Abstract
The cerebellum plays an important role in motor control and is also involved in cognitive processes. Cerebellar function is specialized by location, although the exact topographic functional relationship is not fully understood. The spinocerebellar ataxias are a group of neurodegenerative diseases that cause regional atrophy in the cerebellum, yielding distinct motor and cognitive problems. The ability to study the region-specific atrophy patterns can provide insight into the problem of relating cerebellar function to location. In an effort to study these structural change patterns, we developed a toolbox in MATLAB to provide researchers a unique way to visually explore the correlation between cerebellar lobule shape changes and function loss, with a rich set of visualization and analysis modules. In this paper, we outline the functions and highlight the utility of the toolbox.
The toolbox takes as input landmark shape representations of subjects’ cerebellar substructures. A principal component analysis is used for dimension reduction. Following this, a linear discriminant analysis and a regression analysis can be performed to find the discriminant direction associated with a specific disease type, or the regression line of a specific functional measure can be generated. The characteristic structural change pattern of a disease type or of a functional score is visualized by sampling points on the discriminant or regression line. The sampled points are used to reconstruct synthetic cerebellar lobule shapes. We showed a few case studies highlighting the utility of the toolbox and we compare the analysis results with the literature.
Keywords: cerebellum, cerebellar ataxia, graphical user interface, visualization, structural change, shape analysis, principal component analysis, linear discriminant analysis, regression analysis
1. INTRODUCTION
The cerebellum plays an important role in motor control and is also involved in cognitive processes. Historically thought to be solely involved in motor control, its cognitive roles extend to language, spatial, and memory functions1. The cerebellum is divided into ten smaller regions called lobules, numbered from I to X and cere-bellar function is specialized by location1–3. However, the topographic functional relationship is not yet fully understood1, 4–6.
The spinocerebellar ataxias (SCAs) are a group of neurodegenerative diseases characterized by progressive atrophy of the cerebellum, brainstem, and cerebral cortex7. The different mutations of the disease produce regional cerebellar atrophy, yielding distinctive motor and cognitive problems7, 8. Studying the region-specific atrophy patterns can provide insight into the problem of relating cerebellar function to location. Magnetic resonance (MR) imaging can be used to study structural information of the cerebellum. Clinical evaluations assess function loss by evaluating motor and cognitive tasks performed by patients and assigning outcomes a functional score. Studying the correlation between structural change patterns and functional degeneration can help in understanding the regional function map of the cerebellum. This can also help with the evaluation of disease stage, in predicting function loss and in treatment planning.
In an effort to explore the structural and functional relationships of the cerebellum, we developed a toolbox to visually explore the correlation between cerebellar lobule shape changes and function loss, with a rich set of visualization and analysis modules. We take as input to the toolbox the cerebellar lobule landmark shape representations and clinical evaluation results of a set of subjects. We perform a principal component analysis (PCA) to reduce the dimension of the landmark representation of the cerebellar lobules. Following the PCA, a linear discriminant analysis or a regression analysis can be performed to find the discriminant direction associated with a specific disease type, or the regression line of a specific functional measure. The characteristic structural change pattern of a disease type or of a functional score is visualized by sampling points on the discriminant or regression line. The sampled points are used to reconstruct synthetic cerebellar lobule shapes.
2. METHOD
2.1 Toolbox Design
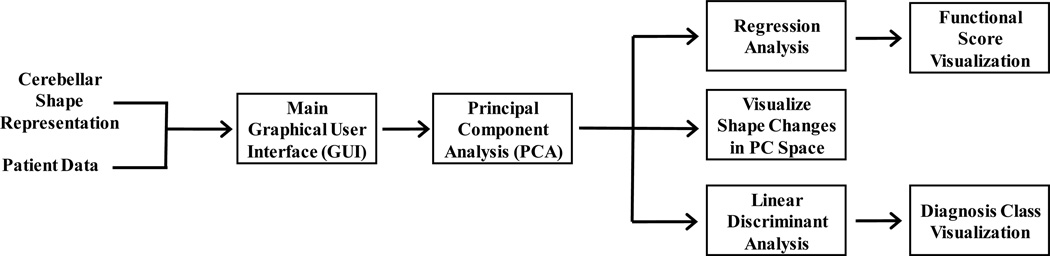
The toolbox was designed as a graphical user interface (GUI) using MathWorks® MATLAB. We used the GUIDE development tool on MATLAB version 2012b, and tested the toolbox on versions up to 2015a. The toolbox functionality is summarized in the figure below. The inputs are the cerebellar landmark shape representations of each subject and spreadsheets containing subject information such as age, diagnosis, and clinical evaluation scores. The main GUI panel is used to load the inputs, visualize the data, and to access all analysis and visualization blocks. A PCA is used for dimension reduction. Following the PCA, a linear discriminant analysis or a regression analysis can be performed to visualize the structural change patterns of a disease type or of functional loss in the cerebellum. The toolbox inputs and the analysis blocks shown on Figure 1 are described in detail in the sections below.
Figure 1.
Toolbox inputs and analysis blocks
2.2 Input Data
Cerebellar shape representation
the input structural information of a subject’s cerebellum is a highly informative shape representation, in particular, we use dense homologous landmarks distributed on the boundary surfaces of cerebellar sub-structures. The landmark shape representation has been widely used in studying shape variation of a population9–12. In our preprocessing to compute the input structural data, the cerebellum was segmented from the MR images and parcellated into 22 sub-structures, the cerebellar lobules13. We use the method proposed by Yang et al. (2015)14 to generate the landmark shape representation of all cerebellar lobules. Let be the ith landmark of the jth subject, and be the spatial coordinates of , then the landmark shape representation of the jth subject
| (1) |
where N is the number of landmarks, and in our case N ≈ 15000.
Patient data
the toolbox takes as input spreadsheets containing patient information such as age, gender, disease diagnosis, clinical evaluation scores, and lobule volumes. The toolbox is flexible and is able to read spreadsheets organized by the user; there are no restrictions on the data to be included in the spreadsheets. Disease diagnosis information enables the user to selectively look at one or multiple disease groups in further analysis. The toolbox displays the inputted information in a simple way, using the columns of the spreadsheets as variable names. This provides the flexibility to the user to upload a variety of numerical data for analysis.
2.3 Main GUI Panel
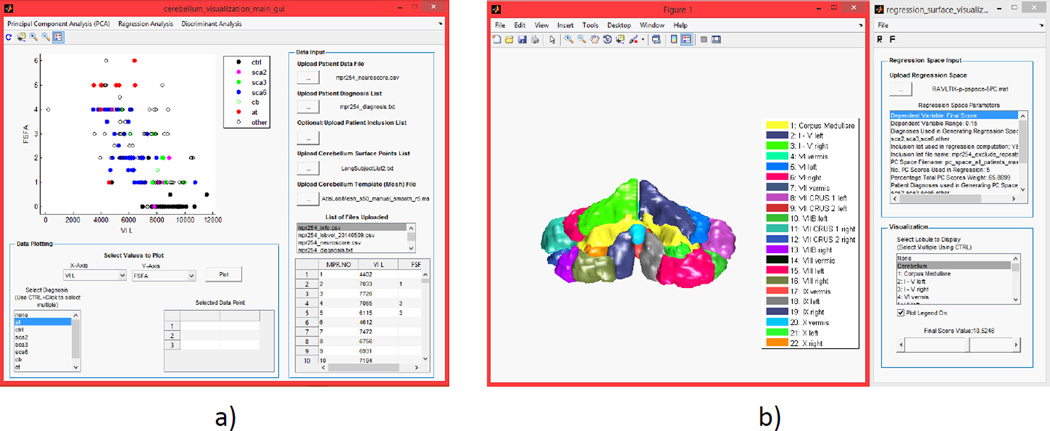
The main GUI panel is used to upload and visualize data, and to access all analysis and visualization panels as shown on Figure 1. Figure 2a shows the main GUI with a plot of a functional score versus left lobule VII volume. The main GUI displays the uploaded data in a table and offers a simple two-dimensional plotting module. The plots can be restricted to specific disease type and a simple regression analysis can be performed to generate a best-fit line and find the correlation between two variables. Figure 2b shows the functional score visualization panel which is accessed from the main GUI. In this panel, a slider can be used to display the cerebellar shape associated with a specific functional score. Functional score visualization is described in detail in Section 2.5.
Figure 2.
Toolbox panels: a)main GUI panel; b) functional score visualization panel
2.4 Principal Component Analysis
We take as input a matrix of the landmark shape representations of m subjects, S=[s1,s2,…,sm]. Since the sample size m is much smaller than the dimension of the landmark shape representation matrix, 3N, regression and linear discriminant analysis become ill-posed problems. We perform a principal component analysis (PCA) to reduce the dimensionality of the data. PCA performs a linear mapping of the data to a lower dimensional space in such a way that the variance of the data in the low-dimensional representation is maximized15. We perform the PCA and obtain a matrix, V of principal component (PC) basis vectors, V=[v1,v2,…,vm−1], where each column of V is a basis vector and these columns are arranged in decreasing order of the contribution to the variance of the original data. We project the landmark shape representation of one or multiple subjects onto the PC space,
| (2) |
Here, the jth row of P represents the jth PC (jth dimension in the PC space) and the ith column represents the PC value for the ith subject. Dimension reduction is then achieved by selectively removing the higher PCs that correspond to minimal variance.
2.4.1 Visualization
The basis vectors of the PC space can be used to visualize the mean shape of the subject set and the modes of geometric variation10. Visualization is done using an inverse projection of (2). Typically, we are interested in the case where P and S are vectors. Given a set of PC values, we construct a vector p=[PC1,PC2,…,PCm-1]T and attempt to find the corresponding vector s. The PC space, V is fixed. Since V is orthogonal (VTV=1), we solve for s,
| (3) |
A cerebellar shape can then be generated given s, as described in Section 2.2. Both the regression and linear discriminant analysis will perform visualization as described in this section.
2.5 Regression Analysis
A linear regression analysis is performed using a cerebellar function test as the regress and and the PCs as the explanatory variables. The regression analysis develops a linear relationship between the function scores and the PCs,
| (4) |
In (4), [a0,a1,… ,ad] represent the regression coefficients, y is the functional test score, PCi is the ith PC value associated with the ith PC (dimension) and d is the number of principal components used in the regression. The regression line is parametrized about the origin as
| (5) |
Following the parametrization, (4) can be rewritten as
| (6) |
Using (6), t can be computed given y. The computed value of t is used in (5) to generate the PC values. Creating a vector using the computed PC values, p=[PC1,PC2,…,PCd]T and substituting the vector in (3), we solve for s to generate a synthetic cerebellar shape to be visualized.
2.6 Linear Discriminant Analysis
We perform a linear discriminant analysis (LDA) to find a linear combination of features that separate two classes of subjects. The toolbox user selects the two classes, and performs an LDA, using the PC values of the classes as the observations. Assuming the classes are normally distributed with identical within-class covariances, the discriminant line direction is computed as w=Σ−1(µ1-µ2), where Σ is the within-class covariance matrix, µ1 and µ2 are the means of class 1 and class 2. The plane orthogonal to the line w separates the two classes. To compute the corresponding PC values, we develop a parametric form of the line w,
| (7) |
In (7), q is the number of PC used in the LDA, r is a linear scale factor and m is the midpoint of the means of the two classes used. Similar to Section 2.5, the PC values are sampled to generate a synthetic cerebellar shape. The cerebellar shapes generated are used to visualize the progression from one class to the other, such as healthy to diseased.
3. CASE STUDIES
This section will describe a few cases highlighting the utility of the toolbox. We highlight the three visualization analysis blocks: visualizing shape changes along PC directions, performing an LDA of two separate disease classes, and performing a regression analysis to compare the atrophy patterns in motor and cognitive function loss. As input to the toolbox, landmark shapes were generated from T1-weighted magnetization prepared rapid gradient echo (MPRAGE) images of 197 subjects (101 female and 96 male) acquired on a 3.0T MR scanner (Intera, Phillips Medical Systems, Netherlands). Landmark shape representation vectors of each subject were generated as described in Section 2.2. Within the 197 subjects, 67 are healthy controls (HC), 87 are patients with diagnosed cerebellar diseases, including different sub-types of spinocerebellar ataxia (SCA), Ataxia-telangiectasia (AT), etc., 43 are subjects with symptoms of cerebellar dysfunction but no diagnosis, or subjects with family history of cerebellar dysfunction. The subjects were 51.0±18.1 years old (mean± standard deviation) at the time of testing.
3.1 Case Study 1: PC space visualization
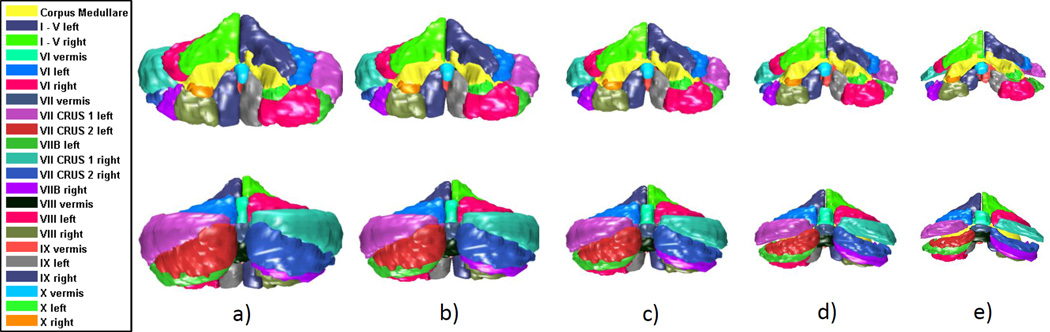
The PC basis vectors provide the opportunity to visualize the geometric variance associated with the cerebellar shape set. The shape changes associated with the 1st and 2 PC basis vectors are shown in Figures 3 and 4, respectively. The mean(µ) shape, , , , and are shown. λi is the eigenvalue of the ith basis vector(vi) and is the corresponding standard deviation. The mean shape of all subjects is µ. From visual observation, the 1st PC space basis vector represents the overall shape of the cerebellum. Moving along the 1st basis vector produces growth or shrinkage in all lobules. The 2nd PC space basis vector (Figure 4) represents shape changes in lobules VII-X and CRUS I/II with minor changes in lobules I-VI and the corpus medulare. The remaining PC basis vectors each produce different change patterns, such as in specific lobules or in the right or left side of the cerebellum. The PC space provides a useful way of analyzing the cerebellar shape changes through the geometric variance in the subject set.
Figure 3.
Trajectory along the first PC space basis vector: a) shape; b); c)µ shape; d) shape; e) shape. The top row is the front view and the bottom is the rear view.
Figure 4.
Trajectory along the second PC space basis vector: a) shape; b); c)µ shape; d) shape; e) shape.
3.2 Case Study 2: Trajectory from healthy to diseased
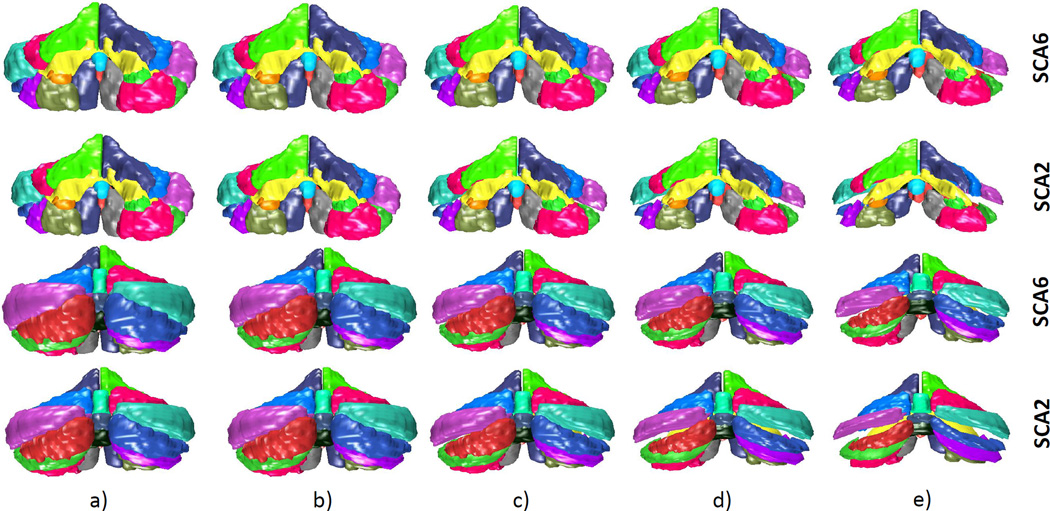
This section will highlight the results of performing an LDA using healthy and diseased classes. The two disease classes used are SCA type 6 and SCA type 2. Of the 87 patients diagnosed with cerebellar disease, 27 were diagnosed with SCA type 6 and 5 with SCA type 2. In the first case, the trajectory from healthy to SCA type 6 is shown. In the second case, the trajectory from healthy to SCA type 2 is shown. Both cases are shown in the same figure for comparison.
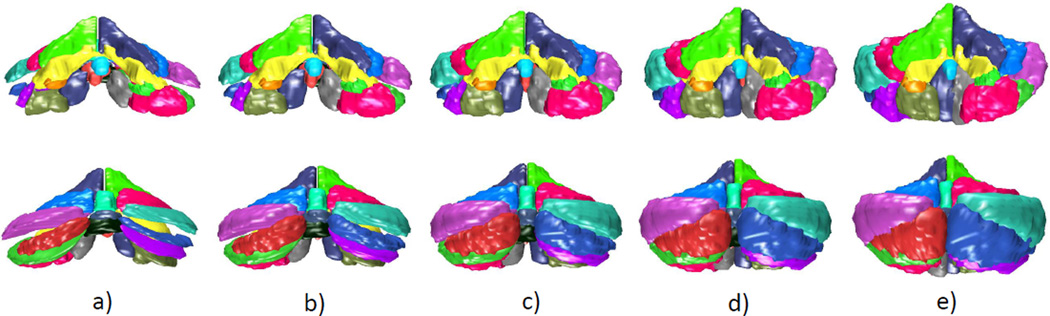
Figure 5 shows the changes from a healthy (control) cerebellum to one that is diagnosed with SCA type 6. The generated cerebellum lie on the discriminant line as described in Section 2.6. In the images produced, significant atrophy patterns are visible in lobule VIII and IX. Lobules VI, X, the anterior lobe (lobules I-V), and VII CRUS I/II undergo minimal decreases in size. The anterior lobe, lobules VIII-IX and parts of lobule VI are believed to be associated with motor functions and lobule VII and CRUS I/II mainly with cognitive functions5. The atrophy pattern here may reveal that SCA type 6 predominantly targets lobules VIII and IX and the corresponding motor outputs are affected. Lack of atrophy in lobule VII and CRUSI/II may hint that SCA type 6 affects little or no cognitive functions. It has been reported that the cognitive defects associated with SCA type 6 are minimal16, 17. In the same figure, the progression from healthy to diseased of SCA type 2 is shown. In comparison to the trajectory of SCA type 6, SCA type 2 produces greater overall atrophy. Additionally, a significant size decrease is observed in lobule VII (with CRUS I/II) in addition to VIII-IX. These results are similar to those observed in a previous MRI study18. Since lobule VII is believed to play an important role in cognitive functions, these atrophy patterns suggest that SCA2 affects both cognitive and motor functionality. A few studies have shown that this is indeed the case17, 19. However, the cognitive impairments in SCA type 2 may be due to extracerebellar degeneration19.
Figure 5.
Trajectory from healthy to diseased (SCA type 6 and SCA type 2): a)projected control class mean; b)midpoint of the control and diseased class means; c)projected disease class mean; d) 2×distance between the projected diseased class mean and the midpoint; e)3×distance between the projected diseased class mean and the midpoint. The first 2 rows are the front view and bottom 2 rows are the rear view.
Performing the LDA has revealed some atrophy patterns associated with two disease types, and similarities and differences can easily be observed. Studying region-specific degeneration in different genotypes of ataxia could provide the researcher the opportunity to predict the functional deficits that may occur. With a better understanding of the topographic functional relationship of the cerebellum, functional loss can be predicted. A linear trajectory from healthy to diseased provides the researcher the opportunity to understand and observe some of the region-specific atrophy patterns. It serves as a useful learning tool to investigate the changes from healthy to diseased, and to compare these with other genotypes. In the toolbox, the flexibility to perform a LDA between user-specified classes, such as between healthy and one type of disease, healthy and multiple types of disease, and disease with disease, serves as a useful tool to understand some of the atrophy patterns associated with cerebellar ataxia.
3.3 Case Study 3: Functional Score Regression
We performed a regression analysis to explore and compare the shape change patterns associated with motor and cognitive function loss. A smaller subset of 103 subjects (59 female and 44 male) aged 53.4±14.0 years were analyzed. Of these subjects, 28 were healthy controls, 33 were diagnosed with cerebellar diseases, and 42 were subjects with symptoms of cerebellar dysfunction but no diagnosis, or subjects with family history of cerebellar dysfunction. The smaller subset was used as both motor and cognitive function testing was performed on this group.
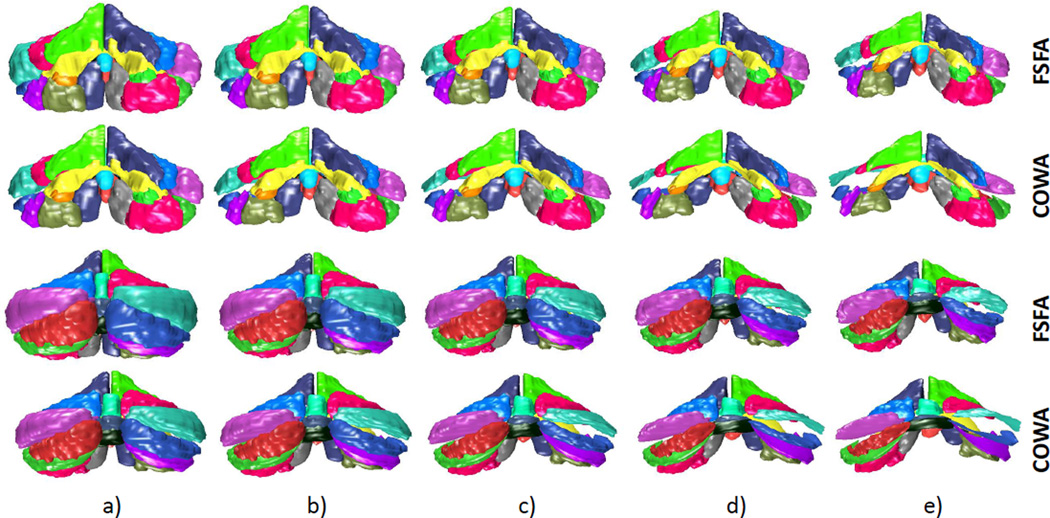
We performed a regression analysis on one motor score, functional staging for ataxia (FSFA), and on one cognitive score, controlled oral word association (COWA) test. These scores provide a comparison of atrophy patterns associated with motor and cognitive impairment. FSFA assess overall patient motility through physical tests of running and jumping20. Patients are assigned a score ranging from 0 to 6. A score of 0 indicates normal movement and 6 indicates complete disability. The COWA test looks at phonemic fluency. Subjects are required to generate as many words as possible in one minute starting from a given letter. Proper names, numbers and repetitions are not allowed. A higher score indicates a greater number of words generated, indicating stronger language cognitive performance. The FSFA scores collected ranged from 0 to 5, and the COWA from 14 to 72. There were no interesting shape patterns beyond a score of 40. The results are shown on Figure 6
Figure 6.
Comparison of regression on FSFA and COWA. Front view (top two rows), rear views (bottom two): a)FSFA=1, COWA=35; b)FSFA=2, COWA=30; c)FSFA=3, COWA=25; d) FSFA=4, COWA=20; e) FSFA=5, COWA=15.
The regression of FSFA results in noticeable atrophy of the cerebellum as a whole. In particular, the anterior lobe (lobules I-V) and lobules VIII-IX have shrunk significantly. The anterior lobe, lobule VIII and parts of lobule VI are responsible for the sensorimotor outputs of the cerebellum1, 4, 5. Lobule IX is also considered essential in the visual guidance of movement4. Atrophy in these regions is expected since FSFA is a purely motor function test. However, there is also atrophy in lobule VII and CRUS I and II. These regions are believed to be involved in cognitive tasks, such as language, emotional processing, and memory1. However, when compared to the COWA regression, the atrophy of lobule VII and CRUS I/II is relatively small. The COWA regression reveals the greatest shrinkage in these lobules in comparison to other regions of the cerebellum. Comparing the FSFA and COWA regression, the COWA case reveals less shrinkage in the motor-related lobules. For example, the anterior lobe undergoes minimal shrinkage, particularly for COWA scores of 35,30 and 25.
There have been many studies attempting to reveal the topographic functional relationship of the cerebellum1. While the lobules responsible for sensorimotor functions are well understood1, 4, those responsible for cognitive functions are not yet fully revealed1, 4–6. Studies investigating language functions have found primarily right-cerebellar activation in lobules VI and CRUS I/II. In particular, studies investigating verb generation5, 6 and noun reading in inner speech6, have found right-cerebellar activation of lobules VI and CRUS I. In the case of a regression on COWA, a language test of word generation, there is a clearly stronger atrophy in the right-side (rear view) of CRUS I and II. The atrophy of lobule VI is less clear. While the COWA test is not the same as the tests used in5, 6, COWA assess similar functionality as patients can generate nouns, verbs, and other types of words.
The regression analysis has revealed some differences between cognitive and motor testing, such as stronger relative atrophy in lobules I-V,VIII, and IX for FSFA, and VII and CRUS I/II for COWA. However, there is not a clear distinction between the two as may be expected. The reason is that we used subjects with a range of cerebellar dysfunction. The patients rarely have only one of motor or cognitive defects. In an attempt to overcome this limitation, the toolbox allows the user to perform a regression analysis using specific groups of subjects, such as disease groups or individual selections. For example, a regression can be performed using subjects with a cerebellar disease that mainly affects motor output to get a better sense of the atrophy patterns associated with different motor function loss. The ability to investigate specific groups of subjects makes the regression analysis a powerful tool to understand the atrophy patterns associated with cerebellar dysfunction.
4. CONCLUSION
We developed a toolbox to allow doctors and researchers to visually explore the cerebellar shape change patterns associated with cerebellar disease and function loss. The toolbox was constructed to be simple and flexible and to contain a rich set of visualization modules to allow researchers to investigate cerebellar shape change patterns in a variety of ways. Through a discriminant analysis or a regression analysis, the user can observe the characteristic change patterns of a disease type or a functional measure. We highlighted the comparison of structural changes associated with SCA types 2 and 6 by performing a linear discriminant analysis. We also compared motor and cognitive functional degeneration through a regression analysis and compared the results with the literature. The cases shown in the paper served to demonstrate the utility of the toolbox. The objective was to use the toolbox as a learning tool to understand the structural changes associated with different cerebellar diseases and with functional loss. By investigating the structural change patterns, we can improve our understanding of the functional topographic relationship of the cerebellum and generate new hypotheses to improve our understanding of the cerebellum and cerebellar disease.
Acknowledgments
This projected was funded by NSF grant #EEC-1460674 and by the NIH through NINDS grant R01 NS056307.
REFERENCES
- 1.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Manni E, Petrosini L. A century of cerebellar somatotopy: a debated representation. Nature Reviews Neuroscience. 2004;5:241–249. doi: 10.1038/nrn1347. [DOI] [PubMed] [Google Scholar]
- 3.Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annual Review of Neuro-science. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 4.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frings M, Dimitrova A, Schorn CF, Elles H-G, Hein-Kropp C, Gizewski ER, Diener HC, Timmann D. Cerebellar involvement in verb generation: an fmri study. Neuroscience letters. 2006;409(1):19–23. doi: 10.1016/j.neulet.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 7.Ying SH, Choi SI, Perlman SL, Baloh RW, Zee DS, Toga AW. Pontine and cerebellar atrophy correlate with clinical disability in SCA2. Neurology. 2006;66(3):424–6. doi: 10.1212/01.wnl.0000196464.47508.00. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Hübsch T, Tezenas du Montcel S, Baliko L, Boesch S, Bonato S, Fancellu R, Giunti P, Globas C, Kang J-S, Kremer B, Mariotti C, Melegh B, Rakowicz M, Rola R, Romano S, Schöls L, Szymanski S, van de Warrenburg BP, Zdzienicka E, Dürr A, Klockgether T. Reliability and validity of the international cooperative ataxia rating scale: A study in 156 spinocerebellar ataxia patients. Movement Disorders. 2006;21(5):699–704. doi: 10.1002/mds.20781. [DOI] [PubMed] [Google Scholar]
- 9.Cootes TF, Hill A, Taylor CJ, Haslam J. The use of active shape models for locating structures in medical images. Information Processing in Medical Imaging. 1993:33–47. [Google Scholar]
- 10.Cootes TF, Taylor CJ, Cooper DH, Graham J. Active shape models-their training and application. Computer vision and image understanding. 1995;61(1):38–59. [Google Scholar]
- 11.de Bruijne M, van Ginneken B, Viergever MA, Niessen WJ. Adapting active shape models for 3d segmentation of tubular structures in medical images. Information Processing in Medical Imaging. 2003:136–147. doi: 10.1007/978-3-540-45087-0_12. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z, Carass A, Chen C, Prince JL. Simultaneous cortical surface labeling and sulcal curve extraction. Proc. SPIE. 2012;8314:831414. doi: 10.1117/12.910552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Ye C, Bogovic JA, Carass A, Jedynak BM, Ying SH, Prince JL. Automated cerebellar lobule segmentation with application to cerebellar structural analysis in cerebellar disease. Neu-roImage. 2015 doi: 10.1016/j.neuroimage.2015.09.032. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z, Abulnaga SM, Carass A, Kansal K, Jedynak BM, Onyike C, Ying SH, Prince JL. Landmark based shape analysis for cerebellar ataxia classification and structural change pattern visualization. Proc. SPIE. 2016:9784. doi: 10.1117/12.2217313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson K. On lines and planes of closest fit to systems of points in space. Philosophical Magazine. 1901;2(11):559–572. [Google Scholar]
- 16.Globas C, Bösch S, Zühlke C, Daum I, Dichgans J, Bürk K. The cerebellum and cognition. Intellectual function in spinocerebellar ataxia type 6 (sca6) Journal of neurology. 2003;250(12):1482–1487. doi: 10.1007/s00415-003-0258-2. [DOI] [PubMed] [Google Scholar]
- 17.Klinke I, Minnerop M, Schmitz-Hübsch T, Hendriks M, Klockgether T, Wüllner U, Helm-staedter C. Neuropsychological features of patients with spinocerebellar ataxia (sca) types 1, 2, 3, and 6. Cerebellum. 2010;9(3):433–442. doi: 10.1007/s12311-010-0183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung B, Choi S, Du A, Cuzzocreo J, Ying H, Landman B, Perlman S, Baloh R, Zee D, Toga A, Prince J, Ying SH. Mri shows a region-specific pattern of atrophy in spinocerebellar ataxia type 2. The Cerebellum. 2012;11(1):272–279. doi: 10.1007/s12311-011-0308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bürk K, Globas C, Bsch S, Klockgether T, Zühlke C, Daum I, Dichgans J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. Journal of Neurology. 2003;250(2):207–211. doi: 10.1007/s00415-003-0976-5. [DOI] [PubMed] [Google Scholar]
- 20.Subramony S, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, Taylor P, Wilson R, Ashizawa T, et al. Measuring friedreich ataxia: interrater reliability of a neurologic rating scale. Neurology. 2005;64(7):1261–1262. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]