Abstract
Viruses can be engineered or adapted for selective propagation in neoplastic tissues and further modified for therapeutic transgene expression to enhance their antitumor potency and druggability. Oncolytic viruses (OVs) can be administered locally or intravenously and spread to a variable degree at sites of tumor growth. OV-infected tumor cells die in situ, releasing viral and tumor antigens that are phagocytosed by macrophages, transported to regional lymph nodes, and presented to antigen-reactive T cells, which proliferate before dispersing to kill uninfected tumor cells at distant sites. Several OVs are showing clinical promise, and one of them, talimogene laherparepvec (T-VEC), was recently granted marketing approval for intratumoral therapy of nonresectable metastatic melanoma. T-VEC also appears to substantially enhance clinical responsiveness to checkpoint inhibitor antibody therapy. Here, we examine the T-VEC paradigm and review some of the approaches currently being pursued to develop the next generation of OVs for both local and systemic administration, as well as for use in combination with other immunomodulatory agents.
Keywords: oncolytic virotherapy, competitive landscape, T-VEC, viro-immuno-oncology
Russell and Peng review the field of oncolytic virotherapy, highlighting the recent approval of intralesional talimogene laherparepvec (T-VEC) for metastatic melanoma and its synergistic interaction with checkpoint inhibitor antibodies. They then explore how newer OVs may further improve treatment outcomes in the post-T-VEC era.
Main Text
Oncolytic virotherapy uses replication-competent viruses that have been adapted to amplify and spread selectively at sites of tumor growth.1, 2 In situ killing of the infected tumor cells, either by the infection or the host immune system, creates a local intratumoral inflammatory milieu containing all the ingredients necessary to boost systemic antitumor immunity (Figure 1). The relative contributions of these two modes of tumor cell killing (direct viral oncolysis and boosted antitumor immunity) is heavily impacted by neutralizing antibodies and virus-reactive T cells generated during previous virus exposures. Virus exposure history is, therefore, an important driver of interindividual variability in oncolytic virus (OV) responses, especially to the first dose of virus administered, and may further lead to progressive changes in the magnitude of response with each successive dose.
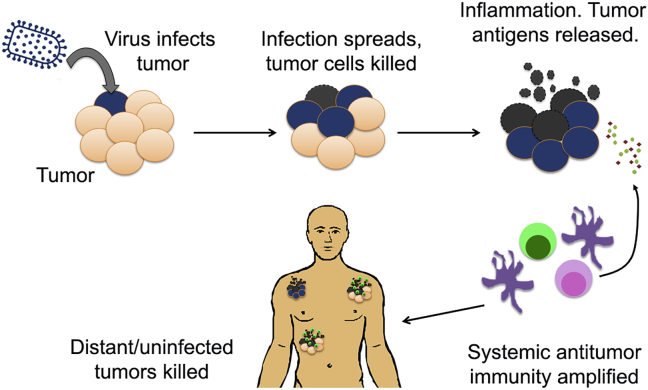
Figure 1.
Oncolytic Virotherapy Is Not Just Immunotherapy
The viro-immunotherapy paradigm involves tumor-selective infection and replication of the virus, followed by cell killing, inducing local inflammation and trafficking of immune cells to the infected tumor nodule, priming and amplifying systemic antitumor immunity, resulting in the induction of tumor-antigen-specific T cells that would participate in the elimination of uninfected or distant metastases.
Talimogene laherparepvec (T-VEC) is an oncolytic herpes simplex virus type 1 (HSV-1) that was recently granted regulatory marketing approval for the treatment of inoperable malignant melanoma.3, 4 Administered intratumorally into one or more accessible (usually cutaneous) lesions every 2 weeks for up to 18 months, the virus produced durable systemic responses in 16% of treated patients,5 and has since shown extraordinarily promising activity in the same disease indication when combined with ipilumumab or pembrolizumab checkpoint antibody therapy.6, 7 Efforts are now underway to explore the potential of T-VEC therapy across a spectrum of different cancers. Unsurprisingly, the successful emergence of T-VEC as an FDA (Food and Drug Administration)-approved drug is accelerating commercial development efforts in the oncolytic virotherapy world and is having a positive energizing effect on numerous academic groups and early-stage companies working with a broad spectrum of OVs deriving from many different virus families and engineered in various ways.
In this review article, to commemorate the 20th anniversary of the journal Molecular Therapy, we will focus initially on T-VEC, giving a brief overview of its origins, design, development, and mechanism of action. We will then address three key assumptions that are driving and sustaining current efforts to develop additional OVs and platforms. The first assumption is that T-VEC is not the last word in local intratumoral virotherapy and that better OVs will come. The second assumption is that systemic virotherapy will prove superior to the local T-VEC treatment paradigm. The third assumption is that, even if viruses with superior activity are not found, a wider selection of OVs will facilitate better treatment outcomes that can be achieved with T-VEC alone; for example, through sequential use of different OVs in a given patient and by selecting OVs based on specific characteristics of a given patient and his or her tumor.
Finally, we will discuss approaches to combination therapy, patient selection, and OV development in veterinary practice, all of which are likely to accelerate progress during the coming years. Our intent is not to provide a comprehensive overview of the field, and we apologize to those many investigators whose outstanding contributions are not discussed. For more comprehensive coverage of specific topics, the interested reader is referred to a number of previously published reviews addressing the early history of the field,8 the design and construction of oncolytic viruses,9 the various approaches to oncolytic immunotherapy,10 and clinical experience to date as well as ongoing trials.11
History of T-VEC: The First FDA-Approved OV
Viruses were first shown to have definite antitumor activity in the 1950s.8 Encouraged by occasional case reports of spontaneous tumor regressions that coincided with viral illnesses, a variety of newly identified viruses were intentionally administered to cancer patients. Not infrequently, treated tumors regressed, but it remained unknown whether this was due to direct oncolysis or amplified antitumor immunity. Whatever their cause, the responses were erratic, short lived, and sometimes associated with unacceptable normal tissue toxicities (e.g., lethal encephalitis). Interest waned but was revived after 1989, when Dr. Robert Martuza reported in Science that a genetically modified HSV-1 virus whose thymidine kinase gene had been inactivated led to tumor control without associated encephalitis when administered into an intracerebral glioma in a mouse model.12 This landmark study set the stage for an oncolytic virotherapy renaissance that has continued to this day, with all manner of viruses being engineered to enhance their tumor specificity, safety, druggability, immunogenicity, and oncolytic potency.
HSV continued to attract considerable attention as a malleable platform from which a variety of tumor-selective variants have been generated. A favorite approach was to destroy the neurovirulence of lab-adapted strains by disrupting both copies of the γ-34.5 gene whose encoded protein normally suppresses key antiviral responses of the host cell. Without γ-34.5, HSV is unable to shut down the host cell interferon response (by suppressing TBK-1 [TANK-binding kinase 1]), block PKR (protein kinase R)-mediated shutoff of protein synthesis in the infected cell, or inhibit autophagy via beclin-1 blockade.13 Some of these γ-34.5-deleted HSVs were tested clinically, most notably in glioma patients, with encouraging but not definitive results.14
T-VEC (or JS1/ICP34.5−/ICP47−/GM-CSF [granulocyte macrophage colony-stimulating factor], the virus that was to become known as T-VEC) was originally described in 2003.15 The thinking behind the design of this virus was that its lab-adapted, γ-34.5-deleted predecessors had been over-attenuated. Thus, T-VEC was derived from a fresh pathogenic virus isolate obtained from the cold sore of a lab worker. Although it was initially attenuated by disrupting both copies of the γ-34.5 gene, the attenuation was partially reversed by engineering US11, whose product also blocks the shutoff of host cell protein synthesis, to be expressed at an earlier stage in the virus infection cycle. In addition to these de-attenuating modifications, the virus was engineered to more effectively boost the antitumor immune response. This was achieved by deleting the ICP47 gene, whose product suppresses antigen presentation by the infected cell, and by inserting two copies of the GM-CSF gene into the virus to activate and promote the differentiation of locally resident antigen-presenting cells (APCs) in the infected tumor.
T-VEC was rapidly advanced to the clinic and shown to be active in malignant melanoma, shrinking injected tumors and sometimes leading to the regression of distant metastatic lesions.16 The phase 3 T-VEC registration trial was launched in May 2009, 2 years before FDA approvals were granted for the anti-CTLA4 antibody ipilumumab and the B-raf inhibitor vemurafenib. Thus, the control randomization arm was subcutaneous GM-CSF, which has very little antimelanoma activity. Between May 2009 and July 2011, 436 patients with unresectable (stage III or IV) melanoma were randomly assigned to intralesional T-VEC or subcutaneous GM-CSF administered every 2 weeks. The durable response rate (responses lasting at least 6 months) was 16.3% in the T-VEC arm and 2.1% in the GM-CSF arm, and T-VEC was associated with a longer overall survival of 23.3 months versus 18.9 months with GM-CSF.5 Based on these positive findings, a biologics license application was filed, and U.S. marketing approval was granted in October 2015, with European and Australian approvals granted shortly thereafter.
Is T-VEC the Last Word in Local Intratumoral Virotherapy?
Arguably, T-VEC is an ideal intratumoral cancer vaccine. It spreads locally within the injected tumor and kills tumor cells by in situ necroptosis, causing them to release tumor antigens, viral antigens, damage-associated molecular patterns (DAMPs), and GM-CSF, providing what is possibly a near-perfect environment for activated APCs to phagocytose a mixture of viral and tumor antigens for presentation to CD4+ helper and CD8+ cytotoxic T cells in the regional lymph nodes.17 Co-presentation of viral and tumor antigens by individual APCs that have “fed” on virus-infected tumor lysate greatly increases the probability that tumor-reactive cytotoxic T lymphocytes (CTLs) recognizing tumor-specific MHC (major histocompatibility complex)-neoantigenic peptide complexes will be stimulated in an environment that is rich in helper T cell cytokines (e.g., from virus-reactive T helper cells), increasing the probability of their amplification, release, and subsequent trafficking back to sites of tumor growth, the basis of the systemic tumor responses.
Can we improve upon this? Will alternative HSV configurations prove superior to T-VEC? There are many new herpes OVs under development encoding matrix-degrading enzymes to enhance their intratumoral spread,18 fusogenic membrane glycoproteins to enhance their potency,19, 20 and/or receptor-targeted attachment proteins,21, 22 microRNA targets,23, 24 and tissue-specific promoters25, 26 to target their tropisms. With the tighter tumor specificity of these targeted HSVs, it is now considered reasonable to further de-attenuate them (e.g., by reconstituting γ-34.5) to more fully restore their antitumor potency.
Will the newer oncolytic HSVs prove superior to T-VEC? Can T-VECs potency be increased simply by increasing the dose or optimizing the intratumoral injection technique? What can be gained by changing the composition of the inflammatory tumor lysate by encoding interleukins, cytokines, and T cell chemokines in the viral genome? Will tumor-reactive T cells be more effectively engaged by viruses encoding proteins that kill uninfected as well as infected tumor cells? The answers to these questions are difficult to predict or to model preclinically, but, judging by the intensity of activity in this area at the present time, answers will soon be forthcoming.
So what about other viruses? HSV is by no means the only platform showing promise as an intratumoral cancer therapy, and melanoma is not the only tumor that responds to oncolytic virotherapy. In brain cancer, for example, in addition to several oncolytic HSVs, patients are being treated with viruses from other families27 and with encouraging results. Examples include a recombinant poliovirus incorporating a neuroattenuating rhinovirus internal ribosome entry site ([PV-RIPO] NCT: 01491893);28 a C-type retrovirus encoding the enzyme cytosine deaminase, which activated the prodrug 5-fluorocytosine to 5-fluorouracil (Toca-511);29 an integrin-targeted serotype 5 adenovirus neuroattenuated by an E1A deletion (DNX-2401);30 and a nonengineered rat parvovirus (H-1PV).31
Also, in non-brain-cancer indications, there are many examples of promising clinical responses following intratumoral administration of non-T-VEC oncolytics. Noteworthy examples include H101, an E1B-deleted serotype 5 adenovirus that was approved by the Chinese FDA in 2005 for intratumoral therapy of head and neck cancer in combination with standard chemotherapy;32, 33 JX-594 (Pexavec), a GM-CSF encoding Wyeth strain vaccinia virus with a disrupted thymidine kinase gene, which showed intratumoral activity in hepatocellular carcinoma;34 and Coxsackievirus A21 (Cavatak), a nonengineered picornavirus showing intratumoral activity in melanoma patients (Viralytics, 2016, Society for the Immunotherapy of Cancer, conference).
Importantly, from a drug development perspective, viruses being developed clinically for non-melanoma indications might never have to prove superiority in a head-to-head comparison with T-VEC, but this depends, to some extent, on whether and how rapidly T-VEC marketing approvals are pursued and granted in other cancer indications. This is a critical question for OV drug developers that must be carefully considered before initiating expensive randomized trials. With each new drug approval for a given cancer, the bar for newer drug approvals is set progressively higher. Newer drugs always have to prove superiority over existing drugs as determined by higher response rates, better durability of response, longer survival, lower toxicity, and/or activity in treatment refractory disease. So with the approval of T-VEC as a melanoma therapy, a high bar has been set for future OV approvals in that indication, and the race is truly on to show clinical benefit of many competing OV platforms in other cancers. However, given the diverse tissue tropisms of naturally occurring viruses, it seems highly unlikely that T-VEC could prove superior to all other intratumoral oncolytic agents across all tumor histologies.
T-VEC does, however, have a theoretical edge over non-herpes OVs in that the natural behavior of HSV-1, the natural precursor of T-VEC, is to reactivate repeatedly throughout life, causing significant local tissue damage in the face of a robust adaptive immune response.35 Thus, as an in situ vaccine that can amplify tumor neoantigen-reactive T cells even in the face of pre-existing antiviral immunity, T-VEC seems like a potentially ideal drug to partner with immune checkpoint antibody therapies. Early clinical data addressing this question are already strongly supportive of the concept, showing greatly improved response rates in patients with stage III/IV melanoma treated with a combination of anti-CTLA4 or anti-PD1 antibody therapy plus T-VEC.6, 7 However, there is emerging evidence for similar synergistic interaction with checkpoint antibody therapy in melanoma patients treated with intratumoral CVA21 (Viralytics, 2016, Society for the Immunotherapy of Cancer, conference), and with an unarmed oncolytic HSV-1 not encoding GM-CSF.36 Responses to the combination of CVA21 plus pembrolizumab were even observed in T-VEC refractory patients (Viralytics, 2016, Society for the Immunotherapy of Cancer, conference).
Will Systemic Virotherapy Be Superior to Local Virotherapy?
Detailed analysis of individual lesion response rates in melanoma patients receiving intratumoral T-VEC showed complete responses in 46.1% of injected lesions, 30.1% of uninjected non-visceral lesions, and only 9.4% of uninjected visceral lesions.5 It is clear from this analysis that tumors respond better to oncolytic virotherapy when they actually get infected with the virus. The rationale for systemic virus delivery to all sites of tumor dissemination is, therefore, compelling, and it is expected that, if adequate systemic delivery can be achieved, response rates will be maximized.
So is efficient systemic virus delivery feasible, and how can it be achieved? The barriers to successful intravenous therapy are well known:1, 37 massive dilution of the virus in the bloodstream; neutralization by antiviral antibodies and complement proteins; virus particle sequestration in liver Kuppfer cells and splenic macrophages; and the limited permeability of tumor neovessels.
Considering these barriers, it is easy to understand why T-VEC is considered a poor candidate for systemic application. It is difficult to manufacture in sufficient quantities for systemic administration and is susceptible to rapid neutralization by circulating anti-HSV-1 antibodies in at least 50% of treatment-eligible patients.38, 39 Also, HSV particles are large (150–200 nm) and, hence, less likely to extravasate from tumor neovessels, even from those with abnormally increased permeability. Viremic dissemination of natural virus infections is typically sustained by the continuous release of progeny particles (e.g., poliovirus, smallpox) or infected cells (e.g., measles-infected lymphocytes and monocytes) from one or more primary sites of infection.40, 41 Also, when viremia is cell associated, dissemination may continue even after the appearance of neutralizing antiviral antibodies.42, 43
Thus, to maximize the efficiency of systemic virus delivery to disseminated cancer cells, we need to achieve high-level viremia, preferably sustained, and/or to develop viruses that can target the endothelial cells lining tumor neovessels. Happily for the field of oncolytic virotherapy, the evidence confirming the feasibility of systemic OV delivery, using a broad-spectrum of viruses and delivery strategies, is overwhelming.
Many published works dating back as far as 1949 have reported the dramatic reduction or elimination of implanted mouse tumors following systemic virus administration.43, 44, 45, 46, 47, 48, 49 Depending on the specific tumor model and the virus used, the mechanisms of tumor destruction may differ. In one simple scenario, the infused virus seeds to the tumor, establishing foci of infection that centrifugally expand and coalesce.50 This mechanism of intratumoral OV spread was delineated in living animals using a recombinant vesicular stomatitis virus (VSV) encoding the thyroidal sodium iodide symporter (VSV-IFNβ [interferon beta]-NIS) to concentrate radioactive iodide, pertechnetate, and/or tetraflouoroborate in virus-infected cells. The nucleation, expansion, and death of individual infectious centers in each tumor and their eventual coalescence were readily apparent from serial high-resolution SPECT/CT (single-photon emission computed tomography/computed tomography) pertechnetate scans in immunocompetent tumor-bearing animals after a single intravenous administration of the virus.51
In a second scenario, some viruses can infect the endothelial cells lining tumor blood vessels, causing intratumoral vascular collapse,52, 53, 54 and in a third scenario, infected tumor cells can release large numbers of progeny particles into the bloodstream driving a sustained viremia that leads to infection and destruction of all tumor deposits.55 This mode of spread is particularly relevant for picornavirus oncolytics due to their high burst size, small particle size, and natural propensity to disseminate in this way,40 and where naked virus particles are neutralized in the bloodstream by preformed antiviral antibodies, efficacy can be fully restored using infected cell carriers to transport them to sites of tumor growth.56, 57 Many cell lineages have been proven suitable as Trojan Horses for several different OVs in preclinical models, and the approach is being tested clinically (NCT: 02068794).58, 59
Clearly then, to achieve efficient systemic delivery in clinical practice, it makes sense to focus on viruses having a low seroprevalence in the human population or to use an appropriate carrier cell that can efficiently traffic to sites of tumor growth. Also, because of intravascular dilution and the unavoidable loss of particles due to sequestration by phagocytic cells in the liver and spleen, the dose of virus required for intravenous efficacy is likely to be two or three orders of magnitude higher than the corresponding intratumoral dose.
Targeting virus tropism is clearly the “sine qua non” of systemic oncolytic virotherapy and has been the subject of a great many important advances over the past 20 years that have been extensively reviewed.1, 60, 61, 62, 63, 64 Key strategies include the use of protein-targeting domains to control receptor specificity,65 the use of tissue-specific promoter enhancer elements to control viral gene expression,64 the use of microRNA target sequences to destabilize viral nucleic acid in nontarget tissues,60 and the disruption of viral mechanisms for controlling the antiviral and apoptotic responses of infected host cells.66
While locoregional delivery has been pursued more aggressively than systemic delivery in human clinical trials to date, several OVs have been administered intravenously. In general, the doses administered in intravenous trials have been higher than for locoregional trials, and the treatment has been well tolerated, although tumor response rates have been disappointingly low. Nonetheless, there have been, occasionally, very encouraging case reports and correlative studies that strongly support the notion that effective systemic oncolytic virotherapy is an achievable goal.
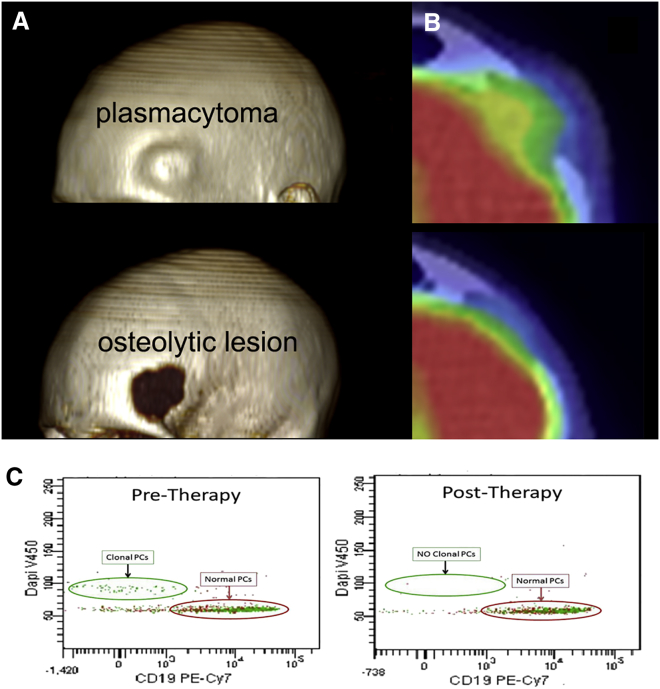
In one particularly compelling case report, a very high dose of the oncolytic measles virus MV-NIS was infused into a 49-year-old woman with multiple myeloma refractory to all available myeloma therapies and rapidly relapsing 8 months after her second autologous stem cell transplant procedure.67 All detectable plasmacytomas (one very prominent on her forehead) regressed completely after a single virus infusion, and her bone marrow, which was diffusely infiltrated with myeloma plasma cells pre-therapy, normalized completely and remains normal 3.5 years later (Figure 2). Importantly, this patient was measles seronegative at the time of virus infusion, and the dose of virus administered was 1011 TCID50 (50% tissue culture infective dose), the maximum feasible dose based on manufacturing process limitations.68
Figure 2.
Complete Remission of Disseminated Multiple Myeloma after One Intravenous Dose of Measles Virus MV-NIS
(A) CT rendering of plasmacytoma on the forehead of the patient showing the tumor protruding from the forehead and osteolytic lesion in the skull. (B) 18F-fludeoxyglucose positron emission tomography image of the glucose avid left frontal plasmacytoma in patient before virus treatment and the lower panel showing resolution of the tumor 7 weeks after MV-NIS treatment. (C) High-sensitivity eight-color plasma cell (PC) flow cytometry on bone marrow plasma cells before and after MV-NIS treatment, showing CD38- and CD138-positive, CD19-negative monoclonal plasma cells (λ-restricted) with hyperdiploid DNA content. In the bone marrow samples obtained 6 weeks after therapy (right panels), the abnormal PCs are not present (adapted from Russell et al.67 Mayo Clinic Proceedings).
The importance of a high virus dose for efficient systemic delivery was further emphasized in another clinical study, where tumor biopsy material was obtained from patients infused with increasing doses of the JX-594 vaccinia virus and then analyzed by PCR for viral genome quantitation.69 The conclusion of this study was that virus could not be detected in tumor biopsies below an infused dose threshold of ∼109 plaque-forming units (PFUs) per patient.
As an alternative to direct intravenous infusion, it may be possible to exploit secondary viremia following intratumoral virus administration to maximize systemic dissemination of an OV infection. Picornaviruses are small (∼30 nm in diameter), and they have a rapid replication cycle (∼6 hr in a susceptible cell) and a large burst size (∼10,000 progeny per infected cell). CVA21 is a picornavirus that shows potent oncolytic activity in preclinical cancer models. A very low dose of lab-adapted CVA21 administered intravenously to mice bearing melanoma or myeloma xenografts can rapidly amplify and disseminate via the bloodstream to mediate complete tumor destruction.47, 70 However, similarly dramatic systemic spread has not yet been seen in human melanoma patients following intratumoral inoculation of this same oncolytic strain of CVA21. However, occasional patients treated by intratumoral OV inoculation have developed sustained high-level viremia due to virus amplification and release from the site of their primary infection. For example, one patient with advanced metastatic colorectal cancer had sustained viremia, resulting in viral spread to uninjected tumors after an oncolytic VSV was injected into one of his hepatic metastases (M. Borad, K.-W.P., and S.J.R., unpublished data).
Will Multiple Viruses Be Better Than a Single Virus?
Naturally occurring viruses are diverse, varying greatly in the size, structure, composition, complexity, and malleability of both their particles and their genomes. Their life cycles, burst sizes, tropisms, modes of transmission, and mechanisms of pathogenesis are correspondingly diverse. It, therefore, seems unlikely that a single viral species/configuration will outperform all others as therapy for anything more than a small subset of cancers.
Another argument supporting parallel development of multiple OV platforms is that, in addition to the heterogeneity of human tumors to be targeted, account must be taken of the heterogeneity of prior virus exposure histories in the human population. Take, for example, HSV-1, vaccinia, and VSV. HSV-1 seroprevalence varies from 0% to 90% based on age, lifestyle, and geographical location.38 Vaccina seroprevalence varies by age according to the year in which the decision was made to discontinue smallpox vaccination and, therefore, has a fairly dramatic age cutoff that varies by country (the last known natural case occurred in Somalia in 1977, and global eradication was declared in 1980, but the U.S. Public Health Service recommended discontinuation in 1971).71 VSV, on the other hand, does not naturally infect humans, so its seroprevalence in cancer patients is generally low except in livestock farmers working in Central America, the southern United States, and other VSV endemic areas.72
It is sometimes argued that prior exposure to a virus does not impact its efficacy as an oncolytic agent.73, 74 While the data available from clinical trials of intratumoral T-VEC show no difference in treatment outcome between HSV-1 seropositive and seronegative individuals,5 there are insufficient efficacy data from intravenous OV trials to judge. However, from all we have learned of the immunology of viruses and vaccines, it seems somewhat unlikely that prior exposure to a virus will fail to impact the outcome of an oncolytic infection or its associated risks. Therefore, we need an explanation for the apparently contradictory T-VEC clinical data. Perhaps the small (106 PFUs) first dose of T-VEC serves to level the playing field by establishing anti-HSV immunity in nonimmune subjects, thus ensuring that all patients are HSV immune before clinically effective dosing at the 108-PFU (100-fold higher) dose level can be safely initiated at the 3-week time point. Another open question is whether the therapeutic gain from each successive 108-PFU dose of T-VEC is equivalent to the first, or whether it diminishes as the anti-HSV response matures further with each successive dose and shuts down the intratumoral infection with increasing speed and efficiency? Considering this question, it may be interesting to determine whether sequential intratumoral administration of OVs from different families can enhance both local and systemic tumor response rates.
Since the antitumor activity of an OV is proportional to the number of tumor cells it kills, there is a strong case to be made that the first dose administered, before the development and maturation of adaptive T and B cell responses, will be the most effective. In a nonimmune individual, there are no preformed antibodies to neutralize the OV particles and no antiviral T cells to kill infected tumor cells before they propagate the virus and release progeny. Hence, there will be more progeny released from each infected cell, faster spread of the infection, and, therefore, more direct oncolytic killing of virus-infected tumor cells. Compared to the T-VEC paradigm, there is a unique set of risks (and barriers) associated with the exploration of this fully oncolytic scenario where the goal is to maximize spread of the virus at sites of tumor growth. For example, the stringency of virus targeting must be greater, the innate immune defenses of the tumor must be compromised (unless already defective), the primary antiviral immune response may need to be slowed, and ancillary therapy may be required to prevent or treat associated tumor lysis syndrome.
Coming back, then, to the question of whether multiple viruses will be better than just one, if the field continues to move in the direction of the oncolytic paradigm and continues to pursue the single-shot cure,44 which is a euphemism for maximally exploiting the first dose of virus, the answer is surely a resounding “yes” since sequential use of immunologically non-cross-reactive viruses (most likely from different families) will likely become the norm.
Additional Issues for the Field
Combination Therapies
Rational combination of small-molecule therapeutics with oncolytic viruses has been explored extensively. One approach to achieving synergy is by using drugs that destroy or suppress innate and/or adaptive antiviral immune responses, thereby prolonging the oncolytic phase of the treatment cycle during which the infection spreads. The other main approach is to use drugs that boost or facilitate the antitumor CTL response so that it more effectively destroys uninfected tumor cells once the oncolytic phase is over.
Combining OVs with Immunosuppressive Drugs
Several drugs (e.g., HDAC, JAK/STAT, and IKK inhibitors) have been shown to antagonize the innate antiviral response, thereby enhancing the susceptibility of tumor cells to the propagation of oncolytic HSVs, VSVs, and other viruses.75, 76, 77 However, while some of these drugs are already clinically approved, their performance in vivo in mouse cancer virotherapy models has, so far, been disappointing, underscoring the need for targeted drug development programs to intentionally develop potent inhibitors of innate immunity.
Recently, a high-throughput screen was applied to identify new compounds that sensitize resistant cancer cells to infection with VSV-MΔ51, an OV highly sensitive to interferon inhibition.78 The lead compound from this assay was shown to dampen the transcriptional response to type 1 interferon and has been further optimized to enhance its potency and druggability. When tested in vivo, the optimized compound, administered by intratumoral injection, prolonged the survival of CT26 tumor-bearing mice that were treated with VSV-MΔ51Luc.79
As an aside, there is an alternative way to equip viruses to combat the innate immune responses of infected cancer cells, namely, by arming them with genes encoding any one of a host of viral immune combat proteins known to interfere with TLR, RIG-I, STING, or interferon signaling pathways or with the functions of the antiviral interferon-stimulated genes.80 However, the risk of this virus-engineering approach is the inadvertent creation of an OV with increased pathogenic potential.
Moving to the adaptive antiviral immune response, there are many clinically approved immunosuppressive drugs in routine use to prevent the rejection of transplanted organs and to treat autoimmune diseases.81 Examples include the glucocorticoids, cyclophosphamide, methotrexate, azathioprine, cyclosporine, mycophenolate, anti-lymphocyte, and anti-TNF (tumor necrosis factor) antibodies. Cyclophosphamide, used for lymphodepletion prior to chimeric antigen receptor (CAR)-T cell infusion, is potently toxic to proliferating T and B lymphocytes and has been shown to suppress immune responses to pathogenic infections in animal models, prolonging virus propagation and worsening outcomes. Cyclophosphamide has also been shown to enhance the potency of OV therapy in murine models by suppressing both primary and anamnestic responses to the therapeutic virus.82, 83 Comparison of different cyclophosphamide regimens to accelerate OV spread and efficacy suggests that the optimal approach is to initiate therapy a day or two prior to virus administration and continue for a short period thereafter.84 A phase 2 trial testing this approach is underway85 (NCT: 02192775).
Combining OVs with Immunotherapies
Checkpoint inhibitor antibodies blocking the interaction of CTLA4 with CD80 and CD86, or of PD-1 with PD-L1, allow tumor-specific CTLs to kill cancer cells. Several of these antibodies have shown superior antitumor activity when compared to conventional cancer therapies in phase 3 drug registration trials, leading to a flurry of recent FDA approvals in a range of cancers such as melanoma; Hodgkin’s lymphoma; and lung, bladder, head and neck, and kidney cancers.86 In general, checkpoint inhibitor antibodies have proven especially effective against tumors with a high mutational burden, which leads to a high neoantigen load, but for most “responsive cancers,” less than half of patients actually do respond (typically those with high mutational burden and abundant tumor-infiltrating T cells), and the responses are not always durable.
So the search is on for companion therapies that enhance the potency of immune checkpoint blockade. The logical partnerships are with vaccination strategies that amplify the tumor-reactive, neoantigen-specific CTLs upon whose existence the checkpoint antibodies rely. Personalized vaccines are currently being generated by analyzing the tumor exome to identify potentially neoantigenic peptides, docking them in silico to model protein structures representing the patient’s known MHC haplotypes, then predicting which of the mutated peptide-MHC complexes are likely targets for T cell recognition.87 This information is then be used to generate a personalized peptide or genetic vaccine specifically designed to boost the immune responses to these computer-predicted targets.88 The approach is appealing, but further work is needed both to improve the veracity of the neoantigen predictions and to speed the process.
In contrast to neoantigen prediction approaches, OVs offer an antigen-agnostic approach in the form of an in situ vaccine that can boost T cell frequencies against tumor antigens whose identity is unknown. A previous concern about the OV approach was that immunodominant viral antigens would obscure the tumor neoantigens, effectively “diverting” the immune response away from the tumor.89 However, this concern has been refuted in recent preclinical studies, which show that OV therapy does, indeed, boost the CTL response to tumor-specific antigens and that OVs do synergize with anti-PD-L1 antibodies.90
Moreover, recent clinical experience with T-VEC provides a very strong endorsement of the approach. Interim analysis of the first 82 patients enrolled in a randomized phase 2 study comparing ipilumumab (anti-CTLA4) single-agent therapy to ipilumumab plus T-VEC in stage III/IV melanoma showed a 27.5% unconfirmed response rate for ipilumumab alone versus 50% with the ipilumumab/T-VEC combination.91 Positive outcomes have also been reported for clinical trials combining CVA21 with ipilumumab (Viralytics, 2016, Society for the Immunotherapy of Cancer, conference), and several additional clinical studies are planned.
In an attempt to build on the antigen-agnostic in situ oncolytic vaccine paradigm, some research groups are developing a derivative approach in which a known tumor antigen is encoded in the OV genome, thereby creating an oncolytic vaccine that may be able to skew the antitumor immune response in a specific (and, possibly, beneficial) direction. In one ongoing clinical study, based on highly promising preclinical data, a prime-boost strategy using a nonreplicating adenovirus and an oncolytic vesiculovirus (Maraba virus), both encoding MAGE-A3, is being pursued in patients with MAGE-A3-positive tumors (NCT: 02285816).
Another concept that is gathering momentum but has not yet been tested clinically is to combine adoptive T cell therapy with the oncolytic vaccine approach.92, 93 Thus, in the B16-ova mouse melanoma model, the antitumor activity of adoptively transferred ovalbumin-specific T cells was substantially boosted by an oncolytic VSV that had been engineered to encode the ova antigen, but not by a comparable VSV construct lacking the ova gene.94 Efforts are underway to advance this promising concept to clinical testing to evaluate a variety of OV-adoptive T cell therapy combinations.
Patient/Target Definition
The decision as to whether to treat a given patient with a given OV will likely need to factor in additional parameters besides those already used to guide cancer treatment decisions. At the present time, cocktails of available anticancer drugs, sometimes in combination with radiotherapy, are selected on the basis of tumor histology; signature metabolic and signaling abnormalities; disease burden; extent of tumor spread, both local and metastatic; age of the patient; and presence of comorbidities affecting vital organ functions.
Additional factors that may be important predictors of the likelihood of response when planning oncolytic virotherapy include previous virus exposure history, serum concentrations of antiviral antibodies, antitumor CTL frequencies, defects in innate immune signaling and/or antigen processing/presentation revealed by gene expression arrays, mutational burden/neoantigen load determined by comparative exome sequencing, and in vitro infectivity assays on tumor biopsy material to demonstrate susceptibility to specific oncolytic viruses.
Comparative Oncology
Preclinical efficacy and toxicity studies in mouse cancer models are notoriously unreliable as predictors of what will transpire in human drug trials. Comparative oncology is a growing field of research (and of veterinary practice) in which naturally occurring cancers in immune-competent pet dogs (and cats) are studied and included in the traditional drug development pathway.95 For certain OVs, the approach offers an excellent opportunity to test the treatment in large animals with naturally occurring cancers and to ask critical questions regarding which factors can predict response to therapy and thus assist in the design of human clinical trials. Many types of spontaneous canine cancers are accepted models for their human counterparts. The dog’s physical size allows serial large-volume biologic sample collections to examine viral shedding, and their inherent tumor heterogeneity allows correlation of tumor- and patient-related factors to clinical outcomes.
Not all OVs are suitable for therapy of companion animals. For OVs derived from human pathogens such as measles, HSV-1, poliovirus, and CVA21, cell-entry receptors and/or intracellular factors required for successful completion of the virus replication cycle may be lacking in canine cells. In general, and for similar reasons, these viruses are also difficult to evaluate in mouse models. However, there are several OVs such as those derived from VSV, vaccinia, Newcastle disease virus, and mengovirus whose tropisms are not species restricted and that are, therefore, suitable candidates for comparative oncology testing and development.96
In one recent comparative oncology study, two versions of VSV-IFNβ-NIS were developed, one encoding human IFNβ and the other encoding canine IFNβ. After completing a formal canine toxicology study,97 these viruses were administered intravenously to eight canine patients with spontaneously arising lymphomas with meaningful clinical responses observed in two of the animals., both with T cell lymphomas. One major advantage of the comparative oncology approach is the ability to initiate therapy at a meaningful dose level so that there can be an expectation of seeing an efficacy signal in potentially responsive tumors, even in a phase 1, first-in-canine trial. Several viruses are being developed with canine veterinary testing in mind,98, 99, 100 and some are even being developed exclusively for canine use, such as the VSV-encoding canine IFNβ and a retinoblastoma gene product (pRb)-responsive, arginine, glycine, asparate (RGD)-modified, hyaluronidase-armed canine adenovirus.100
Conclusions
Since the launch of the journal Molecular Therapy 20 years ago, oncolytic virotherapy has progressed from a promising early-stage technology to a burgeoning new biological drug class. Many oncolytic platforms and products are being pursued, administered by intratumoral, locoregional, or intravenous routes, alone or in combination with immunomodulatory drugs. The field appears to be well on the way to establishing itself as a new cornerstone for future immuno-oncology regimens.
Conflicts of Interest
S.J.R., K.-W.P., and the Mayo Clinic have a financial interest in the technology described in this article. S.J.R. and K.-W.P. are cofounders of Vyriad, Inc., an oncolytic virotherapy company.
Acknowledgments
We acknowledge funding support from NIH/NCI for the Mayo Clinic Myeloma SPORE (P50CA186781 to S.J.R. and R01CA175795 to K.-W.P.), the Mayo Clinic Comprehensive Cancer Center (P30CA015083), the Al and Mary Agnes McQuinn Foundation, and the David F. and Margaret T. Grohne Family Foundation.
References
- 1.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seymour L.W., Fisher K.D. Oncolytic viruses: finally delivering. Br. J. Cancer. 2016;114:357–361. doi: 10.1038/bjc.2015.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the clinic: talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bommareddy P.K., Patel A., Hossain S., Kaufman H.L. Talimogene laherparepvec (T-VEC) and other oncolytic viruses for the treatment of melanoma. Am. J. Clin. Dermatol. 2017;18:1–15. doi: 10.1007/s40257-016-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 6.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene laherparepvec in combination with ipilimumab in previously untreated, unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long G.V., Dummer R., Ribas A., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J.S. Efficacy analysis of MASTERKEY-265 phase 1b study of talimogene lapherparevec (T-VEC) and pembrolizuman (pembro) for unresectable stage IIIB-IV melanoma. J. Clin. Oncol. 2016;34(Suppl.; abstr. 9568) [Google Scholar]
- 8.Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 9.Maroun J., Muñoz-Alía M., Ammayappan A., Schulze A., Peng K.-W., Russell S. Designing and building oncolytic viruses. Future Virol. 2017 doi: 10.2217/fvl-2016-0129. Published online March 31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keller B.A., Bell J.C. Oncolytic viruses—immunotherapeutics on the rise. J. Mol. Med. (Berl.) 2016;94:979–991. doi: 10.1007/s00109-016-1453-9. [DOI] [PubMed] [Google Scholar]
- 11.Lawler S.E., Speranza M.C., Cho C.F., Chiocca E.A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.2064. Published July 21, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Martuza R.L., Malick A., Markert J.M., Ruffner K.L., Coen D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 13.Wilcox D.R., Longnecker R. The herpes simplex virus neurovirulence factor γ34.5: revealing virus-host interactions. PLoS Pathog. 2016;12:e1005449. doi: 10.1371/journal.ppat.1005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaur B., Chiocca E.A., Cripe T.P. Oncolytic HSV-1 virotherapy: clinical experience and opportunities for progress. Curr. Pharm. Biotechnol. 2012;13:1842–1851. doi: 10.2174/138920112800958814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu B.L., Robinson M., Han Z.Q., Branston R.H., English C., Reay P., McGrath Y., Thomas S.K., Thornton M., Bullock P. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 17.Kohlhapp F.J., Kaufman H.L. Molecular pathways: mechanism of action for talimogene laherparepvec, a new oncolytic virus immunotherapy. Clin. Cancer Res. 2016;22:1048–1054. doi: 10.1158/1078-0432.CCR-15-2667. [DOI] [PubMed] [Google Scholar]
- 18.Hong C.S., Fellows W., Niranjan A., Alber S., Watkins S., Cohen J.B., Glorioso J.C., Grandi P. Ectopic matrix metalloproteinase-9 expression in human brain tumor cells enhances oncolytic HSV vector infection. Gene Ther. 2010;17:1200–1205. doi: 10.1038/gt.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamori M., Fu X., Meng F., Jin A., Tao L., Bast R.C., Jr., Zhang X. Effective therapy of metastatic ovarian cancer with an oncolytic herpes simplex virus incorporating two membrane fusion mechanisms. Clin. Cancer Res. 2003;9:2727–2733. [PubMed] [Google Scholar]
- 20.Simpson G.R., Coffin R.S. Construction and characterization of an oncolytic HSV vector containing a fusogenic glycoprotein and prodrug activation for enhanced local tumor control. Methods Mol. Biol. 2009;542:551–564. doi: 10.1007/978-1-59745-561-9_29. [DOI] [PubMed] [Google Scholar]
- 21.Menotti L., Cerretani A., Hengel H., Campadelli-Fiume G. Construction of a fully retargeted herpes simplex virus 1 recombinant capable of entering cells solely via human epidermal growth factor receptor 2. J. Virol. 2008;82:10153–10161. doi: 10.1128/JVI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goins W.F., Hall B., Cohen J.B., Glorioso J.C. Retargeting of herpes simplex virus (HSV) vectors. Curr. Opin. Virol. 2016;21:93–101. doi: 10.1016/j.coviro.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee C.Y., Rennie P.S., Jia W.W. MicroRNA regulation of oncolytic herpes simplex virus-1 for selective killing of prostate cancer cells. Clin. Cancer Res. 2009;15:5126–5135. doi: 10.1158/1078-0432.CCR-09-0051. [DOI] [PubMed] [Google Scholar]
- 24.Mazzacurati L., Marzulli M., Reinhart B., Miyagawa Y., Uchida H., Goins W.F., Li A., Kaur B., Caligiuri M., Cripe T. Use of miRNA response sequences to block off-target replication and increase the safety of an unattenuated, glioblastoma-targeted oncolytic HSV. Mol. Ther. 2015;23:99–107. doi: 10.1038/mt.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kambara H., Okano H., Chiocca E.A., Saeki Y. An oncolytic HSV-1 mutant expressing ICP34.5 under control of a nestin promoter increases survival of animals even when symptomatic from a brain tumor. Cancer Res. 2005;65:2832–2839. doi: 10.1158/0008-5472.CAN-04-3227. [DOI] [PubMed] [Google Scholar]
- 26.Yoo J.Y., Haseley A., Bratasz A., Chiocca E.A., Zhang J., Powell K., Kaur B. Antitumor efficacy of 34.5ENVE: a transcriptionally retargeted and “Vstat120”-expressing oncolytic virus. Mol. Ther. 2012;20:287–297. doi: 10.1038/mt.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foreman P.M., Friedman G.K., Cassady K.A., Markert J.M. Oncolytic virotherapy for the treatment of malignant glioma. Neurotherapeutics. 2017 doi: 10.1007/s13311-017-0516-0. Published March 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goetz C., Dobrikova E., Shveygert M., Dobrikov M., Gromeier M. Oncolytic poliovirus against malignant glioma. Future Virol. 2011;6:1045–1058. doi: 10.2217/fvl.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cloughesy T.F., Landolfi J., Hogan D.J., Bloomfield S., Carter B., Chen C.C., Elder J.B., Kalkanis S.N., Kesari S., Lai A. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016;8:341ra75. doi: 10.1126/scitranslmed.aad9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang F., Conrad C., Gomez-Manzano C.D., Tufaro F., Yung W., Sawaya R., Weinberg J., Prabhu S., Fuller G., Aldape K., Fueyo J. First-in-human phase I clinical trial of oncolytic delta-24-RGD (DNX-2401) with biological endpoints: Implications for viro-immunotherapy. Neuro Oncol. 2014;16(Suppl. 3):iii39. [Google Scholar]
- 31.Geletneky K., Huesing J., Rommelaere J., Schlehofer J.R., Leuchs B., Dahm M., Krebs O., von Knebel Doeberitz M., Huber B., Hajda J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia Z.J., Chang J.H., Zhang L., Jiang W.Q., Guan Z.Z., Liu J.W., Zhang Y., Hu X.H., Wu G.H., Wang H.Q. [Phase III randomized clinical trial of intratumoral injection of E1B gene-deleted adenovirus (H101) combined with cisplatin-based chemotherapy in treating squamous cell cancer of head and neck or esophagus] Chin. J. Cancer. 2004;23:1666–1670. [PubMed] [Google Scholar]
- 33.Yu W., Fang H. Clinical trials with oncolytic adenovirus in China. Curr. Cancer Drug Targets. 2007;7:141–148. doi: 10.2174/156800907780058817. [DOI] [PubMed] [Google Scholar]
- 34.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chentoufi A.A., Benmohamed L. Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections. Clin. Dev. Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andtbacka R.H.I., Ross M.I., Agarwala S.S., Taylor M.H., Vetto J.T., Neves R.I., Daud A., Khong H.T., Ungerleider R.S., Boran A.D.W. Preliminary results from phase II study of combination treatment with HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab in patients with stage IIIb, IIIc, or IV unresectable or metastatic melanoma. J. Clin. Oncol. 2016;34(Suppl.; abstract 9543) [Google Scholar]
- 37.Miller A., Russell S.J. The use of the NIS reporter gene for optimizing oncolytic virotherapy. Expert Opin. Biol. Ther. 2016;16:15–32. doi: 10.1517/14712598.2016.1100162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith J.S., Robinson N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 2002;186(Suppl 1):S3–S28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 39.Xu F., Schillinger J.A., Sternberg M.R., Johnson R.E., Lee F.K., Nahmias A.J., Markowitz L.E. Seroprevalence and coinfection with herpes simplex virus type 1 and type 2 in the United States, 1988-1994. J. Infect. Dis. 2002;185:1019–1024. doi: 10.1086/340041. [DOI] [PubMed] [Google Scholar]
- 40.Virgin S. Pathogenesis of viral infection. In: Knipe D.M., Howley P.M., editors. Volume 1. Lippincott, Williams and Wilkins; Philadelphia, PA: 2007. pp. 327–388. (Fields Virology). [Google Scholar]
- 41.Griffin D.E. Measles virus. In: Knipe D.M., Howley P.M., editors. Volume 2. Lippincott, Williams and Wilkins; Philadelphia, PA: 2007. pp. 1551–1586. (Fields Virology). [Google Scholar]
- 42.Zingher A., Mortimer P. Convalescent whole blood, plasma and serum in the prophylaxis of measles: JAMA, 12 April, 1926; 1180-1187. Rev. Med. Virol. 2005;15:407–418. doi: 10.1002/rmv.480. [DOI] [PubMed] [Google Scholar]
- 43.Moore A.E. The destructive effect of the virus of Russian Far East encephalitis on the transplantable mouse sarcoma 180. Cancer. 1949;2:525–534. doi: 10.1002/1097-0142(194905)2:3<525::aid-cncr2820020317>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Naik S., Nace R., Federspiel M.J., Barber G.N., Peng K.W., Russell S.J. Curative one-shot systemic virotherapy in murine myeloma. Leukemia. 2012;26:1870–1878. doi: 10.1038/leu.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phuangsab A., Lorence R.M., Reichard K.W., Peeples M.E., Walter R.J. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz A.J., Hadac E.M., Nace R.A., Russell S.J. MicroRNA-detargeted mengovirus for oncolytic virotherapy. J. Virol. 2016;90:4078–4092. doi: 10.1128/JVI.02810-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Au G.G., Lindberg A.M., Barry R.D., Shafren D.R. Oncolysis of vascular malignant human melanoma tumors by Coxsackievirus A21. Int. J. Oncol. 2005;26:1471–1476. doi: 10.3892/ijo.26.6.1471. [DOI] [PubMed] [Google Scholar]
- 48.McCart J.A., Ward J.M., Lee J., Hu Y., Alexander H.R., Libutti S.K., Moss B., Bartlett D.L. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 49.Yu L., Baxter P.A., Zhao X., Liu Z., Wadhwa L., Zhang Y., Su J.M., Tan X., Yang J., Adesina A. A single intravenous injection of oncolytic picornavirus SVV-001 eliminates medulloblastomas in primary tumor-based orthotopic xenograft mouse models. Neuro Oncol. 2011;13:14–27. doi: 10.1093/neuonc/noq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey K., Kirk A., Naik S., Nace R., Steele M.B., Suksanpaisan L., Li X., Federspiel M.J., Peng K.W., Kirk D., Russell S.J. Mathematical model for radial expansion and conflation of intratumoral infectious centers predicts curative oncolytic virotherapy parameters. PLoS ONE. 2013;8:e73759. doi: 10.1371/journal.pone.0073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller A., Suksanpaisan L., Naik S., Nace R., Federspiel M., Peng K.W., Russell S.J. Reporter gene imaging identifies intratumoral infection voids as a critical barrier to systemic oncolytic virus efficacy. Mol. Ther. Oncolytics. 2014;1:14005. doi: 10.1038/mto.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breitbach C.J., De Silva N.S., Falls T.J., Aladl U., Evgin L., Paterson J., Sun Y.Y., Roy D.G., Rintoul J.L., Daneshmand M. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 2011;19:886–894. doi: 10.1038/mt.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breitbach C.J., Arulanandam R., De Silva N., Thorne S.H., Patt R., Daneshmand M., Moon A., Ilkow C., Burke J., Hwang T.H. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 2013;73:1265–1275. doi: 10.1158/0008-5472.CAN-12-2687. [DOI] [PubMed] [Google Scholar]
- 54.Benencia F., Courreges M.C., Conejo-García J.R., Buckanovich R.J., Zhang L., Carroll R.H., Morgan M.A., Coukos G. Oncolytic HSV exerts direct antiangiogenic activity in ovarian carcinoma. Hum. Gene Ther. 2005;16:765–778. doi: 10.1089/hum.2005.16.765. [DOI] [PubMed] [Google Scholar]
- 55.Kelly E.J., Hadac E.M., Cullen B.R., Russell S.J. MicroRNA antagonism of the picornaviral life cycle: alternative mechanisms of interference. PLoS Pathog. 2010;6:e1000820. doi: 10.1371/journal.ppat.1000820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Power A.T., Wang J., Falls T.J., Paterson J.M., Parato K.A., Lichty B.D., Stojdl D.F., Forsyth P.A., Atkins H., Bell J.C. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 57.Liu C., Suksanpaisan L., Chen Y.W., Russell S.J., Peng K.W. Enhancing cytokine-induced killer cell therapy of multiple myeloma. Exp. Hematol. 2013;41:508–517. doi: 10.1016/j.exphem.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mader E.K., Maeyama Y., Lin Y., Butler G.W., Russell H.M., Galanis E., Russell S.J., Dietz A.B., Peng K.W. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin. Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mader E.K., Butler G., Dowdy S.C., Mariani A., Knutson K.L., Federspiel M.J., Russell S.J., Galanis E., Dietz A.B., Peng K.W. Optimizing patient derived mesenchymal stem cells as virus carriers for a phase I clinical trial in ovarian cancer. J. Transl. Med. 2013;11:20. doi: 10.1186/1479-5876-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruiz A.J., Russell S.J. MicroRNAs and oncolytic viruses. Curr. Opin. Virol. 2015;13:40–48. doi: 10.1016/j.coviro.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Russell S.J., Peng K.W. Viruses as anticancer drugs. Trends Pharmacol. Sci. 2007;28:326–333. doi: 10.1016/j.tips.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorne S.H., Hermiston T., Kirn D. Oncolytic virotherapy: approaches to tumor targeting and enhancing antitumor effects. Semin. Oncol. 2005;32:537–548. doi: 10.1053/j.seminoncol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Dorer D.E., Nettelbeck D.M. Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv. Drug Deliv. Rev. 2009;61:554–571. doi: 10.1016/j.addr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 64.Beatty M.S., Curiel D.T. Chapter two--Adenovirus strategies for tissue-specific targeting. Adv. Cancer Res. 2012;115:39–67. doi: 10.1016/B978-0-12-398342-8.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura T., Russell S.J. Oncolytic measles viruses for cancer therapy. Expert Opin. Biol. Ther. 2004;4:1685–1692. doi: 10.1517/14712598.4.10.1685. [DOI] [PubMed] [Google Scholar]
- 66.Ilkow C.S., Swift S.L., Bell J.C., Diallo J.S. From scourge to cure: tumour-selective viral pathogenesis as a new strategy against cancer. PLoS Pathog. 2014;10:e1003836. doi: 10.1371/journal.ppat.1003836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Russell S.J., Federspiel M.J., Peng K.W., Tong C., Dingli D., Morice W.G., Lowe V., O’Connor M.K., Kyle R.A., Leung N. Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin. Proc. 2014;89:926–933. doi: 10.1016/j.mayocp.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langfield K.K., Walker H.J., Gregory L.C., Federspiel M.J. Manufacture of measles viruses. Methods Mol. Biol. 2011;737:345–366. doi: 10.1007/978-1-61779-095-9_14. [DOI] [PubMed] [Google Scholar]
- 69.Breitbach C.J., Burke J., Jonker D., Stephenson J., Haas A.R., Chow L.Q., Nieva J., Hwang T.H., Moon A., Patt R. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011;477:99–102. doi: 10.1038/nature10358. [DOI] [PubMed] [Google Scholar]
- 70.Kelly E.J., Hadac E.M., Greiner S., Russell S.J. Engineering microRNA responsiveness to decrease virus pathogenicity. Nat. Med. 2008;14:1278–1283. doi: 10.1038/nm.1776. [DOI] [PubMed] [Google Scholar]
- 71.Strassburg M.A. The global eradication of smallpox. Am. J. Infect. Control. 1982;10:53–59. doi: 10.1016/0196-6553(82)90003-7. [DOI] [PubMed] [Google Scholar]
- 72.Letchworth G.J., Rodriguez L.L., Del cbarrera J. Vesicular stomatitis. Vet. J. 1999;157:239–260. doi: 10.1053/tvjl.1998.0303. [DOI] [PubMed] [Google Scholar]
- 73.Lambright E.S., Kang E.H., Force S., Lanuti M., Caparrelli D., Kaiser L.R., Albelda S.M., Molnar-Kimber K.L. Effect of preexisting anti-herpes immunity on the efficacy of herpes simplex viral therapy in a murine intraperitoneal tumor model. Mol. Ther. 2000;2:387–393. doi: 10.1006/mthe.2000.0133. [DOI] [PubMed] [Google Scholar]
- 74.Ilett E.J., Bárcena M., Errington-Mais F., Griffin S., Harrington K.J., Pandha H.S., Coffey M., Selby P.J., Limpens R.W., Mommaas M. Internalization of oncolytic reovirus by human dendritic cell carriers protects the virus from neutralization. Clin. Cancer Res. 2011;17:2767–2776. doi: 10.1158/1078-0432.CCR-10-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakashima H., Nguyen T., Chiocca E.A. Combining HDAC inhibitors with oncolytic virotherapy for cancer therapy. Oncolytic Virother. 2015;4:183–191. doi: 10.2147/OV.S66081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyên T.L., Abdelbary H., Arguello M., Breitbach C., Leveille S., Diallo J.S., Yasmeen A., Bismar T.A., Kirn D., Falls T. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl. Acad. Sci. USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Forbes N.E., Krishnan R., Diallo J.S. Pharmacological modulation of anti-tumor immunity induced by oncolytic viruses. Front. Oncol. 2014;4:191. doi: 10.3389/fonc.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Diallo J.S., Boeuf F.L., Lai F., Cox J., Vaha-Koskela M., Abdelbary H., Mac Tavish H., Waite K., Falls T., Wang J. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther. 2010;18:1123–1129. doi: 10.1038/mt.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dornan M.H., Krishnan R., Macklin A.M., Selman M., El Sayes N., Son H.H., Davis C., Chen A., Keillor K., Le P.J. First-in-class small molecule potentiators of cancer virotherapy. Sci. Rep. 2016;6:26786. doi: 10.1038/srep26786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Naik S., Russell S.J. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin. Biol. Ther. 2009;9:1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 81.Hartono C., Muthukumar T., Suthanthiran M. Immunosuppressive drug therapy. Cold Spring Harb. Perspect. Med. 2013;3:a015487. doi: 10.1101/cshperspect.a015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dhar D., Spencer J.F., Toth K., Wold W.S. Effect of preexisting immunity on oncolytic adenovirus vector INGN 007 antitumor efficacy in immunocompetent and immunosuppressed Syrian hamsters. J. Virol. 2009;83:2130–2139. doi: 10.1128/JVI.02127-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiao J., Wang H., Kottke T., White C., Twigger K., Diaz R.M., Thompson J., Selby P., de Bono J., Melcher A. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin. Cancer Res. 2008;14:259–269. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng K.W., Myers R., Greenslade A., Mader E., Greiner S., Federspiel M.J., Dispenzieri A., Russell S.J. Using clinically approved cyclophosphamide regimens to control the humoral immune response to oncolytic viruses. Gene Ther. 2013;20:255–261. doi: 10.1038/gt.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Myers R.M., Greiner S.M., Harvey M.E., Griesmann G., Kuffel M.J., Buhrow S.A., Reid J.M., Federspiel M., Ames M.M., Dingli D. Preclinical pharmacology and toxicology of intravenous MV-NIS, an oncolytic measles virus administered with or without cyclophosphamide. Clin. Pharmacol. Ther. 2007;82:700–710. doi: 10.1038/sj.clpt.6100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salama A.K.S., Moschos S.J. Next steps in immuno-oncology: enhancing antitumor effects through appropriate patient selection and rationally designed combination strategies. Ann. Oncol. 2017;28:57–74. doi: 10.1093/annonc/mdw534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yadav M., Jhunjhunwala S., Phung Q.T., Lupardus P., Tanguay J., Bumbaca S., Franci C., Cheung T.K., Fritsche J., Weinschenk T. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–576. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 88.Zhang X., Sharma P.K., Goedegebuure S.P., Gillanders W.E. Personalized cancer vaccines: Targeting the cancer mutanome. Vaccine. 2017;35:1094–1100. doi: 10.1016/j.vaccine.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kloos A., Woller N., Gürlevik E., Ureche C.I., Niemann J., Armbrecht N., Martin N.T., Geffers R., Manns M.P., Gerardy-Schahn R., Kühnel F. PolySia-specific retargeting of oncolytic viruses triggers tumor-specific immune responses and facilitates therapy of disseminated lung cancer. Cancer Immunol. Res. 2015;3:751–763. doi: 10.1158/2326-6066.CIR-14-0124-T. [DOI] [PubMed] [Google Scholar]
- 90.Shen W., Patnaik M.M., Ruiz A., Russell S.J., Peng K.W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood. 2016;127:1449–1458. doi: 10.1182/blood-2015-06-652503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chesney J., Collichio F., Andtbacka R.H., Puzanov I., Glaspy J., Milhem M., Hamid O., Cranmer L., Saenger Y., Ross M. Interim safety and efficacy of a randomized (1:1), open-label phase 2 study of talimogene laherparepvec (T) and ipilimumab (I) vs I alone in unresectable melanoma. Ann. Oncol. 2016;27:379–400. [Google Scholar]
- 92.Shim K.G., Zaidi S., Thompson J., Kottke T., Evgin L., Rajani K.R., Schuelke M., Driscoll C.B., Huff A., Pulido J.S., Vile R.G. Inhibitory receptors induced by VSV viroimmunotherapy are not necessarily targets for improving treatment efficacy. Mol. Ther. 2017 doi: 10.1016/j.ymthe.2017.01.023. Published online February 22, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rommelfanger D.M., Wongthida P., Diaz R.M., Kaluza K.M., Thompson J.M., Kottke T.J., Vile R.G. Systemic combination virotherapy for melanoma with tumor antigen-expressing vesicular stomatitis virus and adoptive T-cell transfer. Cancer Res. 2012;72:4753–4764. doi: 10.1158/0008-5472.CAN-12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wongthida P., Diaz R.M., Pulido C., Rommelfanger D., Galivo F., Kaluza K., Kottke T., Thompson J., Melcher A., Vile R. Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum. Gene Ther. 2011;22:1343–1353. doi: 10.1089/hum.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paoloni M.C., Khanna C. Comparative oncology today. Vet. Clin. North Am. Small Anim. Pract. 2007;37:1023–1032. doi: 10.1016/j.cvsm.2007.08.003. v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gentschev I., Patil S.S., Petrov I., Cappello J., Adelfinger M., Szalay A.A. Oncolytic virotherapy of canine and feline cancer. Viruses. 2014;6:2122–2137. doi: 10.3390/v6052122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.LeBlanc A.K., Naik S., Galyon G.D., Jenks N., Steele M., Peng K.W., Federspiel M.J., Donnell R., Russell S.J. Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Hum. Gene Ther. Clin. Dev. 2013;24:174–181. doi: 10.1089/humc.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sánchez D., Pelayo R., Medina L.A., Vadillo E., Sánchez R., Núñez L., Cesarman-Maus G., Sarmiento-Silva R.E. Newcastle disease virus: potential therapeutic application for human and canine lymphoma. Viruses. 2015;8:3. doi: 10.3390/v8010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gentschev I., Patil S.S., Adelfinger M., Weibel S., Geissinger U., Frentzen A., Chen N.G., Yu Y.A., Zhang Q., Ogilvie G., Szalay A.A. Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered. 2013;4:84–89. doi: 10.4161/bioe.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Laborda E., Puig-Saus C., Rodriguez-García A., Moreno R., Cascalló M., Pastor J., Alemany R. A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol. Ther. 2014;22:986–998. doi: 10.1038/mt.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]