Abstract
Staphylococcus aureus is a major pathogen of varieties of oral mucous infection. Prostaglandin E2 (PGE2) is a pro-inflammatory factor and Cyclooxygenase 2 (COX-2) is a critical enzyme of PGE2 biosynthesis. The purpose of this study is to investigate whether Staphylococcus aureus can increase PGE2 production of oral epithelial cells and how PGE2 functions in the growth and adherence of Staphylococcus aureus. mRNA levels of COX-2, fnbpA and fnbpB were estimated by quantitative PCR. PGE2 production was measured by Enzyme Linked Immunosorbent Assay (ELISA). The binding biomass of Staphylococcus aureus to human fibronectin was investigated by crystal violet staining and confocal laser scanning microscopy and the adherent force was measured by atomic force microscope (AFM). The COX-2 mRNA level and PGE2 production were increased by Staphylococcus aureus. PGE2 promoted the growth and biofilm formation of Staphylococcus aureus, enhanced the attachment of Staphylococcus aureus to the human fibronectin as well as to the HOK cells. The transcription of fnbpB was up-regulated by PGE2 in both early and middle exponential phase but not fnbpA. These results suggest that the activation of COX-2/PGE2 pathway in oral epithelial cell by Staphylococcus aureus can in turn facilitate the growth and the ability to adhere of the pathogen. These findings uncover a new function of PGE2 and may lead to the potential of COX-2/PGE2 targeting in the therapy of inflammation and cancer in both which the COX-2/PGE2 pathway were observed activated.
Introduction
In oral and maxillofacial region, Staphylococcus aureus is a common causative agent of the soft tissue infection and jaw osteomyelitis, both of which can hinder patients from normal diet and thus reduce their life quality [1–3]. Especially, the mouth floor cellulitis, a rampant soft tissue infection in oral and maxillofacial with S. aureus as a main pathogen, can rapidly spread and sometimes develops into life threatening events [4,5]. Additionally, the establishment of the chronic inflammation as a risk factor for carcinogenesis highlights the importance of inflammation prevention and therapy [6,7]. Unfortunately, the routine use of antibiotics in infection therapy often leads to the growing incidence of antibiotic-resistant strains of S. aureus. Therefore, elucidating the pathogenesis of S. aureus induced inflammation in oral and maxillofacial becomes essential to better understand and treat the disease.
Prostaglandin E2 (PGE2) is an oxygenated metabolite of arachidonic acid. Cyclooxygenase (COX) is a restrict enzyme of PGE2 biosynthesis, accounting for the conversion of arachidonic acid to prostaglandin H2 (PGH2) which is subsequently catalyzed by PGE synthase into PGE2 [8]. So far, three forms of COX have been found, COX-l, COX-2 and COX-3, among which COX-2 expression is inducible and is increased in many cases of inflammation and cancer [9–14]. In head and neck squamous cell carcinoma and in the oral mucosa of active smokers, for example, increased levels of COX-2 expression and PGE2 production were detected according to previous reports [15,16]. Although S. aureus was previously shown to induce PGE2 production in some cell lines [17,18], no study on the COX-2 expression in oral epithelial cell suffering from S. aureus infection has been found so far.
As an essential homeostatic factor, PGE2 is generally recognized as a key mediator of immunopathology in chronic infections, regulating many courses of inflammation and multiple functions of some immune cells [19–21]. Accumulated evidence has made the establishment of the paradoxes of PGE2 function. On the one hand, it acts as a pro-inflammatory mediator activating neutrophils, macrophages, and mast cells at early stages of inflammation [20–22]. On the other hand, it has been demonstrated to be a potent immunosuppressant suppressing both innate and specific immunity at the molecular and cellular levels [23–28]. It limits the cytolytic effector functions of NK cells [29,30] as well as inhibits the phagocytosis and pathogen-killing by alveolar macrophages [31,32], for instance. The immunosuppression of PGE2 makes it a potent risk factor in inflammation. Besides, previous studies suggested that chronic inflammation promotes cancer development through COX-2/PGE2 pathway [33,34]. Thus, the functional versatility of PGE2 is increasingly noteworthy.
Although increasingly clear vision of the paradoxical role of PGE2 in various cells in immune responses, little attention has been given to the effect of PGE2 on bacterial pathogen—constitute of the inflammation environment. In C. albicans, PGE2 was demonstrated to induce germ tube formation and involve in biofilm formation [35,36]. Jan Krause et al reported that PGE2 from C. albicans stimulates the growth of S. aureus in mixed biofilms [37]. These results implied a facilitating effect of PGE2 on some pathogens. The pathogenesis of bacteria usually bases on its colonization to host tissues. The ability of S. aureus to adhere is crucial for its early colonization to host tissue and implanted biomaterials. Fibronectin-binding proteins (FnBPs) mediating the binding of S. aureus to mammalian extracellular matrix of fibronectin are important for the adherence of S. aureus in the course of infection [38,39]. fnbpA and fnbpB are genes coding for FnBPs and contribute to the ability of S. aureus adhering to fibronectin-coated surfaces [40,41]. Previous studies showed that the colonization of S. aureus is higher in some infection and cancer tissues which have both been reported to display an inducible COX-2 expression and an increased PGE2 production [9,11,42]. However, whether the higher rate of S. aureus colonization is resulted from the increased PGE2 level has not been known. Therefore, studying the effect of PGE2 on the adherence of S. aureus to cells is beneficial to elucidate the causal relationship between S. aureus colonization and inflammation or caner.
Thus, we here in this study propose that following a challenge with S. aureus, oral mucosal epithelial cell can increase COX-2 expression and PGE2 production and S. aureus can take advantage of the PGE2 to grow and to adhere. To confirm our hypothesis, we investigated the COX-2 mRNA level by qPCR and the PGE2 level by ELISA in HOK cell line with or without S. aureus infection. Also, we investigated the effect of PGE2 on S. aureus growth and adherence. The results indicated that S. arueus can activate the COX-2/PGE2 pathway in HOK and that PGE2 can promote the growth and biofilm formation of S. aureus, facilitate the ability of S. aureus to fibronectin and to HOK cells, and up-regulate the transcriptional level of fnbpB in S. aureus. Our results uncovered a new function of PGE2 in the interaction between S. aureus and oral epithelial cells in the inflammation, directing a new preventive and therapeutic guide for S. aureus infection.
Materials and methods
Cell line, bacterial strain and culture
The cell line of human oral keratinocyte (HOK) was cultured in high glucose Dulbecco’s modified Eagle Medium (DMEM, Hyclone, Logan, UT, USA) containing L-glutamine (2mM) with 10% Fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA), 1% penicillin–streptomycin antibiotic mixture (PS, Hyclone, Logan, UT, USA). The cells were cultured in an incubator with 5% CO2 and 95% air at 37°C. Cells were passaged at regular intervals depending on their growth characteristics using 0.25% trypsin (Hyclone, Logan, UT, USA). Staphylococcus aureus strain ATCC 25923 was routinely cultured in Tryptone soya broth (TSB, Oxoid, Basingstoke, UK) and 1.5% agar was added when needed.
For the infection assay, exponential phase S. aureus was centrifuged at 4000rpm for 15min and washed twice with sterile PBS. The pellet was suspended in fresh DMEM without FBS and PS and the suspension was diluted to the required cell density corresponding to ~1×108 CFUs/mL. HOK was incubated in 6-wells plates for 48h with either 0.025% dimethyl sulfoxide (DMSO) or 20μM NS-398 (Sigma-Aldrich; Saint Louis, Missouri), a specific COX-2 inhibitor, dissolved in DMSO at an optimal dose that was previously determined to provide inhibition of COX-2 [18,43,44]. Then, the NS-398- or DMSO-treated cells were infected with S. aureus at MOI of 100:1. S. aureus suspensions and HOK cells without infection were as negative control and wells without HOK incubation but added with DMEM or S. aureus suspension were as blank control. All the wells were incubated at 37°C, 5% CO2 for 45min. the supernatants were collected and filtrated with 0.22μm microfiltration membrane and stored at -80°C for ELISA or supernatant assay. After being washed with PBS, cells were lysed with TRIzol Reagent (Invitrogen, California, USA) and stored at -80°C for RNA extraction.
RNA extraction and quantitative real-time PCR
To quantify mRNA of COX-2, fnbpA and fnbpB, total RNA was isolated from ~1×106 HOK cells or from ~5×108 bacterial cells following the instructions provided with TRIzol reagent (Invitrogen, California, USA). Total RNA yield and purity were determined by absorbance at 260 nm and 280 nm using a NanoDrop-2000 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). cDNA was then synthesized using the PrimeScript RT reagent Kit with gDNA Eraser (Takara Clontech, Japan) according to the manufacturer’s instructions. Real-time PCR was performed on a C1000 Touch™ Thermal Cycler instrument (Bio-Rad, Philadelphia, PA, USA) with the SYBR reagent (Takara, Dalian, China) following the manufacturer’s instructions. The amplification was performed according to the reported protocol with some modifications [45]. A 25-μl mixture of 12.5 μL SYBR qPCR Mix (Takara, Dalian, China), 2 μL PCR primers mix (10 μM), 2 μL diluted template cDNA, and 8.5 μL deionized distilled water was prepared for each gene and subjected to 40 cycles of three steps consisting of denaturation at 95°C for 3 min, denaturation at 95°C for 5 s, annealing at 60°C for 30 s, followed by a melt curve started at 65°C to 95°C with an increment of 0.5°C for 5S. Relative fold changes of COX-2 were normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression and fnbpA and fnbpB were normalized against S. aureus 16S expression. PCR primers used in this study are listed in Table 1. The amplification efficiency and template specificity for each primer pair were verified and all the assays were conducted with each sample in triplicate.
Table 1. Primers used in this study.
| Genes | PCR primers | Description and product size |
|---|---|---|
| cox-2 | For 5'-TCCTGAAACCCACTCCCAACA-3' | Cyclooxygenase 2 |
| Rev 5'-TGGGCAGTCATCAGGCACAG-3' | 242 bp[46] | |
| GAPDH | For 5'-GTCTTCACTACCATGGAGAAGG-3' | glyceraldehyde-3-phosphate dehydrogenase |
| Rev 5'-TCATGGATGACCTTGGCCAG-3' | 197bp [46] | |
| fnbpA | For 5'-ACCGTCAAACGCAACACAAG-3' | Fibronectin-binding protein A |
| Rev 5'-TTCTGATGCCGTTCTTGGCT-3' | 259bp | |
| fnbpB | For 5'-GCTGCAGCATCGGAACAAAA-3' | Fibronectin binding protein B |
| Rev 5'-TGCTTGCACAGTTTTCGGTG-3' | 201bp | |
| 16S | For 5'-TTGGTCCTGAGGGTGGTTTG-3' | Normalizing internal standard |
| Rev 5'-CGCATACAATGGCGCAGTTT-3' | 113bp |
PGE2 ELISA
The supernatant collection for PGE2 measurement was described above. Simply, HOK cells were cocultured with S. aureus at a MOI of 100:1 in DMEM without FBS for 45 min. the supernatants were harvested, filtrated and the PGE2 content was measured by ELISA (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions with each sample in triplicate [47].
Staphylococcus aureus growth and biofilm formation
Exogenous PGE2 (Sigma-Aldrich) was dissolved in absolute ethanol to prepare the stock solution of 10mg/mL and the aliquots were stored at -20°C protected from light. For experiment, the aliquots of stock solution were diluted in sterile PBS to obtain the desired concentrations. The overnight culture of S. aureus was diluted 1:10 into fresh TSB. Then, PGE2 dilutions were added into the diluted S. aureus cultures at the final concentrations of 0 pg/mL, 20 pg/mL, 50 pg/mL, 100 pg/mL and 500 pg/mL. The mixtures of S. aureus and PGE2 were then transferred into the flat-bottom 96-wells cell culture cluster (Corning, NY, USA). Wells containing equal volume of fresh TSB were used as negative controls. The plates were incubated at 37°C aerobically for 18 h. For the planktonic growth measurement, the plate was incubated in the BioTek microplate reader (Gene Company, American) with shaking per 5 seconds and reading per hour. For the biofilm assay, S. aureus was cultured in TSB medium (containing 5% FBS) added with PGE2 (500 pg/mL) or with PBS and then the culture was transferred into the flat-bottom 96-well cell culture cluster as described above. The plates for biofilm formation were incubated at 37°C statically and after 18h incubation, Liquid medium was removed and the wells were gently rinsed two times with sterile distilled water to remove the planktonic or loosely bound cells. Biofilms were stained with crystal violet (CV) solution as described by Peeters et al with a few modifications [48]. Briefly, biofilms cultured in 96-well plates were fixed with glutaraldehyde. Then, 50 μL of 0.1% crystal violet solution was added to each well and incubated for 20 min at room temperature. Excess CV was removed by washing under running tap water and bounded CV was released by 200 μL of 99% ethanol. Absorbance was measured at 570 nm with a Thermo Scientific Multiskan GO reader (Thermo Fisher Scientific Inc., Waltham, MA, USA)
Fibronectin assay
Binding of S. aureus to solid-phase fibronectin was measured as described previously [49,50] with some modification. Briefly, flat-bottomed polystyrene 96-well plates (Corning, NY, USA) were coated with 100 μL fibronectin (10 μg/mL in PBS) isolated from human plasma (Sigma-Aldrich) for 1 h at 37°C. Fibronectin solution was removed and replaced with a blocking solution of Bovine Serum Albumin (BSA, 2%, w/v) in PBS overnight at 4°C. Wells were washed three times with sterile PBS prior to the addition of bacterial suspension. The overnight S. aureus culture was diluted with fresh TSB that was added with PGE2 (500 pg/mL) or added with PBS at the same volumes. Then, the condition dilutions were cultured to the exponential phase. The cultures were centrifuged at 4°C, 4000 rpm for 15 min. The pellets were washed three times and resuspended with PBS. After adjustment to achieve a similar initial cell density, the cultures were diluted 1:10 with DMEM without FBS and antibiotics. 200μL (approx. 2×106 bacteria) of the diluted cells were transferred in quadruplicate into the wells coated with fibronectin and, as a negative control, into the uncoated wells blocked with BSA. Plates were incubated at 37°C for 1 h. All wells were rinsed three times with PBS to remove unbound bacteria. Adherent bacteria were fixed with glutaraldehyde [200 μL; 2% (v/v) in PBS] for 1 h at room temperature. After rinsing, bacteria in 96-well microtitre plates were stained with 50 μL crystal violet (final concentration 0.01%, w/v) for 15 min. Wells were rinsed three times with sterile water and allowed to air dry. 200 μL 99% ethanol was added to each experimental well and the plates were shaken for several minutes to induce dye release. The quantity of biomass was represented by OD570 measured with a Thermo Scientific Multiskan GO Reader.
Confocal laser scanning microscopy for S. aureus adhesion to fibronectin
A flat-bottom 24-well plate with cover glasses in the bottoms was coated and blocked by the protocol described above for the fibronectin assay. The prepared bacterial suspensions pre-cultured with PGE2 at 0pg/mL or 500pg/mL were added into the prepared wells. The plate was cultivated at 37°C aerobically for 1 h and 3 h without agitation. The glass slides were dyed with a SYTO-9 staining (Molecular Probes, Eugene, OR, USA). After incubation at room temperature in the dark for 15 min, the samples were fixed with mounting oil that protected against fluorescence quenching, immobilized by nail polish on slides and stored at 4°C away from the light before being scanned by a Leica TCS SP2 confocal laser scanning microscopy (CLSM, Leica, German). All the samples were observed by an oil lens and images were at the same magnification of 630×. The excitation/emission for scanning were 480nm/500nm respectively following the instruction and the interval was 1 μm. Images were recorded from signal appeared to signal disappeared and analyzed by a LAS AF Lite software without zoom in.
Measurement of adhesion forces
The cover glasses were coated with human fibronectin as protocol described in the CLSM assay and slides without fibronectin coated were used as control. The preparation of bacterial AFM tips and the measurement of adhesion forces were followed the protocol from study by Chuanyong Wang et al [51]. Briefly, the overnight S. aureus culture was diluted and incubated in fresh TSB broth with (500 pg/mL) or without PGE2 to exponential phase. Then the cultures were centrifuges at 4°C 4000rpm for 15 min and washed twice with PBS and resuspended in adhesion buffer (2 mM potassium phosphate, 50 mM potassium chloride, 1 mM calcium chloride; pH 6.8) [52]. The bacterial suspensions were then sonicated 3 times (10 s working with 5 s waiting each) to scatter bacteria clumps. The CSC38/tipless AFM probe (Ultrasharp, m-Masch, Tallinn, Estonia) were sterilized under UV for 5 min and the half length of the cantilever was dipped into a drop of 0.01% (w/v) poly-L-lysine (Sigma, Poole, UK) for 1 min. After being dried for 2 min in air, the probes were immersed in bacterial suspension for 1 min and were applied for adhesion force measurement immediately. For each probe, two coated and two un-coated slides were measured with 6–8 positions being randomly selected for every slide and each position being repeated at least ten times. Each sample was tested with at least three tips. Every time after used, the tip was detected under a Scanning electron micrograph (SEM) to confirm the intactness of the bacteria layer on the modified cantilever. The force data was disposed once the integrity of bacterial layer was damaged.
Adherent and invasion assay
The adherent and invasion assay was carried out as previously described with some modification [18,53]. Briefly, the suspension of exponential S. aureus in DMEM was prepared as the protocol for the infection assay. The completely confluent cell layers in 24-well plates were rinsed twice with sterile PBS and added with S. aureus suspension for each well at the MOI of 100:1. Equal volumes of S. aureus suspensions were simultaneously added into wells without cells to monitor the growth of S. aureus in DMEM in the experimental periods. Following 45 min cultured, the S. aureus was ten-time step diluted and the dilutions were plated on the TSA agar plates and the HOK cells were washed three times with sterile PBS. For adherent assay, 1 mL of 0.1% Triton X-100 (Amresco, Solon, OH, USA) was added into each well and incubated for 5 min at 37°C to lyse the cells. The lyses were ten-time step diluted and the dilutions were plated on the TSA ager plates. Meanwhile, for the invasion assay, other cells were incubated in DMEM containing gentamicin (100 μg/mL) for 1 h to kill the remaining extracellular bacterial cells and then the cells were lysed and plated after rinsed as the protocol described above for the adherent assay. The plates were incubated at 37°C for 24 h and the single clones were calculated.
The supernatant assay
HOK cells were pretreated with 20 μM NS-398 or with 0.025% DMSO for 48 h and then infected with S. aureus at MOI of 100:1. Cells uninfected were as negative control and DMEM and S. aureus suspension were as blank control. All the wells were incubated at 37°C, 5% CO2 for 45min and the supernatants were collected by centrifuged and filtrated. The overnight culture of S. aureus was diluted with fresh TSB. Then, the diluted cultures were added with the conditional cell supernatants at the final concentration of 20% or added with PGE2 at the concentration of 136.5 pg/mL which equals to the quantity of PGE2 in the supernatants added. After incubated to the exponential phase, the cultures were ten-time step diluted, plated on the TSA agars and incubated at 37°C for 24 h. Or, the cultures were centrifuged at 4000 rpm for 15 min at 4°C, rinsed with sterile PBS and suspended in fresh DMEM. After being adjusted to the same density, the S. aureus suspensions were added into the 96-well plates coated with fibronectin (1 μg per well) for the fibronectin assay or added into 24-well plates with confluent HOK layers for the adherent assay as described above.
Statistical analysis
All experiments were performed in triplicate and results are representative of at least three independent experiments. Comparisons between groups were analyzed by analysis of variance (ANOVA) unless otherwise stated. Data are expressed as means ± SE and results were considered to be statistically significant where P < 0.05.
Results
Induction of COX-2 mRNA expression and PGE2 production in HOK after exposure to S. aureus
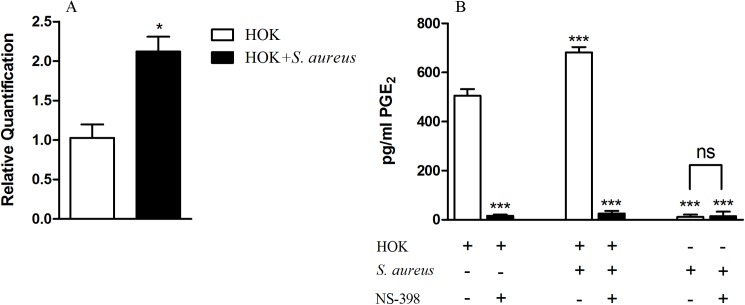
Previous studies showed that S. aureus can induce PGE2 production in nasal fibroblasts and murine osteoblasts [17,18]. To investigate whether COX-2 expression in normal oral epithelial cell can be induced by S. aureus infection, we quantified COX-2 mRNA by qPCR and PGE2 production by ELISA with HOK cell line as a model of normal oral epithelium. As shown in Fig 1, the level of COX-2 mRNA in S. aureus-infected HOK doubles that in HOK without infection (Fig 1A). In accordance with gene regulation, the PGE2 production by the S. aureus-infected HOK was significantly higher than that by the un-infected HOK, 682 pg/mL and 505 pg/mL respectively, that is 35% higher for the infected S. aureus than the un-infected one (Fig 1B).
Fig 1. S. aureus increases the expression of COX-2 mRNA and PGE2 production by HOK.
HOK cells were pretreated with 20μM NS-398 or with 0.025% DMSO for 48 h. Then, the cells were infected with S. aureus at the MOI of 100:1 for 45 min. S. aureus suspension and the HOK without infection were as negative control and DMEM as the blank control. After the infection, the supernatants were collected by centrifuging at 2000 rpm for 25 min and filtrating with 0.22 μM filter membrane and used for PGE2 ELISA. The conditional cells were lysed with TRIzol reagent and used for RNA extraction and qPCR. A. Fold changes of COX-2 mRNA. The level of COX-2 mRNA in S. aureus-infected HOK doubles that in HOK without infection. B. The quantity of PGE2. The PGE2 production by the S. aureus-infected HOK was 682 pg/mL, significantly higher than that by the un-infected HOK which was 505 pg/mL. Data are expressed as means ± standard errors from three independent experiments and asterisks represent significant differences (P < 0.05) compared with HOK.
Considering that COX-2 is not the only restrict enzyme for PGE2 production, we additionally determined whether S. aureus-stimulated increase of PGE2 production was COX-2 derived. By adding NS-398, a small-molecule specific inhibitor of COX-2, into the culture of HOK with or without S. aureus infection, we found that the level of PGE2 both decreased remarkably and the S. aureus-stimulated increase of PGE2 production by HOK infection was disappeared, displaying a similarly low level to that in control cell (Fig 1B). Collectively, these results indicate that S. aureus up-regulates COX-2 transcription which subsequently leads to PGE2 production increased in infected HOK.
Changes in the growth and biofilm formation of S. aureus after treatment with PGE2
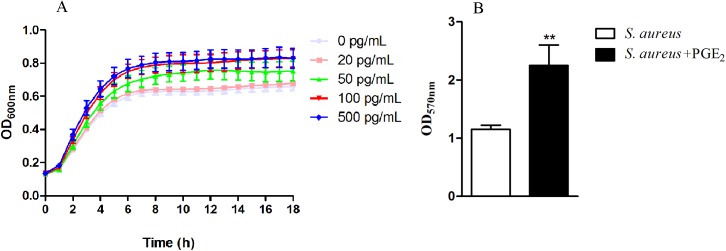
To investigate the impact of PGE2 on S. aureus, we first studied the growth and biofilm formation of S. aureus in presence of different concentration of purified PGE2. As shown in Fig 2A, S. aureus in presence of 20pg/mL PGE2 has a similar growth rate to the control. However, the growth rate of S. aureus treated with 50pg/mL PGE2 was higher than that of the control and the increase in the growth rate was more significantly in S. aureus treated with PGE2 at the concentration of 100 pg/mL and 500 pg/mL. The results indicated that PGE2 facilitates S. aureus growth in a dose-dependent manner.
Fig 2. PGE2 stimulates the growth and biofilm formation of S. aureus.
S. aureus was cocultured with PGE2 at the final concentrations of 0 pg/mL, 20 pg/mL, 50 pg/mL, 100 pg/mL and 500 pg/mL in 96-well plates for 18 h and the growth curves were recorded by the BioTek microplate reader. Or, S. aureus cultured in TSB containing 5% FBS was incubated with PGE2 (500 pg/mL) or with PBS for 18 h and the biofilms were quantified with 0.1% (w/v) crystal violet staining. A. The growth curves of planktonic S. aureus with or without PGE2 treatment. PGE2 facilitated the growth of S. aureus in a dose-dependent manner. The facilitated effect of PGE2 on S. aureus growth was observed at the concentration of 50 pg/mL compared with the control and the facilitation was more significant at the concentration of 100 pg/mL, 200 pg/mL and 500 pg/mL. B. The biofilms quantified by crystal violet staining and measured at the optical density of 570 nm. Biofilm formation of S. aureus in presence of PGE2 was twice as that by S. aureus in absence of PGE2. Results are expressed as means ± standard errors from three replicates per experiment. Asterisks indicate significant (P< 0.05) differences compared to HOK without PGE2 treatment.
Biofilm is the main form in which bacterial exist and function. Thus, we investigated the effect of PGE2 on the biofilm formation of S. aureus. Consistent with the facilitation to the growth in planktonic state, similar increase effect by PGE2 was observed on the biofilm formation of S. aureus. Biofilm formation of S. aureus in presence to PGE2 was twice as that by S. aureus in absence to PGE2 (Fig 2B). These results indicated that PGE2 exerts a facilitated effect on S. aureus growth and biofilm formation.
Changes in the attachment of S. aureus to human fibronectin after treatment with PGE2
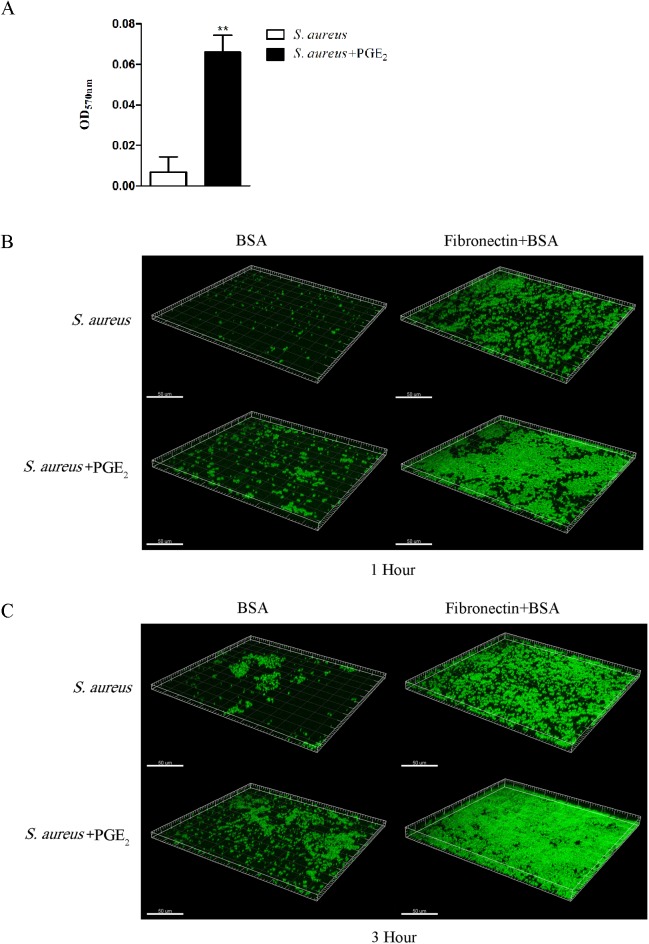
The adherence of S. aureus to cells is one of its pathogenic factors. Previous studies showed that the binding of cell-wall ligands of S. aureus to fibronectin plays a major role in the cause of adherence and invasion [38–41]. Therefore, fibronectin assay was performed to investigate whether PGE2 has an effect on the attachment of S. aureus to human fibronectin. 96-well plates and the cover slips in 24-well plates were coated with fibronectin, at 1μg per well for 96-well plates and 5μg per well for 24-well plates, at 37°C for 1h with uncoated wells and slips as the blank control. Then, plates and cover slips were blocked overnight at 4°C. Exponential S. aureus pretreated with PGE2 (500 pg/mL) or PBS was incubated in the conditional 96- or 24-well plates at 37°C. The adherent biomass of S. aureus was estimated by CV staining at 1 hour after incubation or by CLSM at 1 hour and 3 hour after incubation. As shown in Fig 3A, compared to S. aureus without PGE2 stimulation, the adherent biomass of S. aureus cocultured with PGE2 was significantly more, approximately 6.3-fold increased. Additionally, CLSM assay confirmed the result of CV quantification. As shown in Fig 3B and 3C, after 1 hour or 3 hour cultured, the fluorescent S. aureus on the slides coated with fibronectin showed higher density than that on the slides without fibronectin, confirming the mediated role of fibronectin in the binding between S. aureus and cells. Additionally, the fluorescent dense intensity on the slides by S. aureus cocultured with PGE2 was higher than that by S. aureus in the absence of PGE2 both at the 1 hour and at the 3 hour time points, no matter with or without fibronectin treatment. The CV and CLSM results indicated that PGE2 facilitates the adherence of S. aureus to fibronectin.
Fig 3. PGE2 facilitates the attachment of S. aureus to human fibronectin.
The fibronectin, was coated at 1μg per well in 96-well plates and 5μg per well in 24-well plates set with cover slips at 37°C for 1h, with uncoated wells and slips as the blank control. Then, plates and cover slips were blocked overnight at 4°C. Exponential S. aureus pretreated with PGE2 (500 pg/mL) or PBS was incubated in the conditional 96- or 24-well plates at 37°C. The adherent biomass of S. aureus was estimated by CV staining at 1 hour after incubation or by CLSM at 1 hour and 3 hour after incubation. A. The biomass of attached S. aureus quantified with crystal violent staining and expressed as the optical density at 570 nm. The adherent biomass of S. aureus cocultured with PGE2 was approximately 6.3-fold increased than that by S. aureus without PGE2 treated. Data are expressed as means ± standard errors from three replicates per experiment. Asterisks indicate significant (P< 0.05) differences compared to HOK without PGE2. B. The confocal laser scanning microscopy for attached S. aureus biomass after incubation for 1 hour. C. The confocal laser scanning microscopy for attached S. aureus biomass after incubation for 3 hours. At both 1 h and 3 h time points, slides coated with fibronectin displayed higher fluorescent dense intensity than the uncoated ones and, no matter fibronectin treatment or not, the slides were attached with PGE2-treated S. aureus more than the untreated ones. Samples were observed by an oil lens and images were at the same magnification of 630× and analyzed by the LAS AF Lite software without zoom in. The experiments were performed in triple and three images were randomly captured from each sample.
Changes in the adhesion force of S. aureus to human fibronectin after PGE2 treatment
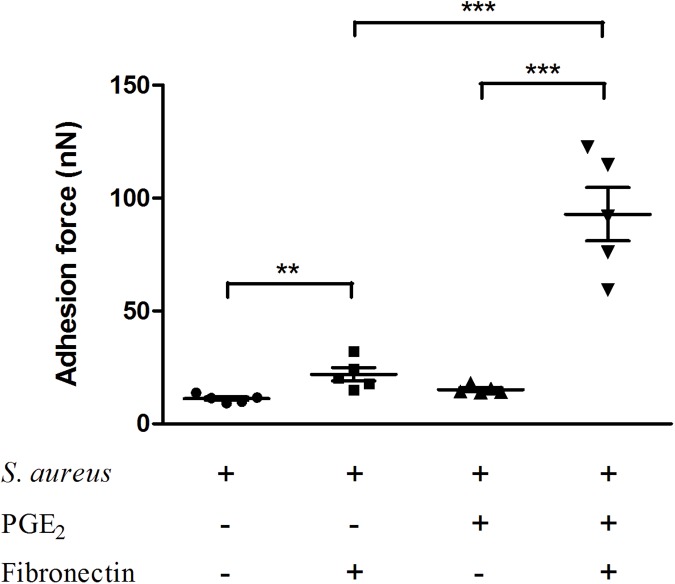
Cover slips were coated or not coated with fibronectin. CSC38/tipless probes were pretreated with 0.01% (w/v) poly-L-lysine and attached with S. aureus treated or untreated with PGE2 (500pg/mL). The adhesion force of S. aureus to human fibronectin or to blank slides was immediately measured by AFM. As shown in Fig 4, the adhesion forces to the control glasses without fibronectin coated are not different between the PGE2 treated and un-treated S. aureus. Both of the two groups displayed a low force value, that is, 11.19 nN for un-treated S. aureus and 15.24 nN for PGE2-treated S. aureus respectively. However, when the cover glasses were coated with human fibronectin, the adhesion force of either un-treated S. aureus or PGE2-treated S. aureus was significantly stronger as compared to their respective control uncoated-glasses, confirming the promotion of fibronectin to S. aureus adhesion force. Notably, the adhesion force of S. aureus with PGE2 treatment (92.87 nN) is remarkably higher than that of all other groups. For S. aureus without PGE2 treatment, although the adhesion force was markedly enhanced by fibronectin (from 11.19 nN of the control group to 21.99 nN of the fibronectin-coated group), the force value is much less than the 92.87 nN of PGE2-treated S. aureus, indicating the significant enhancement of PGE2 to the adhesion force of S. aureus to human fibronectin.
Fig 4. PGE2 enhances the adhesion force of S. aureus to human fibronectin.
Exponential S. aureus cocultured with PGE2 (500 pg/mL) or with PBS was harvested and resuspended in adhesion buffer. The CSC38/tipless AFM probes were coated with 0.01% (w/v) poly-L-lysine for 1min, dried in air for 2min, and then immersed in bacterial suspension for 1 min. The adhesion force of S. aureus to fibronectin or to blank slides was immediately measured by AFM. For S. aureus without PGE2 treatment, the mean adhesion force to the un-coated slides was 11.19 nN and to the fibronectin-coated slides was 21.99 nN. For the PGE2-treated S. aureus, the mean adhesion force to the un-coated slide was 15.24 nN and to the coated slides was 92.87 nN. The adhesion force of S. aureus to fibronectin was stronger than that to the smooth glass slide surface and PGE2 significantly enhanced the force to fibronecin. Each sample was tested with at least three tips and for each probe, two slides were measured. The force data from tip with the integrity of bacterial layer damaged was disposed. Data are expressed as means ± standard errors from three independent experiments and asterisks represent significant differences (P < 0.05).
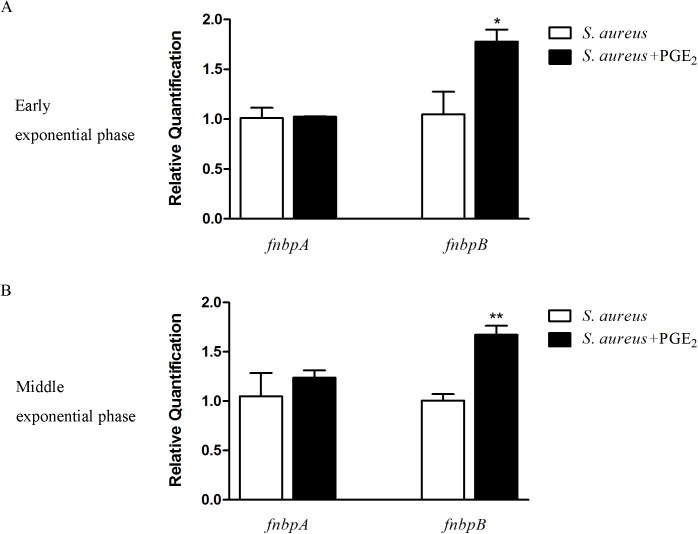
Changes in the mRNA levels of fnbpA and fnbpB after PGE2 treatment
To investigate how PGE2 facilitates the adherent ability of S. aureus, we further examined the transcriptions of fnbpA and fnbpB, two genes coding for the fibronectin-binding proteins that mediate the binding of S. aureus to the mammalian fibronectin. As shown in Fig 5, in both the early and the middle exponential phase, the transcriptional level of fnbpA was not significantly different between the control S. aureus and PGE2-treated S. aureus. However, the fnbpB transcription level of PGE2-treated S. aureus is much higher than that of S. aureus without PGE2 treatment. In detail, the transcriptional level of fnbpB of PGE2-treated S. aureus is 73% higher than that of un-treated S. aureus in the early exponential phase (Fig 5A) and 67% higher in the middle exponential phase (Fig 5B), indicating that PGE2 up-regulated fnbpB but did no regulation to fnbpA.
Fig 5. PGE2 up-regulates the expression of fnbpB but not fnbpA mRNA.
S. aureus were treated with PGE2 (500 pg/mL) or with PBS and bacterial cells were harvested at both the early and the middle exponential phase. Total RNA of S. aureus was isolated from ~5×108 bacterial cells using TRIzol reagent following the manufacturer’s instruction. cDNA was synthesized using the PrimeScript RT reagent Kit and qPCR was performed with the SYBR reagent. A. The transcriptional level of fnbpA and fnbpB mRNA in the early exponential phase. The level of fnbpB mRNA of PGE2-treated S. aureus is 73% higher than that of un-treated S. aureus and the level of fnbpA mRNA had no differences between the two groups. B. The transcriptional level of fnbpA and fnbpB mRNA in the middle exponential phase. The level of fnbpB mRNA of PGE2-treated S. aureus is 67% higher than that of un-treated S. aureus and the level of fnbpA mRNA had no differences between the two groups. All values were normalized against S. aureus 16S rRNA expression. Results are representative of three independent experiments and represent the means ± standard errors for three separate cultures. The asterisk represents significant differences (P < 0.05) compared with the control S. aureus that was not pre-cultured with PGE2.
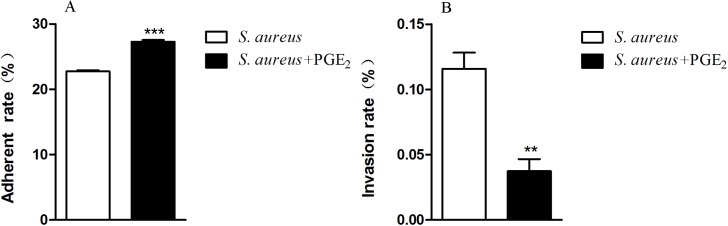
Changes in the adherence and invasion of S. aureus to HOK after PGE2 treatment
Based on the results of fibronectin assay, we further investigated whether PGE2 also has a facilitated effect on the adherence and invasion of S. aureus to HOK cells. S. aureus precultured with or without PGE2 were adjusted to the same CFUs and cocultured with the conflucent HOK cells at a MOI 100:1 for 45min. Cells with or without gentamicin treatment were then lysed with triton X-100 and the lysate was ten-time step diluted with PBS and plated on the TSA plates. As shown in Fig 6A, the adherent rate of PGE2-stimulated S. aureus is significantly higher than that of the control without PGE2 stimulation, 27% and 23% respectively. Inconsistently, the invasion rate of S. aureus in presence of PGE2 is lower than that in absence of PGE2, implying multiple layer of PGE2 functions at different stages of S.aureus infection to HOK cells under a fine regulatory mechanism (Fig 6B). Collectively, the results of the adherent assay indicated that PGE2 facilitates S. aureus to adherent to HOK cells.
Fig 6. PGE2 enhances the adherence but inhibits the invasion of S. aureus to HOK.
Exponential S. aureus treated with PGE2 (500 pg/mL) or PBS was used to infect HOK cells at a MOI of 100:1 for 45 min. Then, the cells were lysed with 1 mL of 0.1% Triton X-100 at 37°C for 5 min, ten-time step diluted and plated on the TSA agar plates. Or, the cells were treated with gentamicin (100 μg/mL) for 1 h and then lysed and plated as above. A. The adherence rate of S. aureus to HOK cells. The adherent rate of PGE2-stimulated S. aureus is 27%, significantly higher than the 23% of the control. B. The invasion rate of S. aureus to HOK cells. The invasion rate of PGE2-treated S. aureus is lower than the control.The invasive S. aureus was represented as the number of S. aureus in lyses of gentamicin-treated cells and the adherent S. aureus was represented as the difference between S. aureus in lyses of cells without gentamicin killing and that in lyses of gentamicin-treated cells. The adherence rate and invasion rate were respectively expressed as the ratio of adherent S. aureus to the total number of incubated S. aureus and the ratio of invasive S. aureus to the total number of incubated S. aureus. Data are expressed as means ± standard errors from three independent experiments and asterisks represent significant differences (P < 0.05) compared with S. aureus without PGE2 preculture.
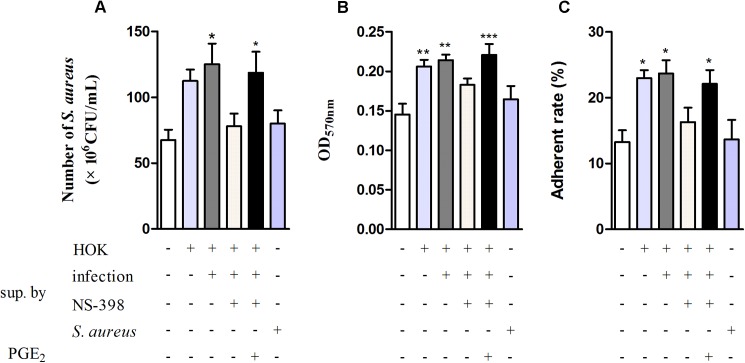
The confirmation for the effect of PGE2 on S. aureus with cell supernatants
As confirmed above, S. aureus infection induces PGE2 production by HOK and the purified PGE2 displayed a facilitated role not only in the planktonic growth but also in the adherence of S. aureus. To further investigate whether the produced PGE2 by HOK has similar facilitated effect on S. aureus, we subsequently performed the supernatant assay. NS-398- or DMSO-treated HOK cells were infected with S. aureus for 45 min at a MOI of 100:1 with uninfected cells as negative control and DMEM and S. aureus suspension as blank control. Then, the supernatants were collected and used, at the final concentration of 20%, for stimulating S. aureus. Or, exogenous PGE2 was added into S. aureus cultures at the concentration of 136.5 pg/mL which equals to the quantity of PGE2 in the infected HOK supernatant. Results from the growth assay by the method of CFUs count revealed that CFUs of S. aureus in presence of the supernatants from uninfected HOK or treated with the supernatants from infected HOK were significantly more than that of the control. And S. aureus treated with supernatants from infected HOK displayed the highest growth rate than others. However, when HOK cells were pretreated with NS-398, the supernatants displayed no effect on S. aureus growth. Notably, the increase in growth rate of S. aureus was recovered by adding the same concentration of exogenous PGE2 into S. aureus cultures. These results indicated that the COX-2 derived PGE2 produced by HOK can promote the growth of S. aureus (Fig 7A).
Fig 7. Supernatants containing PGE2 produced by HOK facilitate the growth and adherence of S. aureus.
NS-398- or DMSO-treated HOK cells were infected with S. aureus for 45 min at a MOI of 100:1, with the uninfected cells as negative control and DMEM and S. aureus suspension as blank controls. Then, the supernatants were collected and used, at the final concentration of 20%, for the stimulation of S. aureus. Or, exogenous PGE2 was added into S. aureus cultures at the concentration of 136.5 pg/mL which equals to the quantity of PGE2 in the infected HOK supernatant added. The growth and adhesion rate to HOK of S. aureus were evaluates with CFU count and the attachment of S. aureus to fibronectin was estimated by CV staining. Supernatants are abbreviated to “sup.” in the shown figures. A. The effect of supernatants on the growth of S. aureus. Sup-H and Sup-Hi can increase the growth of S. aureus but Sup-S and Sup-Hi398 can’t. The addition of PGE2 recovered the promoted effect of Sup-Hi398, which was inhibited by NS-398, on S. aureus growth. B. The effect of supernatants on the adherence of S. aureus to fibronectin. The attached biomass of S. aureus incubated with Sup-H and Sup-Hi was more than that of untreated S. aureus. Sup-S and Sup-Hi398 had no effect on the attachment of S. aureus to fibronectin. However, the addition of PGE2 into Sup-Hi398 made it regain the facilitated effect on the attachment S. aureus to fibronecin. C. The effect of supernatants on the adherence of S. aureus to HOK cells. The adhesion rates of S. aureus simulated with Sup-H and Sup-Hi were ~23% and 24% respectively, higher than that of the untreated S. aureus (~13%). S. aureus incubated with Sup-S and Sup-Hi398 displayed similar adhesion rates, ~16% and ~14% respectively, to the untreated S. aureus. The addition of PGE2 made the Sup-Hi398 regain the facilitated effect on the adherence of S. aureus to HOK cells. Sup-H: supernatant from untreated HOK; Sup-Hi: supernatant from infected HOK without NS-398 treated; Sup-Hi398: supernatant from HOK treated with NS-398 and infection. Sup-S: supernatant from S. aureus suspension. Data are expressed as means ± standard errors from three independent experiments and asterisks represent significant differences (P < 0.05) compared with S. aureus without any supernatant added.
Additionally, the effects of the supernatants on the adherence of S. aureus to fibronectin and HOK cells were also investigated. As shown in Fig 7B and 7C, supernatants from HOK with or without S. aureus infection both enhanced the adherent rate of S. aureus, and the infected HOK supernatant displayed more significant promotion than the uninfected HOK supernatant to S. aureus adherence. Also, the supernatants from NS-398-treated cells had no effect on the adherent of S. aureus to both fibronectin and HOK cells. However, the facilitated effect was reversed when the NS-398-treated cells supernatants were supplemented with exogenous PGE2 in the same concentration of that found in the infected HOK supernatants.The collective results indicated that PGE2 produced by HOK can in turn impact the growth and adherence of S. aureus.
Discussion
S. aureus is the main pathogen of many oral infections among which some can threaten patients’ life [4,5]. Thus, studying the behavior of S. aureus during the infection is helpful to better understand the mechanism in which S. aureus causes inflammation. In the present study, we demonstrated that S. aureus can induce COX-2 transcription and increase PGE2 production in oral epithelial cell line HOK. PGE2 promotes the growth of S. aureus and the binding of S. aureus to fibronectin. Importantly, we demonstrated that PGE2 facilitates the adherence of S. aureus to the oral epithelial cell. So far as we know, our study provides the first evidence for the facilitated role of PGE2 in S. aureus adherence.
Previous studies have indicated that COX-2 expression is increased in some cases of inflammation and cancer [11,13–15]. Increased level of PGE2 has been detected in the head and neck squamous cell carcinoma [54–56]. Report by Dimitrios Moraitis indicated that levels of COX-2 are increased by activating epidermal growth factor receptor (EGFR) in the oral mucosa of active smokers versus never smokers [16]. Here in this study, we first showed that S. aureus up-regulated COX-2 transcription and PGE2 production by normal oral epithelial cell line HOK. Consistent with this finding, increased PGE2 levels resulted from S. aureus infection were detected by Pérez-Novo in nasal tissue fibroblasts [17] and by Somayaji in murine osteoblasts [18]. Considering that PGE2 synthesis is involved with several enzymes, we thus used NS-398, a COX-2 specific inhibitor [57,58], to establish the role of COX-2 with respect to production of PGE2 during S. aureus infection of oral epithelial cell. After being treated with NS-398, both uninfected and S. aureus-infected HOK cells produced a significantly attenuated level of PGE2, which remarkably lower than that by cells without NS-398 treatment. These results indicate that S. aureus can increase COX-2 derived PGE2 production by HOK.
Intensive researches on PGE2 make the paradox and versatility of PGE2 function established. However, few studies hitherto have directly addressed the effects of PGE2 on bacterial pathogens. Recently Jan Krause reported that PGE2 produced by C.albicans displayed a stimulatory effect on the growth of S. aureus [37]. Consistently, we in this study found that both the purified and the produced PGE2 promoted S. aureus growth and biofilm formation at the experimental concentration. The observation that supernatant from HOK treated with NS-398 and infection failed to stimulate S. aureus growth and the addition of PGE2 reversed the facilitated effect that inhibited by NS-398 on S. aureus growth further confirmed that the enhanced growth of S. aureus is resulted from the PGE2 produced by HOK cells.
Fibronectin is a multifunctional extracellular matrix that plays a central role in cell adhesion and in the attachment of varies of microorganisms to human tissues [59,60]. S. aureus is the first bacterium shown to bind to fibronectin [61]. In this study, the binding of S. aureus to the fibronectin-coated surfaces is remarkably more than that to the uncoated surfaces, confirming the promotion of fibronectin to S. aureus attachment. Furthermore, our study first indicated that PGE2 significantly facilitated the ability of S. aureus to adhere to purified fibronectin, which manifested as the increased number of attached bacterial cells and the higher adhesion force value in the PGE2-treated S. aureus group than that in other control groups. Besides, the facilitated effect of PGE2 on the adhesion of S. aureus was also observed in the adherent assay to the oral epithelial cell line HOK. Through incubating S. aureus in absence of cells in the same condition during the adherent assay, we testified that the facilitation of PGE2 to attachment is not resulted from its growth promoting (S1 Fig).
The binding of S. aureus to fibronectin is mediated by cell-wall anchored fibronectin-binding proteins which encoded by two genes of fnbpA and fnbpB. According to study by Greene et al, the double mutant of fnbpA and fnbpB in S. aureus displayed severely impaired adherent ability to coverslips obtained from tissue cages implanted [41]. In this study, the transcriptional level of fnbpB mRNA was remarkably up-regulated by PGE2 both in the early and in the middle exponential phase. However, in the both phases, no change was observed in fnbpA transcription between the PGE2-treated and un-treated S. aureus. The transcriptional results of fnbpA and fnbpB indicated that PGE2 can in part regulate the transcription of genes coding for fibronectin-binding proteins.Several observations supported that S. aureus colonization is significantly higher in some cancer patients than that in the healthy [62–64] and COX-2 appears as frequently upregulated in tumor cells [65,66]. Take the Cutaneous T-cell lymphomas (CTCL) as an example, COX-2 expression has recently been proven in CTCL cells, and treatment with the selective COX-2 inhibitor celecoxib resulted in decreased cell growth and viability [66]. Meanwhile, as Nguyen and Talpur reported, patients with CTCL have a significantly higher rate of S. aureus than the general population [42,67]. Accordingly, our findings, in the present study, that PGE2 facilitates the growth and adherence of S. aureus supplies a reasonable presumption that the high rate of S. aureus colonization in cancers may in part attribute to the promoted impacts of PGE2 to the pathogen. Furthermore, based on the findings from this study and the study by Dimitrios Moraitis who reported that PGE2 production was increased in the oral mucosa of smokers [16], it is conceivable that the elevated PGE2 levels may cause a higher risk of S. aureus colonization in the oral mucosa of smokers. Thus, an active protection from S. aureus infection is essential for smokers.
Through binding to fibronectin which is simultaneously bound to integrin α5β1, S. aureus can be internalized into host cells. In the present study we also showed that PGE2 displayed an inhibitory role in the invasion of S. aureus to HOK cells, contrary to the facilitated impact on the adherence. The inconsistent roles of PGE2 in the attachment and invasion of S. aureus may due to the complicated mechanism by which S. aureus invades to cells. For example, observed evidence indicated that although α5β1 is expressed ubiquitously on human cells, the invasion level varies largely between host cells of different tissues [38,39,53,68,69]. Additionally, previous studies demonstrated that fibronectin promoted the binding of bacterial to polymorphonuclear leukocytes and macrophages but it didn’t facilitate ingestion or killing of the microorganisms [70,71]. Thus, the ability of S. aureus to use PGE2 to enhance its binding to fironectin is of some benefit to its parasites. Besides, as a pro-inflammatory factor, PGE2 was secreted by host cells to activate innate immune system to defend the pathogens impair [20,72,73]. Thus, the inhibition of PGE2 to S. aureus invasion demonstrated the defense reaction of the host to the harmful stimulation by such pathogen.
According to previous reports, chronic inflammation was suggested to promote cancer development by inducing the COX-2/PGE2 pathway and activating NF-κB and Stat3 signals [33,34]. This can be confirmed by the findings that regular use of non-steroidal anti-inflammatory drugs (NSAIDs) can reduce risk of gastrointestinal cancer and the growth of head and neck cancer [74–76] through blockading COX-1 and COX-2 activities, which subsequently suppresses prostaglandin, including PGE2, biosynthesis. In view of our present observations that S. aureus increases PGE2 production by HOK and PGE2 in turn facilitates the growth and adherence of S. aureus, further studies seeking for the probable mediating roles of COX-2/PGE2 pathway in the relationship between inflammation and cancer can be conducted, which may suggest COX-2/PGE2 axis targeting strategies for the prevention and treatment of inflammation and cancer diseases.
Conclusion
To fully realize the potential of PGE2 targeting in the therapy of inflammation and cancer, sufficient investigations on the versatility of PGE2 are considerably essential. Here in this study, we first confirmed that S. aureus can up-regulated the COX-2 transcription and increased PGE2 production by the normal oral epithelial cell line HOK. Using the purified PGE2 and the supernatants, we found that in case of infection, S. aureus can intellectually take advantage of the surrounding PGE2 to increase its growth and adherence. These findings revealed a new look to the pathogenic mechanism of S. aureus and may lead to new therapeutic strategies with higher potency and improved selectivity.
Supporting information
Before incubated in DMEM, the initial number of S. aureus [S. aureus (before)] and S. aureus with PGE2 treated [S. aureus +PGE2 (before)] used for adhesion and invasion assay were approximately 2.63×107 CUFs /mL and 2.57×107 CUFs/mL. After treated in DMEM for 45 min, the numbers for them were 2.38×107 CUFs/mL [S. aureus (after)] and 2.25×107 CUFs/mL [S. aureus + PGE2 (after)] respectively. There was no significantly difference between all the groups.
(TIF)
Acknowledgments
We thank the professor Chaoliang Zhang for his assistant for performing confocal laser scanning microscopy in the fibronecion assay, Liying Hao for assisting with CSC38/tipless probes availability and atomic force microscope scanning. We also thank Xinxuan Zhou for facilitating the obtain of S. aureus and Wei Qiu and Suping Wang for assisting with images acquisition of confocal laser scanning microscopy.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the National Key Research and Development Program of China (http://www.nsfc.gov.cn/) 2016YFC1102700 to X.Z.; National Natural Science Foundation of China grant (http://www.nsfc.gov.cn/) 81372889 to L.C., 81600858 to B.R., 81372890 to M.F. and 81430011 to X.Z.; and Open Fund of State Key Laboratory of Oral Diseases (http://www.sklod.org/CN/index_cn.html) 2040305193002; Recruitment Program for Young Professionals to M.F.. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Walia IS, Borle RM, Mehendiratta D, Yadav AO. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J Maxillofac Oral Surg. 2014;13(1):16–21. doi: 10.1007/s12663-012-0455-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohara-Nemoto Y, Haraga H, Kimura S, Nemoto T. Occurrence of staphylococci in the oral cavities of healthy adults and nasal–oral trafficking of the bacteria. J Med Microbiol. 2008;57(1):95–9. [DOI] [PubMed] [Google Scholar]
- 3.McCormack M, Smith A, Akram A, Jackson M, Robertson D, Edwards G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am J Infect Control. 2015;43(1):35–7. doi: 10.1016/j.ajic.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 4.Zaleckas L, Rasteniene R, Rimkuviene J, Seselgyte R. Retrospective analysis of cellulitis of the floor of the mouth. Stomatologija. 2010;12(1):23–7. [PubMed] [Google Scholar]
- 5.Green A, Flower E, New N. case report: Mortality associated with odontogenic infection! Br Dent J. 2001;190(10):529–30. doi: 10.1038/sj.bdj.4801024a [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 7.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JY, Pillinger MH, Abramson SB. Prostaglandin E 2 synthesis and secretion: the role of PGE 2 synthases. Clin Immunol. 2006;119(3):229–40. doi: 10.1016/j.clim.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 9.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12(12):1063–73. [PubMed] [Google Scholar]
- 10.Griffin ÉW, Skelly DT, Murray CL, Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33(38):15248–58. doi: 10.1523/JNEUROSCI.6361-11.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menter DG, Schilsky RL, DuBois RN. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clin Cancer Res. 2010;16(5):1384–90. doi: 10.1158/1078-0432.CCR-09-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekharan N, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–31. doi: 10.1073/pnas.162468699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sierra JC, Hobbs S, Chaturvedi R, Yan F, Wilson KT, Peek RM, et al. Induction of COX-2 expression by Helicobacter pylori is mediated by activation of epidermal growth factor receptor in gastric epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;305(2):G196–G203. doi: 10.1152/ajpgi.00495.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallo O, Masini E, Bianchi B, Bruschini L, Paglierani M, Franchi A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum Pathol. 2002;33(7):708–14. [DOI] [PubMed] [Google Scholar]
- 16.Moraitis D, Du B, De Lorenzo MS, Boyle JO, Weksler BB, Cohen EG, et al. Levels of cyclooxygenase-2 are increased in the oral mucosa of smokers: evidence for the role of epidermal growth factor receptor and its ligands. Cancer Res. 2005;65(2):664–70. [PubMed] [Google Scholar]
- 17.Pérez-Novo CA, Waeytens A, Claeys C, Van Cauwenberge P, Bachert C. Staphylococcus aureus enterotoxin B regulates prostaglandin E2 synthesis, growth, and migration in nasal tissue fibroblasts. J Infect Dis. 2008;197(7):1036–43. doi: 10.1086/528989 [DOI] [PubMed] [Google Scholar]
- 18.Somayaji SN, Ritchie S, Sahraei M, Marriott I, Hudson MC. Staphylococcus aureus induces expression of receptor activator of NF-κB ligand and prostaglandin E2 in infected murine osteoblasts. Infect Immun. 2008;76(11):5120–6. doi: 10.1128/IAI.00228-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phipps RP, Stein SH, Roper RL. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991;12(10):349–52. doi: 10.1016/0167-5699(91)90064-Z [DOI] [PubMed] [Google Scholar]
- 20.Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188(1):21–8. doi: 10.4049/jimmunol.1101029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi M, Rosenberg DW, editors. Multifaceted roles of PGE2 in inflammation and cancer Semin Immunopathol; 2013. 21: Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama T, Mutsuga N, Yao L, Tosato G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J Leukoc Biol. 2006;79(1):95–104. doi: 10.1189/jlb.0405226 [DOI] [PubMed] [Google Scholar]
- 23.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23(3):144–50. [DOI] [PubMed] [Google Scholar]
- 24.Linnemeyer P, Pollack S. Prostaglandin E2-induced changes in the phenotype, morphology, and lytic activity of IL-2-activated natural killer cells. J Immunol. 1993;150(9):3747–54. [PubMed] [Google Scholar]
- 25.Sreeramkumar V, Fresno M, Cuesta N. Prostaglandin E2 and T cells: friends or foes&quest. Med Humanit. 2012;90(6):579–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agard M, Asakrah S, Morici LA. PGE2 suppression of innate immunity during mucosal bacterial infection. Front Cell Infect Microbiol. 2013;3:45 doi: 10.3389/fcimb.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118(20):5498–505. doi: 10.1182/blood-2011-07-365825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE2-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41(6–7):635–57. doi: 10.3109/08820139.2012.695417 [DOI] [PubMed] [Google Scholar]
- 29.Goto T, Herberman R, Maluish A, Strong D. Cyclic AMP as a mediator of prostaglandin E-induced suppression of human natural killer cell activity. J Immunol. 1983;130(3):1350–5. [PubMed] [Google Scholar]
- 30.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72(6):1407–15. doi: 10.1158/0008-5472.CAN-11-2544 [DOI] [PubMed] [Google Scholar]
- 31.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173(1):559–65. [DOI] [PubMed] [Google Scholar]
- 32.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37(5):562–70. doi: 10.1165/rcmb.2007-0153OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29(6):781–8. doi: 10.1038/onc.2009.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun. 2001;69(5):2957–63. doi: 10.1128/IAI.69.5.2957-2963.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalo-Klein A, Witkin SS. Prostaglandin E2 enhances and gamma interferon inhibits germ tube formation in Candida albicans. Infect Immun. 1990;58(1):260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krause J, Geginat G, Tammer I. Prostaglandin E 2 from Candida albicans stimulates the growth of Staphylococcus aureus in mixed biofilms. PloS one. 2015;10(8):e0135404 doi: 10.1371/journal.pone.0135404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussain M, Haggar A, Peters G, Chhatwal GS, Herrmann M, Flock J-I, et al. More than one tandem repeat domain of the extracellular adherence protein of Staphylococcus aureus is required for aggregation, adherence, and host cell invasion but not for leukocyte activation. Infect Immun. 2008;76(12):5615–23. doi: 10.1128/IAI.00480-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mongodin E, Bajolet O, Cutrona J, Bonnet N, Dupuit F, Puchelle E, et al. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect Immun. 2002;70(2):620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster T. The remarkably multifunctional fibronectin binding proteins of Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 2016:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Greene C, McDevitt D, Francois P, Vaudaux P, Lew DP, Poster T. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin‐binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17(6):1143–52. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen V, Huggins RH, Lertsburapa T, Bauer K, Rademaker A, Gerami P, et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59(6):949–52. doi: 10.1016/j.jaad.2008.08.030 [DOI] [PubMed] [Google Scholar]
- 43.Ouellet M, Percival MD. Effect of inhibitor time-dependency on selectivity towards cyclooxygenase isoforms. Biochem J. 1995;306 (Pt1):247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu YM, Azahri NS, Yu DC, Woll PJ. Effects of COX-2 inhibition on expression of vascular endothelial growth factor and interleukin-8 in lung cancer cells. BMC Cancer. 2008:8:218 doi: 10.1186/1471-2407-8-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi M, Yamamoto D, Ogawa S, Otoi T, Ohtani M, Miyamoto A. Messenger RNA expression of angiotensin-converting enzyme, endothelin, cyclooxygenase-2 and prostaglandin synthases in bovine placentomes during gestation and the postpartum period. Vet J. 2008;177(3):398–404. doi: 10.1016/j.tvjl.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 46.Zbinden C, Stephan R, Johler S, Borel N, Bünter J, Bruckmaier RM, et al. The inflammatory response of primary bovine mammary epithelial cells to Staphylococcus aureus strains is linked to the bacterial phenotype. PloS one. 2014;9(1):e87374 doi: 10.1371/journal.pone.0087374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JH, Perry CJ, Tsui Y-C, Staron MM, Parish IA, Dominguez CX, et al. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat Med. 2015;21(4):327–34. doi: 10.1038/nm.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peeters E, Nelis HJ, Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72(2):157–65. doi: 10.1016/j.mimet.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 49.Peacock S, Day N, Thomas M, Berendt A, Foster T. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J Infect. 2000;41(1):23–31. doi: 10.1053/jinf.2000.0657 [DOI] [PubMed] [Google Scholar]
- 50.Ridley RA, Douglas I, Whawell SA. Differential adhesion and invasion by Staphylococcus aureus of epithelial cells derived from different anatomical sites. J Med Microbiol. 2012;61(12):1654–61. [DOI] [PubMed] [Google Scholar]
- 51.Wang C, Zhao Y, Zheng S, Xue J, Zhou J, Tang Y, et al. Effect of enamel morphology on nanoscale adhesion forces of streptococcal bacteria: an AFM study. Scanning. 2015;37(5):313–21. doi: 10.1002/sca.21218 [DOI] [PubMed] [Google Scholar]
- 52.Mei L, Ren Y, Busscher H, Chen Y, Van der Mei H. Poisson analysis of streptococcal bond-strengthening on saliva-coated enamel. J Dent Res. 2009;88(9):841–5. doi: 10.1177/0022034509342523 [DOI] [PubMed] [Google Scholar]
- 53.Agerer F, Michel A, Ohlsen K, Hauck CR. Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J Biol Chem. 2003;278(43):42524–31. doi: 10.1074/jbc.M302096200 [DOI] [PubMed] [Google Scholar]
- 54.Chan G, Boyle JO, Yang EK, Zhang F, Sacks PG, Shah JP, et al. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999;59(5):991–4. [PubMed] [Google Scholar]
- 55.Gallo O, Franchi A, Magnelli L, Sardi I, Vannacci A, Boddit V, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia. 2001;3(1):53–61. doi: 10.1038/sj/neo/7900127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005;18(1):153–60. doi: 10.1038/modpathol.3800244 [DOI] [PubMed] [Google Scholar]
- 57.Futaki N, Takahashi S, Yokoyama M, Arai I, Higuchi S, Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47(1):55–9. [DOI] [PubMed] [Google Scholar]
- 58.Kim J, Shim M. COX-2 inhibitor NS-398 suppresses doxorubicin-induced p53 accumulation through inhibition of ROS-mediated Jnk activation. Mol Carcinog. 2016. [DOI] [PubMed] [Google Scholar]
- 59.Altrock E, Vasel M, Kawelke N, Dooley S, Sottile J, Nakchbandi I. Inflammatory cell infiltration in experimental liver fibrosis is affected by inhibition of fibronectin matrix assembly. Zeitschrift für Gastroenterologie. 2015;53(01):A1_29. [Google Scholar]
- 60.HOOk M, Switalski L, Wadstrom T, Lindberg M. Interactions of pathogenic microorganisms with fibronectin. Fibronectin. 1989;1:295–308. [Google Scholar]
- 61.McCrae M. Molecular Aspects of Host-Pathogen Interactions: Cambridge University Press; 1997. 50. [Google Scholar]
- 62.Mirvish ED, Pomerantz RG, Geskin LJ. Infectious agents in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;64(2):423–31. doi: 10.1016/j.jaad.2009.11.692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paupière S, Millot F, Grados F, Fardellone P. Primary lymphoma of bone infected with Staphylococcus aureus. Joint Bone Spine. 2013;6(80):669–70. [DOI] [PubMed] [Google Scholar]
- 64.Kullander J, Forslund O, Dillner J. Staphylococcus aureus and squamous cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2009;18(2):472–8. doi: 10.1158/1055-9965.EPI-08-0905 [DOI] [PubMed] [Google Scholar]
- 65.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30(3):377–86. doi: 10.1093/carcin/bgp014 [DOI] [PubMed] [Google Scholar]
- 66.Kopp KLM, Kauczok CS, Lauenborg B, Krejsgaard T, Eriksen KW, Zhang Q, et al. COX-2-dependent PGE2 acts as a growth factor in mycosis fungoides (MF). Leukemia. 2010;24(6):1179–85. doi: 10.1038/leu.2010.66 [DOI] [PubMed] [Google Scholar]
- 67.Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159(1):105–12. doi: 10.1111/j.1365-2133.2008.08612.x [DOI] [PubMed] [Google Scholar]
- 68.Dziewanowska K, Carson AR, Patti JM, Deobald CF, Bayles KW, Bohach GA. Staphylococcal fibronectin binding protein interacts with heat shock protein 60 and integrins: role in internalization by epithelial cells. Infect Immun. 2000;68(11):6321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kintarak S, Whawell SA, Speight PM, Packer S, Nair SP. Internalization of Staphylococcus aureus by human keratinocytes. Infect Immun. 2004;72(10):5668–75. doi: 10.1128/IAI.72.10.5668-5675.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van de Water L, Destree A, Hynes R. Fibronectin binds to some bacteria but does not promote their uptake by phagocytic cells. Science. 1983;220(4593):201–4. [DOI] [PubMed] [Google Scholar]
- 71.Verbrugh HA, Peterson P, Smith D, Nguyen B, Hoidal JR, Wilkinson B, et al. Human fibronectin binding to staphylococcal surface protein and its relative inefficiency in promoting phagocytosis by human polymorphonuclear leukocytes, monocytes, and alveolar macrophages. Infect Immun. 1981;33(3):811–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hara S, Kamei D, Sasaki Y, Tanemoto A, Nakatani Y, Murakami M. Prostaglandin E synthases: understanding their pathophysiological roles through mouse genetic models. Biochimie. 2010;92(6):651–9. doi: 10.1016/j.biochi.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 73.Sakata D, Yao C, Narumiya S. Prostaglandin E2, an immunoactivator. J Pharmacol Sci. 2010;112(1):1–5. [DOI] [PubMed] [Google Scholar]
- 74.Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99(9):2254 doi: 10.1172/JCI119400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ondrey FG, Juhn SK, Adams GL. Inhibition of head and neck tumor cell growth with arachidonic acid metabolism inhibition. Laryngoscope. 1996;106(2):129–34. [DOI] [PubMed] [Google Scholar]
- 76.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9(5):259–67. doi: 10.1038/nrclinonc.2011.199 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Before incubated in DMEM, the initial number of S. aureus [S. aureus (before)] and S. aureus with PGE2 treated [S. aureus +PGE2 (before)] used for adhesion and invasion assay were approximately 2.63×107 CUFs /mL and 2.57×107 CUFs/mL. After treated in DMEM for 45 min, the numbers for them were 2.38×107 CUFs/mL [S. aureus (after)] and 2.25×107 CUFs/mL [S. aureus + PGE2 (after)] respectively. There was no significantly difference between all the groups.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.