Abstract
Opisthorchis viverrini is endemic in the South East Asian region, especially in Cambodia, Lao People's Democratic Republic, Vietnam and Thailand, but there have been no previous records from Myanmar. During stool surveys of rural populations in three regions of Lower Myanmar, Opisthorchis-like eggs were found in 34 out of 364 (9.3%) participants by stool microscopy after using the modified formalin-ether concentration technique. DNA was extracted from these positive stool samples and a portion of the mitochondrial cytochrome c oxidase subunit I (cox1) gene was amplified using the polymerase chain reaction and then sequenced. DNA sequences, successfully obtained from 18 of 34 positive samples (Bago Region, n = 13; Mon State, n = 3; Yangon Region, n = 2), confirmed that the eggs were of O. viverrini. Sequences showed 99.7% identity with O. viverrini mitochondrial cox1 (GenBank accession no. JF739555) but 95%, 88.7%, 82.6% and 81.4% identities with those of Opisthorchis lobatus from Lao People's Democratic Republic (GenBank accession nos. HQ328539-HQ328541), Metorchis orientalis from China (KT239342), Clonorchis sinensis from China (JF729303) and Opisthorchis felineus from Russia (EU921260), respectively. When alignement with other Opisthorchiidae trematodes, 81% similarity with Metorchis bilis from Czech Republic (GenBank accession nos. KT740966, KT740969, KT740970) and Slovakia (GenBank accession nos. KT740971-KT740973), 84.6% similarity with Metorchis xanthosomus from Czech Republic (GenBank accession no. KT740974), 78.6% similarity with M. xanthosomus from Poland (GenBank accession no. KT740968) and 82.2% similarity with Euamphimerus pancreaticus from Czech Republic (GenBank accession no. KT740975) were revealed. This study demonstrated, for the first time, O. viverrini from rural people in Myanmar using molecular methods and is an urgent call for surveillance and control activities against opisthorchiasis in Myanmar.
Introduction
Opisthorchis viverrini, a fish-borne liver fluke, is one of the main public health problems in the South East Asian region, especially along the Mekong River (Cambodia, Lao People's Democratic Republic, Vietnam, and Thailand), where there is a culture of eating raw or undercooked fish that contain encysted metacercariae of the liver fluke [1–3]. Adult flukes are long-lived and feed on epithelial cells of the intrahepatic bile ducts. Although most cases are asymptomatic, complications can include hepatobiliary diseases such as hepatomegaly, cholangitis, cholecystitis, peri-ductal fibrosis and gallstones. Severe chronic infection is a strong risk factor for cholangiocarcinoma [4–6]. More than ten million people are estimated to be infected with O. viverrini: about eight million in Thailand and two million in Lao People's Democratic Republic [7]. The highest prevalences occur in North and Northeast Thailand, especially in rural populations [2,8–9] and in the adjacent southern and central regions of Lao People's Democratic Republic [10–11]. Human infections with O. viverrini and presence of metacercariae in intermediate hosts have been reported in several provinces of Cambodia [12–15]. Miyamoto et al. [13] reported O. viverrini eggs in human fecal samples from 26 out of 55 surveyed villages in five provinces of Cambodia, among which 15 villages had an egg positive rate >10%. Polymerase chain reaction (PCR) amplification and DNA sequencing confirmed the presence of O. viverrini in 30 samples from 5 villages. The parasite is also endemic in southern and central parts of Vietnam [16–17]. A survey conducted in 2015 reported that the overall prevalence of O. viverrini infection was 11.4% in central Vietnam [16]. Although not reported to date from Myanmar, the presence of O. viverrini is likely because of its close proximity to endemic areas in Thailand and because of an open-borders policy that started in 2015 leading to increasing migration among ASEAN Economic Community (AEC) countries (Thailand, Lao People's Democratic Republic, Cambodia, Vietnam and Myanmar) [18].
Opisthorchiasis is usually diagnosed by microscopic examination of eggs in feces, bile or duodenal fluid. However, it is difficult to differentiate opisthorchiid eggs morphologically from those of other trematodes such as lecithodendriids and heterophyids, prompting the development of molecular approaches for this purpose [19–20]. A lack of awareness of potential O. viverrini infection in Myanmar will hinder prevention and control programs against this fluke. This study aims to identify and confirm the presence of O. viverrini in rural parts of Myanmar using conventional and molecular methods. Thus, the results of this study will be helpful to health authorities and researchers in Myanmar for diagnosis of opisthorchiasis and for planning and implementing control strategies.
Materials and methods
Study design and population
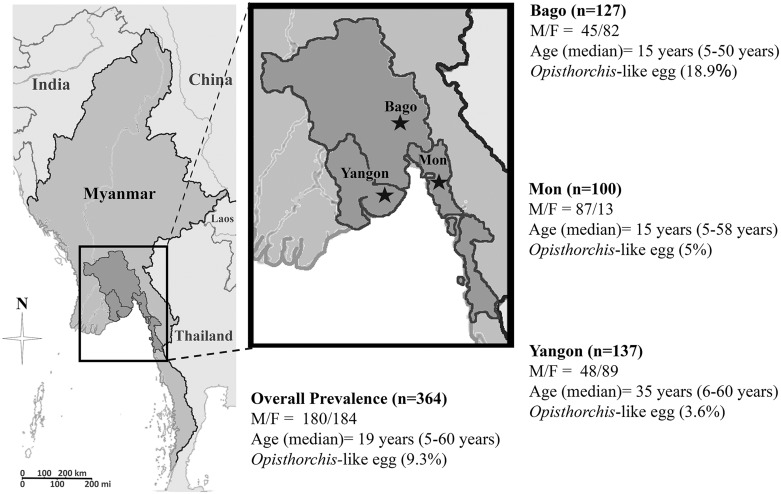
Stool surveys for intestinal parasites in rural populations in three regions of Lower Myanmar (Fig 1) were conducted between June 2015 and March 2016. Sample-size calculation was performed using simple random sampling based on preliminary prevalence data for soil-transmitted helminthiasis [21]. This study was approved by the Research and Ethical Committee of the University of Medicine 1, Yangon, Myanmar (5695/ Research and Ethics 2015) and Khon Kaen University Ethics Committee for Human Research (HE581396). Stool samples were collected from 5 years old up rural people (select the age group has occasion to contact with the soil) including school children, housewife, farmers, plantation workers and gold miners; and excluded the persons who received anthelmintic treatment within 6 months prior to study and persons presenting with diarrhoea at the time of sampling.
Fig 1. Map of the survey areas in Myanmar.
Prevalences (in parentheses) of Opisthorchis-like eggs in Bago, Mon and Yangon Regions in the years 2015–2016. M = Male; F = Female. The Myanmar map was modified from a map in The World Factbook, published by the Central Intelligence Agency [22].
Each participant was informed of study procedures, risks and benefits of the process. Before enrolment, written consent was obtained from all adult participants and from parents or legal guardians of minors.
Stool examination and parasite collection
Stool examination was done using the modified formalin-ether concentration technique at National Health Laboratory, Yangon, Myanmar [23]. Geometric Mean fecal egg counts, expressed as eggs per gram (epg) of feces, were used to indicate intensity of helminth infection. Given the difficulty of distinguishing eggs of many trematode species, such eggs were initially recorded as being Opisthorchis-like (Fig 2A). Samples containing these eggs were preserved in 70% alcohol in a 15 ml centrifuge tubes and shipped to Khon Kaen University for molecular identification.
Fig 2. Showing O. viverrini egg, fish dish and cyprinoid fish.
A) Opisthorchis viverrini egg from stool microscopy. B) The undercooked or fermented fish dish (small raw fish fermented with cooked rice and salt) named “Ngar Lay Chin”. C) Cyprinoid fish (Henicorhynchus sp.) “Nga Khone Ma” (intermediate host of O. viverrini).
DNA extraction, amplification and sequencing
DNA was extracted from the stool samples positive for Opisthorchis-like eggs using a QIAamp® DNA stool mini kit (Qiagen, Hilden, Germany), according to the manufacturer’s instructions. The DNA was eluted in 50 μl of elution buffer, 5 μl of which was used for the PCR reaction. New PCR primers were designed to bind to a conserved portion of the mitochondrial cox1 gene of O. viverrini (GenBank accession number JF739555): COIP4bF (5´- GGGACCCGATTATGTGGTGG- 3´) as a forward primer and OvCOIP4bR (5´- CCCACTGAAAACAAGGCTCG-3´) as a reverse primer, producing an amplicon of about 348 base pairs.
The PCR products were amplified in a GeneAmp PCR system 9700 thermal cycler (Applied Biosystems, Singapore) using the following PCR conditions: initial denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 62°C for 30 seconds and 72°C for 30 seconds, followed by a post-amplification extension for 10 min at 72°C. The PCR product was visualized in a 1% agarose gel to confirm and estimate amplified product length and quantity. DNA sequencing was done in both directions, using the PCR primers as sequencing primers, with an Applied Biosystems 3730 +I DNA Analyzer and an ABI Big Dye Version 3.1 cycle sequencing kit (Foster City, CA). The nucleotide sequences were compared with sequence data for O. viverrini, Clonorchis sinensis, Opisthorchis felineus, and Metorchis orientalis in GenBank by BLAST-N search via NCBI. DNA sequences were aligned using ClustalW. All sequences have been submitted to the GenBank database (GenBank accession nos. KX922693-KX922699).
Statistical analysis
Data were analyzed using SPSS version 22 (SPSS Inc., Chicago, IL) and expressed as numbers and as percentages. Chi-square test was applied to investigate the correlation between parasite infection status and sex. A p-value of less than 0.05 was regarded as statistically significant. For each population, the geometric means and ranges of eggs per gram of faeces were used to express the intensity of infection.
Results
Prevalence of Opisthorchis-like eggs in the study regions by microscopic examination of stool samples
A total of 364 study participants submitted their stool sample for analysis. Of these, 180 (49.5%) were males with median age of 19 years (ranged 5 to 60 years). One-hundred participants were from Mon state, 127 from Bago region and 137 from Yangon (Fig 1). The overall prevalence of Opisthorchis-like eggs was 9.3% with the highest positive rate in the Bago Region (18.9%), followed by Mon State (5%) and Yangon Region (3.6%) (Fig 1). Infection rates did not differ significantly between males and females in any study area. However, a trend towards a higher rate in females (odds ratio 0.53, 95% CI 0.25–1.1). Prevalences differed according to age, with a non-significant trend towards higher prevalences in older people in each study area (Table 1). Fecal counts for Opisthorchis-like eggs ranged from 10 to 150 epg (Geometric Mean = 22.63 epg).
Table 1. Prevalence and intensity of Opisthorchis-like eggs by sex and age group in the three study areas.
| Address | ||||
|---|---|---|---|---|
| Bago n (%) |
Mon n (%) |
Yangon n (%) |
Overall n (%) |
|
| Egg positive cases | 24/127 (18.9) | 5/100 (5) | 5/137 (3.6) | 34/364 (9.3) |
| Sex Male | 7/45 (15.6) | 3/87 (3.4) | 2/48 (4.2) | 12/180 (6.7) |
| Female | 17/82 (20.7) | 2/13 (15.4) | 3/89 (3.4) | 22/184 (12) |
| p-value* | 0.476 | 0.125 | 1.000 | 0.083 |
| Age group | ||||
| 5–11 Years | 5/42 (11.9) | 0/15 (0) | 0/9 (0) | 5/66 (7.5) |
| 12–19 Years | 5/27 (18.5) | 2/70 (2.9) | 1/21 (4.8) | 8/118 (6.8) |
| 20–40 Years | 7/34 (20.6) | 1/5 (20.0) | 3/59 (5.1) | 11/98 (11.2) |
| >41 Years | 7/24 (29.2) | 2/10 (20.0) | 1/48 (2.1) | 10/82 (12.2) |
|
Intensity (Geometric mean, range of eggs per gram) |
19.91, 10–90 | 32.72, 10–150 | 28.95, 10–44 | 22.63, 10–150 |
* p- value less than 0.05 was considered statistically significant
n = number of positive cases/number of total participants
Molecular identification of O. viverrini
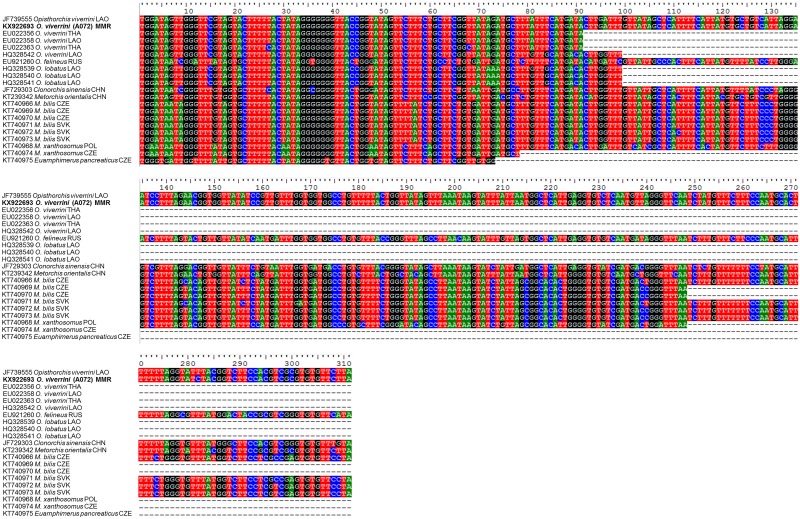
DNA from stool samples that were positive for Opisthorchis-like eggs (n = 34) was used for molecular identification using primers specific for the mitochondrial cox1 gene. DNA sequences, successfully obtained from 18 of 34 positive samples (Bago Region, n = 13; Mon State, n = 3; Yangon Region, n = 2), confirmed that the eggs were of O. viverrini. All partial mitochondrial cox1 gene sequences obtained shared 99.7% similarity with that of O. viverrini (complete mitochondrial genome—GenBank accession no. JF739555). There were also high similarities with O. viverrini cox1 sequences from Lao People's Democratic Republic (96% similarity, coverage 31.8%, GenBank accession no. HQ328542 and 100% similarity, coverage 29.3%, GenBank accession no. EU022358) and Thailand (100% similarity, coverage 29.3%, GenBank accession no. EU022356 and 97.8% similarity, coverage 29.3%, GenBank accession no. EU022363) (Fig 3) Comparisons were also made with other species of Opisthorchiidae trematodes. Our sequences from Myanmar had only 95% similarity with Opisthorchis lobatus from Lao People's Democratic Republic (GenBank accession nos. HQ328539-HQ328541), 82.6% similarity with C. sinensis from China (GenBank accession no. JF729303), 81.4% similarity with O. felineus from Russia (GenBank accession no. EU921260), and 88.7% similarity with M. orientalis from China (GenBank accession no. KT239342), 81% similarity with Metorchis bilis from Czech Republic (GenBank accession nos. KT740966, KT740969, KT740970) and Slovakia (GenBank accession nos. KT740971-KT740973), 84.6% similarity with Metorchis xanthosomus from Czech Republic (GenBank accession no. KT740974), 78.6% similarity with M. xanthosomus from Poland (GenBank accession no. KT740968) and 82.2% similarity with Euamphimerus pancreaticus from Czech Republic (GenBank accession no. KT740975).
Fig 3. Alignment of O. viverrini cox1 nucleotide sequences.
The multiple sequences alignment of representative O. viverrini partial mitochondrial cytochrome c oxidase subunit I (cox1) sequence from Bago Region of Lower Myanmar (KX922693; bold letters) and other Opisthorchiidae trematodes. A, T, G, C nucleotides represent in green, red, black and blue, respectively. Incomplete sequences are represented by dashed lines (-). ISO 3166 Countries Codes are presented (THA, Thailand; LAO, Lao People's Democratic Republic, MMR; Myanmar, VNM; Vietnam, RUS; Russian Federation, CHN; China, CZE; Czech Republic, SVK; Slovakia and POL; Poland).
Discussion
Since the eggs of O. viverrini are difficult to discriminate morphologically from those of small intestinal flukes, PCR-based amplification and sequencing of a partial fragment of the mitochondrial cox1 gene have been used for differentiation of Opisthorchis-like eggs in previous studies [24–25]. The present study demonstrated for the first time the presence of O. viverrini from fecal samples of rural people in Myanmar by this molecular method. PCR products were only obtained from 18 of 34 fecal samples in which Opisthorchis-like eggs were found. There are several explanations for this. The number of eggs (and hence DNA yield) might have been too low in some samples for amplification to occur; the eggs observed might have been shed by members of other trematode families such as Heterophyidae and Lecithodendriidae; inhibitors in some fecal samples might have blocked amplification.
Opisthorchiasis is endemic in neighboring countries throughout the Mekong Basin, including Thailand, Lao People's Democratic Republic, Cambodia and Vietnam [9] but was not previously known from Myanmar. Using molecular methods, we confirmed opisthorchiasis in 18 individuals (4.9%) in rural populations in our study areas. This is considered a low prevalence [9], but our survey was limited and must be regarded as preliminary. In agreement with studies done in Lao People's Democratic Republic and Cambodia, our study found that prevalence did not significantly differ between the sexes but did tend to increase with age [13, 26]. Opisthorchis infection is highly localized, depending on the food habits of local people and the presence of susceptible snail hosts [2]. Prevalence of O. viverrini was much higher in the Bago Region than elsewhere, possibly owing to the local habit of eating raw or semi-cooked or fermented fish dishes (named “Ngar Lay Chin”) in that region (Fig 2B and 2C). The eating of raw or undercooked fish is a known risk factor for opisthorchiasis [26]. Infection can also occur via the contamination of food, hands, surfaces and food preparation utensils with the infective stage of the parasite (i.e., metacercariae) [26]. A high prevalence of O. viverrini infection is strongly positively correlated with the incidence of cholangiocarcinoma in Lao People's Democratic Republic and endemic areas of Thailand [26]. Although human opisthorchiasis control programs have been conducted in endemic areas in the Mekong Basin for a long time, the infection still prominent in many areas [9]. Although opisthorchiasis caused by O. viverrini is common in South East Asian region especially in greater Mekong sub-region countries, we should be aware of possibility of minor agents of Opisthorchiidae trematodes such as O. lobatus, O. felineus, Opisthorchis noverca, M. orientalis, M. bilis, M. xanthosomus, and E. pancreaticus since they are closely related and cannot be easily differentiated with molecular method [27–30]. Understanding of the biology, distribution, transmission and factors influencing O. viverrini infection is required to help establish sustainable human liver fluke prevention and control programs.
Conclusion
In conclusion, we have reported for the first time the presence of O. viverrini in rural people from Myanmar. Identity of the parasite was confirmed by molecular methods. Since there is no accurate diagnosis and reporting of O. viverrini infections in Myanmar, hospital-based studies and larger-scale nation-wide epidemiological studies should be conducted to assess the public health impact. In addition, the roles of intermediate hosts (snails and fish) and definitive hosts (dogs and cats) in transmission of this food-borne trematode infection in Myanmar should be investigated. Combating this disease of poor sanitation should be the focus of a jointly arranged action from government and community sectors.
Acknowledgments
We would like to thank Dr. David Blair through Khon Kaen University Publication Clinic for valuable suggestions and assistance with the presentation of this paper. We also thank the anonymous reviewers for the useful comments.
Data Availability
All relevant data are within the paper. All sequences have been submitted to the GenBank database (GenBank accession no. KX922693-KX922699).
Funding Statement
This study was supported by a TRF Senior Research Scholar Grant, Thailand Research Fund grant number RTA5880001; the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Thailand, through the Health Cluster (SHeP-GMS); the Faculty of Medicine, Khon Kaen University (TR57201); Research and Diagnostic Center for Emerging Infectious Disease, Mekong Health Science Institue, Khon Kaen University through Wanchai Maleewong and Pewpan M. Intapan. Win Pa Pa Aung was partial supported by the Faculty of Medicine, Khon Kaen University (IN59211). Oranuch Sanpool was supported by the Post-Doctoral Training Program from Research Affairs and Graduate School, Khon Kaen University (grant no. 58101). We also certified that all the funders and sources of support received during this specific study (whether external or internal to our organizations) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hung NM, Madsen H, Fried B. Global status of fish-borne zoonotic trematodiasis in humans. Acta Parasitol. 2013; 58: 231–258. 10.2478/s11686-013-0155-5 [DOI] [PubMed] [Google Scholar]
- 2.Sripa B, Bethony JM, Sithithaworn P, Kaewkes S, Mairiang E, Loukas A, et al. Opisthorchiasis and opisthorchis-associated cholangiocarcinoma in Thailand and Laos. Acta Trop. 2011; 120(Suppl 1): S158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008; 24: 349–356. 10.1097/MOG.0b013e3282fbf9b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honjo S, Srivatanakul P, Sriplung H, Kikukawa H, Hanai S, Uchida K, et al. Genetic and environmental determinants of risk for cholangiocarcinoma via Opisthorchis viverrini in a densely infested area in Nakhon Phanom, northeast Thailand. Int J Cancer. 2005;117: 854–860. 10.1002/ijc.21146 [DOI] [PubMed] [Google Scholar]
- 5.IARC. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr Eval Carcinog Risks Hum. 1994; 61: 121–175. [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng S, Zhu Y, Zhao Z, Okanurak K, Lv Z. Liver fluke infection and cholangiocarcinoma: a review. Parasitol Res. 2017; 116: 11–19. 10.1007/s00436-016-5276-y [DOI] [PubMed] [Google Scholar]
- 7.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010; 72: 305–350. 10.1016/S0065-308X(10)72011-X [DOI] [PubMed] [Google Scholar]
- 8.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003; 88: 229–232. [DOI] [PubMed] [Google Scholar]
- 9.Sithithaworn P, Andrews RH, Nguyen VD, Wongsaroj T, Sinuon M, Odermatt P, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol Int. 2012; 61: 10–16. 10.1016/j.parint.2011.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forrer A, Sayasone S, Vounatsou P, Vonghachack Y, Bouakhasith D, Vogt S, et al. Spatial distribution of, and risk factors for, Opisthorchis viverrini infection in southern Lao PDR. PLoS Negl Trop Dis. 2012; 6: e1481 10.1371/journal.pntd.0001481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, et al. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009; 103: 247–254. 10.1016/j.trstmh.2008.10.011 [DOI] [PubMed] [Google Scholar]
- 12.Chai JY, Sohn WM, Na BK, Yong TS, Eom KS, Yoon CH, et al. Zoonotic trematode metacercariae in fish from Phnom Penh and Pursat, Cambodia. Korean J Parasitol. 2014; 52: 35–40. 10.3347/kjp.2014.52.1.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto K, Kirinoki M, Matsuda H, Hayashi N, Chigusa Y, Sinuon M, et al. Field survey focused on Opisthorchis viverrini infection in five provinces of Cambodia. Parasitol Int. 2014; 63: 366–373. 10.1016/j.parint.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Sohn W, Yong TS, Eom KS, Pyo KH, Lee MY, Lim H, et al. Prevalence of Opisthorchis viverrini infection in humans and fish in Kratie Province, Cambodia. Acta Trop. 2012; 124: 215–220. 10.1016/j.actatropica.2012.08.011 [DOI] [PubMed] [Google Scholar]
- 15.Yong TS, Chai JY, Sohn WM, Eom KS, Jeoung HG, Hoang EH, et al. Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011). Korean J Parasitol. 2014; 52: 661–666. 10.3347/kjp.2014.52.6.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dao TTH, Bui TV, Abatih EN, Gabriël S, Nguyen TT, Huynh QH, et al. Opisthorchis viverrini infections and associated risk factors in a lowland area of Binh Dinh Province, Central Vietnam. Acta Trop. 2016; 157: 151–157. 10.1016/j.actatropica.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 17.Dung VT, Waikagul J, Thanh BN, Vo DT, Nguyen DN, Murrell K. Endemicity of Opisthorchis viverrini liver flukes, Vietnam, 2011–2. Emerg Infect Dis. 2014; 20: 152–154. 10.3201/eid2001.130168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ASEAN Economic Community Blueprint. Declaration on the ASEAN Economic Community blueprint 2008. http://asean.org/wp-content/uploads/archive/5187-10.pdf Accessed 4Mar 2017
- 19.Le T, VanDe N, Blair D, Sithithaworn P, McManus DP. Clonorchis sinensis and Opisthorchis viverrini: development of a mitochondrial-based multiplex PCR for their identification and discrimination. Exp Parasitol. 2006; 112: 109–14. 10.1016/j.exppara.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 20.Sanpool O, Intapan PM, Thanchomnang T, Janwan P, Lulitanond V, Doanh PN, et al. Rapid detection and differentiation of Clonorchis sinensis and Opisthorchis viverrini eggs in human fecal samples using a duplex real-time fluorescence resonance energy transfer PCR and melting curve analysis. Parasitol Res. 2012; 111: 89–96. 10.1007/s00436-011-2804-7 [DOI] [PubMed] [Google Scholar]
- 21.Tun A, Myat SM, Gabrielli AF, Montresor A. Control of soil-transmitted helminthiasis in Myanmar: results of 7 years of deworming. Trop Med Int Health. 2013; 18: 1017–1020. 10.1111/tmi.12130 [DOI] [PubMed] [Google Scholar]
- 22.Central Intelligence Agency [US]. The World Factbook. https://www.cia.gov/library/publications/the-world-factbook/geos/bm.html Accesed 4 Mar 2017
- 23.Anamnart W, Pattanawongsa A, Intapan PM, Maleewong W. Factors affecting recovery of Strongyloides stercoralis larvae: an approach to a newly modified formalin-ether concentration technique for diagnosis of strongyloidiasis. J Clin Microbiol. 2010; 48: 97–100. 10.1128/JCM.01613-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buathong S, Leelayoova S, Mungthin M, Naaglor T, Taamasri P, Suwannahitatorn P, et al. Development and evaluation of PCR methods based on cytochrome c oxidase subunit one (cox1) and NADH dehydrogenase subunit one gene (nad1) to detect Opisthorchis viverrini in human fecal samples. Parasitol Res. 2015; 114: 3547–3549. 10.1007/s00436-015-4640-7 [DOI] [PubMed] [Google Scholar]
- 25.Thaenkham U, Visetsuk K, Dung DT, Waikagul J. Discrimination of Opisthorchis viverrini from Haplorchis taichui using COI sequence marker. Acta Trop. 2007; 103: 26–32. [DOI] [PubMed] [Google Scholar]
- 26.Yoon HJ, Ki M, Eom K, Yong TS, Chai JY, Min DY, Rim HJ, Sohn WM, Insisiengmay B, Phommasack B. Risk factors for Opisthorchis viverrini and minute intestinal fluke infections in Lao PDR, 2009–2011. Am J Trop Med Hyg. 2014; 91: 384–388. 10.4269/ajtmh.13-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Na L, Gao JF, Liu GH, Fu X, Su X, Yue DM, et al. The complete mitochondrial genome of Metorchis orientalis (Trematoda: Opisthorchiidae): Comparison with other closely related species and phylogenetic implications. Infect Genet Evol. 2016; 39:45–50. 10.1016/j.meegid.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 28.Thaenkham U, Nuamtanong S, Vonghachack Y, Yoonuan T, Sanguankiat S, Dekumyoy P, et al. Discovery of Opisthorchis lobatus (Trematoda: Opisthorchiidae): a new record of small liver flukes in the Greater Mekong Sub-region. J Parasitol. 2011;97:1152–8. 10.1645/GE-2764.1 [DOI] [PubMed] [Google Scholar]
- 29.Sitko J, Bizos J, Sherrard-Smith E, Stanton DW, Komorová P, Heneberg P. Integrative taxonomy of European parasitic flatworms of the genus Metorchis Looss, 1899 (Trematoda: Opisthorchiidae). Parasitol Int. 2016;65:258–67. 10.1016/j.parint.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Sahu R, Biswal DK, Roy B, Tandon V. Molecular characterization of Opisthorchis noverca (Digenea: Opisthorchiidae) based on nuclear ribosomal ITS2 and mitochondrial COI genes. J Helminthol. 2016;90:607–14. 10.1017/S0022149X15000851 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. All sequences have been submitted to the GenBank database (GenBank accession no. KX922693-KX922699).