Abstract
Chimeric antigen receptors (CARs) are synthetic receptors that reprogram T lymphocytes to target chosen antigens. The targeting of CD19, a cell surface molecule expressed in the vast majority of leukemias and lymphomas, has been successfully translated in the clinic, earning CAR therapy a special distinction in the selection of “cancer immunotherapy” by Science as the breakthrough of the year in 2013. CD19 CAR therapy is predicated on advances in genetic engineering, T cell biology, tumor immunology, synthetic biology, target identification, cell manufacturing sciences, and regulatory compliance—the central tenets of CAR therapy. Here, we review two of these foundations: the genetic engineering approaches and cell types to engineer.
Keywords: CAR, immunotherapy, genetic engineering, T cells, vectors, genome editing
Riviere and Sadelain review the genetic and cellular foundations of CD19 chimeric antigen receptor (CAR) therapy. CAR therapy has made a great impact on the management of relapsed B cell malignancies and prospects for cell and gene therapies in oncology.
Main Text
Chimeric antigen receptors (CARs) are synthetic receptors that target T cells to a chosen antigen and reprogram T cell function, metabolism, and persistence.1, 2 Through their extracellular domain, CARs bind cell surface molecules independently of major histocompatibility complex (MHC), in contrast to the physiological T cell receptor, which engages MHC/peptide complexes. CARs may thus target proteins, carbohydrates, or glycolipids and function irrespective of patient HLA haplotype. Binding to antigen triggers T cell activation, which is commonly mediated by the cytoplasmic domain of the CD3-ζ chain.3, 4, 5, 6, 7 Merely providing T cell activation is, however, not sufficient to direct a productive immune response.8, 9, 10 The CARs that have provided tangible clinical benefits incorporate a costimulatory domain,11 which enables T cells to expand and retain their functionality upon repeated exposure to antigen.12 These receptors have been dubbed second generation CARs13 and are key to the design of persisting engineered T cells that constitute a “living drug” attacking tumors as long as they retain their functionality. Several recent reviews have addressed CAR design,14, 15, 16, 17, 18 CAR prospects for solid tumors,19, 20 and T cell manufacturing.21, 22, 23
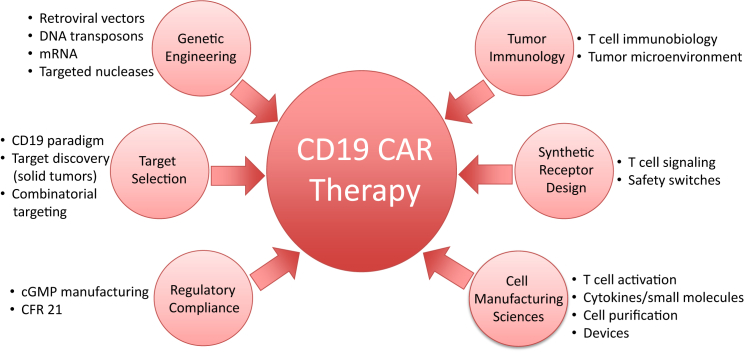
The targeting of CD19,24 a cell surface molecule expressed in the vast majority of leukemias and lymphomas, has been successfully translated in the clinic, earning CAR therapy a special distinction in the selection of “cancer immunotherapy” by Science as the breakthrough of the year in 2013.25 The genesis of CD19 CAR therapy is predicated on the convergence of scientific advances in genetic engineering, T cell biology, tumor immunology, synthetic biology, target identification, cell manufacturing sciences, and regulatory compliance, all of which were needed to enable innovative phase 1 clinical trials (Figure 1). We review here the vectors and cells that are the foundations of present and upcoming CAR therapies.
Figure 1.
Assembling a CAR
CAR therapy is predicated on cell engineering and the convergence of multiple disciplines and technologies.
The Vectors
γ-Retroviral and Lentiviral Vectors
The implementation of T cell engineering begins with forging suitable tools to genetically modify primary lymphocytes. The first successful attempts made use of ecotropic γ-retroviral vectors to transduce mitogen-activated mouse T cells (M. Sadelain and R.C. Mulligan, 1992, Int. Congr. Immunol., abstract). This approach was subsequently adapted to human T cells, incorporating the gibbon ape leukemia virus (GALV) envelope to mediate retroviral vector entry.26, 27, 28, 29, 30 These advances were pivotal for launching mouse and human T cell engineering, which had been hitherto limited to transfection of surrogate leukemia cell lines or hybridomas, which do not recapitulate several critical features of normal T cell proliferation, function, and survival. Receptors and signaling molecules could now be studied in authentic T cells easily harvested from peripheral blood. Retroviral vectors would eventually be the first to be evaluated in the context of T cell-based therapies31, 32, 33, 34, 35, 36, 37, 38 and continue to be relied upon in CAR therapy to this day.39, 40, 41, 42
Retroviral vectors derived from murine leukemia virus require that the target cells divide to allow proviral integration.43, 44 This property was eventually exploited to preferentially transduce cycling T cells within mixed T cell populations.45 In contrast, lentiviral vectors are able to successfully infect nondividing cells, owing to the nuclear translocation capability of the HIV-1 preintegration complex.46, 47, 48, 49 Lentiviruses, however, require that non-dividing cells proceed at least to the G1b stage of the cell cycle to support reverse transcription and allow for completion of retroviral integration.50 Although lentiviral vectors have been reported to transduce cytokine-activated T cells apparently without S phase progression,51, 52 lentiviral vectors are commonly used like γ-retroviral after in vitro activation of T cells.53, 54, 55
γ-retroviral vectors and lentiviral vectors both integrate semi-randomly in the human genome, with similar preference for transcribed genic regions but with some differences (near transcriptional start sites for the former, more evenly distributed intra-genically for the latter).56, 57, 58 This subtle difference is thought to contribute to the lesser genotoxicity of lentiviral vectors in hematopoietic progenitors.59 The relevance of this divergence between retroviral vector types to T cell therapy is, however, uncertain, given the rarity of oncogenic T cell transformation encountered with γ-retroviral vectors.60, 61, 62, 63 Another feature of retroviral vectors is their mutation rate incurred during reverse transcription, which is about 5-fold higher for HIV-1 reverse transcriptase compared with that of Moloney murine leukemia virus (Mo-MLV).64, 65, 66, 67, 68, 69
DNA Transposons
γ-retroviral and lentiviral vectors are complex biological reagents that require expensive biosafety testing and storage. Non-viral approaches would be advantageous, provided that they were as effective. The Sleeping Beauty transposon/transposase system70 has been used to introduce CARs into T cells by electroporation.71 The advantages of this system are its simple manufacturing procedure, relatively low cost, and straightforward release testing. Integration is random, posing a potential oncogenic risk secondary to mutagenesis.72 Ongoing CD19 CAR T cell trials using the Sleeping Beauty transposon/transposase show low T cell toxicity.73 However, the efficacy of CAR T cells generated by this approach remains to be demonstrated.
RNA Transfection
In contrast to the stable and permanent transgene expression afforded by retroviral infection or plasmid DNA transfection, transient expression can be obtained following electroporation or endocytosis of in vitro transcribed messenger RNA (mRNA). This approach eliminates the concerns of genotoxicity and potential generation of a replication-competent retrovirus. RNA transfection allows the expression of the transgene for up to 1 week and has been used to deliver mRNA for physiological T cell receptor (TCR)/CAR, chemokine receptors, and cytokines.74, 75, 76 This approach may be advantageous to screen potentially toxic CAR molecules that could cross-react with normal tissues. Beatty and colleagues77 found that repetitive infusions of mRNA-transduced CAR T cells could elicit an anti-tumor effect in some patients.
Genome Editing
Genome editing technologies are further expanding the landscape of human cell engineering. Four technologies based on the use of targeted nucleases, including meganucleases, zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and CRISPR/Cas9, enable gene disruption in human cells.78, 79, 80, 81, 82, 83 ZFNs and CRISPR/Cas9 are presently the most developed of these tools and have been used, for example, to efficiently target the HIV co-receptor CCR5.84, 85, 86 Gene editing techniques have also been used to disrupt TCR genes and others (e.g., PD-1) in primary T cells,86, 87, 88, 89, 90, 91, 92 further expanding the possibilities of T cell engineering. A first proof-of-concept study reported that the electroporation of TALENs specific for disrupting TCRα and CD52 molecules allows the generation of off-the-shelf CAR T cells from third-party healthy donors.93 We recently demonstrated that knocking in a CAR cDNA to the TCR locus improved CAR expression and signaling, resulting in superior anti-tumor activity.94 T cell genome editing is poised to significantly advance the therapeutic potential of engineered T cells.
The Cells
Autologous T Cells
Autologous T cells are the logical starting point for T cell engineering because they will not attack the recipient or be rejected by the recipient because of their self-origin. Unlike the isolation of tumor-infiltrating T cells, they do not require a surgical intervention and can be simply collected from blood. The engineered T cells have to be able to migrate to tumor sites, expand, and persist in a functional state long enough to eradicate the tumor. While the CAR itself retargets and reprograms the genetically modified lymphocyte, the native properties of the engineered T cell can affect therapeutic potency.95, 96 After leaving the thymus, naive T cells (TN) differentiate into distinct subsets that play specific roles in protective immunity, including memory stem (TSCM), central memory (TCM), effector memory (TEM), tissue resident memory (TRM), and effector (TE) cells, which are endowed with different functional and expansion capabilities.97, 98, 99, 100, 101, 102 At this time, genetic modification of TN, TSCM, and TCM subsets appears to be better suited for enhanced therapeutic efficacy.95, 103, 104, 105, 106, 107, 108, 109
Allogeneic T Cells
Autologous approaches now have a proven track record in the clinic, but personalized manufacture imposes significant logistical constraints and may be challenging in some instances, for example, in patients with chemotherapy or HIV-induced immune deficiency, or in small infants. The promising clinical results of autologous engineered T cell therapy could be further broadened if potent and histocompatible T cells were readily available. Although T cells can be easily harvested from donors, their use is restricted by their alloreactive potential.110 This property underlies the high risk of graft rejection in transplant recipients and of graft-versus-host disease (GVHD) in recipients of donor-derived T cells. To provide an acceptable risk-benefit ratio, donor T cells must be devoid of alloreactive potential. Two strategies are currently in use, based either on the selection of virus-specific T cells or the ablation of TCR expression. Virus-specific T cells lacking alloreactive potential are one potential cellular vehicle for CAR-mediated tumor targeting. Although alloreactivity and unanticipated TCR cross-reactivity cannot be prospectively eliminated with full certainty,111, 112 recent studies suggest that virus-specific T cells can be administered to multiple recipients with limited risk of GVHD.113, 114 Virus-specific T cells (VSTs) may thus serve as cellular vehicles for TCR or CAR therapy. A first trial evaluating VSTs to express CARs found that such T cells expanded in response to viral reactivation, although anti-tumor activity was modest.115 Murine studies have recently revealed the challenges of having both a TCR and a CAR functioning in the same T cell.116 Another approach thus consists in abrogating TCR expression, which is now feasible with targeted nucleases. TCR-deleted lymphocytes cannot mediate GVHD reactivity, but their long-term persistence could potentially be compromised, because homeostatic proliferation is in part dependent on TCR-MHC interactions.117, 118 Furthermore, gene disruption technologies are still in early stages of development and require optimization to afford efficient targeting without genotoxicity at a reasonable cost. Finally, one should be reminded that preventing GVHD potential does not address the opposite response—donor T cell rejection by the recipient—and is thus limited to the treatment of immunocompromised recipients. Donor T cell approaches are thus still labor intensive and constrained by the limited replicative potential of mature T cells.96
Alternative T Cell Sources: Lymphoid Progenitors and Pluripotent Stem Cells
Lymphocyte engineering is not limited to mature T cells and may be performed in lymphoid precursors. T cell progenitors can be generated in vitro119 and be transplanted across MHC barriers, generating T cells that are restricted to host MHC.120 When transduced with a CAR, allogeneic lymphoid progenitors yield tumor-targeted T cells without causing GVHD.121 T cells may also be generated from human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSC) in vitro.122, 123, 124, 125, 126 iPSC-derived T cells expressing a CAR can eradicate tumors in vivo,126 providing a foundation for further exploiting the formidable potential of self-renewing stem cells to engineer therapeutic T cells. The combination of iPSC technology and immune engineering may thus provide an opportunity to generate T cells that uniquely combine favorable attributes including antigen specificity, lack of alloreactivity, enhanced functional properties, and histocompatibility.127
Regulatory T Cells
The potential of engineered T cells may extend beyond cancer to autoimmunity. CAR T cells can eliminate autoreactive B cells as demonstrated in a mouse model of pemphigus vulgaris.128 Engineered T regulatory cells may also be harnessed to dampen immune responses,129, 130 which may be useful in the context of autoimmunity129, 131, 132 and transplantation tolerance.133 This field of application of CAR technology is poised for further development.
Clinical Results: The CD19 Paradigm
To successfully translate CAR therapy in the clinic, one not only needs a powerful CAR but also a suitable target, which ideally is expressed in all tumor cells and absent from all normal cells, or at least vital cells. We identified CD19 as a promising target24 based on its cell-surface expression in most leukemia and lymphomas, and its role in signaling.134, 135, 136 As expected, targeting CD19 induces a B cell aplasia,137, 138, 139, 140 which is clinically manageable especially if it is limited in time. Of interest to us was the added possibility that B cell elimination would prevent the emergence of putative anti-CAR antibodies, which were eventually observed in other CAR T cell trials.141, 142
Our studies on CD19 CARs were the first to demonstrate complete tumor eradication of established, systemic lymphoma following a single infusion of CAR T cells,24 providing the foundation and rationale for subsequent clinical studies. Three groups reported promising early results in three different B cell malignancies: diffuse large B cell lymphoma,143 chronic lymphocytic leukemia (CLL),144 and acute lymphoblastic leukemia (ALL).145 These studies focused on relapsed, chemotherapy refractory patients, and made use of either CD28- or 4-1BB-based CARs.16 Strikingly, despite differences in disease histology, single-chain variable fragment (scFv), vector utilization, and manufacturing process, all groups have reported remarkably high rates of overall and complete response, especially in ALL109, 146, 147, 148, 149, 150 (J.H. Park, et al., 2016, J. Clin. Oncol., abstract) (Table 1). These results have placed T cell engineering and CAR T cells at the center of the cancer immunotherapy revolution that is unfolding today in oncology.25 These clinical results have been extensively reviewed elsewhere.16, 151, 152, 153 They arguably rank among the most compelling clinical achievements obtained through cell and gene therapy to date.
Table 1.
CD19 CAR Therapy for Acute Lymphoblastic Leukemia
| References | Disease | CAR | V | N | T Cells | CR Rate (%) |
|---|---|---|---|---|---|---|
| 147 | ALL (Ad) | CD28 | gRV | 16 | auto | 88 |
| 154 | ALL (Ped) | 4-1BB | LV | 2 | auto | 100 |
| 148 | ALL (Ped) | 4-1BB | LV | 25 | auto | 90 |
| 150 | ALL (Ped) | CD28 | gRV | 21 | auto | 68 |
| J.H. Park, et al., 2016, J. Clin. Oncol., abstract | ALL (Ad) | CD28 | gRV | 46 | auto | 91 |
| C.J. Turtle, et al., 2016, J Clin Invest, abstract | ALL (Ad) | 4-1BB | LV | 29 | 1:14/8 | 93 |
| 93 | ALL (Ped) | 4-1BB | LV | 2 | alloa | 100 |
Ad, adult; allo, allogeneic T cells; auto, autologous T cells; CR, complete remission; gRV, γ-retroviral vector; LV, lentiviral vector; Ped, pediatric; V, vector type; 1:1 CD4/CD8 ratio.
With TALEN-mediated TCR deletion.
CAR Therapy in the Next Decade
Cell and gene engineering technologies have propelled CAR therapy to an effective and tractable therapy, based on the CD19 paradigm.16 CAR therapy has, however, not yet reached its full potential. As reviewed here, there still are numerous questions as how to best harness genetic engineering and cell biology to generate the best medicines. Resolving the toxicities sometimes encountered with engineered T cells and devising how to tackle the vast realm of solid tumors are the next tasks to address. CARs also have the potential to impact on autoimmunity and transplantation tolerance. CD19 CAR therapy has sparked pharmaceutical industry interest more than any other cell therapy before it. The flourishing intellectual and financial capitalization of CAR therapy will likely benefit all other cell engineering therapies. T cell engineering was not easily accepted, but it will now, hopefully, inspire a new generation of scientists and physicians who seek curative medicines through engineered immunity.
References
- 1.Eshhar Z., Bach N., Fitzer-Attas C.J., Gross G., Lustgarten J., Waks T., Schindler D.G. The T-body approach: potential for cancer immunotherapy. Springer Semin. Immunopathol. 1996;18:199–209. doi: 10.1007/BF00820666. [DOI] [PubMed] [Google Scholar]
- 2.Sadelain M., Rivière I., Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 3.Irving B.A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 4.Romeo C., Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell. 1991;64:1037–1046. doi: 10.1016/0092-8674(91)90327-u. [DOI] [PubMed] [Google Scholar]
- 5.Letourneur F., Klausner R.D. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc. Natl. Acad. Sci. USA. 1991;88:8905–8909. doi: 10.1073/pnas.88.20.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eshhar Z., Waks T., Gross G., Schindler D.G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc. Natl. Acad. Sci. USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brocker T., Peter A., Traunecker A., Karjalainen K. New simplified molecular design for functional T cell receptor. Eur. J. Immunol. 1993;23:1435–1439. doi: 10.1002/eji.1830230705. [DOI] [PubMed] [Google Scholar]
- 8.Brocker T., Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J. Exp. Med. 1995;181:1653–1659. doi: 10.1084/jem.181.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gong M.C., Latouche J.B., Krause A., Heston W.D., Bander N.H., Sadelain M. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia. 1999;1:123–127. doi: 10.1038/sj.neo.7900018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood. 2000;96:1999–2001. [PubMed] [Google Scholar]
- 11.Krause A., Guo H.F., Latouche J.B., Tan C., Cheung N.K., Sadelain M. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 1998;188:619–626. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maher J., Brentjens R.J., Gunset G., Rivière I., Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat. Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 13.Sadelain M., Brentjens R., Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr. Opin. Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen M.C., Riddell S.R. Designing chimeric antigen receptors to effectively and safely target tumors. Curr. Opin. Immunol. 2015;33:9–15. doi: 10.1016/j.coi.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Stegen S.J., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadelain M. CAR therapy: the CD19 paradigm. J. Clin. Invest. 2015;125:3392–3400. doi: 10.1172/JCI80010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maus M.V., June C.H. Making better chimeric antigen receptors for adoptive T-cell therapy. Clin. Cancer Res. 2016;22:1875–1884. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esensten J.H., Bluestone J.A., Lim W.A. Engineering therapeutic T cells: from synthetic biology to clinical trials. Annu. Rev. Pathol. 2017;12:305–330. doi: 10.1146/annurev-pathol-052016-100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinrichs C.S., Restifo N.P. Reassessing target antigens for adoptive T-cell therapy. Nat. Biotechnol. 2013;31:999–1008. doi: 10.1038/nbt.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morello A., Sadelain M., Adusumilli P.S. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6:133–146. doi: 10.1158/2159-8290.CD-15-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X., Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol. Ther. Oncolytics. 2016;3:16015. doi: 10.1038/mto.2016.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine B.L., Miskin J., Wonnacott K., Keir C. Global manufacturing of CAR T cell therapy. Mol. Ther. Methods Clin. Dev. 2016;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Rivière I. Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies. Cancer Gene Ther. 2015;22:85–94. doi: 10.1038/cgt.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brentjens R.J., Latouche J.B., Santos E., Marti F., Gong M.C., Lyddane C., King P.D., Larson S., Weiss M., Rivière I., Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat. Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 25.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 26.Miller A.D., Garcia J.V., von Suhr N., Lynch C.M., Wilson C., Eiden M.V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J. Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavilio F., Ferrari G., Rossini S., Nobili N., Bonini C., Casorati G., Traversari C., Bordignon C. Peripheral blood lymphocytes as target cells of retroviral vector-mediated gene transfer. Blood. 1994;83:1988–1997. [PubMed] [Google Scholar]
- 28.Bunnell B.A., Muul L.M., Donahue R.E., Blaese R.M., Morgan R.A. High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA. 1995;92:7739–7743. doi: 10.1073/pnas.92.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallardo H.F., Tan C., Ory D., Sadelain M. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood. 1997;90:952–957. [PubMed] [Google Scholar]
- 30.Sadelain M. Methods for retrovirus-mediated gene transfer into primary T-lymphocytes. In: Robbins P.D., editor. Gene Therapy Protocols. Humana Press; 1997. pp. 241–248. [DOI] [PubMed] [Google Scholar]
- 31.Onodera M., Ariga T., Kawamura N., Kobayashi I., Ohtsu M., Yamada M., Tame A., Furuta H., Okano M., Matsumoto S. Successful peripheral T-lymphocyte-directed gene transfer for a patient with severe combined immune deficiency caused by adenosine deaminase deficiency. Blood. 1998;91:30–36. [PubMed] [Google Scholar]
- 32.Palù G., Li Pira G., Gennari F., Fenoglio D., Parolin C., Manca F. Genetically modified immunocompetent cells in HIV infection. Gene Ther. 2001;8:1593–1600. doi: 10.1038/sj.gt.3301569. [DOI] [PubMed] [Google Scholar]
- 33.Deeks S.G., Wagner B., Anton P.A., Mitsuyasu R.T., Scadden D.T., Huang C., Macken C., Richman D.D., Christopherson C., June C.H. A phase II randomized study of HIV-specific T-cell gene therapy in subjects with undetectable plasma viremia on combination antiretroviral therapy. Mol. Ther. 2002;5:788–797. doi: 10.1006/mthe.2002.0611. [DOI] [PubMed] [Google Scholar]
- 34.Bonini C., Grez M., Traversari C., Ciceri F., Marktel S., Ferrari G., Dinauer M., Sadat M., Aiuti A., Deola S. Safety of retroviral gene marking with a truncated NGF receptor. Nat. Med. 2003;9:367–369. doi: 10.1038/nm0403-367. [DOI] [PubMed] [Google Scholar]
- 35.Muul L.M., Tuschong L.M., Soenen S.L., Jagadeesh G.J., Ramsey W.J., Long Z., Carter C.S., Garabedian E.K., Alleyne M., Brown M. Persistence and expression of the adenosine deaminase gene for 12 years and immune reaction to gene transfer components: long-term results of the first clinical gene therapy trial. Blood. 2003;101:2563–2569. doi: 10.1182/blood-2002-09-2800. [DOI] [PubMed] [Google Scholar]
- 36.Macpherson J.L., Boyd M.P., Arndt A.J., Todd A.V., Fanning G.C., Ely J.A., Elliott F., Knop A., Raponi M., Murray J. Long-term survival and concomitant gene expression of ribozyme-transduced CD4+ T-lymphocytes in HIV-infected patients. J. Gene Med. 2005;7:552–564. doi: 10.1002/jgm.705. [DOI] [PubMed] [Google Scholar]
- 37.Morgan R.A., Dudley M.E., Wunderlich J.R., Hughes M.S., Yang J.C., Sherry R.M., Royal R.E., Topalian S.L., Kammula U.S., Restifo N.P. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershaw M.H., Westwood J.A., Parker L.L., Wang G., Eshhar Z., Mavroukakis S.A., White D.E., Wunderlich J.R., Canevari S., Rogers-Freezer L. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivière I., Brose K., Mulligan R.C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. USA. 1995;92:6733–6737. doi: 10.1073/pnas.92.15.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pule M.A., Savoldo B., Myers G.D., Rossig C., Russell H.V., Dotti G., Huls M.H., Liu E., Gee A.P., Mei Z. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollyman D., Stefanski J., Przybylowski M., Bartido S., Borquez-Ojeda O., Taylor C., Yeh R., Capacio V., Olszewska M., Hosey J. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J. Immunother. 2009;32:169–180. doi: 10.1097/CJI.0b013e318194a6e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kochenderfer J.N., Feldman S.A., Zhao Y., Xu H., Black M.A., Morgan R.A., Wilson W.H., Rosenberg S.A. Construction and preclinical evaluation of an anti-CD19 chimeric antigen receptor. J. Immunother. 2009;32:689–702. doi: 10.1097/CJI.0b013e3181ac6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roe T., Reynolds T.C., Yu G., Brown P.O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajihosseini M., Iavachev L., Price J. Evidence that retroviruses integrate into post-replication host DNA. EMBO J. 1993;12:4969–4974. doi: 10.1002/j.1460-2075.1993.tb06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koehne G., Gallardo H.F., Sadelain M., O’Reilly R.J. Rapid selection of antigen-specific T lymphocytes by retroviral transduction. Blood. 2000;96:109–117. [PubMed] [Google Scholar]
- 46.Zack J.A., Arrigo S.J., Weitsman S.R., Go A.S., Haislip A., Chen I.S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 47.Lewis P.F., Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 49.Zennou V., Petit C., Guetard D., Nerhbass U., Montagnier L., Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 50.Korin Y.D., Zack J.A. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 1998;72:3161–3168. doi: 10.1128/jvi.72.4.3161-3168.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unutmaz D., KewalRamani V.N., Marmon S., Littman D.R. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavalieri S., Cazzaniga S., Geuna M., Magnani Z., Bordignon C., Naldini L., Bonini C. Human T lymphocytes transduced by lentiviral vectors in the absence of TCR activation maintain an intact immune competence. Blood. 2003;102:497–505. doi: 10.1182/blood-2003-01-0297. [DOI] [PubMed] [Google Scholar]
- 53.Humeau L.M., Binder G.K., Lu X., Slepushkin V., Merling R., Echeagaray P., Pereira M., Slepushkina T., Barnett S., Dropulic L.K. Efficient lentiviral vector-mediated control of HIV-1 replication in CD4 lymphocytes from diverse HIV+ infected patients grouped according to CD4 count and viral load. Mol. Ther. 2004;9:902–913. doi: 10.1016/j.ymthe.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Wang X., Chang W.C., Wong C.W., Colcher D., Sherman M., Ostberg J.R., Forman S.J., Riddell S.R., Jensen M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–1263. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levine B.L., Humeau L.M., Boyer J., MacGregor R.R., Rebello T., Lu X., Binder G.K., Slepushkin V., Lemiale F., Mascola J.R. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitchell R.S., Beitzel B.F., Schroder A.R., Shinn P., Chen H., Berry C.C., Ecker J.R., Bushman F.D. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2004;2:E234. doi: 10.1371/journal.pbio.0020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nienhuis A.W., Dunbar C.E., Sorrentino B.P. Genotoxicity of retroviral integration in hematopoietic cells. Mol. Ther. 2006;13:1031–1049. doi: 10.1016/j.ymthe.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Gabriel R., Schmidt M., von Kalle C. Integration of retroviral vectors. Curr. Opin. Immunol. 2012;24:592–597. doi: 10.1016/j.coi.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Montini E., Cesana D., Schmidt M., Sanvito F., Bartholomae C.C., Ranzani M., Benedicenti F., Sergi L.S., Ambrosi A., Ponzoni M. The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J. Clin. Invest. 2009;119:964–975. doi: 10.1172/JCI37630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newrzela S., Cornils K., Li Z., Baum C., Brugman M.H., Hartmann M., Meyer J., Hartmann S., Hansmann M.L., Fehse B., von Laer D. Resistance of mature T cells to oncogene transformation. Blood. 2008;112:2278–2286. doi: 10.1182/blood-2007-12-128751. [DOI] [PubMed] [Google Scholar]
- 61.Cruz C.R., Hanley P.J., Liu H., Torrano V., Lin Y.F., Arce J.A., Gottschalk S., Savoldo B., Dotti G., Louis C.U. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy. 2010;12:743–749. doi: 10.3109/14653241003709686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oliveira G., Ruggiero E., Stanghellini M.T., Cieri N., D’Agostino M., Fronza R., Lulay C., Dionisio F., Mastaglio S., Greco R. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci. Transl. Med. 2015;7:317ra198. doi: 10.1126/scitranslmed.aac8265. [DOI] [PubMed] [Google Scholar]
- 64.Preston B.D., Poiesz B.J., Loeb L.A. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 65.Roberts J.D., Bebenek K., Kunkel T.A. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242:1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 66.Ji J., Loeb L.A. Fidelity of HIV-1 reverse transcriptase copying a hypervariable region of the HIV-1 env gene. Virology. 1994;199:323–330. doi: 10.1006/viro.1994.1130. [DOI] [PubMed] [Google Scholar]
- 67.Mansky L.M., Temin H.M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J. Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.De Angioletti M., Rovira A., Sadelain M., Luzzatto L., Notaro R. Frequency of missense mutations in the coding region of a eukaryotic gene transferred by retroviral vectors. J. Virol. 2002;76:1991–1994. doi: 10.1128/JVI.76.4.1991-1994.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang X., Olszewska M., Capacio V., Stefanski J., Przybylowski M., Samakoglu S., Chang A.H., Sadelain M., Rivière I. Quantitative analysis of clinically relevant mutations occurring in lymphoid cells harboring gamma-retrovirus-encoded hsvtk suicide genes. Gene Ther. 2008;15:1454–1459. doi: 10.1038/gt.2008.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hackett P.B., Ekker S.C., Largaespada D.A., McIvor R.S. Sleeping beauty transposon-mediated gene therapy for prolonged expression. Adv. Genet. 2005;54:189–232. doi: 10.1016/S0065-2660(05)54009-4. [DOI] [PubMed] [Google Scholar]
- 71.Singh H., Figliola M.J., Dawson M.J., Olivares S., Zhang L., Yang G., Maiti S., Manuri P., Senyukov V., Jena B. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8:e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hackett P.B., Largaespada D.A., Switzer K.C., Cooper L.J. Evaluating risks of insertional mutagenesis by DNA transposons in gene therapy. Transl. Res. 2013;161:265–283. doi: 10.1016/j.trsl.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh H., Huls H., Kebriaei P., Cooper L.J. A new approach to gene therapy using Sleeping Beauty to genetically modify clinical-grade T cells to target CD19. Immunol. Rev. 2014;257:181–190. doi: 10.1111/imr.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y., Zheng Z., Cohen C.J., Gattinoni L., Palmer D.C., Restifo N.P., Rosenberg S.A., Morgan R.A. High-efficiency transfection of primary human and mouse T lymphocytes using RNA electroporation. Mol. Ther. 2006;13:151–159. doi: 10.1016/j.ymthe.2005.07.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon S.H., Lee J.M., Cho H.I., Kim E.K., Kim H.S., Park M.Y., Kim T.G. Adoptive immunotherapy using human peripheral blood lymphocytes transferred with RNA encoding Her-2/neu-specific chimeric immune receptor in ovarian cancer xenograft model. Cancer Gene Ther. 2009;16:489–497. doi: 10.1038/cgt.2008.98. [DOI] [PubMed] [Google Scholar]
- 76.Rowley J., Monie A., Hung C.F., Wu T.C. Expression of IL-15RA or an IL-15/IL-15RA fusion on CD8+ T cells modifies adoptively transferred T-cell function in cis. Eur. J. Immunol. 2009;39:491–506. doi: 10.1002/eji.200838594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beatty G.L., Haas A.R., Maus M.V., Torigian D.A., Soulen M.C., Plesa G., Chew A., Zhao Y., Levine B.L., Albelda S.M. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce anti-tumor activity in solid malignancies. Cancer Immunol. Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Urnov F.D., Rebar E.J., Holmes M.C., Zhang H.S., Gregory P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 79.Sander J.D., Joung J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014;32:347–355. doi: 10.1038/nbt.2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim H., Kim J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014;15:321–334. doi: 10.1038/nrg3686. [DOI] [PubMed] [Google Scholar]
- 81.Wright A.V., Nuñez J.K., Doudna J.A. Biology and applications of CRISPR systems: harnessing nature’s toolbox for genome engineering. Cell. 2016;164:29–44. doi: 10.1016/j.cell.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 82.Tsai S.Q., Joung J.K. Defining and improving the genome-wide specificities of CRISPR-Cas9 nucleases. Nat. Rev. Genet. 2016;17:300–312. doi: 10.1038/nrg.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Porteus M. Genome editing: a new approach to human therapeutics. Annu. Rev. Pharmacol. Toxicol. 2016;56:163–190. doi: 10.1146/annurev-pharmtox-010814-124454. [DOI] [PubMed] [Google Scholar]
- 84.Holt N., Wang J., Kim K., Friedman G., Wang X., Taupin V., Crooks G.M., Kohn D.B., Gregory P.D., Holmes M.C., Cannon P.M. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat. Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mandal P.K., Ferreira L.M., Collins R., Meissner T.B., Boutwell C.L., Friesen M., Vrbanac V., Garrison B.S., Stortchevoi A., Bryder D. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15:643–652. doi: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tebas P., Stein D., Tang W.W., Frank I., Wang S.Q., Lee G., Spratt S.K., Surosky R.T., Giedlin M.A., Nichol G. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Provasi E., Genovese P., Lombardo A., Magnani Z., Liu P.Q., Reik A., Chu V., Paschon D.E., Zhang L., Kuball J. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 2012;18:807–815. doi: 10.1038/nm.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Torikai H., Reik A., Liu P.Q., Zhou Y., Zhang L., Maiti S., Huls H., Miller J.C., Kebriaei P., Rabinovich B. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012;119:5697–5705. doi: 10.1182/blood-2012-01-405365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berdien B., Mock U., Atanackovic D., Fehse B. TALEN-mediated editing of endogenous T-cell receptors facilitates efficient reprogramming of T lymphocytes by lentiviral gene transfer. Gene Ther. 2014;21:539–548. doi: 10.1038/gt.2014.26. [DOI] [PubMed] [Google Scholar]
- 90.Poirot L., Philip B., Schiffer-Mannioui C., Le Clerre D., Chion-Sotinel I., Derniame S., Potrel P., Bas C., Lemaire L., Galetto R. Multiplex genome-edited T-cell manufacturing platform for “off-the-shelf” adoptive T-cell immunotherapies. Cancer Res. 2015;75:3853–3864. doi: 10.1158/0008-5472.CAN-14-3321. [DOI] [PubMed] [Google Scholar]
- 91.Schumann K., Lin S., Boyer E., Simeonov D.R., Subramaniam M., Gate R.E., Haliburton G.E., Ye C.J., Bluestone J.A., Doudna J.A., Marson A. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl. Acad. Sci. USA. 2015;112:10437–10442. doi: 10.1073/pnas.1512503112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Menger L., Gouble A., Marzolini M.A., Pachnio A., Bergerhoff K., Henry J.Y., Smith J., Pule M., Moss P., Riddell S.R. TALEN-mediated genetic inactivation of the glucocorticoid receptor in cytomegalovirus-specific T cells. Blood. 2015;126:2781–2789. doi: 10.1182/blood-2015-08-664755. [DOI] [PubMed] [Google Scholar]
- 93.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9:eaaj2013. doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 94.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gattinoni L., Lugli E., Ji Y., Pos Z., Paulos C.M., Quigley M.F., Almeida J.R., Gostick E., Yu Z., Carpenito C. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gattinoni L., Klebanoff C.A., Restifo N.P. Paths to stemness: building the ultimate antitumour T cell. Nat. Rev. Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gattinoni L., Zhong X.S., Palmer D.C., Ji Y., Hinrichs C.S., Yu Z., Wrzesinski C., Boni A., Cassard L., Garvin L.M. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sathaliyawala T., Kubota M., Yudanin N., Turner D., Camp P., Thome J.J., Bickham K.L., Lerner H., Goldstein M., Sykes M. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Buchholz V.R., Flossdorf M., Hensel I., Kretschmer L., Weissbrich B., Gräf P., Verschoor A., Schiemann M., Höfer T., Busch D.H. Disparate individual fates compose robust CD8+ T cell immunity. Science. 2013;340:630–635. doi: 10.1126/science.1235454. [DOI] [PubMed] [Google Scholar]
- 100.Gerlach C., Rohr J.C., Perié L., van Rooij N., van Heijst J.W., Velds A., Urbanus J., Naik S.H., Jacobs H., Beltman J.B. Heterogeneous differentiation patterns of individual CD8+ T cells. Science. 2013;340:635–639. doi: 10.1126/science.1235487. [DOI] [PubMed] [Google Scholar]
- 101.Farber D.L., Yudanin N.A., Restifo N.P. Human memory T cells: generation, compartmentalization and homeostasis. Nat. Rev. Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Graef P., Buchholz V.R., Stemberger C., Flossdorf M., Henkel L., Schiemann M., Drexler I., Höfer T., Riddell S.R., Busch D.H. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8(+) central memory T cells. Immunity. 2014;41:116–126. doi: 10.1016/j.immuni.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 103.Gattinoni L., Klebanoff C.A., Palmer D.C., Wrzesinski C., Kerstann K., Yu Z., Finkelstein S.E., Theoret M.R., Rosenberg S.A., Restifo N.P. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J. Clin. Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berger C., Jensen M.C., Lansdorp P.M., Gough M., Elliott C., Riddell S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hinrichs C.S., Borman Z.A., Cassard L., Gattinoni L., Spolski R., Yu Z., Sanchez-Perez L., Muranski P., Kern S.J., Logun C. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. USA. 2009;106:17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Busch D.H., Fräßle S.P., Sommermeyer D., Buchholz V.R., Riddell S.R. Role of memory T cell subsets for adoptive immunotherapy. Semin. Immunol. 2016;28:28–34. doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sommermeyer D., Hudecek M., Kosasih P.L., Gogishvili T., Maloney D.G., Turtle C.J., Riddell S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492–500. doi: 10.1038/leu.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Afzali B., Lechler R.I., Hernandez-Fuentes M.P. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69:545–556. doi: 10.1111/j.1399-0039.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- 111.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., Grand F., Brewer J.E., Gupta M., Plesa G. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Haque T., Wilkie G.M., Jones M.M., Higgins C.D., Urquhart G., Wingate P., Burns D., McAulay K., Turner M., Bellamy C. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 114.Doubrovina E., Oflaz-Sozmen B., Prockop S.E., Kernan N.A., Abramson S., Teruya-Feldstein J., Hedvat C., Chou J.F., Heller G., Barker J.N. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119:2644–2656. doi: 10.1182/blood-2011-08-371971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cruz C.R., Micklethwaite K.P., Savoldo B., Ramos C.A., Lam S., Ku S., Diouf O., Liu E., Barrett A.J., Ito S. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122:2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghosh A., Smith M., James S.E., Davila M.L., Velardi E., Argyropoulos K.V., Gunset G., Perna F., Kreines F.M., Levy E.R. Donor CD19 CAR T cells exert potent graft-versus-lymphoma activity with diminished graft-versus-host activity. Nat. Med. 2017;23:242–249. doi: 10.1038/nm.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clarke S.R., Rudensky A.Y. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J. Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- 118.Surh C.D., Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 119.Awong G., La Motte-Mohs R.N., Zúñiga-Pflücker J.C. Generation of pro-T cells in vitro: potential for immune reconstitution. Semin. Immunol. 2007;19:341–349. doi: 10.1016/j.smim.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 120.Zakrzewski J.L., Kochman A.A., Lu S.X., Terwey T.H., Kim T.D., Hubbard V.M., Muriglan S.J., Suh D., Smith O.M., Grubin J. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat. Med. 2006;12:1039–1047. doi: 10.1038/nm1463. [DOI] [PubMed] [Google Scholar]
- 121.Zakrzewski J.L., Suh D., Markley J.C., Smith O.M., King C., Goldberg G.L., Jenq R., Holland A.M., Grubin J., Cabrera-Perez J. Tumor immunotherapy across MHC barriers using allogeneic T-cell precursors. Nat. Biotechnol. 2008;26:453–461. doi: 10.1038/nbt1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Timmermans F., Velghe I., Vanwalleghem L., De Smedt M., Van Coppernolle S., Taghon T., Moore H.D., Leclercq G., Langerak A.W., Kerre T. Generation of T cells from human embryonic stem cell-derived hematopoietic zones. J. Immunol. 2009;182:6879–6888. doi: 10.4049/jimmunol.0803670. [DOI] [PubMed] [Google Scholar]
- 123.Kennedy M., Awong G., Sturgeon C.M., Ditadi A., LaMotte-Mohs R., Zúñiga-Pflücker J.C., Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 124.Vizcardo R., Masuda K., Yamada D., Ikawa T., Shimizu K., Fujii S., Koseki H., Kawamoto H. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8(+) T cells. Cell Stem Cell. 2013;12:31–36. doi: 10.1016/j.stem.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 125.Nishimura T., Kaneko S., Kawana-Tachikawa A., Tajima Y., Goto H., Zhu D., Nakayama-Hosoya K., Iriguchi S., Uemura Y., Shimizu T. Generation of rejuvenated antigen-specific T cells by reprogramming to pluripotency and redifferentiation. Cell Stem Cell. 2013;12:114–126. doi: 10.1016/j.stem.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 126.Themeli M., Kloss C.C., Ciriello G., Fedorov V.D., Perna F., Gonen M., Sadelain M. Generation of tumor-targeted human T lymphocytes from induced pluripotent stem cells for cancer therapy. Nat. Biotechnol. 2013;31:928–933. doi: 10.1038/nbt.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Themeli M., Rivière I., Sadelain M. New cell sources for T cell engineering and adoptive immunotherapy. Cell Stem Cell. 2015;16:357–366. doi: 10.1016/j.stem.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ellebrecht C.T., Bhoj V.G., Nace A., Choi E.J., Mao X., Cho M.J., Di Zenzo G., Lanzavecchia A., Seykora J.T., Cotsarelis G. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353:179–184. doi: 10.1126/science.aaf6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Elinav E., Waks T., Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134:2014–2024. doi: 10.1053/j.gastro.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 130.Lee J.C., Hayman E., Pegram H.J., Santos E., Heller G., Sadelain M., Brentjens R. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 2011;71:2871–2881. doi: 10.1158/0008-5472.CAN-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Blat D., Zigmond E., Alteber Z., Waks T., Eshhar Z. Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol. Ther. 2014;22:1018–1028. doi: 10.1038/mt.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Levine A.G., Hemmers S., Baptista A.P., Schizas M., Faire M.B., Moltedo B., Konopacki C., Schmidt-Supprian M., Germain R.N., Treuting P.M., Rudensky A.Y. Suppression of lethal autoimmunity by regulatory T cells with a single TCR specificity. J. Exp. Med. 2017;214:609–622. doi: 10.1084/jem.20161318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boardman D.A., Philippeos C., Fruhwirth G.O., Ibrahim M.A., Hannen R.F., Cooper D., Marelli-Berg F.M., Watt F.M., Lechler R.I., Maher J. Expression of a chimeric antigen receptor specific for donor HLA class I enhances the potency of human regulatory T cells in preventing human skin transplant rejection. Am. J. Transplant. 2016;17:931–943. doi: 10.1111/ajt.14185. [DOI] [PubMed] [Google Scholar]
- 134.Carter R.H., Fearon D.T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- 135.Engel P., Zhou L.J., Ord D.C., Sato S., Koller B., Tedder T.F. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity. 1995;3:39–50. doi: 10.1016/1074-7613(95)90157-4. [DOI] [PubMed] [Google Scholar]
- 136.Rickert R.C., Rajewsky K., Roes J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature. 1995;376:352–355. doi: 10.1038/376352a0. [DOI] [PubMed] [Google Scholar]
- 137.Kochenderfer J.N., Yu Z., Frasheri D., Restifo N.P., Rosenberg S.A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood. 2010;116:3875–3886. doi: 10.1182/blood-2010-01-265041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pegram H.J., Lee J.C., Hayman E.G., Imperato G.H., Tedder T.F., Sadelain M., Brentjens R.J. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davila M.L., Kloss C.C., Gunset G., Sadelain M. CD19 CAR-targeted T cells induce long-term remission and B cell aplasia in an immunocompetent mouse model of B cell acute lymphoblastic leukemia. PLoS ONE. 2013;8:e61338. doi: 10.1371/journal.pone.0061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paszkiewicz P.J., Fräßle S.P., Srivastava S., Sommermeyer D., Hudecek M., Drexler I., Sadelain M., Liu L., Jensen M.C., Riddell S.R., Busch D.H. Targeted antibody-mediated depletion of murine CD19 CAR T cells permanently reverses B cell aplasia. J. Clin. Invest. 2016;126:4262–4272. doi: 10.1172/JCI84813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lamers C.H., Willemsen R., van Elzakker P., van Steenbergen-Langeveld S., Broertjes M., Oosterwijk-Wakka J., Oosterwijk E., Sleijfer S., Debets R., Gratama J.W. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117:72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 142.Maus M.V., Haas A.R., Beatty G.L., Albelda S.M., Levine B.L., Liu X., Zhao Y., Kalos M., June C.H. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 2013;1:26–31. doi: 10.1158/2326-6066.CIR-13-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kalos M., Levine B.L., Porter D.L., Katz S., Grupp S.A., Bagg A., June C.H. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kochenderfer J.N., Dudley M.E., Feldman S.A., Wilson W.H., Spaner D.E., Maric I., Stetler-Stevenson M., Phan G.Q., Hughes M.S., Sherry R.M. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119:2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kochenderfer J.N., Rosenberg S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Park J.H., Geyer M.B., Brentjens R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312–3320. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Singh N., Frey N.V., Grupp S.A., Maude S.L. CAR T cell therapy in acute lymphoblastic leukemia and potential for chronic lymphocytic leukemia. Curr. Treat. Options Oncol. 2016;17:28. doi: 10.1007/s11864-016-0406-4. [DOI] [PubMed] [Google Scholar]
- 154.Grupp S.A., Kalos M., Barrett D., Aplenc R., Porter D.L., Rheingold S.R., Teachey D.T., Chew A., Hauck B., Wright Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]