Abstract
AMP-activated kinase (AMPK) is a key player in energy sensing and metabolic reprogramming under cellular energy restriction. Several studies have linked impaired AMPK function to peripheral metabolic diseases such as diabetes. However, the impact of neurological disorders, such as Alzheimer disease (AD), on AMPK function and downstream effects of altered AMPK activity on neuronal metabolism have been investigated only recently. Here, we report the impact of Aβ oligomers (AβOs), synaptotoxins that accumulate in AD brains, on neuronal AMPK activity. Short-term exposure of cultured rat hippocampal neurons or ex vivo human cortical slices to AβOs transiently decreased intracellular ATP levels and AMPK activity, as evaluated by its phosphorylation at threonine residue 172 (AMPK-Thr(P)172). The AβO-dependent reduction in AMPK-Thr(P)172 levels was mediated by glutamate receptors of the N-methyl-d-aspartate (NMDA) subtype and resulted in removal of glucose transporters (GLUTs) from the surfaces of dendritic processes in hippocampal neurons. Importantly, insulin prevented the AβO-induced inhibition of AMPK. Our results establish a novel toxic impact of AβOs on neuronal metabolism and suggest that AβO-induced, NMDA receptor-mediated AMPK inhibition may play a key role in early brain metabolic defects in AD.
Keywords: Alzheimer disease; AMP-activated kinase (AMPK); ATP; energy metabolism; N-methyl-d-aspartate receptor (NMDA receptor, NMDAR); GLUTs; amyloid-β oligomers
Introduction
AMP-activated kinase (AMPK)3 is a major sensor of cellular energy status and a central regulator of metabolic homeostasis (1). AMPK is activated by elevated intracellular AMP/ATP ratios in response to nutrient deprivation and/or pathological stress (1, 2), and acts pleiotropically to promote metabolic reprogramming (1, 3). Defects in AMPK signaling have been reported in peripheral metabolic disorders (4, 5), and recent evidence suggests that impaired AMPK function may play relevant roles in brain disease, such as Huntington (HD), Parkinson, and Alzheimer (AD) diseases (6). AMPK is highly expressed in the hippocampus (7), a brain region that plays key roles in synaptic plasticity, memory, and cognition, and aberrant AMPK activity has been reported in the brains of transgenic mouse models of AD (8–10) and AD patients (9, 11).
Because AD patients exhibit impaired brain metabolism (12, 13) and AMPK is required for proper metabolism and cell function (2, 3), we aimed to determine the impact of Aβ oligomers (AβOs) on neuronal AMPK. AβOs are toxins that build up in AD brains (14, 15) and in the brains of animal models of AD (16, 17), and are thought to underlie synapse and memory failure (18, 19).
Using in vitro and ex vivo models, we found that AβOs transiently inhibit neuronal AMPK phosphorylation via an NMDA receptor (NMDAR)-mediated mechanism. We further found that AβOs instigate removal of glucose transporters 3 and 4 (GLUT3 and GLUT4, respectively) from the plasma membrane in hippocampal neurons via impaired AMPK activity. AβO-induced AMPK inhibition is accompanied by depletion of intracellular ATP in neurons. Significantly, the metabolic impact initiated by oligomers could be prevented by application of insulin. Current findings demonstrate a deleterious impact of AβOs on AMPK activity that may contribute to initial brain metabolic dysfunction, and suggest that maintaining physiological AMPK activity may be a useful approach to prevent neuronal damage in AD.
Results
AβOs reduce AMPK-Thr(P)172 in cultured hippocampal neurons and in ex vivo human cortical slices
AMPK is a heterotrimeric enzyme comprised of a catalytic (α1 or α2) and two regulatory (β1 or β2, and γ1, γ2, or γ3) subunits. Binding of AMP to the γ subunit leads to AMPK activation by the obligatory phosphorylation at Thr172 (AMPK-Thr(P)172) (2, 3); hence, AMPK-Thr(P)172 is routinely used as a proxy for AMPK activity (9, 20). We initially investigated the impact of ΑβOs on AMPK-Thr(P)172 by immunocytochemistry in mature hippocampal neuronal cultures. AMPK-Thr(P)172 levels were significantly decreased in neurons exposed to 500 nm AβOs for 3 h (Fig. 1, A–C). Scrambled Aβ peptide had no effect on AMPK-Thr(P)172 levels (Fig. 1C). Total AMPK levels were not affected by 3-h exposure to AβOs (Fig. 1, D–F). We further investigated the impact of AβOs on neuronal AMPK-Thr(P)172 levels by Western immunoblotting. Results confirmed that AMPK-Thr(P)172 levels were reduced in cultures exposed to AβOs for 3 or 12 h (Fig. 1, G and H). Decreased AMPK-Thr(P)172 levels were further verified in ex vivo human cortical slices exposed to AβOs for 12 h (Fig. 1I). Results indicate that short-term AβO exposure inhibits AMPK in neurons.
Figure 1.
Short-term exposure to AβOs inhibits neuronal AMPK. A–F, hippocampal cultures were exposed to vehicle (A and D), 500 nm AβOs (B and E), or scrambled Aβ (scrAβ) for 3 h and AMPK-Thr(P)172 or total AMPK levels were determined. C and F, scale bar = 10 μm. Integrated AMPK-Thr(P)172 (C) or total AMPK (F) immunofluorescence levels. Individual symbols represent mean AMPK-Thr(P)172 or total AMPK immunoreactivities from 2 to 4 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (****, p < 0.0001; ***, p < 0.05; ANOVA followed by Tukey's test). G and H, Western blot analysis of AMPK-Thr(P)172 levels in hippocampal cultures exposed to vehicle or 500 nm AβOs for 3 or 12 h, respectively. Lanes were run on the same gel but were noncontiguous. Graphs show densitometric analysis for AMPK-Thr(P)172 normalized by total AMPK levels. Symbols represent densitometric analysis for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (**, p < 0.001 or *, p < 0.05; Student's t test, compared with vehicle-treated cultures). I, AMPK-Thr(P)172 levels in ex vivo human cortical slices exposed to vehicle or 500 nm AβOs for 12 h. Graphs show densitometric analysis for AMPK-Thr(P)172 normalized by total AMPK levels. Symbols represent densitometric analysis for each independent human cortical slice culture used. Horizontal and vertical lines represent mean ± S.E. (p = 0.063; Student's t test, compared with vehicle-treated cultures).
AβOs decrease ATP levels and F0F1-ATPase activity in cultured cortical neurons and in ex vivo human cortical slices
Deregulation of AMPK activity is often followed by metabolic defects. To investigate whether this might take place in an AD context, we measured levels of adenine nucleotides in neurons exposed to 500 nm AβOs. Significant decreases in ATP levels, in line with our previous results (21), but not in AMP levels, were detected in both cultured cortical neurons and ex vivo human cortical slices exposed to AβOs for 12 h (Fig. 2, A and B). Although the ATPase activity of F0F1-ATP synthase was inhibited in neuronal cultures exposed to AβOs for 3 or 12 h (Fig. 2, C and D), the ATP synthase activity of isolated brain mitochondria was not affected by direct AβO exposure (Fig. 2E). This rules out a direct action on mitochondria and suggests that inhibition of neuronal ATP synthase was due to aberrant intracellular signaling triggered by AβOs.
Figure 2.
AβOs decrease intracellular ATP levels and F0F1-ATPase activity, and increase extracellular adenosine in cultured cortical neurons and in ex vivo human cortical slices. A, primary cultured cortical neurons were exposed to vehicle or 500 nm AβOs for 12 h and adenine nucleotides were measured. Symbols represent mean values for each independent culture used. Horizontal and vertical lines represent mean ± S.E. B, human cortical slices were exposed to vehicle or 500 nm AβOs for 12 h. Symbols represent results from tissue for each independent donor. C and D, cortical cultures were exposed to 500 nm AβOs or vehicle for 3 (C) or 12 h (D) and ATP hydrolysis was measured in whole cell homogenates. Symbols represent mean values for each independent cortical culture used. E, ATP hydrolysis in a subcellular, mitochondria-enriched rat brain fraction directly exposed to AβOs (500 nm, 3 h) or vehicle. Symbols represent results for each mitochondrial preparation used. F and G, extracellular adenosine levels in primary cortical cultures exposed to vehicle or 500 nm AβOs for 3 (F) or 12 (G) h. Symbols represent results for each independent culture used. H, MTT reduction measurements in hippocampal cultures exposed to vehicle or 500 nm AβOs for 12 h. Symbols represent mean ± S.E. from experiments with 5 independent cultures. *, p < 0.05, Student's t test, compared with vehicle-exposed cultures.
Besides impaired mitochondrial synthesis of ATP, its extracellular release and catabolism to adenosine could also contribute to decreased ATP levels in cultured neurons under stress (22). Indeed, significant increases in extracellular adenosine were detected in neuronal cultures exposed to AβOs for 3 or 12 h (Fig. 2, F and G). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction measurements showed no inhibition of cellular redox activity in cultures exposed to AβOs for 12 h (Fig. 2H), ruling out the possibility that increased extracellular adenosine levels were due to release from dead or damaged cells. These results indicate that AβO-exposed neurons undergo disturbances in energy homeostasis that include inhibition of mitochondrial synthesis of ATP and stimulation of its extracellular release.
NMDA receptors mediate the decrease in AMPK-Thr(P)172 in cultured hippocampal neurons
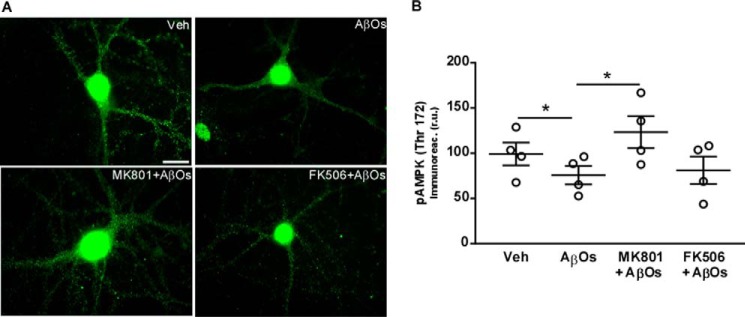
In previous works, we have demonstrated that AβOs cause activation of neuronal NMDARs leading to a rapid increase in intracellular calcium levels and causing abnormal neuronal accumulation of reactive oxygen species, among other pathological consequences (23–26). We thus hypothesized that activation of NMDARs and calcium-dependent protein phosphatase calcineurin/protein phosphatase 2B could mediate the impact of AβOs on AMPK-Thr(P)172 levels. To test this possibility, hippocampal cultures were pre-treated with the NMDAR blocker, MK801, or the calcineurin inhibitor, FK506, prior to the 3-h exposure to AβOs. Results indicate that MK801 prevented the impact of oligomers on AMPK-Thr(P)172, whereas no effect was observed with FK506 (Fig. 3, A and B). These observations suggest that NMDARs, but not calcineurin, are implicated in oligomer-induced AMPK inhibition.
Figure 3.
NMDARs mediate AMPK inhibition by AβOs in cultured neurons. A and B, hippocampal cultures were exposed to vehicle, 500 nm AβΟs, 10 μm MK 801, or 3 μm FK506 (drugs alone, or in the presence or absence of AβOs) for 3 h, and AMPK-Thr(P)172 levels were evaluated by immunocytochemistry. B, integrated AMPK-Thr(P)172 immunofluorescence levels. Symbols represent mean AMPK-Thr(P)172 immunoreactivities from 2 to 3 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (*, p < 0.05, ANOVA followed by Dunn's test). Scale bar = 10 μm.
Pharmacological stimulation of AMPK counteracts its inhibition by AβOs
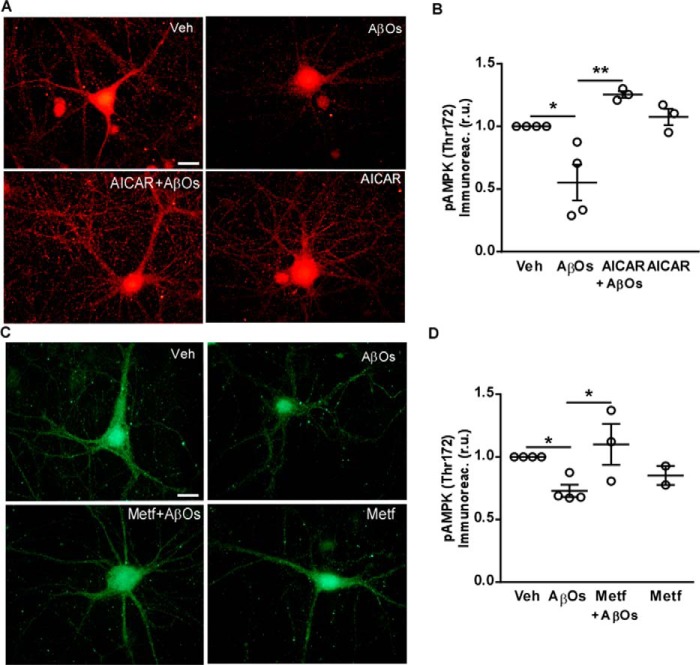
If AβO-induced reductions in AMPK-Thr(P)172 levels indeed mirror alterations in its activity, one might expect that pharmacological agents known to stimulate AMPK activity should be able to counteract AβO actions. In line with this hypothesis, pre-treatment with AICAR, an AMP-mimicking riboside, or with metformin, a known AMPK activator, prevented the decrease in AMPK-Thr(P)172 levels caused by exposure to AβOs in hippocampal neurons (Fig. 4).
Figure 4.
Pharmacological stimulation of AMPK counteracts its inhibition by AβOs. A–D, hippocampal cultures were exposed to vehicle, 500 nm AβOs, 2 mm AICAR, or 2 mm metformin (drugs alone, or in the presence or absence of AβOs) for 3 h, and AMPK-Thr(P)172 levels were evaluated by immunocytochemistry. B and D, integrated AMPK-Thr(P)172 immunofluorescence levels. Symbols represent mean AMPK-Thr(P)172 immunoreactivities from 2 to 4 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (*, p < 0.05, ANOVA followed by Bonferroni's test). Scale bar = 10 μm.
AβOs decrease surface levels of dendritic GLUT3 and GLUT4 in hippocampal neurons
AMPK plays a central role in cellular glucose uptake by facilitating the translocation of glucose transporters to the plasma membrane (27, 28). Because levels of GLUT3 and GLUT4 are decreased in AD brains (29, 30), we investigated the impact of AβOs on surface levels of these transporters in hippocampal cultures. Neurons targeted by AβOs exhibited markedly decreased surface levels of dendritic GLUT3 (Fig. 5, A–G) and GLUT4 (Fig. 5, H–N). As expected, compound C, a pharmacological inhibitor of AMPK, decreased dendritic surface GLUT3 and GLUT4 levels (Fig. 5, E and L). Interestingly, AICAR blocked the decrease in surface levels of GLUTs induced by AβOs (Fig. 5, E and L). Results indicate that AβO-induced decreases in surface levels of glucose transporters are mediated by AMPK inhibition in neurons.
Figure 5.
AβOs induce short-term reductions in surface levels of GLUT3 and GLUT4 in cultured hippocampal neurons. A–G, GLUT3 immunofluorescence (green) in hippocampal cultures exposed to vehicle (A) or 500 nm AβOs (C) for 3 h. B and D, AβO immunolabeling (red; NU4 antibody). F and G, merged double-labeled images of vehicle- or AβO-exposed cultures, respectively. E, quantitative analysis of surface levels of GLUT3 from experiments with 2–5 independent cultures. Symbols represent mean GLUT3 immunoreactivities from 2 to 4 coverslips for each independent culture used. H–N, GLUT4 immunofluorescence (green) in hippocampal cultures exposed to vehicle (H) or 500 nm AβOs (J) for 3 h. I and K, AβO immunolabeling (NU4 antibody; red). M and N, merged double-labeled images of vehicle- or AβO-exposed cultures, respectively. K, quantitative analysis of surface levels of GLUT4 from experiments with 2–3 independent cultures. Symbols represent mean GLUT4 immunoreactivities from 2 to 3 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (**, p < 0.005; ***, p < 0.0001; one-way ANOVA followed by Bonferroni post hoc test compared with vehicle-treated cultures). Scale bar = 10 μm.
Longer term exposure to AβOs does not trigger prolonged metabolic impairment or neuronal death
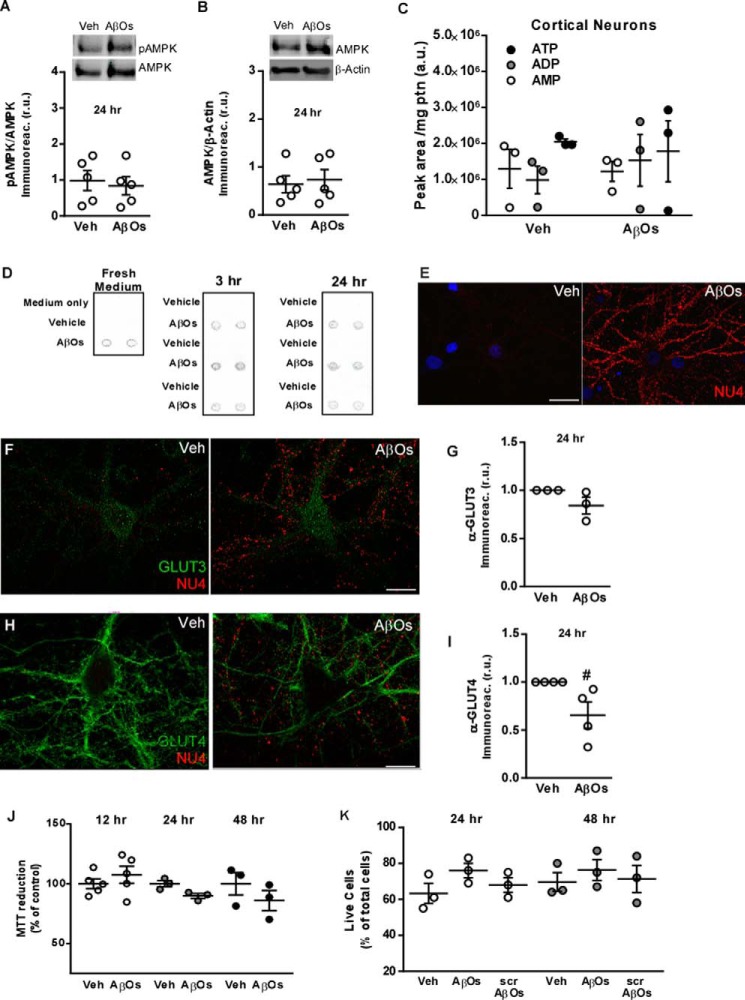
Because prolonged inhibition of AMPK and sustained reductions in ATP and surface GLUT levels could lead to decreased cell viability and cell death, we investigated whether the impact of AβOs on neuronal metabolism would persist upon longer term exposures. We found that levels of AMPK-Thr(P)172 (Fig. 6A), total AMPK (Fig. 6B), and intracellular ATP (Fig. 6C) in neurons exposed to AβOs for 24 h were similar to those in vehicle-treated cultures.
Figure 6.
Longer term exposure to AβOs does not cause metabolic defects or reduce cell viability in cultured neurons. A and B, hippocampal cultures were exposed to vehicle or 500 nm AβOs for 24 h, and AMPK-Thr(P)172 (A) and total AMPK (B) levels were determined by Western blotting. Symbols represent densitometric analysis for each independent culture used. Horizontal and vertical lines represent mean ± S.E. C, levels of ATP, ADP, or AMP are not altered by 24 h exposure to AβOs in cultured cortical neurons. Symbols represent analyses for each independent culture used. D, immunoblot dot analysis of culture medium from 3 independent cultures (carried out in duplicate) exposed to vehicle or 500 nm AβOs for 3 or 24 h. AβOs (500 nm) added to fresh Neurobasal medium alone was used as a control. E, representative image of AβO binding to hippocampal cultures after 24 h; cultures were exposed to vehicle or 500 nm AβOs for 24 h and AβO immunoreactivity was detected using the NU4 anti-oligomer monoclonal antibody. F–I, exposure to 500 nm AβOs for 24 h does not significantly impact surface levels of GLUT3 (F and G) and GLUT4 (H and I) in hippocampal neurons. Neurons were double-labeled for GLUTs (green) and AβOs (NU4 antibody; red). Symbols represent mean GLUT immunoreactivities from 2 to 3 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (#, p = 0.087, Student's t test). J, hippocampal neurons showed preserved redox capacity (assessed by the MTT reduction assay) upon exposure to AβOs for 12, 24, or 48 h. K, no changes in cell viability were detected by a live/dead assay in hippocampal cultures exposed to AβOs or scrambled Aβ (scrAβ) for 24 or 48 h. Symbols represent analysis for each independent culture used. Horizontal and vertical lines represent means ± S.E.
To examine the possibility that restoration of AMPK-Thr(P)172 levels after 24 h of exposure to oligomers was due to reduced levels of AβOs in the culture medium, we aimed to determine whether extracellular AβO levels were maintained for the duration of the 24-h incubation period. We exposed neuronal cultures to 500 nm AβOs for 3 or 24 h. Culture medium from 3 independent cultures was then collected, and immunoblot dot analysis was carried out using the NU4 anti-oligomer monoclonal antibody (31). Similar AβO immunoreactivities were observed at 3 or 24 h (compared with 500 nm AβOs in Neurobasal medium alone, used as a control), suggesting that restored levels of AMPK-Thr(P)172 were not related to depletion of AβOs from the medium upon longer (24 h) incubation with neuronal cultures (Fig. 6D). We further found that oligomers remain bound to neuronal processes 24 h after addition of AβOs to cultures (Fig. 6E), with a binding pattern identical to that observed at 3 h (Fig. 5, D and K).
Furthermore, no significant impact on surface levels of GLUT3 (Fig. 6, F and G) was verified after 24 h of exposure of neurons to AβOs. A nonstatistically significant (p = 0.08) trend of decrease in surface levels of GLUT4 was observed after 24 h of exposure to AβOs (Fig. 6, H and I).
Control experiments showed that exposure to 500 nm AβOs for 12, 24, or 48 h had no effect on MTT reduction in hippocampal cultures (Fig. 6J, see also Ref. 32), indicating preserved cellular redox activity under these conditions. No reduction in cell viability was further detected in hippocampal neurons exposed to AβOs or scrambled Aβ for 24 or 48 h, as measured using a live/dead assay (Fig. 6K). Collectively, results indicate that AβOs trigger transient inhibition of AMPK with early deleterious consequences in terms of neuronal energy metabolism (decreased ATP and surface levels of GLUTs), and that compensatory mechanisms operate to re-establish homeostasis and prevent further metabolic damage and cell death upon longer term exposure to AβOs.
Insulin prevents AβO-induced AMPK inhibition and adenosine release in hippocampal neurons
Stimulation of brain insulin signaling has been suggested as a promising therapeutic approach in AD (33–35). We have previously shown that insulin prevents inhibition of brain insulin signaling, activation of aberrant cellular stress mechanisms, and synapse deterioration induced by AβOs (36–38). We thus hypothesized that bolstering insulin signaling might protect neurons from the inhibition of AMPK by AβOs.
We found that pre-treatment of hippocampal cultures with insulin prevented the decrease in neuronal AMPK-Thr(P)172 levels (Fig. 7, A and B) induced by a 3-h exposure to AβOs. Insulin alone had no effect on neuronal AMPK-Thr(P)172 levels (Fig. 7B). Insulin further prevented the increase in extracellular adenosine levels induced by 3- or 12-h exposure of hippocampal cultures to AβOs (Fig. 7, C and D). Results indicate that insulin prevents the inhibition of AMPK and metabolic deregulation induced by AβOs, thereby extending the grounds toward the translational potential of insulin in AD.
Figure 7.
Insulin prevents AβO-induced AMPK inhibition and adenosine release in hippocampal cultures. A, hippocampal cultures were exposed to vehicle or 500 nm AβOs (in the absence or presence of insulin) for 3 h, and AMPK-Thr(P)172 levels were evaluated by immunocytochemistry. Scale bar = 10 μm. B, integrated AMPK-Thr(P)172 immunofluorescence levels. Insulin alone had no effect on AMPK-Thr(P)172 levels. Symbols represent mean AMPK-Thr(P)172 immunoreactivities from 2 to 4 coverslips for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (*, p < 0.05; **, p < 0.001; one-way ANOVA followed by Bonferroni test). C and D, extracellular adenosine levels in hippocampal cultures exposed for 3 (C) or 12 (D) h to 500 nm AβOs, in the absence or presence of insulin (1 μm). Symbols represent analyses for each independent culture used. Horizontal and vertical lines represent mean ± S.E. (*, p < 0.05, one-way ANOVA followed by Newman-Keuls test).
Discussion
Using in vitro and ex vivo models of AD, we have found that AβOs transiently inhibit neuronal AMPK through a NMDA receptor-dependent mechanism, and decrease ATP and glucose transporter levels. Previous observations indicate that AβOs are implicated in different processes leading to neuronal dysfunction, including oxidative stress, abnormal Tau phosphorylation, and synapse loss (32, 39–42). The discovery that AβOs interfere with AMPK activity indicates a new mechanism contributing to neuronal metabolic defects that are germane to AD.
AMPK has been implicated in other brain disorders, including stroke (43), HD (44), and tauopathies (11). Overactivation of AMPK was reported in the striatum of Huntington patients, and further implicated in brain atrophy, neuronal loss, and formation of huntingtin aggregates in a HD transgenic mouse model (45). Thus, altered AMPK may comprise a common feature leading to impaired neuronal metabolic function in neurodegenerative diseases.
Our findings are in line with recent reports suggesting that soluble Aβ species reduce neuronal AMPK-Thr(P)172 in vitro (20, 46). On the other hand, previous studies have shown that Aβ rapidly and transiently (from 10 to 60 min) stimulates AMPK-Thr(P)172 in cultured neurons (20, 47). At first sight, such an oscillating behavior of AMPK-Thr(P)172 could be interpreted as conflicting observations in the literature. Nevertheless, both AMPK activation within minutes and the subsequent longer term inhibition induced by Aβ have been linked to increased Tau phosphorylation and attenuated protein synthesis in neuronal cultures through distinct mechanisms (20, 46, 47).
Our current results indicate preserved cell viability even after a 24-h exposure to AβOs. Therefore, reductions in AMPK activity reported here likely reflect aberrant signaling mechanisms rather than cell death. We further provide initial insight into the mechanism underlying transient AMPK inhibition following exposure to AβOs. We show that it involves signaling via NMDA receptors, whereas calcineurin does not appear to play a significant role in this process. Overactivation of NMDARs has been shown to mediate oligomer-induced neuronal defects, including oxidative stress and synapse loss (23–26, 48). Deregulation of intracellular calcium homeostasis initiated by AβO-NMDAR signaling could lead to aberrant activation of the calcium-dependent phosphatase calcineurin/protein phosphatase 2B (49–51). It is thus possible that NMDAR-triggered pathways alter the kinase-phosphatase balance, ultimately leading to reduced AMPK activity and early impairments in neuronal metabolism. Future studies are warranted to fully elucidate the intracellular mechanisms linking AβO/NMDAR signaling to impaired AMPK and defective energy metabolism.
We found that neurons targeted by AβOs present an acute reduction in surface levels of GLUT3 and GLUT4, suggestive of a short-term metabolic defect. Interestingly, pharmacological activation of AMPK prevents the impact of AβOs on GLUTs. AβO-induced AMPK inhibition is further accompanied by decreased intracellular levels of ATP. These results add novel components to the metabolic stress landscape mediated by AMPK-Thr(P)172 fluctuations across AD progression. In this regard, an interesting recent study identified GLUT4 as a key metabolic modulatory system that up-regulates glycolysis in firing neurons to meet activity-driven ATP demand at nerve terminals (52). Significantly, AMPK was found to drive GLUT4 regulatory activity, essential for sustained synaptic transmission. It is conceivable, therefore, that AβO-induced AMPK inhibition and reduction in surface levels of neuronal GLUT4 comprises a mechanism leading to reduced or defective synapse function in AD.
Abnormal ATP release to the extracellular medium in neuronal cultures exposed to AβOs may be an important mediator of neurotoxicity via activation of purinergic receptors in AD brains. Indeed, a recent report demonstrated that AβO-induced ATP leakage activates P2X receptors to cause excitotoxicity (53). Furthermore, adenosine resulting from extracellular ATP degradation could also serve as a messenger mediating communication between neurons and glia, likely via activation of glial purinergic receptors, to exacerbate brain and memory dysfunction, as suggested (54–57).
In line with neuropathology studies in AD (9, 11) and in transgenic models of AD (8–10, 58), experimental evidence suggests a role for chronic AMPK activation in synapse dysfunction in AD transgenic models (20, 58). Our findings in neuronal cultures establish that AβOs cause early inhibition of AMPK activity, but this impact is reversed in longer time frames (24 h). Previous studies by our group and others have indicated that the neuronal impact of AβOs persist after several hours or even days of exposure of neurons to oligomers (38, 40, 59–61). Our current results suggest that, despite restored AMPK activity, AβOs remain bound to neurons after 24 h, and no considerable signal decay is observed in oligomer binding to neurons at 3 versus 24 h. These findings suggest the recovery in AMPK activity is not related to reduced levels of AβOs in the medium or desensitization of AβO-activated signaling pathways.
On the other hand, current results show a sharp decrease in ATP levels in both rodent and human cortical neurons exposed to AβOs for 12 h. As a key cellular energy sensor, AMPK is activated in response to such a decrease in energy status. We feel this homeostatic response to reduced energy levels is responsible for the recovery of AMPK activity observed 24 h following application of AβOs. It is, thus, possible that accumulation of AβOs in pre-AD brains initially inhibits AMPK activity, triggering early brain and cognitive damage. As disease progresses, however, homeostatic mechanisms may lead to persistent and excessive brain AMPK activity that could, in turn, further compromise synapse integrity and function.
The existence of compensatory mechanisms to control AMPK activity finds support in that pharmacological manipulation of brain AMPK shows only transient and limited action in promoting benefits in mice (62). Taken together, these findings suggest that the prolonged action of a homeostatic mechanism targeting AMPK function could lead to its overactivity and drive brain damage in AD. Preventing initial AMPK deregulation could thus be an approach to avoid extensive brain damage in AD.
Bolstering brain insulin signaling has been suggested as a promising therapeutic approach in AD (35, 63–65). Intranasal insulin treatment improves memory performance in early AD patients (33, 34). Furthermore, insulin has been found to protect against synapse damage and neurotoxicity induced by AβOs (36–38). Current results indicate a beneficial action of insulin against AβO-induced AMPK inhibition, extending the grounds supporting neuroprotection by insulin in animal models and human brains. Although we acknowledge that physiological concentrations of insulin would be lower than the micromolar range used in our study, neuroprotection by micromolar concentrations of insulin has also been verified in several other experimental approaches and conditions (22, 36–38, 66, 67). We note, however, that we cannot exclude the possibility that insulin activates related receptors, including IGF-1R, at the concentration used in our study. Pharmacological distinction between possible neuroprotective actions of insulin via insulin or IGF-1 receptors is made difficult by the lack of drugs that specifically target only one of these receptors. Further compounding this issue, insulin and IGF-1 receptors may form hybrid dimers (68, 69). Nonetheless, our findings may help to explain the neuroprotective actions of insulin in the context of metabolic brain dysfunction in neurodenegerative diseases (70, 71). Further investigation of the mechanisms of insulin action in the brain will provide a more complete view on neuroprotection by insulin, notably when such information is combined with results from ongoing clinical trials.
In conclusion, our current results establish that Aβ oligomers transiently reduce neuronal AMPK activity in an NMDAR-mediated mechanism that leads to reduced surface exposure of GLUT3 and GLUT4. In addition to previously reported AMPK signaling defects in neurons (9, 11, 20, 58), impaired energy sensing and adequate metabolic shift could render neurons more vulnerable to stress, as recently pointed out (72). Furthermore, the recent demonstration that activity-dependent, AMPK-mediated synaptic GLUT4 mobilization is essential for synapse function (52) suggests a novel mechanism by which AβOs may lead to synapse dysfunction. Thus, the effects of AβOs on AMPK may contribute to AD-linked brain metabolic and synaptic defects. AMPK is a key regulator of metabolic homeostasis and therefore could be a target for novel therapeutic opportunities in AD.
Experimental procedures
Materials
Synthetic Aβ(1–42) peptide was from American Peptide (Sunnyvale, CA). Culture media/reagents, Alexa Fluor-labeled secondary antibodies, and ProLong anti-fade reagent were from Invitrogen. Fluorescent secondary antibodies for Western blotting were from LiCor (Lincoln, NE). Antibodies against pAMPK (Thr172) and AMPK were from Santa Cruz Biotechnology (Santa Cruz, CA) or Cell Signaling (Danvers, MA). Antibodies against GLUT3, GLUT4, and β-actin were from Abcam. The oligomer-selective antibody NU4 (31) was produced in Dr. Klein's laboratory. SuperSignal West Femto Maximum Sensitivity substrate, protease/phosphatase inhibitors were from Thermo-Pierce (Rockford, IL). Tetrabutylammonium bromide was from Fluka (Steinheim/Switzerland). Metformin (1,1-dimethylbiguanide hydrochloride), compound C, and AICA-riboside were from Calbiochem (Darmstadt, Germany). Insulin was from Eli Lilly (Indianapolis, IL). FK506 was from Tocris Biosciences (Bristol, UK). MK801 was from Sigma.
Aβ oligomers
AβOs were prepared from synthetic Aβ(1–42) as previously described (32, 37). Oligomer preparations were routinely characterized by size exclusion HPLC and, occasionally, by Western blots using oligomer-sensitive NU4 monoclonal antibody (31), and comprised a mixture of Aβ dimers, trimers, tetramers, and higher molecular weight oligomers. Protein concentration was determined using the BCA assay (Thermo-Pierce).
Mature cortical and hippocampal neuronal cultures
Cortices from 14-day-old rat embryos or hippocampi from 18-day-old embryos were dissected and cultured as previously described (73, 74) with minor modifications. Briefly, dissociated cells were suspended in Neurobasal medium supplemented with B27, Glutamax, and antibiotics, and were plated on glass coverslips previously coated with 1.5 μg/ml of poly-l-lysine. Cultures were maintained at 37 °C in a humidified atmosphere containing 5% CO2 for 18–21 days prior to use. When present, insulin (1 μm), MK-801 (10 μm), FK506 (3 μm), AICAR (2.5 mm), compound C (10 μm), or metformin (2 mm) were added to cultures 30 min before application of vehicle or AβOs (at 250 or 500 nm, as indicated under “Results”).
Ex vivo human cortical slices
Adult human cortical slices were prepared as described (75). Healthy cortical tissue was obtained from patients with drug-resistant epilepsy subjected to temporal lobectomy for removal of hippocampal epileptic foci. After dissection under sterile conditions, 0.4-mm thick slices were prepared using a McIlwain tissue chopper and maintained in supplemented Neurobasal A medium (2% B27, 0.5 mm glutamine, 5 ng/ml of FGF2, 2 μm dehydroepiandrosterone sulfate, 2 nm BDNF) with 50 μg/ml of gentamicin. One-third of the medium was replaced after 3 days in culture. After 5 days at 37 °C in a 5% CO2 atmosphere, cultures were exposed to vehicle or AβOs (500 nm) for 12 h.
Neuronal viability and MTT assays
Neuronal viability was measured using the live/dead assay (Thermo-Pierce), which relies on fluorescent calcein or ethidium incorporation to discriminate populations of cells with preserved esterase activity and membrane integrity. Experiments were performed according to manufacturer's instructions, as described (21). Briefly, primary cultured hippocampal neurons were exposed to AβOs for the indicated time points and then loaded with 2 μm calcein and 1 μm ethidium. Cells were imaged on a Nikon Eclipse TE 2000-U fluorescence microscope and the proportion of live (calcein-positive) or dead (ethidium-positive) cells were determined relative to the total number of cells. Three experiments with independent neuronal cultures were performed.
The MTT reduction assay (Roche Applied Science) was used to evaluate cellular metabolic redox activity in 18-day old cortical neuronal cultures. Three experiments with independent neuronal cultures (each using 3 wells per experimental condition) were carried out as described (75).
Immunocytochemistry
Cells were fixed and blocked as described previously (37) and probed with anti-pThr172-AMPK (1:250), anti-AMPK (1:100) and oligomer-sensitive NU4 antibody (31). For immunodetection of surface-exposed GLUT3 or GLUT4, cells were double-labeled with oligomer-sensitive NU4 mouse monoclonal antibody and either GLUT3 (Abcam, 1:750) or GLUT4 (Abcam, 1:500) rabbit polyclonal antibodies under non-permeabilizing conditions. Alexa Fluor-conjugated secondary antibodies were used. Coverslips were imaged on a Nikon Eclipse TE 2000-U fluorescence microscope (for AMPK-Thr(P)172 and AMPK labeling) and Zeiss Axio Observer Z1 microscope (for GLUT imaging). Omission of primary antibodies eliminated all labeling.
Measurement of intracellular adenine nucleotide levels
Adenine nucleotide contents in 18–21-day-old in vitro rat cortical neurons were analyzed by ion-paired reverse phase liquid chromatography. Cultures were exposed to vehicle, 500 nm AβOs, or insulin + AβOs for 12 h. When present, insulin (1 μm) was added to cultures 30 min before AβOs. The culture medium was removed and liquid nitrogen was used to disrupt cells and stop cellular metabolism. The plates were kept in an ice bath until the liquid nitrogen evaporated completely. Cells were homogenized and proteins were precipitated with 6% trichloroacetic acid, and the suspension was neutralized by adding a small aliquot of 1 m Tris solution and centrifuged at 20,800 × g for 5 min at 4 °C. Protein content was determined using the BCA assay. Aliquots were injected into an HPLC system using a Supelguard column (Supelco) coupled to a Supercosil C-18 carrier (particle size 5 μm, Supelco) column. Isocratic elution was performed at a flow-rate of 1 ml/min at room temperature with 50 mm KH2PO4 buffer, pH 6, methanol (90/10), and 4 mm tetrabutylammonium bromide. Nucleotide levels were measured by UV absorbance at 254 nm (ϵ = 15,400 m−1 cm−1). ATP, ADP, AMP, and adenosine peaks were identified by co-injecting 2-nmol standards in an independent run. Results were expressed by normalizing peak areas by the total amount of protein.
Western and dot immunoblotting
Cultures were harvested with RIPA buffer (25 mm Tris-HCl, pH 7.6, 150 mm NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 5 mm EDTA, and 1% Triton X-100) plus protease/phosphatase inhibitors. Lysates were incubated on ice for 30 min with 5-s vortexing every 5 min and were centrifuged at 10,000 × g for 5 min at 4 °C. Protein concentration in the supernatant was determined by the BCA assay. Thirty micrograms of protein were resolved on 10% SDS-PAGE and analyzed by Western blot using anti-pThr172-AMPK (1:500) or anti-AMPKα1–2 (1:2,000) antibodies. β-Actin (1:50,000) was used as a loading control. For dot immunoblot analyses, culture media were removed from hippocampal cultures after treatments and immediately frozen until analyses. Samples (4 μl) were spotted onto nitrocellulose membranes and incubated in blocking solution (3% BSA) for 1 h. Membranes were then incubated with the oligomer-selective antibody NU4 (31) (1:1,000) overnight. Immunoblots were developed either by chemiluminescence using HRP-conjugated secondary antibodies (1:20,000), or by fluorescence using infrared dye-conjugated secondary antibodies (1:5,000–1:10,000; LiCor) for Odyssey.
F0F1-ATPase activity
Cortical cultures were exposed to 500 nm AβOs or vehicle (2% DMSO in PBS) for 3 or 12 h (as indicated under “Results”). Cultures were washed twice and lysed in ice-cold PBS. Lysates were centrifuged at 14,000 rpm for 5 min at 4 °C. The supernatant was discarded and the pellet was resuspended in buffer containing 0.32 m sucrose, 1 mm EDTA, 1 mm EGTA, and 10 mm Tris-HCl, pH 7.4. Experiments using mitochondria-enriched preparations were performed using pellets obtained through successive centrifugation steps. We measured the mitochondrial ATPase activity in the absence or presence of 5 mm NaN3 in reaction medium containing 50 mm MOPS-Tris, pH 7.0, 4 mm MgCl2, and 1 mm ATP. The mitochondrial F0-F1-ATPase is the difference between activities in the presence and absence of azide, and is referred to as azide-sensitive ATPase. Inorganic phosphate (Pi) derived from ATP hydrolysis was determined by a colorimetric method with minor modifications (76).
Image analysis
AMPK-Thr(P)172, total AMPK, GLUT3, and GLUT4 immunofluorescence intensities were analyzed in a total of 3–6 experiments (see figure legends) using independent neuronal cultures. Immunofluorescence of each neuron, including cell bodies and dendrites, was quantified in each experimental condition. Quantitative analysis of immunofluorescence data were carried out by histogram analysis of the fluorescence intensity at each pixel across the images using Image J (NIH; Windows version). Briefly, the program analyzes a grayscale image by plots of the intensity histogram and by calculation of average intensity and standard deviation. Appropriate thresholding was employed to eliminate background signal in the images before histogram analysis, and fluorescence intensity was normalized by the number of immunoreactive cells in each field. To exclude the possibility that exposure to AβOs could lead to alterations in neuronal cell area, which could potentially bias pAMPK analyses, we determined the areas of MAP2 (used as an independent and unbiased neuronal marker that is not affected by oligomers) immunoreactive cells in four independent cultures, and found that AβOs do not cause significant variations in neuronal area across cultures (not shown). In each experiment, 20–30 images were acquired from 3 coverslips in each experimental condition. The results of the analysis of images were then combined to allow quantitative estimates of changes in neuronal levels of AMPK-Thr(P)172, total AMPK, GLUT3, or GLUT4.
Statistics
Statistical significances were assessed by Student's t test (for comparison between two experimental conditions) or ANOVA (for multiple comparisons) followed by appropriate post hoc tests, using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). See legends for specific details on statistical analyses.
Study approval
Experiments involving hippocampal cultures were performed in certified facilities under protocols approved by the Institutional Animal Care and Use Committee of the Federal University of Rio de Janeiro (protocol number IBQM 077). All procedures involving human cortical tissue were approved and regulated by the Committee for Research Ethics of the Clementino Fraga Filho University Hospital (protocol number 0069.0.197.000-05). Donors gave written informed consent for use of brain tissue that would otherwise have been discarded.
Author contributions
G. S. S. S., H. M. M., M. V. L., S. T. F., and F. G. F. designed the research. G. S. S., H. M. M., M. V. L., N. M. L. S., M. B. C., S. A. L., J. M. S., and W. S. S. performed research and analyzed data. W. L. K. and W. S. S. contributed reagents/analytical tools. F. G. F., M. V. L., G. S. S., and S. T. F. wrote the manuscript.
This work was supported by grants from Human Frontiers Science Program (HFSP) and John Simon Guggenheim Foundation (to F. G. F.), the National Institute for Translational Neuroscience (INNT/Brazil), the Brazilian funding agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (to S. T. F. and F. G. F.), and pre-doctoral fellowships from CNPq or FAPERJ (to G. S. S., H. M. M., M. V. L., and N. L. S.). W. L. K. is co-founder of Acumen Pharmaceuticals, which has been licensed by Northwestern University to develop ADDL technology for AD therapeutics and diagnostics.
- AMPK
- AMP-activated kinase
- HD
- Huntington's disease
- AD
- Alzheimer's disease
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- AβO
- Aβ oligomer
- GLUT
- glucose transporter
- NMDAR
- NMDA receptor
- IGF
- insulin-like growth factor
- ANOVA
- analysis of variance.
References
- 1. Zhang B. B., Zhou G., and Li C. (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407–416 [DOI] [PubMed] [Google Scholar]
- 2. Kahn B. B., Alquier T., Carling D., and Hardie D. G. (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 1, 15–25 [DOI] [PubMed] [Google Scholar]
- 3. Steinberg G. R., and Kemp B. E. (2009) AMPK in health and disease. Physiol. Rev. 89, 1025–1078 [DOI] [PubMed] [Google Scholar]
- 4. Saha A. K., Xu X. J., Lawson E., Deoliveira R., Brandon A. E., Kraegen E. W., and Ruderman N. B. (2010) Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes 59, 2426–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang J., Ma H., Tong C., Zhang H., Lawlis G. B., Li Y., Zang M., Ren J., Nijland M. J., Ford S. P., Nathanielsz P. W., and Li J. (2010) Overnutrition and maternal obesity in sheep pregnancy alter the JNK-IRS-1 signaling cascades and cardiac function in the fetal heart. FASEB J. 24, 2066–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramamurthy S., and Ronnett G. (2012) AMP-activated protein kinase (AMPK) and energy-sensing in the brain. Exp. Neurobiol. 21, 52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Culmsee C., Monnig J., Kemp B. E., and Mattson M. P. (2001) AMP-activated kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J. Mol. Neurosci. 17, 45–58 [DOI] [PubMed] [Google Scholar]
- 8. Lopez-Lopez C., Dietrich M. O., Metzger F., Loetscher H., and Torres-Aleman I. (2007) Disturbed crosstalk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J. Neurosci. 27, 824–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ma T., Chen Y., Vingtdeux V., Zhao H., Viollet B., Marambaud P., and Klann E. (2014) Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid β. J. Neurosci. 34, 12230–12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Son S. M., Jung E. S., Shin H. J., Byun J., and Mook-Jung I. (2012) Aβ-induced formation of autophagosomes is mediated by RAGE-CaMKKβ-AMPK signaling. Neurobiol. Aging 33, 1006 e1011–1023 [DOI] [PubMed] [Google Scholar]
- 11. Vingtdeux V., Davies P., Dickson D. W., and Marambaud P. (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's disease and other tauopathies. Acta Neuropathol. 121, 337–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoyer S. (2004) Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur. J. Pharmacol. 490, 115–125 [DOI] [PubMed] [Google Scholar]
- 13. Mattson M. P. (2004) Pathways towards and away from Alzheimer's disease. Nature 430, 631–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong Y., Chang L., Viola K. L., Lacor P. N., Lambert M. P., Finch C. E., Krafft G. A., and Klein W. L. (2003) Alzheimer's disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc. Natl. Acad. Sci. U.S.A. 100, 10417–10422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xia W., Yang T., Shankar G., Smith I. M., Shen Y., Walsh D. M., and Selkoe D. J. (2009) A specific enzyme-linked immunosorbent assay for measuring β-amyloid protein oligomers in human plasma and brain tissue of patients with Alzheimer disease. Arch. Neurol. 66, 190–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lesné S., Kotilinek L., and Ashe K. H. (2008) Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neuroscience 151, 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tomiyama T., Matsuyama S., Iso H., Umeda T., Takuma H., Ohnishi K., Ishibashi K., Teraoka R., Sakama N., Yamashita T., Nishitsuji K., Ito K., Shimada H., Lambert M. P., Klein W. L., and Mori H. (2010) A mouse model of amyloid-β oligomers: their contribution to synaptic alteration, abnormal tau phosphorylation, glial activation and neuronal loss in vivo. J. Neurosci. 30, 4845–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Selkoe D. J. (2011) Resolving controversies on the path to Alzheimer's therapeutics. Nat. Med. 17, 1060–1065 [DOI] [PubMed] [Google Scholar]
- 19. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., and Klein W. L. (1998) Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoon S. O., Park D. J., Ryu J. C., Ozer H. G., Tep C., Shin Y. J., Lim T. H., Pastorino L., Kunwar A. J., Walton J. C., Nagahara A. H., Lu K. P., Nelson R. J., Tuszynski M. H., and Huang K. (2012) JNK3 perpetuates metabolic stress induced by Aβ peptides. Neuron 75, 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saraiva L. M., Seixas da Silva G. S., Galina A., da-Silva W. S., Klein W. L., Ferreira S. T., and De Felice F. G. (2010) Amyloid-β triggers the release of neuronal hexokinase 1 from mitochondria. PloS One 5, e15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duarte A. I., Proença T., Oliveira C. R., Santos M. S., and Rego A. C. (2006) Insulin restores metabolic function in cultured cortical neurons subjected to oxidative stress. Diabetes 55, 2863–2870 [DOI] [PubMed] [Google Scholar]
- 23. De Felice F. G., Velasco P. T., Lambert M. P., Viola K., Fernandez S. J., Ferreira S. T., and Klein W. L. (2007) Aβ oligomers induce neuronal oxidative stress through an N-methyl-d-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 282, 11590–11601 [DOI] [PubMed] [Google Scholar]
- 24. Decker H., Jürgensen S., Adrover M. F., Brito-Moreira J., Bomfim T. R., Klein W. L., Epstein A. L., De Felice F. G., Jerusalinsky D., and Ferreira S. T. (2010) N-Methyl-d-aspartate receptors are required for synaptic targeting of Alzheimer's toxic amyloid-β peptide oligomers. J. Neurochem. 115, 1520–1529 [DOI] [PubMed] [Google Scholar]
- 25. Decker H., Lo K. Y., Unger S. M., Ferreira S. T., and Silverman M. A. (2010) Amyloid-β peptide oligomers disrupt axonal transport through an NMDA receptor-dependent mechanism that is mediated by glycogen synthase kinase 3β in primary cultured hippocampal neurons. J. Neurosci. 30, 9166–9171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paula-Lima A. C., Adasme T., SanMartín C., Sebollela A., Hetz C., Carrasco M. A., Ferreira S. T., and Hidalgo C. (2011) Amyloid β-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid. Redox Signal. 14, 1209–1223 [DOI] [PubMed] [Google Scholar]
- 27. Thong F. S., Bilan P. J., and Klip A. (2007) The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 56, 414–423 [DOI] [PubMed] [Google Scholar]
- 28. Cidad P., Almeida A., and Bolaños J. P. (2004) Inhibition of mitochondrial respiration by nitric oxide rapidly stimulates cytoprotective GLUT3-mediated glucose uptake through 5′-AMP-activated protein kinase. Biochem. J. 384, 629–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y., Liu F., Iqbal K., Grundke-Iqbal I., and Gong C.-X. (2008) Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 582, 359–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simpson I. A., Chundu K. R., Davies-Hill T., Honer W. G., and Davies P. (1994) Decreased concentrations of GLUT1 and GLUT3 glucose transporters in the brains of patients with Alzheimer's disease. Ann. Neurol. 35, 546–551 [DOI] [PubMed] [Google Scholar]
- 31. Lambert M. P., Velasco P. T., Chang L., Viola K. L., Fernandez S., Lacor P. N., Khuon D., Gong Y., Bigio E. H., Shaw P., De Felice F. G., Krafft G. A., and Klein W. L. (2007) Monoclonal antibodies that target pathological assemblies of Aβ. J. Neurochem. 100, 23–35 [DOI] [PubMed] [Google Scholar]
- 32. De Felice F. G., Wu D., Lambert M. P., Fernandez S. J., Velasco P. T., Lacor P. N., Bigio E. H., Jerecic J., Acton P. J., Shughrue P. J., Chen-Dodson E., Kinney G. G., and Klein W. L. (2008) Alzheimer's disease-type neuronal tau hyperphosphorylation induced by Aβ oligomers. Neurobiol. Aging 29, 1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reger M. A., Watson G. S., Green P. S., Wilkinson C. W., Baker L. D., Cholerton B., Fishel M. A., Plymate S. R., Breitner J. C., DeGroodt W., Mehta P., and Craft S. (2008) Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 70, 440–448 [DOI] [PubMed] [Google Scholar]
- 34. Craft S., Baker L. D., Montine T. J., Minoshima S., Watson G. S., Claxton A., Arbuckle M., Callaghan M., Tsai E., Plymate S. R., Green P. S., Leverenz J., Cross D., and Gerton B. (2012) Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol. 69, 29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Craft S. (2012) Alzheimer disease: insulin resistance and AD, extending the translational path. Nat. Rev. Neurol. 8, 360–362 [DOI] [PubMed] [Google Scholar]
- 36. Bomfim T. R., Forny-Germano L., Sathler L. B., Brito-Moreira J., Houzel J. C., Decker H., Silverman M. A., Kazi H., Melo H. M., McClean P. L., Holscher C., Arnold S. E., Talbot K., Klein W. L., Munoz D. P., Ferreira S. T., and De Felice F. G. (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer's disease-associated Aβ oligomers. J. Clin. Invest. 122, 1339–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Felice F. G., Vieira M. N., Bomfim T. R., Decker H., Velasco P. T., Lambert M. P., Viola K. L., Zhao W. Q., Ferreira S. T., and Klein W. L. (2009) Protection of synapses against Alzheimer's-linked toxins: insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc. Natl. Acad. Sci. U.S.A. 106, 1971–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lourenco M. V., Clarke J. R., Frozza R. L., Bomfim T. R., Forny-Germano L., Batista A. F., Sathler L. B., Brito-Moreira J., Amaral O. B., Silva C. A., Freitas-Correa L., Espírito-Santo S., Campello-Costa P., Houzel J. C., Klein W. L., et al. (2013) TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metab. 18, 831–843 [DOI] [PubMed] [Google Scholar]
- 39. Forny-Germano L., Lyra e Silva N. M., Batista A. F., Brito-Moreira J., Gralle M., Boehnke S. E., Coe B. C., Lablans A., Marques S. A., Martinez A. M., Klein W. L., Houzel J. C., Ferreira S. T., Munoz D. P., and De Felice F. G. (2014) Alzheimer's disease-like pathology induced by amyloid-β oligomers in nonhuman primates. J. Neurosci. 34, 13629–13643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Figueiredo C. P., Clarke J. R., Ledo J. H., Ribeiro F. C., Costa C. V., Melo H. M., Mota-Sales A. P., Saraiva L. M., Klein W. L., Sebollela A., De Felice F. G., and Ferreira S. T. (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight Aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J. Neurosci. 33, 9626–9634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roselli F., Tirard M., Lu J., Hutzler P., Lamberti P., Livrea P., Morabito M., and Almeida O. F. (2005) Soluble β-amyloid 1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J. Neurosci. 25, 11061–11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin M., Shepardson N., Yang T., Chen G., Walsh D., and Selkoe D. J. (2011) Soluble amyloid-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc. Natl. Acad. Sci. U.S.A. 108, 5819–5824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J., Zeng Z., Viollet B., Ronnett G. V., and McCullough L. D. (2007) Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38, 2992–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mochel F., Durant B., Meng X., O'Callaghan J., Yu H., Brouillet E., Wheeler V. C., Humbert S., Schiffmann R., and Durr A. (2012) Early alterations of brain cellular energy homeostasis in Huntington disease models. J. Biol. Chem. 287, 1361–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ju T. C., Chen H. M., Lin J. T., Chang C. P., Chang W. C., Kang J. J., Sun C. P., Tao M. H., Tu P. H., Chang C., Dickson D. W., and Chern Y. (2011) Nuclear translocation of AMPK-1 potentiates striatal neurodegeneration in Huntington's disease. J. Cell Biol. 194, 209–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park H., Kam T. I., Kim Y., Choi H., Gwon Y., Kim C., Koh J. Y., and Jung Y. K. (2012) Neuropathogenic role of adenylate kinase-1 in Aβ-mediated tau phosphorylation via AMPK and GSK3beta. Hum. Mol. Genet. 21, 2725–2737 [DOI] [PubMed] [Google Scholar]
- 47. Thornton C., Bright N. J., Sastre M., Muckett P. J., and Carling D. (2011) AMP-activated protein kinase (AMPK) is a tau kinase, activated in response to amyloid β-peptide exposure. Biochem. J. 434, 503–512 [DOI] [PubMed] [Google Scholar]
- 48. Talantova M., Sanz-Blasco S., Zhang X., Xia P., Akhtar M. W., Okamoto S., Dziewczapolski G., Nakamura T., Cao G., Pratt A. E., Kang Y. J., Tu S., Molokanova E., McKercher S. R., Hires S. A., et al. (2013) Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl. Acad. Sci. U.S.A. 110, E2518–E2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao W. Q., Santini F., Breese R., Ross D., Zhang X. D., Stone D. J., Ferrer M., Townsend M., Wolfe A. L., Seager M. A., Kinney G. G., Shughrue P. J., and Ray W. J. (2010) Inhibition of calcineurin-mediated endocytosis and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid β oligomer-induced synaptic disruption. J. Biol. Chem. 285, 7619–7632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu H. Y., Hudry E., Hashimoto T., Kuchibhotla K., Rozkalne A., Fan Z., Spires-Jones T., Xie H., Arbel-Ornath M., Grosskreutz C. L., Bacskai B. J., and Hyman B. T. (2010) Amyloid-β induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 30, 2636–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jürgensen S., Antonio L. L., Mussi G. E., Brito-Moreira J., Bomfim T. R., De Felice F. G., Garrido-Sanabria E. R., Cavalheiro É. A., and Ferreira S. T. (2011) Activation of D1/D5 dopamine receptors protects neurons from synapse dysfunction induced by amyloid-β oligomers. J. Biol. Chem. 286, 3270–3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ashrafi G., Wu Z., Farrell R. J., and Ryan T. A. (2017) GLUT4 mobilization supports energetic demands of active synapses. Neuron 93, 606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sáez-Orellana F., Godoy P. A., Bastidas C. Y., Silva-Grecchi T., Guzmán L., Aguayo L. G., and Fuentealba J. (2016) ATP leakage induces P2XR activation and contributes to acute synaptic excitotoxicity induced by soluble oligomers of beta-amyloid peptide in hippocampal neurons. Neuropharmacology 100, 116–123 [DOI] [PubMed] [Google Scholar]
- 54. Sanz J. M., Chiozzi P., Ferrari D., Colaianna M., Idzko M., Falzoni S., Fellin R., Trabace L., and Di Virgilio F. (2009) Activation of microglia by amyloid β requires P2X7 receptor expression. J. Immunol. 182, 4378–4385 [DOI] [PubMed] [Google Scholar]
- 55. Orr A. G., Hsiao E. C., Wang M. M., Ho K., Kim D. H., Wang X., Guo W., Kang J., Yu G. Q., Adame A., Devidze N., Dubal D. B., Masliah E., Conklin B. R., and Mucke L. (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat. Neurosci. 18, 423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pamplona R., Dalfó E., Ayala V., Bellmunt M. J., Prat J., Ferrer I., and Portero-Otín M. (2005) Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation: effects of Alzheimer disease and identification of lipoxidation targets. J. Biol. Chem. 280, 21522–21530 [DOI] [PubMed] [Google Scholar]
- 57. Terni B., Boada J., Portero-Otin M., Pamplona R., and Ferrer I. (2010) Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer's disease pathology. Brain Pathol. 20, 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mairet-Coello G., Courchet J., Pieraut S., Courchet V., Maximov A., and Polleux F. (2013) The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through tau phosphorylation. Neuron 78, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Madeira C., Lourenco M. V., Vargas-Lopes C., Suemoto C. K., Brandão C. O., Reis T., Leite R. E., Laks J., Jacob-Filho W., Pasqualucci C. A., Grinberg L. T., Ferreira S. T., and Panizzutti R. (2015) d-Serine levels in Alzheimer's disease: implications for novel biomarker development. Transl. Psychiatry 5, e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Alberdi E., Sánchez-Gómez M. V., Cavaliere F., Pérez-Samartín A., Zugaza J. L., Trullas R., Domercq M., and Matute C. (2010) Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47, 264–272 [DOI] [PubMed] [Google Scholar]
- 61. Dineley K. T., Kayed R., Neugebauer V., Fu Y., Zhang W., Reese L. C., and Taglialatela G. (2010) Amyloid-β oligomers impair fear conditioned memory in a calcineurin-dependent fashion in mice. J. Neurosci. Res. 88, 2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Guerrieri D., and van Praag H. (2015) Exercise-mimetic AICAR transiently benefits brain function. Oncotarget 6, 18293–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ferreira S. T., Clarke J. R., Bomfim T. R., and De Felice F. G. (2014) Inflammation, defective insulin signaling and neuronal dysfunction in Alzheimer's disease. Alzheimer's Dementia 10, S76–S83 [DOI] [PubMed] [Google Scholar]
- 64. De Felice F. G., Lourenco M. V., and Ferreira S. T. (2014) How does brain insulin resistance develop in Alzheimer's disease? Alzheimer's Dementia 10, S26–S32 [DOI] [PubMed] [Google Scholar]
- 65. De Felice F. G. (2013) Alzheimer's disease and insulin resistance: translating basic science into clinical applications. J. Clin. Invest. 123, 531–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Grillo C. A., Piroli G. G., Hendry R. M., and Reagan L. P. (2009) Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 1296, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dixon-Salazar T. J., Fourgeaud L., Tyler C. M., Poole J. R., Park J. J., and Boulanger L. M. (2014) MHC class I limits hippocampal synapse density by inhibiting neuronal insulin receptor signaling. J. Neurosci. 34, 11844–11856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Slaaby R., Schäffer L., Lautrup-Larsen I., Andersen A. S., Shaw A. C., Mathiasen I. S., and Brandt J. (2006) Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J. Biol. Chem. 281, 25869–25874 [DOI] [PubMed] [Google Scholar]
- 69. Soos M. A., Whittaker J., Lammers R., Ullrich A., and Siddle K. (1990) Receptors for insulin and insulin-like growth factor-I can form hybrid dimers: characterisation of hybrid receptors in transfected cells. Biochem. J. 270, 383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fernandez A. M., and Torres-Alemán I. (2012) The many faces of insulin-like peptide signalling in the brain. Nat. Rev. Neurosci. 13, 225–239 [DOI] [PubMed] [Google Scholar]
- 71. Ghasemi R., Haeri A., Dargahi L., Mohamed Z., and Ahmadiani A. (2013) Insulin in the brain: sources, localization and functions. Mol. Neurobiol. 47, 145–171 [DOI] [PubMed] [Google Scholar]
- 72. Lourenco M. V., Ferreira S. T., and De Felice F. G. (2015) Neuronal stress signaling and eIF2α phosphorylation as molecular links between Alzheimer's disease and diabetes. Prog. Neurobiol. 129, 37–57 [DOI] [PubMed] [Google Scholar]
- 73. De Felice F. G., Vieira M. N., Saraiva L. M., Figueroa-Villar J. D., Garcia-Abreu J., Liu R., Chang L., Klein W. L., and Ferreira S. T. (2004) Targeting the neurotoxic species in Alzheimer's disease: inhibitors of Aβ oligomerization. FASEB J. 18, 1366–1372 [DOI] [PubMed] [Google Scholar]
- 74. Paula-Lima A. C., De Felice F. G., Brito-Moreira J., and Ferreira S. T. (2005) Activation of GABAA receptors by taurine and muscimol blocks the neurotoxicity of β-amyloid in rat hippocampal and cortical neurons. Neuropharmacology 49, 1140–1148 [DOI] [PubMed] [Google Scholar]
- 75. Sebollela A., Freitas-Correa L., Oliveira F. F., Paula-Lima A. C., Saraiva L. M., Martins S. M., Mota L. D., Torres C., Alves-Leon S., de Souza J. M., Carraro D. M., Brentani H., De Felice F. G., and Ferreira S. T. (2012) Amyloid-β oligomers induce differential gene expression in adult human brain slices. J. Biol. Chem. 287, 7436–7445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fiske C. H., and Subbarow Y. (1925) The colorimetric determination of phosphorus. J. Biol. Chem. 66, 375–400 [Google Scholar]