Abstract
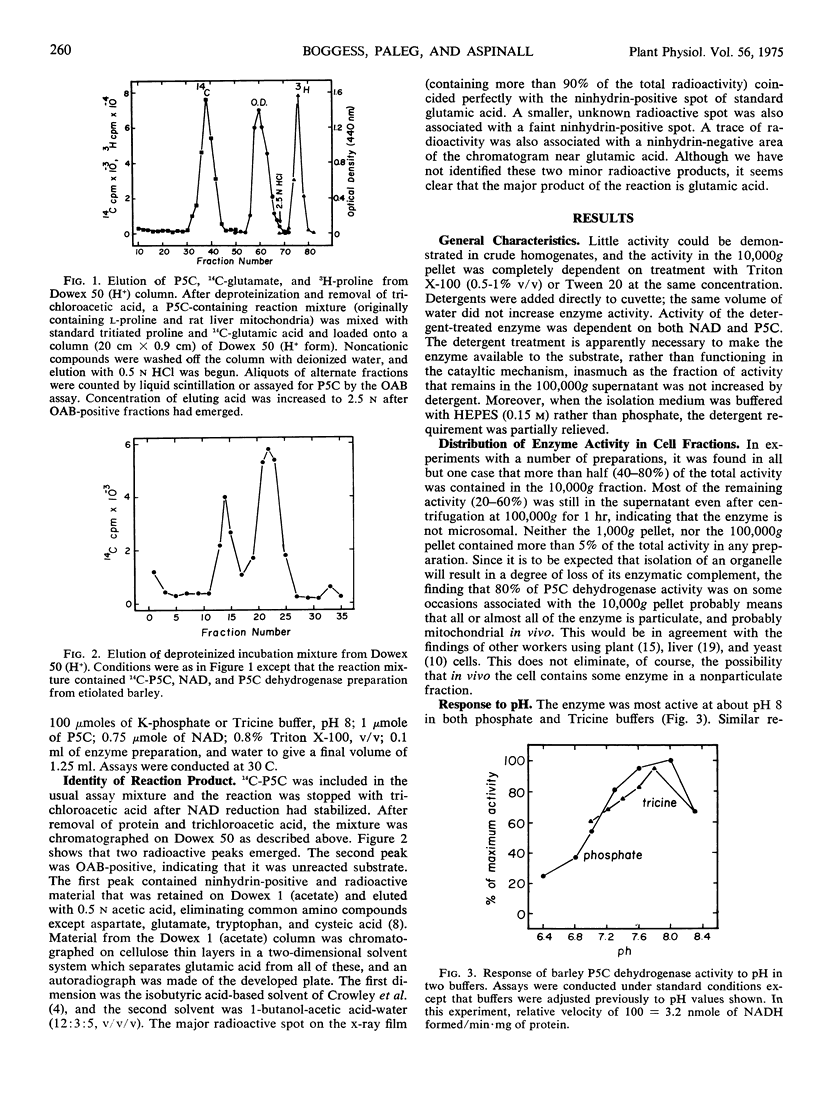
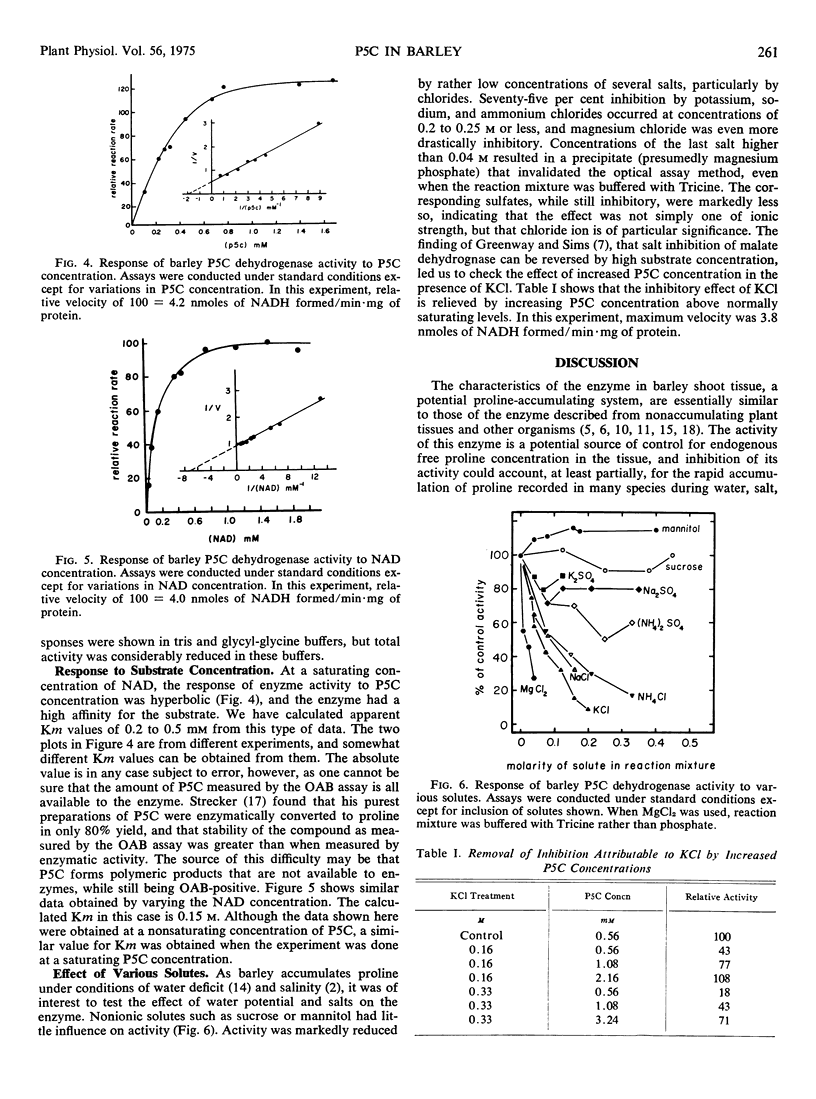
The characteristics of the enzyme Δ1-pyrroline-5-carboxylic acid dehydrogenase from etiolated barley (Hordeum distichum) shoots have been examined. The bulk of the enzyme activity was found in the 10,000g pellet fraction, this activity being displayed only after detergent treatment of the suspended pellet. The enzyme was most active at pH 8, and activity was NAD-dependent. Enzyme activity was unaffected by either mannitol or sucrose in the reaction mixture up to a concentration of 0.45 m but was strongly inhibited by Cl− and, to a lesser extent, SO42−. The inhibition attributable to KCl was reversed by increasing the concentration of Δ1-pyrroline-5-carboxylic acid in the reaction mixture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWLEY G. J., MOSES V., ULLRICH J. A VERSATILE SOLVENT TO REPLACE PHENOL FOR THE PAPER CHROMATOGRAPHY OF RADIOACTIVE INTERMEDIARY METABOLITES. J Chromatogr. 1963 Oct;12:219–228. doi: 10.1016/s0021-9673(01)83673-6. [DOI] [PubMed] [Google Scholar]
- FRANK L., RANHAND B. PROLINE METABOLISM IN ESCHERICHIA COLI. 3. THE PROLINE CATABOLIC PATHWAY. Arch Biochem Biophys. 1964 Aug;107:325–331. doi: 10.1016/0003-9861(64)90338-8. [DOI] [PubMed] [Google Scholar]
- JOHNSON A. B., STRECKER H. J. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J Biol Chem. 1962 Jun;237:1876–1882. [PubMed] [Google Scholar]
- LING C. M., HEDRICK L. R. PROLINE OXIDASES IN HANSENULA SUBPELLICULOSA. J Bacteriol. 1964 Jun;87:1462–1470. doi: 10.1128/jb.87.6.1462-1470.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren D. W., Ogur M. Inhibition of yeast 1 -pyrroline-5-carboxylate dehydrogenase by common amino acids and the regulation of proline catabolism. Biochim Biophys Acta. 1973 Feb 28;297(2):246–257. doi: 10.1016/0304-4165(73)90071-8. [DOI] [PubMed] [Google Scholar]
- McNamer A. D., Stewart C. R. Nicotinamide Adenine Dinucleotide-dependent Proline Dehydrogenase in Chlorella. Plant Physiol. 1974 Mar;53(3):440–444. doi: 10.1104/pp.53.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Thompson J. F., Johnson C. M. Metabolism of Glutamic Acid and N-Acetylglutamic Acid in Leaf Discs and Cell-free Extracts of Higher Plants. Plant Physiol. 1969 Jul;44(7):1023–1026. doi: 10.1104/pp.44.7.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRECKER H. J., MELA P. The interconversion of glutamic acid and proline. Biochim Biophys Acta. 1955 Aug;17(4):580–581. doi: 10.1016/0006-3002(55)90422-4. [DOI] [PubMed] [Google Scholar]
- STRECKER H. J. The interconversion of glutamic acid and proline. I. The formation of delta1-pyrroline-5-carboxylic acid from glutamic acid in Escherichia coli. J Biol Chem. 1957 Apr;225(2):825–834. [PubMed] [Google Scholar]
- STRECKER H. J. The interconversion of glutamic acid and proline. II. The preparation and properties of delta 1-pyrroline-5-carboxylic acid. J Biol Chem. 1960 Jul;235:2045–2050. [PubMed] [Google Scholar]


