Abstract
A spinal dural arteriovenous fistula is an abnormally layered connection between radicular arteries and venous plexus of the spinal cord. This vascular condition is relatively rare with an incidence of 5–10 cases per million in the general population. Diagnosis of spinal dural arteriovenous fistula is differentiated by contrast-enhanced magnetic resonance angiography or structural magnetic resonance imaging, but a definitive diagnosis requires spinal angiography methods. Here, we report a case of a 67-year-old female with a spinal dural arteriovenous fistula, provide a pertinent clinical history to the case nosology, and discuss the biology of adhesive proteins, chemotactic molecules, and transcription factors that modify the behavior of the vasculature to possibly cause sensorimotor deficits.
Keywords: Angiography, MRI, Sensorimotor, Transcription, Venous system
Introduction
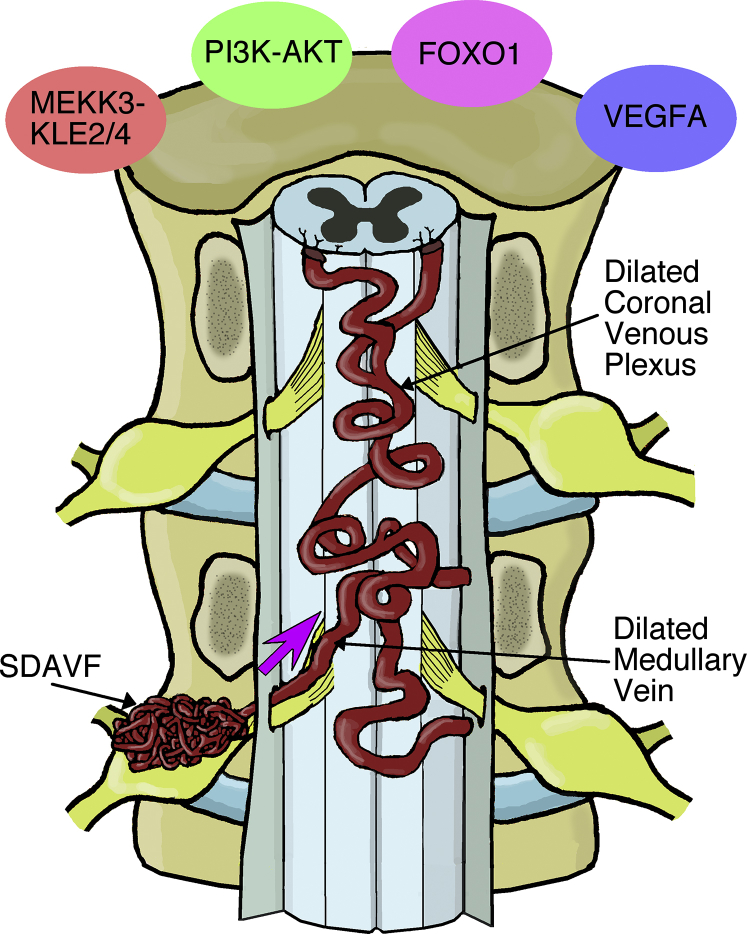
The spinal cord is composed of distinct cell types, including those cells that make up the anterior and posterior spinal arteries, spinal branches, and spinal veins. Vascular cells in the spinal cord depend on adhesive membrane proteins expressed, particularly, by endothelial cells or local extracellular chemotactic proteins for sculpting and maintaining a connective layered scaffold between the arterial and venous systems [1]. This requires that during central nervous system (CNS) development, differentiated cells become dispersed and positioned correctly, often over long distances, from sites in the early neural tube to final sites in the segmented spinal cord [2]. If this cell-to-cell guidance milestone fails to materialize, it is conceivable that a hugely disorganized and dilated vascular network generates upon full spinal cord maturation with serious pathologic implications (Fig. 1). For instance, during the formation of spinal dural arteriovenous fistula (SDAVF), an abnormally aligned connection between radicular arteries and venous plexus is established within ventral (anterior; motor) and dorsal (posterior; sensory) aspects of nerve roots [3].
Fig. 1.
SDAVFs are localized to dural sleeves of nerve roots. This simplified schematic diagram shows that under pathologic conditions, blood is supplied to a SDAVF lesion by dural arteries (not shown) and then drained in a retrograde manner to normal venous flow through a medullary vein into the coronal venous plexus. Due to the aberrant arteriovenous architecture, the venous system (coronal venous plexus via the medullary vein) receives arterial blood that is “arterialized.” This leads to venous congestion, which can put pressure on the spinal cord to produce clinical symptoms. Although direct evidence is missing, a modified vascular phenotype may be the result of changes in adhesive properties or the expression of specific differentiation-promoting (e.g., FOXO1) or differentiation-inhibiting factors that code for uncoordinated vascular hyperplasia (e.g., VEGFA; PI3K-AKT), vessel diameter malformation, and/or aberrant endothelial cell growth (e.g., MEKK3-KLF2/4). FOXO1, fork-head box O; VEGFA, vascular endothelial growth factor A.
As its name implies, SDAVF is localized to the dural sleeve of a nerve root and the striking hyperplastic feature of the fistula leads blood from the arterial system to directly be shunted into the venous plexus with reduced vascular resistance and, importantly, without the inclusion of the capillary network to regulate unidirectional blood flow [4]. The end result of this abnormal blood flow is the “arterialization” of the venous plexus with subsequent impediment bouts of venous drainage that commonly lead to venous congestion, venous hypertension, intramedullary edema, and localized nerve cell hypoxia [3], [4], [5], [6]. If this particular clinical phenotype goes unrecognized and left untreated, SDAVF can cause serious venous infarction, subarachnoid hemorrhage, and irreversible progressive ascending myelopathy [3], [7]. Understanding the mechanisms of such variable expressivity will undoubtedly provide critical pathophysiological clues [8].
Rigorous attempts to diagnose SDAVF require using structural magnetic resonance imaging (MRI) approaches to reveal dilation of the perimedullary veins and discrete spinal cord enlargement gradients [9]. In addition, T2 signal abnormalities within the conus medullaris can also be used for precision diagnosis [3], [5], [6], [7]. Although MRI is useful in identifying structural differences in spinal cord appearance among patients, spinal angiography is the gold standard in localizing vascular malformations and confirming degrees of arterial drainage outflow [3], [6], [7], [10]. Although these imaging advances do not yet deliver a complete picture of the architecture of SDAVFs, there is sufficient information to draw some general conclusions.
SDAVFs are also known as type I spinal vascular malformations with a relatively low incidence of clinical presentations (5–10 individuals per million in the general population) [3], [7]. Although small effect size common variants to larger effect size rare mutations are thought to provide casual anchors from which to understand SDAVFs, it is not known whether other, as-of-yet-unknown factors, may contribute to spinal cord vascular pathology with parent-of-origin effects. However, we cannot exclude the possibility of sporadic rather than familial estimates, contributing to disease onset and disease progression. Regardless of heritable estimates or rates of de novo point mutations, 80% of clinical cases are diagnosed in males, with 66% of these patients presenting with manifestations of vascular disease in their sixth or seventh decade [5], [7]. Against this background, we describe here the clinical history of a female patient with SDAVF, highlight the challenges reflected in the heterogeneity of the syndromic diagnoses, and discuss treatment options and complications of disease pathology characterized by abnormally aligned endothelial blood vessels.
Case report
A 67-year-old white female presented to the emergency department with an acute onset of numbness and tingling in the hips and legs after going shopping. She then drove to work and was not able to exit her car upon arrival due to muscle weakness. This was accompanied by a short episode of 5/10 midsternal chest pain and anxiety. Two weeks prior, she had a similar episode of sensorimotor symptoms, which resolved spontaneously. The patient also reported a 1-month history of ON and OFF cold-like symptoms and oral/buccal cold sores. Past medical history included hyperlipidemia which was controlled with appropriate diet.
The patient was awake, alert, and oriented with signs of tachycardia with otherwise stable vital signs on physical examination. The neurologic examination in the emergency department was significant for decreased muscle strength (0/5) and decreased sensation (4/5) in the lower extremities, as well as decreased rectal tone. A lack of sensation in the lower extremities was found with the greatest degree of sensation loss in the toes. An additional physical examination finding was obesity with a body mass index of 27.8.
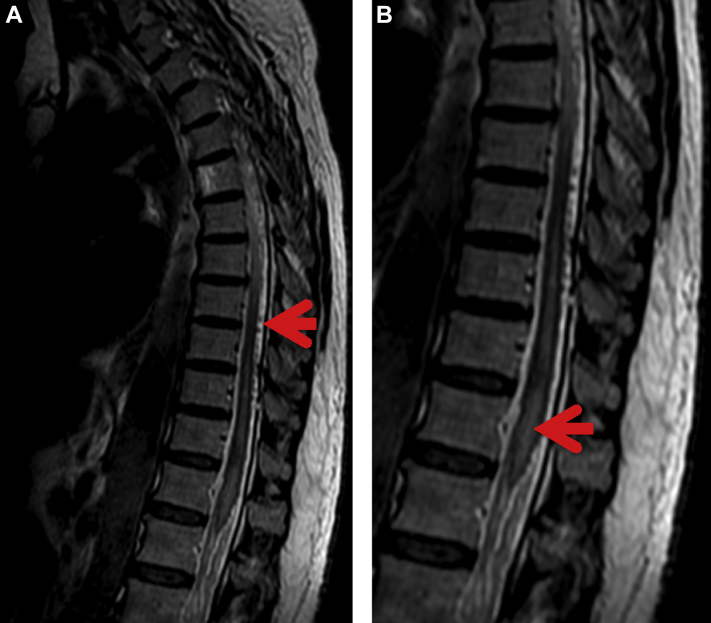
Consultations were requested from Cardiology, Neurology, and Neurosurgery departments. Laboratory testing revealed elevated troponins with a normal echocardiogram. Initial testing with unenhanced MRI of the lumbar spine demonstrated abnormal thoracic spinal cord expansion in the region of T11–T12, increased fluid signal within the cord at these levels as well as crowding of the cauda equina nerve roots. Non–contrast-enhanced MRI of the lumbar spine was also performed and demonstrated the same findings at T11–T12 levels with no significant pathology within the lumbar spine (Figs 2A and B).
Fig. 2.
(A) Sagittal T2 MRI of the spine depicting multiple low-signal flow voids posterior to the spinal cord (red arrow), representing dilated veins secondary to the fistula shunt. (B) Magnified view of the image on the left demonstrates an abnormal high signal within the cauda equina (red arrow), suggestive of edema. MRI, magnetic resonance imaging.
Initial diagnostic impression was spinal cord infarct versus transverse myelitis. The patient was placed on steroids for spinal cord edema and observed closely. Elevated troponins were attributed to myocarditis as opposed to acute coronary syndrome. The patient, accordingly, was placed on statins, beta-blocker, and continuous EKG monitoring.
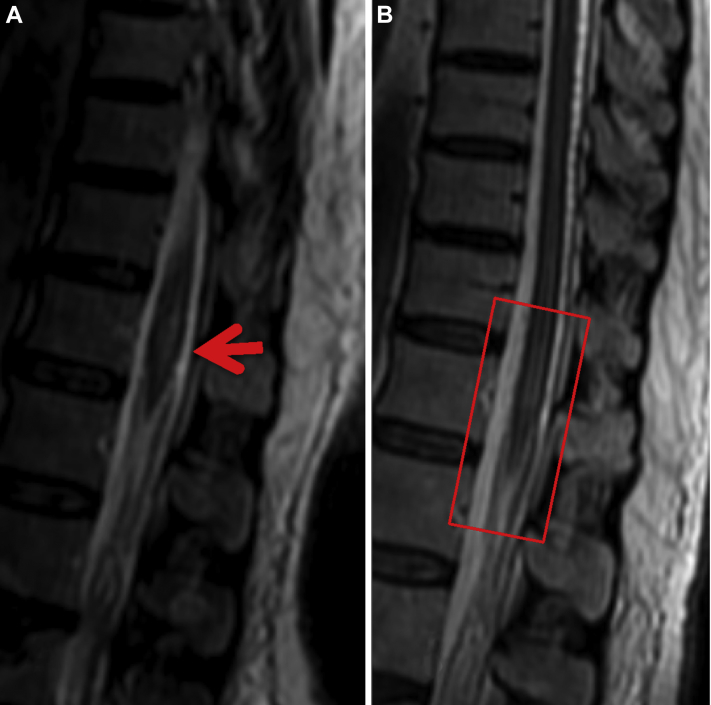
Further workup included a lumbar puncture with cerebrospinal fluid analysis, which was unremarkable. A contrast-enhanced MRI of the entire spine was performed, which redemonstrated edema within the spinal cord at T11–T12 levels. The MRIs additionally revealed numerous abnormally dilated vascular structures within the thoracic and lumbar epidural space, consistent with SDAVF syndrome (Figs 3A and B). The topographic location of the SDAVF explained the spinal cord edema seen at T11–T12 levels, as well as the patient's sensorimotor complaints and physical examination findings in the lower extremities and anal sphincter. Abnormal flow dynamics within the spinal cord, secondary to the arteriovenous fistula, was presumed to be causing cord ischemia and edema. The patient was transferred to an outside facility for spinal angiogram to further define specific vessel involvement and to formulate an effective therapeutic treatment.
Fig. 3.
Magnified sagittal T2 preintervention (A) and postintervention (B) MRIs of the spine demonstrate increased signal (red arrow) representing edema in the thoracic cord secondary to venous hypertension, which improved after treatment (square red box). MRI, magnetic resonance imaging.
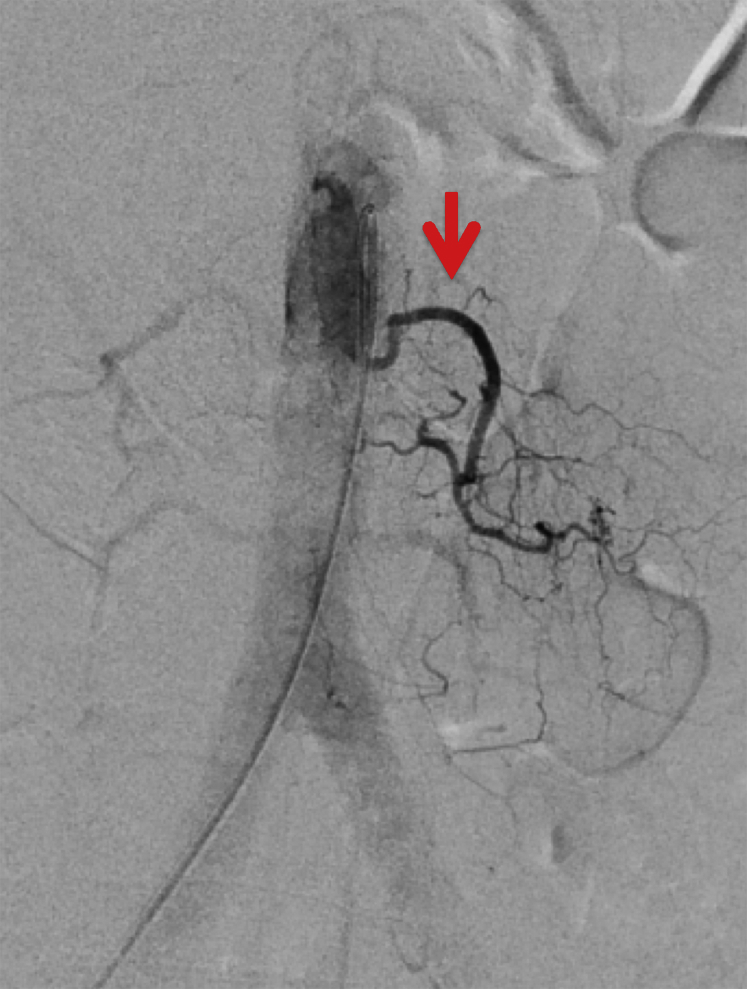
The patient was sedated with general anesthesia for the spinal angiogram procedure. A right groin common femoral artery puncture was performed for vascular access, using a micropuncture kit and 5-French sheath. Selective spinal angiography was initiated using a 4-French Shepherd Hook catheter, at the levels of L2–L3, bilaterally (Fig. 4). In a second stage, selective angiography of the bilateral intercostal arteries from T8–T12 was performed. Normal spinal cord vascular anatomy was observed, including a radiculomedullary branch off of the left T9 artery supplying the anterior spinal axis. Multiple dilated anterior and posterior spinal veins were observed in the thoracic spinal canal. Specific significant findings during angiography were an epidural fistula supplied by the right L1 and L2 arteries draining into the right L2 radicular veins. Associated venous hypertension was demonstrated by observing slow emptying of the anterior spinal artery and slow filling of the spinal cord veins at the above spinal levels. These findings were discussed with referring neurologists and neurosurgeons, and the decision was made to treat the aforementioned patient with embolization.
Fig. 4.
Frontal digital subtraction angiogram during catheter injection of the left L2 lumbar artery shows normal filling of the main trunk (red arrow).
In the same procedure, super-selective angiography and endovascular embolization with N-Butyl Cyanoacrylate and platinum microcoils of the leading L1 and L2 arteries were performed. Complete angiographic obliteration of the fistula was successful. An angiogram at the end of the procedure demonstrated resolution of the epidural fistula and improvement in venous hypertension (Figs 5A and B). The patient also experienced immediate improvement in sensorimotor symptoms, which progressed with each postoperative recovery day. She regained full lower extremity muscle strength, sensation, and anal sphincter tone. A postoperative MRI demonstrated expected improvement in spinal cord edema as well as decreased abnormal spinal vascular engorgement. The patient was discharged a few days after treatment, on her lipid-lowering medications. She does not have complaints related to her prior sensorimotor symptoms and continues to follow with her primary physician.
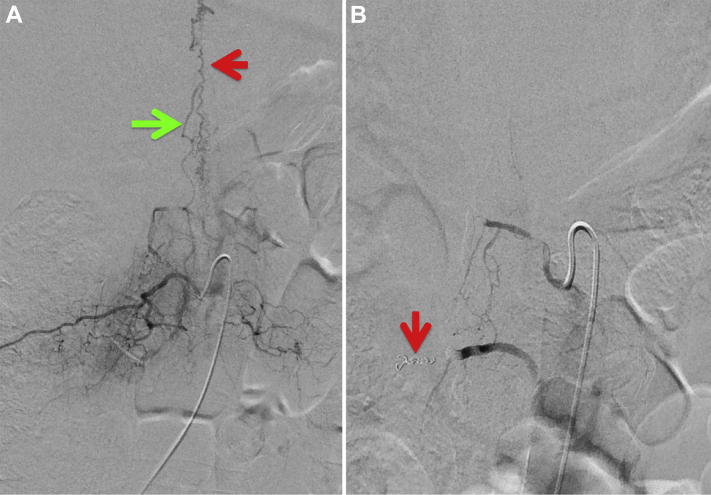
Fig. 5.
Frontal digital subtraction angiograms: preintervention (A) and postintervention (B) with both liquid embolization and microcoils (red arrow). Preintervention image (A) during right L2 lumbar artery contrast injection shows abnormal filling of a dilated right radicular ascending vein (green arrow) and dilated epidural veins (red arrow in B). Postintervention image (B) displays interval resolution of the arteriovenous fistula and dilated epidural venous plexus.
Discussion
Our female patient presented to the emergency department with nonspecific symptoms of paresthesia (numbness and tingling) in the hips and legs. This initial self-report is by itself highly ambiguous as the aforementioned symptoms might also be indicative of sciatic nerve compression or sciatic nerve palsy, restless legs syndrome, peripheral diabetic neuropathy, chronic low back pain with radiculopathy, Guillain-Barré syndrome, or Sjogren's syndrome. All of these should be considered in the differential diagnosis of SDAVF as they are specifically characterized by numbness and tingling of the limbs, particularly if there is compression of the lumbar nerve roots. To confirm or rule out SDAVF, structural MRI and neurophysiological tests (e.g., electromyography) are essential tools to discriminate idiopathic sensorimotor-signaling disorders. In our clinical case, we did not apply electromyography or nerve conduction tests to interrogate sensory abnormalities of the bilateral lower extremities. Although electrophysiological examination is thought to be the best diagnostic technique in phenotyping diseases with pure sensory involvement [7], [9], numbness and tingling phenomena as a result of lesions to the dorsal column spinal cord lemniscus pathway can only be seen in structural MRIs [9], [11].
Neurologic examination of our female patient revealed nonexisting muscle strength in the lower extremities relative to the upper extremities, where muscle strength thresholds were sufficiently noted. Decreased muscle strength with restricted range of motion, spasticity, and/or edema is often symptomatic of corticospinal tract impairment within the anterior lumbar columns of the spinal cord [9]. Again, accurately diagnosis of discrete lesions to lumbar spine regions requires CNS imaging to initially characterize sensorimotor-signaling disorders with relative precision. Unenhanced MRI of the lumbar region of the spinal cord indeed revealed active vasogenic edema in our female patient, a neurologic finding that supports compression of spinal cord and adjacent nerve roots. Structural MRI analyses with contrast-enhanced methods further buttressed the above neurologic finding as aberrant dilated, and sprouting vascular vessels within the spinal canal were visualized in the T2-weighted images. The specificity of structural MRIs along with certain pathologic features, particularly the presence of tortuous blood vessel fiber arrangement and microscopy edema, is highly indicative of SDAVF pathology.
It should be noted that our female patient also exhibited a clinical history of hyperlipidemia and elevated serum levels of troponins as well as cold sores and anxiety-like symptoms. Troponins may be indicative of myocardial necrosis in acute cardiovascular syndromes [12]. However, their implication in the absence of clinical evidence of cardiac pathology was not established in our female patient. Along the same lines, facial dermatoses (e.g., cold sores) and anxiety-like symptoms might be viewed as accessory symptoms to self-reporting acute episodes of paresthesia. In this context, stress, mood states, and changes in immune and neuroendocrine physiology might significantly impact psychomotor indices of sensorimotor-signaling disorders [13]. Indeed, there is evidence of an inflammatory constituent present in major depressive and anxiety disorders that may contribute to the development or exacerbation of a number of medical diseases, perhaps including, SDAVF [14], [15]. Regardless, cognitive impairment and depression/anxiety phenomena not only significantly impact therapy outcome(s), but might also be representative indices of vascular pathology before objective confirmation of SDAVF can be made.
An interesting fact about the incidence of SDAVF pathology is that it is predominantly biased toward males [3]. Sex-dependent differences are obvious phenotypic traits across the spectrum of health and disease, particularly in areas such as pain and pain perception, autoimmune disorders, aging and longevity, vascular disease, and affective disorders [16], [17], [18], [19]. The brain is indeed highly dimorphic in terms of functionality, and although sex-dependent differences vary according to neuroanatomic location, interaction among genes, gonadal, and brain-borne steroid hormones, epigenetic, and environmental factors play critical roles in determining how certain diseases become differentially established in the genotype of male or female nervous systems. SDAVF might be one of the nervous system diseases that is qualitatively different between male and female patients. The reasons for this are not entirely clear, but there is compelling evidence for steroid hormones modulating CNS networks and contributing to repair mechanisms within damaged neural circuits [16]. Our female patient at the time of presentation was 67 years of age, with significant decreases in estrogen levels which combined with cellular aging pathways may have increased sex-specific periods of vulnerability to SDAVF. Understanding the potential underlying mechanisms driving sex-dependent differences and their relevance to SDAVF pathology is critical for guiding the development of novel therapies.
As discussed in the introductory paragraphs, the formation of blood vessels in the brain and spinal cord requires proliferation of endothelial cells [2]. In this context, blood vessels must sprout, elongate, form lumens, and regress in an orderly fashion, a key developmental program that appears to be driven, at least in part, by the fork-head box O (FOXO1) transcription factor [20]. If FOXO1 is deleted, at least in experimental animals, hyperplasia and blood vessel dilation is produced suggesting that FOXO1 is a critical molecule for endothelial growth and vascular expansion. Other signaling molecules involved in angiogenesis, the process through which new blood vessels arise from existing ones, also include vascular endothelial growth factor A (VEGFA) and the PI3K-AKT pathway, a signaling pathway downstream of VEGFA [20]. VEGFA and PI3K-AKT, in addition to adhesive proteins and chemotactic factors, appear to contribute substantially to the full differentiation of the CNS vasculature [21].
As these molecules precisely guide vascular architecture during embryonic development, what is the relevance of signaling molecules to SDAVF pathology? At the present time, it is unclear the mechanistic role of adhesive molecules, vascular cells, or large-scale chemotactic networks to the pathogenesis of SDAVF. However, in cerebral cavernous malformation, an inherited and sporadic vascular disease that promotes strokes and seizures in young patients, the causal mechanism appears to be related to gain of MEKK3-KLF2/4 signaling in the brain endothelium [22]. Thus, transcriptional signaling events that specifically drive fiber caliber and lumen formation may contribute to the burden of vascular malformations more broadly than previously appreciated. Continued investigation of such causal mechanisms is essential for progress in understanding the architecture of blood vessels formation and their relationship to sensorimotor-signaling disorders.
In conclusion, SDAVF is a discrete lesion within the spinal cord with numerous signaling cellular pathways proposed to have casual roles. This inherited or sporadic vascular malformation typically presents in males in the fifth to sixth decade, with progressive paraplegia. Similar to the case of our patient, there is usually an associated bladder or bowel dysfunction with the progression of disease. At the gross or macroscopic level, SDAVF is thought to arise as a result of extradural venous thrombosis causing progressive pressure gradients on the spinal venous system. Clinical outcome depends on the duration of symptoms and whether there is irreversible damage, such as spinal cord infarcts [23]. Our patient seems to have presented to the emergency department quite early in the course of her disease, with a short history of sensorimotor symptoms, as well as reversible pathology as demonstrated by the results of diagnostic and therapeutic spinal angiogram(s). Furthermore, postprocedural MRI demonstrated immediate improvement in spinal cord signaling events, suggesting the reversibility of the patient's condition. Given her rapid improvement in anal sphincter tone, she appears to have fared better than most patients who suffer from this disorder, as they usually do not fully recover from bladder or bowel dysfunctions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Sharma H.S. Elsevier Academic Press; San Diego (CA): 2003. Blood-spinal cord and brain barriers in health and disease. [Google Scholar]
- 2.Sadler T.W. 13th ed. Lippincott, Williams, Wilkins; Baltimore (MD): 2014. Langman’s medical embryology. [Google Scholar]
- 3.Fugate J.E., Lanzino G., Rabinstein A.A. Clinical presentation and prognostic factors of spinal dural arteriovenous fistulas: an overview. Neurosurg Focus. 2012;32(5):E17. doi: 10.3171/2012.1.FOCUS11376. [DOI] [PubMed] [Google Scholar]
- 4.Hassler W., Thron A., Grote E.H. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg. 1989;70(3):360–370. doi: 10.3171/jns.1989.70.3.0360. [DOI] [PubMed] [Google Scholar]
- 5.Krings T., Geibprasert S. Spinal dural arteriovenous fistulas. Am J Radiol. 2009;30:639–648. doi: 10.3174/ajnr.A1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeng Y., Chen D.Y.-T., Hsu H.-L., Huang Y.-L., Chen C.-J., Tseng Y.-C. Spinal dural arteriovenous fistula: imaging features and its mimics. Korean J Radiol. 2015;16(5):1119–1131. doi: 10.3348/kjr.2015.16.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koch C. Spinal dural arteriovenous fistula. Curr Opin Neurol. 2006;19(1):69–75. doi: 10.1097/01.wco.0000200547.22292.11. [DOI] [PubMed] [Google Scholar]
- 8.Mehesry T.H., Shaikh N., Malmstrom M.F., Marcus M.A., Khan A. Ruptured spinal arteriovenous malformation: presenting as stunned myocardium and neurogenic shock. Surg Neurol Int. 2015;6(Suppl 16):S424–S427. doi: 10.4103/2152-7806.166180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Lim Y.M., Suh D.C., Rhim S.C., Kim S.J., Kim K.K. Clinical presentation, imaging findings, and prognosis of spinal dural arteriovenous fistula. J Clin Neurosci. 2016;26:105–109. doi: 10.1016/j.jocn.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Jellema K., Tijssen C., van Giin J. Spinal dural arteriovenous fistulas: a congestive myelopathy that initially mimics a peripheral nerve disorder. Brain. 2006;129(12):150–3164. doi: 10.1093/brain/awl220. [DOI] [PubMed] [Google Scholar]
- 11.Maimon S., Luckman Y., Strauss I. Spinal dural arteriovenous fistula: a review. Adv Tech Stand Neurosurg. 2016;43:111–137. doi: 10.1007/978-3-319-21359-0_5. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K., Alpert J.S., White H.D. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 13.Goldsmith D.R., Haroon E., Woolwine B.J., Jung M.Y., Wommack E.C., Harvey P.D. Inflammatory markers are associated with decreased psychomotor speed in patients with major depressive disorder. Brain Behav Immun. 2016;56:281–282. doi: 10.1016/j.bbi.2016.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsuchopharmacol Biol Psychiatry. 1995;19(1):11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 15.Vogelzangs N., Beekman A.T.F., de Jonge P., Penninx B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangasser D.A., Valentino R.J. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35(3):303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendrek A., Fattore L. Sex differences in drug-induced psychosis. Curr Opin Behav Sci. 2017;13:152–157. [Google Scholar]
- 18.Goldstone A., Mayhew S.D., Przezdzik I., Wilson R.S., Hale J.R., Bagshaw A.P. Gender specific re-organization of resting-state networks in older age. Front Aging Neurosci. 2016;8:285. doi: 10.3389/fnagi.2016.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shansky R.M., Woolley C.S. Considering sex as a biological variable will be valuable for neuroscience research. J Neurosci. 2016;36(47):11817–11822. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilheim K., Happel K., Eelen G., Schoors S., Oellerich M.F., Lim R. FOXO1 couples metabolic activity and growth state in vascular endothelium. Nature. 2016;529(7585):216–220. doi: 10.1038/nature16498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai J.L., Lee Y.M., Pan C.Y., Lee A.Y. The novel VEGF121-VEGF165 fusion attenuates angiogenesis and drug resistance via targeting VEGFR2-HIF-1α-VEGF165/Lon signaling through PI3K-AKT-mTOR pathway. Curr Cancer Drug Targets. 2016;16(3):275–286. doi: 10.2174/156800961603160206125352. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z., Tang A.T., Wong W.Y., Bamezai S., Goddard L.M., Shenkar R. Cerebral cavernous malformations arise from endothelial gain of MEKK3-KLF2/4 signaling. Nature. 2016;532(7597):122–126. doi: 10.1038/nature17178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmetz M.P., Chow M.M., Krishnaney A.A., Andrews-Hinders D., Benzel E.C., Masaryk T.J. Outcome after the treatment of spinal dural arteriovenous fistulae: a contemporary single-institution series and meta-analysis. Neurosurgery. 2004;55(1):77–87. doi: 10.1227/01.neu.0000126878.95006.0f. [DOI] [PubMed] [Google Scholar]