Abstract
A 24-year-old man, with past medical history significant only for nocturnal enuresis until the age of 12 years, presented to the emergency department with acute abdominal pain after an episode of difficulty with micturition in the middle of the night. On presentation, physical examination was suggestive of ascites and laboratories revealed an elevated serum creatinine of 1.88 mg/dL. He was subsequently found to have a ruptured bladder, without any inciting trauma, which required surgical repair. His only surgical history is an unknown, apparently urologic, surgery when he was 11-12 years old. The patient's unique presentation prompts discussion of bladder rupture and its manifestations, the role of clinical information in informing imaging protocol, and the importance of sagittal images in identifying pathology.
Keywords: Bladder rupture, Ascites, Renal failure, Urology
Introduction
There are multiple reports in the medical literature regarding traumatic urinary bladder rupture as well as peripartum urinary retention with subsequent bladder rupture [1], [2], [3], [4]. There are also several case reports and literature reviews of nontraumatic bladder rupture, but these cases are often associated with malignancy, radiation therapy, or other causes of obstructive pathology [5], [6], [7], [8]. Previous reports of spontaneous bladder rupture without a known inciting event are rare—the most recent being in 2014 [9], [10], [11], [12], [13], [14]. No matter the etiology, bladder rupture may present as renal pseudo-failure due to reabsorption of creatinine across the peritoneal membrane. Renal pseudo-failure can be defined as elevated creatinine with an etiology that does not fall under the traditional categories of prerenal, renal, or postrenal kidney injury. In renal pseudo-failure, there is no underperfusion of the kidney, no increased pressure in the glomerulus caused by obstruction, and no intrinsic disease of the kidney. Rather, the kidneys are functioning normally, but there is an increase in measured serum creatinine due to increased production or reabsorption.
Before presenting the case at hand, a brief review of nighttime urine incontinence in the pediatric population is prudent. Nocturnal enuresis can be thought of as primary if there has never been any period of nighttime continence or secondary if there has been a period of 6 months of dry nights followed by re-emergence of bed-wetting. At the age of 5 years, approximately 1 in 5 children will have primary nighttime incontinence that improves to roughly 1 in 100 children by the age of 17 years. Generally, the process is self-limited and thought to be due to delayed neurological maturation of micturition pathways. However, in patients with secondary enuresis or persistent daytime symptoms, there is increased prevalence of underlying organic or psychological pathology to include parental divorce, detrusor overactivity, antidiuretic hormone hyposecretion, or physiologic obstruction. If the diagnostic workup does not reveal any underlying abnormality, the cornerstone of therapy for enuresis includes educating the family about voiding habits, limiting afternoon and evening fluid intake, and bed-wetting alarms. Desmopressin, with or without oxybutynin, is the most common pharmacologic therapy but is of limited value given the high rate of symptom recurrence after cessation of therapy [15].
Case report
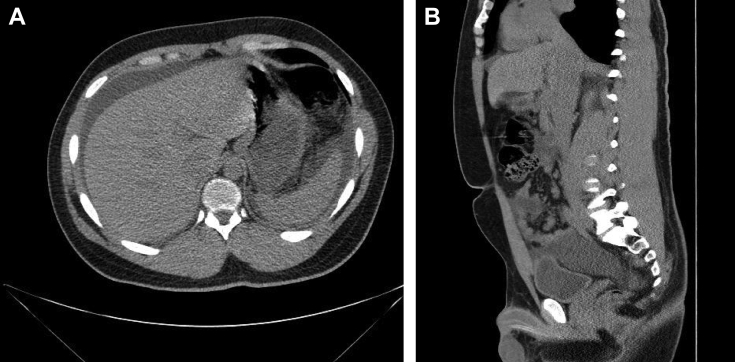
A 24-year-old healthy man, with past medical history significant only for primary nocturnal enuresis until the age of 12 years, presented to the emergency department with diffuse abdominal pain, nausea, vomiting limiting his oral intake, and difficulty with micturition. He reports waking up in the middle of the night before presentation and trying to urinate, after which he experienced sudden, intense abdominal pain that worsened over several hours prompting him to present to the emergency department. On presentation, he was noted to be agitated and was unable to find a comfortable position. Examination revealed suprapubic fullness and tenderness with associated mild abdominal distention and no other abnormalities noted. An indwelling urinary catheter was placed and approximately 1000 mL of nonbloody, translucent, yellow urine rapidly drained. The laboratory values on admission revealed apparent acute kidney injury with a creatinine of 1.88 mg/dL that resolved over the next 24 hours to 0.9 mg/dL with persistent indwelling urinary catheterization and intravenous fluids. A computerized tomography (CT) scan identified large-volume ascites (Fig. 1A). The day after admission, his abdominal pain had spontaneously resolved, laboratory abnormalities normalized, and the patient tolerated oral intake. The patient was discharged with plans for therapeutic paracentesis 2 days later as an outpatient.
Fig. 1.
(A) Transverse slice of CT abdomen without contrast on day 1 showing free fluid collections around the liver and spleen. (B) Sagittal slice CT abdomen without contrast on day 1 notable for disruption of smooth demarcation of bladder wall in the dome of the bladder sagittal reconstruction showing findings consistent with bladder rupture. CT, computed tomography.
The patient underwent paracentesis as scheduled. The procedure yielded 2000 mL of yellow, slightly turbid fluid. Further examination of the fluid revealed 378 WBC/μL and 113 RBC/μL. The serum-ascites albumin gradient value was greater than 3, consistent with a transudative or nonperitoneal cause of ascites. After paracentesis, he had minimal abdominal pain and was sent home.
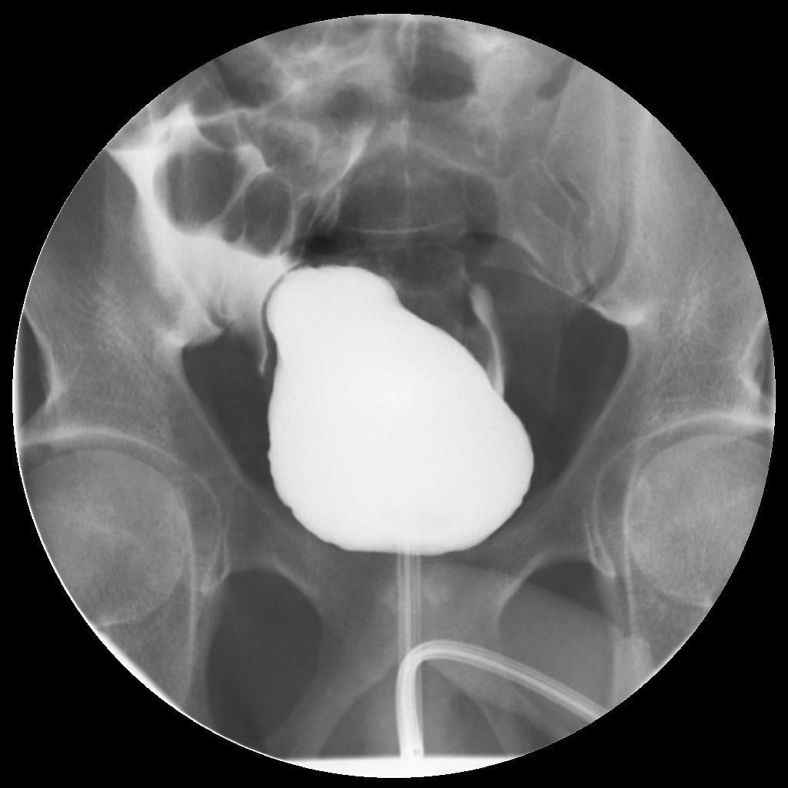
Three days after paracentesis, he presented to the ED once again with recurrent abdominal pain similar in quality to that which prompted the first hospitalization. A comprehensive metabolic panel and complete blood count revealed a creatinine of 11.44 mg/dL, hemoglobin of 18.1 g/dL, and urinalysis was notable for 500 mg/dL of proteinuria. An indwelling urinary catheter was placed, promptly yielding 1400 mL of pale, yellow urine, and aggressive fluid resuscitation with normal saline at 200 mL/hour was initiated. His creatinine dropped precipitously to 6.43 mg/dL after 10 hours and then to 0.87 mg/dL after 36 hours. Hemoglobin dropped to 13.1 g/dL during this same interval. Twenty-four–hour urine collection demonstrated 212 mg/day of protein. At this point, a postrenal etiology for elevated creatinine was suspected and the patient underwent fluoroscopic cystourethrogram that revealed contrast moving outside the bladder into the peritoneal space (Fig. 2). He subsequently underwent laparotomic surgical repair of the ruptured bladder without complications. The surgeon noted that in addition to a small perforation in the dome of the bladder, which was repaired with 3 layers of sutures, there was also damage to the anterior peritoneum, seromuscular tears of the small intestine, and a web of adhesions. The seromuscular tears were sutured without incident. A cystoscopy performed 5 days postoperatively revealed no evident cause for obstruction—no congenital or acquired anatomic anomaly was identified. The urologist did note prominent trabeculae of the urinary bladder suggestive of chronic work of the detrusor muscle against resistance.
Fig. 2.
Fluoroscopic cystourethrogram on day 7 after original admission. Findings: Transit of contrast outside the bounds of the bladder and ureters was noted from the right side of the urinary bladder.
On further questioning, the patient reports that he did have prior surgery when he was 11-12 years old, the details of which are uncertain. Apparently, he had nocturnal enuresis and abdominal pain until his preteen years that resolved with the operation. Records of this surgery are unavailable as the patient is unsure which hospital this was performed at and he is not in contact with the family members who were taking care of him at that time.
The patient was discharged with a Foley catheter in place for several weeks to prevent increased bladder pressure that would inhibit healing. He was followed by urology as an outpatient and, as of 6 months after hospitalization, has had no recurrence of obstructive symptomatology or difficulty with micturition.
Discussion
The patient presented with severe abdominal pain and distention after difficult micturition prompting protracted diagnostic evaluation before identification and surgical repair of spontaneously ruptured bladder. Spontaneously ruptured bladder with associated renal pseudo-failure and uroperitoneum is a rare cause of abdominal pain and ascites. Most cases are associated with trauma, pregnancy, or bladder cancer. However, the presentation of uroperitoneum as renal pseudo-failure and proteinuria is consistent with previous cases that have been reported [16]. The concept that creatinine and toxins equilibrate across the peritoneal membrane is a familiar one, as it is the foundation for peritoneal dialysis. In this patient, equilibration promoted the extremely high levels of creatinine found in the patient's blood that corrected quickly with catheter placement and fluid resuscitation. Renal function was never truly compromised.
The mildly elevated proteinuria noted on 24-hour urine collection may have been postrenal in nature due to inflammation of the urinary tract and peritoneum. The patient was noted to have volume contraction and dehydration due to poor fluid intake, which likely also promoted proteinuria. Adhesions of the small intestine found during surgery are thought to be the result of previous urologic surgery. The young patient's bladder rupture was secondary to an unknown obstructive pathology—possibly sympathetic over activity or congenital urologic anomaly, although none was identified on cystoscopy.
The history of urologic surgery at the age of 12 years may provide clues to the etiology of the patient's presentation. One possibility is that the patient had posterior urethral valves, one of the more common congenital abnormalities of the male urethra. It usually requires surgery, causes obstructive symptoms, and can lead to trabeculated bladder. Intervention, however, is required earlier in life, making this less likely. Congenital or acquired urethral strictures are associated with enuresis and can present in older children, but the correction is transurethral incision rather an abdominal surgery. Other potential causes of obstruction and incontinence include urethral polyps and anterior urethral valves, but again these would likely not require abdominal surgical approach. The history of surgery and nocturnal enuresis in this patient seem to be unavoidably linked to his bladder rupture—the true nature of this connection, however, is hazy. Without more definitive medical records, one can only conjecture and take solace in his swift recovery without evidence of permanent end-organ damage [17].
As with many rare conditions, the history obtained at initial presentation is vital to making the correct diagnosis. Those details provide the contextual evidence to suggest such an anomalous diagnosis. Our patient had sudden onset of abdominal pain after experiencing difficulty with micturition followed by the development of ascites. Although uroperitoneum is a rare cause of ascites, such a direct correlation between difficult micturition and onset of symptoms should raise red flags. Unfortunately, the initial CT ordered on the patient's ED visit did not include contrast-enhanced delayed imaging to allow passage of contrast to the bladder—and possibly facilitating the diagnosis of bladder rupture on the patient's first hospital day. Although documentation indicates that the ED physician was suspicious of bladder injury, this was not apparently conveyed to the radiology department, where the indication for the examination was “abdominal pain.” This suboptimal communication highlights the need for clinical information during both the protocol and interpretation phases of radiologic examinations. Creating a culture of open communication and frequent interaction between the ordering clinician and the radiologist may help to mitigate potential errors. Radiologists can potentiate this culture by openly encouraging communication and making the radiology suite visible through attending committees, tumor boards, and interacting with patients. Furthermore, if spontaneous ruptured bladder is suspected, the radiologist can recommend options aside from further imaging. Specifically, the ascitic fluid can be analyzed for creatinine, electrolytes, and cell counts, which can be compared to urine levels. Similarity of the fluid to urine can help confirm the diagnostic suspicion, which can then be established with plain film fluoroscopic cystogram or CT, resulting in prompt urologic intervention.
From a radiologic interpretation perspective, patients with abdominal pain and ascites are often assessed with abdominal CT. In the case of this patient, the abnormality of the bladder is much more easily identified on sagittal imaging—even if intraperitoneal contrast is not identified because of limited study protocol. On the CT scan of our patient, there is some disruption of the normal smooth, well-demarcated contour of the bladder wall that is more evident in some of the sagittal reconstructions (Fig. 1B). These findings are difficult to appreciate in the transverse slices and were not noted until examined retrospectively. If appropriate clinical suspicion had been communicated effectively, identification of this subtle abnormality may have aided in the more expedient diagnosis and repair of the patient's spontaneously ruptured bladder.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Consent: This report is made with the written consent of the patient. All identifying information has been removed from the case.
References
- 1.Johnsen N.V., Young J.B., Reynolds S.W., Kaufman M.R., Milam D.F., Guillamondegui O.D. Evaluating the role of operative repair of extraperitoneal bladder rupture following blunt pelvic trauma. J Urol. 2016;195(3):661–665. doi: 10.1016/j.juro.2015.08.081. [DOI] [PubMed] [Google Scholar]
- 2.Duenas-Garcia O.F., Rico H., Gorbea-Sanchez V., Herrerias-Canedo T. Bladder rupture caused by postpartum urinary retention. Obstet Gynecol. 2008;112(2 Pt 2):481–482. doi: 10.1097/AOG.0b013e31817997a4. [DOI] [PubMed] [Google Scholar]
- 3.Parry N.G., Rozycki G.S., Feliciano D.V., Tremblay L.N., Cava R.A., Voeltz Z. Traumatic rupture of the urinary bladder: is the suprapubic tube necessary? J Trauma. 2003;54(3):431–436. doi: 10.1097/01.TA.0000053196.19218.4F. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor R. Two-layer laparoscopic repair of intraperitoneal bladder rupture in blunt abdominal trauma: a case report with literature review. Surg Laparosc Endosc Percutan Tech. 2012;22(4):e204–e205. doi: 10.1097/SLE.0b013e31824ea67e. [DOI] [PubMed] [Google Scholar]
- 5.Oray D., Limon O., Ertan C., Ugurhan A. Spontaneous bladder rupture and pelvic fracture due to bladder cancer. Turk J Emerg Med. 2014;14(3):139–141. doi: 10.5505/1304.7361.2014.55707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin J.Y., Yoon S.M., Choi H.J., Lee SN, Kim H.B., Joo W.C. A case of post-radiotherapy urethral stricture with spontaneous bladder rupture, mimicking obstructive uropathy due to cancer metastasis. Electrolyte Blood Press. 2014;12(1):26–29. doi: 10.5049/EBP.2014.12.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarmah P.B., Noah A., Kelly B.D., Ryan P.G. Asymptomatic ureteral rupture secondary to chronic urinary retention from massive prostatic enlargement. J Surg Case Rep. 2015;2015(11):rjv135. doi: 10.1093/jscr/rjv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ficarra V., Beltrami P., Giusti G., Tontodonati M., Zanon G., Malossini G. Spontaneous bladder perforation due to eosinophilic cystitis: a case report. Prog Urol. 1997;7(6):1012–1014. [PubMed] [Google Scholar]
- 9.Cusano A., Abarzua-Cabezas F., Meraney A. Spontaneous bladder perforation unrealted to trauma or surgery. BMJ Case Rep. 2014;2014:2119–2121. doi: 10.1136/bcr-2014-204161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppenheimer G.D., Druckerman L.J. Recurrent non-traumatic intraperitoneal rupture of the urinary bladder; recovery. J Urol. 1974;57(2):238–241. doi: 10.1016/S0022-5347(17)69622-8. [DOI] [PubMed] [Google Scholar]
- 11.Evans R.A., Reece R.W., Smith M.J. Idiopathic rupture of the bladder. J Urol. 1976;116(5):565–567. doi: 10.1016/s0022-5347(17)58913-2. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F.S., Pense S., Christenson S. Spontaneous intraperitoneal rupture of the bladder. J Med Liban. 1994;42(3):149–154. [PubMed] [Google Scholar]
- 13.Heyns C.F., Rimington P.D. Recurrent spontaneous bladder rupture. A case report. S Afr Med J. 1989;75(9):445–446. [PubMed] [Google Scholar]
- 14.Christensen J.B., Wara P., Hillstom C. Spontaneous rupture of the urine bladder. Report of three cases. Scand J Urol Nephrol. 1986;20(1):73–74. doi: 10.3109/00365598609024484. [DOI] [PubMed] [Google Scholar]
- 15.Arda E., Cakiroglu B., Thomas D. Primary nocturnal enuresis: a review. Nephrourol Mon. 2016;8(4):e35809. doi: 10.5812/numonthly.35809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wystrychowski A., Nowicki M., Kokot F. Hyponatraemic renal pseudofailure – don’t forget the possibility of uroperitoneum. Nephrol Dial Transplant. 1996;11:2491–2492. doi: 10.1093/oxfordjournals.ndt.a027222. [DOI] [PubMed] [Google Scholar]
- 17.Levin T., Han B., Little B. Congenital anomalies of the male urethra. Pediatr Radiol. 2007;37(9):851–862. doi: 10.1007/s00247-007-0495-0. [DOI] [PMC free article] [PubMed] [Google Scholar]