Main Text
Combination antiretroviral therapy (cART) is currently the only sustainable strategy to prevent AIDS-related morbidity and mortality in the millions of individuals living with HIV. However, even profound suppression of HIV viremia is insufficient to achieve a cure. A long-lived latent viral reservoir that harbors replication-competent provirus enables HIV to escape the effects of cART and re-establish active infection as soon as therapy is interrupted.1 Various strategies to eliminate the reservoir, or at least to achieve permanent viral remission in the absence of cART, are under investigation. One such approach, the use of endonucleases such as the clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 system to disable or excise HIV-1 proviruses by targeting the long terminal repeat (LTR) or essential viral genes, has shown promise in vitro. In this issue of Molecular Therapy, Yin and colleagues take an important step in moving this approach to in vivo application by investigating the feasibility of Cas9-mediated provirus excision in three murine models of HIV-1 infection.2 Their ability to deliver an all-in-one adeno-associated virus (AAV) vector containing multiple single-guide RNAs (sgRNAs) in tandem with Staphylococcus aureus (sa)Cas9 and to detect HIV-1provirus excision in multiple tissues and organs in models, including bone marrow-liver-thymus (BLT) mice with chronic HIV-1 infection, represents an exciting milestone in moving the field toward human trials.
There is an abundance of in vitro data supporting the potential of endonuclease-based gene editing therapies toward the cure for chronic viral infections.3 For HIV, the preponderance of evidence suggests that provirus excision from infected cells is particularly efficacious and precise.4, 5 However, in vivo data supporting gene editing of viral genomes is lacking, largely attributed to limitations in vector delivery. Only a single previous study has shown successful targeting of an established viral infection, with herpes simplex virus, in vivo.6 The report by Yin and colleagues2 now expands this list to include established HIV infection by demonstrating successful delivery and activity of HIV-specific endonuclease therapy in vivo.
Yin et al.2 demonstrated that an oversized cargo of 5.7 kb, encoding four multiplexed sgRNAs along with saCas9, can be efficiently packaged into AAV-DJ and AAV-DJ/8 vectors and delivered in vivo. AAV was a logical choice of gene transfer vector for this application. Its wide use in clinical trials, non-pathogenicity, and varied tropism are all attractive attributes. The use of AAV-DJ and AAV-DJ/8 is also logical as a proof of concept. Both of these engineered AAV vectors contain hybrid capsids derived from multiple AAV serotypes, allowing them to transduce a broad range of tissues and cell types.7 The ability to deliver and express multiple sgRNAs along with saCas9 from the same vector in the same cell also has significant advantages over single sgRNA treatment. Recent in vitro studies have demonstrated that suboptimal mutagenic DNA repair after endonuclease cleavage at a single HIV loci inevitably leads to rapid viral escape.8 Complementary evidence also suggests that a combinatorial approach targeting two or more regions of the HIV genome that can facilitate higher proviral mutagenesis could circumvent viral escape. In the present study, Yin et al.2 showed that combining sgRNAs targeting LTR and structural proteins resulted in higher levels of both small indels and excision of HIV segments between target sites, resulting in higher overall disruption efficiency. These properties will play crucial roles as HIV genome editing progresses toward clinical trials by minimizing concerns about HIV diversity and the emergence of resistance.5, 9, 10
As a first step towards demonstrating the viability of saCas9-mediated HIV-1 proviral excision in vivo, the investigators opted for the HIV-1 Tg26 transgenic mouse. This model is widely used to study HIV-related pathology. Each cell of the Tg26 transgenic mouse contains a tandem repeat of 10–20 copies of a replication incompetent 7.4 kb pNL4-3 proviral construct. Yin et al.2 administered multiplexed saCas9 AAV-DJ or AAV-DJ/8 through tail vein injection and demonstrated efficient expression of saCas9 in various solid tissues and organs throughout the mice as early as 1 week. 4 weeks later, the researchers also observed fragmentation of the integrated HIV provirus by PCR genotyping in various tissues, including brain, heart, liver, lung, kidney, and spleen. A notable finding was the consistently quantifiable difference in relative Env, Gag, and Tat mRNA expression levels in treated versus control mice across multiple organs/tissues. This suggests that given efficient delivery and expression, saCas9 and sgRNAs are able to mediate proviral cleavage events, leading to meaningful reduction in transcription of genes associated with target sites.
With definitive evidence that quadruplexed saCas9 is able to excise integrated provirus in vivo, Yin et al.2 then investigated the potential utility of saCas9/sgRNA during systemic HIV-1 infection. For this purpose, the team inoculated the NCr strain of nude mice injected with an EcoHIV-eLuc reporter virus and AAVDJ/8 through sequential retro-orbital injection and followed dissemination of EcoHIV-eLuc infection through longitudinal bioluminescence imaging. EcoHIV-eLuc is a chimeric HIV-1 that contains the gp80 envelope protein of murine leukemia virus-1 in place of gp120. It is able to productively infect murine lymphocytes and is often utilized to study HIV-associated neurocognitive deficits. Supporting the earlier observations in Tg26 mice, varying degrees of excision were observed in multiple tissues and organs, including up to 96% by qPCR in liver, perhaps attributable to the high level of AAV transduction in that organ.
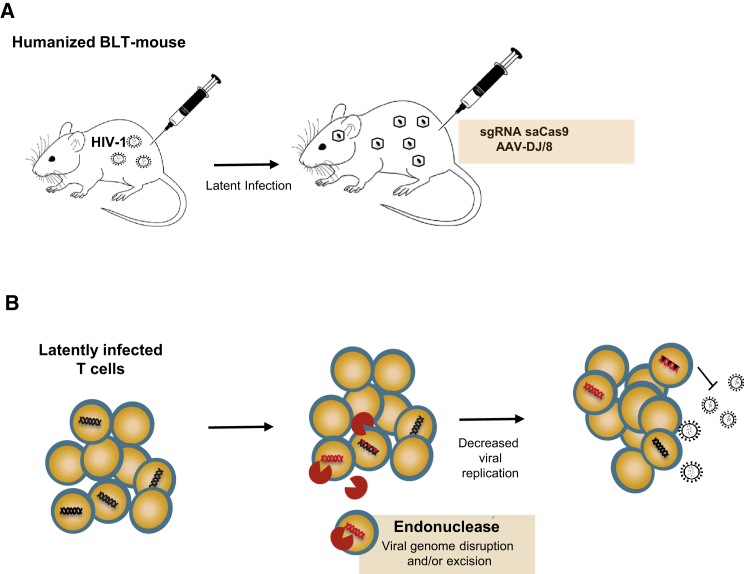
Memory CD4+ T cells constitute a well-established HIV cellular reservoir that is likely the major contributor to HIV latency.11 Eliminating the provirus from this cellular compartment might pave the road to an HIV cure or unlock heretofore unknown cellular reservoirs for the virus. Thus, the most relevant in vivo models for HIV infection must closely mimic these reservoirs and viral latency and recapitulate viral rebound after treatment interruption. Hence, the third and final model used by Yin et al.,2 the BLT mouse, is particularly important. The BLT mouse is a uniquely useful in vivo model of HIV latency, generated by transplanting NOD SCID mice with human fetal liver, thymus, and CD34+ cells, thereby allowing tissue-wide human T cell distribution. Human hematopoietic cells are present in all mouse tissues, including peripheral blood, lymphoid, and mucosal tissues, and latent HIV infection can be established.12 In the present study, Yin et al.2 established latency in BLT mice through either vaginal mucosal transmission or intraperitoneal injection with an R5-, M tropic HIV molecular clone, NL-Bal-eLuc. After intravenous and intravaginal administration of AAV-DJ/8 carrying qudraplex sgRNA with saCas9, they were able to detect Cas9-mediated excision events in various tissues and organs, including in the human thymic organoid. Thus, Yin et al.2 have demonstrated that saCas9 driven excision of latent HIV infection can be successfully achieved in vivo (Figure 1).
Figure 1.
In Vivo Excision of Latent HIV
(A) HIV infection, establishment of latency in humanized BLT mouse, and delivery of sgRNA saCas9 through AAV. (B) Endonuclease viral genome excision and provirus disruption leading to reduction in reservoir.
These promising results suggest that, given the opportunity for efficient delivery and expression, saCas9 can efficiently cleave the HIV genome in various anatomical locations. However, several issues remain to be addressed prior to clinical trials. While an AAV serotype with broad tropism is ideal for proof-of-concept studies, replication-competent HIV is rare (present only in one of every 10,000 to 1,000,000 CD4+ T cells), and thus identifying delivery vectors with high specificity to the HIV reservoir remains a significant hurdle. There is currently no known viral or non-viral agent that is capable of efficiently and selectively delivering and expressing transgenes in these cells. An ideal delivery candidate should possess the ability to carry a relatively large cargo to relevant reservoir cells and facilitate pharmacologically significant enzymatic activity. It should also exhibit little to no toxicity irrespective of the duration of its presence in vivo, whether transient or long term.
There are other limitations that the field, as a whole, needs to overcome as it moves toward clinical trials. Mathematical modeling suggests that greater than a 10,000-fold reduction of the reservoir may be necessary to completely prevent viral rebound during the lifetime of an infected individual.13, 14 Therefore, quantifying the level of reservoir reduction following gene therapy remains essential for evaluating treatment efficacy and determining when treatment can be safely interrupted. Such quantification was limited in this study, especially in the BLT mouse model. The long-term effects of Cas9/sgRNA therapy, such as potential off-target genomic effects, also need to be evaluated comprehensively in increasingly relevant animal models, such as non-human primates. Nevertheless, the in vivo findings of Yin et al.2 provide a significant milestone in the application of genome editing technology against HIV infection. Taken together with progress in other strategies against HIV, the work supports continued optimism that a cure for HIV can be achieved.
References
- 1.Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T., Smith K., Lisziewicz J., Lori F., Flexner C. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999;5:512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 2.Yin C., Zhang T., Qu X., Zhang Y., Putatunda R., Xiao X., Li F., Xiao W., Zhao H., Dai S. In vivo excision of HIV-1 provirus by saCas9 and multiplex single-guide RNAs in animal models. Mol. Ther. 2017;25:1168–1186. doi: 10.1016/j.ymthe.2017.03.012. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone D., Niyonzima N., Jerome K.R. Genome editing and the next generation of antiviral therapy. Hum. Genet. 2016;135:1071–1082. doi: 10.1007/s00439-016-1686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G., Zhao N., Berkhout B., Das A.T. A Combinatorial CRISPR-Cas9 Attack on HIV-1 DNA Extinguishes All Infectious Provirus in Infected T Cell Cultures. Cell Rep. 2016;17:2819–2826. doi: 10.1016/j.celrep.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 5.Lebbink R.J., de Jong D.C.M., Wolters F., Kruse E.M., van Ham P.M., Wiertz E.J.H.J., Nijhuis M. A combinational CRISPR/Cas9 gene-editing approach can halt HIV replication and prevent viral escape. Sci. Rep. 2017;7:41968. doi: 10.1038/srep41968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aubert M., Madden E.A., Loprieno M., DeSilva Feelixge H.S., Stensland L., Huang M.-L., Greninger A.L., Roychoudhury P., Niyonzima N., Nguyen T. In vivo disruption of latent HSV by designer endonuclease therapy. JCI Insight. 2016;1:e88468. doi: 10.1172/jci.insight.88468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm D., Lee J.S., Wang L., Desai T., Akache B., Storm T.A., Kay M.A. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J. Virol. 2008;82:5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G., Zhao N., Berkhout B., Das A.T. CRISPR-Cas9 can inhibit HIV-1 replication but NHEJ repair facilitates virus escape. Mol. Ther. 2016;24:522–526. doi: 10.1038/mt.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva Feelixge H.S., Stone D., Pietz H.L., Roychoudhury P., Greninger A.L., Schiffer J.T., Aubert M., Jerome K.R. Detection of treatment-resistant infectious HIV after genome-directed antiviral endonuclease therapy. Antiviral Res. 2016;126:90–98. doi: 10.1016/j.antiviral.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Pan Q., Gendron P., Zhu W., Guo F., Cen S., Wainberg M.A., Liang C. CRISPR/Cas9-derived mutations both inhibit HIV-1 replication and accelerate viral escape. Cell Rep. 2016;15:481–489. doi: 10.1016/j.celrep.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 11.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B., Kovacs C., Gange S.J., Siliciano R.F. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 12.Denton P.W., Olesen R., Choudhary S.K., Archin N.M., Wahl A., Swanson M.D., Chateau M., Nochi T., Krisko J.F., Spagnuolo R.A. Generation of HIV latency in humanized BLT mice. J. Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill A.L., Rosenbloom D.I., Fu F., Nowak M.A., Siliciano R.F. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc. Natl. Acad. Sci. USA. 2014;111:13475–13480. doi: 10.1073/pnas.1406663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill A.L., Rosenbloom D.I., Goldstein E., Hanhauser E., Kuritkes D.R., Silicano R.F., Henrich T.J. Real-time predictions of reservoir size and rebound time during antiretroviral therapy interruption trials for HIV. PLoS Pathog. 2016;12:e1005535. doi: 10.1371/journal.ppat.1005535. [DOI] [PMC free article] [PubMed] [Google Scholar]