Abstract
Individuals with schizophrenia display notable deficits in social functioning. Research indicates that neural connectivity within the default mode network (DMN) is related to social cognition and social functioning in healthy and clinical populations. However, the association between DMN connectivity, social cognition, and social functioning has not been studied in schizophrenia. For the present study, we used resting-state neuroimaging data to evaluate connectivity between the main DMN hubs (i.e., the medial prefrontal cortex (mPFC) and the posterior cingulate cortex-anterior precuneus (PPC)) in individuals with schizophrenia (n=28) and controls (n=32). We also examined whether DMN connectivity was associated with social functioning via social attainment (measured by the Specific Levels of Functioning Scale) and social competence (measured by the Social Skills Performance Assessment), and if social cognition mediates the association between DMN connectivity and these measures of social functioning. Results revealed that DMN connectivity did not differ between individuals with schizophrenia and controls. However, connectivity between the mPFC and PCC hubs was significantly associated with social competence and social attainment in individuals with schizophrenia but not in controls as reflected by a significant group-by-connectivity interaction. Social cognition did not mediate the association between social functioning and DMN connectivity in individuals with schizophrenia. Our findings suggest that fronto-parietal DMN connectivity in particular may be differentially associated with social functioning in schizophrenia and controls. As a result, DMN connectivity may be used as a neuroimaging marker to monitor treatment response or as a potential target for interventions that aim to enhance social functioning in schizophrenia.
Keywords: Default Mode Network Connectivity, Resting-State fMRI, Social Competence, Social Attainment, Schizophrenia
Social functioning measures the way we engage with family, friends, co-workers, and service providers in conversation, shared decision-making, compromise, and social activities (Kalin et al., 2015). Social functioning is notably impaired in schizophrenia and is considered by many to be a fundamental characteristic of the disorder (Hafner, Nowotny, Loffler, & an der Heiden, 1995). Restoring an individual with schizophrenia’s social functioning via their social attainment (i.e., one’s social relationships and participation in community activities) and social competence (i.e., one’s capacity to effectively socialize with others) is a cornerstone of functional recovery. To date, research has evaluated several interventions aimed at improving social functioning (Horan et al., 2009; Horan et al., 2011; Kurtz & Mueser, 2008; Liberman, Mueser, & Wallace, 1986; Penn, Roberts, Combs, & Sterne, 2007). However, the effects have been modest, and as such, interventions targeting the social dysfunction associated with schizophrenia could benefit from additional optimization (Kurtz & Mueser, 2008; Kurtz & Richardson, 2012). One approach to optimize these interventions is to identify the mechanisms that underlie social functioning impairments, such as the neural systems that support key social behaviors in schizophrenia. Therefore, research is needed to understand the associations between neural networks and social functioning among individuals with schizophrenia.
The “dysconnectivity hypothesis” is a promising theory from which to investigate the neural basis of social functioning. This hypothesis suggests that abnormal communication between neural networks is responsible for the cognitive and clinical symptoms of schizophrenia, including social functioning deficits (Bullmore, Frangou, & Murray, 1997; Friston, 1994; Friston & Frith, 1995; Stephan, Baldeweg, & Friston, 2006). For instance, several studies observed aberrant connectivity within the default mode network (DMN) among individuals with schizophrenia (Alonso-Solis et al., 2012; Bluhm et al., 2007; Camchong, MacDonald, Bell, Mueller, & Lim, 2011; Chai et al., 2011; Liemburg et al., 2012; Liu et al., 2012; Ongur et al., 2010; Whitfield-Gabrieli et al., 2009; Zhou et al., 2007). Moreover, this aberrant DMN connectivity has been associated with deficits in social cognition (e.g., social perception, mentalizing) among individuals with schizophrenia (Brunet, Sarfati, Hardy-Bayle, & Decety, 2003; Delaveau et al., 2010; Fett et al., 2011; Mars et al., 2012; Mitchell, Banaji, & Macrae, 2005; Pelletier-Baldelli, Bernard, & Mittal, 2015; Shi et al., 2015; Spreng & Grady, 2010; Uddin, Lacoboni, Lange, & Keenan, 2007; Walter et al., 2009). Specifically, social perception is the ability to perceive social cues such as facial affect, tone, or gestures, while mentalizing is the ability to ascertain others’ emotions, beliefs, and intentions (Green, Horan, & Lee, 2015). In turn, a meta-analysis suggests that social cognition is the most proximal factor to social functioning among individuals with schizophrenia (Fett et al., 2011). Thus, social cognition may represent an underlying mechanism that could explain the association between DMN connectivity and social functioning.
Alternatively, prior studies suggest that functional connectivity of the default mode network (DMN) is directly related to social functioning in various clinical and healthy populations (Che et al., 2014; Dodell-Feder, DeLisi, & Hooker, 2014; Jung et al., 2014; Schreiner et al., 2013; Washington & VanMeter, 2015; Yerys et al., 2015). Specifically, increased functional connectivity within the DMN has been associated with better social functioning in healthy controls (Che et al., 2014; Jung et al., 2014; Washington & VanMeter, 2015), and decreased DMN functional connectivity has been associated with social impairment in individuals with autism and 22q11.2 deletion syndrome (Jung et al., 2014; Schreiner et al., 2013; Yerys et al., 2015). Most recently, Dodell-Feder and colleagues found that reduced resting-state DMN connectivity was associated with poorer social functioning in healthy controls and first-degree relatives of individuals with schizophrenia (Dodell-Feder et al., 2014). Thus, the DMN’s aberrant connectivity in individuals with schizophrenia, its relation to social cognition, and its relation to social functioning in various populations suggest that the DMN may be directly associated with social functioning in individuals with schizophrenia. To our knowledge, prior research has not investigated the associations between DMN functional connectivity and social functioning or the underlying mechanisms for such associations in schizophrenia. Thus, a direct investigation could elucidate whether DMN connectivity is a potential treatment target for functional recovery.
The DMN consists of a frontal hub (i.e., highly connected area within the brain) located in the medial prefrontal cortex and a posterior hub located in the posterior cingulate cortex and precuneus that are highly connected with each other and other areas of the brain (Andrews-Hanna, 2012; Laird et al., 2011). In the current paper, we define DMN functional connectivity as inter-network connectivity (i.e., correlations between the timeseries of data-derived neural networks) between the medial prefrontal cortex (mPFC) and posterior cingulate cortex and anterior precuneus (PPC) hubs. Prior research suggests inter-network connectivity of the mPFC and PPC hubs is related to social functioning among healthy individuals and neuropsychiatric populations (Che et al., 2014; Dodell-Feder et al., 2014; Jung et al., 2014; Schreiner et al., 2013; Washington & VanMeter, 2015; Yerys et al., 2015). Specifically, inter-network connectivity between the mPFC and PPC hubs activates during self-reflection (Amodio & Frith, 2006; Andrews-Hanna, 2012; Ochsner et al., 2004; Schmitz & Johnson, 2007) and when a person is presented with information about people who are important to them (e.g., friends, relatives, etc.) (Andrews-Hanna, 2012; Bartels & Zeki, 2004; Ochsner et al., 2005). This inter-network connectivity also activates when a person is anticipating a social reward or threat, such as positive or negative affect (Andrews-Hanna, 2012; Kober et al., 2008; Maddock, 1999).
Additionally, DMN connectivity can be observed during both resting and task-activated neural states. Meta-analytic results suggest that the spatial topographies of intrinsic connectivity networks (ICNS) at rest are similar to networks derived during task (Fox & Raichle, 2007; Laird et al., 2011; Smith et al., 2009). However, researchers can use resting-state methods to overcome certain limitations that are associated with task-based approaches (e.g., individual differences in attention, effort, or comprehension) (Callicott et al., 2000; Callicott et al., 2003; Hooker, Bruce, Lincoln, Fisher, & Vinogradov, 2011). Additionally, resting-state methods may be useful for capturing brain activity related to broader constructs that include multiple processes (e.g., social functioning) as this method does not depend on specific task demands (Abram et al., 2015). Thus, resting-state methods may be particularly useful for examining associations between connectivity and social functioning.
A common approach for evaluating patterns of resting-state connectivity is probabilistic independent component analysis (pICA) (McKeown et al., 1998), which is a data-driven procedure that separates multivariate signals into statistically independent sources, or group-level spatial maps. More specifically, the group-level spatial maps reflect temporally synchronized fluctuations in fMRI signals (Beckmann & Smith, 2004). As a data-driven procedure, pICA generates components that represent functionally integrated brain regions as opposed to structurally defined brain regions. In comparison to other connectivity approach (e.g., seed-based methods), pICA reduces bias by parsing variance related to artifacts (i.e., head motion, cardiac function) from neural activity of interest (Beckmann, DeLuca, Devlin, & Smith, 2005). We therefore used pICA to derive group-level spatial maps for the current study.
pICA can also help establish whether individuals with schizophrenia are characterized by either hypo- or hyper-DMN connectivity when compared to healthy controls as current findings are mixed (Alonso-Solís et al., 2015; Chang et al., 2014; Jafri, Pearlson, Stevens, & Calhoun, 2008; Li et al., 2015; Liang et al., 2006; Liemburg et al., 2012; Mingoia et al., 2012; Ongur et al., 2010; Schilbach et al., 2016; Whitfield-Gabrieli & Ford, 2012; Zhou et al., 2007). The inconsistent findings may be attributed to methodological differences, such as seed versus pICA analyses, or due to differences in the specific networks that were examined (Jafri et al., 2008; Whitfield-Gabrieli & Ford, 2012; Zhou et al., 2007). Many studies that evaluated resting-state DMN connectivity in schizophrenia using pICA reported on the connectivity between the main DMN hubs and other brain networks (Alonso-Solís et al., 2015; Jafri et al., 2008; Liang et al., 2006; Ongur et al., 2010; Zhou et al., 2007). Of the studies examining DMN connectivity (Alonso-Solís et al., 2015; Chang et al., 2014; Jafri et al., 2008; Li et al., 2015; Liang et al., 2006; Liemburg et al., 2012; Mingoia et al., 2012; Ongur et al., 2010; Schilbach et al., 2016; Zhou et al., 2007), only two studies directly examined connectivity between the DMN hubs (Chang et al., 2014; Liemburg et al., 2012). One of these studies found that individuals with schizophrenia exhibited decreased DMN inter-network connectivity as compared to controls (Liemburg et al., 2012), and one study did not observe group differences in DMN inter-network connectivity (Chang et al., 2014). Due to conflicting findings in the literature, further research comparing DMN inter-network connectivity of individuals with schizophrenia to controls is needed.
The aims of the current study were threefold. First, we compared resting-state DMN inter-network connectivity between individuals with schizophrenia and controls. We hypothesized that individuals with schizophrenia, as compared to controls, would either show decreased or similar connectivity between the DMN hubs. Second, we assessed between-group differences in DMN connectivity as well as the relationship between DMN connectivity and social functioning via measures of social attainment and social competence. We hypothesized that DMN connectivity would be positively associated with social attainment and social competence in healthy controls and individuals with schizophrenia. Third, we investigated whether social cognition (i.e., social perception and mentalizing) mediated the association between DMN connectivity and social functioning among individuals with schizophrenia.
Method
Participants and Procedure
Individuals with schizophrenia (n=28) and controls (n=32) ages 18–45 were group-matched for age, gender, and race. Participants completed a clinical interview, neuropsychological battery, and resting-state functional magnetic resonance imaging scan. Participants were recruited from the Northwestern University Schizophrenia Research Group. Details on recruitment can be found in Smith et al. (2015).1 Individuals with schizophrenia were included in the study if they 1) met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria for a diagnosis of schizophrenia, 2) were clinically stable (i.e., their symptoms remained unchanged for at least 2 weeks) (Rastogi-Cruz & Csernansky, 1997), and 3) were currently on antipsychotic medication. Individuals were excluded from the study if they 1) met DSM-IV criteria for intellectual disability, 2) met substance abuse or dependence DSM-IV criteria in the past 6 months, 3) had a documented neurological injury or disorder, or 4) had a severe medical disorder. Controls were also excluded from the study if they 1) had a lifetime history of a DSM-IV Axis I psychiatric disorder or 2) had a first-degree relative with a psychotic disorder. Additionally, we excluded nine participants from the analysis (five individuals with schizophrenia and four controls), for excessive in-scanner motion (mean absolute displacement above 1.5 mm, or any absolute displacement (translations or rotations) above 3 mm/degrees). The institutional review board at Northwestern University Feinberg School of Medicine approved the study procedures, and all participants provided informed consent (IRB approval number: STU00013034).
Measures
Demographic and clinical measures
DSM-IV diagnosis was determined through the Structured Clinical Interview for DSM Disorders (SCID-IV) (First, Spitzer, Gibbon, & Williams). The SCID-IV was conducted by masters- or PhD-level research staff and validated through a semi-structured interview by a study psychiatrist. All antipsychotic medication dosages were converted to chlorpromazine equivalents (CPZeq) (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010). Clinical symptoms of schizophrenia were assessed using the Scale for the Assessment of Positive Symptoms (SAPS) (Andreasen, 1983) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1983). Global ratings for hallucinations, delusions, bizarre behavior, positive formal thought disorder, affective flattening, alogia, avolition, and anhedonia were provided by masters- or PhD-level research staff. We generated global scores for positive, negative, and disorganized symptoms by calculating the mean of positive SAPS items, negative SANS items, and disorganized SANS items. Mean parental socioeconomic status (SES) was assessed using the Barrett Simplified Measure of Social Status (Barratt, 2005).
Neurocognitive assessments
Participants completed a battery of tests used to generate four neurocognitive domains (Nuechterlein et al., 2004), including: 1) Crystallized IQ from scores on the Vocabulary subtest of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) (Wechsler, 1997a), 2) Working Memory from scores on the Continuous Performance Task (CPT) (Barch et al., 2004) and the Digit-Span, Spatial-Span, and Letter-Number Sequencing subtests of the Wechsler Memory Scale-Third Edition (WMS-III) (Wechsler, 1997b), 3) Episodic Memory from scores on the Logical Memory I and Family Pictures I subtests of the WMS-III and the free recall score from the first five trials of the California Verbal Learning Test (CVLT) (Delis, Kramer, Kaplan, & Ober, 1983), 4) Executive Functioning from scores on the Wisconsin Card Sort Task (WCST) (Heaton, 2003), Letter and Animal Fluency (Benton, Hamsher, & Sivan, 1994), the Trail Making Test Part B (Reitan & Wolfson, 1985), and the Matrix Reasoning subtest of the WAIS-III. Domain scores were calculated by first standardizing individual subtests (using control group data) and then averaging across these standardized scores within each domain (Smith et al., 2012). A global neurocognitive score was computed using the average of these four domains.
Social Cognition
Social Perception
We assessed social perception using a facial affect perception task and a social perception task. The facial affect perception task presented participants with 30 faces displaying happiness, sadness, fear, disgust, anger, or neutrality, and participants selected the correct emotional expression from two emotion labels (Smith et al., 2014). We used the half-profile of nonverbal sensitivity (PONS) to measure social perception. Specifically, the task presented participants with 110 video scenes containing facial expressions, voice intonations, and/or gestures. Following the scene, participants were presented with two labels and chose which label best described the social cue (Ambady, Hallahan, & Rosenthal, 1995; Rosenthal et al., 1979). We mean-centered accuracy rates to the control group, and averaged transformed scores from both tasks to obtain a social perception domain score.
Mentalizing
We assessed mentalizing using cognitive empathy tasks, specifically the Awareness of Social Inference Test (TASIT) Part III (McDonald, 2002) and an emotional perspective-taking task (Smith et al., 2014). The TASIT-III presented participants with 16 video vignettes of common social interactions. Each vignette contained an untrue comment presented as either a lie or sarcasm. Participants answered yes/no questions about the thoughts, intentions, beliefs, and feelings of the people in the vignette. The emotional perspective-taking task presented participants with 60 pictures of two person interactions depicting happiness, sadness, fear, disgust, anger, or neutrality. In each picture, one person’s face was masked and participants chose which of two faces depicted the emotion of the masked actor. We mean-centered accuracy rates to the control group, and then averaged the transformed accuracy scores to obtain the mentalizing domain score.
Social functioning
Social attainment
We assessed social attainment using the interview version of the Specific Levels of Functioning Scale (SLOF), which is a 30 item interview-based measure that assesses the following domains of social attainment: interpersonal relationships, social acceptability, activities of daily living, and work skills (Schneider & Struening, 1983). Higher scores on the SLOF indicate better social attainment (range = 84–150). Variance in SLOF scores did not significantly differ between groups (F27,31 = 1.38, P = .39). The SLOF is considered the gold standard for assessing current functioning based on findings from the VALERO Study (Harvey et al., 2011; Leifker, Patterson, Heaton, & Harvey, 2011).
Social competence
We assessed social competence using the Social Skills Performance Assessment (SSPA) (Patterson, Moscona, McKibbin, Davidson, & Jeste, 2001), which is comprised of 2 role-play scenes between a trained actor and the participant that are video-recorded. The scenes involve meeting a new neighbor and making a maintenance request to a landlord. Two trained research assistants rated the performance on a 5-point scale across 8 criteria for the first scene and 9 criteria for the second scene. Additional details on the use of this measure can be found here: Smith et al., 2014. We calculated a final score by averaging the scores for each scene (ICC = .97 for 2 blinded raters on 25% of the videos). Higher scores on the SSPA indicate better social competence (range = 1.66–5.00). Variance in SSPA scores did not significantly differ between groups (F22,26 = 1.89, P = .12). The SSPA is considered the gold standard for assessing social competence in schizophrenia (Harvey, Velligan, & Bellack, 2007; Kalin et al., 2015).
fMRI data acquisition and pICA
Resting-state scans were acquired on a 3T TIM Trio system (Siemens Medical Systems) scanner at Northwestern University’s Feinberg School of Medicine for the study sample. The scanning parameters included: gradient-echo echo-planar imaging of 164 volumes; repetition time (TR) = 2.5 s; echo time (TE) = 20 ms; flip angle = 80°; voxel size = 1.7 × 1.7 × 3 mm. The group-level spatial maps were derived from resting-state scans of an independent community sample of 218 volunteers collected at the University of Minnesota (mean age = 26 years [range 20 to 39], 49% male) (Abram et al., 2015). Data were preprocessed using the following preprocessing steps in the MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components) toolkit in FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC): brain extraction, motion correction, and high-pass temporal filtering (threshold of 0.1 Hz) (Wisner, Patzelt, Lim, & MacDonald, 2013). As a final step, the data underwent motion regression outside of FSL using participant-specific movement parameters generated during registration (Moodie, Wisner, & MacDonald, 2014; Wisner, Atluri, Lim, & MacDonald, 2013).
As noted previously, the group-level spatial maps were generated from a larger dataset of 218 community volunteers using a meta-melodic spatial pICA pipeline (Abram et al., 2015). More specifically, the MELODIC function in FSL was used to run 25 temporal concatenation (model-free and multi-subject) group-level pICAs. Each ICA included a random order of 80 participants as inputs to decrease the likelihood of over-fitting, with a dimensionality constraint of 60 based on reliability research (Poppe et al., 2013) and findings that large-scale networks, such as the DMN, fractionate at higher dimensionalities (Ray et al., 2013; Wisner, Atluri, et al., 2013; Wisner, Patzelt, et al., 2013). The 60 components from each ICA were merged into one file which was used as the input to a meta-level MELODIC (meta-ICA). The meta-ICA generated the 60 most consistent group-level components. Visual inspection was done to identify artifactual components, which included components likely to show homeostatic fluctuations, white matter tracts, or movement (Kelly et al., 2010). 27 non-artifactual components were identified following these procedures.
As recommended by prior research, we used maps from this external sample because maps generated from larger datasets are likely to be more robust and these maps were unbiased to the schizophrenia or control groups used in the present study (Griffanti et al., 2016). We also used ICA maps from this larger dataset rather than mPFC and PCC atlas masks because we did not want to make assumptions regarding exactly which voxels may contribute to a network (Esposito et al., 2005; Ramnani, Behrens, Penny, & Matthews, 2004). By using maps from an external sample, we were able to assess functional connectivity within and between functionally-derived networks as opposed to structurally-defined networks. We applied the group-level spatial maps from the external dataset to our current dataset through FSL’s dual-regression function. This enabled us to derive individual timeseries and corresponding spatial maps for the current participants. We used the participant-specific timeseries and spatial maps to compute connectivity metrics for each participant.
Inter-network connectivity metric computations
We used dual regression to create connectivity maps and time courses for each subject based on the group-level spatial maps from the meta-ICA (Filippini et al., 2009; Wisner, Patzelt, et al., 2013; Zuo et al., 2010). We calculated inter-network connectivity metrics between all group-level spatial maps using the participant-level timeseries data from the dual regression (Wisner, Patzelt, et al., 2013). These values were Pearson correlations between all pairs of participant-level timeseries (i.e., for all combinations of the 60 networks), and reflect the temporal association between network pairs. Group-level means were calculated using Fischer-Z values, and were transformed back to r-values for reporting.
In the current study, we were interested in connectivity between the mPFC and the PPC (see Supplemental Figure 1). Of the 60 group-level spatial maps (Abram et al., 2015), three non-artifactual components included mPFC and twelve non-artifactual components included PPC (see Supplemental Table 1). We examined the 4 components that included an mPFC or PPC-overlap of at least 750 voxels in our analyses to ensure appropriate mPFC or PPC contribution. These components included the following compositions: the PPC, the mPFC, the precuneus (P), and the orbitofrontal cortex (OFC). Other areas such as the OFC and angular gyrus (included in the PPC component) likely emerged through this analysis because they are highly functionally connected to the main DMN hubs and are related to social functioning (Andrews, Wang, Csernansky, Gado, & Barch, 2014; Andrews-Hanna, 2012). The inter-network connectivity metrics were then as follows: 1) PPC-to-P metric, 2) mPFC-to-PPC metric, 3) OFC-to-PPC metric, 4) mPFC-to-P metric, 5) OFC-to-P metric, and 6) mPFC-to-OFC metric (see Table 1 for a full description of these metrics).
Table 1.
Inter-Network Connectivity Metrics
| Hubs | Inter-network Connectivity Metric |
|---|---|
| Posterior Cingulate Cortex/Anterior Precuneus with additional Precuneus areas | PPC-to-P metric |
| Medial Prefrontal Cortex with Posterior Cingulate Cortex/Anterior Precuneus | mPFC-to-PPC metric |
| Orbitofrontal Cortex with Posterior Cingulate Cortex/Anterior Precuneus | OFC-to-PPC metric |
| Medial Prefrontal Cortex with Precuneus | mPFC-to-P metric |
| Orbitofrontal Cortex with Precuneus | OFC-to-P metric |
| Medial Prefrontal Cortex with Orbitofrontal Cortex | mPFC-to-OFC metric |
Potential movement confounds
Movement was calculated as the root mean square (RMS) absolute and incremental movement for each group (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012). We did not detect group differences in absolute (x̄absCON = 0.36, x̄absSCZ = 0.31, t45= −0.90, P = 0.37) or incremental (x̄incCON = 0.05, x̄incSCZ = 0.05, t45= −0.73, P = 0.47) movement. RMS incremental movement was significantly correlated with the PPC-to-P metric (r = 0.31, P < .05). Neither RMS absolute nor incremental movement was correlated with the other inter-network connectivity metrics, social attainment, or social competence (all P > .10). Therefore, we only included RMS incremental movement as a covariate in models that contained the PPC-to-P metric.
Statistical Analyses
Demographic and behavioral analyses
Group differences for demographic variables, neurocognitive performance, social perception, mentalizing, social attainment, and social competence were evaluated using t-tests for continuous variables and chi-square (χ2) tests for categorical variables.
Connectivity analyses
We used multivariate analysis of variance (MANOVA) to evaluate whether individuals with schizophrenia differed from controls with respect to the DMN connectivity metrics; in this model the PPC-to-P, mPFC-to-PPC, OFC-to-PPC, mPFC-to-P, OFC-to-P, and mPFC-to-OFC metrics served as the dependent variables.
Connectivity and social functioning analyses
Main Effects Model
We used robust linear regression to assess the associations between our DMN connectivity variables and social attainment and social competence. More specifically, we created two models with social attainment as the dependent variable in the first model, social competence as the dependent variable in the second model, and the PPC-to-P, mPFC-to-PPC, OFC-to-PPC, mPFC-to-P, OFC-to-P, and mPFC-to-OFC metrics and group status as the independent variables in both models. We used the modeling package ‘robust’ in R (Wang et al., 2008) to adjust for the presence of multivariate outliers (Field, 2009). To rule out RMS incremental movement as confounds, we re-assessed the association between DMN connectivity and social attainment and social competence while including the aforementioned variable as a covariate.
Follow-up Interaction Models
We used robust linear regression to examine between-group differences in the associations between DMN connectivity and social attainment and social competence. To conserve power, we only included DMN connectivity metrics that were significantly associated with social attainment or social competence in the main effects models. We again created two models with social attainment as the dependent variable in the first model, social competence as the dependent variable in the second model, and the mPFC-to-PPC metric, group status, and a group-by-connectivity interaction term as the independent variables in both models. Based on prior research suggesting that social status may be associated with neural activity in the main DMN hubs (Muscatell et al., 2012), we included parental SES as a covariate in both models. To account for the association between neurocognitive and social deficits observed in schizophrenia (Fett et al., 2011; Ventura et al., 2015), we also included mean global neurocognition as a covariate in both models. Finally, we included gender and age as covariates in both models given research that shows gender and age-related resting-state connectivity differences (Damoiseaux et al., 2008; Satterthwaite et al., 2014; Tian, Wang, Yan, & He, 2011).
Mediation analyses
In order for a variable to be a mediator, it must be related to both the independent and dependent variables (Baron & Kenny, 1986). Therefore, to determine if mediation analyses were appropriate for our proposed mediators (i.e., social perception and mentalizing), we first examined Pearson correlations to see whether the proposed mediators were significantly correlated with the social functioning variables and DMN connectivity in individuals with schizophrenia. For any significant associations, we performed mediation analyses to see if social perception or mentalizing mediated these associations.
Connectivity and activities of daily living analysis
As a final step, we assessed the specificity of any DMN connectivity and social functioning associations. Specifically, we tested for associations between DMN connectivity and a performance-based daily living skills measure (UPSA-B).
Results
Participant Characteristics
As shown in Table 2, individuals with schizophrenia and controls did not differ according to age, gender, or race. However, the groups differed with regard to parental SES (P < .01), global neurocognition (P < .001), social perception (P < .01), and mentalizing (P < .001). Individuals with schizophrenia also scored lower than controls on the social attainment and social competence measures (both P < .001). Descriptive data for duration of illness, CPZeq, and clinical symptoms are also presented in Table 2.
Table 2.
Study Sample Characteristics
| CON (n = 32) | SCZ (n = 28) | Χ2/t Statistics | |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) | 31.46 (8.06) | 33.17 (6.63) | 0.90 |
| Gender (% male) | 53.13 | 64.29 | 0.38 |
| Parental SES, mean (SD)a | 30.08 (9.21) | 23.15 (9.87) | −2.75** |
| Duration of illness, mean years (SD) | --- | 14.57 (6.34) | --- |
| CPZeq, mean (SD) | --- | 329.79 (207.31) | --- |
| Race | |||
| % Caucasian | 50.00 | 42.90 | 0.31 |
| % African American | 34.40 | 39.30 | |
| % Other | 15.63 | 17.86 | |
| Neurocognitive Function | |||
| Global neurocognition, mean (SD) | 0.00 (0.64) | −0.90 (0.58) | −5.64*** |
| Clinical Symptomsb | |||
| Positive symptoms, mean (SD) | --- | 2.57 (1.86) | --- |
| Negative symptoms, mean (SD) | --- | 2.86 (1.08) | --- |
| Disorganized symptoms, mean (SD) | --- | 1.80 (1.29) | --- |
| Social Cognitive Measures | |||
| Social Perception, mean (SD) | 0.00 (0.86) | −0.77 (1.05) | −3.08** |
| Mentalizing, mean (SD) | 0.00 (0.87) | −1.34 (1.09) | −5.18*** |
| Functioning Measures | |||
| Social Attainment, mean (SD) | 141.34 (12.72) | 125.43 (14.93) | −4.41*** |
| Social Competence, mean (SD)c | 4.49 (0.63) | 3.19 (0.87) | −5.96*** |
Note: SCZ, schizophrenia participants; CON, controls; SES, socioeconomic status; CPZeq, chlorpromazine equivalent.
Completed by n = 31 CON and n = 27 SCZ
Based off of SAPS SANS Global Ratings
Completed by n = 27 CON and n = 23 SCZ
P < .05,
P < .01,
P < .001
Between-Group Connectivity Analysis
Table 3 reports the between-group connectivity results. MANOVA revealed that individuals with schizophrenia and controls did not differ with respect to any of the connectivity variables: PPC-to-P metric, mPFC-to-PPC metric, OFC-to-PPC metric, mPFC-to-P metric, OFC-to-P metric, or the mPFC-to-OFC metric (F6,53 = 0.49, P = .81).
Table 3.
Between-group comparisons of inter-network connectivity metrics
| CON (n = 32) | SCZ (n = 28) | F Statistic | P-value | |
|---|---|---|---|---|
| Mean PPC-to-P metric (SD) | 0.69 (0.0.21) | 0.66 (0.22) | 0.49 | 0.81 |
| Mean mPFC-to-PPC metric (SD) | 0.26 (0.27) | 0.25 (0.30) | ||
| Mean OFC-to-PPC metric (SD) | −0.03 (0.22) | −0.05 (0.20) | ||
| Mean mPFC-to-P metric (SD) | 0.31 (0.21) | 0.24 (0.26) | ||
| Mean OFC-to-P metric (SD) | −0.06 (0.17) | −0.11 (0.21) | ||
| Mean mPFC-to-OFC metric (SD) | 0.39 (0.20) | 0.38 (0.24) |
Note: SCZ, schizophrenia participants; CON, controls; PPC-to-P metric, inter-network connectivity between the posterior cingulate cortex-anterior precuneus and the precuneus; mPFC-to-PPC metric, inter-network connectivity between the medial prefrontal cortex and the posterior cingulate cortex-anterior precuneus; OFC-to-PPC metric, inter-network connectivity between the orbital frontal cortex and the posterior cingulate cortex-anterior precuneus; mPFC-to-P metric, inter-network connectivity between the medial prefrontal cortex and the precuneus; OFC-to-P metric, inter-network connectivity between the orbital frontal cortex and the precuneus; mPFC-to-OFC metric, inter-network connectivity between the medial prefrontal cortex and the orbital frontal cortex
Between-Group Robust Linear Regression Analyses
Main Effects Models
The overall model evaluating associations between all inter-network connectivity metrics and social attainment was significant (F7,52 = 7.56, P < .001) (See Table 4), with main effects of the mPFC-to-PPC metric and group status (both P < .001). None of the other connectivity metrics were associated with social attainment (all P > .10) and, therefore, were not used in subsequent analyses. The exploratory covariate (i.e., RMS incremental movement) was non-significant within the model (P > .10). Thus, we did not include it in the final model to optimize statistical power.
Table 4.
Inter-network connectivity metrics associated with social attainment and social competence (between-group model)
| β (SE) | t-statistic | P-value | |
|---|---|---|---|
| Social Attainment Robust Linear Regression Model | |||
| Inter-network Connectivity | |||
| PPC-to-P metric | −0.07 (0.10) | −0.71 | 0.48 |
| mPFC-to-PPC metric | 0.50 (0.16) | 3.19 | < 0.001** |
| OFC-to-PPC metric | −0.23 (0.14) | −1.66 | 0.10 |
| mPFC-to-P metric | −0.09 (0.14) | −0.60 | 0.55 |
| OFC-to-P metric | −0.03 (0.12) | −0.29 | 0.77 |
| mPFC-to-OFC metric | 0.10 (0.09) | 1.13 | 0.26 |
| Group | 1.11 (0.17) | 6.43 | < 0.001*** |
|
|
|||
| β (SE) | t-statistic | P-value | |
| Social Competence Robust Linear Regression Modela | |||
| Inter-network Connectivity | |||
| PPC-to-P metric | −0.01 (0.12) | −0.86 | 0.40 |
| mPFC-to-PPC metric | 0.44 (0.17) | 2.53 | 0.02* |
| OFC-to-PPC metric | −0.03 (0.15) | −0.17 | 0.87 |
| mPFC-to-P metric | −0.18 (0.17) | −1.12 | 0.27 |
| OFC-to-P metric | −0.04 (0.14) | −0.26 | 0.79 |
| mPFC-to-OFC metric | 0.21 (0.11) | 1.85 | 0.07+ |
| Group | 1.39 (0.19) | 7.16 | < 0.001*** |
Note: PPC-to-P metric, inter-network connectivity between the posterior cingulate cortex-anterior precuneus and the precuneus; mPFC-to-PPC metric, inter-network connectivity between the medial prefrontal cortex and the posterior cingulate cortex-anterior precuneus; OFC-to-PPC metric, inter-network connectivity between the orbital frontal cortex and the posterior cingulate cortex-anterior precuneus; mPFC-to-P metric, inter-network connectivity between the medial prefrontal cortex and the precuneus; OFC-to-P metric, inter-network connectivity between the orbital frontal cortex and the precuneus; mPFC-to-OFC metric, inter-network connectivity between the medial prefrontal cortex and the orbital frontal cortex
n = 27 CON and n = 23 SCZ
P < .10,
P < .05,
P < .01,
P < .001
The overall model evaluating associations between all inter-network connectivity metrics and social competence was also significant (F7,42 = 9.32, P < .001) (See Table 4), with main effects of the mPFC-to-PPC metric and group status (both P < .001). Although the OFC-to-P metric had a trend level association with social competence (P = .07), it was not used in subsequent analyses. None of the other connectivity metrics were significantly associated with social attainment (all P > .10) and, were also discarded from further analysis. The exploratory covariate (i.e., RMS incremental movement) was non-significant within the model (P > .10). Thus, we did not include it in the final model to optimize statistical power.
Follow-up Interaction Models
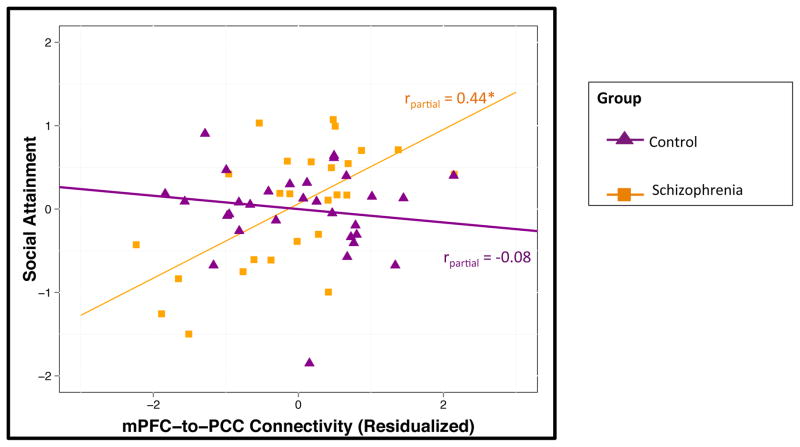
The overall model evaluating associations between connectivity and social attainment was significant (F7,50 = 11.68, P < .001). There were significant main effects of the mPFC-to-PPC metric (P < .001), group status (P < .001), parental SES (P < .05), and global neurocognition (P < .05) (see Table 5). We observed an interaction between group and DMN connectivity (P < .01). A plot of the group-by-DMN connectivity interaction is presented in Figure 1. The other a priori covariates (i.e., age and gender) were not significantly associated with social attainment (both P > .10). Follow-up within group analysis indicated that DMN connectivity was positively correlated with social attainment in individuals with schizophrenia (partial r = 0.44, P < .05) but was not associated in controls (partial r = −0.08, P > .10). See Supplemental Table 2 for zero-order correlations.
Table 5.
Inter-network connectivity associated with social attainment and social competence (between-group model)
| β (SE) | t-statistic | P-value | |
|---|---|---|---|
| Social Attainment Robust Linear Regression Modela | |||
| Inter-network Connectivity | |||
| mPFC-to-PPC metric | 0.46 (0.10) | 4.38 | < 0.001*** |
| Group | 0.92 (0.19) | 4.77 | < 0.001*** |
| Age | 0.08 (0.08) | 0.98 | 0.33 |
| Gender | < 0.01(0.08) | 0.05 | 0.96 |
| Parental SES | −0.20 (0.09) | −2.24 | 0.03* |
| Global neurocognition | 0.23 (0.11) | 2.15 | 0.04* |
| Interaction Term | |||
| mPFC-to-PCC x Group | −0.52 (0.15) | −3.45 | 0.001** |
|
|
|||
| β (SE) | t-statistic | P-value | |
| Social Competence Robust Linear Regression Modelb | |||
| Inter-network Connectivity | |||
| mPFC-to-PPC metric | 0.46 (0.11) | 4.16 | < 0.001*** |
| Group | 1.01 (0.21) | 4.87 | < 0.001*** |
| Age | 0.02 (0.08) | 0.26 | 0.80 |
| Gender | −0.12 (0.09) | −1.34 | 0.19 |
| Parental SES | 0.07 (0.10) | 0.71 | 0.48 |
| Global neurocognition | 0.23 (0.11) | 2.07 | 0.05* |
| Interaction Term | |||
| mPFC-to-PCC x Group | −0.50 (0.17) | −2.97 | < 0.01** |
Note: mPFC-to-PPC metric, inter-network connectivity between the medial prefrontal cortex and the posterior cingulate cortex-anterior precuneus
n = 31 CON and n = 27 SCZ
n = 26 CON and n = 22 SCZ
P < .05,
P < .01,
P < .001
Fig. 1.
Functional connectivity is associated with social attainment in schizophrenia
*p < 0.05
Social Attainment, Scores on the Specific Levels of Functioning Scale with higher scores indicating better social attainment; mPFC-to-PPC Metric, Inter-network connectivity of medial prefrontal cortex and posterior cingulate cortex-anterior precuneus with higher scores indicating more connectivity between networks
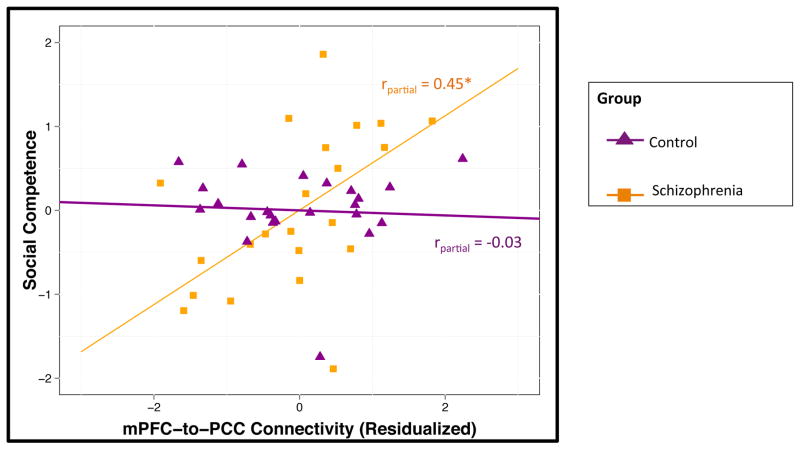
The overall model evaluating associations between connectivity and social competence was significant (F7,42 = 14.07, P < .001). There were significant main effects of the mPFC-to-PPC metric (P < .001), group status (P < .001), and global neurocognition (P < .05) (see Table 4). There was a significant interaction between group and DMN connectivity (P < .01). A plot of the group-by-DMN connectivity interaction is presented in Figure 2. The other a priori covariates (i.e., age, gender, and parental SES) were not significantly associated with social competence (all P > .10). Follow-up within group analysis indicated that DMN connectivity was positively correlated with social competence in individuals with schizophrenia (partial r = 0.45, P < .05) but not correlated in controls (partial r = −0.03, P > .10). See Supplemental Table 2 for zero-order correlations.
Fig. 2.
Functional connectivity is associated with social competence in schizophrenia
*p < 0.05
Social Competence, Scores on the Social Skills Performance Assessment with higher scores indicating better social competence; mPFC-to-PPC Metric, Inter-network connectivity of medial prefrontal cortex and posterior cingulate cortex-anterior precuneus with higher scores indicating more connectivity between networks
Mediation Analyses
Social Attainment
Pearson correlations indicated that social perception and mentalizing were not significantly correlated with social attainment (all P > .10). Since neither of our possible mediators were significantly correlated with both social attainment and DMN connectivity, we did not conduct a mediation analysis.
Social Competence
Pearson correlations indicated that mentalizing was significantly correlated with social competence (r = 0.45, P < .05) in individuals with schizophrenia, but social perception was not significantly correlated with social competence (P > .10). Mentalizing was not significantly correlated with the mPFC-to-PCC metric in individuals with schizophrenia (r = 0.09, P > .10). Since neither of our possible mediators were significantly correlated with both social competence and DMN connectivity, we did not run a mediation analysis.
Within-Group Specificity Analysis
Follow-up analyses indicated specificity for the associations between mPFC-to-PPC inter-network connectivity with social attainment and social competence. In particular, this metric was not correlated with the UPSA-B in individuals with schizophrenia (r = 0.02, P > .10). Additionally, the correlation between social attainment and the mPFC-to-PPC metric was significantly stronger than the correlation between UPSA-B performance and the mPFC-to-PPC metric (Meng’s z = 2.06, P = .04). The correlation between social competence and the mPFC-to-PPC metric was stronger than the correlation between UPSA-B performance and the mPFC-to-PPC metric at trend level (Meng’s z = 1.66, P = .09).
Discussion
In the current study, we were interested in the association between DMN connectivity and social functioning in controls and individuals with schizophrenia and whether this association was mediated by social cognition. Specifically, we evaluated whether connectivity between DMN components differed between individuals with schizophrenia and controls, and whether specific DMN connectivity metrics were associated with two measures of social functioning (i.e., social attainment or social competence). We controlled for global neurocognition, age, gender, and parental SES. Our results suggested that the groups did not differ with respect to average DMN connectivity magnitude. However, connectivity between the mPFC and PPC hubs was differentially related to social attainment and social competence in individuals with schizophrenia and controls as indicated by a significant group-by-connectivity interaction. Follow-up tests revealed that DMN inter-network connectivity was positively associated with measures of social attainment and social competence among individuals with schizophrenia but was not associated with measures of social attainment or social competence in controls. Given non-significant associations between connectivity metrics with social perception or mentalizing, we did not conduct subsequent mediation analyses.
These findings build on our prior research from this dataset that examined associations between social cognition and social functioning (Abram et al., 2014; Karpouzian, Alden, Reilly, & Smith, 2016; Smith et al., 2014; Smith et al., 2012), and associations between neural activation during a mentalizing task and social functioning (Smith et al., 2015). In particular, the current findings indicate that resting state DMN connectivity is associated social functioning in individuals with schizophrenia, and that social cognition does not mediate this particular association. Additionally, these findings add to the emerging literature examining the behavioral correlates of DMN connectivity (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010; Brunet et al., 2003; Das, Lagopoulos, Coulston, Henderson, & Malhi, 2012; Dodell-Feder et al., 2014; Laird et al., 2009; Mitchell, Neil Macrae, & Banaji, 2005; Spreng & Grady, 2010; Walter et al., 2009; Welborn & Lieberman, 2015). Furthermore, to our knowledge, our study is the first to examine the associations between DMN inter-network connectivity and social attainment and social competence among individuals with schizophrenia.
Connectivity Predictor of Social Attainment and Social Competence
We observed that stronger temporal synchrony between the mPFC and PPC was associated with better social attainment and social competence in individuals with schizophrenia, but this connectivity was not associated with social functioning in controls. Moreover, these findings remained significant after controlling for age, gender, global neurocognition, and parental SES. Therefore, these neural-behavior associations were not simply due to neurocognitive impairment or demographic characteristics, and neural data may provide additional information on social attainment and social competence beyond what is captured with background characteristics and neuropsychological tests (MacDonald, 2013; Sprong, Schothorst, Vos, Hox, & van Engeland, 2007).
Our findings are consistent with a recent study that found stronger DMN subsystem connectivity was related to better social functioning in first-degree relatives of individuals with schizophrenia (Dodell-Feder et al., 2014). However, the null correlations between our measure of DMN connectivity and the two measures of social cognition were not consistent with prior studies showing stronger connectivity between the mPFC and PPC was related to better social cognition (Andrews-Hanna et al., 2010; Mitchell et al., 2005; Spreng & Grady, 2010; Welborn & Lieberman, 2015). Our inability to identify a mechanism underlying the association between DMN connectivity and social functioning is not unexpected given that current understanding of mechanisms underlying the association between neural connectivity and functioning is limited.
One possible explanation for the null correlation findings between DMN connectivity and social cognition is that there may be an indirect association between DMN connectivity and social functioning through social cognitive functions other than mentalizing and social perception. For example, stronger connectivity between the mPFC and PPC may facilitate enhanced self-reflection (Andrews-Hanna et al., 2010), and self-reflection has been linked with social functioning (Brune, Dimaggiob, & Lysaker, 2011). Therefore, self-reflection may mediate the association between DMN connectivity and social functioning in schizophrenia. Cognitive insight is another possible mediator for the association between DMN connectivity and social functioning in schizophrenia. Research suggests that cognitive insight may be positively associated with DMN connectivity in individuals with schizophrenia (Liemburg et al., 2012), and a treatment study demonstrated that improvements in cognitive insight through metacognitive training resulted in increased social functioning in individuals in the early stages of schizophrenia (Ussorio et al., 2016). Alternatively, there may be no underlying mechanism for the association between DMN connectivity and social functioning, because DMN connectivity may be a direct neural signature for social functioning.
Connectivity Alterations between Groups
The lack of a difference in connectivity between the main DMN hubs in individuals with schizophrenia compared to controls is consistent with work by Chang et al. (Chang et al., 2014), but differs from one study that reported connectivity differences between individuals with schizophrenia and controls (Liemburg et al., 2012). There are various explanations for the inconsistent findings. All three of these studies (including the current study) had relatively small sample sizes (individuals with schizophrenia, n < 35), so power issues may contribute to the differences in findings. Additionally, Liemburg and colleagues suggest that cognitive insight may affect DMN connectivity such that patients with poorer insight exhibit decreased DMN connectivity compared to patients with higher insight (Liemburg et al., 2012). However, only one study assessed insight so future studies may benefit from evaluating insight in this context. Based on these results, future research using similar methods (e.g., pICA) with larger sample sizes is needed to verify DMN connectivity patterns in schizophrenia.
Finally, although individuals with schizophrenia and controls did not differ with respect to DMN connectivity, individuals with schizophrenia had distinctly poorer social attainment and social competence than controls. Given that DMN connectivity was only associated with social functioning for individuals with schizophrenia, it appears that similar communication between neural hubs may have differential outcomes across controls and individuals with schizophrenia. Future research could evaluate whether strengthening DMN connectivity beyond the level of connectivity observed in controls yields improvement in social attainment and social competence in individuals with schizophrenia.
Study Implications
Our results suggest that DMN connectivity was associated with social attainment and social competence in schizophrenia. Therefore, DMN connectivity could be assessed as a treatment target for interventions focused on improving social attainment and social competence in schizophrenia. Such interventions are emerging and are much needed given the poor quality of life experienced by individuals with schizophrenia (Bradshaw, 2000; Gibson et al., 2014; Granholm et al., 2005; Ntoutsia, Katsamagkos, & Economou, 2013). Based on our results, interventions that strengthen connectivity between the mPFC and PPC may be especially efficacious. To support the feasibility of testing such a hypothesis, cognitive remediation and certain types of pharmacotherapy have been shown to alter DMN connectivity in individuals with schizophrenia (Bor et al., 2011; Eack, Newhill, & Keshavan, 2016; Penades et al., 2014; Sambataro et al., 2010). Additionally, rTMS and EEG neurofeedback methods have altered DMN connectivity among healthy controls and among individuals with major depressive disorder (Kluetsch et al., 2014; Liston et al., 2014; Ros et al., 2013; van der Werf, Sanz-Arigita, Menning, & van den Heuvel, 2010). Thus, future research should examine whether these and other interventions targeting social attainment and social competence are strengthening DMN connectivity. Additionally, DMN connectivity metrics could be used as neuroimaging markers to monitor the success of treatments that target social functioning. For example, the observation of early changes in DMN resting-state connectivity could predict the effectiveness of cognitive remediation or social skills training targeting schizophrenia (Subramaniam & Vinogradov, 2013).
Limitations
The current study had several limitations. First, cross-sectional studies cannot infer causality. Thus, longitudinal research is needed to determine whether DMN connectivity is a stable neuroimaging marker of social attainment and social competence. Second the sample size was relatively small. Thus, replication and further exploration with a larger sample is needed. Third, our schizophrenia sample consisted of chronically ill individuals. Therefore, future research on the association between DMN connectivity and social attainment and social competence among first episode patients could help address the generalizability of the findings. Lastly, we did not evaluate occupational functioning as an outcome, and future studies could examine the potential association between DMN connectivity and occupational functioning.
Conclusions
In conclusion, we observed that greater connectivity between structures of the DMN was associated with better social attainment and social competence in individuals with schizophrenia, despite the lack of group differences in average DMN connectivity. Additionally, we found no evidence to support social cognition as a mediator for the association between DMN connectivity and social functioning in individuals with schizophrenia. Our findings support the general hypothesis that DMN connectivity could potentially be a novel treatment target and a neuroimaging marker for monitoring treatments aimed to enhance social attainment and social competence in schizophrenia.
Supplementary Material
General Scientific Summary.
This study suggests that individuals with schizophrenia and healthy controls do not differ in default mode network (DMN) connectivity. However, default mode network connectivity is differentially associated with social functioning in individuals with schizophrenia and healthy controls. Social cognition does not underlie the relationship between DMN connectivity and social functioning in individuals with schizophrenia.
Acknowledgments
Funding Details: National Institute of Mental Health, Grant Number: R01 MH056584, Recipient: John G. Csernansky, M.D.
Footnotes
One of our prior publications (Abram et al., 2016) uses the same sample that is presented in the current manuscript.
References
- Abram SV, Karpouzian TM, Reilly JL, Derntl B, Habel U, Smith MJ. Accurate perception of negative emotions predicts functional capacity in schizophrenia. Psychiatry Research. 2014;216(1):6–11. doi: 10.1016/j.psychres.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Abram SV, Wisner KM, Fox JM, Barch DM, Wang L, Csernansky JG, … Smith MJ. Fronto-temporal connectivity predicts cognitive empathy deficits and experiential negative symptoms in schizophrenia. Human Brain Mapping. 2016 doi: 10.1002/hbm.23439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abram SV, Wisner KM, Grazioplene RG, Krueger RF, MacDonald AW, III, DeYoung CG. Functional coherence of insula networks Is associated with externalizing behavior. Journal of Abnormal Psychology. 2015 doi: 10.1037/abn000007810.1037/abn0000078.supp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solis A, Corripio I, de Castro-Manglano P, Duran-Sindreu S, Garcia-Garcia M, Proal E, … Castellanos FX. Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophrenia Research. 2012;139(1):13–18. doi: 10.1016/j.schres.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Solís A, Vives-Gilabert Y, Grasa E, Portella MJ, Rabella M, Sauras RB, … Pérez V. Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophrenia Research. 2015;161(2):261–268. doi: 10.1016/j.schres.2014.10.047. [DOI] [PubMed] [Google Scholar]
- Ambady N, Hallahan M, Rosenthal R. On judging and being judged accurately in zero-acquaintance situations. Journal of Personality and Social Psychology. 1995;69(3):518. doi: 10.1037/0022-3514.69.3.518. [DOI] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1983. [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biological Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Wang L, Csernansky JG, Gado MH, Barch DM. Abnormalities of thalamic activation and cognition in schizophrenia. American Journal of Psychiatry. 2014;163(3):463–469. doi: 10.1176/appi.ajp.163.3.463. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain’s default network and its adaptive role in internal mentation. The Neuroscientist. 2012;18(3):251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/J.Neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. Journal of Abnormal Psychology. 2004;113(4):556–568. doi: 10.1037/0021-843x.113.4.556. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barratt W. The Barratt Simplified Measure of Social Status (BSMSS): Measuring SES. Indiana State University, Department of Educational Leadership, Administration, and Foundations; Terre Haute, IN: 2005. [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(3):1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B-Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/Rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD, Sivan AB. Multilingual aphasia examination: Manual of instructions. AJA Association; 1994. [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, … Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophrenia Bulletin. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Brunelin J, d’Amato T, Costes N, Suaud-Chagny MF, Saoud M, Poulet E. How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatry Research-Neuroimaging. 2011;192(3):160–166. doi: 10.1016/J.Pscychresns.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Bradshaw W. Integrating cognitive-behavioral psychotherapy for persons with schizophrenia into a psychiatric rehabilitation program: Results of a three year trial. Community Mental Health Journal. 2000;36(5):491–500. doi: 10.1023/a:1001911730268. [DOI] [PubMed] [Google Scholar]
- Brune M, Dimaggiob G, Lysaker PH. Metacognition and social functioning in schizophrenia: Evidence, mechanisms of influence and treatment implications. Current Psychiatry Reviews. 2011;7(3):239–247. doi: 10.2174/157340011797183210. [DOI] [Google Scholar]
- Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Frangou S, Murray R. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophrenia Research. 1997;28(2):143–156. doi: 10.1016/S0920-9964(97)00114-X. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJP, Duyn J, Coppola R, … Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. The American Journal of Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, III, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophrenia Bulletin. 2011;37(3):640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XQJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JDE, Castanon AN, McCarthy JM, … Ongur D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36(10):2009–2017. doi: 10.1038/Npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Shen H, Wang L, Liu Z, Xin W, Hu D, Miao D. Altered default mode and fronto-parietal network subsystems in patients with schizophrenia and their unaffected siblings. Brain Research. 2014;1562:87–99. doi: 10.1016/j.brainres.2014.03.024. [DOI] [PubMed] [Google Scholar]
- Che X, Zhang Q, Zhao J, Wei D, Li B, Guo Y, … Liu Y. Synchronous activation within the default mode network correlates with perceived social support. Neuropsychologia. 2014;63:26–33. doi: 10.1016/j.neuropsychologia.2014.07.035. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJS, Barkhof F, Scheltens P, Stam CJ, … Rombouta SARB. Reduced resting-state brain activity in the ‘default network’ in normal aging. Cerebral Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: A functional MRI study. Schizophrenia Research. 2012;134(2–3):158–164. doi: 10.1016/j.schres.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson’s disease. European Neuropsychopharmacology. 2010;20(11):784–792. doi: 10.1016/j.euroneuro.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test, Research Edition. The Psychological Corporation; Cleveland, OH: 1983. [Google Scholar]
- Dodell-Feder D, DeLisi LE, Hooker CI. The relationship between default mode network connectivity and social functioning in individuals at familial high-risk for schizophrenia. Schizophrenia Research. 2014;156(1):87–95. doi: 10.1016/j.schres.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eack SM, Newhill CE, Keshavan MS. Cognitive enhancement therapy improves resting-state functional connectivity in early course schizophrenia. Journal of the Society for Social Work and Research. 2016;7(2) doi: 10.1086/686538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, … Di Salle F. Independent component analysis of fMRI group studies by self-organizing clustering. Neuroimage. 2005;25(1):193–205. doi: 10.1016/j.neuroimage.2004.10.042. [DOI] [PubMed] [Google Scholar]
- Fett AKJ, Viechtbauer W, Dominguez M-d-G, Penn DL, van Os J, Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: A meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering statistics using SPSS. Sage publications; 2009. [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, … Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proceedings of the National Academy of Sciences. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping. 1994;2(1–2):56–78. doi: 10.1002/hbm.460020107. [DOI] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome. Clinical Neuroscience. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Gibson CM, Penn DL, Smedley KL, Leserman J, Elliott T, Pedersen CA. A pilot six-week randomized controlled trial of oxytocin on social cognition and social skills in schizophrenia. Schizophrenia Research. 2014;156(2–3):261–265. doi: 10.1016/j.schres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- Granholm E, McQuaid JR, McClure FS, Auslander LA, Perivoliotis D, Pedrelli P, … Jeste DV. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. The American Journal of Psychiatry. 2005;162(3):520–529. doi: 10.1176/appi.ajp.162.3.520. [DOI] [PubMed] [Google Scholar]
- Green MF, Horan WP, Lee J. Social cognition in schizophrenia. Nature Reviews Neuroscience. 2015;16:620–631. doi: 10.1038/nrn4005. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Rolinski M, Szewczyk-Krolikowski K, Menke RA, Filippini N, Zamboni G, … Mackay CE. Challenges in the reproducibility of clinical studies with resting state fMRI: An example in early Parkinson’s disease. Neuroimage. 2016;124:704–713. doi: 10.1016/j.neuroimage.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner H, Nowotny B, Loffler W, an der Heiden W. When and how does schizophrenia produce social deficits? European Archives of Psychiatry and Clinical Neuroscience. 1995;246(1):17–28. doi: 10.1007/BF02191811. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Raykov T, Twamley EW, Vella L, Heaton RK, Patterson TL. Validating the measurement of real-world functional outcomes: Phase I results of the VALERO study. American Journal of Psychiatry. 2011;168:1195–1201. doi: 10.1176/appi.ajp.2011.10121723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: Usefulness in clinical treatment studies. Schizophrenia Bulletin. 2007;33(5):1138–1148. doi: 10.1093/schbul/sbm040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK. Computerized Wisconsin Card Sort Task Version 4 Research Edition. Psychological Assessment Resources; Odessa, FL: 2003. [Google Scholar]
- Hooker CI, Bruce L, Lincoln SH, Fisher M, Vinogradov S. Theory of mind skills are related to gray matter volume in the ventromedial prefrontal cortex in schizophrenia. Biological Psychiatry. 2011;70(12):1169–1178. doi: 10.1016/j.biopsych.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: An initial efficacy study of stabilized outpatients. Schizophrenia Research. 2009;107(1):47–54. doi: 10.1016/j.schres.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Kern RS, Tripp C, Hellemann G, Wynn JK, Bell M, … Green MF. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. Journal of Psychiatric Research. 2011;45(8):1113–1122. doi: 10.1016/j.jpsychires.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/J.Neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M, Kosaka H, Saito DN, Ishitobi M, Morita T, Inohara K, … Tomoda A. Default mode network in young male adults with autism spectrum disorder: Relationship with autism spectrum traits. Molecular Autism. 2014;5(1):1. doi: 10.1186/2040-2392-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin M, Kaplan S, Gould F, Pinkham AE, Penn DL, Harvey PD. Social cognition, social competence, negative symptoms and social outcomes: Inter-relationships in people with schizophrenia. Journal of Psychiatric Research. 2015;68:254–260. doi: 10.1016/j.jpsychires.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpouzian TM, Alden EC, Reilly JL, Smith MJ. High functioning individuals with schizophrenia have preserved social perception but not mentalizing abilities. Schizophrenia Research. 2016;171(1):137–139. doi: 10.1016/j.schres.2016.01.029. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Jr, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, … Hoptman MJ. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. Journal of Neuroscience Methods. 2010;189(2):233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch RC, Ros T, Theberge J, Frewen PA, Calhoun VD, Schmahl C, … Lanius RA. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatrica Scandinavia. 2014;130(2):123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical–subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz MM, Mueser KT. A meta-analysis of controlled research on social skills training for schizophrenia. Journal of Consulting and Clinical Psychology. 2008;76(3):491–504. doi: 10.1037/0022-006x.76.3.491. [DOI] [PubMed] [Google Scholar]
- Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophrenia Bulletin. 2012;38(5):1092–1104. doi: 10.1093/schbul/sbr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. The Journal of Neuroscience. 2009;29(46):14496–14505. doi: 10.1523/jneurosci.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, … Fox PT. Behavioral interpretations of intrinsic connectivity networks. Journal of Cognitive Neuroscience. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifker FR, Patterson TL, Heaton RK, Harvey PD. Validating measures of Real-World Outcome: The results of the VALERO expert survey and RAND panel. Schizophrenia Bulletin. 2011;37(2):334–343. doi: 10.1093/schbul/sbp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Deng W, He Z, Wang Q, Huang C, Jiang L, … Li T. A splitting brain: Imbalanced neural networks in schizophrenia. Psychiatry Research: Neuroimaging. 2015;232(2):145–153. doi: 10.1016/j.pscychresns.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. NeuroReport: For Rapid Communication of Neuroscience Research. 2006;17(2):209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liberman RP, Mueser KT, Wallace CJ. Social skills training for schizophrenic individuals at risk for relapse. The American Journal of Psychiatry. 1986;143(4):523–526. doi: 10.1176/ajp.143.4.523. [DOI] [PubMed] [Google Scholar]
- Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, Aleman A. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS ONE. 2012;7(8):e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, … Dubin MJ. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biological Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EYH, … Liu Z. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophrenia Bulletin. 2012;38(2):285–294. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AW., III . What kind of a thing is schizophrenia? Specific causation and general failure modes. In: Silverstein SM, Moghaddam B, Wykes T, editors. Strungmann forum reports. Cambridge, MA: MIT Press; 2013. pp. 25–47.pp. xipp. 390 [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: New insights from functional neuroimaging of the human brain. Trends in Neurosciences. 1999;22(7):310–316. doi: 10.1016/S0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Mars RB, Neubert F-X, Noonan MP, Sallet J, Toni I, Rushworth MFS. On the relationship between the ‘default mode network’ and the ‘social brain’. Vol. 6. Switzerland: Frontiers Research Foundation; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S. Awareness of Social Inference Test: Manual. Thames Valley Test Company; 2002. [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping. 1998;6(3):160–188. doi: 10.1002/(Sici)1097-0193(1998)6:3<160::Aid-Hbm5>3.0.Co;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, … Nenadic I. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophrenia Research. 2012;138(2–3):143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–1315. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Neil Macrae C, Banaji MR. Forming impressions of people versus inanimate objects: Social-cognitive processing in the medial prefrontal cortex. Neuroimage. 2005;26(1):251–257. doi: 10.1016/j.neuroimage.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Moodie CA, Wisner KM, MacDonald AW., 3rd Characteristics of canonical intrinsic connectivity networks across tasks and monozygotic twin pairs. Human Brain Mapping. 2014;35(11):5532–5549. doi: 10.1002/hbm.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscatell KA, Morelli SA, Falk EB, Way BM, Pfeifer JH, Galinsky AD, … Eisenberger NI. Social status modulates neural activity in the mentalizing network. Neuroimage. 2012;60(3):1771–1777. doi: 10.1016/j.neuroimage.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntoutsia P, Katsamagkos A, Economou M. The efficacy of social skills training for individuals with schizophrenia. Psychology: The Journal of the Hellenic Psychological Society. 2013;20(1):34–53. [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophrenia Research. 2004;72(1):29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, Cooper JC, Gabrieli JD, Kihsltrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. 2005;28(4):797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: Neural systems supporting the cognitive down-and up-regulation of negative emotion. Neuroimage. 2004;23(2):483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Research: Neuroimaging. 2010;183(1):59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophrenia Research. 2001;48(2–3):351–360. doi: 10.1016/s0920-9964(00)00109-2. [DOI] [PubMed] [Google Scholar]
- Pelletier-Baldelli A, Bernard JA, Mittal VA. Intrinsic functional connectivity in salience and default mode networks and aberrant social processes in youth at ultra-high risk for psychosis. PLoS ONE. 2015;10(8):e0134936. doi: 10.1371/journal.pone.0134936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penades R, Pujol N, Catalan R, Massana G, Rametti G, Garcia-Rizo C, … Junque C. ‘Brain effects of cognitive remediation therapy in schizophrenia: A structural and functional neuroimaging study’: Erratum. Biological Psychiatry. 2014;75(5):425. doi: 10.1016/j.biopsych.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Penn DL, Roberts DL, Combs D, Sterne A. The development of the social cognition and interaction training program for schizophrenia spectrum disorders. Psychiatric Services. 2007;58(4):449–451. doi: 10.1176/appi.ps.58.4.449. [DOI] [PubMed] [Google Scholar]
- Poppe AB, Wisner K, Atluri G, Lim KO, Kumar V, MacDonald AW., III Toward a neurometric foundation for probabilistic independent component analysis of fMRI data. Cognitive, Affective & Behavioral Neuroscience. 2013;13(3):641–659. doi: 10.3758/s13415-013-0180-8. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56(9):613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Rastogi-Cruz D, Csernansky J. Adult Psychiatry. Mosby, Inc; St. Louis: 1997. Clinical rating scales; pp. 45–52. [Google Scholar]
- Ray KL, McKay DR, Fox PM, Riedel MC, Uecker AM, Beckmann CF, … Laird AR. ICA model order selection of task co-activation networks. Frontiers in Neuroscience. 2013;7:237. doi: 10.3389/fnins.2013.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neurpsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Ros T, Theberge J, Frewen PA, Kluetsch R, Densmore M, Calhoun VD, Lanius RA. Mind over chatter: Plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Neuroimage. 2013;65:324–335. doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal R, Hall JA, DiMatteo MR, Rogers PL, Archer D. Sensitivity to Nonverbal Communication: The PONS Test. Johns Hopkins University Press; Baltimore, MD: 1979. [Google Scholar]
- Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, … Bertolino A. Treatment with olanzapine is associated with modulation of the default mode network in patients with schizophrenia. Neuropsychopharmacology. 2010;35(4):904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Roalf DR, Ruparel K, Erus G, Vandekar S, … Gur RC. Linked sex differences in cognition and functional connectivity in youth. Cerebral Cortex. 2014 doi: 10.1093/cercor/bhu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L, Hoffstaedter F, Müller V, Cieslik E, Goya-Maldonado R, Trost S, … Sommer I. Transdiagnostic commonalities and differences in resting state functional connectivity of the default mode network in schizophrenia and major depression. NeuroImage: Clinical. 2016;10:326–335. doi: 10.1016/j.nicl.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, Struening EL. SLOF: A behavioral rating scale for assessing the mentally ill. Social Work Research & Abstracts. 1983;19(3):9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- Schreiner MJ, Karlsgodt KH, Uddin LQ, Chow C, Congdon E, Jalbrzikowski M, Bearden CE. Default mode network connectivity and reciprocal social behavior in 22q11. 2 deletion syndrome. Social Cognitive and Affective Neuroscience. 2013;9(9):1261–1267. doi: 10.1093/scan/nst114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Wang X, Yi J, Zhu X, Zhang X, Yang J, Yao S. Default mode network alterations during implicit emotional faces processing in first-episode, treatment-naive major depression patients. Frontiers in Psychology. 2015;6:1198. doi: 10.3389/fpsyg.2015.01198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Cobia DJ, Karpouzian TM, Fox JM, Reilly JL, Breiter HC. Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophrenia Bulletin. 2014;40(4):824–834. doi: 10.1093/schbul/sbt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophrenia Research. 2012;137(1–3):196–202. doi: 10.1016/j.schres.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Schroeder MP, Abram SV, Goldman MB, Parrish TB, Wang X, … Breiter HC. Alterations in brain activation during cognitive empathy are related to social functioning in schizophrenia. Schizophrenia Bulletin. 2015;41(1):211–222. doi: 10.1093/schbul/sbu023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of Cognitive Neuroscience. 2010;22(6):1112–1123. doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Sprong M, Schothorst P, Vos E, Hox J, van Engeland H. Theory of mind in schizophrenia. The British Journal of Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry. 2006;59(10):929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Vinogradov S. Cognitive training for psychiatric disorders. Neuropsychopharmacology. 2013;38(1):242–243. doi: 10.1038/npp.2012.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Wang J, Yan C, He Y. Hemisphere- and gender-related differences in small-world brain networks: A resting-state functional MRI study. Neuroimage. 2011;54(1):191–202. doi: 10.1016/j.neuroimage.2010.07.066. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Lacoboni M, Lange C, Keenan JP. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Sciences. 2007;11(4):153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ussorio D, Giusti L, Wittekind CE, Bianchini V, Malavolta M, Pollice R, … Roncone R. Metacognitive training for young subjects (MCT young version) in the early stages of psychosis: Is the duration of untreated psychosis a limiting factor? Psychology and Psychotherapy: Theory, Research and Practice. 2016;89(1):50–65. doi: 10.1111/papt.12059. [DOI] [PubMed] [Google Scholar]
- van der Werf YD, Sanz-Arigita EJ, Menning S, van den Heuvel OA. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neuroscience. 2010;11(1):1. doi: 10.1186/1471-2202-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Ered A, Gretchen-Doorly D, Subotnik KL, Horan WP, Hellemann GS, Nuechterlein KH. Theory of mind in the early course of schizophrenia: Stability, symptom and neurocognitive correlates, and relationship with functioning. Psychological Medicine. 2015;45(10):2031–2043. doi: 10.1017/s0033291714003171. [DOI] [PubMed] [Google Scholar]
- Walter H, Ciaramidaro A, Adenzato M, Vasic N, Ardito RB, Erk S, Bara BG. Dysfunction of the social brain in schizophrenia is modulated by intention type: An fMRI study. Social Cognitive and Affective Neuroscience. 2009;4(2):166–176. doi: 10.1093/scan/nsn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zamar R, Marazzi A, Yohai V, Salibian-Barrera M, Maronna R, … Konis K. Robust: insightful Robust library. R package version 0.3-4. 2008 http://CRAN.R-project.org/package=robust.
- Washington SD, VanMeter JW. Anterior-posterior connectivity within the default mode network increases during maturation. International Journal of Medical and Biological Frontiers. 2015;21(2):207. [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) The Psychological Corporation; San Antonio, TX: 1997a. [Google Scholar]
- Wechsler D. Wechsler memory scale (WMS-III) The Psychological Corporation; San Antonio, TX: 1997b. [Google Scholar]
- Welborn BL, Lieberman MD. Person-specific theory of mind in medial pFC. Journal of Cognitive Neuroscience. 2015;27(1):1–12. doi: 10.1162/jocn_a_00700. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, … Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Atluri G, Lim KO, MacDonald AW., III Neurometrics of intrinsic connectivity networks at rest using fMRI: Retest reliability and cross-validation using a meta-level method. Neuroimage. 2013;76:236–251. doi: 10.1016/j.neuroimage.2013.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW., III An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. The American Journal of Drug and Alcohol Abuse. 2013;39(6):403–413. doi: 10.3109/00952990.2013.848211. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, … Vaidya CJ. Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. NeuroImage: Clinical. 2015;9:223–232. doi: 10.1016/j.nicl.2015.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang TZ, Tian LX, Liu Y, Liu ZN, … Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience Letters. 2007;417(3):297–302. doi: 10.1016/J.Neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.