Main Text
Cellular immunotherapy using chimeric antigen receptor (CAR)-bearing T cells has shown promise in early clinical trials, especially for the treatment of CD19+ B cell malignancies.1 Despite impressive remission rates in this clinical setting, associated toxicity has been observed.2 CAR T cells directed against solid tumor antigens have fared less well, with few durable clinical responses but still associated toxicity.3 Toxicity results in part from the “on-target, off-tumor” effects of CAR-T cells, as the tumor-targeting ectodomain of the CAR molecule cannot discern target molecules expressed on tumors versus normal tissues. In this issue of Molecular Therapy, Fisher et al.4 describe a unique approach using γδ-T cells as the platform for expression of a novel co-stimulatory CAR, arguing for the potential of these uniquely engineered cells to mediate tumor-specific killing with far less off-tumor toxicity.
γδ-T cells are a minor subset of peripheral lymphocytes in humans (<5%). Unlike αβ-T cells (the major circulating T cell subset, which are commonly utilized in creating CAR T cell immunotherapy products), γδ-T cells do not require major histocompatibility complex (MHC) class I or II molecules for recognizing antigens.5 γδ-T cells respond to non-peptide phosphoantigens generated in the eukaryotic mevalonate metabolic pathway. The most abundant γδ-T cells express the Vγ9Vδ2 T cell receptor (TCR) that recognizes isopentenyl pyrophosphate (IPP), which is overproduced in cancer cells.5 The dysregulated mevalonate pathway in tumors leads to higher concentrations of IPP, which is sensed by γδ-TCR as a “danger signal.” Hence, γδ-T cells can discriminate tumor cells with dysregulated metabolism, which express these danger signals, from healthy cells, which do not.
γδ-T cells are thought to help bridge the innate and adaptive immune systems, and, thus, functionally and phenotypically share components of both.6 For example, γδ-T cells express numerous receptors typically found on innate immune effectors, such as natural killer (NK) cells, which play crucial roles in anti-tumor responses. Natural killer group 2 member protein D (NKG2D) expressed on Vγ9Vδ2 T cells binds to non-classical MHC molecules (MICA/B and the ULBPs) expressed on tumor cells. Ligand binding to NKG2D activates γδ-T cells via the intracellular signaling molecule, DAP10, with subsequent release of anti-tumor cytokines and enhanced cytotoxicity.
The unique tumor recognition function of γδ-T cells that provides broad reactivity to many different types of tumors, combined with recent success in their large-scale expansion for adoptive transfer into humans, has created a renewed interest to explore their anti-tumor therapeutic potential. The safety and efficacy of unmanipulated ex vivo-expanded γδ-T cells have been evaluated in several clinical trials.7 Introduction of CAR expression on γδ-T cells has resulted in enhanced cytotoxicity in pre-clinical models,8 but has not yet been evaluated in clinical trials.
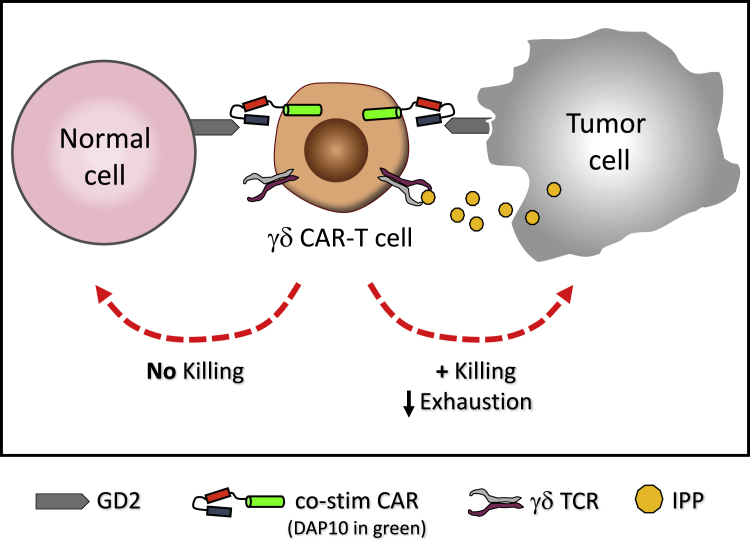
Traditional CARs comprise an ectodomain that recognizes a tumor-associated antigen fused to endodomains that provide stimulatory signals. When expressed in αβ-T cells, CAR endodomains contain CD3ζ (signal 1 for T cell activation), which provides cytotoxic capacity, in addition to one (2nd generation) or two (3rd generation) co-stimulatory endodomains, such as CD28 or 41BB (signal 2 for T cell activation), that foster expansion and viability.9 Unfortunately, this all-in-one signaling moiety, which is completely independent of the native TCR, has led to unexpected toxicity, mostly owing to CAR-T cell activity in response to off-tumor antigen expression.3 In order to limit this toxicity against normal tissue, Fisher et al.4 devised a CAR design in which signals 1 and 2 for γδ-T cell activation are provided by separate receptors. The cytotoxic capacity of CD3ζ (signal 1) is mediated through the native γδ-TCR recognizing the tumor-associated danger signal, IPP, while co-stimulation (signal 2) is provided by a CAR recognizing the GD2 solid tumor antigen with an endodomain containing the innate NKG2D signaling molecule, DAP10. In this schema, the CAR-γδ T cell can be stimulated only after recognition of two separate and distinct tumor-associated molecules, whereas recognition of either alone would result in no activation (Figure 1). In this manner, CAR recognition of a tumor-associated antigen on normal (off-tumor) tissue would not result in unwanted toxicity.
Figure 1.
“Co-stimulation Only” CAR-γδ T Cell Activation after Recognition of Two Separate Tumor-Associated Molecules
The cytotoxic capacity of CD3ζ (signal 1) is mediated through the native γδ-TCR recognizing the tumor-associated danger signal, IPP, while co-stimulation (signal 2) is provided by a CAR recognizing the GD2 solid tumor antigen with an endodomain consisting of the innate NKG2D signaling molecule, DAP10. Normal healthy tissue does not express IPP and thus does not activate γδ T cells through their TCR.
The investigators compared the effects of two retroviral CAR constructs (a traditional 2nd generation CAR directed against GD2 and the γδ “co-stimulation only” CAR with anti-GD2 ectodomain and DAP10 endodomain) on γδ-T cell antitumor efficacy. Using in vitro cytokine secretion and cytotoxicity against human tumor lines as markers for γδ-T cell activation, the investigators showed that, while the traditional GD2 CAR-mediated activation of γδ-T cells in the presence of the CAR antigen alone, the “co-stimulation only” CAR-expressing γδ-T cells required both tumor-associated IPP and the CAR antigen for activation. This effect was not seen in αβ-T cells expressing the “co-stimulation only” CAR or against GD2− tumor targets, confirming the specificity of both signals in γδ-T cells. Interestingly, when the human GD2 tumor antigen was expressed in a murine tumor line that failed to engage the human γδ-TCR but would still be able to elicit a CAR response, γδ-T cells expressing the traditional CAR exhibited killing of tumor targets, whereas the “co-stimulation only” CAR-expressing γδ-T cells did not. The investigators argued that this was a method to mimic “healthy tissue” that expresses GD2 antigen to show the potential for decreased “off-tumor” toxicity. Finally, the authors compared the expression of exhaustion markers on γδ-T cells expressing the traditional CAR versus the “co-stimulation only” CAR. They showed that while “co-stimulation only” CAR expression induced upregulation of the exhaustion makers, PD-1 and TIM-3, they were far more pronounced when the traditional CAR was expressed. The authors thus provide a proof-of-principle of their concept that by using γδ-T cells expressing a “co-stimulation only” CAR, as opposed to γδ-T cells expressing a traditional CAR or αβ-T cells expressing a “co-stimulation only” CAR, activation can occur only in the presence of two distinct tumor-associated signals.
The findings of the current study inspire a few key points that deserve additional consideration. First, the concept of “off-tumor” toxicity is dependent on normal tissue not expressing danger signals, such as IPP, which are capable of activating the native γδ-TCR. Although these danger signals are known to not be expressed in healthy adults, patients with a highly pro-inflammatory disease, such as cancer, may express these signals from “normal” tissues at various time points in the disease process. Indeed, other stress-induced danger signals, such as the NKG2D ligands MICA/B, are known to be upregulated in normal tissues in patients with cancer,10 making off-tumor toxicity possible even when employing “co-stimulation only” CAR γδ-T cells. Although it has been difficult for the CAR-T cell field in general to develop animal models that mimic “off-tumor” toxicity, testing an approach such as this certainly would require either development of such a model or early implementation of phase 1 trials to test the concept in patients with cancer.
Second, as the authors themselves concede, the concept of a co-stimulatory CAR approach in which signals 1 and 2 of T cell activation are provided by separate receptors has been proposed and investigated previously in αβ-T cells. Lanitis et al.11 developed a trans signaling CAR strategy where signal 1 (CD3ζ) was physically dissociated from co-stimulatory signal 2 (CD28) in two separate CARs of differing antigen specificity. These trans-signaling CAR-T cells showed weak cytokine secretion and cytotoxicity against target cells expressing only one antigen but showed enhanced activity against tumor cells expressing both antigens. To address the possibility that a CAR containing signal 1 could “overpower” the need for co-stimulation from the second CAR, depending on the density and binding avidity of the tumor antigens, Kloss et al.12 reported a combinatorial antigen recognition and balanced signaling approach in which the CAR containing signal 1 (CD3ζ) was constructed to be deliberately inefficient at killing and, only when combined with signal 2 (a separate CD28-containing CAR), would lead to optimal T cell activation. More recently, Roybal et al.13 reported a combinatorially activated T cell circuit in which a synthetic Notch receptor for one tumor antigen induced the expression of a CAR for a second tumor antigen. These dual receptor “AND-gate” T cells were only armed/activated in the presence of dual antigen-expressing tumor targets. All these approaches, however, were employed in αβ-T cells in which the contributions of the native TCR were essentially ignored. The approach detailed in the current study by Fisher et al.4 utilizes the endogenous signaling cascade of the native γδ-TCR, which potentially has the benefit of more robust and controlled signaling. Importantly, use of danger signals, as opposed to a tumor-associated antigen, allows the γδ-T cell approach to be utilized for myriad tumor types, some of which may not have more than one tumor-associated antigen target available. Further modeling both in vitro and in vivo is needed to clarify several points about universality and safety.
In an era when, for some specific cancer types, the clinical efficacy of CAR-T cell adoptive immunotherapy is being established, approaches to improve tumor specificity and avoidance of off-tumor toxicity are now needed. Certainly, the approach detailed in the current report by Fisher et al.4 represents a unique method that offers the possibility of an alternative T cell product that can deliver dual targeted anti-tumor activity with minimal toxicity.
References
- 1.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morgan R.A., Yang J.C., Kitano M., Dudley M.E., Laurencot C.M., Rosenberg S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher J., Abramowski P., Wisidagamage Don N.D., Flutter B., Capsomidis A., Cheung G.W.K., Gustafsson K., Anderson J. Avoidance of on-target off tumor activation using a costimulation-only chimeric antigen receptor. Mol. Ther. 2017;25:1234–1247. doi: 10.1016/j.ymthe.2017.03.002. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morita C.T., Jin C., Sarikonda G., Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol. Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 6.Mak T.W., Ferrick D.A. The gammadelta T-cell bridge: linking innate and acquired immunity. Nat. Med. 1998;4:764–765. doi: 10.1038/nm0798-764. [DOI] [PubMed] [Google Scholar]
- 7.Chiplunkar S., Dhar S., Wesch D., Kabelitz D. gammadelta T cells in cancer immunotherapy: current status and future prospects. Immunotherapy. 2009;1:663–678. doi: 10.2217/imt.09.27. [DOI] [PubMed] [Google Scholar]
- 8.Deniger D.C., Moyes J.S., Cooper L.J. Clinical applications of gamma delta T cells with multivalent immunity. Front. Immunol. 2014;5:636. doi: 10.3389/fimmu.2014.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raulet D.H., Gasser S., Gowen B.G., Deng W., Jung H. Regulation of ligands for the NKG2D activating receptor. Annu. Rev. Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanitis E., Poussin M., Klattenhoff A.W., Song D., Sandaltzopoulos R., June C.H., Powell D.J., Jr. Chimeric antigen receptor T Cells with dissociated signaling domains exhibit focused antitumor activity with reduced potential for toxicity in vivo. Cancer Immunol. Res. 2013;1:43–53. doi: 10.1158/2326-6066.CIR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kloss C.C., Condomines M., Cartellieri M., Bachmann M., Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat. Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roybal K.T., Rupp L.J., Morsut L., Walker W.J., McNally K.A., Park J.S., Lim W.A. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164:770–779. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]