Abstract
Each year approximately 13,000 Veterans transition to maintenance dialysis, mostly in the traditional form of thrice-weekly hemodialysis from the start. Among >6,000 dialysis units nationwide, there are currently approximately 70 Veterans Affairs (VA) dialysis centers. Given this number of VA dialysis centers and their limited capacity, only 10% of all incident dialysis Veterans initiate treatment in a VA center. Evidence suggests that, among Veterans, receipt of care within the VA system is associated with favorable outcomes, potentially due to enhanced access to healthcare resources. Data from the United States Renal Data System Special Study Center “Transition-of-Care-in-CKD” suggest that Veterans who receive dialysis in a VA unit exhibit greater survival compared to non-VA centers. Substantial financial expenditures arise from the high volume of outsourced care and higher dialysis reimbursement paid by the VA than by Medicare to outsourced providers. Given the exceedingly high mortality and abrupt decline in residual kidney function (RKF) in the first dialysis year, it is possible that incremental transition to dialysis through an initial twice-weekly hemodialysis regimen preserves RKF, prolongs vascular access longevity, improves patients’ quality of life, and is a more patient-centered approach and consistent with “personalized” dialysis. Broad implementation of incremental dialysis may also result in more Veterans receiving care within a VA dialysis unit. Controlled trials are urgently needed to examine safety and efficacy of incremental hemodialysis in Veterans and other populations, and the administrative and health care as well as provider structure within the VA system would facilitate the performance of such trials.
Keywords: Veterans, residual kidney function, twice-weekly hemodialysis, survival, end-stage renal disease
Veterans Heath Care in the United States
The term “Veterans” typically refers to persons who previously served in the armed services. According to the United States (US) government, a Veteran is defined as a person who served in the active military (army, naval, marine corps, air service, or coast guard) and who was discharged or released under conditions other than those that were dishonorable.1 In the US, members of the National Guard and Reserve may also qualify as Veterans.1 Whereas approximately 90% of the US Veteran population is presently male, it is estimated that in the next decade the proportion of females will rise to 18 to 20%.2, 3 Minority Veterans currently comprise about 22% of the overall Veterans’ population, among whom the majority are of Black or African American race (12% of all Veterans) and Hispanic or Latino ethnicity (7% of all veterans).4, 5
While most Veterans are healthy upon joining the armed services due to recruitment requirements, some have subsequently been exposed to military or occupational factors, including but not limited to participation in combat operations, that later affect their physical and mental health with enduring consequences. For instance, Veterans are reported to have a higher rate of post-traumatic stress disorder than the general population.6 In order to receive federal health care benefits from the Veterans Health Administration (VHA) of the Department of Veterans Affairs (VA) including the VA Healthcare System and VA medical centers, Veterans must have served a minimum pre-defined period of time and have met other qualifications. Services provided by the VHA facilities, which is the nation’s largest integrated health care delivery system,7 include comprehensive medical care, life insurance, disability compensation, home loans, educational benefits, pensions, and vocational rehabilitation training. In the US, there are more than 20 million living Veterans including nine million enrolled in the VHA, among whom roughly six million Veterans receive healthcare in the VA Healthcare System.8–10
The VHA facility network consists of approximately 150 medical centers, 820 community-based outpatient clinics, 300 Veterans’ centers, and 71 dialysis centers. These dialysis centers serve Veterans with advanced chronic kidney disease (CKD) whose status is deemed as “end-stage renal disease” (ESRD).5
Veterans with Advanced CKD and ESRD
Each year 12,000 to 14,000 Veterans with advanced CKD, i.e., estimated glomerular filtration rate (eGFR) <25 ml/min/1.73m2, transition to ESRD to receive renal replacement therapy, mostly in the form of maintenance dialysis treatment (see Table 1).9 Hence, Veterans comprise 11% of the nation’s incident dialysis population, given that 110,000 to 120,000 persons initiate maintenance dialysis treatment each year in the US.9, 10 Out of 450,000 US Americans who currently undergo maintenance dialysis treatment, approximately 35,000 or more are Veterans, reflecting a higher ESRD prevalence among Veterans than in the general US population (604 vs. 187 per 100,000, respectively).11 The majority of these patients undergo thrice-weekly hemodialysis from Day 1 of treatment. The vast majority of VA dialysis centers are based in VA hospitals, with the exception of a few off-campus dialysis units that have recently been established.12 Hence, the national VA dialysis system can be considered a quasi “medium dialysis organization” or chain with a somewhat more homogeneous practice pattern. Most of the VA hospital-based dialysis units provide both maintenance outpatient and acute inpatient dialysis treatments in the same location simultaneously, including dialysis treatment for Veterans with ESRD who are admitted to the hospital as well as those with acute kidney injury (AKI) requiring renal replacement therapy. To that end, a VA dialysis center is unique, as compared to other non-VA dialysis clinics where, until December 2016, inpatient dialysis treatments might not be performed according to the regulations governed by the Center for Medicare and Medicaid Services (CMS).13
Table 1.
Incidence and rates of end-stage renal disease (ESRD) among United States (US) Veterans, from 2008 to 2013, with comparison to the entire US population. Note that the denominators may not be commensurate given the older age of Veterans. Adopted from the United States Renal Data System 2015/2016 Annual Data Report.9
| Calendar Year | 2008 | 2009 | 2010 | 2011 | 2012 |
|---|---|---|---|---|---|
| Veterans with incident ESRD | 13,529 | 13,842 | 13,448 | 12,755 | 12,869 |
| All Veterans | 22,424,712 | 21,854,374 | 21,798,077 | 21,458,427 | 21,230,865 |
| Incidence rate of ESRD in Veterans, PM | 2,760 | 2,874 | 2,792 | 2,680 | 2,716 |
|
| |||||
| Incident ESRD in US | 112,483 | 116,178 | 116,580 | 114,613 | 116,615 |
| Adult US Population | 230,478,491 | 232,666,095 | 233,971,134 | 236,462,244 | 238,925,961 |
| ESRD incidence rate in the US, PM | 3,992 | 4,036 | 3,992 | 3,826 | 3,772 |
The VHA provides comprehensive medical care for patients with kidney disease, including all stages of CKD as well as AKI. Any enrolled Veteran who develops ESRD is eligible to receive renal replacement therapy from the VHA. Dialysis care is a covered benefit under VA’s Medical Benefits Package for Veterans enrolled in the VA, irrespective of their service connectedness.13 For patients requiring in-center dialysis treatment, the VHA provides dialysis both through units maintained and operated by individual VA facilities (hence usually hospital based dialysis centers), or by outsourcing dialysis services to private dialysis providers. This may happen in cases where the distance from a VA facility is prohibitive for thrice-weekly dialysis therapy, when there is a lack of home dialysis resources or expertise, or when the capacity of the VA facility-operated dialysis unit is exceeded.13
Given the relatively small number of VA-based dialysis centers in the nation and their limited capacity (each usually has only 10 to 20 dialysis stations), only 10% of all Veterans with advanced CKD initiate dialysis treatment in a VA dialysis center, while 90% are receive care in outside (non-VA) dialysis units, some under a subcontracted system. Recent data including reports from the United States Renal Data System (USRDS) (see below) suggest that Veterans who receive care within a VA dialysis center exhibit greater survival than those in non-VA dialysis units.9, 10 Furthermore, substantial financial expenditures arise for outsourcing Veterans’ dialysis treatments in non-VA dialysis centers given higher dialysis reimbursement paid by the VA than by CMS to non-VA dialysis providers.11 Hence, there are compelling reasons to explore effective ways to expand access to and capacity within VA dialysis programs to permit more Veterans with ESRD to receive care within the VA healthcare system.
Transition of Veterans to Renal Replacement Therapy
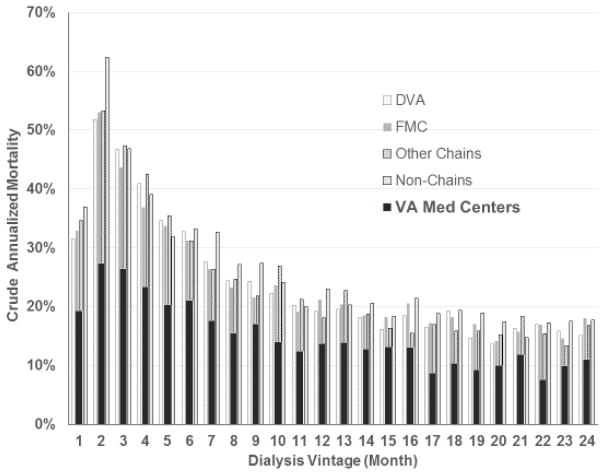
In 2014 a new USRDS Special Study Center under the designation “Transition of Care in CKD” (TC-CKD) was created to focus on examining patients who transition to renal replacement therapy, with special attention to risk factors in the pre-dialysis period. Among others, the TC-CKD Special Study Center has examined and annually reported data on all Veterans who have transitioned to ESRD starting from the fiscal year 2007 and onwards. The initial TC-CKD reports included 52,172 Veterans who transitioned to ESRD over a four-year period (10/2007–9/2011) (Table 2).10 Within this cohort, 83% and 78% were assigned to in-center hemodialysis on Day 1 and Day 90 of treatment, respectively, while those receiving home hemodialysis remained at only 0.5%. Peritoneal dialysis comprised 5% of the modalities. A total of 589 Veterans (1.1%) received pre-emptive kidney transplantation. Most notably, however, 5,348 or 10% of Veterans died in the first 90 days of dialysis treatment, translating into an equivalent annualized mortality of 41% in the first three months, while 1,798 (3.5%) regained kidney function and stopped dialysis treatment, likely after resolution of presumed AKI (see Table 2). The high early dialysis mortality is also shown in Figure 1 and discussed further (see below).10
Table 2.
Renal replacement therapy modalities on Days 1, 30, 60 and 90 of end-stage renal disease (ESRD) in 52,172 Veterans who transitioned to ESRD over a four-year period (from 10/1/207 through 9/30/2011). Adopted from the United States Renal Data System 2014/2015 Annual Data Report.10
| Dialysis Modality | Day 1 | Day 30 | Day 60 | Day 90 |
|---|---|---|---|---|
| In-Center HD | 43,242 (83%) | 43,244 (83%) | 43,149 (83%) | 40,905 (78%) |
| Home HD | 260 (0.5%) | 260 (0.5%) | 259 (0.5%) | 258 (0.5%) |
| CAPD | 1,405 (2.7%) | 1,405 (2.7%) | 1,398 (2.7%) | 1,302 (2.5%) |
| CCPD | 1,165 (2.2%) | 1,165 (2.2%) | 1,173 (2.3%) | 1,387 (2.7%) |
| Other PD | 9 (<0.1$) | 9 (<0.5%) | 9 (<0.1%) | 8 (<0.1%) |
| Uncertain modality | 5,301 (10%) | 3,509 (6.7%) | 626 (1.2%) | 460 (0.9%) |
| Death | 201 (0.4%) | 1,561 (2.9%) | 3,672 (7.1%) | 5,348 (10.3%) |
| Transplant | 589 (1.1%) | 654 (1.3%) | 679 (1.3%) | 701 (1.3%) |
| Loss to follow-up | n/a | 3 (<0.1%) | 3 (<0.1%) | 5 (<0.1%) |
| Recovery of kidney function | n/a | 362 (0.7%) | 1,204 (2.3%) | 1,798 (3.5%) |
|
| ||||
| Total N | 52,172 | 52,172 | 52,172 | 52,172 |
Abbreviations:
HD: hemodialysis, PD; peritoneal dialysis; CAPD, chronic ambulatory peritoneal dialysis; CCPD: automated (chronic cyclic) peritoneal dialysis
Figure 1.
High mortality rate during the first 12 months of hemodialysis therapy in 52,000 incident ESRD Veterans across all states and territories of the United States over four years, i.e., 10/1/2007–9/30/2011 (adapted from the Transition of Care in Chronic Kidney Disease Chapter of the United States Renal Data System 2014/2015 Annual Data Report). 10
Only 5,157 of these 52,172 Veterans (9.9%) received dialysis therapy in a dialysis unit based in a VA Medical Center Table 3; the majority (90%) were outsourced to private dialysis providers, including large for-profit dialysis organizations (27.6% and 24.5% of Veterans treated within Fresenius and DaVita dialysis units, respectively), other dialysis chains (13.1%), or an independent dialysis center (21.1%). After three months of treatment, among Veterans continuing to require renal replacement therapy for ESRD, 52.4% still received dialysis therapy in a for-profit large dialysis organization, 13.2% in other dialysis chains, 21.1% in independent (here refed to as “non-chain”) dialysis centers, 2.5% in non-specified (unknown) facilities, and 10.7% in VA medical center based dialysis units, suggesting that the dialysis provider proportions remain relatively constant over time.
Table 3.
Dialysis providers on Day 1 of treatment in 52,172 Veterans who transitioned to end-stage renal disease (ESRD) over four years (from 10/1/207 through 9/30/2011). Note that providers could not be identified on Day 1 in 2,010 (3.9%) of Veterans. Patients were on average 70.3 ± 12.1 years old and included 5.7% women, 24.1% Blacks, and 41.7% diabetics. Adopted from the Unites States Renal Data System 2014/2015 Annual Data Report. 10
| VA Med Centers | DaVita | Fresenius Med Care | Other Dialysis Chains | Non-Chain units | |
|---|---|---|---|---|---|
| Incident patients (N) | 5,157 (9.9%) | 12,766 (24.5%) | 14,380 (27.6%) | 6,850 (13.1%) | 11,007 (21.1%) |
| Age, yrs (SD) | 64.6±11.4 | 70.3±12.1 | 70.7±11.8 | 71.2±11.9 | 72.1±11.8 |
| Elderly proportion (%) | |||||
| Age >65 yrs | 43.4 | 66.7 | 68.6 | 70.0 | 72.6 |
| Age >75 yrs | 21.4 | 42.6 | 44.5 | 46.9 | 49.6 |
| Age >85 yrs | 4.6 | 11.3 | 11.1 | 12.2 | 14.3 |
| Females (%) | 2.5 | 6.0 | 6.1 | 6.1 | 5.7 |
| Race and ethnicity (%) | |||||
| Native American | 0.7 | 1.1 | 0.8 | 0.8 | 1.5 |
| Asian | 1.8 | 1.7 | 1.3 | 3.4 | 1.2 |
| Black | 41.3 | 24.9 | 23.4 | 22.1 | 18.5 |
| White (non-Hispanic) | 55.7 | 72.1 | 74.3 | 73.6 | 78.6 |
| Hispanic | 8.8 | 5.4 | 5.9 | 6.1 | 5.3 |
| Comorbidities (%) | |||||
| Diabetes | 47.0 | 42.7 | 42.6 | 41.7 | 40.1 |
| Hypertension | 22.2 | 32.8 | 34.2 | 32.6 | 32.3 |
| Glomerulonephritis | 7.8 | 4.9 | 5.1 | 5.6 | 5.0 |
| Cystic Kidney | 1.6 | 1.5 | 1.5 | 1.3 | 1.4 |
| Dialysis modality (%) | |||||
| Hemodialysis | 90.6 | 84.6 | 84.8 | 84.9 | 78.2 |
| Peritoneal dialysis | 3.6 | 5.1 | 4.9 | 4.9 | 5.7 |
| Laboratory data (mean±SD) | |||||
| Hemoglobin, g/d | 9.8±1.6 | 10.0±1.6 | 10.0±1.6 | 10.1±1.6 | 10.1±1.5 |
| Albumin, g/dL | 3.2±0.7 | 3.2±0.7 | 3.2±0.7 | 3.2±0.7 | 3.2±0.7 |
| Creatinine, mg/dL | 6.9±3.1 | 6.0±2.9 | 6.0±2.9 | 5.9±2.9 | 5.8±2.8 |
| eGFR at start (ml/min/1.73m2) | 10.9±4.4 | 12.2±5.1 | 12.1±5.1 | 12.3±5.2 | 12.4±5.2 |
| Body mass index (kg/m2) | 28.6±6.8 | 28.2±6.6 | 28.6±6.9 | 28.0±6.6 | 28.0±6.6 |
Abbreviations: eGFR, estimated glomerular filtration rate.
Veterans who received dialysis treatment in a VA medical center were on average five to seven years younger than Veterans in non-VA dialysis clinics, such that only 42.4% of the former patients were older than 65 years as compared to 64.8% of all Veterans with incident ESRD Table 3. VA Medical Centers had a higher prevalence of African Americans (42.6%) as compared to all Veterans with incident ESRD (25.9%). Dialysis patients who received treatment in a dialysis unit based in a VA hospital exhibited a greater survival at any given month over the first 24 months of dialysis initiation or vintage Figure 1. Furthermore, the high early mortality surge notable in the first several months of treatment across all dialysis providers is attenuated in VA dialysis units. Preliminary analyses shows that this survival superiority of VA dialysis units persists even after multivariate adjustment for case-mix characteristics.14
Disparities Among Veterans with Incident ESRD Across the Nation
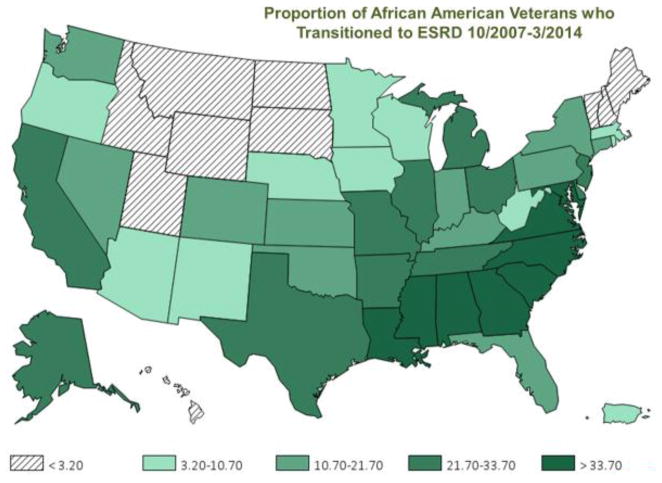
The TC-CKD Special Study Center chapter in the 2016 USRDS Annual Data Report15 highlights the characteristics of 85,505 Veterans with incident ESRD who transitioned to dialysis over the period of 10/1/2007 to 3/31/2014, i.e., over six-and-a-half years. The cohort’s mean ± SD age was 70.1 ± 12.0 years, and included 25% patients of Black race and 6% of Hispanic ethnicity. The main causes of ESRD were diabetes mellitus (42%) or hypertension (31%). Across the nation, the proportion of Black Veterans with incident ESRD varied by state and region. Southern states such as Alabama, Georgia, Louisiana, Mississippi, and Washington D.C. had the highest prevalences of Black Veterans who transitioned to ESRD, whereas northeastern and northwestern states had lower proportions Figure 2).15
Figure 2.
Distribution of Black Veterans with incident end-stage renal disease (ESRD) (%) among 85,505 incident ESRD Veterans across states and territories of the United State over six-and-half years, i.e., 10/1/2007–3/31/2014 (adapted from Figure 8.1. in Vol 1 of the Transition of Care in Chronic Kidney Disease chapter of the 2016/2017 United States Renal Data System Annual Data Report).15 The figure depicts states and territories of the United State.
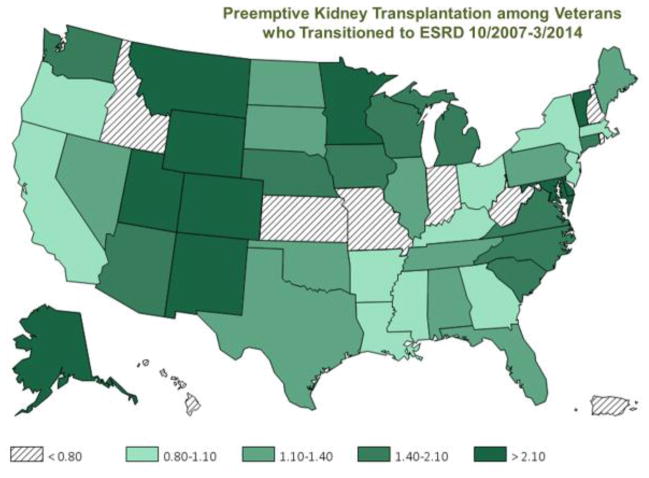
The national rate of pre-emptive kidney transplantation among Veterans with advanced CKD over the same period is described in Figure 3.15 The rates were calculated based on the number of pre-emptive transplants divided by the total number of Veterans with incident ESRD in that state or territory (n=1,133 pre-emptive transplantations over six-and-a-half years in the entire nation). The states with the highest pre-emptive kidney transplantation rates among Veterans (>2.1%) were Alaska, Colorado, Delaware, Maryland, Minnesota, Montana, New Mexico, Utah, Vermont, and Wyoming. Juxta-positioning these two sets of figures highlight potential areas of disparity for pre-emptive kidney transplantation across race, implying that there are opportunities to more accurately identify and address the discrepancies related to renal replacement therapy modalities and distribution across Veterans throughout the nation.
Figure 3.
Distribution of pre-emptive kidney transplantation rate among 85,505 incident end-stage renal disease (ESRD) Veterans across states and territories of the United States over six-and-a-half years, i.e., 10/1/2007–3/31/2014 (adapted from Figure 8.3. in Vol 1 of the Transition of Care in Chronic Kidney Disease chapter of the 2016/2017 United States Renal Data System Annual Data Report).15 The figure depicts states and territories of the United States.
A recent landmark study by Kovesdy et al4 showed that, by having equal access to healthcare resources in the VHA system, African Americans have lower all-cause mortality and incidence of coronary heart disease as well as a similar incidence of ischemic stroke, which stands in contrast to the higher mortality observed among Black individuals in the general US population outside of the VA system. Hence, Veterans’ increased opportunity to receive dialysis care within an integrated healthcare system could yield improved understanding of drivers of disparities in CKD care. In addition, leveraging the VHA’s considerable research infrastructure would permit discovery of additional health service delivery innovations to reduce disparities and improve outcomes of people with ESRD.
Incremental Dialysis Initiation for Veterans
Whereas prevalent dialysis patients have an exceptionally high mortality rate of 15 to 20% per year nationwide, worse than most malignancies,16 mortality is even higher in the first several months of dialysis therapy with the annualized mortality surpassing 40% as discussed above (see Figure 1 and Table 3). Although a limited number of controlled trials have examined the extent to which clinical outcomes can be improved among prevalent dialysis patients, interventions among incident dialysis patients have largely been overlooked despite their high mortality, morbidity, and marked decrements in health-related quality of life. There is an urgent need to address these crises of care, including the exceptionally high death rate of incident dialysis patients. Two potential factors that may be related to high early dialysis mortality are (1) loss of residual kidney function and (2) decline in health-related quality of life, both of which may be direct consequences of abrupt initiation of thrice-weekly hemodialysis treatment. It has been suggested, although not unequivocally proven, that thrice-weekly or more frequent hemodialysis upon transition to renal replacement therapy may accelerate loss of residual kidney function whereas a more gradual transition to dialysis, e.g. once to twice-weekly hemodialysis in the first several months of dialysis initiation, may preserve residual kidney function. Moreover, a less frequent initial hemodialysis schedule may allow patients to more easily adapt to renal replacement therapy with less disruption and strain upon their lifestyles. Indeed, residual kidney function and elevated health-related quality of life are among the two strongest predictors of survival in the first year of dialysis therapy.17
Although not currently the standard of care in the US, there is increasing evidence to suggest that initiation of hemodialysis using a twice-weekly schedule may offer substantial benefits, even if transition to thrice-weekly hemodialysis is later required.17–19 This incremental hemodialysis approach provides a more gradual and tolerable transition to renal replacement therapy, thus resulting in better health-related quality of life, longer preservation of the residual kidney function,20, 21 reduction of hemodialysis-induced inflammatory and oxidative stress,22 decreased frequency of intra-dialytic hypotension, reduced erythropoiesis-stimulating agent dose, and reduced morbidity and mortality.23, 24 Most importantly, dialysis initiation with twice-weekly hemodialysis may be a more patient-centered approach, as it may improve quality of life by mitigating interruptions in day-to-day living, employment, and relationships; avert post-dialysis fatigue; as well as contain costs. Hence, incremental hemodialysis initiation is a more patient-centered approach and consistent with “personalized” dialysis, where the provision of renal replacement therapy is based on the residual clearance and whole person.
Twice-weekly hemodialysis initiation builds upon the concept of “Incremental Dialysis” initially described among peritoneal dialysis patients. In patients who start with peritoneal dialysis, residual kidney function is critical to solute clearance, fluid balance, and survival, and is preserved by avoiding nephrotoxic agents and by pharmacotherapeutic modulation of the renin angiotensin aldosterone pathway.25 Although widely accepted as a treatment strategy among peritoneal dialysis patients, the current US paradigm is to initiate “full-dose,” thrice-weekly hemodialysis therapy without prescription adjustment irrespective of presence of residual kidney function and changes over time.
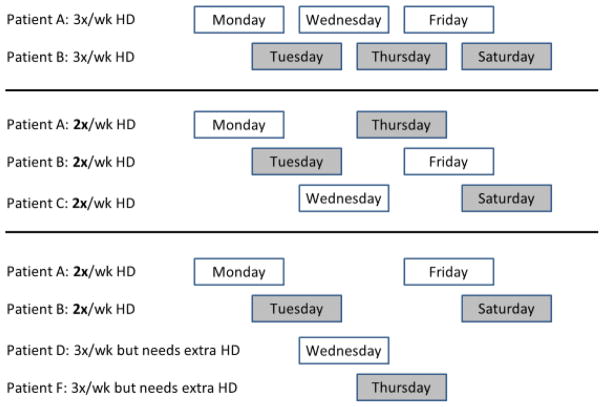
If twice-weekly hemodialysis were to be widely implemented among incident Veterans with advanced CKD who are approaching ESRD, it would allow more Veterans to receive care that is provided within VA-based dialysis units where Veterans benefit from even greater survival (Figure 1 and Table 3). Evidence suggests that over half to two-thirds or more of new ESRD patients have adequate residual kidney function for initiation of incremental hemodialysis initiation. According to the 2006 Kidney Disease Outcome Quality Initiative dialysis adequacy guidelines, a urea clearance (KRU) greater than 3 ml/min/1.73m2 is sufficient for twice-weekly hemodialysis therapy, whereas a decline in KRU to below 2 ml/min/1.73m2 is suggested as a transition point for increasing frequency to thrice-weekly treatment.26 The twice-weekly hemodialysis practice model would allow facilities to start three patients on treatment using the same resources that are needed to for two patients on thrice-weekly hemodialysis (Figure 4). According to our simulations, if each year 60% of the 12,000 to 13,000 Veterans starting thrice-weekly hemodialysis are instead initiated on twice-weekly hemodialysis, and assuming that the median time to the needed transition from twice-weekly to thrice-weekly HD is nine months based on suggested criteria (Box 1), then each year 300 to 500 more Veterans can receive care in a VA based dialysis unit.
Figure 4.
Hemodialysis treatment practice models suggesting that instead of two thrice-weekly patients, three twice-weekly patients can be treated using same resources, or as an alternative scenario, two twice-weekly patients can allow two extra treatment spots to be available for two more frequent (e.g. for two four-times-a-week) hemodialysis patients.
Box 1. Proposed Decision Support System with 11 criteria for initiating and maintaining incremental (twice-weekly) hemodialysis (HD) treatment upon transition to end-stage renal disease (adapted from Kalantar-Zadeh et al.17 and Rhee et al.33).
Incremental (Twice-Weekly) Hemodialysis Treatment Criteria*
|
Implementation Strategies
|
The proposed criteria may be refined for use in clinical trials and clinical decision making.
The minimum required urine output to initiate twice-weekly has been changed to 600 ml/day in this adaptation, while >500 ml/day is needed to maintain twice-weekly regimen.
Lack of systolic dysfunction (EF>40%) and no major coronary intervention over the past three months.
Criterion #11 in this adaptation.
For the incremental hemodialysis regimen to be broadly implemented among Veterans, a pragmatic randomized controlled trial across multiple VA dialysis centers can be readily conducted27 to examine the hypothesis that Veterans who require initiation of maintenance dialysis therapy and who have reasonable residual kidney function, i.e., >500 ml/day urine output and >3 ml/min1.73m2 urea clearance, twice-weekly incremental hemodialysis during the first 12 months of dialysis therapy will slow the rate of residual kidney function decline and improve health-related quality of life. Other relevant endpoints to be examined include the safety of twice-weekly hemodialysis and its potential benefits including attenuation of the very high dialysis mortality in the first several months of dialysis therapy, reduction in inflammatory and oxidative stress, enhancement of patient and dialysis staff adherence and satisfaction, augmentation of dialysis vascular access longevity, and reduction in the hospitalization and readmission risk, as well as other several clinically relevant questions such as the roles of loop diuretics administration and avoidance of nephrotoxic agents in preserving residual kidney function longer.
How to Implement and Monitor Incremental Hemodialysis
In 2014, the blueprint of a Decision Support System for implementation and monitoring of incremental hemodialysis was developed by a group of experts in the setting of a consensus article (Box 1).17 This Decision Support System was based on ten clinical metrics to guide the initial hemodialysis dose and frequency as well as the transition points for escalation from twice-weekly to thrice-weekly hemodialysis, and was tailored for dialysis practice in resource limited economies where KRU may not be readily available. In the current review paper, we have added KRU (>3 ml/min/1.73m2 to start twice-weekly hemodialysis and < 2 ml/min/1.73m2 to transition to thrice-weekly hemodialysis) as the 11th metric (see Box 1). These metrics should be re-examined in individual patients periodically (e.g. monthly to quarterly) to determine the dose and frequency of hemodialysis treatment individually. RKF, including the modified urine volume criterion, (>600 ml/day as the minimum urine output to consider twice-weekly hemodialysis and <500 ml/day as the transition point for thrice-weekly hemodialysis) along with the above mentioned KRU threshold levels, is perhaps the most important determinant of dialysis dose and frequency.17 The proposed modified Decision Support System criteria (Box 1) can be used to this end until the results of additional comparative effectiveness studies or randomized controlled trials are available.
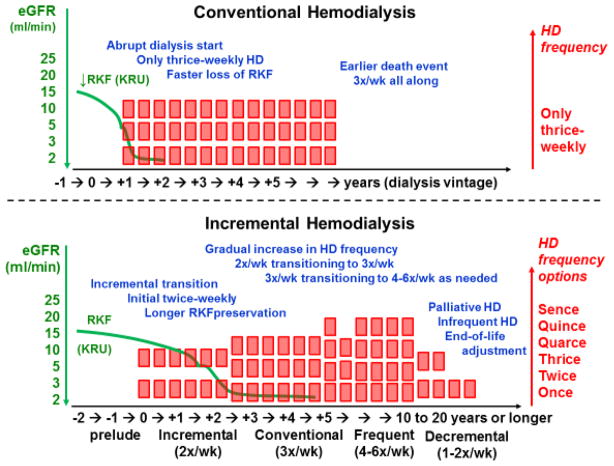
How to Reconcile Incremental with Conventional and More Frequent Hemodialysis?
There is a general misconception that the objectives of incremental hemodialysis conflicts with conventional thrice-weekly and more frequent hemodialysis regimens. Indeed, the incremental hemodialysis approach is based on the premise that almost all patients who have initiated renal replacement therapy with a twice-weekly regimen will eventually need to transition to thrice-weekly and more frequent hemodialysis schedules given the inevitable decline in residual kidney function with the passage of time among most ESRD patients. Hence, incremental hemodialysis offers a unique opportunity to expand the spectrum of dialysis frequency (leftward on Figure 5) and to better implement individualized or personalized dialysis therapy among patients. Traditional and novel urea kinetics models with more accurate incorporation of RRF can be employed or developed and evolved to more effectively explore as to how incremental versus conventional hemodialysis regimens can be evaluated for commensurate urea clearance.28 Given recent data suggesting limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial,29 more clearance may not necessarily equate with more effective uremia management,30. To date there has been no controlled trials of incident ESRD patients transitioning to hemodialysis based on the collective of urea kinetics of the dialysis therapy and residual kidney function combined.
Figure 5.
Juxtaposition of conventional (only thrice-weekly) and incremental (with initial twice-weekly) hemodialysis regimens. The incremental or progressive approach maintains residual kidney function (RKF) longer and better integrates the residual urea clearance (KRU), while it transitions to more frequent treatment with the decline in RKF over time. Note that a palliative or decremental approach is included as an optional scenario for end-of-life adjustment, where twice-weekly hemodialysis regimen can be reutilized.
Veterans’ Preference of Dialysis Treatment
According to some of the coauthors of this and other articles17 who serve as VA staff nephrologists, clinicians caring for Veterans with ESRD often encounter patients who choose to initiate twice-weekly hemodialysis instead of the routine thrice-weekly protocol as their preferance. There are also patients who request once-weekly to twice-weekly hemodialysis as a palliative and end-of-life approach as discussed above. Some VA staff nephrologists may not object to this arrangement with the caveat that Veterans are clearly informed that their choice is not considered conventional.17 Patients may elect less frequent dialysis because of reluctance to travel inconvenient distances; skepticism about their need for more frequent hemodialysis therapy or any dialysis at all; involvement with ill family members and children requiring time and attention; or adverse symptoms related to hemodialysis therapy such as post-dialysis fatigue.17 Moreover, some elderly Veterans are substantially debilitated, with malignancies or end-stage liver disease and other comorbidities, whose quality of life or life expectancy are unlikely to benefit from more frequent hemodialysis; these patients are sometimes concerned that more frequent dialysis will be aburden on their family members. Hence, many Veterans benefit from having the option of twice-weekly hemodialysis both upon initiation of dialysis therapy and discussions related to dialysis treatment withdrawal.
Conclusions and Future Steps
The overarching objective of implementing incremental and twice-weekly dialysis approaches in the VA dialysis system is to ensure that US Veterans with advanced CKD are given the opportunity for expanded choices of renal replacement therapy, especially in the first 12 months of their transition to ESRD when mortality is the highest (Figure 1), as well as for end-of-life issue considerations (Figure 5). The incremental dialysis approach can leverage the existing resources of the VA system under experienced clinicians, researchers, and dialysis staff across over 71 VA medical center affiliated dialysis clinics nationwide. Thus, based on emerging evidence this innovative and pragmatic dialysis treatment approach is posited to improve outcomes of veterans with ESRD, a vulnerable and under-studied population, while helping to overcome existing VA dialysis resource constraints. By implementing incremental dialysis, more Veterans with incident ESRD can be retained in VA dialysis units (Figure 4), while also potentially improving patients’ outcomes and containing excess purchased care costs.
There are other important indications for incremental hemodialysis including gradually breaking in new vascular accesses (e.g., arteriovenous fistulas or grafts) to prolong their longevity, and to allow Veterans and their families to better adapt physically, mentally, and emotionally to a dialysis-dependent lifestyle. It also provides a potential compromise to the ongoing debate of early versus later initiation of maintenance dialysis, since it offers an alternative to the abrupt transition to conventional thrice-weekly treatments in lieu of the traditional all-or-none approach of dialysis therapy.17 It may also allow a more gradual escalation from a low protein to high protein diet (for pre-dialysis chronic kidney disease and ESRD patients, respectively). Notably, according to an Italian dialysis experience report, patients who started dialysis therapy on a once-weekly hemodialysis schedule continued a low protein diet on non-dialysis days, while on dialysis days a high protein diet was provided.31
We understand that the incremental dialysis approach challenges the current standard of care of conventional thrice-weekly hemodialysis. Indeed, it is less likely that the incremental dialysis approach will be supported by non-governmental dialysis organizations, given perceived reduction in dialysis treatment revenue if twice-weekly hemodialysis is to be implemented among most incident dialysis patients. However, the innovative concept of incremental hemodialysis may lead to an imminent and more patient-centered paradigm shift. We envision that a randomized controlled trial of incremental versus conventional thrice-weekly dialysis in VA dialysis centers will examine its safety and efficacy including preservation of residual kidney function and improvement in patient-centered outcomes and functional status such as health-related quality of life and relevant symptoms related to dialysis treatment.32 The ultimate long-term objective is to provide personalized dialysis therapy that is tailored to patients’ individual characteristics in order to optimize survival, health-related quality of life, and other patient-centered outcomes. The efficient use of resources within VA dialysis units are well-suited for timely implementation of a large, multi-center controlled trial to address this urgent unmet need in tens of thousands of Veterans nationwide and the results could potentially impact millions of people with advanced CKD globally.
Acknowledgments
Funding:
This work has been supported by United States Renal Data System Special Study Center grant U01 DK102163. KKZ has been supported by the NIH/NIDDK mid-career award K24-DK091419. KKZ and CPK have been supported by the NIH/NIDDK grant R01-DK096920. CMR has been supported by the NIH/NIDDK early career award K23-DK102903. KCN has been supported by NIH grants P20-MD000182, UL1TR000124, and P30AG021684. SB has been supported by NIH grants R01-DK091437 and R21-DK106574. TS has been supported by R03-DK-104012 and R01-HL-132372.
This study is supported by grant U01-DK102163 from the National Institute of Health (NIH) to CPK and KKZ, and by resources from the US Department of Veterans Affairs. The data reported here have been supplied by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (Project Numbers SDR 02-237 and 98-004). Opinions expressed in this presentation are those of the authors and do not represent the official opinion of the US Department of Veterans Affairs.
Footnotes
Conflict of Interest and Disclosure:
KKZ has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO Oncology, Chugai, DaVita, Fresenius, Genentech, Haymarket Media, Hofstra Medical School, International Federation of Kidney Foundations, International Society of Hemodialysis, International Society of Renal Nutrition & Metabolism, Japanese Society of Dialysis Therapy, Hospira, Kabi, Keryx, Novartis, National Institutes of Health, National Kidney Foundation, OPKO, Pfizer, Relypsa, Resverlogix, Sandoz, Sanofi, Shire, Vifor, UpToDate, and ZS-Pharma. KN has received support from Abbvie and OPKO. TV has received support from Abbvie, GSK, and UpToDate. TS has received consulting fees from Siemens.
References
- 1.Dandeker C, Wessely S, Iversen A, Ross J. What’s in a Name? Defining and Caring for “Veterans” The United Kingdom in International Perspective. Armed Forces & Society. 2006;32(2):161–177. http://www.repwavets.org/uploads/4/1/7/6/41764559/r42324.pdf. [Google Scholar]
- 2.Street AE, Vogt D, Dutra L. A new generation of women veterans: stressors faced by women deployed to Iraq and Afghanistan. Clin Psychol Rev. 2009;29(8):685–94. doi: 10.1016/j.cpr.2009.08.007. http://www.ncbi.nlm.nih.gov/pubmed/19766368. [DOI] [PubMed] [Google Scholar]
- 3.Wong ES, Wang V, Liu CF, Hebert PL, Maciejewski ML. Do Veterans Health Administration Enrollees Generalize to Other Populations? Medical care research and review : MCRR. 2016;73(4):493–507. doi: 10.1177/1077558715617382. http://www.ncbi.nlm.nih.gov/pubmed/26589675. [DOI] [PubMed] [Google Scholar]
- 4.Kovesdy CP, Norris KC, Boulware LE, Lu JL, Ma JZ, Streja E, Molnar MZ, Kalantar-Zadeh K. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation. 2015;132(16):1538–48. doi: 10.1161/CIRCULATIONAHA.114.015124. http://www.ncbi.nlm.nih.gov/pubmed/26384521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Affairs DoV. National center for veterans analysis and statistics. 2012 [Google Scholar]
- 6.Polusny MA, Erbes CR, Thuras P, Moran A, Lamberty GJ, Collins RC, Rodman JL, Lim KO. Mindfulness-Based Stress Reduction for Posttraumatic Stress Disorder Among Veterans: A Randomized Clinical Trial. JAMA. 2015;314(5):456–65. doi: 10.1001/jama.2015.8361. http://www.ncbi.nlm.nih.gov/pubmed/26241597. [DOI] [PubMed] [Google Scholar]
- 7.Shulkin DJ. Beyond the VA Crisis--Becoming a High-Performance Network. N Engl J Med. 2016;374(11):1003–5. doi: 10.1056/NEJMp1600307. https://www.ncbi.nlm.nih.gov/pubmed/26981931. [DOI] [PubMed] [Google Scholar]
- 8.Selim AJ, Berlowitz D, Kazis LE, Rogers W, Wright SM, Qian SX, Rothendler JA, Spiro A, 3rd, Miller D, Selim BJ, Fincke BG. Comparison of health outcomes for male seniors in the Veterans Health Administration and Medicare Advantage plans. Health Serv Res. 2010;45(2):376–96. doi: 10.1111/j.1475-6773.2009.01068.x. http://www.ncbi.nlm.nih.gov/pubmed/20050934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saran R, Li Y, Robinson B, Abbott KC, Agodoa LY, Ayanian J, Bragg-Gresham J, Balkrishnan R, Chen JL, Cope E, Eggers PW, Gillen D, Gipson D, Hailpern SM, Hall YN, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Kalantar-Zadeh K, Kovesdy CP, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, Nguyen DV, O’Hare AM, Plattner B, Pisoni R, Port FK, Rao P, Rhee CM, Sakhuja A, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, White S, Woodside K, Hirth RA. US Renal Data System 2015 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2016;67(3 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.12.014. http://www.ncbi.nlm.nih.gov/pubmed/26925525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;66(1 Suppl 1):Svii, S1–305. doi: 10.1053/j.ajkd.2015.05.001. http://www.ncbi.nlm.nih.gov/pubmed/26111994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang V, Maciejewski ML, Patel UD, Stechuchak KM, Hynes DM, Weinberger M. Comparison of outcomes for veterans receiving dialysis care from VA and non-VA providers. BMC health services research. 2013;13:26. doi: 10.1186/1472-6963-13-26. http://www.ncbi.nlm.nih.gov/pubmed/23327632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Relations VNKP-OoPAM. Learn About Kidney Disease: VA Kidney Disease and Dialysis Services Fact Sheet (2015) Veterans Health Administration. 2015 http://wwwvagov/health/services/renal/learnasp. http://www.va.gov/health/services/renal/learn.asp.
- 13.Watnick S, Crowley ST. ESRD care within the US Department of Veterans Affairs: a forward-looking program with an illuminating past. Am J Kidney Dis. 2014;63(3):521–9. doi: 10.1053/j.ajkd.2013.10.046. http://www.ncbi.nlm.nih.gov/pubmed/24331978. [DOI] [PubMed] [Google Scholar]
- 14.Streja E, Soohoo M, Rhee C, Ravel V, Chen JLT, Jing J, Kovesdy CP, Kalantar-Zadeh K. Association of Dialysis Provider Assignment with Early Dialysis Mortality in US Veterans: A Transition of Care in CKD Study. J Am Soc Nephrol. 2015 abstract ASN. [Google Scholar]
- 15.Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Soohoo M, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2017 doi: 10.1053/j.ajkd.2016.12.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35(6):548–58. doi: 10.1159/000338673. http://www.ncbi.nlm.nih.gov/pubmed/22677686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalantar-Zadeh K, Unruh M, Zager PG, Kovesdy CP, Bargman JM, Chen J, Sankarasubbaiyan S, Shah G, Golper T, Sherman RA, Goldfarb DS. Twice-weekly and incremental hemodialysis treatment for initiation of kidney replacement therapy. Am J Kidney Dis. 2014;64(2):181–6. doi: 10.1053/j.ajkd.2014.04.019. http://www.ncbi.nlm.nih.gov/pubmed/24840669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew A, Obi Y, Rhee CM, Chen JL, Shah G, Lau WL, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Treatment frequency and mortality among incident hemodialysis patients in the United States comparing incremental with standard and more frequent dialysis. Kidney Int. 2016;90(5):1071–1079. doi: 10.1016/j.kint.2016.05.028. http://www.ncbi.nlm.nih.gov/pubmed/27528548. [DOI] [PubMed] [Google Scholar]
- 19.Obi Y, Streja E, Rhee CM, Ravel V, Amin AN, Cupisti A, Chen J, Mathew AT, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Incremental Hemodialysis, Residual Kidney Function, and Mortality Risk in Incident Dialysis Patients: A Cohort Study. Am J Kidney Dis. 2016;68(2):256–65. doi: 10.1053/j.ajkd.2016.01.008. http://www.ncbi.nlm.nih.gov/pubmed/26867814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obi Y, Rhee CM, Mathew AT, Shah G, Streja E, Brunelli SM, Kovesdy CP, Mehrotra R, Kalantar-Zadeh K. Residual Kidney Function Decline and Mortality in Incident Hemodialysis Patients. J Am Soc Nephrol. 2016;27(12):3758–3768. doi: 10.1681/ASN.2015101142. http://www.ncbi.nlm.nih.gov/pubmed/27169576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int. 2016;90(2):262–71. doi: 10.1016/j.kint.2016.02.037. http://www.ncbi.nlm.nih.gov/pubmed/27182000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafi T, Jaar BG, Plantinga LC, Fink NE, Sadler JH, Parekh RS, Powe NR, Coresh J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) Study. Am J Kidney Dis. 2010;56(2):348–58. doi: 10.1053/j.ajkd.2010.03.020. Epub 2010/07/08 http://www.ncbi.nlm.nih.gov/pubmed/20605303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee CM, Unruh M, Chen J, Kovesdy CP, Zager P, Kalantar-Zadeh K. Infrequent dialysis: a new paradigm for hemodialysis initiation. Semin Dial. 2013;26(6):720–7. doi: 10.1111/sdi.12133. http://www.ncbi.nlm.nih.gov/pubmed/24016197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obi Y, Eriguchi R, Ou SM, Rhee CM, Kalantar-Zadeh K. What Is Known and Unknown About Twice-Weekly Hemodialysis. Blood Purif. 2015;40(4):298–305. doi: 10.1159/000441577. http://www.ncbi.nlm.nih.gov/pubmed/26656764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolesnyk I, Noordzij M, Dekker FW, Boeschoten EW, Krediet RT. Treatment with angiotensin II inhibitors and residual renal function in peritoneal dialysis patients. Perit Dial Int. 2011;31(1):53–9. doi: 10.3747/pdi.2009.00088. Epub 2010/06/05 http://www.ncbi.nlm.nih.gov/pubmed/20522672. [DOI] [PubMed] [Google Scholar]
- 26.Initiative; NKFKD-DOQ. KDOQI clinical practice guidelines and clinical practice recommendations for 2006 updates: Hemodialysis adequacy, peritoneal dialysis adequacy and vascular access. Am J Kidney Dis. 2006;48(Suppl 1):S1–S322. [Google Scholar]
- 27.Crowley ST, Chertow GM, Vitale J, O’Connor T, Zhang J, Schein RM, Choudhury D, Finkel K, Vijayan A, Paganini E, Palevsky PM. Lessons for successful study enrollment from the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study. Clin J Am Soc Nephrol. 2008;3(4):955–61. doi: 10.2215/CJN.05621207. Epub 2008/04/04 CJN.05621207 [pii] http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=18385390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obi Y, Kalantar-Zadeh K, Streja E, Daugirdas JT. Prediction equations for calculating residual kidney urea clearance using urine collections for different hemodialysis treatment frequencies and interdialytic intervals. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfw473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirich TL, Fong K, Larive B, Beck GJ, Chertow GM, Levin NW, Kliger AS, Plummer NS, Meyer TW Frequent Hemodialysis Network Trial G. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney Int. 2017 doi: 10.1016/j.kint.2016.11.002. https://www.ncbi.nlm.nih.gov/pubmed/28089366. [DOI] [PubMed]
- 30.Meyer TW, Sirich TL, Fong KD, Plummer NS, Shafi T, Hwang S, Banerjee T, Zhu Y, Powe NR, Hai X, Hostetter TH. Kt/Vurea and Nonurea Small Solute Levels in the Hemodialysis Study. J Am Soc Nephrol. 2016;27(11):3469–3478. doi: 10.1681/ASN.2015091035. https://www.ncbi.nlm.nih.gov/pubmed/27026365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolasco P, Cupisti A, Locatelli F, Caria S, Kalantar-Zadeh K. Dietary Management of Incremental Transition to Dialysis Therapy: Once-Weekly Hemodialysis Combined With Low-Protein Diet. J Ren Nutr. 2016;26(6):352–359. doi: 10.1053/j.jrn.2016.01.015. http://www.ncbi.nlm.nih.gov/pubmed/26936151. [DOI] [PubMed] [Google Scholar]
- 32.Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, Switzer GE. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27(3):226–40. doi: 10.1016/j.jpainsymman.2003.07.004. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15010101. [DOI] [PubMed] [Google Scholar]
- 33.Rhee CM, Ghahremani-Ghajar M, Obi Y, Kalantar-Zadeh K. Incremental and infrequent hemodialysis: a new paradigm for both dialysis initiation and conservative management. Panminerva Med. 2017 doi: 10.23736/S0031-0808.17.03299-2. epub http://www.ncbi.nlm.nih.gov/pubmed/28090764. [DOI] [PMC free article] [PubMed]