Abstract
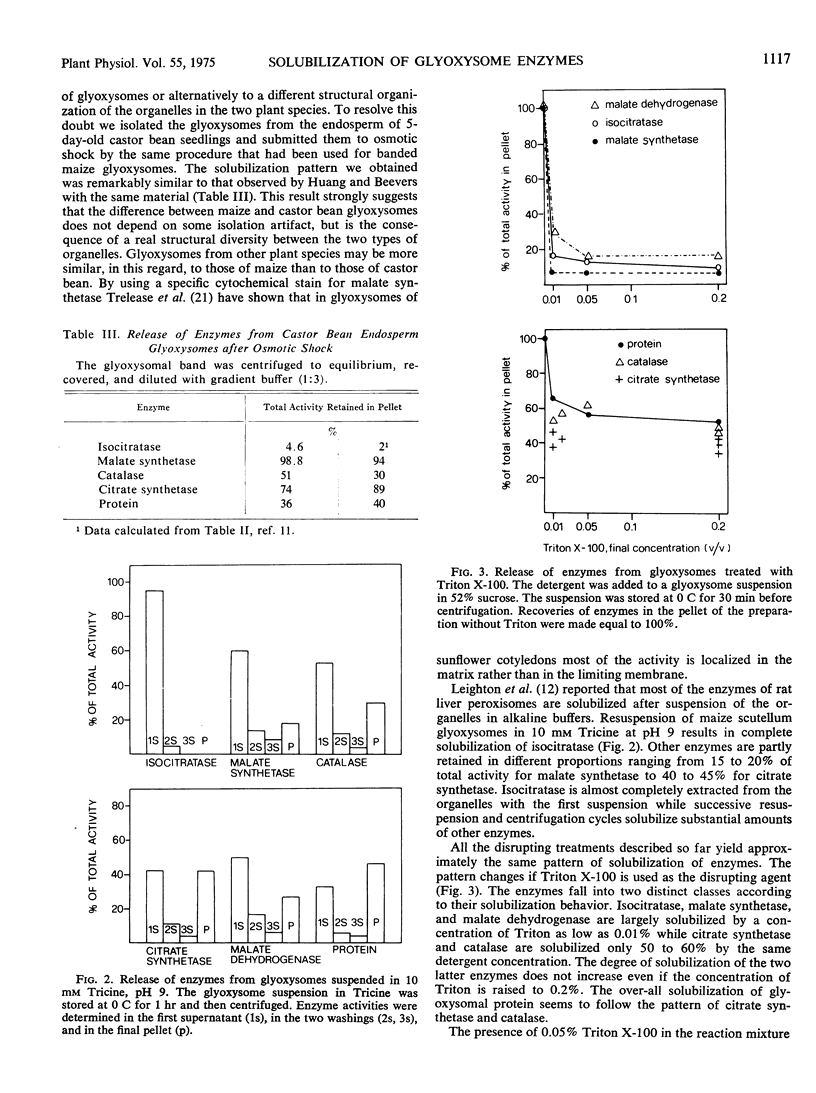
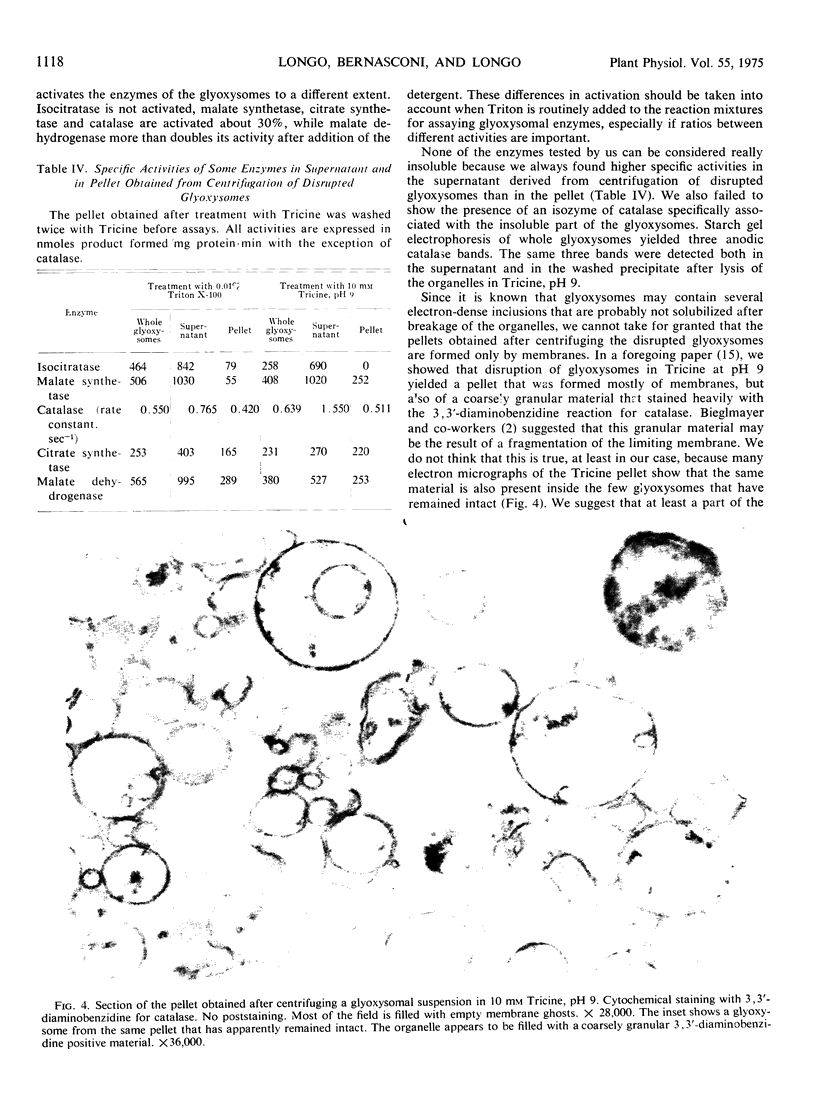
Glyoxysomes isolated from maize scutella (Zea mays L.) were subjected to several disruptive treatments (osmotic shock, resuspension in an alkaline medium, addition of detergent). The damaged glyoxysomes were centrifuged at 89,500g for 40 minutes and several enzymic activities (isocitratase, malate synthetase, catalase, citrate synthetase, malate dehydrogenase) were measured in the supernatant fraction and in the pellet. Isocitratase is the most easily released of all glyoxysomal enzymes closely followed by malate synthetase. Citrate synthetase is in all instances the most insoluble enzyme. All of the enzymes had higher specific activities in the supernatant than in the pellet. These findings suggest that in corn scutellum glyoxysomes none of these enzymes is truly membrane-bound.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbareschi D., Longo G. P., Servettaz O., Zulian T., Longo C. P. Citrate synthetase in mitochondria and glyoxysomes of maize scutellum. Plant Physiol. 1974 Jun;53(6):802–807. doi: 10.1104/pp.53.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieglmayer C., Graf J., Ruis H. Membranes of glyoxysomes from castor-bean endosperm. Enzymes bound to purified-membrane preparations. Eur J Biochem. 1973 Sep 3;37(3):553–562. doi: 10.1111/j.1432-1033.1973.tb03018.x. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt B. P., Beevers H. Developmental studies on glyoxysomes in Ricinus endosperm. J Cell Biol. 1970 Jan;44(1):94–102. doi: 10.1083/jcb.44.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt B., Beevers H. Influence of sucrose on protein determination by the Lowry procedure. Anal Biochem. 1968 Aug;24(2):337–339. doi: 10.1016/0003-2697(68)90187-5. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Suga T., Ninobe S. Studies on peroxisones. 3. Further studies on the intraparticulate localization of peroxisomal components in the liver of the rat. Biochim Biophys Acta. 1973 Jan 24;297(1):110–119. doi: 10.1016/0304-4165(73)90054-8. [DOI] [PubMed] [Google Scholar]
- Huang A. H., Beevers H. Localization of enzymes within microbodies. J Cell Biol. 1973 Aug;58(2):379–389. doi: 10.1083/jcb.58.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton F., Poole B., Lazarow P. B., De Duve C. The synthesis and turnover of rat liver peroxisomes. I. Fractionation of peroxisome proteins. J Cell Biol. 1969 May;41(2):521–535. doi: 10.1083/jcb.41.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo C. P., Longo G. P. The development of glyoxysomes in peanut cotyledons and maize scutella. Plant Physiol. 1970 Mar;45(3):249–254. doi: 10.1104/pp.45.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Dragonetti C., Longo C. P. Cytochemical localization of catalase in glyoxysomes isolated from maize scutella. Plant Physiol. 1972 Oct;50(4):463–468. doi: 10.1104/pp.50.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo G. P., Longo C. P. The development of glyoxysomes in maize scutellum: changes in morphology and enzyme compartmentation. Plant Physiol. 1970 Oct;46(4):599–604. doi: 10.1104/pp.46.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Burke J. J. Cytochemical localization of malate synthase in glyoxysomes. J Cell Biol. 1974 Feb;60(2):483–495. doi: 10.1083/jcb.60.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigil E. L. Structure and function of plant microbodies. Subcell Biochem. 1973;2(3):237–285. [PubMed] [Google Scholar]



