorbance at 260 nm vs. time for PVAS incubated with 200 μM KO2. Error bars represent standard deviation of two trials.
One important question about the mechanism of depolymerization is whether the generation of monomer is based on direct reaction with ROS or from initiation of depolymerization after ROS is generated. To test the kinetics of this process, PVAS was mixed with potassium superoxide in DMSO, and UV-Vis spectra were taken at various time points, showing the increase in sulfur dioxide absorbance (Figure 4b). The depolymerization appears to follow two phases. In the initial phase, absorbance increases quickly, following first-order kinetics (Figure S10). This is likely to be the initial reaction of the superoxide at different places in the polymer chain, which would be pseudo first order with superoxide. The second phase also follows first-order kinetics but with an apparent rate of about one order of magnitude slower, which corresponds to deplymerization. A similar reaction profile was observed for reaction with sodium hypochlorite (Figure S9b). Without superoxide, the polymer remains intact (Figure S11). While a specific rate constant could not be calculated due to difficulties in preparing a calibration curve with SO2, this evidence indicates that both superoxide and hypochlorite are able to initiate depolymerization of PVAS.
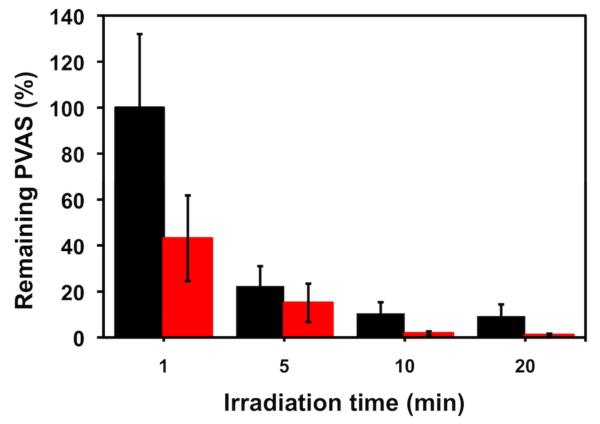
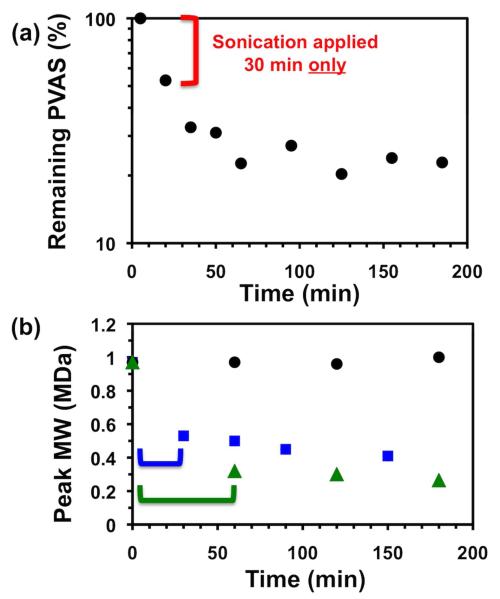
Finally, the reactivity of PVAS was demonstrated against mechanical activation by ultrasonication. Ultrasonication generates transient solvent cavities that collapse to produce shear forces capable of breaking higher molecular weight polymers. Weaker chemical bonds are more susceptible to breakage, and carbon-sulfur bonds have lower bond dissociation energies than carbon-carbon or carbon oxygen bonds.27,28 Most polymers simply break into smaller fragments until a critical molecular weight (~ 30 kDa) is reached, but no further. However, if PVAS could undergo depolymerization by mechanical activation, the polymer would continue to disintegrate even after ultrasound was removed. To show that PVAS was susceptible to ultrasonication degradation, PVAS was dissolved in acetone-d6 and subjected to probe sonication under argon (see SI for more details). The polymer solution was probe sonicated for 30 min only, and the generation of monomer was measured for a total of 4 h. As shown in Figure 5, monomer was generated quickly during the sonication process, but even after sonication was finished the monomer peaks continued to increase over time. Since the temperature of the sonication solution did not increase by more than 2°C for the entire experiment, the temperature stayed well within the previously reported limits of Tc (−20°C) and decomposition temperature (140°C).7 Thus, PVAS not only undergoes chain scission but also continues to generate free monomer after sonication is finished, indicating a depolymerization mechanism.
Figure 5.
(a) Remaining PVAS vs. time for probe sonication in acetone-d6. Sonication was applied for first 30 min only. (b) Peak molecular weight for larger molecular weight fraction vs. time in DMSO as measured by GPC (vs. PMMA). Blue squares: sonication applied for first 30 min only; green triangles: sonication applied for first 60 min only; black circles: no sonication applied.
To further support a mechanically-activated depolymerization mechanism, similar sonication experiments were run in DMSO and the polymer molecular weight was monitored by DMSO GPC. Prior to sonication the GPC trace of the polymer is bimodal, with one peak at about 1 MDa (vs. PMMA) and another at 14 kDa, and no peaks in the small molecule regime (Figure S13). Upon sonication, the peak molecular weight of the higher species decreases significantly as a function of sonication time (Figure S14). More tellingly, each molecular weight continues to decrease after sonication as well, indicating a depolymerization mechanism (Figure 5b). At the same time, a peak in the small molecule regime, presumably free monomer, increases, while the 14 kDa peak does not change significantly (Figure S15, Figure S16). Without sonication, none of these peaks change over the same time period (Figure S17). Since it is well-known from the mechanochemistry literature that larger molecular weight polymers are more susceptible to mechanical activation than smaller,16,31 these experiments support a hypothesis of mechanical activation rather than ROS-generated activation. This mechanism of ultrasonic activation may find use in drug delivery applications in which therapy can be initiated within tissue but controlled slow release follows.
In conclusion, poly(vinyl acetate-alt-sulfur dioxide) (PVAS) was found to undergo depolymerization in response to Reactive Oxygen Species and ultrasound. PVAS is most reactive to superoxide, followed by hypochlorite, then hydroxyl radical. Kinetic studies of the hypochlorite-induced depolymerization showed that the reaction proceeded through a two-part mechanism in which monomer is generated rapidly first, then slowly. The PVAS shows reactivity to superoxide with a detection limit of 200 μM. Finally, PVAS shows reactivity to ultrasonication through an initiation/depolymerization mechanism. Current studies are focusing on adapting the alternating copolymers of sulfur dioxide as ROS-sensitive drug delivery vehicles.
Supplementary Material
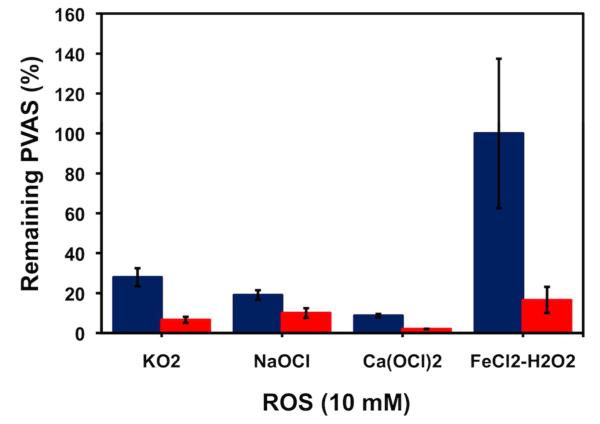
Figure 3.
Conversion of PVAS to VA for various ROS at 10 mM. Blue bars indicate 20 min incubation, red bars: 24 h incubation. Error bars represent standard deviation of three trials.
ACKNOWLEDGMENT
This work was supported by a NIH Director’s New Innovator Award (A.P.G., DP2 EB020401) and University of Colorado Boulder start-up funds. We thank Prof. Chris Bowman for use of his UV irradiation apparatus and his GPC. The GPC was obtained via a NSF MRSEC grant (DMR 1420736). We would also like to thank Chen Wang for his help with setting up GPC experiments. Finally, we thank Dr. Richard Shoemaker for help with setting up the NMR spectroscopy kinetics experiments, Prof. Jennifer Cha for helpful discussions and Ms. Della Shin for help with synthesis.
ABBREVIATIONS
- DMSO
dimethyl sulfoxide
- GPC
gel permeation chromatography
- NMR
Nuclear Magnetic Resonance
- PVAS
poly(vinyl acetate-alt-sulfone)
- ROS
Reactive Oxygen Species
- TBHP
t-butyl hydroperoxide
Footnotes
Supporting Information. Experimental methods, 1H NMR spectra, sonication setup, GPC spectra, and UV-Vis spectra. This information is available free of charge via the Internet at http://pubs.acs.org/journal/amlccd.
Author Contributions
The manuscript was written through contributions of all authors.
REFERENCES
- (1).Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N. Angewandte Chemie International Edition. 2009;48:299. doi: 10.1002/anie.200804851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. J. Biol. Chem. 2003;278:3170. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- (3).Miller EW, Tulyathan O, Isacoff EY, Chang CJ. Nat. Chem. Biol. 2007;3:349. doi: 10.1038/nchembio871. [DOI] [PubMed] [Google Scholar]
- (4).Miller EW, Albers AE, Pralle A, Isacoff EY, Chang CJ. J. Am. Chem. Soc. 2005;127:16652. doi: 10.1021/ja054474f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Willson CG, Ito H, Frechet JMJ, Tessier TG, Houlihan FM. J. Electrochem. Soc. 1986;133:181. [Google Scholar]
- (6).Ito H, Willson CG. Polym. Eng. Sci. 1983;23:1012. [Google Scholar]
- (7).Jiang Y, Frechet JMJ. Macromolecules. 1991;24:3528. [Google Scholar]
- (8).Olah MG, Robbins JS, Baker MS, Phillips ST. Macromolecules. 2013;46:5924. [Google Scholar]
- (9).Seo W, Phillips ST. J. Am. Chem. Soc. 2010;132:9234. doi: 10.1021/ja104420k. [DOI] [PubMed] [Google Scholar]
- (10).Phillips ST, DiLauro AM. Acs Macro Letters. 2014;3:298. doi: 10.1021/mz5000784. [DOI] [PubMed] [Google Scholar]
- (11).Kaitz JA, Possanza CM, Song Y, Diesendruck CE, Spiering AJH, Meijer EW, Moore JS. Polymer Chemistry. 2014;5:3788. [Google Scholar]
- (12).Hernandez HL, Kang SK, Lee OP, Hwang SW, Kaitz JA, Inci B, Park CW, Chung SJ, Sottos NR, Moore JS, Rogers JA, White SR. Adv. Mater. 2014;26:7637. doi: 10.1002/adma.201403045. [DOI] [PubMed] [Google Scholar]
- (13).Diesendruck CE, Peterson GI, Kulik HJ, Kaitz JA, Mar BD, May PA, White SR, Martinez TJ, Boydston AJ, Moore JS. Nature Chemistry. 2014;6:624. doi: 10.1038/nchem.1938. [DOI] [PubMed] [Google Scholar]
- (14).Larsen MB, Boydston AJ. J. Am. Chem. Soc. 2013;135:8189. doi: 10.1021/ja403757p. [DOI] [PubMed] [Google Scholar]
- (15).Fan B, Trant JF, Wong AD, Gillies ER. J. Am. Chem. Soc. 2014;136:10116. doi: 10.1021/ja504727u. [DOI] [PubMed] [Google Scholar]
- (16).Peterson GI, Boydston AJ. Macromol. Rapid Commun. 2014;35:1611. doi: 10.1002/marc.201400271. [DOI] [PubMed] [Google Scholar]
- (17).Shamis M, Lode HN, Shabat D. J. Am. Chem. Soc. 2004;126:1726. doi: 10.1021/ja039052p. [DOI] [PubMed] [Google Scholar]
- (18).Amir RJ, Pessah N, Shamis M, Shabat D. Angewandte Chemie-International Edition. 2003;42:4494. doi: 10.1002/anie.200351962. [DOI] [PubMed] [Google Scholar]
- (19).Fomina N, McFearin C, Sermsakdi M, Edigin O, Almutairi A. J. Am. Chem. Soc. 2010;132:9540. doi: 10.1021/ja102595j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lux CD, McFearin CL, Joshi-Barr S, Sankaranarayanan J, Fomina N, Almutairi A. Acs Macro Letters. 2012;1:922. doi: 10.1021/mz3002403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Sasaki T, Yaguchi H. J Polym Sci Pol Chem. 2009;47:602. [Google Scholar]
- (22).Lawrie KJ, Blakey I, Blinco JP, Cheng HH, Gronheid R, Jack KS, Pollentier I, Leeson MJ, Younkin TR, Whittaker AK. J. Mater. Chem. 2011;21:5629. [Google Scholar]
- (23).Chen L, Goh YK, Cheng HH, Smith BW, Xie P, Montgomery W, Whittaker AK, Blakey I. J Polym Sci Pol Chem. 2012;50:4255. [Google Scholar]
- (24).Sasaki T, Yoneyama T, Hashimoto S, Takemura S, Naka Y. J Polym Sci Pol Chem. 2013;51:3873. [Google Scholar]
- (25).Lobez JM, Swager TM. Angewandte Chemie-International Edition. 2010;49:95. doi: 10.1002/anie.200904936. [DOI] [PubMed] [Google Scholar]
- (26).Lobez JM, Swager TM. Macromolecules. 2010;43:10422. [Google Scholar]
- (27).Fitch KR, Goodwin AP. Chem. Mater. 2014;26:6771. [Google Scholar]
- (28).Kerr JA. Chem. Rev. 1966;66:465. [Google Scholar]
- (29).Tanaka N, Sato E, Matsumoto A. Macromolecules. 2011;44:9125. [Google Scholar]
- (30).Herr DS, Noyes WA. J. Am. Chem. Soc. 1940;62:2052. [Google Scholar]
- (31).Ribas-Arino J, Marx D. Chem. Rev. 2012;112:5412. doi: 10.1021/cr200399q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.