Abstract
Glucose metabolism promotes insulin secretion in β-cells via metabolic coupling factors that are incompletely defined. Moreover, chronically elevated glucose causes β-cell dysfunction, but little is known about how cells handle excess fuels to avoid toxicity. Here we sought to determine which among the candidate pathways and coupling factors best correlates with glucose-stimulated insulin secretion (GSIS), define the fate of glucose in the β-cell, and identify pathways possibly involved in excess-fuel detoxification. We exposed isolated rat islets for 1 h to increasing glucose concentrations and measured various pathways and metabolites. Glucose oxidation, oxygen consumption, and ATP production correlated well with GSIS and saturated at 16 mm glucose. However, glucose utilization, glycerol release, triglyceride and glycogen contents, free fatty acid (FFA) content and release, and cholesterol and cholesterol esters increased linearly up to 25 mm glucose. Besides being oxidized, glucose was mainly metabolized via glycerol production and release and lipid synthesis (particularly FFA, triglycerides, and cholesterol), whereas glycogen production was comparatively low. Using targeted metabolomics in INS-1(832/13) cells, we found that several metabolites correlated well with GSIS, in particular some Krebs cycle intermediates, malonyl-CoA, and lower ADP levels. Glucose dose-dependently increased the dihydroxyacetone phosphate/glycerol 3-phosphate ratio in INS-1(832/13) cells, indicating a more oxidized state of NAD in the cytosol upon glucose stimulation. Overall, the data support a role for accelerated oxidative mitochondrial metabolism, anaplerosis, and malonyl-CoA/lipid signaling in β-cell metabolic signaling and suggest that a decrease in ADP levels is important in GSIS. The results also suggest that excess-fuel detoxification pathways in β-cells possibly comprise glycerol and FFA formation and release extracellularly and the diversion of glucose carbons to triglycerides and cholesterol esters.
Keywords: glucose metabolism, insulin secretion, lipid metabolism, metabolomics, mitochondrial metabolism, glucodetoxification, pancreatic β cells
Introduction
Insulin secretion by pancreatic β-cells in response to glucose and other nutrients regulates fuel homeostasis (1). Glucose is the main driver for insulin secretion via its intracellular metabolism in the β-cell. Enhanced glucose metabolism results in an elevation of the β-cell ATP/ADP ratio, closure of ATP-sensitive K+ (KATP)3 channels, depolarization of the plasma membrane, and activation of voltage-dependent Ca2+ channels followed by a rise in intracellular Ca2+ that triggers insulin exocytosis (2–6). However, this classical pathway alone does not explain how other nutrient stimuli such as free fatty acids (FFAs) increase glucose-stimulated insulin secretion (GSIS). Experimental evidence supports the existence of other metabolic processes called KATP-independent or amplifying pathways that enhance GSIS (7–9).
We have proposed that the glycerolipid (GL)/FFA cycle with its lipogenesis and lipolysis arms regulates GSIS via the production of lipid signaling molecules (10) and recently shown that saturated long chain 1-monoacylglycerol acts as a metabolic coupling factor (MCF) that amplifies GSIS via activation of the exocytosis protein munc13-1 (11–13). Additional metabolites derived from glucose metabolism, such as ATP (4), malonyl-CoA (14, 15), glutamate (16), mitochondrial GTP (17), reactive oxygen species (18, 19), and NADPH (20, 21) possibly via redox-dependent deSUMOylation (22) have been proposed to act as MCFs for GSIS. However, the relative importance of each of these metabolites for GSIS remains to be established.
Short-term exposure of β-cells to FFA increases GSIS, but chronic exposure to elevated concentrations of FFA results in β-cell dysfunction and apoptosis (23). Growing interest has been expressed in the concepts of glucotoxicity (24), lipotoxicity (25), and glucolipotoxicity (26, 27) in type 2 diabetes, and their roles in β-cell failure have been widely studied. However, the question whether the β-cell has mechanisms to protect against such fuel excess toxicity remains. In response to chronic fuel surfeit, many cells are able to defend themselves against nutrient-induced toxicity. For example, in skeletal and cardiac muscle, insulin normally promotes nutrient uptake and storage. Nonetheless, chronically elevated fuel supply induces insulin resistance (28–30). This is likely a beneficial adaptive process to prevent tissue dysfunction from fuel overload (31, 32). Excess fuel can be stored as more “inert” triglycerides (TGs) in adipose tissue and as glycogen and TG in liver. The response of islet β-cells to chronic fuel excess, however, is different. To maintain glucose homeostasis, the β-cell has to continuously sense fuel load, particularly glucose, and respond with precise insulin secretion. β-Cells cannot protect themselves by blocking glucose uptake to avoid excess nutrient load, and they have limited capacity to store fuel excess. Thus, β-cells must remove or divert glucose carbons rapidly toward various metabolic pathways to continuously maintain glucose flux entering the cell for insulin secretion and to protect from fuel surfeit.
The GL/FFA cycle is not only implicated in lipid signaling for insulin secretion (11, 33) but may also at elevated glucose play a role in preventing metabolic stress in β-cells and provide a means for nutrient-excess detoxification because it is an ATP-consuming futile cycle (34). Of relevance, we estimated that glucose carbon flux through the glycerol backbone of GL accounts for ∼25% of the total islet glucose utilization in Zucker fatty rat islets (35). Thus, this pathway is able to divert significant quantities of glucose entering the cell away from mitochondrial oxidation. Another means is to limit the buildup of potentially harmful lipid intermediates such as lysophosphatidic acid or to divert FFA from de novo ceramide synthesis (23) by increasing TG deposition in lipid droplets. In fact, a higher capacity of islets to accumulate TG has been shown to be associated with reduced FFA-induced cytotoxicity in islets (36). However, no systematic examination of such candidate pathways of fuel-excess detoxification has been carried out in β-cells. Thus, identification of these pathways that allow β-cells to cope with fuel surfeit may prove important for a better understanding of the molecular nature of β-cell metabolic stress and glucolipotoxicity and provide avenues for the treatment of type 2 diabetes.
We hypothesized that putative fuel detoxification pathways should be active at elevated glucose levels even beyond those concentrations where insulin secretion is maximal. Therefore, one way to identify candidate glucose-excess detoxification pathways is to measure metabolic pathways and metabolites at various glucose concentrations and to identify those that still increase beyond maximal concentrations of glucose for GSIS. By doing so, we expected to discriminate between pathways/metabolites implicated in GSIS from those involved or also implicated in glucose detoxification. To accurately discriminate between these two, we decided to use an enriched incubation buffer (basal glucose of 4 mm in the presence of 2 mm glutamine (Gln) in rat islets and 2 mm glucose plus 2 mm Gln in INS-1(832/13) cells) that mimics more closely the physiological milieu in terms of basal nutrient supply. We recently realized that during standard in vitro insulin secretion experiment with 0–2.8 mm glucose β-cells are in fact energy-depleted, and thus upon glucose stimulation most metabolites are increased (37–40), making it difficult to discern metabolites that act as MCFs from those that increased upon the glucose supply simply because they were depleted due to very low metabolic rate under basal condition (1, 41). In the present study, we aimed to determine which among the candidate metabolic pathways and MCFs best correlate with GSIS, define the fate of glucose in the β-cell, and identify pathways that may be involved in excess-fuel detoxification.
Results
Glucose-induced insulin secretion and its correlations with glucose and mitochondrial metabolism and the production of ROS, glycerol, and glycogen
To better define the fate of glucose metabolism in the β-cell and to distinguish metabolites and pathways involved in GSIS versus others that may also be implicated in the detoxification of excess glucose, we compared the glucose dose dependence of islets for insulin secretion with the profile of various metabolic processes, metabolites, and candidate MCFs under the same incubation conditions. Isolated rat islets were incubated for 60 min at 4, 10, 16, and 25 mm glucose for measuring insulin release as well as glucose utilization and oxidation, oxygen consumption, ATP and ROS production, glycerol release, and glycogen content. As expected GSIS increased between 4 and 16 mm glucose before reaching a plateau between 16 and 25 mm (Fig. 1A). Similarly, glucose oxidation (Fig. 1C), oxygen consumption (Fig. 1D), and ATP production (Fig. 1E) all showed a saturation plateau between 16 and 25 mm. Mitochondrial uncoupling/proton leak did not vary with the glucose concentration (Fig. 1F). However, glucose utilization (Fig. 1B), glycerol release (Fig. 1I), and glycogen content (Fig. 1L) did not show such saturation and increased almost linearly between 4 and 25 mm glucose. This suggested that the latter metabolic pathways and molecules (Fig. 1, B, I, and L) are possibly linked to excess-fuel detoxification. In particular, release of glycerol (three-carbon compound) by islets showed a molar equivalency to the amount of glucose (six-carbon compound) utilized, indicating that at high glucose concentration almost one-third of glucose used gave rise to glycerol, which is released out of the cell. Thus, glucose utilization is measured by the release of labeled H2O occurring at the enolase step that lies downstream of dihydroxyacetone phosphate in the glycolysis pathway and of its direct derivative glycerol 3-phosphate (Gro3P). Hence, glycolysis flux in the early part of the pathway corresponds to glucose utilization calculated on the basis of enolase reaction plus glycerol release. Note that Gro3P can be directly hydrolyzed to glycerol by a Gro3P phosphatase that we have recently identified in mammalian cells (42) or esterified to form glycerolipids before being released as glycerol following lipolysis (43). Also, data showed that glucose utilization was quantitatively much higher than oxidation, which reached a plateau at 16 mm glucose, with a rate of 25 nmol of glucose oxidized/mg of protein/h. Thus, glucose utilization is not entirely related to insulin secretion, and a significant portion of utilized glucose carbons is likely redirected to other pathways with some possibly linked to fuel-excess detoxification.
Figure 1.
Glucose-induced insulin secretion in isolated rat islets and its correlation with various parameters of glucose and energy metabolism. A, insulin secretion measured in islets incubated at 4 (4G), 10 (10G), 16 (16G), and 25 (25G) mm glucose for 1 h. Glucose utilization (B) and oxidation (C) in islets incubated for 1 h with d-[U-14C] glucose and d-[5-3H] glucose are shown. D–F, respiration and mitochondrial function. Oxygen consumption rate, ATP production, and proton leak were calculated from individual traces. Superoxide (O2˙̄) (G and J) and H2O2 production (H and K) in dispersed rat islet cells incubated for 1 h are shown. J and K, ROS data with ROS inhibitor N-acetyl-l-cysteine (NAC; 0.4 mm) and the positive controls H2O2 (10 μm) and 16 mm glucose (16G) + rotenone (Rot; 1 μm). Glycerol release (I) and islet glycogen content (L) after 1 h incubation are shown. Error bars represent means ± S.E. of six to nine islet incubations in three separate experiments except for glycogen determinations, which were performed in two experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus 4 mm glucose; one way-ANOVA.
Previous studies have demonstrated that hyperglycemia-induced intracellular ROS mediates β-cell apoptosis (44, 45). However, at relatively low concentrations, ROS could be beneficial having signaling roles in various cellular processes (46), including insulin secretion in β-cells. We found that production of superoxide (Fig. 1, G and J) and hydrogen peroxide (Fig. 1, H and K) were significantly increased at 10 mm glucose compared with 4 mm in isolated islets but decreased rapidly at higher glucose concentration (16 and 25 mm), suggesting that cellular ROS levels are regulated to prevent potentially “toxic” accumulation (45, 46). We then examined the importance of the mitochondrial electron transport chain (ETC) in the intracellular ROS generation by exposing islets to high glucose in the presence of an inhibitor of the ETC or a ROS scavenger (Fig. 1, J and K). Results showed that ROS scavenger N-acetylcysteine lowered the production of superoxide and hydrogen peroxide at elevated glucose, whereas rotenone, a complex I inhibitor, increased ROS production, indicating that site I of the ETC is implicated in ROS production. Overall, the results indicate that GSIS correlates with glucose-dependent respiration and ATP production (Fig. 1, A, D, and E) and that a major fate of glucose metabolism is formation and release of glycerol and to a much lower extent glycogen (see nmol of glucose utilization, glycerol release, and glycogen) (Fig. 1, B, I, and L).
Glucose linearly increases the production and release of FFA as well as cellular content of TG and total and esterified cholesterol
Considering that excess glucose carbons can be directed toward cholesterol, FFA, and glycerolipid synthesis, we examined the effect of increasing glucose level on the accumulation of these classes of lipids in rat islets. Total FFA content and release (Fig. 2, A and B) increased linearly with glucose concentration up to 25 mm glucose. More specifically, the main FFA species that accumulated in islets were myristate (C14:0), palmitate (C16:0), oleate (C18:1), and stearate (C18:0) (Fig. 2D). Except myristate, all of these FFAs increased linearly with glucose concentration, and glucose quantitatively caused the accumulation of long-chain saturated FFA. Similar results were obtained for FFA release even though oleate release did not linearly increase with glucose concentration. Furthermore, stearate and palmitate were the predominant FFA species that accumulated in islets and released in the incubation medium (Fig. 2, D and E). Octanoate, decanoate, laureate, palmitoleate, arachidonate, and linoleate were also measured but did not show any change after glucose stimulation (not shown). Similarly, TG (Fig. 2C), total cholesterol, cholesterol esters, and free cholesterol (Fig. 2, F–H) all linearly increased with glucose concentration.
Figure 2.
Glucose dose-dependently enhances FFA release and causes FFA, triglyceride, and cholesterol deposition in isolated rat islets without saturation at concentrations maximal for secretion. A and B, cellular content and release of total FFA by islets at 4 (4G), 10 (10G), 16 (16G), and 25 (25G) mm glucose. C, TG content. D and E, cellular content and release of different FFA species. Total cholesterol (Total Chol) (F), cholesterol ester (Chol Esters) (G), and free cholesterol (Free Chol) (H) contents are shown. Error bars represent means ± S.E. of six to nine islet incubations from of two to three independent experiments. Incubation time, 1 h. C14:0, myristic acid; C16:0, palmitic acid; C18:1, oleic acid; C18:0, stearic acid. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus 4 mm glucose; one way-ANOVA.
A major fate of glucose metabolism in islets is glycerol release and lipid synthesis
The results in supplemental Fig. 1 are the raw data used for the calculations of the changes in flux of metabolic pathways and metabolites from 4 to 16 mm and from 16 to 25 mm glucose that are reported in Fig. 3. As discussed above, glucose usage measures flux in the lower part of the pathway at the enolase step (2-phosphoglycerate to phosphoenolpyruvate) and we named it in supplemental Fig. 1 and Fig. 3 “lower glycolysis.” Glycolysis flux in the upper part of the pathway below glucose 6-phosphate was estimated as the carbons related to glucose usage plus those of glycerol release, and we named it “upper glycolysis.” Indeed, glycerol release comes from the dihydroxyacetone phosphate-derived Gro3P either via lipogenesis and then lipolysis or via direct hydrolysis of Gro3P by glycerol-3-phosphate phosphatase (42). “Calculated glycolysis” in Fig. 3, C and D, is upper glycolysis plus the carbon in glycogen that is derived from glucose 6-phosphate. It reflects the calculated flux through glucokinase, the first step in the pathway in β-cells. As glucose carbons have several cellular fates, we examined the absolute levels of various metabolites compared with glycolysis following glucose stimulation (Fig. 3, A and B). To better quantitatively assess pathways possibly involved in excess glucose detoxification, glucose-derived metabolite levels were plotted as net differences between 4 and 16 mm glucose (Fig. 3A) and between 16 and 25 mm glucose (Fig. 3B), assuming that the differences noted beyond 16 mm glucose would reflect candidate pathways involved in excess-glucose detoxification. Importantly, although it is recognized that the assessment is indirect because we did not directly measure metabolic flux derived from glucose carbons with isotope labeling, the measurements of the levels in various metabolites likely largely correspond to glucose-derived carbons because glucose was the only exogenous fuel that varied under our experimental condition. Fig. 3, A and B, show that glycerol release and to a lesser degree total FFA were the two parameters that increased the most in terms of nmol/mg of protein /h when glucose concentration was increased from 4 to 16 mm and from 16 to 25 mm, whereas total cholesterol (the addition of cholesterol esters and free cholesterol) and glycogen were lower in comparison followed by TG content. However, when the data were analyzed in carbon equivalents (see “Experimental procedures”) (Fig. 3, C and D), which take into account the carbon numbers of each metabolite, total FFA, TG, and total cholesterol became significant recipients for the glucose carbons with approximately equivalent accumulation. Nonetheless, released glycerol remained the quantitatively higher metabolite of glucose-derived carbons at very high glucose concentrations (Fig. 3D). For easier quantitative comparison of the various pathways and metabolites with glycolysis flux, Fig. 3, E and F, report the same data expressed as carbon equivalents as a percentage of calculated glycolysis. Thus, an important fate of glucose carbons in rat islets is formation and release of glycerol as well as lipids, in particular total FFA, total cholesterol, and TG.
Figure 3.
A major fate of glucose metabolism in islets is glycerol release and lipid molecules. A and B, changes in the flux of metabolic pathways and in the levels of various metabolites compared with lower glycolysis in isolated islets following glucose stimulation from 4 to 16 mm (A) and from 16 to 25 mm (B). C and D, transformation in carbon equivalents, taking into account the number of carbons of each molecule, of the changes in the levels of various metabolites found in A and B following glucose stimulation from 4 to 16 mm (C) and from 16 to 25 mm (D). E and F report the same data of carbon equivalents expressed as percentages of calculated glycolysis. Carbon equivalents were calculated as described under “Experimental procedures.” The terms lower glycolysis, upper glycolysis, and calculated glycolysis are explained under “Experimental procedures” and the corresponding “Results” section. Incubation time, 1 h. Error bars represent means ± S.E. of six to 12 islets incubations in two to four independent experiments. Chol, cholesterol.
Changes in the levels of metabolites in response to glucose in INS-1(832/13) cells and correlation with GSIS
To enhance our understanding of metabolic signaling of glucose in the β-cell, we then used a targeted metabolomics approach to identify the metabolites that best correlate with the dose dependence of GSIS. Various classes of metabolites and candidate MCFs were measured by LC-MS/MS in INS-1(832/13) cells in a medium with 2 mm glucose and 2 mm Gln for the basal fuel condition. INS-1(832/13) cells were chosen for this study instead of isolated islets due to measurements sensitivity issues, and 2 mm glucose and not 4 mm (islet studies above) was chosen because the dose dependence of GSIS in INS-1(832/13) cells is shifted to the left in comparison with islets. Insulin secretion increased with glucose concentration between 2 and 11 mm glucose (Fig. 4A) and reached a plateau at 11 mm glucose. The half-maximal effect was observed at lower concentrations of glucose in INS-1(832/13) compared with isolated islets as reported before (47, 48).
Figure 4.
Insulin secretion and its correlation with the levels of glycolysis-related metabolites and citric acid cycle intermediates in response to increasing glucose concentrations in INS-1(832/13) cells. A, insulin secretion at 2, 4, 12, and 20 mm glucose. At the end of the 1-h incubation, the following metabolites were extracted from the cells and analyzed by LC-MS/MS: DHAP (B), Gro-3-P (C), pyruvate (D), lactate (E), isocitrate + citrate (F), α-ketoglutarate (G), succinate (H), fumarate (I), and malate (J). n.d., not detected. Error bars represent means ± S.E. of 12 cell incubations in four independent experiments. **, p < 0.01; ***, p < 0.001 versus 2 mm glucose; one way-ANOVA.
All measured glycolysis-related and Krebs cycles intermediates increased in INS-1(832/13) cells in response to elevated glucose concentration (Fig. 4). In accordance with a key role of anaplerosis (replenishment of Krebs cycle intermediates) in glucose signaling, several cycle intermediates correlated well with GSIS, in particular citrate/isocitrate, fumarate, and malate. Thus, as for GSIS, their level at 20 mm glucose was not significantly different from that at 11 mm. Also, in accordance with a role of GL/FFA cycling (1, 43) and the Gro3P shuttle (8) in GSIS, the level of Gro3P correlated with insulin secretion because its concentration at 20 mm glucose was not significantly different from that at 11 mm. Figs. 5–7 show metabolite data from the same experiments as in Fig. 4.
Figure 5.
Changes in the levels of some amino acids and short-chain CoA derivatives in response to increasing glucose concentrations in INS-1(832/13) cells. Experimental conditions are those of Fig. 4. A, glutamine. B, glutamate. C, aspartate. D, leucine. E, alanine. F, acetoacetyl-CoA. G, acetyl-CoA. H, malonyl-CoA. I, HMG-CoA. J, insulin secretion (the data are the same data as shown in Fig. 4A and are used for comparison of the dose dependence of metabolites levels with insulin release). Error bars represent means ± S.E. of 12 cell incubations in four independent experiments. ***, p < 0.001 versus 2 mm glucose; one way-ANOVA.
Figure 6.
Effect of increasing glucose concentrations on the levels of nicotinamide adenine dinucleotides and glutathione derivatives in INS-1(832/13) cells. Experimental conditions are those of Fig. 4. A, NAD+. B, NADH. C, NADP+. D, NADPH. E, GSH. F, GSSG. G, NADH/NAD. H, NADPH/NADP. I, GSH/GSSG. J, DHAP/Gro-3-P. K, pyruvate/lactate. L, insulin secretion (the data shown are the same data shown in Fig. 4A and are used for comparison of the dose dependence of metabolites levels with insulin release). n.d., not detected. Error bars represent means ± S.E. of 12 cell incubations in four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus 2 mm glucose; one way-ANOVA.
Figure 7.
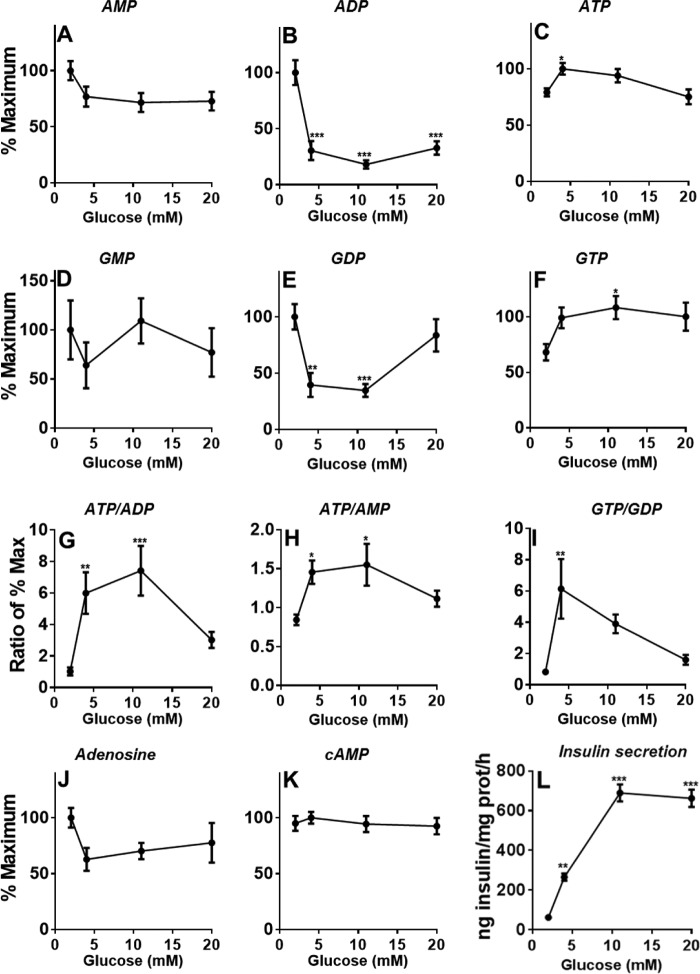
Effect of increasing glucose concentrations on the levels of adenine, guanine, and cyclic nucleotides in INS-1(832/13) cells. Experimental conditions are those of Fig. 4. A, AMP. B, ADP. C, ATP. D, GMP. E, GDP. F, GTP. G, ATP/ADP. H, ATP/AMP. I, GTP/GDP. J, adenosine. K, cAMP. L, insulin secretion (the data shown are the same data shown in Fig. 4A and are used for comparison of the dose dependence of metabolites levels with insulin release). Error bars represent means ± S.E. of 12 cell incubations in four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus 2 mm glucose; one way-ANOVA.
The levels of glutamine, glutamate, and acetoacetyl-CoA (Fig. 5, A, B, and F) remained unchanged with increasing glucose concentration. As described before, leucine and alanine levels slightly increased with glucose concentration (40, 49) (Fig. 5, D and E). Acetyl-CoA (Fig. 5G) levels showed a tendency to increase with glucose concentration. In accordance with the observation that an important fate of glucose in the β-cell is cholesterol, the first intermediate in the pathway of cholesterol biosynthesis, hydroxymethylglutaryl-CoA (HMG-CoA) (Fig. 5I), was reduced perhaps due to HMG-CoA reductase activation due to AMP kinase inhibition at elevated glucose (50). We found a significant reduction in aspartate (Fig. 5C) levels as a function of glucose similar to previous reports (14, 37). Following glucose exposure, malonyl-CoA levels rose markedly (Fig. 5H) and correlated well with insulin secretion (Fig. 5J) as described previously (37).
The increase in NADH upon glucose stimulation was very prominent (Fig. 6B), whereas that of NADPH was modest and did not reach statistical significance (Fig. 6D). Reduced glutathione (GSH) levels (Fig. 6E) rose only at 20 mm glucose, whereas it was unchanged at 11 mm where secretion was maximal (Fig. 6L). NAD+ (Fig. 6A) and NADP+ (Fig. 6C) did not vary, but there was a decrease in oxidized glutathione (GSSG) after glucose stimulation (Fig. 6F) and no change in the GSH/GSSG ratio at concentrations of glucose (4 and 11 mm) that promoted effective insulin secretion. NADPH and GSH have been proposed to be implicated in the control of insulin exocytosis via thioredoxin and glutaredoxin (21, 40, 51). The results obtained using our richer medium in terms of basal fuels do not provide support to the view that the glutathione redox systems regulate GSIS at least in INS cells, although the data do not discount this possibility.
The contents of NADH and NAD+ and their ratio may not indicate the oxidation-reduction state in subcellular compartments in the β-cell. The ratio of dihydroxyacetone phosphate (DHAP) to Gro3P (DHAP/Gro3P) is linked to the cytosolic free NAD+/NADH ratio by the NAD+-dependent cytosolic l-Gro3P dehydrogenase. Indeed, this enzyme is well expressed in the normal β-cell and is thought to catalyze a near equilibrium reaction (52). We found that glucose dose-dependently increases the DHAP/Gro3P ratio in INS-1(832/13) cells, indicating a more oxidized state of NAD in the cytosol upon glucose stimulation. A high cytosolic NAD/NADH ratio would favor glycolysis by promoting flux through glyceraldehyde-3-phosphate dehydrogenase. This is in agreement with a previous islet study reporting a similar effect in pure pancreatic rat β-cells during GSIS (52). Also consistent with this conclusion is the finding that the pyruvate to lactate ratio rose dose-dependently upon glucose stimulation (Fig. 6K). Thus, the rise in total NADH caused by elevated glucose (Fig. 6B) likely reflects NADH in the mitochondrial compartment.
KATP channel closure via a rise in the ATP/ADP ratio is important for GSIS. In agreement, ATP levels (at least at 4 and 10 mm glucose; Fig. 7C) slightly increased, and AMP (Fig. 7A) and ADP (Fig. 7B) decreased with glucose concentrations. Even though the absolute increase in ATP levels was very modest (Fig. 7C), both ADP and AMP fell markedly (Fig. 7, A and B), thereby significantly altering the ATP/ADP (Fig. 7G) and ATP/AMP (Fig. 7H) ratios, and earlier observations indicated that KATP channel may be more influenced by a reduction in ADP levels (53) than an increase in ATP. Studies have provided evidence for a role for mitochondrial GTP in GSIS (17). Although there was a small increase in total GTP levels (Fig. 7F), which includes both the smaller pool of mitochondrial GTP and the larger GTP pool in the cytosol, the precise changes in the mitochondrial pool may not be detectable (48). GDP and adenosine levels decreased at intermediate glucose levels (Fig. 7, E and J), whereas GMP (Fig. 7D) and cAMP (Fig. 7K) levels did not vary. There was a prominent increase in the GTP/GDP ratio (Fig. 7I) at stimulatory concentrations of glucose for insulin secretion (Fig. 7L).
Discussion
In the present study, using rat islets, we examined the fate of glucose and metabolic pathways and corresponding metabolites involved in stimulus secretion coupling and possibly excess-fuel detoxification processes and whether these pathways are shared or different. Why would the β-cell need pathways related to fuel-excess detoxification? One reason is that, due to its intrinsic glucose-sensing properties, unlike most cell types it immediately equilibrates glucose levels across the plasma membrane due to the presence of the high Km and Vmax transporter Glut2 such that hyperglycemia translates into elevated concentrations of glucose in the β-cell cytoplasm. Additionally, the high Km hexokinase IV (glucokinase) acts as a glucose trap via its phosphorylation because glucose-6-phosphate phosphatase is found at low levels in the β-cell (1). As a result, the fate of glucose once it has entered the β-cell is its intracellular metabolism, and excess glucose should be eliminated in pathways to prevent the formation of toxic lipids, mitochondrial dysfunction, or endoplasmic reticulum stress (1).
There is a clear dichotomy in the glucose concentration dependence profiles of GSIS and glucose oxidation, which showed saturation by 16 mm glucose, as compared with glucose utilization, glycerol release, glycogen synthesis, FFA synthesis and release, and synthesis and storage of TG and cholesterol, which increased linearly up to 25 mm glucose without any indication of saturation. An ideal detoxification pathway is expected to operate continuously with less or no saturation so that the toxicity due to the excessive amount of substrate is alleviated effectively. In light of this, it can be viewed that the major fate and pathways of glucose metabolism in rat islet, besides its oxidation, that are possibly involved in fuel-excess detoxification (Fig. 3D) are glycerol formation and release, TG formation and storage, FFA synthesis and release, formation of free cholesterol and cholesterol ester, and glycogen synthesis and storage, the latter being quantitatively less important. Below we discuss the candidate roles of these pathways in fuel-excess detoxification versus glucose signaling for secretion.
Glycerol release, which linearly increases with glucose concentration, likely arises primarily from the direct hydrolysis of Gro3P by the newly discovered glycerol-3-phosphate phosphatase (42) at least at elevated glucose concentrations. Thus, contribution to glycerol release by glucose-stimulated lipolysis is important only at glucose concentrations <10 mm (42). We recently reported that overexpression of glycerol-3-phosphate phosphatase promotes glycerol release from rat islets and INS-1(832/13) cells and reduces the apoptotic effect of chronic elevated glucose (glucotoxicity), whereas down-regulation of the enzyme had opposite effects. Thus, glycerol release is likely an important pathway of glucodetoxification in the β-cell (42).
Glucose utilization, which increases linearly with increasing glucose concentration, is reflected in the increase in FFA (cellular and release), total cholesterol, and TG. Several studies have also shown changes in TG content (54, 55), FFA content and release (55, 56), and cholesterol content (55) in response to increasing glucose concentrations in islets and in other cell types (57). However, this is the first islet study to examine, in the same setting, many pathways, metabolites, and candidate MCFs such that they can be quantitatively compared.
Glucose carbons can be directed toward de novo biosynthesis of FFA and FFA elongation in various cells, including β-cells, and this proportion increases with increased glucose concentrations (58, 59). Our results indicate that islets convert a significant amount of glucose to FFAs and export nearly 50% of them out of the cell. Stearic and palmitic acids are the predominant FFAs that are synthesized and released into the medium. Similar results were obtained by Martins et al. (56) who measured changes in fatty acid composition caused by glucose in pancreatic islets. Because a large portion of FFA is released from the islets at high glucose, fatty acid synthesis and release are a strong candidate pathway for glucodetoxification in the β-cell. However, the released fatty acid could also act as an autocrine and paracrine signal in the lipid amplification pathway for GSIS via the fatty acid receptor FFAR1 (1). Similarly, the rise in intracellular FFA may help to drive the GL/FFA cycle for the formation of lipid signaling molecules for secretion, in particular monoacylglycerol (1).
Free cholesterol is essential for many cellular functions and membrane fluidity but has toxic effects when present in excess (60, 61). Toxicity of accumulating cholesterol is normally controlled via its esterification with FFA by acyl-CoA:cholesterol acyltransferase-1 to produce cholesterol esters (CE) (62) and its efflux via ATP-binding cassette transporter A1 (ABCA1). It has been shown that deletion of ABCA1 and/or ABCG1 genes that facilitate cholesterol efflux from β-cells causes accumulation of total cholesterol and its derivatives and reduced insulin secretion (63, 64). We noticed that much of the glucose carbons are directed toward CE and free cholesterol in islets. These results are in accordance with an earlier study by MacDonald et al. (55) who used labeled glucose and reported that at high glucose ∼15% of the glucose carbons are incorporated into CE. Because cholesterol can be secreted from cells and CE are considered as a biologically inert storage (detoxification) form of cholesterol and because inhibition of cholesterol efflux causes β-cell dysfunction, this pathway is also potentially involved in excess-fuel detoxification in the β-cell. This is not to dismiss that chronic accumulation of free cholesterol in the β-cell contributes to β-cell failure in diabetes (50, 65).
Elevated glucose also caused significant accumulation of TG that in various cell types constitutes a relatively inert form of lipid accumulation in the form of droplets of various sizes. Thus, TG formation in islets may contribute to glucodetoxification, and consistent with this view an inverse relationship between cytotoxicity of chronic elevated FFA in pancreatic islet cells and β-cell TG accumulation has been reported (36).
Glycogen also significantly accumulated in islets at high glucose, although it was quantitatively less than total FFA, cholesterol, and TG. Similar to TG, glycogen is considered as a relatively inert form of fuel excess energy storage in various cell types, and thus this pathway may also contribute to nutrient-excess detoxification. However, upon chronic exposure to marked hyperglycemia, like cholesterol, the massive accumulation of glycogen could contribute to β-cell dysfunction because the β-cell glycogen content appears to correlate with apoptosis in a mouse model of human neonatal diabetes (66).
In sum, this study identifies glycerol and FFA release as well as cholesterol ester, triglyceride, and glycogen deposition as potential pathways of nutrient-excess detoxification in the β-cell. Additional work is required to directly test the relative importance of these pathways to protect the β-cell from hyperglycemia.
It is somewhat unexpected that glucose utilization in rat islets continues to increase linearly as the concentration of glucose increases. The rate-controlling transporters/enzymes for these processes, notably glucokinase, have Km values of ∼10 mm, and one might therefore expect some tailing off of the rates as glucose increases from 11 to 25 mm. The possible explanation of the lack of tailing off of glucose usage is that endogenous glucokinase under our experimental conditions in intact cells has perhaps higher Km than the purified enzyme. It is important to keep in mind that the Km calculated for purified glucokinase cannot be readily applied to whole-cell studies. Glucokinase in the whole cell (β-cells) is regulated/activated by its association with other components, for example PFK2/FBPase-2 (67). Nonetheless, the lack of tailing off of glucose usage is consistent with the lack of tailing off of anaplerosis-cataplerosis-derived FFA, cholesterol, and TG synthesis.
Another important aspect of this study concerns the coupling mechanisms of GSIS, and this is discussed below. Inasmuch as there is a significant correlation between overall glucose oxidation and GSIS in islets, we examined whether the formation of any specific glucose oxidation-derived product(s) follows the same glucose dose dependence as GSIS and can be accounted as a potential MCF contributor. Thus, production of ATP, needed for KATP channel inhibition, follows the same saturation kinetics as GSIS and agrees with the widely accepted role of ATP as an MCF (1). Even though ROS were proposed to be MCFs, no close correlation with GSIS was noticed in our results. Interestingly, the decline in ROS levels above 10 mm glucose may be an indication of their detoxification (23). The decrease in ROS at higher glucose concentration is in accordance with the metabolomics data in INS-1(832/13) cells showing an increase in NADPH and GSH at high glucose concentration because NADPH is used by the glutathione redox system to eliminate ROS (68, 69). Several studies have shown enhanced ROS levels at elevated concentration of glucose in β-cells (19, 70–72), but to our knowledge none so far have reported the glucose dose dependence of the effect. Thus, these studies likely missed the “bell shape” of ROS production as a function of glucose levels, particularly for hydrogen peroxide (H2O2).
To gain further insight into signaling metabolites, we used a targeted metabolomics approach in INS-1(832/13) cells. Fumarate, malate, citrate/isocitrate, succinate, and malonyl-CoA levels correlated well with GSIS and thus could be candidate MCFs. Thus, several studies implicated anaplerosis and the citrate/isocitrate/malate-pyruvate cycles as pathways for GSIS (1, 8, 33, 73). Citrate cataplerosis generates malonyl-CoA, which by inhibiting FFA oxidation (74) was shown to play a role in GSIS. The marked reduction in HMG-CoA with elevated glucose, as we observed before in HIT cells (15), may be due to the reduced fatty acid oxidation and ketogenesis at elevated glucose because HMG-CoA is an intermediate of this pathway or the consumption of this metabolite for cholesterol synthesis, which linearly increased with glucose concentration in islets.
Dihydroxyacetone phosphate, Gro3P, pyruvate, lactate, α-ketoglutarate, and NADH correlate better with glucose utilization than with GSIS. It is interesting to note that significant amounts of cataplerotic metabolites, in particular citrate (59, 75) and α-ketoglutarate (76), can efflux from cells and thus contribute to excess-fuel detoxification. In fact, we previously observed that as much as 20% of the glucose carbons entering glycolysis exit from β-cells in the form of citrate (77). Thus, citrate by virtue of its participation in pyruvate cycling processes and malonyl-CoA production and by its ability to exit the cell in relatively large amounts is a common metabolite involved in pathways for GSIS as well as for glucose detoxification.
The steady rise in dihydroxyacetone phosphate and Gro3P with glucose underlies the linear increase in glycerol release, likely through the hydrolysis of Gro3P by glycerol-3-phosphate phosphatase, but may not be directly related to GSIS. However, it is likely involved in excess-fuel detoxification as discussed above. Although we noticed a glucose-dependent linear non-saturating increase in pyruvate and lactate in INS-1(832/13) cells, this may not be of significance for excess-fuel detoxification in primary β-cells, which have very low amounts of lactate dehydrogenase (78).
The large increase in malate after glucose stimulation accompanied with a decrease in aspartate is consistent with the idea that the “malate-aspartate shuttle” plays an important role in the transfer of NADH across mitochondrial membrane and in the anaplerotic supply of oxaloacetate in mitochondria from aspartate. These results are consistent with the initial proposal by Simpson et al. (79) that aspartate is consumed during GSIS and forms the primary non-pyruvate carboxylase-derived anaplerotic substrate oxaloacetate for the tricarboxylic acid cycle during GSIS.
Glutamate has been proposed to participate in GSIS through the action of mitochondrial glutamate dehydrogenase (α-ketoglutarate to glutamate direction) (80). However, our results indicate unchanged levels of glutamate with increasing glucose concentrations in INS-1(832/13) cells. The results do not support a role of glutamate as an MCF, but the possibility that there is a change of glutamate in a signaling pool without a change in total glutamate cannot be ruled out. Another proposed MCF for GSIS is NADPH, which showed parallel changes with insulin secretion and is consistent with the idea that NADPH or the NADPH/NADP ratio is a potential MCF for GSIS (51, 73, 81, 82).
The overall regulation of KATP channels by adenine nucleotides depends on the net inhibitory effect of ATP on Kir6.2 and the activating effect of MgADP on the SUR1 component of the channel (83, 84). Although the increase in ATP levels at elevated glucose was rather subtle, the simultaneous decrease in ADP was marked, and this probably results in an optimal ATP/ADP ratio that facilitates the closure of KATP channels. The data suggest that variations in ADP upon glucose stimulation are more important than those of ATP to modulate KATP channels and insulin secretion.
A limitation with this and earlier metabolomics studies in the β-cell and other cell types is that the bulk measurements of metabolites in cell populations provide no information on intercellular and subcellular heterogeneity or on the free and bound concentrations in a single compartment (41). In terms of MCFs, it would be informative to know the cytosolic content of metabolites or their concentrations in the vicinity of the plasma membrane close to the exocytosis process; hopefully technological developments will allow this in the future.
In conclusion, the results support the concept that in the β-cell some metabolic pathways play a role in fuel-excess detoxification. These possible detoxification processes could involve the storage of high energy currencies (TG, glycogen, and cholesterol esters) and the extracellular release of metabolites derived from glucose (glycerol, cholesterol, and FFA). Furthermore, the data indicate that besides glucose oxidation major fates of glucose-derived metabolites are glycerol, FFA, cholesterol, and TG. Also, a comprehensive study of islet metabolism and targeted metabolomics in INS-1(832/13) cells showed that glucose oxidation, oxygen consumption, ATP production, some citric acid cycle intermediates, malonyl-CoA, and a lowering of ADP closely correlated with insulin secretion, whereas glucose usage, glycerol release, glycogen content, FFA content and release, and TG and cholesterol content increased almost linearly with glucose concentration. By contrast, the dose dependence of GSIS did not correlate well with ATP, cAMP, ROS, and glutamate that have been proposed to act as MCFs for insulin secretion. Overall, the data support some prevailing hypotheses of β-cell metabolic signaling, in particular a role for accelerated oxidative mitochondrial metabolism, anaplerosis, and malonyl-CoA/lipid signaling and suggest that a decrease in ADP levels plays an important role in GSIS.
Experimental procedures
Islet isolation and culture
All procedures involving animals were approved by the Institutional Committee for the Protection of Animals at the Centre de Recherche du Centre Hospitalier de l'Université de Montréal. Pancreatic islets were isolated from male Wistar rats from Charles River (St-Constant, Quebec, Canada) as described before (85) by collagenase (type XI from Sigma-Aldrich) digestion of total pancreas. Isolated islets were handpicked under a stereoscope and cultured overnight at 37 °C in RPMI 1640 medium with sodium bicarbonate supplemented with 10% fetal calf serum, 10 mm HEPES (pH 7.4), 2 mm l-glutamine, 1 mm sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin at 11.1 mm glucose in a Petri dish before the start of the experiments.
Insulin secretion in isolated islets
Islets were transferred to RPMI 1640 medium with 4 mm glucose for 2 h. Then batches of 100 islets were washed in Krebs-Ringer buffer-HEPES (KRBH) at pH 7.4 containing 4 mm glucose and 0.5% defatted BSA and preincubated for 45 min in KRBH containing 4 mm glucose, 0.5% defatted BSA, 50 μm l-carnitine, 2 mm glutamine, and various pharmacological agents or DMSO. Islets were then incubated for 60 min in KRBH with 0.5% defatted BSA, 50 μm l-carnitine, and 2 mm glutamine containing different concentrations of glucose in the presence or absence of pharmacological agents. At the end of the incubations, media were collected. Total insulin released into medium and total insulin content were determined by radioimmunoassay with a kit (Linco Research, St. Charles, MO) or by AlphaLISA assay (PerkinElmer Life Sciences) using human insulin (Sigma-Aldrich) as a standard.
Glycerol assay
One hundred freshly isolated rat islets were incubated for 60 min in KRBH as described above at various concentrations of glucose. At the end of the incubation, media were kept to measure glycerol release. A radiometric glycerol assay was used to measure glycerol in the medium as described (42, 86). The assay is based on glycerol phosphorylation by glycerokinase in the presence of [γ-32P]ATP (Perkin Elmer Life Sciences).
Determination of free fatty acids
For FFA determinations, isolated rat islets were incubated for 60 min in KRBH at various glucose concentrations as described above. FFAs accumulated in the islet cells and released into the medium were extracted separately by a modified Dole-Meinertz extraction procedure (87). FFAs released into the medium were measured using 0.5 ml of incubation media. For cellular FFA content determinations, medium was rapidly removed, and islets were rinsed once in PBS and immediately frozen in liquid nitrogen. Then the frozen islets were resuspended in 0.5 ml of water, taken for extraction, derivatized, and measured by HPLC as described before (11). Briefly, samples (0.5 ml) in Pyrex glass tubes were mixed with the internal standard [2H31]palmitic acid and extracted with 2.5 ml of a solvent mixture containing isopropanol, n-heptane, and 2 m phosphoric acid (40:10:1 by volume). After thorough mixing by vortexing, tubes were introduced in a bath sonicator (Branson Ultrasonic) and sonicated for 2 min with 30-s intervals, avoiding sample heating. After rigorous mixing by vortexing, samples were incubated at room temperature for 10 min before the addition of 1 ml of heptane and 1.5 ml of water. Tubes were then again thoroughly vortexed and sonicated for 1 min. Tubes were centrifuged at 1000 × g for 10 min at 4 °C. An aliquot of 1.5 ml (88% of the total organic phase) from the top layer was transferred to 2.0-ml Reacti-Vials (Supelco) and dried under nitrogen (N-Evap, Organomation, Berlin, MA). The dried fatty acids were derivatized with phenacyl bromide and quantified by reverse phase HPLC using a Zorbax Eclipse Plus XDB analytical C18 column (4.6 × 250 mm, 5 μm; Agilent Technology). The FFAs were eluted using methanol/water (92.5:7.5 by volume) at a flow rate of 1.5 ml/min, and the absorbance of eluting FFA was measured at 242 and 254 nm. The peaks were identified by comparing their retention times with fatty acid standards, and the concentrations of individual FFAs were calculated by the internal standard method from peak area using the standard curves of individual FFAs.
Islet glucose metabolism
Groups of 20 freshly isolated islets, cultured and preincubated as described for insulin secretion, were incubated at 37 °C for 90 min in KRBH with 0.5% defatted BSA containing 0.5 μCi of d-[5-3H]glucose (16 Ci/mmol), 1 μCi/ml d-[U-14C]glucose (250 mCi/mmol) (PerkinElmer Life Sciences), and different concentrations of glucose. Incubation was stopped by the addition of citrate/NaOH buffer (400 mm, pH 4.9) containing antimycin A (10 μm), rotenone (10 μm), and potassium cyanide (5 mm) as described previously (88). Glucose oxidation was measured by following the generation of 14CO2 trapped in potassium hydroxide after 60-min incubation at room temperature. Glucose utilization was determined by measuring the amount of 3H2O (89).
Islet triglyceride and cholesterol
Triglyceride (54) and cholesterol (65) contents were measured in batches of 100 isolated rat islets. Briefly, for islet triglyceride determinations, isolated islets were subjected to liquid-liquid extraction using a chloroform/methanol (2:1, v/v) mixture. Organic phases (chloroform) were transferred into new glass tubes and dried under nitrogen (N-Evap). For quantification, the dried material was resuspended in isopropanol, and triglycerides were measured enzymatically with a commercial kit (GPO Trinder, Sigma). Triolein (Sigma), dissolved in chloroform and processed similarly to samples, was used as a standard. For islet cholesterol determinations, the same method was used to extract lipids, and cholesterol was measured using a commercial kit (Amplex® Red cholesterol assay kit, Molecular Probes).
Oxygen consumption and mitochondrial function
Oxygen consumption was measured at 37 °C from isolated rat islets after overnight recovery using a Seahorse XF24 analyzer (Seahorse Bioscience, Billerica, MA). Islets were seeded at a density of 75 islets/well. After basal respiration measurement for 20 min, glucose levels were elevated to 10, 16, or 25 mm. After incubation for 20 min, oligomycin, rotenone, and antimycin were added by three successive injections to assess uncoupled respiration and non-mitochondrial respiration. ATP production was calculated by measuring the decrease in oxygen consumption rate upon injection of oligomycin (90).
Reactive oxygen species determination
Superoxide (O2˙̄) levels were measured in dispersed cells from freshly isolated islets (200 islets per determination) that had been incubated at different concentrations of glucose. Superoxide was detected by FACS measurement of hydroethidine (HE) oxidation as described before (91, 92). Following 40-min incubations, 2.5 μm HE was added, and cells were incubated at 37 °C for an additional 20 min prior to analysis of HE-derived red fluorescence (690-nm bandpass filter). For H2O2 measurement, intact rat islets were used and treated as described above, and an Amplex Red hydrogen peroxide/peroxidase assay kit (ThermoFisher Scientific, Waltham, MA) was used for detection. Amplex Red reagent (100 μm) was added 20 min prior to the termination of the 60-min incubations to react with intracellular H2O2, and the resulting red fluorescence oxidation product, resorufin, was measured using a Fluostar Optima (BMG Biotechnology, Germany) with the filters at 570 nm for excitation and 595 nm for emission.
Glycogen content
Islet glycogen content following 60-min incubations was determined as glucose units analyzed fluorometrically using a glycogen assay kit (Abcam, Toronto, Ontario, Canada).
Insulin secretion and targeted metabolomics in INS-1(832/13) cells
Rat insulinoma INS-1(832/13) cells (47) (passages 54–63) were cultured at 11.1 mm glucose in RPMI 1640 medium supplemented with 10% (w/v) fetal bovine serum, 10 mm HEPES, 2 mm glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol (complete RPMI) at 37 °C in a humidified atmosphere (5% CO2, 95% air). Cells were seeded at 4 × 105 cells in 6-well culture plates for 2 days to reach 60–70% confluence at the day of treatment. Cells were then preincubated in complete RPMI containing 2 mm glucose followed by two successive incubations in KRBH as described under “Insulin secretion in isolated islets” except that 2 mm glucose + glutamine was the basal value for glucose. At the end of the 60-min incubation period with different concentrations of glucose, media were rapidly removed and kept for measurement of secreted insulin, and cell metabolism was rapidly quenched by transferring culture plates in liquid nitrogen. Metabolites were extracted as described previously (37) with the following modifications. Cells were scraped on ice and collected in 675 μl of ice-cold extraction buffer (80% methanol, 13.7 mm ammonium acetate, pH 9.0, with 10 μm [13C10,15N5]adenosine 5′-monophosphate lithium salt (Sigma-Aldrich) as internal standard; transferred into polypropylene tubes; and sonicated in a cup-horn sonicator (Q700 sonicator, Qsonica, Newtown, CT) at 150 watts for 2 min (cycles of 10 s on, 10 s off) in an ethanol-ice bath. Cell extracts were centrifuged at 4 °C for 10 min at 25,830 × g, and supernatants were collected in ice-cold 2-ml polypropylene tubes to which 250 μl of water were added. Polar metabolites were extracted with 1080 μl of chloroform:heptane (3:1, v/v) by 2 × 10-s vortexing followed by 10-min incubation on ice and 15-min centrifugation at 4 °C at 12,500 × g. From the upper phase, 600 μl were collected without carrying out any interface material and transferred into new cold 2-ml polypropylene tubes. These tubes were centrifuged again, and 400 μl of supernatant were collected into cold 1.5-ml polypropylene tubes. Samples were frozen in liquid nitrogen and dried in two steps: first, in a SpeedVac concentrator for ∼2 h (Savant; maximal vacuum, no heat) at 4 °C to remove most of the methanol; second, by lyophilization for 90 min (FreeZone, Labconco, Kansas City, MO), and then stored at −80 °C until used. Samples were reconstituted in 14 μl of Milli-Q water, and injections of 3 μl were performed in duplicate on an LC-electrospray ionization-MS/MS system composed of an Agilent 1200 SL (93) and a triple-quadrupole mass spectrometer (4000Q TRAP MS/MS, Sciex). Samples were separated by gradient elution for 12 min on a Poroshell 120 EC-C18, 2.1 × 75-mm, 2.7-μm column (Agilent Technologies) using a mobile phase consisting of an aqueous solvent A (10 mm tributylamine, 15 mm acetic acid, pH 5.20) and an organic solvent B (95% acetonitrile in water, 0.1% formic acid) at a flow rate of 0.75 ml/min. Column oven temperature during the separation was maintained at 40 °C. The MS system was operated in negative electrospray ionization mode using a turbo ion spray source. Transitions used and quantifications were described previously (37). Peak areas were used for relative quantification of identified metabolites.
Calculation of carbon equivalents in metabolite determination studies
To provide an estimate of the fate of glucose carbons upon increasing concentrations of glucose (Fig. 3, C and D), carbon equivalents for the analyzed metabolites were determined by taking into account the carbon content of each metabolite. Briefly, the carbon equivalent for lower glycolysis was calculated by multiplying the nmol/mg of protein/h glucose used by 6, corresponding to the number of carbons found in glucose. Glycerol has three carbons; thus we multiplied the nmol of glycerol released by 3. For FFA, the palmitate (16 carbons) was taken as a benchmark; thus we multiplied the nmol of FFA content and released by 16. For TG, we considered tripalmitin as the reference molecule with three palmitates providing 48 carbons (16 carbons × 3) and then added three carbons for the glycerol backbone for a total of 51 carbons. Glycogen content is expressed as nmol of glucose, and therefore we multiplied the nmol of glucose produced by 6. Finally, for cholesterol contents, we considered 27 carbons, and for cholesterol esters we considered 45 carbons, corresponding to the esterification of an oleate (18 carbons) onto cholesterol. The carbon equivalent values for total cholesterol content correspond to the addition of carbon equivalents of free cholesterol and cholesterol esters. Upper glycolysis corresponds to the addition of carbon equivalents of lower glycolysis and glycerol. Calculated glycolysis corresponds to the addition of carbon equivalents of upper glycolysis and glycogen (see also “Calculation of carbon equivalents in metabolite determination studies” under “Results”).
Author contributions
M. P., Y. M., E. J., and S. R. M. M. conceived and designed the experiments. Y. M., S. Z., J. L., and A. A.-M. performed the experiments. Y. M., M.-L. P., E. J., S. R. M. M., and M. P. analyzed the data. Y. M., E. J., S. R. M. M., B. E. C., and M. P. wrote the paper.
Supplementary Material
Acknowledgments
We thank the Metabolomics and Cellular Physiology Core Facilities of the Centre de Recherche du Centre Hospitalier de l'Université de Montréal and the Montreal Diabetes Research Center for performing the metabolite and insulin determinations.
This work was supported in part by grants from the Canadian Institutes of Health Research (to M. P. and S. R. M. M.) and fellowships from Fonds de Recherche du Québec-Santé, the department of Nutrition of Université de Montréal, and Diabète Québec (to Y. M.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains supplemental Fig. 1.
- KATP
- ATP-sensitive K+
- GSIS
- glucose-stimulated insulin secretion
- FFA
- free fatty acid
- GL
- glycerolipid
- MCF
- metabolic coupling factor
- SUMO
- small ubiquitin-like modifier
- Gro3P
- glycerol 3-phosphate
- ROS
- reactive oxygen species
- ETC
- electron transport chain
- DHAP
- dihydroxyacetone phosphate
- CE
- cholesterol esters
- ABC
- ATP-binding cassette transporter
- KRBH
- Krebs-Ringer buffer-HEPES
- HE
- hydroethidine.
References
- 1. Prentki M., Matschinsky F. M., and Madiraju S. R. (2013) Metabolic signaling in fuel-induced insulin secretion. Cell Metab. 18, 162–185 [DOI] [PubMed] [Google Scholar]
- 2. Henquin J. C. (2011) The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res. Clin. Pract. 93, Suppl. 1, S27–S31 [DOI] [PubMed] [Google Scholar]
- 3. Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P. 4th, Boyd A. E. 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., and Nelson D. A. (1995) Cloning of the β cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science 268, 423–426 [DOI] [PubMed] [Google Scholar]
- 4. Ashcroft F. M., Harrison D. E., and Ashcroft S. J. (1984) Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature 312, 446–448 [DOI] [PubMed] [Google Scholar]
- 5. Straub S. G., and Sharp G. W. (2002) Glucose-stimulated signaling pathways in biphasic insulin secretion. Diabetes Metab. Res. Rev. 18, 451–463 [DOI] [PubMed] [Google Scholar]
- 6. Ashcroft F. M. (1991) Ca2+ channels and excitation-contraction coupling. Curr. Opin. Cell Biol. 3, 671–675 [DOI] [PubMed] [Google Scholar]
- 7. Henquin J. C., Nenquin M., Ravier M. A., and Szollosi A. (2009) Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes. Metab. 11, Suppl. 4, 168–179 [DOI] [PubMed] [Google Scholar]
- 8. Jitrapakdee S., Wutthisathapornchai A., Wallace J. C., and MacDonald M. J. (2010) Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53, 1019–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muoio D. M., and Newgard C. B. (2008) Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 193–205 [DOI] [PubMed] [Google Scholar]
- 10. Prentki M., and Madiraju S. R. (2008) Glycerolipid metabolism and signaling in health and disease. Endocr. Rev. 29, 647–676 [DOI] [PubMed] [Google Scholar]
- 11. Zhao S., Mugabo Y., Iglesias J., Xie L., Delghingaro-Augusto V., Lussier R., Peyot M. L., Joly E., Taïb B., Davis M. A., Brown J. M., Abousalham A., Gaisano H., Madiraju S. R., and Prentki M. (2014) α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 19, 993–1007 [DOI] [PubMed] [Google Scholar]
- 12. Zhao S., Poursharifi P., Mugabo Y., Levens E. J., Vivot K., Attane C., Iglesias J., Peyot M. L., Joly E., Madiraju S. R., and Prentki M. (2015) α/β-Hydrolase domain-6 and saturated long chain monoacylglycerol regulate insulin secretion promoted by both fuel and non-fuel stimuli. Mol. Metab 4, 940–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pearson G. L., Mellett N., Chu K. Y., Boslem E., Meikle P. J., and Biden T. J. (2016) A comprehensive lipidomic screen of pancreatic β-cells using mass spectroscopy defines novel features of glucose-stimulated turnover of neutral lipids, sphingolipids and plasmalogens. Mol Metab. 5, 404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Corkey B. E., Glennon M. C., Chen K. S., Deeney J. T., Matschinsky F. M., and Prentki M. (1989) A role for malonyl-CoA in glucose-stimulated insulin secretion from clonal pancreatic β-cells. J. Biol. Chem. 264, 21608–21612 [PubMed] [Google Scholar]
- 15. Prentki M., Vischer S., Glennon M. C., Regazzi R., Deeney J. T., and Corkey B. E. (1992) Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. J. Biol. Chem. 267, 5802–5810 [PubMed] [Google Scholar]
- 16. Maechler P., and Wollheim C. B. (1999) Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 402, 685–689 [DOI] [PubMed] [Google Scholar]
- 17. Kibbey R. G., Pongratz R. L., Romanelli A. J., Wollheim C. B., Cline G. W., and Shulman G. I. (2007) Mitochondrial GTP regulates glucose-stimulated insulin secretion. Cell Metab. 5, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leloup C., Tourrel-Cuzin C., Magnan C., Karaca M., Castel J., Carneiro L., Colombani A. L., Ktorza A., Casteilla L., and Pénicaud L. (2009) Mitochondrial reactive oxygen species are obligatory signals for glucose-induced insulin secretion. Diabetes 58, 673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pi J., Bai Y., Zhang Q., Wong V., Floering L. M., Daniel K., Reece J. M., Deeney J. T., Andersen M. E., Corkey B. E., and Collins S. (2007) Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56, 1783–1791 [DOI] [PubMed] [Google Scholar]
- 20. MacDonald M. J. (1995) Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 270, 20051–20058 [PubMed] [Google Scholar]
- 21. Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in 't Veld P., Renström E., and Schuit F. C. (2005) Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes 54, 2132–2142 [DOI] [PubMed] [Google Scholar]
- 22. Ferdaoussi M., and MacDonald P. E. (2017) Toward connecting metabolism to the exocytotic site. Trends Cell Biol. 27, 163–171 [DOI] [PubMed] [Google Scholar]
- 23. El-Assaad W., Buteau J., Peyot M. L., Nolan C., Roduit R., Hardy S., Joly E., Dbaibo G., Rosenberg L., and Prentki M. (2003) Saturated fatty acids synergize with elevated glucose to cause pancreatic β-cell death. Endocrinology 144, 4154–4163 [DOI] [PubMed] [Google Scholar]
- 24. Bensellam M., Laybutt D. R., and Jonas J. C. (2012) The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol. Cell. Endocrinol. 364, 1–27 [DOI] [PubMed] [Google Scholar]
- 25. Unger R. H., and Zhou Y. T. (2001) Lipotoxicity of β-cells in obesity and in other causes of fatty acid spillover. Diabetes 50, Suppl. 1, S118–S121 [DOI] [PubMed] [Google Scholar]
- 26. Poitout V., Amyot J., Semache M., Zarrouki B., Hagman D., and Fontés G. (2010) Glucolipotoxicity of the pancreatic β cell. Biochim. Biophys. Acta 1801, 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prentki M., and Corkey B. E. (1996) Are the β-cell signaling molecules malonyl-CoA and cystolic long-chain acyl-CoA implicated in multiple tissue defects of obesity and NIDDM? Diabetes 45, 273–283 [DOI] [PubMed] [Google Scholar]
- 28. Kraegen E. W., Cooney G. J., Ye J. M., Thompson A. L., and Furler S. M. (2001) The role of lipids in the pathogenesis of muscle insulin resistance and β cell failure in type II diabetes and obesity. Exp. Clin. Endocrinol. Diabetes 109, Suppl. 2, S189–S201 [DOI] [PubMed] [Google Scholar]
- 29. Krebs M., and Roden M. (2004) Nutrient-induced insulin resistance in human skeletal muscle. Curr. Med. Chem. 11, 901–908 [DOI] [PubMed] [Google Scholar]
- 30. Tremblay F., Lavigne C., Jacques H., and Marette A. (2007) Role of dietary proteins and amino acids in the pathogenesis of insulin resistance. Annu. Rev. Nutr. 27, 293–310 [DOI] [PubMed] [Google Scholar]
- 31. Nolan C. J., Ruderman N. B., and Prentki M. (2013) Intensive insulin for type 2 diabetes: the risk of causing harm. Lancet Diabetes Endocrinol. 1, 9–10 [DOI] [PubMed] [Google Scholar]
- 32. Nolan C. J., Ruderman N. B., Kahn S. E., Pedersen O., and Prentki M. (2015) Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 64, 673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nolan C. J., and Prentki M. (2008) The islet β-cell: fuel responsive and vulnerable. Trends Endocrinol. Metab. 19, 285–291 [DOI] [PubMed] [Google Scholar]
- 34. Nolan C. J., Madiraju M. S., Delghingaro-Augusto V., Peyot M. L., and Prentki M. (2006) Fatty acid signaling in the β-cell and insulin secretion. Diabetes 55, Suppl. 2, S16–S23 [DOI] [PubMed] [Google Scholar]
- 35. Nolan C. J., Leahy J. L., Delghingaro-Augusto V., Moibi J., Soni K., Peyot M. L., Fortier M., Guay C., Lamontagne J., Barbeau A., Przybytkowski E., Joly E., Masiello P., Wang S., Mitchell G. A., and Prentki M. (2006) β cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia 49, 2120–2130 [DOI] [PubMed] [Google Scholar]
- 36. Cnop M., Hannaert J. C., Hoorens A., Eizirik D. L., and Pipeleers D. G. (2001) Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes 50, 1771–1777 [DOI] [PubMed] [Google Scholar]
- 37. Guay C., Joly E., Pepin E., Barbeau A., Hentsch L., Pineda M., Madiraju S. R., Brunengraber H., and Prentki M. (2013) A role for cytosolic isocitrate dehydrogenase as a negative regulator of glucose signaling for insulin secretion in pancreatic ss-cells. PLoS One 8, e77097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasan N. M., Longacre M. J., Seed Ahmed M., Kendrick M. A., Gu H., Ostenson C. G., Fukao T., and MacDonald M. J. (2010) Lower succinyl-CoA:3-ketoacid-CoA transferase (SCOT) and ATP citrate lyase in pancreatic islets of a rat model of type 2 diabetes: knockdown of SCOT inhibits insulin release in rat insulinoma cells. Arch. Biochem. Biophys. 499, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spégel P., Malmgren S., Sharoyko V. V., Newsholme P., Koeck T., and Mulder H. (2011) Metabolomic analyses reveal profound differences in glycolytic and tricarboxylic acid cycle metabolism in glucose-responsive and -unresponsive clonal β-cell lines. Biochem. J. 435, 277–284 [DOI] [PubMed] [Google Scholar]
- 40. Gooding J. R., Jensen M. V., and Newgard C. B. (2016) Metabolomics applied to the pancreatic islet. Arch. Biochem. Biophys. 589, 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicholls D. G. (2016) The pancreatic β-cell: a bioenergetic perspective. Physiol. Rev. 96, 1385–1447 [DOI] [PubMed] [Google Scholar]
- 42. Mugabo Y., Zhao S., Seifried A., Gezzar S., Al-Mass A., Zhang D., Lamontagne J., Attane C., Poursharifi P., Iglesias J., Joly E., Peyot M. L., Gohla A., Madiraju S. R., and Prentki M. (2016) Identification of a mammalian glycerol-3-phosphate phosphatase: role in metabolism and signaling in pancreatic β-cells and hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 113, E430–E439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Prentki M., and Madiraju S. R. (2012) Glycerolipid/free fatty acid cycle and islet β-cell function in health, obesity and diabetes. Mol. Cell. Endocrinol. 353, 88–100 [DOI] [PubMed] [Google Scholar]
- 44. Prentki M., and Nolan C. J. (2006) Islet β cell failure in type 2 diabetes. J. Clin. Investig. 116, 1802–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gehrmann W., Elsner M., and Lenzen S. (2010) Role of metabolically generated reactive oxygen species for lipotoxicity in pancreatic β-cells. Diabetes Obes. Metab. 12, Suppl. 2, 149–158 [DOI] [PubMed] [Google Scholar]
- 46. Maechler P., Li N., Casimir M., Vetterli L., Frigerio F., and Brun T. (2010) Role of mitochondria in β-cell function and dysfunction. Adv. Exp. Med. Biol. 654, 193–216 [DOI] [PubMed] [Google Scholar]
- 47. Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., and Newgard C. B. (2000) Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes 49, 424–430 [DOI] [PubMed] [Google Scholar]
- 48. Lorenz M. A., El Azzouny M. A., Kennedy R. T., and Burant C. F. (2013) Metabolome response to glucose in the β-cell line INS-1 832/13. J. Biol. Chem. 288, 10923–10935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Huang M., and Joseph J. W. (2012) Metabolomic analysis of pancreatic β-cell insulin release in response to glucose. Islets 4, 210–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pepin É., Al-Mass A., Attané C., Zhang K., Lamontagne J., Lussier R., Madiraju S. R., Joly E., Ruderman N. B., Sladek R., Prentki M., and Peyot M. L. (2016) Pancreatic β-cell dysfunction in diet-induced obese mice: roles of AMP-kinase, protein kinase Cϵ, mitochondrial and cholesterol metabolism, and alterations in gene expression. PLoS One 11, e0153017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferdaoussi M., Dai X., Jensen M. V., Wang R., Peterson B. S., Huang C., Ilkayeva O., Smith N., Miller N., Hajmrle C., Spigelman A. F., Wright R. C., Plummer G., Suzuki K., Mackay J. P., et al. (2015) Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J. Clin. Investig. 125, 3847–3860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Matschinsky F. M., Ghosh A. K., Meglasson M. D., Prentki M., June V., and von Allman D. (1986) Metabolic concomitants in pure, pancreatic β cells during glucose-stimulated insulin secretion. J. Biol. Chem. 261, 14057–14061 [PubMed] [Google Scholar]
- 53. Fridlyand L. E., Ma L., and Philipson L. H. (2005) Adenine nucleotide regulation in pancreatic β-cells: modeling of ATP/ADP-Ca2+ interactions. Am. J. Physiol. Endocrinol. Metab. 289, E839–E848 [DOI] [PubMed] [Google Scholar]
- 54. Zhou Y. P., Ling Z. C., and Grill V. E. (1996) Inhibitory effects of fatty acids on glucose-regulated B-cell function: association with increased islet triglyceride stores and altered effect of fatty acid oxidation on glucose metabolism. Metabolism 45, 981–986 [DOI] [PubMed] [Google Scholar]
- 55. MacDonald M. J., Dobrzyn A., Ntambi J., and Stoker S. W. (2008) The role of rapid lipogenesis in insulin secretion: insulin secretagogues acutely alter lipid composition of INS-1 832/13 cells. Arch. Biochem. Biophys. 470, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martins E. F., Miyasaka C. K., Newsholme P., Curi R., and Carpinelli A. R. (2004) Changes of fatty acid composition in incubated rat pancreatic islets. Diabetes Metab. 30, 21–27 [DOI] [PubMed] [Google Scholar]
- 57. Xue J. H., Yuan Z., Wu Y., Liu Y., Zhao Y., Zhang W. P., Tian Y. L., Liu W. M., Liu Y., and Kishimoto C. (2010) High glucose promotes intracellular lipid accumulation in vascular smooth muscle cells by impairing cholesterol influx and efflux balance. Cardiovasc. Res. 86, 141–150 [DOI] [PubMed] [Google Scholar]
- 58. Sandberg M. B., Fridriksson J., Madsen L., Rishi V., Vinson C., Holmsen H., Berge R. K., and Mandrup S. (2005) Glucose-induced lipogenesis in pancreatic β-cells is dependent on SREBP-1. Mol. Cell. Endocrinol. 240, 94–106 [DOI] [PubMed] [Google Scholar]
- 59. Brun T., Roche E., Assimacopoulos-Jeannet F., Corkey B. E., Kim K. H., and Prentki M. (1996) Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic β-cell nutrient signaling. Diabetes 45, 190–198 [DOI] [PubMed] [Google Scholar]
- 60. Kellner-Weibel G., Jerome W. G., Small D. M., Warner G. J., Stoltenborg J. K., Kearney M. A., Corjay M. H., Phillips M. C., and Rothblat G. H. (1998) Effects of intracellular free cholesterol accumulation on macrophage viability: a model for foam cell death. Arterioscler. Thromb. Vasc. Biol. 18, 423–431 [DOI] [PubMed] [Google Scholar]
- 61. Lu X., Liu J., Hou F., Liu Z., Cao X., Seo H., and Gao B. (2011) Cholesterol induces pancreatic β cell apoptosis through oxidative stress pathway. Cell Stress Chaperones 16, 539–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yokoyama S. (2000) Release of cellular cholesterol: molecular mechanism for cholesterol homeostasis in cells and in the body. Biochim. Biophys. Acta 1529, 231–244 [DOI] [PubMed] [Google Scholar]
- 63. Brunham L. R., Kruit J. K., Verchere C. B., and Hayden M. R. (2008) Cholesterol in islet dysfunction and type 2 diabetes. J. Clin. Investig. 118, 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kruit J. K., Wijesekara N., Westwell-Roper C., Vanmierlo T., de Haan W., Bhattacharjee A., Tang R., Wellington C. L., LütJohann D., Johnson J. D., Brunham L. R., Verchere C. B., and Hayden M. R. (2012) Loss of both ABCA1 and ABCG1 results in increased disturbances in islet sterol homeostasis, inflammation, and impaired β-cell function. Diabetes 61, 659–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Peyot M. L., Pepin E., Lamontagne J., Latour M. G., Zarrouki B., Lussier R., Pineda M., Jetton T. L., Madiraju S. R., Joly E., and Prentki M. (2010) β-Cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced β-cell mass. Diabetes 59, 2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brereton M. F., Rohm M., Shimomura K., Holland C., Tornovsky-Babeay S., Dadon D., Iberl M., Chibalina M. V., Lee S., Glaser B., Dor Y., Rorsman P., Clark A., and Ashcroft F. M. (2016) Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat. Commun. 7, 13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lenzen S. (2014) A fresh view of glycolysis and glucokinase regulation: history and current status. J. Biol. Chem. 289, 12189–12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Freeman H., Shimomura K., Horner E., Cox R. D., and Ashcroft F. M. (2006) Nicotinamide nucleotide transhydrogenase: a key role in insulin secretion. Cell Metab. 3, 35–45 [DOI] [PubMed] [Google Scholar]
- 69. Wong N., Blair A. R., Morahan G., and Andrikopoulos S. (2010) The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology 151, 96–102 [DOI] [PubMed] [Google Scholar]
- 70. Llanos P., Contreras-Ferrat A., Barrientos G., Valencia M., Mears D., and Hidalgo C. (2015) Glucose-dependent insulin secretion in pancreatic β-cell islets from male rats requires Ca2+ release via ROS-stimulated ryanodine receptors. PLoS One 10, e0129238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kim W. H., Lee J. W., Suh Y. H., Lee H. J., Lee S. H., Oh Y. K., Gao B., and Jung M. H. (2007) AICAR potentiates ROS production induced by chronic high glucose: roles of AMPK in pancreatic β-cell apoptosis. Cell. Signal. 19, 791–805 [DOI] [PubMed] [Google Scholar]
- 72. Sarre A., Gabrielli J., Vial G., Leverve X. M., and Assimacopoulos-Jeannet F. (2012) Reactive oxygen species are produced at low glucose and contribute to the activation of AMPK in insulin-secreting cells. Free Radic. Biol. Med. 52, 142–150 [DOI] [PubMed] [Google Scholar]
- 73. Jensen M. V., Joseph J. W., Ronnebaum S. M., Burgess S. C., Sherry A. D., and Newgard C. B. (2008) Metabolic cycling in control of glucose-stimulated insulin secretion. Am. J. Physiol. Endocrinol. Metab. 295, E1287–E1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Roduit R., Nolan C., Alarcon C., Moore P., Barbeau A., Delghingaro-Augusto V., Przybykowski E., Morin J., Massé F., Massie B., Ruderman N., Rhodes C., Poitout V., and Prentki M. (2004) A role for the malonyl-CoA/long-chain acyl-CoA pathway of lipid signaling in the regulation of insulin secretion in response to both fuel and nonfuel stimuli. Diabetes 53, 1007–1019 [DOI] [PubMed] [Google Scholar]
- 75. Schuit F., De Vos A., Farfari S., Moens K., Pipeleers D., Brun T., and Prentki M. (1997) Metabolic fate of glucose in purified islet cells. Glucose-regulated anaplerosis in β cells. J. Biol. Chem. 272, 18572–18579 [DOI] [PubMed] [Google Scholar]
- 76. Yang L., Kombu R. S., Kasumov T., Zhu S. H., Cendrowski A. V., David F., Anderson V. E., Kelleher J. K., and Brunengraber H. (2008) Metabolomic and mass isotopomer analysis of liver gluconeogenesis and citric acid cycle. I. Interrelation between gluconeogenesis and cataplerosis; formation of methoxamates from aminooxyacetate and ketoacids. J. Biol. Chem. 283, 21978–21987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Farfari S., Schulz V., Corkey B., and Prentki M. (2000) Glucose-regulated anaplerosis and cataplerosis in pancreatic β-cells: possible implication of a pyruvate/citrate shuttle in insulin secretion. Diabetes 49, 718–726 [DOI] [PubMed] [Google Scholar]
- 78. Sekine N., Cirulli V., Regazzi R., Brown L. J., Gine E., Tamarit-Rodriguez J., Girotti M., Marie S., MacDonald M. J., Wollheim C. B., and Rutter G. A. (1994) Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic β-cells. Potential role in nutrient sensing. J. Biol. Chem. 269, 4895–4902 [PubMed] [Google Scholar]
- 79. Simpson N. E., Khokhlova N., Oca-Cossio J. A., and Constantinidis I. (2006) Insights into the role of anaplerosis in insulin secretion: a 13C NMR study. Diabetologia 49, 1338–1348 [DOI] [PubMed] [Google Scholar]
- 80. Maechler P., and Wollheim C. B. (2001) Mitochondrial function in normal and diabetic β-cells. Nature 414, 807–812 [DOI] [PubMed] [Google Scholar]
- 81. MacDonald M. J., Fahien L. A., Brown L. J., Hasan N. M., Buss J. D., and Kendrick M. A. (2005) Perspective: emerging evidence for signaling roles of mitochondrial anaplerotic products in insulin secretion. Am. J. Physiol. Endocrinol. Metab. 288, E1–E15 [DOI] [PubMed] [Google Scholar]
- 82. Reinbothe T. M., Ivarsson R., Li D. Q., Niazi O., Jing X., Zhang E., Stenson L., Bryborn U., and Renström E. (2009) Glutaredoxin-1 mediates NADPH-dependent stimulation of calcium-dependent insulin secretion. Mol. Endocrinol. 23, 893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Drews G., Krippeit-Drews P., and Düfer M. (2010) Electrophysiology of islet cells. Adv. Exp. Med. Biol. 654, 115–163 [DOI] [PubMed] [Google Scholar]
- 84. Proks P., de Wet H., and Ashcroft F. M. (2010) Activation of the KATP channel by Mg-nucleotide interaction with SUR1. J. Gen. Physiol. 136, 389–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lamontagne J., Pepin E., Peyot M. L., Joly E., Ruderman N. B., Poitout V., Madiraju S. R., Nolan C. J., and Prentki M. (2009) Pioglitazone acutely reduces insulin secretion and causes metabolic deceleration of the pancreatic β-cell at submaximal glucose concentrations. Endocrinology 150, 3465–3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bradley D. C., and Kaslow H. R. (1989) Radiometric assays for glycerol, glucose, and glycogen. Anal. Biochem. 180, 11–16 [DOI] [PubMed] [Google Scholar]
- 87. Püttmann M., Krug H., von Ochsenstein E., and Kattermann R. (1993) Fast HPLC determination of serum free fatty acids in the picomole range. Clin. Chem. 39, 825–832 [PubMed] [Google Scholar]
- 88. Massa M. L., Borelli M. I., Del Zotto H., and Gagliardino J. J. (2001) Changes induced by sucrose administration on glucose metabolism in pancreatic islets in normal hamsters. J. Endocrinol. 171, 551–556 [DOI] [PubMed] [Google Scholar]
- 89. Peyot M. L., Guay C., Latour M. G., Lamontagne J., Lussier R., Pineda M., Ruderman N. B., Haemmerle G., Zechner R., Joly E., Madiraju S. R., Poitout V., and Prentki M. (2009) Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. J. Biol. Chem. 284, 16848–16859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wikstrom J. D., Sereda S. B., Stiles L., Elorza A., Allister E. M., Neilson A., Ferrick D. A., Wheeler M. B., and Shirihai O. S. (2012) A novel high-throughput assay for islet respiration reveals uncoupling of rodent and human islets. PLoS One 7, e33023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Castedo M., Ferri K., Roumier T., Métivier D., Zamzami N., and Kroemer G. (2002) Quantitation of mitochondrial alterations associated with apoptosis. J. Immunol. Methods 265, 39–47 [DOI] [PubMed] [Google Scholar]
- 92. Rothe G., and Valet G. (1990) Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J. Leukoc. Biol. 47, 440–448 [PubMed] [Google Scholar]
- 93. Rancoule C., Attané C., Grès S., Fournel A., Dusaulcy R., Bertrand C., Vinel C., Tréguer K., Prentki M., Valet P., and Saulnier-Blache J. S. (2013) Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia 56, 1394–1402 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.